Abstract
Background:
Accurate Sezary cell detection in peripheral blood of mycosis fungoides/Sezary syndrome (MF/SS) patients by flow cytometry can be difficult due to overlapping immunophenotypes with normal T cells using standard markers. We assessed the utility of programmed death-1 (PD-1/CD279), a transmembrane protein expressed in some hematopoietic cells, for identification and quantitation of circulating Sezary cells among established markers using flow cytometry.
Methods:
50 MF/SS and 20 control blood samples were immunophenotyped by flow cytometry. Principal component analysis (PCA) assessed contributions of antigens to separation of abnormal from normal T cell populations. PD-1 was assessed over time in blood and bone marrow of available MF/SS cases.
Results:
Normal CD4+ T cells showed dim/intermediate to absent PD-1 expression. PD-1 in Sezary cells was informatively brighter (≥1/3 log) than internal normal CD4+ T cells in 39/50 (78%) cases. By PCA, PD-1 ranked 3rd behind CD7 and CD26 in population separation as a whole; it ranked in the top 3 markers in 32/50 (64%) cases and 1st in 4/50 (8%) cases when individual abnormal populations were compared to total normal CD4+ T cells. PD-1 clearly separated Sezary from normal CD4+ T cells in 15/26 (58%, 30% of total) cases with few and subtle alterations of pan T-cell antigens/CD26 and was critical in 6 (12% of total), without which identification and quantification were significantly affected or nearly impossible. PD-1 remained informative in blood/bone marrow over time in most patients.
Conclusions:
PD-1 significantly contributes to accurate flow cytometric Sezary cell assessment in a routine Sezary panel.
Keywords: PD-1, Sezary syndrome, mycosis fungoides, flow cytometry
Introduction
Mycosis fungoides (MF) and Sezary syndrome (SS) are related subtypes of cutaneous T cell lymphoma with overlapping histologic and immunophenotypic characteristics as well as a shared clinical staging system and treatment paradigm (1–4). Accurate assessment of neoplastic T cells (termed “Sezary cells”) in peripheral blood of MF/SS patients is critical as Sezary cell burden is used for clinical staging (2), therapeutic decision making and assessment of therapeutic responses (4, 5).
Current recommendations for detection and quantification of Sezary cells involve identification of homogeneous T cells clusters with immunophenotypes different from normal utilizing multiple antigens. This method is more sensitive and accurate than former ones like cytomorphologic peripheral blood smear review and simplified calculations purported to correlate with Sezary cell burden by flow cytometry, such as elevated CD4:CD8 ratios or expanded CD4+/CD7- and/or CD4+/CD26- T cell populations (6–9). This method requires a comprehensive panel of antigens, which is currently limited to pan-T cell antigens and CD26 in many laboratories, to determine a composite phenotype as Sezary cells often overlap immunophenotypically with normal T cells and lack specific markers. Indeed, although diminished CD7 and CD26 are the most frequent aberrancies in MF/SS, they can also be reduced in subsets of normal T cells (10, 11), while alterations of other pan-T cell antigens are often subtle (8, 10, 12, 13). Given this gating strategy shift and the limitations of current markers, novel antigens that can differentiate Sezary cells from normal T cells are increasingly important.
Programmed death-1 (PD-1/CD279) is a transmembrane protein within the CD28 receptor family that inhibits T cell function (14). It is expressed in T cells, some B cells and a subset of myeloid cells upon activation (14, 15) and in certain cell types, like T follicular helper cells, at constitutively high levels (16). Although studies have demonstrated increased PD-1 expression in MF/SS (8, 13, 17–27), most were limited to immunohistochemical evaluation of skin biopsies. Such studies suffered from difficulties in definitively characterizing tumor cells due to admixed reactive inflammatory cells and differing thresholds for defining positivity, consequently showing conflicting results (13, 17–22). Large scale studies evaluating PD-1 by flow cytometry in circulating Sezary cells identified by composite immunophenotype are rare (8, 23–25) and none to our knowledge have comprehensively assessed the utility of PD-1 in the context of established markers for accurate Sezary cell detection. In addition, a recent international guideline could not reach a consensus on whether to recommend PD-1 for Sezary cell evaluation by flow cytometry, likely due to limited experience with this maker in many laboratories (12). Given the limited data, we systematically evaluated the additive value of PD-1 to standard T cell antigens for accurate identification of circulating Sezary cells using flow cytometry.
Materials and methods
Patient and sample selection:
Fifty patients with MF or SS and peripheral blood samples evaluated by flow cytometry at Memorial Sloan Kettering Cancer Center between 12/2015 and 9/2020 were identified. The first blood sample from each patient in which PD-1 was utilized in the flow cytometry panel with an aberrant T cell population comprising ≥0.1% of total white cells was evaluated. The diagnosis of MF/SS was established based on multidisciplinary clinical assessment, histologic evaluation of skin, lymph node and/or bone marrow biopsies, flow cytometric analysis of peripheral blood and, when needed, T cell receptor (TCR) gene rearrangement analysis. Subsequent available peripheral blood and bone marrow samples containing aberrant T cell populations comprising ≥0.1% of total white cells were evaluated in patients with at least 2 such samples. Twenty control peripheral blood samples from patients without a T cell lymphoproliferative disorder were analyzed. Clinical and laboratory data was obtained from electronic medical records. The study was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center.
Flow cytometry:
High sensitivity multiparameter flow cytometric immunophenotyping was performed with a FACS Canto 10-color instrument (BD Biosciences, San Jose, CA) using the following antibodies: CD45-V500C (2D1, BD Biosciences), CD3-PC7 (UCHT1, Beckman-Coulter, Miami, FL), CD2-BV421 (RPA-2.10, BD Horizon, San Jose, CA), CD5-APC (L17F12, BD Biosciences), CD7-BB515 (M-T701, BD Horizon), CD4-APC-A700 (13B8.2, Beckman-Coulter), CD8-APC-H7 (SK1, BD Biosciences), CD56-PE (N901, Beckman-Coulter), CD26-PerCP-Cy5.5 (BA5B, BioLegend, San Diego, CA) and CD279 (PD-1)-BV605 (EH12.2H7, BioLegend). At least 200,000 cells were acquired and at least 20 events were required for a positive result. The data was analyzed using custom software (“Woodlist,” gift of B.L. Wood, University of Washington, Seattle, WA). Abnormal T cell populations were identified by visual assessment based on aberrant antigen expression. Median fluorescent intensity (MFI) of markers was evaluated. Abnormal populations were considered “dim” or “bright” for a given marker if the fluorescent intensity was ≥1/3 log different from that of corresponding internal normal CD4+ T cells. In select cases, clonality of T cell populations was assessed with a Beta Mark TCR Vβ Repertoire Kit (Beckman-Coulter) using a modified, single-tube method as described previously (25).
Molecular analysis:
TCR gene rearrangement was performed by polymerase chain reaction-based capillary electrophoresis fragment analysis on select blood and tissue samples during clinical work up. Briefly, genomic DNA was extracted and PCR performed using commercially available fluorescently-labeled primers (Invivoscribe, Inc. BIOMED-2, San Diego, CA) to amplify conserved regions within the TCR β and TCR γ genes. Capillary electrophoresis with an ABI 3730 DNA analyzer (Thermo Fisher Scientific, Waltham, MA) was used to determine fragment lengths of the PCR products and the results were interpreted according to established guidelines (28).
Statistical analysis:
Principal component analysis (PCA) was performed with Infinicyt™ flow cytometry data analysis software (Cytognos, Salamanca, Spain), which utilizes an automatic population separator based on principal component analysis, to evaluate the relative contributions of each antibody utilized in the flow panel (CD45/CD3/CD2/CD5/CD7/CD4/CD8/CD56/CD26/CD279 (PD-1)) to the separation of normal and abnormal T cell clusters. Three database groups were created and compared: a normal CD4+ T cell group, an abnormal T cell group and an internal normal CD4+ T cell group. The 20 control peripheral blood samples were used for the normal CD4+ T cell group. The internal normal group utilized the background immunophenotypically normal CD4+ T cells from the MF/SS patient blood samples. All groups were created using equal numbers of events from each case. Each antigen’s contribution of variation to the principal component 1 (PC1) and principal component 2 (PC2) vectors separating normal from abnormal T cells was documented. Paired t test was used to compare abnormal and internal normal populations’ MFI values while unpaired Mann-Whitney tests were utilized for all other comparative analyses.
Results
Sezary cells in MF/SS commonly express brighter PD-1 than normal CD4+ T cells in peripheral blood
In all 20 control blood samples, normal CD4+ T cells showed absent or, less commonly, a continuum of PD-1 expression from dim/intermediate to absent without a distinctly positive population (Figure 1A). PD-1 expression in abnormal and residual internal normal CD4+ T cells in blood samples from 50 unique MF/SS patients was then evaluated. The median age at sample collection was 65 (range 31–86) years with a male:female ratio of 1:0.85. Sixteen of the MF/SS samples were obtained at initial diagnosis and 34 at relapse or persistent/progressive disease. Based on World Health Organization-European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphomas guidelines (3, 29), 23 patients were classified as SS and 27 as MF. No patient had previously received anti-PD-1 or anti-PD-L1 therapy.
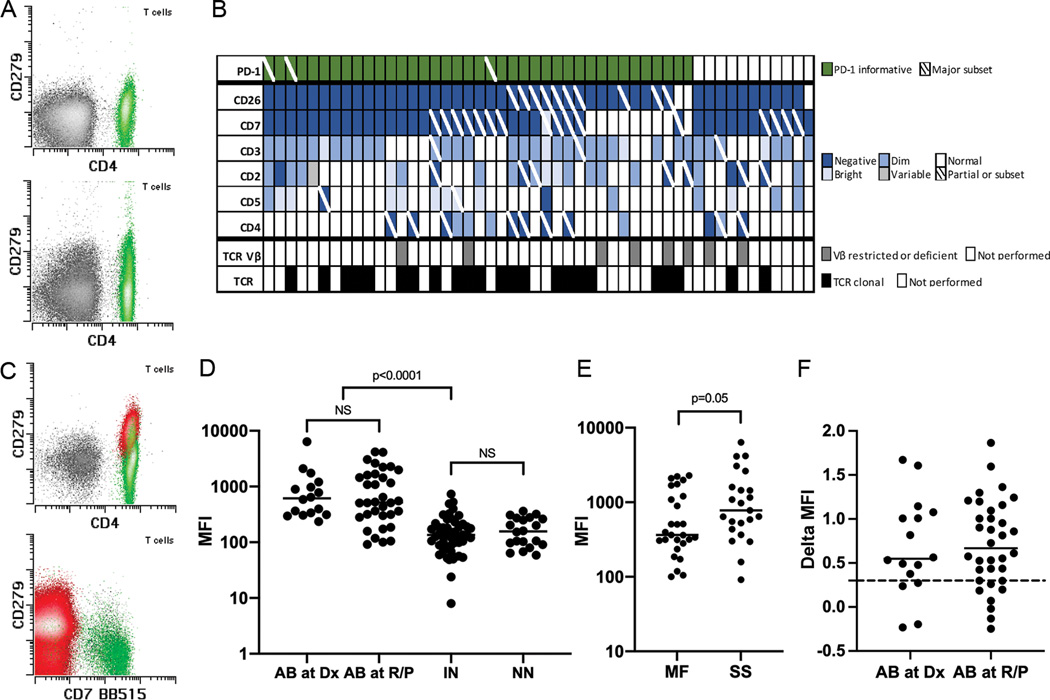
Figure 1. PD-1 expression in normal CD4+ T cells and Sezary cells and full Sezary cell immunophenotype.
A. Example normal CD4+ T cell populations (green events) from normal controls showing absent (top panel) or dim/intermediate to absent PD-1 (CD279) expression (bottom panel). B. Immunophenotypic and clonality results of the 50 MF/SS cases. Each column represents 1 case. C. Example Sezary cell populations (red events) from initial samples from 2 MF/SS patients (top and bottom panels) exhibiting brighter PD-1 expression (major subset in bottom panel) than corresponding internal normal CD4+ T cells (green events). D. PD-1 median fluorescent intensity (MFI) of abnormal T cells (AB) from the 50 initial MF/SS cases obtained at initial diagnosis (Dx) and at relapsed/persistent disease (R/P), internal normal CD4+ T cells from MF/SS cases (IN) and normal CD4+ T cells from the control group (NN). E. PD-1 MFI of neoplastic T cells from MF and SS patients. F. Log difference in PD-1 MFI between neoplastic and corresponding internal normal CD4+ T cell populations in each MF/SS case (“Delta MFI”) obtained at diagnosis and at relapsed/persistent disease. Solid horizontal lines indicate medians and dotted horizontal line indicates the 1/3 log level. TCR Vβ indicates T cell receptor Vβ repertoire analysis by flow cytometry (8/8 abnormal); TCR, T cell receptor gene rearrangement by molecular analysis (23/23 abnormal).
Neoplastic T cells comprised a median of 12.0% of total white cells (range 0.43–88.4%) and a median of 75.5% of total CD4+ T cells (or of Sezary cells + CD4+ T cells in CD4 negative cases) (range 2.56–98.9%). The neoplastic T cells accounted for a median of 50,944 events (range 2,041–385,938). The most commonly altered antigens in the MF/SS cases were CD26 (47/50 cases [94%], including 37/50 [74%] with complete and 10/50 [20%] with partial absence) and CD7 (41/50 [82%], including 25/50 [50%] with complete and 15/50 [30%] with partial absence and 1/50 [2%] with partial bright expression), consistent with the literature (8, 12, 23) (Figure 1B). The number of cases showing altered expression of other antigens were as follows: CD3: 36/50 (72%); CD2: 25/50 (50%); CD5: 16/50 (32%); CD4: 14/50 (28%) (Figure 1B). All cases were CD8 negative.
Overall, abnormal T cells in the 50 initial samples from the MF/SS patients showed significantly higher PD-1 expression than internal normal CD4+ T cells (median [central 95% range] MFI: 538.3 [93.8–5777.9] vs 135.1 [12.4–675.7], p<0.0001) (Figure 1C–D). Among the 50 initial MF/SS samples, no significant difference in PD-1 expression was seen between samples obtained at initial diagnosis and those at relapse/persistent disease (Figure 1D). The residual normal CD4+ T cells in the MF/SS cases showed similar levels of PD-1 expression as the controls (median [central 95% range] MFI: 135.1 [12.4–675.7] vs 156.7 [58.8–360.7], p=NS) (Figure 1D). A strong trend towards higher PD-1 MFI was seen among SS cases as compared to MF cases (median [central 95% range] MFI: 776.8 [91.4–6381.6] vs 363.9 [100.1–2279.4], p=0.05) (Figure 1E). No significant difference in PD-1 MFI was identified between MF/SS cases with a high absolute burden of circulating neoplastic T cells (≥1000/μL) and a low (<1000/μL) one (median [central 95% range] MFI: 615.0 [91.4–6381.6] vs 526.3 [100.1–4186.5], p=NS) among cases with available complete blood cell count information (n=49).
On a case-by-case basis, we found PD-1 to be informative in differentiating abnormal from normal T cell populations when it was expressed at least 1/3 log brighter than corresponding internal normal CD4+ T cells (Figure 1C, 1F). PD-1 expression in the neoplastic T cells was seen at or above this level in 39/50 (78%) of the MF/SS cases (entire population in 36 cases and a distinct major subset [>50%] in 3 cases), making PD-1 the third most common aberrantly expressed antigen behind CD26 and CD7 (Figure 1B, 1F).
PD-1 differentiates Sezary cells from normal CD4+ T cells and significantly aids in accurately detecting Sezary cells in the context of established markers
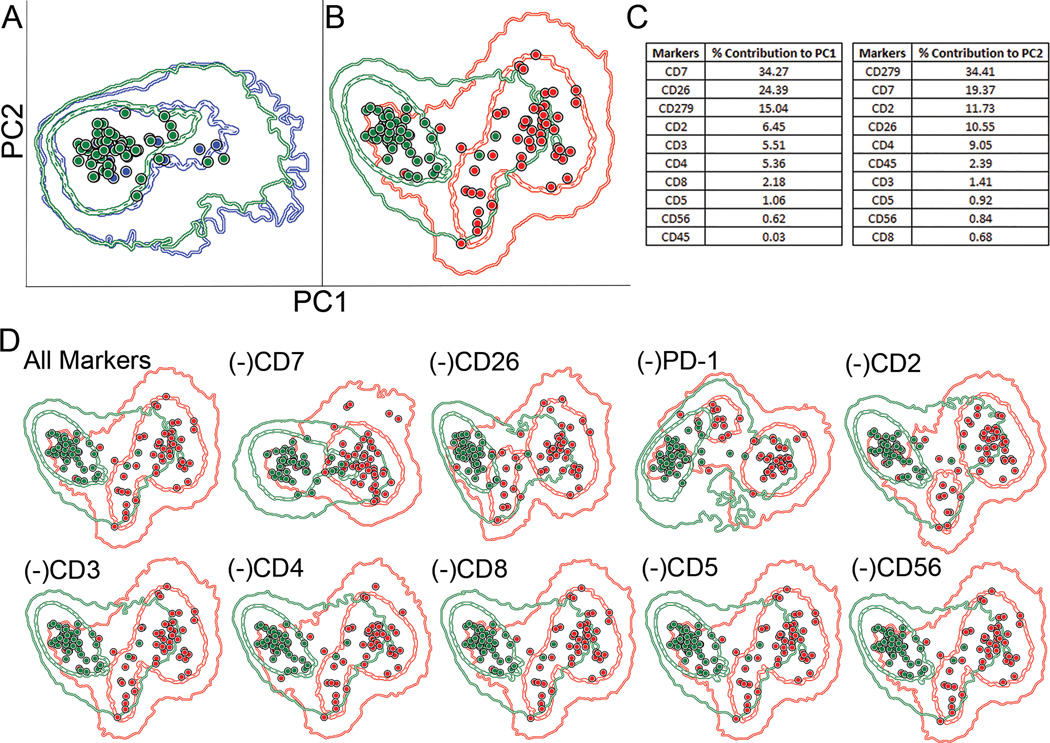
To assess the utility of PD-1 amongst the pan-T cell antigens and CD26, we first performed a principal component analysis (PCA) to calculate the relative contribution of each antigen in the panel to the separation of abnormal from internal normal CD4+ T cell populations in the MF/SS cases. First, the internal normal CD4+ T cell populations from the MF/SS cases were compared to those in the control samples. These two groups did not show significant separation with PCA (Figure 2A), supporting the internal normal CD4+ T cells as an appropriate control. PCA demonstrated separation of the abnormal T cell populations as a whole from the internal normal CD4+ T cell populations (Figure 2B). The percent contribution of each antigen to the separation of these two groups was evaluated and by PC1, PD-1 ranked 3rd behind CD7 and CD26 (Figure 2C). The PCA was again performed with the removal of one of each of the markers. The degree of separation between the abnormal and internal normal groups visibly decreased when CD7, CD26 and PD-1 were removed from the analysis while no visible change in separation was seen with removal of the other antigens (Figure 2D). The analysis was again performed comparing the abnormal T cell populations from each individual MF/SS case to the internal normal CD4+ T cell populations a whole. By PC1, PD-1 ranked in the top 3 markers in 32/50 (64%) cases and as 1st in 4/50 (8%) cases to the separation of these populations (Supplemental Table 1).
Figure 2. PD-1 contribution to standard Sezary cell markers by principal component analysis (PCA).
A. Comparison of normal control CD4+ T cell populations (each blue dot indicates one case [median], blue circles indicate the first and second standard deviations) to internal normal CD4+ T cells from the MF/SS cases (green dots and circles) showed no significant separation by PCA analysis. B. Separation of the internal normal CD4+ T cells from the abnormal T cell populations (red dots and circles) was identified. C. Relative contributions of the markers utilized to the principal component 1 (PC1) and principal component 2 (PC2) vectors. D. PCA analysis was performed after removal of 1 of each of the antigens. The top left plot demonstrates the analysis using all markers while the remaining plots exhibit analyses in which the marker indicated above was removed.
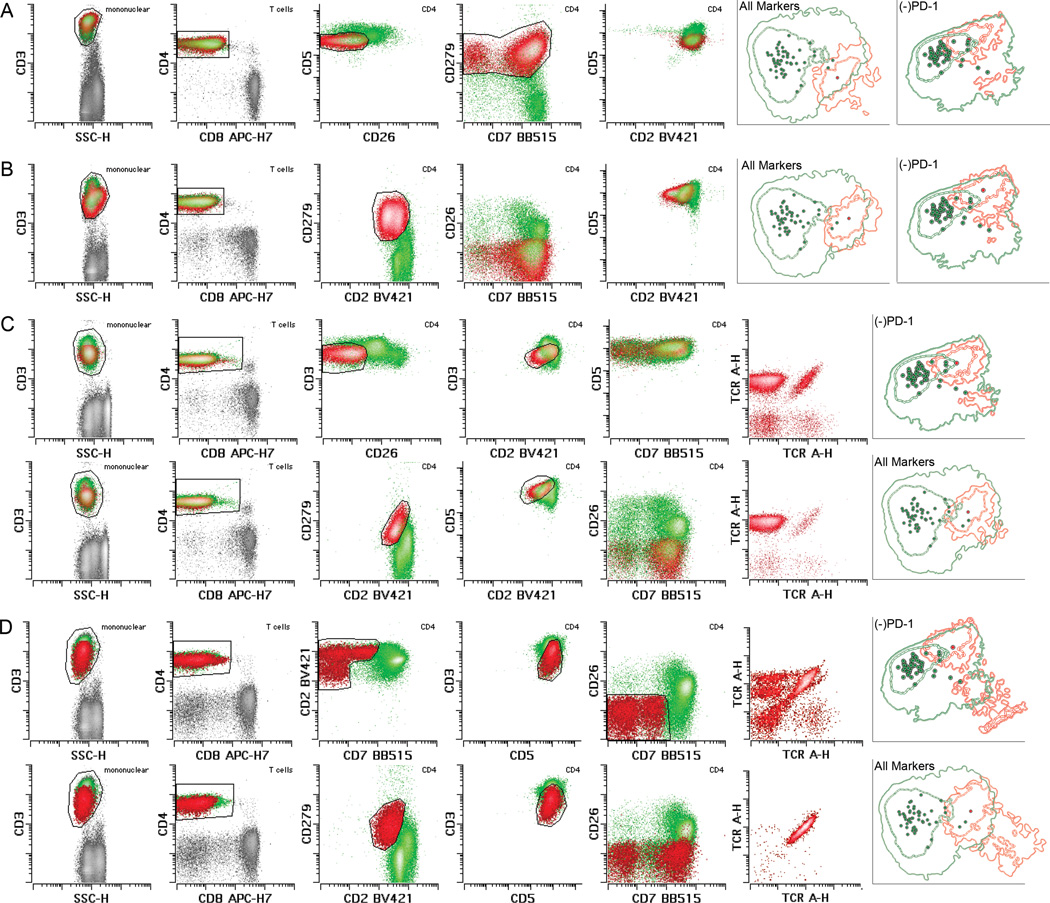
We next assessed PD-1 expression in MF/SS cases in which population separation was limited using standard Sezary markers. We identified 26 MF/SS cases in our cohort in which the abnormal T cell populations demonstrated few (≤4) and, aside from CD7 and CD26, subtle aberrations in the remaining markers in our panel (Figure 3). PD-1 overexpression clearly distinguished the aberrant T cell populations from internal normal CD4+ ones in 15 of these 26 more challenging cases (58%, 30% of total cases) (Figure 3). Among these 15 cases, 9, 3 and 1 cases demonstrated aberrations in only 3, 2 or 1 antigens (apart from PD-1), respectively. Additionally, 8 and 1 of these 15 cases showed largely normal CD7 and CD26 expression, respectively, although in all cases some overlap in CD7 and CD26 expression between normal and abnormal populations was seen. In 6 of these 15 cases (12% of total cases), the remaining phenotypic alterations were so subtle that the aberrant populations could easily have been missed without PD-1. Indeed, when these 6 cases were evaluated and gated both with and without PD-1 in a blinded fashion, a >10% difference in the size of the gated populations was identified in 2 cases (4% of total) (Figure 3C) while in 2 other cases (4%), gating was virtually impossible without the use of PD-1 (Figure 3D). Of note, when these 15 challenging cases were individually compared to the internal normal CD4+ T cell group using PCA, PD-1 ranked 1st in 4 cases, 2nd in 5 cases and 3rd in 6 cases to population separation, supporting our visual observations.
Figure 3. Utility of PD-1 in subtle MF/SS cases.
A, B, C and D each depict a case in which PD-1 provided clear visual separation between Sezary cells (red [emphasized in D]) and normal CD4+ T cells (green) while alterations of the remaining antigens were few and subtle, including normal CD7 expression. C and D depict example cases in which gating with the baseline panel without PD-1 in a blinded fashion (shown in the top rows) resulted in more inaccurate Sezary cell identification and enumeration compared to when PD-1 was included and used for gating (shown in the bottom rows), which was confirmed with TCR Vβ analysis (right most histograms). The sizes (percentage of total white cells) of the gated populations (red) in each case were: A: 16.9%; B: 11.4%; C top row (without PD-1): 4.87% (includes normal CD4+ T cells); C bottom row (with PD-1): 4.32% (largely pure Sezary cell population); D top row (without PD-1): 1.39% (includes normal CD4+ T cells); D bottom row (with PD-1): 2.0% (pure Sezary cell population). In each illustrated case, PCA (right most panels) demonstrated separation of the individual abnormal populations (red) from total internal normal CD4+ T cells (green). The degree of separation diminished when PD-1 was removed from the analysis (indicated by “(−)PD-1”). PD-1 in these cases provided clear, rapid and more efficient identification of the Sezary cell populations, improved separation of abnormal from normal populations, and aid in distinguishing normal T cell subsets with differential antigen expression from the truly abnormal populations.
TCR Vβ analysis of the aberrant T cell populations by flow cytometry was performed on 8 patients (concurrent sample in 2 patients, prior/subsequent sample in 6), including 5 with subtle phenotypes, all of which showed restricted (5/8) or deficient (3/8) Vβ usage, supporting monoclonality (Figures 1B, 3C–D). TCR gene rearrangement performed on 23 patients (concurrent sample in 15, prior/subsequent blood sample in 8) all demonstrated clonal rearrangements with clonal peaks similar to those identified in skin in 16 of 17 patients in which TCR gene rearrangement was performed on a skin sample (1 skin biopsy lacked a detectable clonal TCR gene rearrangement) (Figure 1B).
PD-1 expression in Sezary cells is largely stable over time in blood and bone marrow
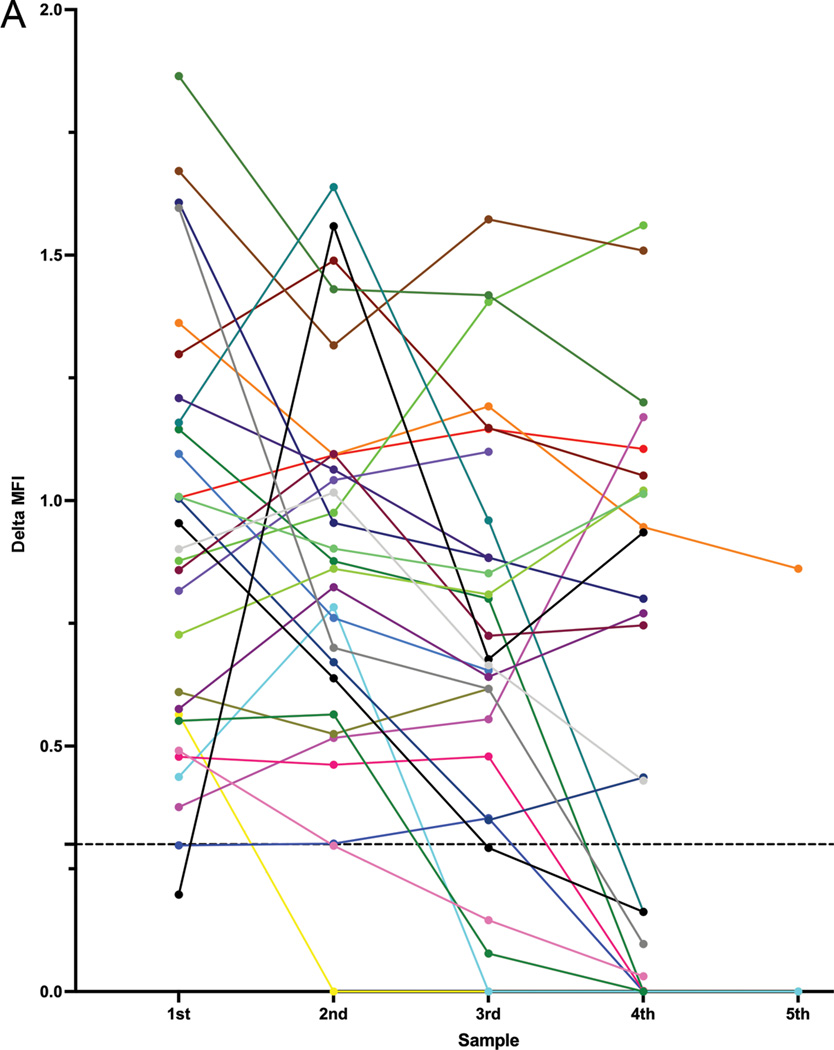
Lastly, we assessed the stability of PD-1 over time in blood and bone marrow samples (Figure 4). In total, 34 patients had at least 2 subsequent peripheral blood and/or bone marrow samples containing similar aberrant T cell populations available for analysis. In 18 of the 28 patients (64%) in which PD-1 was informative (≥1/3 log brighter than internal normal CD4+ T cells) in the initial sample, PD-1 expression in the aberrant T cell populations remained informative in all subsequent samples. In 4 patients, PD-1 was ~1/3 to ~1/2 log brighter than corresponding internal normal CD4+ T cells in the initial sample, which diminished below 1/3 log over time. In 3 patients, new PD-1 negative subsets emerged in subsequent samples while the original PD-1 positive population proportionally diminished. In 1 patient, PD-1 positive and PD-1 negative subsets were present initially, the proportions of which fluctuated over time, with the PD-1 positive subset being undetectable in rare cases. In 1 of the 6 patients (17%) in which PD-1 was not informative in the initial sample, PD-1 expression increased (>1/3 log brighter than internal normal CD4+ T cells) in subsequent samples. In total, PD-1 expression status remained the same over time in 68% of patients.
Figure 4. PD-1 expression in Sezary cells over time.
A. Log difference in PD-1 MFI between Sezary cell and internal normal CD4+ T cell populations in MF/SS patients. Each colored line represents 1 patient and up to 5 representative samples are depicted in chronological order. The 5 patients in which PD-1 remained uninformative over time are not depicted. Dotted horizontal line indicates the 1/3 log level.
Discussion
Although accurate detection of circulating Sezary cells is critical for prognostic and therapeutic evaluation in MF/SS, it can be difficult with recommended flow cytometric techniques and standard markers. Recent guidelines from a panel of flow cytometry experts recommended use of composite immunophenotypes to identify and quantify Sezary cells using a minimum of 6 markers (CD45, CD3, CD4, CD8, CD7, CD26) (12). However, even with such strategies, accurate Sezary cell detection can be challenging for several reasons, including the fact that standard markers (aside from CD7 and CD26) are often subtly altered and aberrations of virtually all routine markers (particularly CD7 and CD26) can be seen in normal T cell subsets. Indeed, despite having a large panel that could accommodate all pan T cell antigens and CD26, we found that over half of our cases were challenging due to few and subtle alterations of these markers. As we found no clear, distinct overexpression of PD-1 in normal T cells in blood, such aberration was highly suggestive of a neoplastic population, which then required confirmation and gate refinement with the remaining antigens. As such, PD-1 overexpression quickly and efficiently indicated the presence of an abnormal population, helped distinguish normal T cell subsets with differential expression of the other markers from a truly abnormal population, and provided better separation of abnormal from normal populations for improved gating and quantification when used in conjunction with the remaining antigens. Indeed, in 12% of cases, PD-1 was critical for accurate Sezary cell identification, without which accurate gating and quantification were significantly affected or nearly impossible. Our PCA also supported a high level of contribution of PD-1 to our panel of markers for distinguishing Sezary cells from normal CD4+ T cells both globally and on an individual case basis. While we recognize that a PCA may not fully reflect the practical utility of the markers in this context, it can act as another means to assess relative contributions of multiple antigens in an objective and quantitative manner and support our visual assessment, as has been done similarly in other studies (30, 31). We also note that while such automatic population separator (APS) tools can provide such type of valuable data, we do not believe APS gating is sufficient for identification of abnormal populations. More sophisticated computational tools such as artificial intelligence or stochastic neighbor embedding (SNE) variations, as used by other groups (32, 33), may be helpful but were not attempted in this study.
Peripheral blood flow cytometric analysis is also important for distinguishing patients with MF/SS from those with benign inflammatory skin conditions, which in some cases can be challenging using clinical presentation and skin histology alone. As some increased PD-1 expression has been shown in reactive T cells in inflammatory conditions, including infections (34), autoimmune disease (35) and other cancers (36), it was prudent to ensure PD-1 overexpression was not a feature of reactive change. Our control group consisted entirely of patients with dermatitis (n= 9) and/or a history of other cancer (n=14), which are inflammatory conditions. As previously mentioned, we did not observe clear, distinct populations of normal CD4+ T cells with PD-1 overexpression in our control group and PD-1 expression did not significantly differ between the control and internal normal CD4+ T cells (which similarly may be expected to have reactive change in the setting of concurrent MF/SS) in MFI or by PCA. Given these findings, our control populations are likely adequately reflective of reactive states.
Flow cytometric clonality assays, including TCR Vβ repertoire and, more recently, TRBC1 have shown to be useful tools for Sezary cell assessment (37, 38). These assays use restricted or deficient expression of the variable or constant regions of the T cell receptor to infer clonality. While these assays are useful for confirming the clonality of an immunophenotypically aberrant population, they can be problematic when used alone as clonal but reactive (non-neoplastic) T cell populations are not uncommon, particularly in reactive conditions such as a chronic rash, and can include CD4+/CD7-/CD26- populations (38–40). As flow cytometry is important for distinguishing patients with MF/SS from those with benign inflammatory skin conditions, extreme care must be taken to not interpret such benign clonal populations as abnormal in these patients. Thus, accurate Sezary cell detection still requires phenotypic assessment and markers like PD-1 that can aid in distinguishing abnormal from normal populations.
Our longitudinal analysis found that PD-1 expression remained stable over time in most patients. The etiology of the PD-1 status change seen in a minority of patients is uncertain (discussed below), but we saw no obvious association with timing or types of therapy. We saw a variable effect on Sezary cell detection among the few cases in which PD-1 resolution diminished over time. Population identification was most troublesome in the cases in which the remainder of the phenotypic alterations were subtle and the populations small. However, abnormal populations could be identified mostly because they had other alterations and a previously defined abnormal phenotype. In the most challenging cases, TCR Vβ repertoire or TRBC1 analysis may be helpful.
Interestingly, we found a strong trend towards higher PD-1 expression in clinically defined SS compared to MF, which did not reach statistical significance, but no difference in PD-1 expression between high and low-level disease or between initial and relapse/persistent disease. A greater degree of PD-1 positivity in SS as compared to MF has been described (17), although other studies have demonstrated similar levels of PD-1 expression (18, 22, 23, 41). However, even among studies showing overall similar PD-1 expression levels, some have reported trends toward higher PD-1 positivity in SS cases (22, 23, 41). While it has been suggested that this difference in PD-1 expression may reflect a differing underlying biology of these two entities (17), a recent study demonstrated genetic commonalities between MF and SS, suggesting they may represent two extremes of the same disease (42). This study also showed a correlation between lower-level PD-1 protein expression, a proliferative phenotype, and a high frequency of alterations of PDCD1, the gene that encodes PD-1, in MF and SS while higher level PD-1 expression correlated with a non-proliferative phenotype and a low frequency of PDCD1 alterations. PD-1 deletions also increased in frequency with increasing stage and were associated with shorter overall survival in both MF and SS. The authors suggested that PD-1 expression may in fact indicate disease progression and aggressiveness rather than a unique cell of origin in MF and SS. Although the aim of our study was the diagnostic rather than the prognostic value of PD-1, these results may explain the somewhat heterogeneous PD-1 expression seen in our cases as well as the diminishment of PD-1 over time in a few patients. While it suggests PD-1 could function as an important prognostic biomarker, further work and additional validation are needed to assess the prognostic or biologic utility of PD-1.
In summary, we show that PD-1 is commonly overexpressed in circulating Sezary cells, significantly contributes to accurate Sezary cell detection by flow cytometry in the context of pan T cell antigens and CD26, and is largely stable over time in blood and bone marrow. We recommend use of PD-1 in flow cytometry panels for Sezary cell assessment.
Supplementary Material
Acknowledgements:
This work was supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748, NIH/NCI MSK Lymphoma SPORE P50 CA192937 (A.D.) and Farmer Family Foundation (A.D.).
N.E.L. consults and is on an advisory board for United States Drug Testing Laboratories. A.J.M. has received research support from ADC Therapeutics, Beigene, Miragen, Seattle Genetics, Merck, Bristol-Myers Squibb, and Incyte and honoraria from Janpix Ltd., Merck, Seattle Genetics, and Takeda. S.M.H has received research support for clinical trials from ADC Therapeutics, Affimed, Celgene, Daiichi Sankyo, Kyowa Hakko Kirin, Millennium /Takeda, Seattle Genetics, Trillium Therapeutics, and Verastem/SecuraBio and has consulted for, received honorarium from, or participated on advisory boards for Acrotech Biopharma, C4 Therapeutics, Kyowa Hakko Kirin, Myeloid Therapeutics, ONO Pharmaceuticals, Seattle Genetics, SecuraBio, Shoreline Biosciences, Inc., Takeda, Trillium Therapeutics, Tubulis, and Vividion Therapeutics. A.D. has received personal consultancy fees from Roche, Corvus Pharmaceuticals, Physicians’ Education Resource, Seattle Genetics, Peerview Institute, Abbvie, Takeda and EUSA Pharma and research support from Roche and Takeda. M.R. participates in a scientific advisory board for and has equity in Auron Therapeutics, Inc., has received research support from Cellularity, Roche-Genentech, Beat AML, and Bayer and has received consultancy fees from Peerview Institute, Celgene and Physicians’ Education Resource.
Footnotes
Conflicts of Interest: The remaining authors declare no competing financial interests related to this work.
References
- 1.Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105(10):3768–85. [DOI] [PubMed] [Google Scholar]
- 2.Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110(6):1713–22. [DOI] [PubMed] [Google Scholar]
- 3.Vonderheid EC, Bernengo MG, Burg G, Duvic M, Heald P, Laroche L, et al. Update on erythrodermic cutaneous T-cell lymphoma: report of the International Society for Cutaneous Lymphomas. J Am Acad Dermatol. 2002;46(1):95–106. [DOI] [PubMed] [Google Scholar]
- 4.Jawed SI, Myskowski PL, Horwitz S, Moskowitz A, Querfeld C. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. Prognosis, management, and future directions. J Am Acad Dermatol. 2014;70(2):223.e1–17; quiz 40–2. [DOI] [PubMed] [Google Scholar]
- 5.Guitart J. Sézary syndrome and mycosis fungoides flow cytometric evaluation: The clinicians’ perspective. Cytometry B Clin Cytom. 2021;100(2):129–31. [DOI] [PubMed] [Google Scholar]
- 6.Horna P, Deaver DM, Qin D, Moscinski LC, Sotomayor EM, Glass LF, et al. Quantitative flow cytometric identification of aberrant T cell clusters in erythrodermic cutaneous T cell lymphoma. Implications for staging and prognosis. Journal of clinical pathology. 2014;67(5):431–6. [DOI] [PubMed] [Google Scholar]
- 7.Craig FE. It is time to adopt a multicolor immunophenotyping approach to evaluate blood for Sézary syndrome and mycosis fungoides. Cytometry B Clin Cytom. 2021;100(2):125–8. [DOI] [PubMed] [Google Scholar]
- 8.Novelli M, Fava P, Sarda C, Ponti R, Osella-Abate S, Savoia P, et al. Blood flow cytometry in Sézary syndrome: new insights on prognostic relevance and immunophenotypic changes during follow-up. Am J Clin Pathol. 2015;143(1):57–69. [DOI] [PubMed] [Google Scholar]
- 9.Lyapichev KA, Bah I, Huen A, Duvic M, Routbort MJ, Wang W, et al. Determination of immunophenotypic aberrancies provides better assessment of peripheral blood involvement by mycosis fungoides/Sézary syndrome than quantification of CD26- or CD7- CD4+ T-cells. Cytometry B Clin Cytom. 2021;100(2):183–91. [DOI] [PubMed] [Google Scholar]
- 10.Boonk SE, Zoutman WH, Marie-Cardine A, van der Fits L, Out-Luiting JJ, Mitchell TJ, et al. Evaluation of Immunophenotypic and Molecular Biomarkers for Sézary Syndrome Using Standard Operating Procedures: A Multicenter Study of 59 Patients. J Invest Dermatol. 2016;136(7):1364–72. [DOI] [PubMed] [Google Scholar]
- 11.Jones D, Dang NH, Duvic M, Washington LT, Huh YO. Absence of CD26 expression is a useful marker for diagnosis of T-cell lymphoma in peripheral blood. Am J Clin Pathol. 2001;115(6):885–92. [DOI] [PubMed] [Google Scholar]
- 12.Horna P, Wang SA, Wolniak KL, Psarra K, Almeida J, Illingworth AJ, et al. Flow cytometric evaluation of peripheral blood for suspected Sézary syndrome or mycosis fungoides: International guidelines for assay characteristics. Cytometry B Clin Cytom. 2021;100(2):142–55. [DOI] [PubMed] [Google Scholar]
- 13.Çetinözman F, Jansen PM, Willemze R. Expression of programmed death-1 in skin biopsies of benign inflammatory vs. lymphomatous erythroderma. Br J Dermatol. 2014;171(3):499–504. [DOI] [PubMed] [Google Scholar]
- 14.Fife BT, Pauken KE. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann N Y Acad Sci. 2011;1217:45–59. [DOI] [PubMed] [Google Scholar]
- 15.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 2011;29:621–63. [DOI] [PubMed] [Google Scholar]
- 17.Cetinözman F, Jansen PM, Vermeer MH, Willemze R. Differential expression of programmed death-1 (PD-1) in Sézary syndrome and mycosis fungoides. Arch Dermatol. 2012;148(12):1379–85. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen GH, Olson LC, Magro CM. Upregulation of inhibitory signaling receptor programmed death marker-1 (PD-1) in disease evolution from cutaneous lymphoid dyscrasias to mycosis fungoides and Sezary’s syndrome. Ann Diagn Pathol. 2017;28:54–9. [DOI] [PubMed] [Google Scholar]
- 19.Bosisio FM, Cerroni L. Expression of T-follicular helper markers in sequential biopsies of progressive mycosis fungoides and other primary cutaneous T-cell lymphomas. Am J Dermatopathol. 2015;37(2):115–21. [DOI] [PubMed] [Google Scholar]
- 20.Kantekure K, Yang Y, Raghunath P, Schaffer A, Woetmann A, Zhang Q, et al. Expression patterns of the immunosuppressive proteins PD-1/CD279 and PD-L1/CD274 at different stages of cutaneous T-cell lymphoma/mycosis fungoides. Am J Dermatopathol. 2012;34(1):126–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JH, Han JH, Kang HY, Lee ES, Kim YC. Expression of follicular helper T-cell markers in primary cutaneous T-cell lymphoma. Am J Dermatopathol. 2014;36(6):465–70. [DOI] [PubMed] [Google Scholar]
- 22.Wada DA, Wilcox RA, Harrington SM, Kwon ED, Ansell SM, Comfere NI. Programmed death 1 is expressed in cutaneous infiltrates of mycosis fungoides and Sézary syndrome. American journal of hematology. 2011;86(3):325–7. [DOI] [PubMed] [Google Scholar]
- 23.Horna P, Moscinski LC, Sokol L, Shao H. Naïve/memory T-cell phenotypes in leukemic cutaneous T-cell lymphoma: Putative cell of origin overlaps disease classification. Cytometry B Clin Cytom. 2019;96(3):234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray D, McMurray JL, Eldershaw S, Pearce H, Davies N, Scarisbrick JJ, et al. Progression of mycosis fungoides occurs through divergence of tumor immunophenotype by differential expression of HLA-DR. Blood Adv. 2019;3(4):519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yabe M, Gao Q, Ozkaya N, Huet S, Lewis N, Pichardo JD, et al. Bright PD-1 expression by flow cytometry is a powerful tool for diagnosis and monitoring of angioimmunoblastic T-cell lymphoma. Blood Cancer J. 2020;10(3):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samimi S, Benoit B, Evans K, Wherry EJ, Showe L, Wysocka M, et al. Increased programmed death-1 expression on CD4+ T cells in cutaneous T-cell lymphoma: implications for immune suppression. Arch Dermatol. 2010;146(12):1382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saulite I, Ignatova D, Chang YT, Fassnacht C, Dimitriou F, Varypataki E, et al. Blockade of programmed cell death protein 1 (PD-1) in Sézary syndrome reduces Th2 phenotype of non-tumoral T lymphocytes but may enhance tumor proliferation. Oncoimmunology. 2020;9(1):1738797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langerak AW, Groenen PJ, Brüggemann M, Beldjord K, Bellan C, Bonello L, et al. EuroClonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations. Leukemia. 2012;26(10):2159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willemze R, Cerroni L, Kempf W, Berti E, Facchetti F, Swerdlow SH, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133(16):1703–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Q, Yellapantula V, Fenelus M, Pichardo J, Wang L, Landgren O, et al. Tumor suppressor CD99 is downregulated in plasma cell neoplasms lacking CCND1 translocation and distinguishes neoplastic from normal plasma cells and B-cell lymphomas with plasmacytic differentiation from primary plasma cell neoplasms. Mod Pathol. 2018;31(6):881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Dongen JJ, Lhermitte L, Böttcher S, Almeida J, van der Velden VH, Flores-Montero J, et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26(9):1908–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goshaw JM, Gao Q, Wardrope J, Dogan A, Roshal M. 14-Color single tube for flow cytometric characterization of CD5+ B-LPDs and high sensitivity automated minimal residual disease quantitation of CLL/SLL. Cytometry B Clin Cytom. 2021;100(4):509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amir el AD, Davis KL, Tadmor MD, Simonds EF, Levine JH, Bendall SC, et al. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat Biotechnol. 2013;31(6):545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jubel JM, Barbati ZR, Burger C, Wirtz DC, Schildberg FA. The Role of PD-1 in Acute and Chronic Infection. Front Immunol. 2020;11:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang S, Wang L, Li M, Zhang F, Zeng X. The PD-1/PD-L pathway in rheumatic diseases. J Formos Med Assoc. 2021;120(1 Pt 1):48–59. [DOI] [PubMed] [Google Scholar]
- 36.Xie M, Huang X, Ye X, Qian W. Prognostic and clinicopathological significance of PD-1/PD-L1 expression in the tumor microenvironment and neoplastic cells for lymphoma. Int Immunopharmacol. 2019;77:105999. [DOI] [PubMed] [Google Scholar]
- 37.Feng B, Jorgensen JL, Jones D, Chen SS, Hu Y, Medeiros LJ, et al. Flow cytometric detection of peripheral blood involvement by mycosis fungoides and Sézary syndrome using T-cell receptor Vbeta chain antibodies and its application in blood staging. Mod Pathol. 2010;23(2):284–95. [DOI] [PubMed] [Google Scholar]
- 38.Horna P, Shi M, Jevremovic D, Craig FE, Comfere NI, Olteanu H. Utility of TRBC1 Expression in the Diagnosis of Peripheral Blood Involvement by Cutaneous T-Cell Lymphoma. J Invest Dermatol. 2021;141(4):821–9.e2. [DOI] [PubMed] [Google Scholar]
- 39.Shi M, Jevremovic D, Otteson GE, Timm MM, Olteanu H, Horna P. Single Antibody Detection of T-Cell Receptor αβ Clonality by Flow Cytometry Rapidly Identifies Mature T-Cell Neoplasms and Monotypic Small CD8-Positive Subsets of Uncertain Significance. Cytometry B Clin Cytom. 2020;98(1):99–107. [DOI] [PubMed] [Google Scholar]
- 40.Shi M, Olteanu H, Jevremovic D, He R, Viswanatha D, Corley H, et al. T-cell clones of uncertain significance are highly prevalent and show close resemblance to T-cell large granular lymphocytic leukemia. Implications for laboratory diagnostics. Mod Pathol. 2020;33(10):2046–57. [DOI] [PubMed] [Google Scholar]
- 41.Khodadoust MS, Rook AH, Porcu P, Foss F, Moskowitz AJ, Shustov A, et al. Pembrolizumab in Relapsed and Refractory Mycosis Fungoides and Sézary Syndrome: A Multicenter Phase II Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020;38(1):20–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park J, Daniels J, Wartewig T, Ringbloom KG, Martinez-Escala ME, Choi S, et al. Integrated genomic analyses of cutaneous T-cell lymphomas reveal the molecular bases for disease heterogeneity. Blood. 2021;138(14):1225–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.