Abstract
Background and Objectives
Neurocognitive outcomes after surgery for temporal lobe epilepsy in childhood are variable. Postoperative changes are not directly predicted by seizure freedom, and associations between epilepsy, neuropsychological function, and developing neural networks are poorly understood. Here, we leveraged whole-brain connectomic profiling in magnetoencephalography (MEG) to retrospectively study associations between brain connectivity and neuropsychological function in children with temporal lobe epilepsy undergoing resective surgery.
Methods
Clinical and MEG data were retrospectively analyzed for children who underwent temporal lobe epilepsy surgery at the Hospital for Sick Children from 2000 to 2021. Resting-state connectomes were constructed from neuromagnetic oscillations via the weighted-phase lag index. Using a partial least-squares (PLS) approach, we assessed multidimensional associations between patient connectomes, neuropsychological scores, and clinical covariates. Bootstrap resampling statistics were performed to assess statistical significance.
Results
A total of 133 medical records were reviewed, and 5 PLS analyses were performed. Each PLS analysis probed a particular neuropsychological domain and the associations between its baseline and postoperative scores and the connectomic data. In each PLS analysis, a significant latent variable was identified, representing a specific percentage of the variance in the data and relating neural networks to clinical covariates, which included changes in rote verbal memory (n = 41, p = 0.01, σ2 = 0.38), narrative/verbal memory (n = 57, p = 0.00, σ2 = 0.52), visual memory (n = 51, p = 0.00, σ2 = 0.43), working memory (n = 44, p = 0.00, σ2 = 0.52), and overall intellectual function (n = 59, p = 0.00, σ2 = 0.55). Children with more diffuse, bilateral intrinsic connectivity across several frequency bands showed lower scores on all neuropsychological assessments but demonstrated a greater propensity for gains after resective surgery.
Discussion
Here, we report that connectomes characterized by diffuse connectivity, reminiscent of developmentally immature networks, are associated with lower preoperative cognition and postoperative cognitive improvement. These findings provide a potential means to understand neurocognitive function in children with temporal lobe epilepsy and expected changes postoperatively.
The temporal lobe is critical to cognition, mediating learning and memory of verbal and nonverbal information and language. While resection of the temporal lobe is an effective treatment for pediatric temporal lobe epilepsy,1 the effects of surgery on temporal lobe–mediated cognition are difficult to predict, with previous works reporting postoperative cognitive improvement, preservation, and deterioration.2 A substantial body of literature suggests that memory is most vulnerable to decline after surgery for temporal lobe epilepsy, while measures of intelligence tend to remain stable.3 However, intellectual gains and improvements in long-term memory also have previously been demonstrated in individuals who underwent temporal lobe surgery in childhood.4 Likewise, inconsistent findings have been reported for postoperative outcomes in attention and working memory.3
The heterogeneity of postoperative cognitive outcomes in temporal lobe epilepsy is likely influenced by a multitude of factors, including age at seizure onset, hemispheric laterality, and preoperative cognitive ability.5,6 It has previously been suggested that children may experience greater overall cognitive improvement after temporal lobe epilepsy surgery relative to adults, which could potentially be attributed to the plasticity and compensational capacity of the developing brain, although this work was limited by short-term follow-up.5 Furthermore, the manifestation of postoperative cognitive deficits differs between children and adults.2 In adults, there is evidence of lateralized, material-specific memory deficits after temporal lobectomy: (1) left temporal lobectomy is associated with a decline in language and verbal memory, and (2) right temporal lobectomy is associated with a decline in visual memory. In children, these findings are less conclusive.2
Given the variability and inconsistency of findings in the literature, there is a critical need to better understand neural correlates of postoperative cognitive outcomes in children with temporal lobe epilepsy, which can be used to inform clinical decision-making and postoperative expectations. Recently, the use of connectomic neuroimaging data has emerged as a promising predictor of cognitive outcome after temporal lobe surgery.7 To date, however, there has been no concerted effort to leverage brain network connectomics to understand postoperative cognitive outcomes in children with temporal lobe epilepsy.
In the current work, we analyzed preoperative connectomic profiles of children computed from magnetoencephalography (MEG) recordings to elucidate the relationship between brain connectivity and cognitive outcomes in children who underwent surgery for temporal lobe epilepsy. We conducted a retrospective review of a large and unique database of MEG recordings from the Hospital for Sick Children spanning >20 years. We performed a partial least-squares (PLS) analysis to probe multidimensional associations between brain connectivity and clinical patient phenotypes, including preoperative and postoperative cognitive scores. The results of this work provide a potential means to understand neurocognitive changes in patients with temporal lobe epilepsy and postoperative course through the use of brain network connectomics.
Methods
Population
We retrospectively reviewed the medical records of children who underwent resection for the treatment of drug-resistant temporal lobe epilepsy at the Hospital for Sick Children from 2000 to 2021. Patients were excluded if they (1) required sedation for MEG, (2) demonstrated excessive head motion during scanning (i.e., >5-mm head displacement), (3) exhibited abnormalities on MRI that precluded accurate image registration, or (4) lacked preoperative or postoperative neuropsychological assessment scores in the neuropsychological measure of interest.
Standard Protocol Approvals, Registrations, and Patient Consents
The current study complies with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and was approved by the research ethics boards of the Hospital for Sick Children. Institutional waiver of informed consent was obtained.
Neuropsychological Measures
Patients underwent detailed preoperative and postoperative neuropsychological assessments. Postoperative neuropsychological assessments occurred 1 year after surgery. Measures of intellectual function, working memory, verbal memory, and nonverbal memory were selected for the present study. Measures of Full Scale IQ (FSIQ) and the Working Memory Index were obtained from the Wechsler Preschool and Primary Scale of Intelligence,8 Reynolds Intellectual Assessment Scale,9 Wechsler Intelligence Scale for Children,10 or the Wechsler Adult Intelligence Scale III or IV.11,12 These were standard scores with a mean of 100 and an SD of 15. Immediate and delayed memory scores for faces and stories were obtained from the Children's Memory Scale13 and the Wechsler Memory Scale.14 These were scaled scores with a mean of 10 and an SD of 3. In addition, immediate and delayed rote verbal memory for a list of words was obtained from the California Verbal Learning Test–Second Edition15 and Children's Auditory Verbal Learning Task–Second Edition.16 These were converted to standard scores with a mean of 100 and an SD of 15.
Classification by Seizure Outcome and Extent of Epilepsy
Seizure outcome was determined at 1 year postoperatively, approximately at the time of postoperative neuropsychological assessment. Children were classified as seizure-free (Engel class IA) or not seizure-free (other than Engel class IA). Children were further classified by the extent of their epilepsy beyond the temporal lobe (i.e., temporal lobe or temporal lobe plus). Children were identified as having temporal lobe plus epilepsy if they underwent both temporal and extratemporal resections. Conversely, children were identified as having temporal lobe epilepsy if they underwent temporal resections and were subsequently deemed seizure-free. Children who underwent temporal resections and had recurrence of seizures (i.e., not seizure-free) required further postoperative investigation to be classified because recurrence of seizures could be the result of residual epileptogenic foci within the temporal lobe or unrecognized temporal lobe plus epilepsy. This determination was based on the best available evidence from all noninvasive modalities, including MEG, EEG, or PET, as well as subsequent postsurgical outcome.
MEG Acquisition
Patients underwent MEG with a whole-head gradiometer-based CTF system (151 channels, CTF MEG International Services, Coquitlam, British Columbia, Canada) in a magnetically shielded room. Fifteen 2-minute segments of resting-state data were recorded with concurrent MEG and 10-20 scalp EEG. The data were sampled at 625 Hz, as previously published,17,18 and for a minority of patients, the data were sampled at 1,250 Hz and down-sampled to 625 Hz. Each epoch of data was classified by state of vigilance (i.e., awake or drowsy/asleep) by an experienced neurophysiologist. Only epochs with patients awake were used in subsequent analyses. Nine children were sedated for MEG acquisition and were therefore excluded from analysis. In addition, head positions were measured at the beginning and end of each 2-minute segment, and data from 2-minute segments with >5 mm of head displacement between the beginning and end of the recording were discarded.
The data were imported into MATLAB (MathWorks, Natick, MA) with the FieldTrip toolbox and preprocessed with a fourth-order Butterworth filter (1–150 Hz) and a notch filter (60 Hz and harmonics) to remove line noise. Artifactual channels were removed, while electrocardiographic and electrooculographic artifacts were detected and removed with independent component analysis.
Construction of Connectomes
We used SPM12 to nonlinearly register each patient's individual T1 scan to the standard ICBM152 template in Montreal Neurologic Institute space.19 In addition, single-shell head models were derived by fitting spheres to each patient's inner skull, scalp, and brain surface.20 The centroid of each region in the Desikan-Killiany DK60 atlas21 was transformed from template space into individual headspaces of MEG acquisitions. The regional MEG signals were extracted from each brain region using vector beamforming; virtual sensors within each atlas region were estimated with a linearly constrained minimum variance beam former implemented in FieldTrip.22 This involved a spatial filtering method that passed neuronal activity within the region of interest while maximally attenuating all neuronal activity from outside the target region. This method assumed that no 2 areas of the brain were highly temporally correlated. Tikhonov regularization of the covariance matrix with 1% of the maximal eigenvector value was performed to generate broadband time series from each source location and to increase robustness of the source estimates.
Using filters designed with fir1 in MATLAB, we filtered reconstructed MEG data from each source into the canonical frequency bands, including delta (1–4 Hz), theta (4–7 Hz), alpha (8–12 Hz), beta (15–30 Hz), and gamma (>30 Hz). These neural oscillations mediate functional connectivity, which facilitates interregional communication that is critical to neurocognition.23 Neural oscillations at different frequencies serve specialized functions in neurocognition. For example, synchronization in high frequencies is thought to underlie stimulus-driven, bottom-up processing. Conversely, low-frequency synchronization is thought to underlie context-driven, top-down processing.24 Indeed, previous work in resting-state MEG demonstrates that enhanced connectivity in the theta band is related to better cognitive performance in working memory and attention in particular.25 On the other hand, studies leveraging MEG with children who were born very preterm or children with autism show reduced theta connectivity in cortical areas involved in working memory and attention, respectively.26,27
The analytic signal, H(t), was obtained from the filtered signal, f(t), using the Hilbert transform for each source and frequency. The weighted-phase lag index (wPLI)28 is used to estimate the phase synchrony between each source pair, calculated as follows:
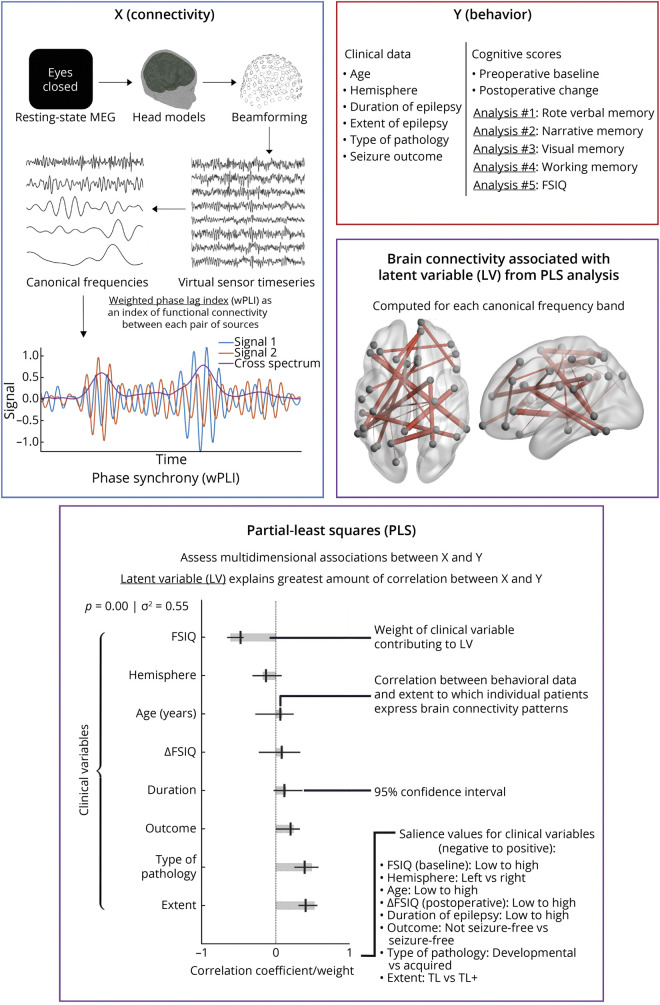
where E{} is the expected value operator and T(X) is the imaginary component of the cross-spectrum. The values of the wPLI served as an index of functional connectivity between each pair of sources, ranging from 0 (representing no phase synchrony) to a maximal value of 1 (representing 2 signals that are 90° out of phase). These values were used to generate an adjacency matrix for each patient. A schematic of the experimental methodology, including illustration of the MEG pipeline, is depicted in Figure 1.
Figure 1. Schematic of the Overall Experimental Methodology Involving Analysis of Clinical and MEG Data From Children With Temporal Lobe Epilepsy.
Resting-state data from magnetoencephalography (MEG) were acquired and connectomes were derived from neuromagnetic oscillations with the weighted-phase lag index (wPLI). Clinical and neuropsychological data were acquired through retrospective chart review and administration of preoperative and postoperative neuropsychological tests. Multidimensional associations between patient connectomes, neurocognitive function (e.g., rote verbal memory, narrative memory, visual memory, working memory, and Full Scale IQ [FSIQ]), and clinical covariates were probed with a partial least-squares (PLS) analysis.
PLS Analysis
PLS analysis29 was used to discover multivariate relationships within our connectomic and clinical patient phenotype dataset. PLS is a mathematical technique that identifies joint correlates within and between 2 sets of variables (i.e., brain and behavioral results) and is particularly valuable when used on datasets with hypothesized multicollinearity, e.g., a battery of psychometric test scores. Conceptually, similar to the principal component analysis, PLS reduces the signal into a set of subcomponents. The goal of PLS, however, is to maximize explained variance in the dependent variable within a minimal number of latent variables (LVs).30 An illustration of PLS and its components is included in Figure 1. Here, the clinical variables of interest included age at the time of MEG data acquisition; cause of epilepsy (e.g., acquired in cases of gliosis, cavernomas/cavernous hemangiomas, tumors, mesial temporal sclerosis, hemorrhagic infarction, or chronic encephalitis; developmental in cases of cortical dysplasia, architectural cortical lamination abnormalities, patchy heterotopic gray matter/ectopic neurons, microcortical dysgenesis, tuberous sclerosis, polymicrogyria, or augmented population of oligodendrocyte-like cells)31-35; extent of epilepsy beyond the temporal lobe (i.e., temporal lobe vs temporal lobe plus); proportion of life with epilepsy; hemisphere of seizure laterality (i.e., left vs right); seizure outcome (i.e., not seizure-free vs seizure-free); preoperative neuropsychological score; and change in neuropsychological score after surgery.
After data acquisition, the data were separated into predictor matrix X, representing the independent variables (i.e., the connectomic data) and behavior matrix Y, representing the dependent variables (i.e., the clinical patient phenotypes). Each row of X contained patient-specific wPLI data for all frequency bands, whereas the columns of Y included data-centered clinical patient phenotypes.
We used the open-source PLS MATLAB toolbox from the Rotman Research Institute (Toronto, Ontario, Canada) to perform the PLS analysis. As previously described,36 PLS calculates the covariance between X and Y (equation 2) and then uses the singular value decomposition (SVD) to decompose the resulting heterogeneous covariance matrix, R, as shown in equation 3 and Figure 1. The SVD produces components, known as LVs, which explain the greatest amount of correlation between X and Y.
R = YTX (2)
USVT = RT (3)
In equation 3, U is an SVD matrix that contains the LVs representing the connectomic data, and S is an SVD matrix that contains the LVs representing the clinical data and neuropsychological scores. Last, V is a matrix that holds the relative weights of these variables as they relate to the PLS model. In analysis of the contribution of an individual component to the overall variance in R, the effect size and amount of variance explained by each may be derived by calculating the ratio of a single squared value from the U or S matrix to the sum of all squared values within the matrices. By projecting weights onto an individual patient's data, we determine how closely an individual patient's data aligns with the PLS model created.
Statistical Analysis
Monte Carlo permutation methods were used for statistical analysis of the PLS model. First, we assessed the probability of the LV arising by chance using a permutation test. Second, we identified stable clinical/neuropsychological and connectomic contributors to the LV through bootstrapping.
In the permutation test, we generated a distribution of singular values under the null hypothesis of no association between the connectomic and clinical/neuropsychological data. The rows of X were randomly permuted 500 times, and each time the SVD was recomputed. The resulting distribution provided an estimate of the probability of each LV arising by chance under the null hypothesis. Significance was determined using a value of p = 0.05.
The stability of the source saliences was tested by bootstrapping, a process of sampling with replacement. Bootstrapping was performed 500 times to identify elements of U (connectivity) that were stable regardless of the patients in the sample. For each bootstrap, the data were rotated by a Procrustes rotation to align and scale the recomputed U and V to the SVD of the original nonbootstrapped data. The resampling distribution was used to calculate standard errors and 95% CIs for the contributions of each covariate to the component. A bootstrap ratio, the ratio of each element in U to its bootstrap-estimated standard error (similar to a z score), was calculated to estimate the statistical reliability of each source weighted by its contribution to the overall LV. Significance was determined using |z| > 2.58, corresponding to p < 0.01 under the assumption of an approximately normal distribution.
A total of 5 PLS analyses were performed, and the results were corrected for multiple comparisons with the Bonferroni method.37 Each PLS analysis probed a particular neuropsychological domain and the associations between its baseline and postoperative scores and the connectomic data. The 5 neuropsychological domains were analyzed in separate PLS models on the basis of the assumption that different patterns of intrinsic brain connectivity could foreseeably be associated with each domain and its postoperative trajectory.
Data Availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Results
Clinical Covariates
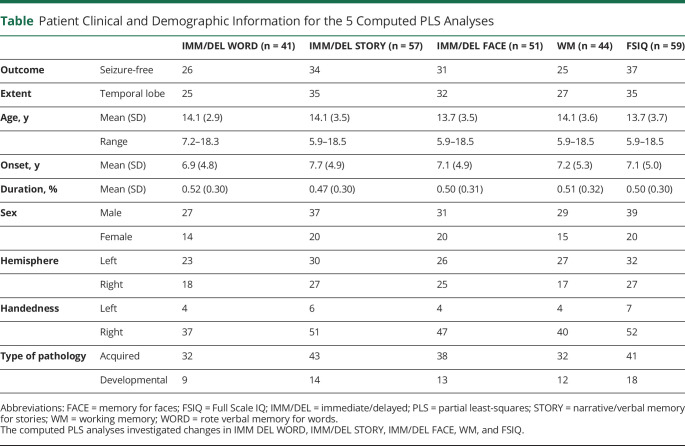
One hundred thirty-three children who had undergone temporal lobe epilepsy surgery at the Hospital for Sick Children were initially identified. From this cohort, 41 children (mean age 14.1 years, 25 with temporal lobe epilepsy, 26 seizure-free, 23 with left hemisphere resections) were included in the first analysis of rote verbal memory, which was indexed by immediate and delayed memory for a list of words. The average score for baseline immediate rote verbal memory was 90 (SD 19), and the average score for baseline delayed rote verbal memory was 96 (SD 19). Second, 57 children (mean age 14.1 years, 35 with temporal lobe epilepsy, 34 seizure-free, 30 with left hemisphere resections) were included in the analysis of narrative/verbal memory, which was indexed by immediate and delayed memory for short stories. The average score for baseline immediate narrative memory was 8 (SD 3), and the average score for baseline delayed narrative memory was 8 (SD 3). Third, 51 children (mean age 13.7 years, 32 with temporal lobe epilepsy, 31 seizure-free, 26 with left hemisphere resections) were included in the analysis of visual memory, which was indexed by immediate and delayed memory for faces. The average score for baseline immediate visual memory was 8 (SD 4), and the average score for baseline delayed visual memory was 9 (SD 3). Fourth, 44 children (mean age 14.1 years, 27 with temporal lobe epilepsy, 25 seizure-free, 27 with left hemisphere resections) were included in the analysis of working memory. The average score for working memory was 90 (SD 19). Fifth, 59 children (mean age 13.7 years, 35 with temporal lobe epilepsy, 37 seizure-free, 32 with left hemisphere resections) were included in the analysis of FSIQ. The average score for baseline FSIQ was 85 (SD 18). The clinical and demographic information of the patients is summarized in the Table. It is notable that no patients in this cohort had bitemporal epilepsy.
Table.
Patient Clinical and Demographic Information for the 5 Computed PLS Analyses
Verbal Memory
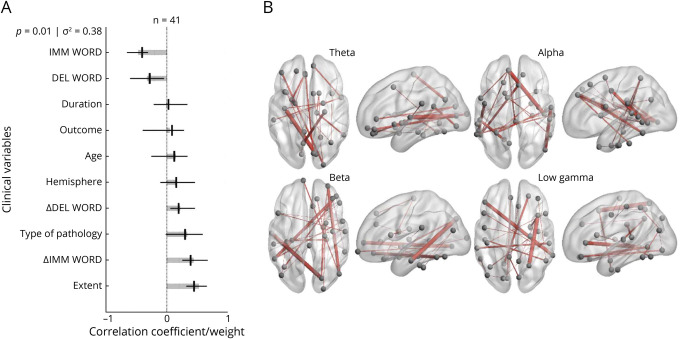
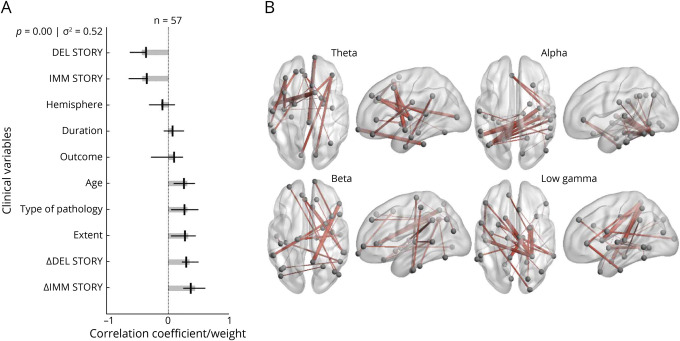
On assessment of verbal memory, we identified an association between diffuse, or anatomically widespread,38-41 bilateral connectivity in multiple frequency bands and lower baseline verbal memory function, improvement in verbal memory after resection, and greater extent of epilepsy (temporal lobe plus vs temporal lobe). For rote verbal memory, a single significant LV was identified, representing 38% of the variance in the data (Figure 2A). Significant contributions to this LV included temporal lobe plus epilepsy, lower preoperative scores in immediate and delayed word list memory, and improved postoperative scores in immediate and delayed word list memory. The connectomic profiles that were associated with this LV were characterized by bilateral connections that were diffusely distributed in the theta, alpha, beta, and gamma bands across several important nodes, including the superior frontal gyri, left insula, left fusiform, left pars triangularis, right precuneus, right temporal lobe, and right inferior parietal lobe (Figure 2B). For narrative/verbal memory, we identified a single significant LV representing 52% of the variance in the data (Figure 3A). Significant contributions to this component included temporal lobe plus epilepsy, developmental cause of epilepsy, older age, lower preoperative scores in immediate and delayed story memory, and improved postoperative scores in immediate and delayed story memory. The connectomic profiles that were associated with this LV were marked by strong bitemporal connectivity in the alpha band (Figure 3B).
Figure 2. PLS Analysis Investigating Associations Between Rote Verbal Memory Scores, Clinical Covariates, and Connectomic Data.
(A) Contributions of clinical covariates and rote verbal memory scores to the latent variable (LV). LV accounts for 38% of the total variance and is significant (p = 0.01). LV weights are in gray. Correlations (95% CIs) between the behavioral data and the extent to which individual patients express the brain connectivity patterns shown in panel B are in black. Preoperative and postoperative scores for rote verbal memory and extent of epilepsy are significant behavioral components of the LV. (B) Brain activity associated with the LV. Important nodes include the left insula and right precuneus in the theta band, the left superior frontal gyrus and right temporal lobe in the alpha band, the right superior frontal gyrus and left fusiform in the beta band, and the left pars triangularis and right inferior parietal lobe in the low gamma band. DEL = delayed; IMM = immediate; PLS = partial least-squares; WORD = rote verbal memory for words.
Figure 3. PLS Analysis Investigating Associations Between Narrative Verbal Memory Scores, Clinical Covariates, and Connectomic Data.
(A) Contributions of clinical covariates and narrative/verbal memory scores to the latent variable (LV). LV accounts for 52% of the total variance and is significant (p = 0.00). LV weights are in gray. Correlations (95% CI) between the behavioral data and the extent to which individual patients express the brain connectivity patterns shown in panel B are in black. Preoperative and postoperative scores for narrative memory, age, type of pathology, and extent of epilepsy are significant behavioral components of the LV. (B) Brain activity associated with the LV. Important nodes include the left temporal lobe in the left insula and right superior frontal gyrus in the theta band, the left fusiform and right temporal lobe in the alpha band, the right precuneus and right pars triangularis in the beta band, and the left temporal lobe and right lingual gyrus in the low gamma band. DEL = delayed; IMM = immediate; PLS = partial least-squares; STORY = narrative/verbal memory for stories.
Visual Memory
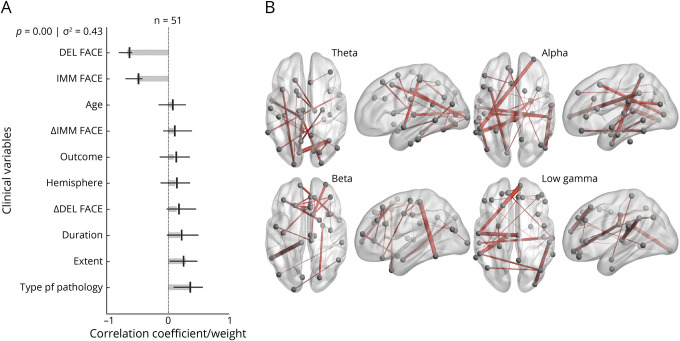
The PLS analysis probing visual memory demonstrated that diffuse, bilateral connectivity in the network was significantly associated with lower visual memory function in children with temporal lobe plus epilepsy. A single significant LV was identified, representing 43% of the variance in the data (Figure 4A). Significant contributions to this LV included temporal lobe plus, developmental cause of epilepsy, and lower preoperative scores in immediate and delayed facial memory. The connectomic profiles that were associated with this LV were characterized by bilateral connections that were diffusely distributed in the theta, alpha, beta, and gamma bands across several important nodes, including the right fusiform, right rostral middle frontal lobe, right medial orbital frontal lobe, left lingual gyrus, left superior parietal lobe, left inferior temporal lobe, left paracentral lobe, and left supramarginal gyrus (Figure 4B).
Figure 4. PLS Analysis Investigating Associations Between Visual Memory Scores, Clinical Covariates, and Connectomic Data.
(A) Contributions of clinical covariates and visual memory scores to the latent variable (LV). LV accounts for 43% of the total variance and is significant (p = 0.00). LV weights are in gray. Correlations (95% CI) between the behavioral data and the extent to which individual patients express the brain connectivity patterns shown in panel B are in black. Preoperative scores for visual memory, type of pathology, and extent of epilepsy are significant behavioral components of the LV. (B) Brain activity associated with the LV. Important nodes include the left lingual gyrus and right fusiform in the theta band, the left superior parietal lobe and right rostral middle frontal lobe in the alpha band, the left inferior temporal lobe and left paracentral lobe in the beta band, and the left supramarginal gyrus and right medial orbital frontal lobe in the low gamma band. FACE = memory for faces; DEL = delayed; IMM = immediate; PLS = partial least-squares.
Working Memory
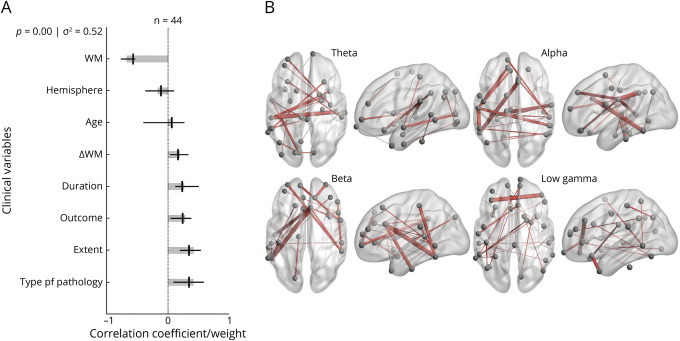
The PLS analysis examining working memory demonstrated that diffuse, bilateral connectivity in the network was significantly associated with lower working memory function in children with temporal lobe plus epilepsy and improved working memory scores after resection. We identified a single significant LV representing 52% of the variance in the data (Figure 5A). Significant contributions to this component included temporal lobe plus epilepsy, developmental cause of epilepsy, longer duration of epilepsy, lower preoperative scores in working memory, seizure freedom after surgery, and improved postoperative scores in working memory. The connectomic profiles that were associated with this LV were marked by frontal and bitemporal connections across frequency bands (Figure 5B).
Figure 5. PLS Analysis Investigating Associations Between Working Memory Scores, Clinical Covariates, and Connectomic Data.
(A) Contributions of clinical covariates and working memory scores to the latent variable (LV). LV accounts for 52% of the total variance and is significant (p = 0.00). LV weights are in gray. Correlations (95% CI) between the behavioral data and the extent to which individual patients express the brain connectivity patterns shown in panel B are in black. Preoperative and postoperative scores for working memory, duration of epilepsy, seizure freedom outcome, extent of epilepsy, and type of pathology are significant behavioral components of the LV. (B) Brain activity associated with the LV. Important nodes include the left supramarginal gyrus and right transverse temporal lobe in the theta band, the left inferior temporal lobe and right caudal anterior cingulate in the alpha band, the left inferior temporal lobe and right caudal anterior cingulate in the beta band, and the left rostral middle frontal lobe and right medial orbital frontal lobe in the low gamma band. PLS = partial least-squares; WM = working memory.
Full Scale IQ
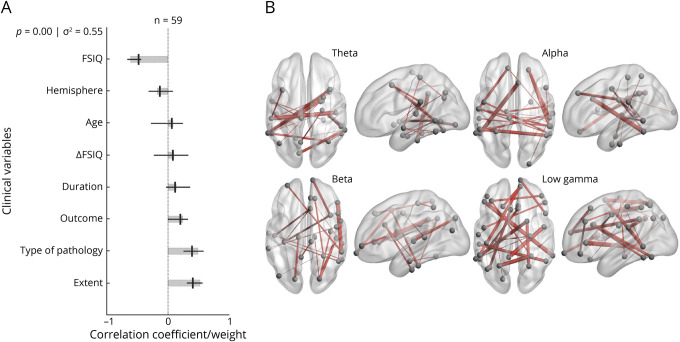
The PLS analysis probing FSIQ demonstrated that diffuse, bilateral connectivity in the network was significantly associated with lower FSIQ in children with temporal lobe plus epilepsy. A single significant LV was identified, representing 55% of the variance in the data (Figure 6A). Significant contributions to this component included temporal lobe plus epilepsy, developmental cause of epilepsy, and lower preoperative scores in FSIQ. The connectomic profiles that were associated with this LV were marked by diffuse bilateral connections in the theta, alpha, and gamma bands across several important nodes, including the left supramarginal gyrus, left inferior temporal lobe, left superior frontal lobe, right transverse temporal lobe, right caudal anterior cingulate, right superior parietal lobe, right pars triangularis, and right inferior parietal lobe (Figure 6B).
Figure 6. PLS Analysis Investigating Associations Between FSIQ Scores, Clinical Covariates, and Connectomic Data.
(A) Contributions of clinical covariates and Full Scale IQ (FSIQ) scores to the latent variable (LV). LV accounts for 55% of the total variance and is significant (p = 0.00). LV weights are in gray. Correlations (95% CI) between the behavioral data and the extent to which individual patients express the brain connectivity patterns shown in panel B are in black. Preoperative scores for FSIQ, type of pathology, and extent of epilepsy are significant behavioral components of the LV. (B) Brain activity associated with the LV. Important nodes include the left supramarginal gyrus and right transverse temporal lobe in the theta band, the left inferior temporal lobe and right caudal anterior cingulate in the alpha band, the right superior parietal lobe and right pars triangularis in the beta band, and the left superior frontal lobe and right inferior parietal lobe in the low gamma band. PLS = partial least-squares.
Discussion
Postoperative neurocognitive outcomes in children with temporal lobe epilepsy are variable, and there is great interest in elucidating preoperative markers that could identify children who are at risk of adverse neurocognitive sequalae.42 In the current work, we used a multivariable, multidimensional approach, PLS, to probe associations between preoperative intrinsic brain connectivity and neuropsychological changes after temporal lobe surgery in children. We report 3 main findings: (1) children with greater impairments in neurocognitive function manifest more widespread and bilateral intrinsic connectivity networks across multiple frequency bands; (2) these diffuse networks also relate to a greater propensity for postoperative improvement in neuropsychological function; and (3) various features of epilepsy, previously known to relate to neuropsychological function, including extent of epileptic network and developmental causes of epilepsy, are collinear with the neural networks we identify. These findings highlight the utility of connectomic and neuropsychological profiling in understanding neurocognitive function in children with temporal lobe epilepsy and expected neurocognitive changes postoperatively. A limitation of the current study is the lack of an external validation cohort. Future work aimed at validating these potential preoperative markers would be valuable. Validation of these markers would provide a potential means to elucidate expected clinical course in children undergoing temporal lobe epilepsy surgery and to identify children who are at risk of adverse outcomes.
Previous work suggests that preoperative cognitive ability could serve as a marker of postoperative neurocognitive outcome. Indeed, it is widely reported that patients with higher preoperative cognitive ability are at greater risk of postoperative cognitive decline, whereas patients with lower preoperative cognitive ability are most likely to experience postoperative cognitive improvement. Collectively, such observations have previously been interpreted as evidence supporting the functional adequacy hypothesis, which posits that cognitive outcomes in temporal lobe epilepsy surgery are predicted by the functional integrity of the local tissue that is resected.6 Our findings provide evidence of large-scale differences that portend neuropsychological outcomes with implications for neuroplasticity and recovery by highlighting an association between lower cognitive function and greater propensity for improvement in children with diffuse network structure.
A hallmark of typical development is increasingly localized neural networks and sharper gradients between intrinsic connections of the brain.43-46 Conventionally, brain regions associated with the same network strengthen in connectivity and become more integrated with age, whereas brain regions associated with different networks become more segregated.46 For instance, during adolescence and early adulthood, connectivity gradients in the task-negative default mode network sharpen.45 Concurrently, decreased correlation between the default mode network and task-positive attention control networks is evident.45 Collectively, these changes are thought to give rise to more mature and functionally specialized networks in adulthood. By extension, the failure of this internetwork segregation and intranetwork integration across development results in less mature networks. In the current work, the diffuse or anatomically widespread, bilateral intrinsic connectivity that is evident in children with lower neuropsychological function is reminiscent of developmentally immature network structures. These network signatures share a collinear phenotype with temporal lobe plus epilepsy and developmental etiology of epilepsy, conceivably because the broader wider epileptic networks are associated with a greater disruption of internetwork segregation and intranetwork integration.
The functional status of the temporal lobe or temporal lobe-associated network could be indexed by performance on temporal lobe–mediated cognitive tasks; impaired performance reflects dysfunctional temporal lobe tissue or network activity. Not surprisingly, patients with temporal lobe epilepsy have impairments in memory and demonstrate pathology or dysfunction in cortical and subcortical areas within the temporal lobe network that are important for memory such as reduced thalamic, hippocampal, and caudate volumes compared to controls.47 In the current work, we find that lower preoperative function in verbal memory, visual memory, working memory, and FSIQ is associated with diffuse, bilateral connectivity without spectral specificity (i.e., across multiple frequency bands). Such sparse or developmentally immature networks would fail to support functions such as memory that are increasingly localized with typical development. Indeed, the impaired development of connectivity gradients in children with epilepsy has previously been associated with worse neurocognitive outcomes.43
Conversely, postoperative improvement in cognitive scores could be attributed to greater compensational capacity and plasticity in children with temporal lobe epilepsy.5 For instance, preoperative reorganization across a bilateral network could preserve working memory and verbal memory function postoperatively.48 In addition, ongoing maturation of the temporal lobes during the second decade of life, as indicated by EEG, MRI, and postmortem studies, could enable temporal lobe networks to further reorganize and recover after temporal lobe epilepsy surgery.5 Thus, the functional implications of the findings from the PLS analyses probing working memory and verbal memory are particularly notable; given that both abilities underpin academic learning and are associated with health-related quality of life,24,42 elucidating markers for favorable postoperative outcomes in working memory and verbal memory functions is critical.
Plasticity and compensational capacity in the developing brain could be limited to certain cognitive domains. In the current study, we find that low preoperative visual memory function is not associated with postoperative improvement in visual memory for faces. It is important to note that facial recognition appears to be lateralized from infancy.49 Consequently, the early specialization and anatomic localization of facial memory could portend reduced plasticity and compensational capacities for this cognitive domain when perturbed by epileptic activity, leading to more durable impairments. In addition, we find that low preoperative FSIQ is not associated with postoperative improvement in FSIQ. This result is in line with findings from previous follow-up studies spanning up to 2 years after temporal lobe surgery that show little or no group changes in intellectual function.50,51 However, it has been postulated that intelligence may show late changes after surgery. Indeed, long-term follow-up studies demonstrate improved intellectual outcome in children after temporal lobectomy.4 Given that these changes were seen only after a postoperative period of 6 years in 1 cohort, it is conceivable that intellectual function requires a prolonged interval of cognitive recovery and network reorganization.4
Glossary
- FSIQ
Full Scale IQ
- LV
latent variable
- MEG
magnetoencephalography
- PLS
partial least-squares
- SVD
singular value decomposition
- wPLI
weighted-phase lag index
Appendix. Authors
Study Funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7(6):525-537. [DOI] [PubMed] [Google Scholar]
- 2.Mabbott DJ, Smith ML. Memory in children with temporal or extra-temporal excisions. Neuropsychologia. 2003;41:995-1007. [DOI] [PubMed] [Google Scholar]
- 3.Jambaqué I, Dellatolas G, Fohlen M, et al. Memory functions following surgery for temporal lobe epilepsy in children. Neuropsychologia. 2007;45(12):2850-2862. [DOI] [PubMed] [Google Scholar]
- 4.Skirrow C, Cross JH, Cormack F, Harkness W, Vargha-Khadem F, Baldeweg T. Long-term intellectual outcome after temporal lobe surgery in childhood. Neurology. 2011;76(15):1330-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gleissner U, Sassen R, Schramm J, Elger CE, Helmstaedter C, Gleissner U. Greater functional recovery after temporal lobe epilepsy surgery in children. Brain. 2005;128:2822-2829. [DOI] [PubMed] [Google Scholar]
- 6.Chelune G. Hippocampal adequacy versus functional reserve: predicting memory functions following temporal lobectomy. Arch Clin Neuropsychol. 1995;10(5):413-432. [PubMed] [Google Scholar]
- 7.Osipowicz K, Sperling MR, Sharan AD, Tracy JI. Functional MRI, resting state fMRI, and DTI for predicting verbal fluency outcome following resective surgery for temporal lobe epilepsy. J Neurosurg. 2016;124(4):929-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wechsler D. The Wechsler Preschool and Primary Scale of Intelligence, 3rd ed. Psychological Corporation; 2002. [Google Scholar]
- 9.Reynolds CR, Kamphaus RW. Reynolds Intellectual Assessment Scales. Psychological Assessment Resources; 2003. [Google Scholar]
- 10.Wechsler D. Wechsler Intelligence Scale for Children, 4th ed. Psychological Corporation; 2003. [Google Scholar]
- 11.Wechsler D. Wechsler Adult Intelligence Scale, 3rd ed. Psychological Corporation; 1997. [Google Scholar]
- 12.Wechsler D. Wechsler Adult Intelligence Scale, 4th ed. Psychological Corporation; 2008. [Google Scholar]
- 13.Cohen MJ. Children's Memory Scale (CMS). Psychological Corporation; 1997. [Google Scholar]
- 14.Wechsler D. Wechsler Memory Scale, 3rd ed. Psychological Corporation; 1997. [Google Scholar]
- 15.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test-Second Edition (CVLT-II), 2nd ed. Psychological Corporation; 2000. [Google Scholar]
- 16.Talley JL. CAVLT-2: Children's Auditory Verbal Learning Test-2. Psychological Assessment Resources, Inc; 1993. [Google Scholar]
- 17.Ibrahim GM, Cassel D, Morgan BR, et al. Resilience of developing brain networks to interictal epileptiform discharges is associated with cognitive outcome. Brain. 2014;137(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ochi A, Go CY, Otsubo H. Clinical MEG analyses for children with intractable epilepsy. Magnetoencephalography. InTech; 2011. [Google Scholar]
- 19.UCL. Statistical Parametric Mapping. Accessed September 3, 2019. fil.ion.ucl.ac.uk/spm/
- 20.Nolte G. The magnetic lead field theorem in the quasi-static approximation and its use for magnetoenchephalography forward calculation in realistic volume conductors. Phys Med Biol. 2003;48(22):3637-3652. [DOI] [PubMed] [Google Scholar]
- 21.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968-980. [DOI] [PubMed] [Google Scholar]
- 22.Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9(10):474-480. [DOI] [PubMed] [Google Scholar]
- 24.Arski ON, Young JM, Smith M-L, Ibrahim GM. The oscillatory basis of working memory function and dysfunction in epilepsy. Front Hum Neurosci. 2021;14:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douw L, Schoonheim MM, Landi D, et al. Cognition is related to resting-state small-world network topology: an magnetoencephalographic study. Neuroscience. 2011;175:169-177. [DOI] [PubMed] [Google Scholar]
- 26.Ye AX, Aucoin-Power M, Taylor MJ, Doesburg SM. Disconnected neuromagnetic networks in children born very preterm: disconnected MEG networks in preterm children. Neuroimage Clin. 2015;9:376-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doesburg SM, Vidal J, Taylor M.J. Reduced theta connectivity during set-shifting in children with autism. Front Hum Neurosci. 2013;0(Nov):785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vinck M, Oostenveld R, Van Wingerden M, Battaglia F, Pennartz CMA. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage. 2011;55(4):1548-1565. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan A, Williams LJ, McIntosh AR, Abdi H. Partial least squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage. 2011;56(2):455-475. [DOI] [PubMed] [Google Scholar]
- 30.Wu J, Quinlan EB, Dodakian L, et al. Connectivity measures are robust biomarkers of cortical function and plasticity after stroke. Brain. 2015;138(pt 8):2359-2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shorvon SD. The etiologic classification of epilepsy. Epilepsia. 2011;52(6):1052-1057. [DOI] [PubMed] [Google Scholar]
- 32.Shorvon SD. The causes of epilepsy: changing concepts of etiology of epilepsy over the past 150 years. Epilepsia. 2011;52(6):1033-1044. [DOI] [PubMed] [Google Scholar]
- 33.Griessenauer CJ, Salam S, Hendrix P, et al. Hemispherectomy for treatment of refractory epilepsy in the pediatric age group: a systematic review. J Neurosurg Pediatr. 2015;15(1):34-44. [DOI] [PubMed] [Google Scholar]
- 34.Devlin AM, Cross JH, Harkness W, et al. Clinical outcomes of hemispherectomy for epilepsy in childhood and adolescence. Brain. 2003;126(3):556-566. [DOI] [PubMed] [Google Scholar]
- 35.Ramantani G, Kadish NE, Brandt A, et al. Seizure control and developmental trajectories after hemispherotomy for refractory epilepsy in childhood and adolescence. Epilepsia. 2013;54(6):1046-1055. [DOI] [PubMed] [Google Scholar]
- 36.Arski ON, Wong SM, Warsi NM, et al. Spectral changes following resective epilepsy surgery and neurocognitive function in children with epilepsy. J Neurophysiol. 2021;126(5):1614-1621. [DOI] [PubMed] [Google Scholar]
- 37.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6(2):65-70. [Google Scholar]
- 38.Englander ZA, Pizoli CE, Batrachenko A, et al. Diffuse reduction of white matter connectivity in cerebral palsy with specific vulnerability of long range fiber tracts. Neuroimage Clin. 2013;2(1):440-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hawellek DJ, Hipp JF, Lewis CM, Corbetta M, Engel AK. Increased functional connectivity indicates the severity of cognitive impairment in multiple sclerosis. Proc Natl Acad Sci USA. 2011;108(47):19066-19071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner KC, Frost L, Linsenbardt D, McIlroy JR, Müller RA. Atypically diffuse functional connectivity between caudate nuclei and cerebral cortex in autism. Behav Brain Funct. 2006;2(1):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fjell AM, Sneve MH, Grydeland H, et al. Functional connectivity change across multiple cortical networks relates to episodic memory changes in aging. Neurobiol Aging. 2015;36(12):3255-3268. [DOI] [PubMed] [Google Scholar]
- 42.Law N, Benifla M, Rutka J, SmithLou M. Verbal memory after temporal lobe epilepsy surgery in children: do only mesial structures matter? Epilepsia. 2017;58(2):291-299. [DOI] [PubMed] [Google Scholar]
- 43.Ibrahim GM, Cassel D, Morgan BR, et al. Resilience of developing brain networks to interictal epileptiform discharges is associated with cognitive outcome. Brain. 2014;137(10):2690-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ibrahim GM, Morgan BR, Lee W, et al. Impaired development of intrinsic connectivity networks in children with medically intractable localization-related epilepsy. Hum Brain Mapp. 2014;35(11):5686-5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson JS, Ferguson MA, Lopez-Larson M, Yurgelun-Todd D. Connectivity gradients between the default mode and attention control networks. Brain Connect. 2011;1(2):147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dosenbach NU, Nardos B, Cohen AL, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329(5997):1358-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Longo CA, Kerr EN, SmithLou M. Executive functioning in children with intractable frontal lobe or temporal lobe epilepsy. Epilepsy Behav. 2013;26(1):102-108. [DOI] [PubMed] [Google Scholar]
- 48.Bell B, Lin JJ, Seidenberg M, Hermann B. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat Rev Neurol. 2011;7(3):154-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzalez LM, Anderson VA, Wood SJ, Mitchell LA, Harvey AS. The localization and lateralization of memory deficits in children with temporal lobe epilepsy. Epilepsia. 2007;48(1):124-132. [DOI] [PubMed] [Google Scholar]
- 50.Williams J, Griebel ML, Sharp GB, Boop FA. Cognition and behavior after temporal lobectomy in pediatric patients with intractable epilepsy. Pediatr Neurol. 1998;19(3):189-194. [DOI] [PubMed] [Google Scholar]
- 51.Westerveld M, Sass KJ, Chelune GJ, et al. Temporal lobectomy in children: cognitive outcome. J Neurosurg. 2000;92(1):24-30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.