Abstract
OBJECTIVE
This study evaluated the effects of continuous glucose monitoring (CGM) combined with family behavioral intervention (CGM+FBI) and CGM alone (Standard-CGM) on glycemic outcomes and parental quality of life compared with blood glucose monitoring (BGM) in children ages 2 to <8 years with type 1 diabetes.
RESEARCH DESIGN AND METHODS
This was a multicenter (N = 14), 6-month, randomized controlled trial including 143 youth 2 to <8 years of age with type 1 diabetes. Primary analysis included treatment group comparisons of percent time in range (TIR) (70–180 mg/dL) across follow-up visits.
RESULTS
Approximately 90% of participants in the CGM groups used CGM ≥6 days/week at 6 months. Between-group TIR comparisons showed no significant changes: CGM+FBI vs. BGM 3.2% (95% CI −0.5, 7.0), Standard-CGM vs. BGM 0.5% (−2.6 to 3.6), CGM+FBI vs. Standard-CGM 2.7% (−0.6, 6.1). Mean time with glucose level <70 mg/dL was reduced from baseline to follow-up in the CGM+FBI (from 5.2% to 2.6%) and Standard-CGM (5.8% to 2.5%) groups, compared with 5.4% to 5.8% with BGM (CGM+FBI vs. BGM, P < 0.001, and Standard-CGM vs. BGM, P < 0.001). No severe hypoglycemic events occurred in the CGM+FBI group, one occurred in the Standard-CGM group, and five occurred in the BGM group. CGM+FBI parents reported greater reductions in diabetes burden and fear of hypoglycemia compared with Standard-CGM (P = 0.008 and 0.04) and BGM (P = 0.02 and 0.002).
CONCLUSIONS
CGM used consistently over a 6-month period in young children with type 1 diabetes did not improve TIR but did significantly reduce time in hypoglycemia. The FBI benefited parental well-being.
Introduction
Management of type 1 diabetes in youth is challenging, with considerable demands placed on families of young children. Care of these children is difficult due to their unpredictable behaviors and eating patterns, inability to articulate symptoms of out-of-range blood glucose (BG) levels (1,2), and frequent intercurrent illnesses. These variations may result in acute complications of severe hypoglycemia and severe hyperglycemia and even lead to diabetic ketoacidosis (DKA). Traditional management in young children has emphasized avoidance of hypoglycemia based on scant data on adverse central nervous system outcomes related to youth experiencing recurrent seizure/loss of consciousness (3–5). Yet, emerging data indicate that hyperglycemia also adversely impacts central nervous system structure and function in young children (1,6–8), creating a need for a paradigm shift in diabetes management. Recent evidence indicates that, possibly due to the pervasiveness of parental fear of hypoglycemia, particularly overnight (9), the overwhelming majority of young children with type 1 diabetes currently have suboptimal glycemic control. Only 24% of young children in the T1D Exchange Registry achieved an HbA1c <7.5% (58 mmol/mol) (10).
At the time we began this study, there were no data demonstrating improved glycemic control with continuous glucose monitoring (CGM) use in young children (1,11) and no randomized controlled trials of CGM compared with BG monitoring (BGM) of 6 months’ duration in very young children. The burdens associated with data overload, the disruptions to sleep and activities from device alarms, and the inclination (on the part of parents and medical care providers) to avoid hypoglycemia at all costs created barriers to effective CGM use (12). Recent improvements in CGM wearability and performance characteristics may have reduced these barriers in this age-group. This study aimed to evaluate whether CGM both with and without a novel family behavioral intervention (FBI) improved glycemic outcomes and parental quality of life compared with traditional fingerstick BGM among young children 2 to <8 years of age with type 1 diabetes.
Research Design and Methods
The Strategies to Enhance New CGM Use in Early Childhood (SENCE) trial was conducted at 14 pediatric endocrinology practices in the U.S. Each site’s institutional review board approved the protocol and Health Insurance Portability and Accountability Act–compliant informed consent forms. Written informed consent was obtained from each parent or legal guardian prior to enrollment, and assent was obtained from the participant as applicable. An independent Data and Safety Monitoring Board provided trial oversight for safety. Protocol details are available at ClinicalTrials.gov (clinical trial reg. no. NCT02912728) and summarized below.
Study Participants
Major eligibility criteria included clinical diagnosis of type 1 diabetes, age 2 to <8 years, total daily insulin requirement ≥0.3 units/kg/day, no use of real-time CGM in 30 days prior to enrollment, and HbA1c 7.0– <10.0% (53– <86 mmol/mol) within 30 days prior to consent or at time of screening. Initially, eligibility was restricted to children with diabetes duration ≥6 months and consistent use of either an insulin pump or multiple daily injections for 3 months; however during the study these conditions were changed to ≥3 months and 1 month, respectively, to facilitate timely subject recruitment (Supplementary Table 1 outlines all inclusion and exclusion criteria).
Each participant was required to complete a 14- to 21-day prerandomization phase using a masked Dexcom G4 PLATINUM Professional CGM (masked receivers were configured to record glucose concentrations not visible to the participant, and there were no alerts for out-of-range glucose excursions). All study participants had a 30-min in-person session to review CGM insertion and blinded CGM use. To be eligible for the randomized trial, participants needed to have at least 200 h (equivalent to 8.3 days) of available masked CGM data and to have performed an average of at least three BG meter checks per day during the prerandomization phase.
Twenty-two enrolled individuals did not enter the randomized trial because the participant was deemed ineligible after informed consent (N = 5), the run-in phase was not successfully completed (N = 6), the participant elected not to proceed with randomization (N = 10), or the participant was lost to follow-up prior to randomization (N = 1) (Supplementary Fig. 1).
Study Design and Procedures
Following eligibility verification, each study participant was randomly assigned to one of three groups 1:1:1 (with use of a block design stratified by clinical center and screening HbA1c <8.5% [<69 mmol/mol] and ≥8.5% [≥69 mmol/mol]): CGM with standardized training and a FBI (CGM+FBI group), CGM with standardized training (Standard-CGM group), or fingerstick BGM without CGM (BGM group).
Participants in the two CGM groups were provided with a Dexcom G5 Mobile Continuous Glucose Monitor that included a transmitter, receiver, and sensors. Participants/caregivers with compatible smartphones were permitted to use the phone as the display device instead of or in addition to the receiver. All participants used their own BGM meter and test strips during the study. All study groups had scheduled in-clinic visits at 6, 13, 19, and 26 weeks and phone calls at 10, 16, and 22 weeks following randomization. At 1 and 3 weeks, the two CGM groups had an in-clinic visit for training and the BGM group had a phone call.
Training of participant families in the two CGM groups involved four ∼30-min sessions of standardized CGM training, incorporating the need for two daily BG CGM calibrations. These were delivered at the randomization visit and at the 1-, 3-, and 6-week visits by an experienced diabetes educator (see Supplementary Table 2 for description of sessions). Once families became comfortable with CGM use, they were encouraged to use the sensor glucose values and trend arrows nonadjunctively for dosing insulin at times when calibrations were not required and were provided with safety recommendations regarding when a confirmatory BGM check should be performed. Remote monitoring of CGM data by caregivers using the Dexcom Share/Follow applications also was encouraged if feasible.
Caregivers of participants in the CGM+FBI group also participated in standardized ∼30-min interactive training sessions at the 1-, 3-, 6-, 13-, and 19-week visits delivered by a research assistant trained by the study team. The FBI sessions were developed based on data from a qualitative study consisting of interviews with 80 families with young children with type 1 diabetes (9,12–14). Based on these qualitative data, psychologists, social workers, and physicians on the study team designed the FBI content to address family feelings, attitudes, and behaviors that can be barriers to CGM use and to teach skills to manage behavioral barriers to CGM use (e.g., relaxation, problem-solving, communication strategies [see Supplementary Table 3 for description of sessions]). FBI sessions were delivered in person or by telephone, based on participant preference.
For 1 week after the 6-, 13-, and 19-week visits and 1 week prior to the 26-week visit, participants in the BGM group wore a masked Dexcom G4 PLATINUM Professional CGM for collection of sensor glucose data. The masked CGM glucose data were not available to the clinical team or study staff; instead, they were used for statistical analyses of glucose outcomes after study completion.
Central laboratory HbA1c was measured at randomization, 13 weeks, and 26 weeks by the University of Minnesota with the Tosoh A1c 2.2 Plus Glycohemoglobin Analyzer. A parent or legal guardian completed questionnaires either on paper or electronically using a study laptop or iPad at randomization and 26 weeks.
Outcomes
The primary outcome was percentage of time spent in target range 70–180 mg/dL (TIR). Prespecified secondary glycemic outcomes included mean glucose, glucose variability (coefficient of variation), time in hyperglycemic (>180 mg/dL, >250 mg/dL, >300 mg/dL), and hypoglycemic (<70 mg/dL, <60 mg/dL, <54 mg/dL) ranges, rate of hypoglycemic events per week, and HbA1c at 26 weeks. A CGM-measured hypoglycemic event was defined as 15 consecutive minutes with a sensor glucose value <54 mg/dL; the hypoglycemic event was considered as “ended” once at least 15 consecutive minutes had elapsed with a sensor glucose concentration ≥70 mg/dL (15).
Baseline CGM-measured outcomes were calculated for all participants using the masked sensor data collected during the 14- to 21-day prerandomization phase. For the BGM group, follow-up CGM-measured outcomes were calculated by pooling of data from the four 1-week time periods when they wore a masked device. For the CGM groups, follow-up CGM-measured outcomes were calculated by selection of real-time sensor data from the same 1-week time periods that the BGM group wore a masked sensor.
Primary caregiver-reported outcomes included scores for the following questionnaires: the 5-item World Health Organization Well-Being Index (WHO-5) (16), Problem Areas in Diabetes Survey - Parent Revised version (PAID-PR) (17), Diabetes Family Impact Scale (DFIS) (18), hypoglycemia fear survey for parents of young children (HFS-PYC) (19), and Diabetes Technology Questionnaire (DTQ) (20).
Safety outcomes included severe hypoglycemia (defined as an event that required assistance from another person due to altered consciousness to administer carbohydrate, glucagon, or other resuscitative actions), hyperglycemia resulting in treatment at a health care facility or that involved DKA (as defined by the Diabetes Control and Complications Trial [21]), all device-related events, and all serious adverse events regardless of causality. Skin reactions from sensor placement were reportable if severe or requiring treatment.
Statistical Methods
A total sample size of 126 was determined to have 90% power to detect a treatment group difference in mean percent TIR 70–180 mg/dL, assuming a population difference of 10% and an SD adjusted for baseline of 13%. Since the primary analysis would involve three pairwise treatment group comparisons, the two-sided type 1 error rate was set to be 0.0167. The sample size was increased to 150 to ensure that at least 126 participants would complete the randomized trial and have CGM data for analysis.
Statistical analyses were performed on an intention-to-treat basis, and all participants were included in the primary analysis and in all secondary analyses. Sensor use was calculated for the CGM groups with use of data from the 28 days prior to each visit.
The primary analysis of TIR included three pairwise treatment group comparisons of CGM-measured percent TIR 70–180 mg/dL during follow-up using a linear mixed effects regression model with adjustment for baseline value, baseline HbA1c (used to stratify randomization), and clinical center. The analyses of the secondary CGM-measured outcomes, HbA1c, and parent-reported outcomes paralleled the primary analysis. TIR 70–180 mg/dL and the secondary outcome of time <54 mg/dL were also assessed separately during daytime (6:00 a.m. to 9:59 p.m.) and nighttime (10:00 p.m. to 5:59 a.m.) hours. We compared the number of severe hypoglycemic events and occurrences of DKA between treatment groups using Fisher exact test.
Modification of the treatment effect by additional prespecified participant characteristics was assessed in an exploratory analysis by inclusion of an interaction term in a model as described above for the primary outcome. Two sensitivity analyses were performed for the primary outcome. To check for confounding, we adjusted the primary outcome model for any baseline characteristics where there was an observed imbalance by chance between the treatment groups. In addition, the primary analysis was repeated including only participants who met the following per-protocol criteria: 1) had ≥336 h of follow-up CGM data, 2) had a 26-week visit within 28 days of the target date, 3) did not start using a nonstudy CGM device, 4) had an average of ≥5 days per week of real-time CGM use (in the CGM groups), and 5) had an average of four or more BGM measurements per day in the BGM group.
A post hoc analysis was performed for comparison of TIR 70–180 mg/dL between treatment groups calculated using CGM data collected at the time of the 19- and 26-week visits, as these visits occurred following completion of the FBI interventions.
For the primary analysis, P values and 95% CIs were adjusted for multiple treatment group comparisons using the Benjamini-Hochberg linear step-up approach. For each secondary/exploratory analysis, the same method was used for adjustments for multiple treatment group comparisons. No adjustments were made for multiple secondary outcomes. All P values are two sided and reported as adjusted values. Analyses were conducted with SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
Between February 2017 and August 2018, 143 participants and their caregivers were randomly assigned to the CGM+FBI group (N = 50), the Standard-CGM group (N = 44), or the BGM group (N = 49). Of enrolled children, 50% were female and 68% non-Hispanic White; mean ± SD age was 5.7 ± 1.8 years and mean duration of diabetes 2.3 ± 1.9 years. Thirty-five percent used an insulin pump. Mean HbA1c at randomization was 8.2% ± 0.8% (66 ± 8.7 mmol/mol). Participant characteristics according to treatment randomization group are shown in Table 1.
Table 1.
Baseline characteristics of study participants by treatment group
| Overall (N = 143)¶ | CGM+FBI (N = 50) | Standard-CGM (N = 44) | BGM (N = 49) | |
|---|---|---|---|---|
| Age at enrollment (years) | ||||
| Mean ± SD | 5.7 ± 1.8 | 5.7 ± 1.7 | 5.2 ± 1.8 | 6.2 ± 1.7 |
| Range | 2.0–8.0 | 2.3–8.0 | 2.0–7.9 | 2.1–8.0 |
| Diabetes duration at enrollment (years) | ||||
| Mean ± SD | 2.3 ± 1.9 | 2.4 ± 1.9 | 1.9 ± 1.7 | 2.6 ± 1.9 |
| Range | 0.2–6.8 | 0.3–6.8 | 0.3–6.2 | 0.2–6.3 |
| Female sex (child) | 72 (50) | 29 (58) | 17 (39) | 26 (53) |
| Race/ethnicity (child) | ||||
| Non-Hispanic White | 95 (68) | 32 (65) | 33 (77) | 30 (63) |
| Non-Hispanic Black | 21 (15) | 10 (20) | 4 (9) | 7 (15) |
| Hispanic or Latino | 16 (11) | 5 (10) | 5 (12) | 6 (13) |
| Asian | 1 (<1) | 0 (0) | 0 (0) | 1 (2) |
| Other/more than one race | 7 (5) | 2 (4) | 1 (2) | 4 (8) |
| Highest parent education | ||||
| High school or less | 32 (24) | 13 (28) | 10 (25) | 9 (18) |
| Some college/Associate degree | 47 (35) | 13 (28) | 14 (35) | 20 (41) |
| Bachelor degree or higher | 57 (42) | 21 (45) | 16 (40) | 20 (41) |
| Annual household income | ||||
| <$35,000 | 25 (19) | 9 (20) | 11 (26) | 5 (12) |
| $35,000 to <$75,000 | 54 (41) | 19 (41) | 19 (45) | 16 (37) |
| ≥$75,000 | 52 (40) | 18 (39) | 12 (29) | 22 (51) |
| Health insurance | ||||
| Private | 87 (62) | 29 (58) | 27 (61) | 31 (66) |
| Medicaid/other | 52 (37) | 20 (40) | 16 (36) | 16 (34) |
| None | 2 (1) | 1 (2) | 1 (2) | 0 (0) |
| Prior CGM use | ||||
| Yes, but no recent use | 17 (12) | 5 (10) | 5 (11) | 7 (14) |
| Never | 126 (88) | 45 (90) | 39 (89) | 42 (86) |
| Insulin pump use | 50 (35) | 15 (30) | 13 (30) | 22 (45) |
| Screening HbA1c, % (mmol/mol)† | ||||
| Mean ± SD | 8.3 ± 0.7 (67 ± 7.7) | 8.3 ± 0.8 (67 ± 8.7) | 8.3 ± 0.8 (67 ± 8.7) | 8.3 ± 0.7 (67 ± 7.7) |
| Range | 7.0–9.9 (53–85) | 7.0–9.7 (53–83) | 7.1–9.7 (58–83) | 7.0–9.9 (53–85) |
| Randomization HbA1c, % (mmol/mol)‡ | ||||
| Mean ± SD | 8.2 ± 0.8 (66 ± 8.7) | 8.2 ± 0.8 (66 ± 8.7) | 8.2 ± 0.8 (66 ± 8.7) | 8.2 ± 0.7 (66 ± 7.7) |
| Range | 6.5–10.0 (48–86) | 7.0–9.9 (53–85) | 6.5–9.9 (48–85) | 6.7–10.0 (50–86) |
| Total daily insulin dose/kg, median (Q1, Q3) | 0.7 (0.5, 0.8) | 0.7 (0.5, 0.8) | 0.6 (0.5, 0.8) | 0.7 (0.5, 0.8) |
| ≥1 severe hypoglycemia event in the past 12 months§ | 14 (10) | 2 (4) | 5 (11) | 7 (14) |
| ≥1 DKA event in the past 12 months‖ | 34 (24) | 10 (20) | 13 (30) | 11 (22) |
Q, quartile. Data are N (%) unless otherwise indicated.
Screening HbA1c measured by point-of-care device or local laboratory and used to determine eligibility.
Randomization HbA1c measured by central laboratory.
Severe hypoglycemia was defined as an event that required assistance from another person to administer carbohydrate or glucagon or other resuscitative actions.
DKA was defined as an episode when the participant had ketosis that necessitated treatment in a health care facility.
Missing data: race/ethnicity 3 (2%), total daily insulin 1 (<1%), income 12 (8%), parent education 7 (5%), health insurance 2 (1%), randomization HbA1c 2 (1%). No missing data for all other characteristics.
Visit Completion
The 6-month trial was completed by 48 participants in the CGM+FBI group (96.0%), 43 in the Standard-CGM group (97.7%), and 46 in the BGM Group (93.9%) (Supplementary Fig. 2). Unscheduled clinic visits and contacts by treatment group are shown in Supplementary Table 4.
Intervention Adherence
Sensor use was high throughout the study in both CGM groups (Supplementary Fig. 3 and Supplementary Table 5). In the 4 weeks prior to the 26-week visit, 90% of participants in the CGM+FBI group and 89% of participants in the Standard-CGM group wore the device ≥6 days per week. Besides the two participants (one in each group) who withdrew, all were using CGM at 6 months.
In the CGM+FBI group, all participants except one completed all five FBI sessions. Eighty-four percent of families in the CGM+FBI completed three or more of the FBI sessions in person. Of note, two participants assigned to the BGM group initiated real-time CGM within 1 month following randomization.
There were 81 reported device issues over the 26-week follow-up (Supplementary Table 6), including two in which the sensor tip remained under the participant’s skin after sensor removal (both resolved without intervention).
CGM Glycemic Outcomes
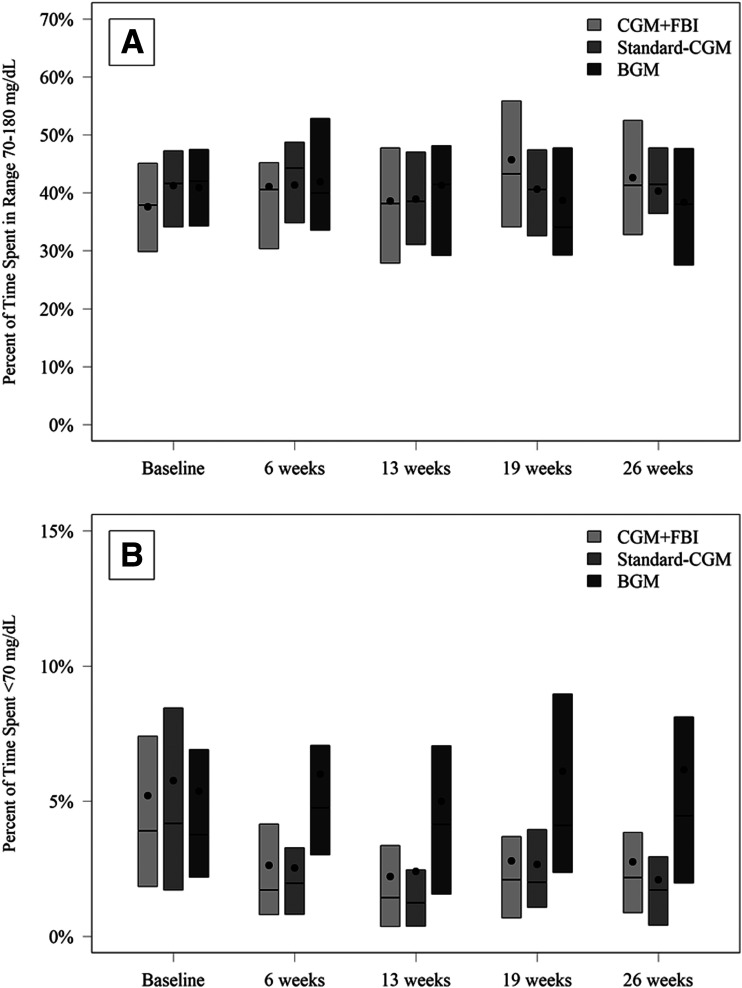
Mean TIR 70–180 mg/dL was 38% at baseline and 42% during follow-up in the CGM+FBI group, 41% and 40% in the Standard-CGM group, and 41% and 40% in the BGM group (adjusted mean difference for CGM+FBI vs. BGM 3.2% [95% CI −0.5 to 7.0], Standard-CGM vs. BGM 0.5% [−2.6 to 3.6], and CGM+FBI vs. Standard-CGM 2.7% [−0.6 to 6.1]) (Table 2 and Fig. 1). There was no significant interaction of the effect of treatment on the TIR outcome according to baseline age, sex, socioeconomic variables, duration of diabetes, insulin delivery method, TIR, or HbA1c (Supplementary Table 7).
Table 2.
Glycemic outcomes by treatment group †
| CGM+FBI | Standard-CGM | BGM | CGM+FBI vs. BGM | Standard-CGM vs. BGM | CGM+FBI vs. Standard-CGM | ||||
|---|---|---|---|---|---|---|---|---|---|
| Adjusted difference (95% CI), CGM+FBI − BGM§ | P § | Adjusted difference (95% CI), Standard-CGM − BGM§ | P § | Adjusted difference (95% CI), CGM+FBI − Standard-CGM§ | P § | ||||
| CGM metrics, N | |||||||||
| Baseline | 50 | 44 | 49 | ||||||
| Follow-up | 49 | 44 | 45 | ||||||
| Hours of CGM data | |||||||||
| Baseline | 315 ± 59 [206, 467] | 315 ± 61 [202, 471] | 320 ± 73 [204, 486] | ||||||
| Follow-up | 581 ± 80 [299, 661] | 584 ± 89 [137, 655] | 556 ± 135 [168, 955] | ||||||
| Glucose control | |||||||||
| % TIR 70–180 mg/dL‡ | |||||||||
| Baseline | 38 ± 12 | 41 ± 10 | 41 ± 10 | ||||||
| Follow-up | 42 ± 10 | 41 ± 9 | 40 ± 9 | 3.2 (−0.5, 7.0) | 0.11 | 0.5 (−2.6, 3.6) | 0.75 | 2.7 (−0.6, 6.1) | 0.12 |
| Mean glucose (mg/dL) | |||||||||
| Baseline | 211 ± 37 | 199 ± 28 | 199 ± 24 | ||||||
| Follow-up | 205 ± 22 | 205 ± 22 | 201 ± 24 | −0.5 (−7.8, 6.8) | 0.89 | 3.0 (−5.2, 11.1) | 0.66 | −3.5 (−11.8, 4.9) | 0.66 |
| Coefficient of variation (%) | |||||||||
| Baseline | 43 ± 7 | 45 ± 7 | 44 ± 7 | ||||||
| Follow-up | 41 ± 5 | 40 ± 5 | 45 ± 6 | −3.6 (−5.6, −1.7) | <0.001 | −5.6 (−7.9, −3.3) | <0.001 | 2.0 (0.2, 3.8) | 0.034 |
| % time with hyperglycemia | |||||||||
| >180 mg/dL | |||||||||
| Baseline | 57 ± 15 | 53 ± 13 | 54 ± 11 | ||||||
| Follow-up | 55 ± 10 | 57 ± 10 | 54 ± 10 | 0.0 (−3.3, 3.3) | 0.99 | 2.8 (−0.9, 6.5) | 0.16 | −2.8 (−6.5, 0.9) | 0.16 |
| >250 mg/dL | |||||||||
| Baseline | 34 ± 15 | 28 ± 11 | 28 ± 10 | ||||||
| Follow-up | 29 ± 11 | 28 ± 10 | 29 ± 10 | −2.4 (−6.5, 1.7) | 0.45 | −1.3 (−4.7, 2.3) | 0.52 | −1.1 (−4.3, 2.3) | 0.52 |
| >300 mg/dL | |||||||||
| Baseline | 21 ± 14 | 15 ± 8 | 15 ± 7 | ||||||
| Follow-up | 16 ± 8 | 14 ± 8 | 17 ± 8 | −2.6 (−5.4, −0.1) | 0.046 | −2.7 (−5.5, 0.0) | 0.046 | 0.1 (−2.2, 2.4) | 0.91 |
| % time with hypoglycemia | |||||||||
| <70 mg/dL | |||||||||
| Baseline | 5.2 ± 4.2 | 5.8 ± 5.3 | 5.4 ± 4.6 | ||||||
| Follow-up | 2.6 ± 1.6 | 2.5 ± 1.9 | 5.8 ± 3.3 | −2.6 (−3.8, −1.6) | <0.001 | −2.8 (−4.2, −1.7) | <0.001 | 0.2 (−0.3, 0.7) | 0.40 |
| <60 mg/dL | |||||||||
| Baseline | 3.2 ± 3.1 | 3.6 ± 4.1 | 3.4 ± 3.7 | ||||||
| Follow-up | 1.2 ± 1.0 | 1.2 ± 1.0 | 3.5 ± 2.3 | −1.9 (−2.6, −1.1) | <0.001 | −1.9 (−2.8, −1.1) | <0.001 | 0.0 (−0.2, 0.3) | 0.79 |
| <54 mg/dL | |||||||||
| Baseline | 2.3 ± 2.5 | 2.6 ± 3.4 | 2.4 ± 3.1 | ||||||
| Follow-up | 0.7 ± 0.7 | 0.7 ± 0.7 | 2.4 ± 1.7 | −1.4 (−2.0, −0.8) | <0.001 | −1.4 (−1.9, −0.8) | <0.001 | −0.1 (−0.2, 0.1) | 0.49 |
| Rate of hypoglycemic events per week | |||||||||
| Baseline | 2.5 ± 2.0 | 2.9 ± 2.8 | 2.7 ± 2.6 | ||||||
| Follow-up | 1.1 ± 1.1 | 1.4 ± 1.3 | 2.9 ± 1.8 | −1.5 (−2.2, −0.9) | <0.001 | −1.4 (−2.1, −0.8) | <0.001 | −0.1 (−0.5, 0.1) | 0.37 |
| HbA1c, N | |||||||||
| Baseline | 49 | 44 | 48 | ||||||
| 26 weeks | 48 | 43 | 46 | ||||||
| Mean HbA1c | |||||||||
| Baseline | 8.2 ± 0.8 | 8.2 ± 0.8 | 8.2 ± 0.7 | ||||||
| 26 weeks | 8.1 ± 0.9 | 8.2 ± 0.8 | 8.1 ± 0.8 | −0.1 (−0.3, 0.2) | 0.58 | 0.1 (−0.2, 0.3) | 0.58 | −0.2 (−0.5, 0.1) | 0.58 |
Unless otherwise indicated, data are means ± SD with or without [minimum, maximum].
Follow-up includes data pooled from the 6-, 13-, 19-, and 26-week time points.
Primary outcome. Similar results were observed when we adjusted primary analysis for potential baseline imbalances between treatment groups such as age, diabetes duration, sex, pump use, and income or when we only included those who met the per-protocol criteria (defined in the research design and methods section).
Outcomes were analyzed in a linear mixed-effects model that adjusts for baseline value, baseline HbA1c, and clinical center as a random effect. % time with glucose level >250 mg/dL, >300 mg/dL, <70 mg/dL, <60 mg/dL, and <54 mg/dL and rate of hypoglycemia events had skewed distributions and so were modeled with use of a rank-based transformation. For these skewed outcomes, we calculated point estimates and CIs for the treatment group difference using the technique described by Hodges and Lehmann (29). We adjusted P values and 95% CIs for multiple treatment group comparisons using the Benjamini-Hochberg linear step-up approach. Note that this adjustment results in some of the P values being identical.
Figure 1.
TIR 70–180 mg/dL (A) and time with glucose level <70 mg/dL (B) by treatment group and visit.
In an exploratory post hoc analysis, the CGM+FBI group showed improvement in TIR 70–180 mg/dL compared with the Standard-CGM or BGM groups for TIR calculated with only the CGM data from the 19 and 26 weeks (after FBI content delivery completion) (adjusted mean difference for CGM+FBI vs. Standard CGM 5.4% [95% CI 1.1–9.7], P = 0.012, and CGM+FBI vs. BGM 6.9% [2.0–11.7], P = 0.002) (Supplementary Table 8).
Both CGM groups reduced the time spent in a hypoglycemic state compared with the BGM group (Table 2 and Fig. 1). Mean percent time with glucose level <70 mg/dL was 5.2% (75 min) at baseline and 2.6% (37 min) at follow-up in the CGM+FBI group, 5.8% (84 min) at baseline, and 2.5% (36 min) at follow-up in the Standard-CGM group vs. 5.4% (78 min) at baseline and 5.8% (84 min) at follow-up in the BGM group (adjusted difference for CGM+FBI vs. BGM −2.6% [95% CI −3.8 to −1.6], P < 0.001, and Standard-CGM vs. BGM −2.8% [−4.2 to −1.7], P < 0.001). The difference in percent time spent with glucose level <70 mg/dL between CGM+FBI and Standard-CGM was not significant (CGM+FBI vs. Standard-CGM 0.2% [−0.3 to 0.7], P = 0.40) (Table 2). Similar results favoring both CGM groups were observed for percent time with glucose level <60 mg/dL, percent time with glucose level <54 mg/dL, and the rate of the CGM-measured hypoglycemic events per week (Table 2).
Both CGM groups also saw reductions in glucose variability (coefficient of variation) compared with the BGM group (adjusted difference for CGM+FBI vs. BGM −3.6% [95% CI −5.6 to −1.7], Standard-CGM vs. BGM −5.6% [−7.9 to −3.3], and CGM+FBI vs. Standard-CGM 2.0% [0.2–3.8]) (Table 2).
No significant treatment group differences were observed for mean glucose or percent time with glucose level >180 mg/dL or >250 mg/dL (Table 2). However, there was a small treatment group difference of time spent in the upper extreme, >300 mg/dL, in the CGM groups versus the BGM groups (adjusted difference for CGM+FBI vs. BGM −2.6% [95% CI −5.4 to −0.1], P = 0.046, and Standard-CGM vs. BGM −2.7% [−5.5 to 0.0], P = 0.046).
Separate analyses of CGM metrics during daytime and nighttime paralleled the overall study findings (Supplementary Table 9).
HbA1c
HbA1c was relatively unchanged from baseline to 26 weeks in all three treatment groups (Table 2). At baseline, the percentage of participants with an HbA1c at or below the American Diabetes Association target during the study (22) of <7.5% (<58 mmol/mol) was 20%, 20%, and 13% in the CGM+FBI, Standard-CGM, and BGM groups, respectively, and 29%, 16%, and 28% at the 26-week visit (P value for CGM+FBI vs. BGM group = 0.79, Standard-CGM vs. BGM = 0.10, and CGM+FBI vs. Standard-CGM = 0.10).
Primary Caregiver-Reported Outcomes
Caregiver-reported outcomes indicated significant benefit with the CGM+FBI intervention. At 26 weeks, primary caregivers in the CGM+FBI group reported lower diabetes burden, as measured by the PAID-PR, compared with both the BGM group (P = 0.015) and the Standard-CGM group (P = 0.008) (Table 3). Caregivers in the CGM+FBI group also reported lower hypoglycemia fear (HFS-PYC) scores at 26 weeks compared with the BGM group (P = 0.002) and the Standard-CGM group (P = 0.037). Finally, CGM+FBI group caregivers at the 26-week visit reported better experience with diabetes technology (higher DTQ scores) compared with the BGM group (P = 0.006) and tended to report higher scores compared with the Standard-CGM group (P = 0.08). There were no significant pairwise treatment group differences in well-being (WHO-5) score or diabetes family impact (DFIS) scores.
Table 3.
Primary caregiver-reported outcomes by treatment group
| CGM+FBI | Standard-CGM | BGM | CGM+FBI vs. BGM P value# | Standard-CGM vs. BGM P value# | CGM+FBI vs. Standard-CGM P value# | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | N | Mean ± SD | ||||
| WHO-5* | |||||||||
| Baseline | 48 | 67 ± 16 | 42 | 66 ± 18 | 48 | 65 ± 20 | |||
| 26 weeks | 45 | 66 ± 18 | 41 | 65 ± 15 | 46 | 63 ± 16 | 0.66 | 0.66 | 0.77 |
| PAID-PR† ‡ | |||||||||
| Baseline | 50 | 45 ± 17 | 43 | 48 ± 15 | 48 | 49 ± 17 | |||
| 26 weeks | 45 | 40 ± 19 | 41 | 52 ± 16 | 46 | 51 ± 16 | 0.015 | 0.61 | 0.008 |
| DFIS† § | |||||||||
| Baseline | 49 | 20 ± 14 | 43 | 25 ± 16 | 47 | 27 ± 17 | |||
| 26 weeks | 44 | 21 ± 16 | 40 | 24 ± 15 | 45 | 27 ± 19 | 0.79 | 0.79 | 0.79 |
| HFS-PYC total† ‖ | |||||||||
| Baseline | 49 | 39 ± 17 | 42 | 44 ± 17 | 47 | 42 ± 17 | |||
| 26 weeks | 45 | 35 ± 15 | 40 | 44 ± 16 | 46 | 46 ± 16 | 0.002 | 0.24 | 0.037 |
| HFS-PYC worry subscale† ‖ | |||||||||
| Baseline | 49 | 36 ± 22 | 42 | 41 ± 22 | 47 | 38 ± 19 | |||
| 26 weeks | 45 | 31 ± 20 | 40 | 41 ± 21 | 46 | 43 ± 20 | 0.004 | 0.23 | 0.08 |
| DTQ Now¶ | |||||||||
| Baseline | 50 | 56 ± 17 | 44 | 50 ± 18 | 48 | 52 ± 19 | |||
| 26 weeks | 45 | 61 ± 20 | 40 | 53 ± 17 | 46 | 49 ± 18 | 0.006 | 0.08 | 0.26 |
| DTQ Change¶ | |||||||||
| Baseline | — | — | — | — | — | — | |||
| 26 weeks | 45 | 72 ± 18 | 40 | 69 ± 12 | — | — | — | — | 0.47 |
WHO-5: measure of recent (past 2 weeks) subjective well-being, which is a component of overall quality of life. Scale 0–100 where higher score indicates better well-being.
Higher score indicates a worse outcome.
PAID-PR assesses parents’ current experiences with diabetes-specific emotional burden. Scale 0–100 where higher score indicates more parental burden.
DFIS measures the impact of diabetes on members of the family over the past year. Scale 0–100 where higher score indicates more negative family impact.
HFS-PYC: this version of the hypoglycemia fear survey is adapted for the parents of young children. The worry scale consists of 16 items that measure anxiety and fear surrounding hypoglycemia, and the behavior scale consists of 10 items that measure behaviors involved in avoidance and overtreatment of hypoglycemia. Scale 0–100 where higher score indicates more fear.
DTQ evaluates respondents’ feelings/attitudes about and experiences with diabetes technologies. Scale 0–100 where higher indicates less of a problem.
Outcomes were analyzed using ranks in a linear mixed-effects model with adjustment for baseline score (where applicable), baseline HbA1c, and clinical center as a random effect. We adjusted P values for multiple treatment group comparisons using the Benjamini-Hochberg linear step-up approach.
Safety Outcomes
No severe hypoglycemia events occurred in the CGM+FBI group, whereas one occurred in the Standard-CGM group and five occurred in the BGM group (P value for CGM+FBI vs. BGM group = 0.03, Standard-CGM vs. BGM = 0.21, and CGM+FBI vs. Standard-CGM = 0.47) (Supplementary Table 10). Three of the five participants who had a severe hypoglycemia event in the BGM group experienced seizure or loss of consciousness. The one participant who had an event in the Standard-CGM group required only active assistance from a family member to administer oral carbohydrate. DKA occurred in one participant in each of the CGM groups and none in the BGM group (P = NS).
Effects of CGM on Insulin Dosing and BGM Frequency
At the 1-week visit, 38% of the CGM+FBI group and 40% of the Standard-CGM group reported dosing insulin based on a CGM reading without confirmatory BGM. This increased to 79% and 95% in the CGM+FBI and Standard-CGM groups, respectively, by the 26-week visit. The median number of daily finger-stick BG measurements, derived from the 7 days prior to each visit, decreased over time in the CGM groups compared with BGM group, with frequency of 6.0/day at baseline and 4.0/day over follow-up in the CGM+FBI group, 6.0/day and 4.3/day in the Standard-CGM group, and 6.0/day and 6.1/day in the BGM group (P value for CGM+FBI vs. BGM group <0.001, Standard-CGM vs. BGM <0.001, and CGM+FBI vs. Standard-CGM =0.90).
CGM Remote Monitoring
At the 26-week visit, 52% of the CGM+FBI group and 40% of the Standard-CGM group reported using the Share feature for remote monitoring of CGM glucose data.
Conclusions
In this 6-month randomized controlled study in very young children with type 1 diabetes, real-time CGM use alone or combined with a behavioral intervention targeting barriers to CGM use did not improve TIR. Additionally, we did not observe an impact on HbA1c in either CGM intervention group or the BGM group, with most children remaining well above the American Diabetes Association target of <7.5% (<58 mmol/mol), the recommended target at the time of study enrollment. However, CGM use in both the Standard-CGM and CGM+FBI intervention arms was associated with significant improvements in three critical glycemic indices—time spent in a state of hypoglycemia, number of severe hypoglycemic events, and glucose variability—as well as parents’ psychosocial functioning. Notably, the CGM+FBI and Standard-CGM groups had reduced time spent with glucose level <70 mg/dL by 37 min and 40 min per day, respectively, on average. This demonstrates that caregivers likely used CGM to focus on avoiding hypoglycemia rather than on reducing hyperglycemia or increasing glucose time in range.
The behavioral intervention for parents resulted in additional benefits beyond the reductions in hypoglycemia. Specifically, the CGM+FBI arm experienced improved parental psychosocial outcomes, including lower diabetes distress and less fear of hypoglycemia, compared with CGM alone or BGM. In addition, caregivers in the CGM+FBI group also reported better experience with diabetes technology use than the BGM group with a trend toward better experience than the Standard-CGM group. Given evidence of persistent diabetes-related mood concerns and specifically worries about detecting and managing low glucoses in parents of young children with diabetes (23,24), improvement in these key factors is clinically meaningful. Moreover, we previously reported multiple aspects of parental concern specifically related to diabetes management with devices including CGM (9,12–14), which the CGM+FBI arm was able to successfully address above and beyond the introduction of CGM without behavioral support. These findings indicate that a brief behavioral intervention for parents, tailored to the specific burdens of care and CGM use in young children, may be helpful for alleviating some of the challenges associated with having a very young child with diabetes and may contribute to successful uptake and sustained use of advanced technologies in young children. This was the first behavioral intervention specifically designed to support CGM use in this population, highlighting the degree to which these critical psychosocial findings advance the field.
The only prior large CGM randomized trial in this age-group was conducted by the Diabetes Research in Children Network (DirecNet) 10 years ago (1). In that trial of 146 children age 4–8 years old with type 1 diabetes, glycemic outcomes were not improved, even in children who wore the CGM on a nearly daily basis. Only 41% used CGM six or more days/week at 6 months, indicating markedly reduced long-term CGM use compared with the current trial. Enhancements in CGM technology over the last 10 years have reduced the burden of using CGM, which may help explain the greater usage found in the current trial (25,26). Since the DirecNet trial was published, limited observational data have suggested improvements in glycemic variability and HbA1c with CGM use in young children (27).
The failure of CGM to improve TIR in the current study indicates the need for investigation of additional tools to assist families using CGM to reduce hyperglycemia. For example, there can be tools that tailor the use of CGM according to family needs and concerns. There can be strategies to promote insulin dosing prior to meals for parents fearful that their young child may not eat and to optimize dosing for high glucose levels as well as hyperglycemia between meals and strategies for medical nutrition therapy to ensure adequate coverage of food; for encouraging physical activity without increasing fear of hypoglycemia that can lead to overtreatment with carbs that, in turn, increases risk of hyperglycemia; and for additional caregiver education regarding benefits of increased glucose TIR. Also, there are needs for improved insulin delivery methods to better match glycemic excursions around meals and activity. Given the promising results of the CGM+FBI intervention arm, additional research to intensify or further enhance the behavioral intervention’s focus on the behavioral and psychosocial contributors to these management issues may strengthen the impact on TIR and clinically meaningful outcomes beyond HbA1c (28).
The strengths of this study include its multicenter randomized design to assign treatment interventions, high participant adherence to the assigned intervention, high retention, and enrollment of an ethnically and socioeconomically diverse cohort that included 32% racial/ethnic minority participants with 38% possessing nonprivate health insurance. Since 88% of this diverse cohort was CGM naïve at study entry, our results suggest that the education and intervention provided could be generalized to the larger population of families of young children with type 1 diabetes. Additionally, the use of trained, nonmedical providers to deliver the FBI lends itself to scaling of the intervention and implementation in routine practice.
The study also had notable limitations. The CGM used in the trial required twice a day calibrations with fingerstick BG measurements, whereas this is no longer required with contemporary factory-calibrated devices. The lack of need for finger sticks and easier insertion with current sensors could result in less burden. In addition, the results may not apply to children with type 1 diabetes who have HbA1c outside the eligibility range of 7.0–9.9% (53–85 mmol/mol). Finally, the informed consent process and run-in phase also had the potential to exclude individuals who might have more barriers to CGM use than the studied cohort.
In summary, while real-time CGM use alone or combined with an FBI did not improve TIR for young children with type 1 diabetes, it did result in reduced hypoglycemia, fewer severe hypoglycemia events, and less time spent with glucose level >300 mg/dL. The addition of the brief FBI, which focused on supporting parents to effectively manage CGM benefits and barriers, was associated with two key psychosocial challenges that are commonly experienced by parents of young children with diabetes: improved caregiver-reported outcomes including reduced disease burden. Future studies should explore interventions that assist families in more effectively using CGM, likely in combination with other advanced diabetes technologies, to improve glycemic management for young children.
Appendix
Writing Commttee: Linda A. DiMeglio (Indiana University School of Medicine, Indianapolis, IN), Lauren G. Kanapka (Jaeb Center for Health Research, Tampa, FL), Daniel J. DeSalvo (Baylor College of Medicine and Texas Children’s Hospital, Houston, TX), Marisa E. Hilliard (Baylor College of Medicine and Texas Children’s Hospital), Lori M. Laffel (Joslin Diabetes Center, Harvard Medical School, Boston, MA), William V. Tamborlane (Yale School of Medicine, New Haven, CT), Michelle A. Van Name (Yale School of Medicine), Stephanie Woerner (Indiana University School of Medicine), Saleh Adi (Diabetes Center at University of California San Francisco, San Francisco, CA), Anastasia Albanese-O’Neill (University of Florida, Gainesville, FL), G. Todd Alonso (Barbara Davis Center for Childhood Diabetes, Aurora, CO), Barbara J. Anderson (Baylor College of Medicine and Texas Children’s Hospital), Sarah D. Corathers (Cincinnati Children’s Hospital Medical Center and University of Cincinnati, Cincinnati, OH), Kelly Fegan-Bohm (Baylor College of Medicine and Texas Children’s Hospital), Greg P. Forlenza (Barbara Davis Center for Childhood Diabetes), Rachelle G. Gandica (Naomi Berrie Diabetes Center at Columbia University, New York, NY), Robin S. Goland (Naomi Berrie Diabetes Center at Columbia University), Michael J. Haller (University of Florida), Anat Hanono (Joslin Diabetes Center, Harvard Medical School), Kara R. Harrington (Joslin Diabetes Center, Harvard Medical School), Heba M. Ismail (Indiana University School of Medicine), Heather A. Jolivette (Indiana University School of Medicine), Jennifer C. Kelley (Vanderbilt University Medical Center, Nashville, TN), Suzanne Kingery (University of Louisville, Louisville, KY), Sarah A. MacLeish (Rainbow Babies and Children’s Hospital, Cleveland, OH), Shideh Majidi (Barbara Davis Center for Childhood Diabetes), Kellee M. Miller (Jaeb Center for Health Research), Pantea P. Minnock (Children’s Hospital of Philadelphia, Philadelphia, PA), Brandon M. Nathan (University of Minnesota, Minneapolis, MN), Nicole M. Sheanon (Cincinnati Children’s Hospital Medical Center and University of Cincinnati), Jill H. Simmons (Vanderbilt University Medical Center), Muna Sunni (University of Minnesota), R. Paul Wadwa (Barbara Davis Center for Childhood Diabetes), Sara E. Watson (University of Louisville), Kate Weyman (Yale School of Medicine), Kristen M. Williams (Naomi Berrie Diabetes Center at Columbia University), Steven M. Willi (Children’s Hospital of Philadelphia, Perelman School of Medicine, University of Pennsylvania), Kupper A. Wintergerst (University of Louisville), and Jamie Wood (Rainbow Babies and Children’s Hospital).
Article Information
Funding. This study was supported by The Leona M. And Harry B. Helmsley Charitable Trust. Dexcom provided nonfinancial support in the form of devices.
Duality of Interest. D.J.D. reports consulting fees from Dexcom outside the submitted work. L.M.L. reports grants and person fees from Dexcom outside the submitted work. R.P.W. reports grants and personal fees from Dexcom outside the submitted work. S.M.W. reports personal fees from Roche Diagnostics and Boehringer Ingelheim outside the submitted work. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. L.A.D. was involved in study design, researched data, and wrote and edited the manuscript. L.G.K. performed statistical analyses and wrote and edited the manuscript. S.W., L.M.L., K.R.H., B.J.A., M.E.H., D.J.D., W.V.T., M.A.V.N., R.P.W., B.M.N., A.A.-O., J.C.K., R.S.G., S.M.W., S.D.C., S.A.M., K.A.W., G.P.F., S.M., G.T.A., M.S., M.J.H., A.H., J.H.S., K.M.W., R.G.G., P.P.M., H.A.J., H.M.I., S.A., K.F.-B., N.M.S., K.W., J.W., S.E.W., S.K., and K.M.M. were involved in study design, researched data, contributed to discussion, and reviewed and edited the manuscript. K.M.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
Footnotes
The members of the Writing Committee are listed in the Appendix, and a full listing of the members of the Strategies to Enhance New CGM Use in Early Childhood (SENCE) Study Group is included in the online supplementary material.
Clinical trial reg no. NCT02912728, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.13148741.
Contributor Information
Collaborators: Strategies to Enhance New CGM Use in Early Childhood (SENCE) Study Group:, Lori Laffel, Kara Harrington, Anat Hanono, Nisha Naik, Louise Ambler-Osborn, Alan Schultz, Linda DiMeglio, Stephanie Woerne, Heather Jolivette, Heba Ismail, Megan Tebbe, America Newman, Megan Legge, William Tamborlane, Michelle Van Name, Kate Weyman, Jennifer Finnegan, Amy Steffen, Melinda Zgorski, Daniel DeSalvo, Marisa Hilliard, Kylie DeLaO, Cicilyn Xie, Wendy Levy, R. Paul Wadwa, Greg Forlenza, Shideh Majidi, Guy Alonso, Isabel Weber, Michelle Clay, Emily Simmons, Brandon Nathan, Muna Sunni, Jessica Sweet, Beth Pappenfus, Anne Kogler, Marrissa Ludwig, Brittney Nelson, Anne Street, Darcy Weingartner, Anastasia Albanese-O’Neill, Michael Haller, Janey Adams, Miriam Cintron, Nicole Thomas, Jennifer Kelley, Jill Simmons, George William, Faith Brendle, Robin Goland, Kristen Williams, Rachelle Gandica, Sarah Pollak, Emily Casciano, Elizabeth Robinson, Steven Willi, Pantea Minnock, Diana Olivos, Cathy Carchidi, Brian Grant, Jenise C. Wong, Saleh Adi, Sarah Corathers, Nicole Sheanon, Cathy Fox, Tammy Weis, Sarah MacLeish, Jamie Wood, Terri Casey, Wendy Campbell, Paul McGuigan, Kupper Wintergerst, Sara Watson, Suzanne Kingery, Gwen Pierce, Heather Ruch, Lauren Rayborn, Manuel Rodriguez-Luna, and Amy Deuser
References
- 1. Mauras N, Beck R, Xing D, et al.; Diabetes Research in Children Network (DirecNet) Study Group . A randomized clinical trial to assess the efficacy and safety of real-time continuous glucose monitoring in the management of type 1 diabetes in young children aged 4 to <10 years. Diabetes Care 2012;35:204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sundberg F, Forsander G. Detection and treatment efficacy of hypoglycemic events in the everyday life of children younger than 7 yr. Pediatr Diabetes 2014;15:34–40 [DOI] [PubMed] [Google Scholar]
- 3. Rovet JF, Ehrlich RM. The effect of hypoglycemic seizures on cognitive function in children with diabetes: a 7-year prospective study. J Pediatr 1999;134:503–506 [DOI] [PubMed] [Google Scholar]
- 4. Rovet JF, Ehrlich RM, Hoppe M. Specific intellectual deficits in children with early onset diabetes mellitus. Child Dev 1988;59:226–234 [DOI] [PubMed] [Google Scholar]
- 5. Ryan CM. Searching for the origin of brain dysfunction in diabetic children: going back to the beginning. Pediatr Diabetes 2008;9:527–530 [DOI] [PubMed] [Google Scholar]
- 6. Barnea-Goraly N, Raman M, Mazaika P, et al.; Diabetes Research in Children Network (DirecNet) . Alterations in white matter structure in young children with type 1 diabetes. Diabetes Care 2014;37:332–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marzelli MJ, Mazaika PK, Barnea-Goraly N, et al.; Diabetes Research in Children Network (DirecNet) . Neuroanatomical correlates of dysglycemia in young children with type 1 diabetes. Diabetes 2014;63:343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Šuput Omladič J, Slana Ozimič A, Vovk A, et al. Acute hyperglycemia and spatial working memory in adolescents with type 1 diabetes. Diabetes Care 2020;43:1941–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Name MA, Hilliard ME, Boyle CT, et al. Nighttime is the worst time: parental fear of hypoglycemia in young children with type 1 diabetes. Pediatr Diabetes 2018;19:114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tsalikian E, Fox L, Weinzimer S, et al.; Diabetes Research in Children Network Study Group . Feasibility of prolonged continuous glucose monitoring in toddlers with type 1 diabetes. Pediatr Diabetes 2012;13:301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hilliard ME, Levy W, Anderson BJ, et al. Benefits and barriers of continuous glucose monitoring in young children with type 1 diabetes. Diabetes Technol Ther 2019;21:493–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Commissariat PV, Harrington KR, Whitehouse AL, et al. “I’m essentially his pancreas”: parent perceptions of diabetes burden and opportunities to reduce burden in the care of children <8 years old with type 1 diabetes. Pediatr Diabetes 2020;21:377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Commissariat PV, Whitehouse AL, Hilliard ME, et al. Sources and valence of information impacting parents’ decisions to use diabetes technologies in young children <8 years old with type 1 diabetes. Diabetes Technol Ther 2020;22:697–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Topp CW, Østergaard SD, Søndergaard S, Bech P. The WHO-5 Well-Being Index: a systematic review of the literature. Psychother Psychosom 2015;84:167–176 [DOI] [PubMed] [Google Scholar]
- 17. Markowitz JT, Volkening LK, Butler DA, Antisdel-Lomaglio J, Anderson BJ, Laffel LM. Re-examining a measure of diabetes-related burden in parents of young people with type 1 diabetes: the Problem Areas in Diabetes Survey - Parent Revised version (PAID-PR). Diabet Med 2012;29:526–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Katz ML, Volkening LK, Dougher CE, Laffel LM. Validation of the Diabetes Family Impact Scale: a new measure of diabetes-specific family impact. Diabet Med 2015;32:1227–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care 1987;10:617–621 [DOI] [PubMed] [Google Scholar]
- 20. Wysocki T, Reeves G, Kummer M, Ross J, Yu M. Pyschometric validations of the Diabetes Technology Questionnaire(Abstract). Diabetes 2015;64:A633 [Google Scholar]
- 21. Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 22. American Diabetes Association . 13. Children and adolescents: Standards of Medical Care in Diabetes—2020 . Diabetes Care 2020;43(Suppl. 1):S163–S182 [DOI] [PubMed] [Google Scholar]
- 23. Driscoll KA, Raymond J, Naranjo D, Patton SR. Fear of hypoglycemia in children and adolescents and their parents with type 1 diabetes. Curr Diab Rep 2016;16:77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Streisand R, Monaghan M. Young children with type 1 diabetes: challenges, research, and future directions. Curr Diab Rep 2014;14:520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beck RW, Bergenstal RM, Laffel LM, Pickup JC. Advances in technology for management of type 1 diabetes. Lancet 2019;394:1265–1273 [DOI] [PubMed] [Google Scholar]
- 26. Laffel L. Improved accuracy of continuous glucose monitoring systems in pediatric patients with diabetes mellitus: results from two studies. Diabetes Technol Ther 2016;18(Suppl. 2):S223–S233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dovc K, Cargnelutti K, Sturm A, Selb J, Bratina N, Battelino T. Continuous glucose monitoring use and glucose variability in pre-school children with type 1 diabetes. Diabetes Res Clin Pract 2019;147:76–80 [DOI] [PubMed] [Google Scholar]
- 28. Agiostratidou G, Anhalt H, Ball D, et al. Standardizing clinically meaningful outcome measures beyond HbA1c for type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care 2017;40:1622–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hodges JL, Lehmann EL. Estimates of location based on rank tests. Ann Math Stat 1963;34:598–611 [Google Scholar]