Abstract
Background and Objectives
Physical activity has been associated with a decreased risk for dementia, but the mechanisms underlying this association remain to be determined. Our objective was to assess whether cardiovascular risk factors mediate the association between physical activity and brain integrity markers in older adults.
Methods
At baseline, participants from the Age-Well study completed a physical activity questionnaire and underwent cardiovascular risk factors collection (systolic blood pressure, body mass index [BMI], current smoker status, and high-density lipoprotein cholesterol, total cholesterol, and insulin levels) and multimodal neuroimaging (structural MRI, diffusion MRI, FDG-PET, and florbetapir PET). Multiple regressions were conducted to assess the association among physical activity, cardiovascular risk factors, and neuroimaging. Mediation analyses were performed to test whether cardiovascular risk factors mediated the associations between physical activity and neuroimaging.
Results
A total of 134 cognitively unimpaired older adults (≥65 years) were included. Higher physical activity was associated with higher gray matter (GM) volume (β = 0.174, p = 0.030) and cerebral glucose metabolism (β = 0.247, p = 0.019) but not with amyloid deposition or white matter integrity. Higher physical activity was associated with lower insulin level and BMI but not with the other cardiovascular risk factors. Lower insulin level and BMI were related to higher GM volume but not to cerebral glucose metabolism. When controlling for insulin level and BMI, the association between physical activity and cerebral glucose metabolism remained unchanged, while the association with GM volume was lost. When insulin level and BMI were entered in the same model, only BMI remained a significant predictor of GM volume. Mediation analyses confirmed that insulin level and BMI mediated the association between physical activity and GM volume. Analyses were replicated within Alzheimer disease–sensitive regions and results remained overall similar.
Discussion
The association between physical activity and GM volume is mediated by changes in insulin level and BMI. In contrast, the association with cerebral glucose metabolism seems to be independent from cardiovascular risk factors. Older adults engaging in physical activity experience cardiovascular benefits through the maintenance of a lower BMI and insulin level, resulting in greater structural brain integrity. This study has implications for understanding how physical activity affects brain health and may help in developing strategies to prevent or delay age-related decline.
Trial Registration Information
EudraCT: 2016-002,441-36; IDRCB: 2016-A01767-44; ClinicalTrials.gov Identifier: NCT02977819.
Physical activity is important in preventing pathologic aging and dementia, including Alzheimer disease (AD).1 Growing evidence indicates that older adults engaging in a higher amount of physical activity have greater brain integrity,2,3 including increased gray matter (GM) volume,4 cerebral glucose metabolism,5-7 and white matter (WM) microstructural integrity,8 along with reduced amyloid burden6,7,9 and WM hyperintensities (WMH).8 However, the relationship between physical activity and brain structure and function is still not fully understood as some inconsistencies remain10 and the mechanisms by which physical activity exerts its benefits on brain health remain to be determined. Animal studies suggest that physical activity promotes neurogenesis, cell survival, expression of neurotrophic factors, and synaptic plasticity,3 while human studies also propose indirect effects of physical activity via a reduction in cardiovascular risk factors.11,12 Physical activity has been proven to efficiently reduce cardiovascular risk factors,13,14 which are known to increase dementia risk and negatively affect brain structure and function.15 Nevertheless, the role of cardiovascular risk factors on the association between physical activity and brain integrity remains unknown. Most of the studies only controlled for cardiovascular risk factors and found no changes after such correction,16 but contradictory findings have also been reported.17
The scarcity of studies combining several neuroimaging modalities in the same sample and exploring the role of cardiovascular risk factors in the association between physical activity and neuroimaging biomarkers makes it difficult to understand how physical activity contributes to brain health. We investigated whether the association between physical activity and multimodal neuroimaging, including GM volume, cerebral glucose metabolism, amyloid burden, WMH load, and WM microstructure, is mediated by cardiovascular risk factors.
Methods
Participants
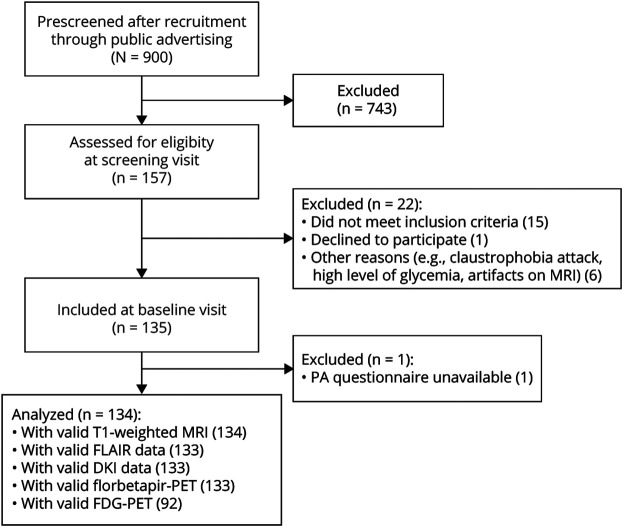
Participants from the baseline visit of the Age-Well randomized clinical trial (Medit-Ageing European Project)18 were included (Figure 1). Individuals were recruited from the general population from November 2016 until March 2018. They were all native French speakers, retired for at least 1 year, had at least 7 years of education, and performed within the normal range on standardized cognitive tests. The main exclusion criteria were antecedent of major neurologic or psychiatric disorders (including alcohol or drug abuse), presence of a chronic disease or acute unstable illness (including cardiovascular and metabolic), and current or recent treatments that may interfere with cognitive functioning. All participants included underwent structural MRI, 18F-florbetapir-PET, and 18F-fluorodeoxyglucose (FDG)–PET scans, along with a physical activity questionnaire, blood sampling, and a clinical examination including information on cardiovascular risk factors, within a 3-month period. Baseline data were collected from November 2016 until April 2018.
Figure 1. Flow Diagram of the Inclusion Process.
DKI = diffusion kurtosis imaging; FDG = 18F-fluorodeoxyglucose; FLAIR = fluid-attenuated inversion recovery; WMH = white matter hyperintensities.
Standard Protocol Approvals, Registrations, and Patient Consents
The Age-Well randomized clinical trial was approved by the local ethics committee (Comité de Protection des Personnes Nord-Ouest III, Caen, France; trial registration number: EudraCT: 2016-002,441-36; IDRCB: 2016-A01767-44; ClinicalTrials.gov Identifier: NCT02977819) and all participants gave written informed consent prior to the examinations.
Image Acquisition
All participants were scanned on the same MRI (Philips Achieva 3.0T scanner) and PET (Discovery RX VCT 64 PET-CT scanner; General Electric Healthcare) cameras at the Cyceron Center (Caen, France).
High-resolution T1-weighted structural images using a 3D fast-field echo sequence (sagittal; repetition time [TR] 7.1 ms, echo time [TE] 3.3 ms, field of view [FOV] 256 × 256 mm2, 180 slices, voxel size 1 × 1 × 1 mm3) and a 3D fluid-attenuated inversion recovery (FLAIR; sagittal; TR 4,800 ms, TE 272 ms, inversion time 1,650 ms; FOV 250 × 250 mm2, 180 slices, voxel size 0.98 × 0.98 × 1 mm3) were acquired for 133 participants. An echoplanar imaging/spin echo diffusion-weighted sequence (diffusion kurtosis imaging [DKI]) was then performed at multiple shells for 133 participants: 3b values (0, 1,000, 2,000 seconds/mm2) (axial; 30 directions, TR 6,100 ms, TE 101 ms, flip angle 90°, FOV 216 × 216 mm2, 48 slices, voxel size 2.7 × 2.7 × 2.7 mm3) and additional blips images with b = 0 seconds/mm2 (number of signal averages 9) were acquired in reverse phase encoding direction for susceptibility distortion.
Florbetapir-PET and FDG-PET scans were acquired in 2 separate sessions, with a resolution of 3.76 × 3.76 × 4.9 mm3 (FOV 157 mm). Forty-seven planes with a voxel size of 1.95 × 1.95 × 3.27 mm3 were obtained. Before the PET acquisition, a transmission scan was performed for attenuation correction. For florbetapir-PET, each participant (n = 133) underwent a 10-minute PET scan beginning 50 minutes after the IV injection of ∼4 MBq/Kg of florbetapir. For the FDG-PET scan, participants (n = 92) fasted for at least 6 hours before scanning. After a 30-minute resting period in a quiet and dark environment, ∼180 MBq of FDG was injected IV as a bolus and a 10-minute PET acquisition scan was acquired 50 minutes after injection.
Image Processing
T1-weighted MRIs were segmented using FLAIR images and normalized to the Montreal Neurologic Institute (MNI) space. GM normalized segments were modulated to correct for nonlinear warping effects using the Statistical Parametric Mapping (SPM12) software multiple channels segmentation procedure (fil.ion.ucl.ac.uk/spm/software/spm12).
Raw FLAIR images were coregistered onto their corresponding native space T1-weighted MRI and WMH were segmented by the lesion prediction algorithm (LPA)19 implemented in the Lesion Segmentation Toolbox version 2.0.15 (statistical-modelling.de/lst.html) for SPM12. A minimum extend threshold of 0.01 cm³ was set. Lesion probability maps were binarized by applying a threshold of 0.5 and lesion masks were thus generated. Lesion masks were then visually inspected and corrected for false-positives in corticospinal tracts if necessary, using a specific corticospinal tract mask for each participant.20
DKI images were corrected for susceptibility artifacts, eddy current distortions, and subject motion using Functional Magnetic Resonance Imaging of the Brain diffusion toolbox (FSL 5.0.9). DKI data were then processed using MATLAB R2012b (MathWorks) and diffusional kurtosis estimator software (DKE, version 2.6) to estimate the diffusional kurtosis tensor.21 Images were smoothed with a 3.375 × 3.375 × 3.375 mm full-width at half-maximum Gaussian filter. Mean kurtosis (MK) and fractional anisotropy (FA) maps, reflecting WM microstructural integrity,22 were then extracted from the DKE. These maps were coregistered to the T1-weighted MRI and normalized to the MNI template by applying the deformation parameters from the corresponding T1-weighted MRI.
PET data were coregistered onto their corresponding MRI, corrected for partial volume effects (PVE) using the Muller-Gartner method, and normalized using the deformation parameters defined from the T1-weighted procedure. Resulting images were quantitatively normalized using the cerebellar GM as the reference region.
Averaged global neuroimaging values were obtained by applying a binary mask of GM for preprocessed T1-weighted and PET images and a binary mask of WM for preprocessed DKI images. The cerebellum was excluded from both masks, and only voxels with a probability >60% of being GM and WM were included, to exclude non-GM or WM voxel and reduce the probability of overlap with other tissue classes.
For FLAIR data, the total volume of WMH was extracted, as fully described elsewhere.20
To investigate how the results could apply to AD, neuroimaging values were also extracted in AD-sensitive regions. More specifically, we extracted GM volume in the hippocampus (region of interest), glucose metabolism standardized uptake value ratio in the precuneus (from the automated anatomical atlas [AAL]) and in posterior cingulate and temporoparietal regions previously determined,23 as well as FA in the cingulum (from the JHU WM atlas).
Physical Activity
Physical activity over the past 12 months was assessed using a French version of the Modifiable Activity Questionnaire,24 adapted to be self-administered.25 This version of the questionnaire allows assessing the amount of physical activity, including both leisure activities and work activities. Because participants were retired, only leisure-related physical activity was considered in the current study. Participants had to report, for each activity they did at least 10 times over the past 12 months (e.g., walking, hiking, biking, gardening, jogging), how much time they spent doing that activity (i.e., how many months per year, time per month, and minutes each time). The total physical activity score corresponds to the average hours per week of leisure-time physical activity over the past 12 months. The average hours per week was calculated for each activity as ([number of months per year] × [number of times per month] × [minutes per time]/60 [min])/52 (weeks per year) and summed to obtain a total score of physical activity.
Cardiovascular Risk Factors
Cardiovascular risk factor measures included insulin level, cholesterol (total and high-density lipoprotein [HDL]), systolic blood pressure, body mass index (BMI), and smoking habits.
Fasting blood samples were obtained from all participants. The plasma concentration of insulin level (pmol/L) was performed by chemiluminescence assay on automated analyzer COBAS 6000 (Roche Diagnostics), using ready-made commercial reagent kits (insulin level; Roche). The quantitative determination of total cholesterol (mmol/L) and HDL cholesterol (mmol/L) concentrations in serum were carried out by an enzymatic staining test in Beckman Coulter Clinical Chemistry AU analyzers. Systolic blood pressure (SBP; mm Hg) was averaged over 3 consecutive assessments at 2 different times in a seated position at rest. The BMI was objectively obtained during the medical interview and was calculated as weight in kilograms divided by height in meters squared (kg/m2). Participants were questioned on their smoking habits and were classified as current smokers (yes) or not (no).
Statistical Analysis
We carried out multiple linear regressions in order to assess whether there was a specific association between (1) physical activity and each neuroimaging value separately, (2) physical activity and each cardiovascular risk factor separately, and (3) cardiovascular risk factors and neuroimaging values that were significantly associated with physical activity. Next, to better understand the specific implication of each cardiovascular risk factor for these effects, we replicated the same multiple regression by including cardiovascular risk factors of interest (i.e., found to be associated in the previous analyses) in the same model. All analyses were controlled for age, sex, and education. Analyses with insulin level, total and HDL cholesterol, and SBP were further controlled for glycemic, cholesterol, and blood pressure treatments, respectively.
Models with polynomial terms of different orders for physical activity were considered in order to assess which function best described the association between physical activity and neuroimaging global values (eMaterials, links.lww.com/WNL/B888).
Analyses were conducted using R software (R Core Team; 2019) and considered significant at a p < 0 .05.
We used causal mediation analyses to examine whether the cardiovascular risk factors mediated these associations.
We tested the mediation effect using mediation analysis and reported the average direct effects and average causal mediation effect estimated using nonparametric bootstrapping based on 5,000 bootstrap samples (p < 0 .05).
Data Availability
Data are available on request following a formal data sharing agreement and approval by the consortium and executive committee. The data sharing request form can be downloaded online.26
Results
Data of 134 cognitively unimpaired older adults (≥65 years) were analyzed.
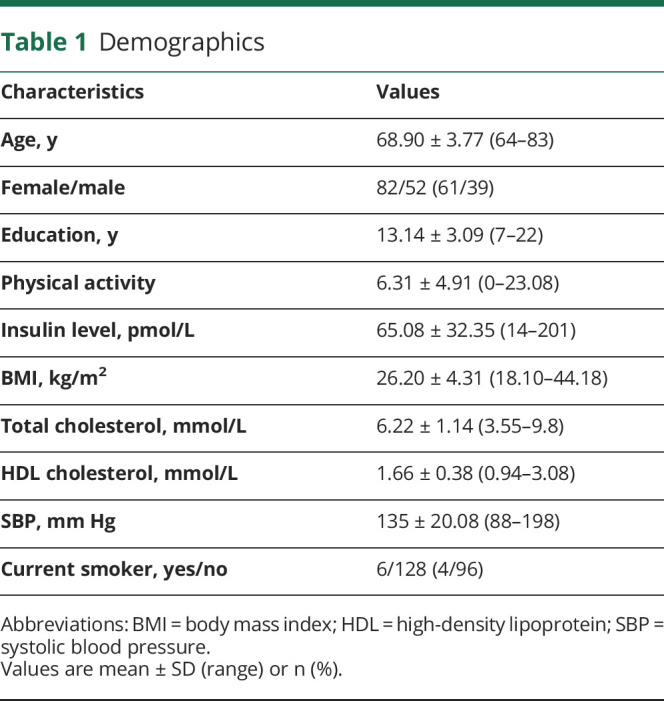
Participants' characteristics are detailed in Table 1. Because physical activity, insulin level, and BMI were not normally distributed, values were log-transformed. One participant had a physical activity score of 0, which cannot be log-transformed. As a result, to be able to include this participant in the analyses, a constant of 1 was added to all physical activity scores before transformation.
Table 1.
Demographics
Association Between Physical Activity and Global Neuroimaging Measures
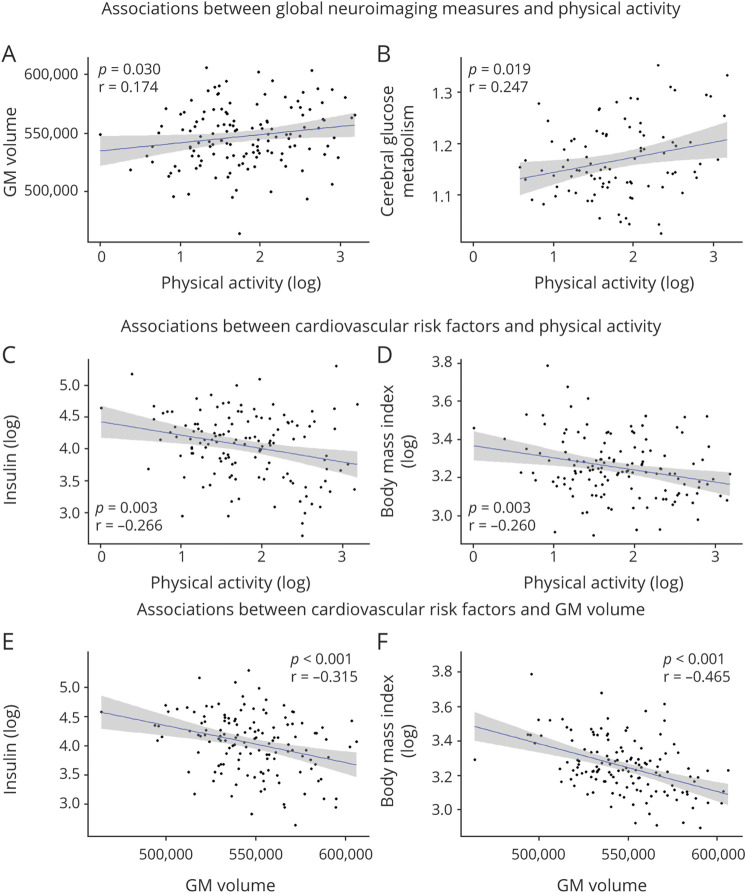
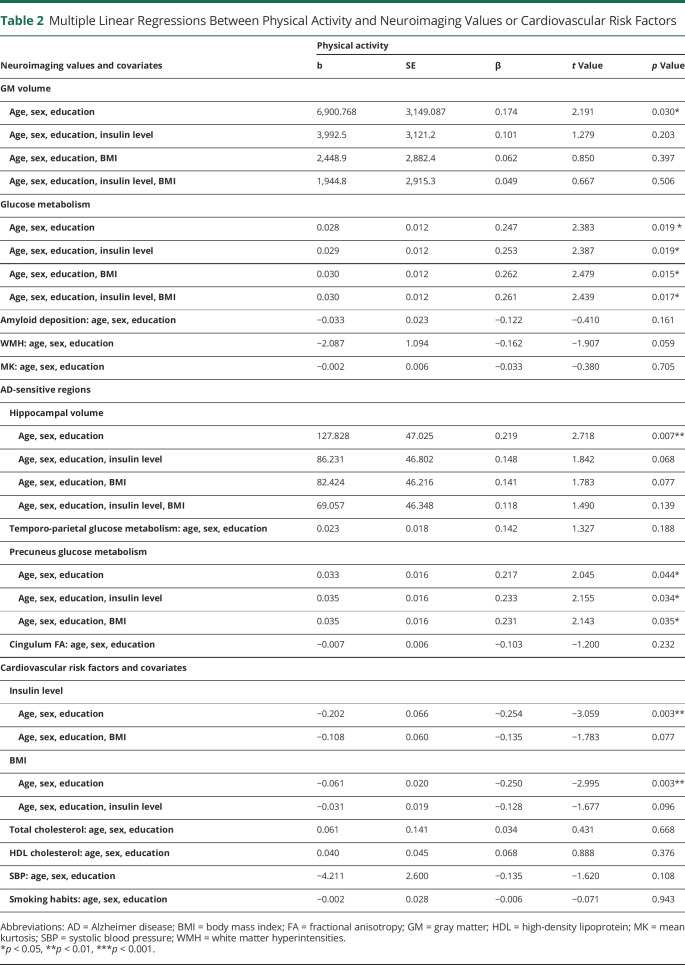
Higher physical activity was correlated to higher global GM volume (Figure 2A) and cerebral glucose metabolism (Figure 2B), but not amyloid burden, WMH volume, or WM microstructural integrity (MK; Table 2).
Figure 2. Associations Between Physical Activity, Neuroimaging Values, and Cardiovascular Risk Factors.
Physical activity is associated with global neuroimaging values of gray matter (GM) volume (A) and glucose metabolism (B), as well as insulin level (C) and body mass index (D). Global neuroimaging values of GM volume are associated with insulin level (G) and body mass index (H). Raw data (i.e., unadjusted) are plotted. Solid lines represent estimated regression lines and shaded areas represent 95% CIs. Statistical values were obtained using multiple linear regressions controlling for age, sex, and education. Physical activity, insulin level, and body mass index values are log-transformed.
Table 2.
Multiple Linear Regressions Between Physical Activity and Neuroimaging Values or Cardiovascular Risk Factors
The association between physical activity and GM volume was best described by a linear function. The association between glucose metabolism and physical activity also seemed better described by a linear association, even though some indices suggest that the quadratic model cannot be completely rejected (eTable 1, links.lww.com/WNL/B888).
Association Between Physical Activity and Cardiovascular Risk Factors
Higher physical activity was associated with lower insulin level (Figure 2C) and BMI (Figure 2D) but not with total and HDL cholesterol, SBP, or current smoker status (Table 2). Results remained similar when further controlling for treatments (eTable 2, links.lww.com/WNL/B888).
When insulin level and BMI were entered in the same model, they were no longer associated with physical activity, suggesting that the link of physical activity with insulin level and BMI is not independent one from another. Consistently, insulin level and BMI were highly correlated.
Association Between Cardiovascular Risk Factors and Global Neuroimaging Measures
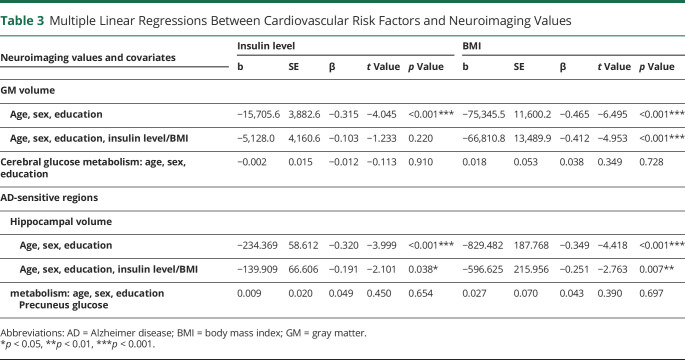
We then examined whether the cardiovascular risk factors (insulin level, BMI) and the global neuroimaging values (GM volume and cerebral glucose metabolism) found to be associated with physical activity were correlated to each other. Lower insulin level (Figure 2E) and BMI (Figure 2F) were both correlated with higher GM volume, but neither of them was associated with cerebral glucose metabolism (Table 3). When BMI and insulin level were included in the same model, the correlation of GM volume with insulin level was lost, while the association with BMI remained unchanged, suggesting that the association between insulin level and GM volume was driven by BMI. Results remained similar when further controlling for treatments (eTable 1, links.lww.com/WNL/B888).
Table 3.
Multiple Linear Regressions Between Cardiovascular Risk Factors and Neuroimaging Values
Influence of Cardiovascular Risk Factors on the Association Between Physical Activity and Neuroimaging
When the association between physical activity and GM volume was controlled for insulin level and BMI separately, or simultaneously, the association was lost. On the other hand, the association with cerebral glucose metabolism remained significant when controlling for insulin level, BMI or both.
Mediation Analyses
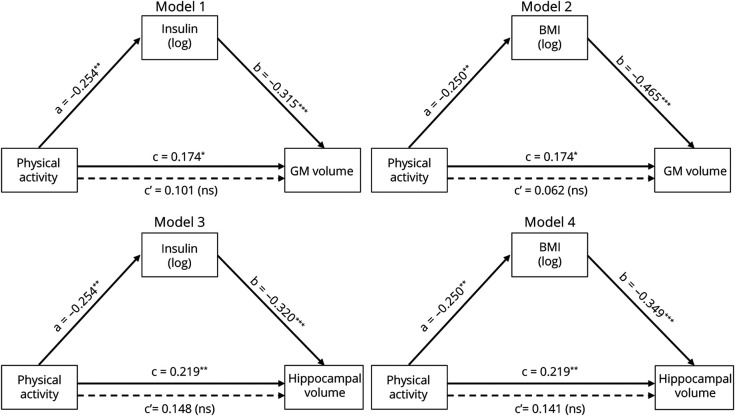
To directly assess whether the association between physical activity and global GM volume was mediated by insulin level and BMI, we performed mediation analyses. The relationship between physical activity and GM volume was fully mediated by insulin level (Figure 3, model 1) and BMI (Figure 3, model 2). The indirect effects of insulin level and BMI separately were significant (Table 4).
Figure 3. Causal Mediation Analyses of the Association Between Physical Activity and Global and Hippocampal Gray Matter Volume.
Direct effects in filled arrows (simple regressions between variables) are expressed as standardized regression coefficients and indirect effects in dotted arrows (multiple regressions in which the predictor and the mediator are both added in the model) as partial correlation coefficients. All regressions are adjusted for age, sex, and education. Physical activity, insulin level, and body mass index (BMI) values are log-transformed. *p < 0.05, **p < 0.01, ***p < 0.001. Abbreviation: GM = gray matter.
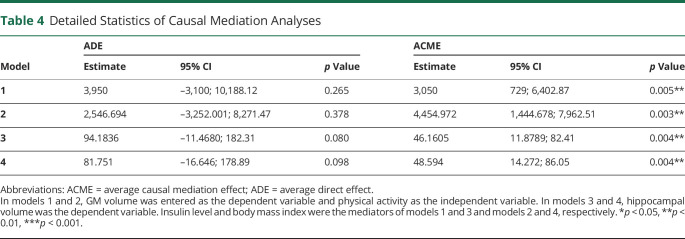
Table 4.
Detailed Statistics of Causal Mediation Analyses
AD-Sensitive Region of Interest
To further investigate whether the effect of physical activity, cardiovascular risk factors, and the mediations were present in regions specifically involved in AD, analyses were replicated on GM volume, glucose metabolism, and WM microstructural integrity in AD-sensitive regions of interest.
As depicted in Table 1, higher physical activity was correlated to higher hippocampal volume and glucose metabolism in the precuneus (eFigure 1, A and B, links.lww.com/WNL/B888), but not to global glucose metabolism, suggesting that the link found in the main analyses involves AD-sensitive regions only in part.
We then examined whether the cardiovascular risk factors (insulin level, BMI) and the neuroimaging values (hippocampal volume and glucose metabolism of the precuneus) found to be associated with physical activity were correlated to each other. Lower insulin level and BMI were correlated with higher hippocampal volume (Table 2 and eFigure 1, C and D, links.lww.com/WNL/B888). Of note, when adding insulin level and BMI into the model, BMI was the best predictor of hippocampal volume. On the other hand, neither insulin level nor BMI was associated with glucose metabolism of the precuneus.
When insulin level and BMI were added separately or simultaneously (Table 2) in the regression model between physical activity and hippocampal volume, the association was no longer significant (although close to the threshold of 0.05).
Mediation analyses confirmed that insulin level (Figure 2, model 1) and BMI (Figure 2, model 2) fully mediated the association between physical activity and hippocampal volume. The indirect effects of insulin level and BMI separately were significant (Table 3).
Complementary Analyses
Analyses were replicated on FDG-PET data not corrected for PVE and show similar results (eTable 3, eTable 4, and eFigure 2, links.lww.com/WNL/B888).
In addition, to further investigate whether other lifestyle factors drive the association between physical activity and brain integrity, analyses were replicated adjusting for Mediterranean diet adherence and lifetime cognitive activity. Results remained similar (eTables 5 and 6, links.lww.com/WNL/B888), suggesting that the associations previously highlighted are not driven by other lifestyle factors.
Discussion
Our multimodal neuroimaging study provides an integrated view of the differential association between physical activity and complementary measures of brain integrity. In cognitively unimpaired older adults, we found that physical activity was directly associated with cerebral glucose metabolism, whereas the association with global GM volume was mediated by cardiovascular risk factors, and more specifically insulin level and BMI. Similar results were found when focusing on AD-sensitive brain regions.
The finding that physical activity is associated with higher GM volume is in line with previous literature in cognitively unimpaired older adults using self-reported physical activity and related measures such as exercise and fitness.6,27-29 Importantly, this association was mediated by the effect of insulin level and BMI. These results tie well with prior studies showing that increased physical activity in older adults was accompanied by lower insulin level9 and BMI30 and other evidence showing that lower insulin level and BMI31 were linked to greater GM volume in older adults. One conceivable explanation is that physical activity leads to better cardiovascular health, which could in turn lead to a greater preservation of brain structure.32
Obesity and insulin level dysfunction may develop with aging33 and are risk factors for brain atrophy, cognitive impairment, and dementia, including AD.31,34 Obesity is the most common cause of insulin resistance33,35 and they are both sources of inflammation and oxidative stress36 that negatively affect brain health and increase AD risk.37 Insulin action in the brain includes neuroprotective, neurotrophic, and neuromodulatory functions and its perturbation may accelerate brain atrophy in AD.34 Insulin resistance is the main characteristic of type 2 diabetes, a disease associated not only with aging, obesity, and physical inactivity,38 but also with brain atrophy and increased dementia risk.39 Recently, active lifestyle has been found to protect against the deleterious effects of diabetes on dementia over 12 years, likely through its positive effects on reduced brain volume loss.40 Insulin level and BMI were strongly associated in our study, and when insulin level and BMI were included in the same model to predict GM volume, BMI remained the only predictor. Therefore, maintenance of lower BMI through physical activity could help prevent disturbed insulin metabolism observed in aging, thus promoting brain health.
To date, only one study specifically looked at and found insulin sensitivity to mediate the relation between physical activity and anterior cingulate cortex volume.41 Our study extends these results to a global measure of GM volume. Only a small number of studies accounted for the effect of BMI on the association between physical activity and GM volume,6,16,28 but they failed to find a dependent effect of BMI on this association. This contradictory finding might be due in part to shorter physical activity assessment periods (e.g., 7 days) considered in other research,16 which may have hampered an effect on BMI and, by extension, any possible mediation on the link between physical activity and GM volume. Others included a smaller28 or younger sample than the one included here.6 Our findings go beyond previous reports, providing evidence of indirect, cardiovascular risk factor–dependent association between physical activity and GM volume.
As a second key finding, physical activity was associated with greater cerebral glucose metabolism in a subsample of 92 participants, in agreement with FDG-PET neuroimaging modality,5-7 but see also reference 10. The relation between physical activity and cerebral glucose metabolism did not depend on insulin level or BMI. The benefits of physical activity on cerebral glucose metabolism may rather act through more direct neuronal effects, such as increased neurogenesis, cell survival, expression of neurotrophic factors, and synaptic plasticity.3 The increased nutrient and energy demands of these neural processes are met with increased expression of enzymes implicated in glucose use and metabolism.13 Alternatively, benefits of physical activity on cerebral glucose metabolism could be mediated by other factors not measured here. With regards to insulin level, the literature is controversial. Some animal studies highlighted the role of insulin in the regulation of brain glucose uptake and metabolism.42 However, evidence in humans is contradictory, with studies reporting an association of brain glucose uptake with insulin43 and insulin resistance,44 whereas others found no correlation.45 Our results align with prior observations showing that neuron glucose uptake, transport, and use can be influenced by, but would not depend on, insulin.34 Similarly, evidence on the link between BMI and cerebral glucose metabolism in aging individuals is weak and heterogeneous. Higher BMI has been found to be associated with both increased46 and reduced5 cerebral glucose metabolism, preventing clear conclusions. Only a few studies previously examined the association between physical activity and cerebral glucose metabolism in older participants.5-7 Among them, none controlled for insulin level, making it difficult to assess whether it might have influenced that relationship. On the other hand, in the studies controlling for BMI, the association between physical activity and cerebral glucose metabolism remained significant.16 Overall, it remains unclear how much insulin level and BMI are involved in the regulation of cerebral glucose metabolism. In the current study, we did not find these cardiovascular risk factors to be related to cerebral glucose metabolism or to mediate the link between physical activity and cerebral glucose metabolism.
Taken together, these 2 key findings are important to help our understanding of the mechanisms by which physical activity benefits brain health. As a plausible interpretation, some authors proposed that direct and indirect mechanisms are likely interconnected via the modulation of growth factor signaling.13 Accordingly, physical activity directly induces the release of neurotrophic factors, which are supposed to activate a cascade of brain responses (i.e., neurogenesis, synaptic plasticity) that independently protect the brain. Physical activity also helps reduce proinflammatory conditions (induced by risk factors such as obesity and related insulin disturbances), which in turn indirectly improves growth factor signaling13 and mitigates their deleterious effects on brain health.37 Nevertheless, whereas the results discussed were all statistically significant, they were of relatively modest magnitude (small effect sizes).
Our multimodal study suggests that self-reported physical activity is associated with GM volume and cerebral glucose metabolism but does not correlate with amyloid or markers of WM integrity (WMH and WM microstructure). Even if the effect size (β coefficient) of the link between physical activity and WMH was close to that of the link with GM volume, they did not reach the statistical threshold selected in this study. Previous literature has produced mixed results.8,10 Methodologic differences with our study,6-9 including instruments (fitness test, accelerometry, exercise interventions), time intervals (physical activity in the past 7 days), and mean age of participants (younger than our sample) could possibly explain the divergent findings. Moreover, whereas higher physical activity was associated with reduced insulin level and BMI, we found no associations with SBP, total and HDL cholesterol, or smoking status. Despite prior studies suggesting benefits of physical activity on SBP, total and HDL cholesterol,14 and smoking habits,47 evidence in older individuals is scarce48 and conflicting data have been also reported.14 Furthermore, only a minority of studies examined these cardiovascular risk factors as primary outcomes. Further research is warranted to disentangle the influence of physical activity on amyloid burden, WM integrity, and cardiovascular risk factors.
Results were also present when focusing on AD-sensitive brain regions. More specifically, physical activity was associated with higher hippocampal volume. This is in agreement with previous literature in cognitively unimpaired older adults using both self-reported and objective measures of physical activity.7,16 Physical activity was also associated with cerebral glucose metabolism in the precuneus, which is in line with previous studies.49 Our study further indicates that the association between physical activity and hippocampal volume is mediated by a reduction in insulin level and BMI, whereas it was not the case for glucose metabolism in the precuneus. This suggests that results previously discussed for global neuroimaging measures could be extended to AD-sensitive regions. However, physical activity was not associated with temporo-parietal cerebral glucose metabolism or with WM integrity of the cingulum (FA), suggesting that the results could not be generalized to all AD-related regions.
Our study has some limitations. First, we used a subjective (self-reported questionnaire) instead of an objective measure of physical activity. However, self-reported physical activity has been extensively used in the literature and questionnaires have an acknowledged clinical validity. The one that we used in this study evaluates physical activity over the past year, whereas objective measures often assess physical activity over a short time interval, which might not be enough to detect changes in the variables examined. Even though imprecision in the measure is likely to occur, validity and reliability of the questionnaire have been previously established.24 In addition, as participants are cognitively unimpaired, it seems unlikely that major recollection issues affected the questionnaire. Second, we used BMI as indicator of obesity, in line with most of the studies. However, controversy exists in the literature; BMI might not be a sensitive index at older ages as it could be protective.50 BMI is nonetheless considered a valuable measure that is widely used, allowing for better comparison with previous studies. Third, the cross-sectional nature of the design prevents us from inferring causal relationships regarding the association of physical activity with GM volume and cerebral glucose metabolism. Thus, we cannot exclude that older adults with better brain health stay more physically active. Although mediation analyses provide statistical support to theoretically driven hypotheses, they do not explain the biological mechanisms underlying these associations. Studies including longitudinal and interventional designs should facilitate answering these questions and further understanding of the factors explaining the effects of physical activity on brain health.
Accumulating evidence highlights the effects of midlife cardiovascular risk factors on brain health. However, we did not address this issue, because only current cardiovascular risk factors were available.51 The population of our study is relatively healthy, with no severe cardiovascular risk factors. This could prevent generalizability of the results and explain discrepancy with previous studies including individuals with more severe cardiovascular diseases. Finally, no correction was made for multiple comparisons.
Overall, our results suggest that the associations between physical activity and global GM including hippocampal volume are mediated by changes in insulin level and BMI. Thus, older adults practicing physical activity receive cardiovascular benefit through the maintenance of lower insulin level and BMI, which could in turn promote greater structural brain integrity. In contrast, the association with global and precuneus cerebral glucose metabolism seems to be independent of cardiovascular risk factors. This study has strong implications into understanding how physical activity affects brain health and may aid with development of strategies to prevent or delay age-related brain decline.
Acknowledgment
The authors thank Marine Faure, Jeanne Lepetit, Marie Saville, and the Cyceron MRI-PET staff members for help with recruitment and neuroimaging data acquisition; INSERM administrative, financial, and legal departments; INSERM Transfert (Delphine Smagghe) and Aurélia Cognet for administrative support; the sponsor (Pole de Recherche Clinique at INSERM, Dr. Hélène Espérou); the Euclid Team (Dr Eric Frison) for data management; and the participants of the study and their families.
Glossary
- AD
Alzheimer disease
- BMI
body mass index
- DKE
diffusional kurtosis estimator
- DKI
diffusion kurtosis imaging
- FA
fractional anisotropy
- FDG
fluorodeoxyglucose
- FLAIR
fluid-attenuated inversion recovery
- FOV
field of view
- GM
gray matter
- HDL
high-density lipoprotein
- MK
mean kurtosis
- MNI
Montreal Neurologic Institute
- PVE
partial volume effect
- SBP
systolic blood pressure
- TE
echo time
- TR
repetition time
- WM
white matter
- WMH
white matter hyperintensities
Appendix 1. Authors
Appendix 2. Coinvestigators

Footnotes
CME Course: NPub.org/cmelist
Editorial, page 825
Study Funding
The Age-Well randomized clinical trial is part of the Medit-Ageing project and is supported by the European Union's Horizon 2020 Research and Innovation Program (grant 667696), Region Normandie (Label d’Excellence), and Fondation d’Entreprise MMA des Entrepreneurs du Futur. Institut National de la Santé et de la Recherche Médicale (INSERM) is the sponsor. F.F. was funded by the European Union's Horizon 2020 Research and Innovation Program (grant 667,696) and the MMA IARD SA.
Disclosure
G. Chételat reports grants, personal fees, and nonfinancial support from INSERM, grants from European Union's Horizon 2020 research and innovation programme (grant 667,696), and Fondation d'Entreprise MMA des Entrepreneurs du Future during conduction of the study; personal fees from Fondation Entrepreneurs MMA; grants and personal fees from Fondation Alzheimer; and grants from Région Normandie (Label d’Excellence), Fondation Recherche Alzheimer, and Association France Alzheimer outside the submitted work. The other authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10(9):819-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen FT, Hopman RJ, Huang CJ, et al. The effect of exercise training on brain structure and function in older adults: a systematic review based on evidence from randomized control trials. J Clin Med. 2020;9(4):914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erickson KI, Gildengers AG, Butters MA. Physical activity and brain plasticity in late adulthood. Dialogues Clin Neurosci. 2013;15(1):99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Domingos C, Pêgo JM, Santos NC. Effects of physical activity on brain function and structure in older adults: a systematic review. Behav Brain Res. 2021;402:113061. [DOI] [PubMed] [Google Scholar]
- 5.Neth BJ, Graff-Radford J, Mielke MM, et al. . Relationship between risk factors and brain reserve in late middle age: implications for cognitive aging. Front Aging Neurosci. 2020;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthews DC, Davies M, Murray J, et al. . Physical activity, Mediterranean diet and biomarkers-assessed risk of Alzheimer’s: a multi-modality brain imaging study. Adv Mol Imaging. 2014;4(4):43-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okonkwo OC, Schultz SA, Oh JM, et al. Physical activity attenuates age-related biomarker alterations in preclinical AD. Neurology. 2014;83(19):1753-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sexton CE, Betts JF, Demnitz N, Dawes H, Ebmeier KP, Johansen-Berg H. A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. Neuroimage. 2016;131:81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown BM, Peiffer JJ, Taddei K, et al. Physical activity and amyloid-β plasma and brain levels: results from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Mol Psychiatry. 2013;18(8):875-881. [DOI] [PubMed] [Google Scholar]
- 10.Vemuri P, Lesnick TG, Przybelski SA, et al. Effect of lifestyle activities on Alzheimer disease biomarkers and cognition. Ann Neurol. 2012;72(5):730-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes JN, Corkery AT. Exercise improves vascular function, but does this translate to the brain?. Brain Plast. 2018;4(1):65-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walhovd KB, Storsve AB, Westlye LT, Drevon CA, Fjell AM. Blood markers of fatty acids and vitamin D, cardiovascular measures, body mass index, and physical activity relate to longitudinal cortical thinning in normal aging. Neurobiol Aging. 2014;35(5):1055-1064. [DOI] [PubMed] [Google Scholar]
- 13.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464-472. [DOI] [PubMed] [Google Scholar]
- 14.Strasser B. Physical activity in obesity and metabolic syndrome. Ann NY Acad Sci. 2013;1281(1):141-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox SR, Lyall DM, Ritchie SJ, et al. Associations between vascular risk factors and brain MRI indices in UK Biobank. Eur Heart J. 2019;40(28):2290-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamer M, Sharma N, Batty GD. Association of objectively measured physical activity with brain structure: UK Biobank study. J Intern Med. 2018;284(4):439-443. [DOI] [PubMed] [Google Scholar]
- 17.Castro MG, Venutolo C, Yau PL, Convit A. Fitness, insulin level sensitivity, and frontal lobe integrity in adults with overweight and obesity. Obesity. 2016;24(6):1283-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poisnel G, Arenaza-Urquijo E, Collette F, et al. The Age-Well randomized controlled trial of the Medit-Ageing European project: effect of meditation or foreign language training on brain and mental health in older adults. Alzheimers Dement. 2018;4:714-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt P. Bayesian Inference for Structured Additive Regression Models for Large-Scale Problems with Applications to Medical Imaging [Online]. 2017. Accessed December 27, 2018. edoc.ub.uni-muenchen.de/20373/ [Google Scholar]
- 20.Garnier-Crussard A, Bougacha S, Wirth M, et al. . White matter hyperintensities across the adult lifespan: relation to age, Aβ load, and cognition. Alzheimers Res Ther. 2020;12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabesh A, Jensen JH, Ardekani BA, Helpern JA. Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn Reson Med. 2011;65(3):823-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falangola MF, Jensen JH, Babb JS, et al. Age-related non-Gaussian diffusion patterns in the prefrontal brain. J Magn Reson Imaging. 2008;28(6):1345-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Besson FL, Joie RL, Doeuvre L, et al. . Cognitive and brain profiles associated with current neuroimaging biomarkers of preclinical Alzheimer's disease. J Neurosci. 2015;35(29):10402-10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kriska AM, Knowler WC, LaPorte RE, et al. . Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13(4):401-411. [DOI] [PubMed] [Google Scholar]
- 25.Vuillemin A, Oppert J-M, Guillemin F, et al. . Self-administered questionnaire compared with interview to assess past-year physical activity. Med Sci Sports Exerc. 2000;32(6):1119-1124. [DOI] [PubMed] [Google Scholar]
- 26.Silver Santé Study. Data Sharing. silversantestudy.eu/2020/09/25/data-sharing/
- 27.Raichlen DA, Klimentidis YC, Bharadwaj PK, Alexander GE. Differential associations of engagement in physical activity and estimated cardiorespiratory fitness with brain volume in middle-aged to older adults. Brain Imaging Behav. 2020;14(5):1994-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arenaza-Urquijo EM, de Flores R, Gonneaud J, et al. . Distinct effects of late adulthood cognitive and physical activities on gray matter volume. Brain Imaging Behav. 2017;11(2):346-356. [DOI] [PubMed] [Google Scholar]
- 29.Boots EA, Schultz SA, Oh JM, et al. . Cardiorespiratory fitness is associated with brain structure, cognition, and mood in a middle-aged cohort at risk for Alzheimer's disease. Brain Imaging Behav. 2015;9(3):639-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gennuso KP, Gangnon RE, Matthews CE, Thraen-Borowski KM, Colbert LH. Sedentary behavior, physical activity, and markers of health in older adults. Med Sci Sports Exerc. 2013;45(8):1493-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raji CA, Ho AJ, Parikshak NN, et al. Brain structure and obesity. Hum Brain Mapp. 2010;31(3):353-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho AJ, Raji CA, Becker JT, et al. The effects of physical activity, education, and body mass index on the aging brain. Hum Brain Mapp. 2011;32(9):1371-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan AS. Insulin level resistance with aging. Sport Med. 2000;30(5):327-346. [DOI] [PubMed] [Google Scholar]
- 34.Arnold SE, Arvanitakis Z, Macauley-Rambach SL, et al. Brain insulin level resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018;14(3):168-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sripetchwandee J, Chattipakorn N, Chattipakorn SC. Links between obesity-induced brain insulin level resistance, brain mitochondrial dysfunction, and dementia. Front Endocrinol. 2018;9:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin Y-A. How does obesity and physical activity affect aging? Focused on telomere as a biomarker of aging. J Obes Metab Syndr. 2019;28(2):92-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corlier F, Hafzalla G, Faskowitz J, et al. Systemic inflammation as a predictor of brain aging: contributions of physical activity, metabolic risk, and genetic risk. Neuroimage. 2018;172:118-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de la Monte SM, Wands JR. Alzheimer's disease is type 3 diabetes: evidence reviewed. J Diabetes Sci Technol. 2008;2(6):1101-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biessels GJ, Reijmer YD. Brain changes underlying cognitive dysfunction in diabetes: what can we learn from MRI? Diabetes. 2014;63:2244-2252. [DOI] [PubMed] [Google Scholar]
- 40.Marseglia A, Darin-Mattsson A, Kalpouzos G, et al. . Can active life mitigate the impact of diabetes on dementia and brain aging? Alzheimers Dement. 2020;16(11):1534-1543. [DOI] [PubMed] [Google Scholar]
- 41.Castro MG, Venutolo C, Yau PL, Convit A. Fitness, insulin level sensitivity, and frontal lobe integrity in adults with overweight and obesity. Obesity. 2016;24(6):1283-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lucignani G, Namba H, Nehlig A, Porrino LJ, Kennedy C, Sokoloff L. Effects of insulin level on local cerebral glucose utilization in the rat. J Cereb Blood Flow Metab. 1987;7(3):309-314. [DOI] [PubMed] [Google Scholar]
- 43.Byun MS, Kim HJ, Yi D, et al. Region-specific association between basal blood insulin level and cerebral glucose metabolism in older adults. Neuroimage Clin. 2019;22:101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willette AA, Bendlin BB, Starks EJ, et al. Association of insulin level resistance with cerebral glucose uptake in late middle-aged adults at risk for Alzheimer disease. JAMA Neurol. 2015;72(9):1013-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishibashi K, Onishi A, Fujiwara Y, Ishiwata K, Ishii K. Effects of glucose, insulin level, and insulin level resistance on cerebral 18F-FDG distribution in cognitively normal older subjects. PLoS One. 2017;12(7):e0181400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pegueroles J, Pané A, Vilaplana E, et al. Obesity impacts brain metabolism and structure independently of amyloid and tau pathology in healthy elderly. Alzheimers Dement. 2020;12(1):e12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swan JH, Brooks JM, Amini R, Moore AR, Turner KW. Smoking predicting physical activity in an aging America. J Nutr Health Aging. 2018;22(4):476-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.PJ Herrod, Doleman B, Blackwell JEM, et al. . Exercise and other nonpharmacological strategies to reduce blood pressure in older adults: a systematic review and meta-analysis. J Am Soc Hypertens. 2018;12(4):248-267. [DOI] [PubMed] [Google Scholar]
- 49.Dougherty RJ, Schultz SA, Kirby TK, et al. . Moderate physical activity is associated with cerebral glucose metabolism in adults at risk for Alzheimer’s disease. J Alzheimers Dis. 2017;58(4):1089-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66(3):336-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Debette S, Seshadri S, Beiser A, et al. . Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request following a formal data sharing agreement and approval by the consortium and executive committee. The data sharing request form can be downloaded online.26