Abstract
Background and Objectives
Most primary progressive aphasia (PPA) literature is based on English language users. Linguistic features that vary from English, such as logographic writing systems, are underinvestigated. The current study characterized the dysgraphia phenotypes of patients with PPA who write in Chinese and investigated their diagnostic utility in classifying PPA variants.
Methods
This study recruited 40 participants with PPA and 20 cognitively normal participants from San Francisco, Hong Kong, and Taiwan. We measured dictation accuracy using the Chinese Language Assessment for PPA (CLAP) 60-character orthographic dictation test and examined the occurrence of various writing errors across the study groups. We also performed voxel-based morphometry analysis to identify the gray matter regions correlated with dictation accuracy and prevalence of writing errors.
Results
All PPA groups produced significantly less accurate writing responses than the control group and no significant differences in dictation accuracy were noted among the PPA variants. With a cut score of 36 out of 60 in the CLAP orthographic dictation task, the test achieved sensitivity and specificity of 90% and 95% in identifying Chinese participants with PPA vs controls. In addition to a character frequency effect, dictation accuracy was affected by homophone density and the number of strokes in semantic variant PPA and logopenic variant PPA groups. Dictation accuracy was correlated with volumetric changes over left ventral temporal cortices, regions known to be critical for orthographic long-term memory. Individuals with semantic variant PPA frequently presented with phonologically plausible errors at lexical level, patients with logopenic variant PPA showed higher preponderance towards visual and stroke errors, and patients with nonfluent/agrammatic variant PPA commonly exhibited compound word and radical errors. The prevalence of phonologically plausible, visual, and compound word errors was negatively correlated with cortical volume over the bilateral temporal regions, left temporo-occipital area, and bilateral orbitofrontal gyri, respectively.
Discussion
The findings demonstrate the potential role of the orthographic dictation task as a screening tool and PPA classification indicator in Chinese language users. Each PPA variant had specific Chinese dysgraphia phenotypes that vary from those previously reported in English-speaking patients with PPA, highlighting the importance of language diversity in PPA.
Primary progressive aphasia (PPA) represents a family of neurodegenerative syndromes in which speech or language impairments are the most salient features of the disease.1 Previous studies have demonstrated the presence of writing impairment in PPA, with dysgraphia phenotypes varying across different PPA variants.2,3 The most frequently reported dysgraphia errors in English-speaking individuals with PPA have been surface and phonologic dysgraphia.1-5 Individuals with surface dysgraphia typically spell words based on graphene–phoneme correspondence (i.e., the sublexical route) due to their failure to access the orthographic whole-word forms (i.e., the lexical route), thereby causing spelling difficulties with exceptional sound-to-spelling correspondence (i.e., irregular) while relatively sparing the ability to spell regular and pseudowords.2-5 Surface dysgraphia typically generates phonetically plausible spelling errors and is most commonly reported in individuals with semantic variant PPA (svPPA).4 In contrast, phonologic dysgraphia is considered a product of selective impairment over the phonologic route that associates phonemes with corresponding orthographic symbols at the sublexical level. Given that the lexical route remains intact, these individuals preserve their ability to spell existing regular and irregular words but struggle with the spelling of pseudowords that lack lexical representations.4,6 Both nonfluent/agrammatic PPA (nfvPPA) and logopenic variant PPA (lvPPA) have been reported to result in phonologic dysgraphia, with the former exhibiting a higher predilection for phonetically plausible than implausible errors.3,4,7 Another infrequently discussed dysgraphia pattern is the graphemic buffer disorder that occurs due to deterioration in orthographic working memory. It typically presents with length-dependent letter omissions, substitutions, and additions. Few case reports have described graphemic buffer disorder in all 3 PPA variants, although rarely as the dominant dysgraphia type.2,5
The literature to date on PPA has mostly involved speakers of English.8 The cross-linguistic validity of the language symptoms exclusively derived from English-speaking individuals with PPA has been inadequately understood, especially when other languages have distinct linguistic features. One typical example is the orthographic system of the Chinese language. English words rely on alphabets to construct words and provide phonologic representations, whereas the Chinese language adopts a logographic script in which the orthographic logograms are formed by strokes (e.g., “一” and “丿”) and radicals (i.e., graphical units with semantic or phonologic information, e.g., “氵” and “工”). Chinese logograms are generally referred to as Chinese characters and modern Chinese words commonly exist in compound words format with 2 or more characters (e.g., the word computer [電腦] consist of the characters for electric [電] and brain [腦]). Modern Chinese characters mainly comprise compound characters with 2 or more radicals, typically a semantic and a phonetic radical, commonly referred to as semantic–phonetic compound characters.9 However, there is a substantial portion of Chinese characters that are graphical depictions of objects (i.e., pictographic characters, such as “日” for sun), abstract notions (i.e., ideographic characters, such as “三” for 3), or compound characters that lack phonetic radicals (e.g., “印” for seal). The orthographical structures of these characters do not carry phonologic information; thus, their pronunciations cannot be decoded sublexically. Even with compound characters possessing phonetic radicals, only 19%–39% of semantic–phonetic compound characters are pronounced similarly to their phonetic radicals.10,11 Consequently, Chinese language is generally regarded as having weak graphene–phoneme correspondence. In addition, Chinese characters typically have an abundance of homophones, averaging ≈15 homophones per character.12 Modern Chinese commonly adopts disyllabic/2-character word form to reduce the number of compatible words (e.g., when “sun” is paired with “flower” to form “sunflower,” the pronunciation /ˈflaʊ.ɚz/ is easily discernible as “flower” instead of “flour”).13 Moreover, modern Chinese characters exist in >16 forms of radical spatial configurations, with the number of strokes ranging from 1 to 64 per character.14,15
Given the large differences in orthographic structures, we hypothesized that the dysgraphia patterns in patients with PPA differ between English and Chinese language users. Indeed, the few case reports that have described dysgraphia in Chinese patients with PPA revealed orthographic errors less depicted in English language users, specifically homophone errors, orthographically similar errors, and reversal of compound word errors.16-18 Although enlightening, these case reports mainly provide impressionistic findings that only involve one of the PPA variants. Based on the cognitive architecture of orthographic processing and the neuroanatomical changes specific to the 3 PPA variants, we speculated that (1) Chinese patients with svPPA would show a higher occurrence of phonologically plausible errors attributed to lexical–semantic knowledge loss; (2) orthographically similar errors would be more prevalent in Chinese patients with lvPPA due to visuospatial impairment; and (3) Chinese individuals with nfvPPA would commonly present with compound word errors secondary to executive dysfunction.2,4,19-21
Methods
Participants
This study included 60 native Chinese speakers who received 6 years or more of formal education in the Chinese language. Participants who received less than 6 years of formal education were included when their education was conducted exclusively in the Chinese language. Among them, 40 participants fulfilled the diagnostic criteria for PPA1 and 20 participants were determined to have normal cognition. Participants with PPA were classified into svPPA (n = 10), nfvPPA (n = 9), or lvPPA (n = 21) according to the 2011 consensus criteria.1 Participants with a history of other neurodegenerative diseases, brain surgery, major brain trauma requiring intensive care, oropharyngeal disorders that affected articulation, severe hearing impairment, or a Mini-Mental State Examination (MMSE) score22 lower than 10 were excluded. Participants were recruited from 7 study sites across Hong Kong, Taiwan, and the United States between March 2019 and March 2021. The study protocol was approved by the relevant institutional review boards and written informed consent was obtained from all participants or their medical proxies consistent with Declaration of Helsinki guidelines. The study flow diagram is provided in eFigure 1 (links.lww.com/WNL/B962).
Neuropsycholinguistic Assessment
All neuropsycholinguistic assessments were conducted by board-certified native Chinese-speaking neuropsychologists, speech-language pathologists, or supervised research staff. Although the cognitive assessment protocol varied between different study sites, all participants received the MMSE22 and the Chinese Language Assessment for PPA (CLAP) battery.23 The CLAP battery encompassed tests involving picture naming, single-word comprehension, semantic association, syntax comprehension, repetition, and motor speech. In the picture naming tests, participants were asked to name 48 items (objects or animals) with high concreteness and varying word frequency. Single-word comprehension was evaluated by reading participants the names of 15 objects or animals and then asking participants to identify each target stimulus out of 6 pictures that belonged to the same category. The design of the CLAP semantic association tests was analogous to the Pyramid and Palm Tree test.24 Participants were presented with 30 sets of 3 words or pictures, with the target stimulus placed on top of the other 2 stimuli. Participants were then tasked to identify the words or pictures that best matched the target stimulus. These tasks aimed to reflect the semantic and naming abilities of the participants. The syntax comprehension test was adapted from the Northwestern Assessment of Verbs and Sentences test.25 Participants were shown 30 Chinese sentences (active sentences, passive sentences, multiclause sentences, and serial-verb sentences) together with pictures varying in syntactic constructional meanings. Participants were then tasked with matching the sentence to the corresponding picture, primarily based on the syntactic structure of the sentence. For the repetition task, participants are asked to repeat sensical and nonsensical phrases and sentences ranging from 3 to 11 characters in length. For the motor speech evaluation, participants were tasked with repeating 4-character phrases that varied in lexical tone, place, or manner of articulation for 5 times each, with 5 stimuli in each category, for a total of 15 phrases.
Orthographic Dictation Task
To examine the orthographic dictation performance of Chinese patients with PPA, we developed a 60-character writing dictation list that consisted of 12 pictographic, 12 ideographic, and 36 semantic–phonetic compound characters.23 The characters were designed to vary in character frequency, homophone density, and stroke number. The character frequency was based on the Chinese character frequency database established by the Human Cognition Project.26 Homophone density, which represents the number of homophones each target character has, was derived from the Chinese character database of Taiwan Academia Sinica27 and the Chinese University of Hong Kong.28 To standardize these lexical variables across databases, the word frequency, which was measured in parts per million, was standardized using Zipf law29; homophone density was represented in z score measures. Because Chinese characters generally have numerous homophones, the Chinese characters were read aloud in either Mandarin or Cantonese (according to participant preference) in a 2-character format to specify the exact character for dictation (e.g., 西瓜的瓜, the word “melon” in “watermelon”). Given that Chinese language users from Taiwan and Hong Kong adopt Traditional Chinese script, the writing responses were reviewed based on the Traditional Chinese writing system.
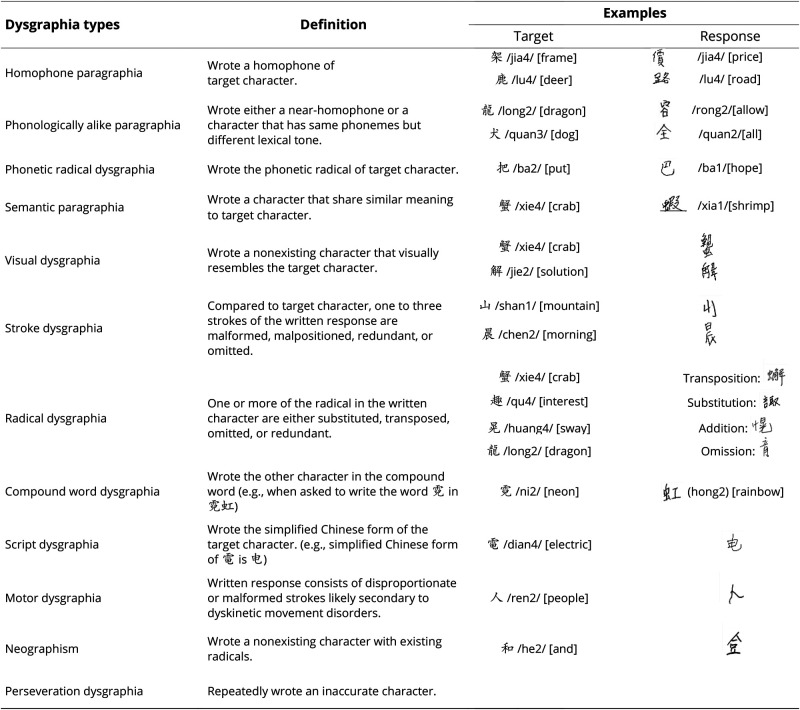
Aside from orthographic dictation accuracy, the writing errors were further categorized by a board-certified speech-language pathologist (L.Y.L.K.C.), a neurologist (B.L.T.), and research staff, all of whom were native Chinese speakers and received more than 16 years of formal education in the Chinese language. Based on the previous literature30-32 and a group consensus, we identified 12 types of Chinese dysgraphia in our PPA cohort, with their definitions and examples illustrated in Figure 1.
Figure 1. Definition and Examples of the Writing Errors Found in the Study Participants (n = 53).
The alphabets written within // represent the Pinyin pronunciation (the official romanization system of Chinese language) of the Chinese characters (e.g., /xie/) and the numerals within the // denote the lexical tones of the Chinese character (e.g., /2/); English translations of the Chinese characters are provided in square brackets.
Statistical Analysis
For demographic variables and neuropsychological, speech, and language measures, the group differences between the 3 PPA variants and control groups were analyzed using analysis of variance (ANOVA) with Bonferroni post hoc analysis for continuous variables and Pearson χ2 test for categorical variables. Among the 12 forms of dysgraphia identified by the 3 raters, 6 of them were found to be statistically significantly different in the numbers of occurrences between the 4 study groups. Principal component analysis (PCA) with direct Oblimin rotation and Kaiser normalization settings33 was conducted using the occurrence of these 6 dysgraphia types. PCA factors with an eigenvalue higher than 1.0 were extracted and dysgraphia types with a loading weight of more than 0.5 were considered to contribute significantly to the factors. We calculated the occurrence of each factor by summing the occurrence of dysgraphia types that carry a factor loading higher than 0.5. Thereafter, we studied the occurrence of each factor across the 3 PPA variants and control groups. The correlations between factors and other speech and language measures were determined using Pearson correlations. We also examined the effects of character frequency, homophone density, stroke number, and phonologic regularity on the accuracy of the writing dictation task using a general linear model. Receiver operating characteristic (ROC) curve analysis was performed to assess the diagnostic utility of the orthographic dictation task in differentiating patients with PPA from cognitively normal controls. We used IBM SPSS Statistics 26.0 to conduct statistical analyses and p < 0.05 was used as the criterion for statistical significance (with Bonferroni correction where applicable).
Neuroimaging Acquisition and Data Processing
A total of 35 participants (cognitively normal, n = 13; svPPA, n = 6; nfvPPA, n = 5; lvPPA, n = 11) completed a brain MRI scan within 3 months of their speech and language assessment. Given the multisite nature of this study, images were acquired from 3T MRI scanners following the Alzheimer's Disease Neuroimaging Initiative 3 acquisition protocol measures. Neuroimaging data were preprocessed using the Computational Anatomy Toolbox (CAT12) in Statistical Parametric Mapping software (SPM12) operating under MATLAB 2017b (MathWorks). The T1-weighted images were bias-field corrected, skull-stripped, and classified as gray matter (GM), white matter, or CSF using a segmentation approach based on an adaptive maximum a posteriori technique without the need for a priori information on the tissue probabilities. GM probability maps were nonlinearly normalized to the Montreal Neurologic Institute space using DARTEL, modulated by the Jacobian determinant of the deformations derived from the spatial normalization and smoothed with an isotropic Gaussian kernel of 8 mm full-width at half-maximum.34
The smoothed and modulated GM tissue probability maps were used to examine the association between GM volume and dictation accuracy and prevalence of dysgraphia types in all 35 participants using voxel-wise multiple linear regression models. The prevalence of each dysgraphia type was calculated using the number of occurrences divided by the total number of writing errors, excluding the blank responses. Age, education, total GM volume, diagnosis, and overall dictation accuracy were entered as nuisance variables in each regression model. A significance threshold of p < 0.05 family-wise error corrected at cluster level and k > 80 was used to detect areas of GM atrophy associated with the linguistic scores. Additional information on the GM atrophy pattern is available in eFigure 2 (links.lww.com/WNL/B962).
Data Availability
Anonymized data are available from the corresponding author upon reasonable request.
Results
Demographic and Neuropsychological Data
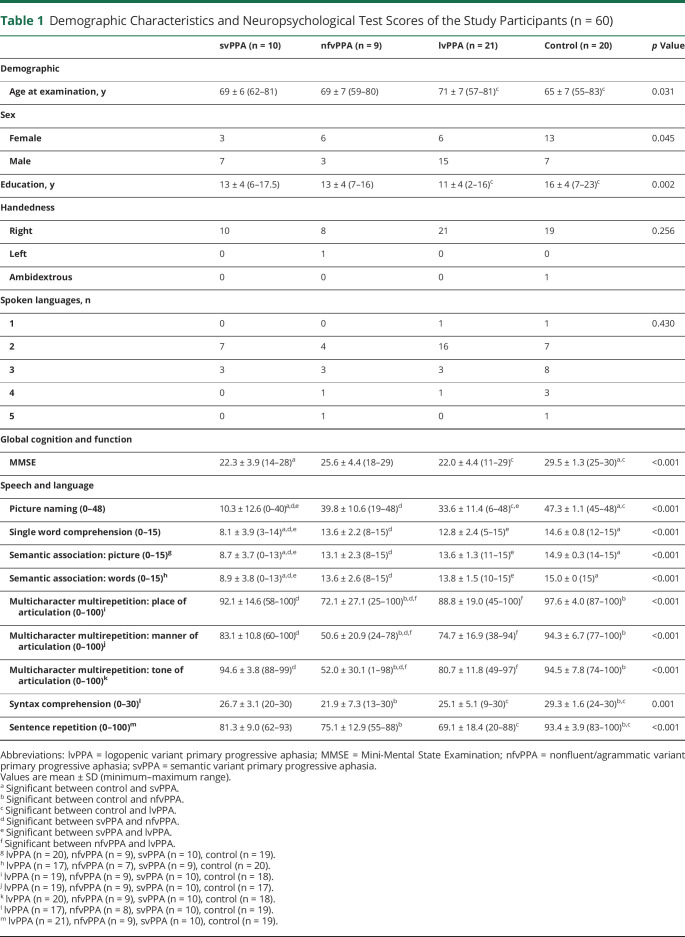
Demographic, neuropsychological, speech, and language data are summarized in Table 1. There were no significant differences among the 3 PPA variants and the healthy control groups in terms of sex and handedness ratios. Compared with controls, participants with lvPPA were older (p = 0.031) and had a lower education level (p = 0.002). As anticipated, MMSE scores were significantly lower in the PPA groups when compared with healthy controls (p < 0.0001). For speech and language assessments, individuals with svPPA scored significantly lower than controls and individuals with other PPA variants in semantic-based tasks, specifically the picture naming (p < 0.0001), single-word comprehension (p < 0.0001), and semantic association tests (word: p < 0.0001; picture: p < 0.0001). For the motor speech evaluation, participants with nfvPPA produced fewer accurate verbal responses when repeating phrases that differed in tones, place, and manner of articulations in comparison with controls and other PPA groups (tone: p < 0.0001; place: p < 0.0001; manner: p < 0.0001). On the syntax comprehension and repetition tests, the scores were significantly lower in nfvPPA and lvPPA when compared with the control group (p = 0.001 and p < 0.0001, respectively).
Table 1.
Demographic Characteristics and Neuropsychological Test Scores of the Study Participants (n = 60)
Orthographic Dictation Accuracy
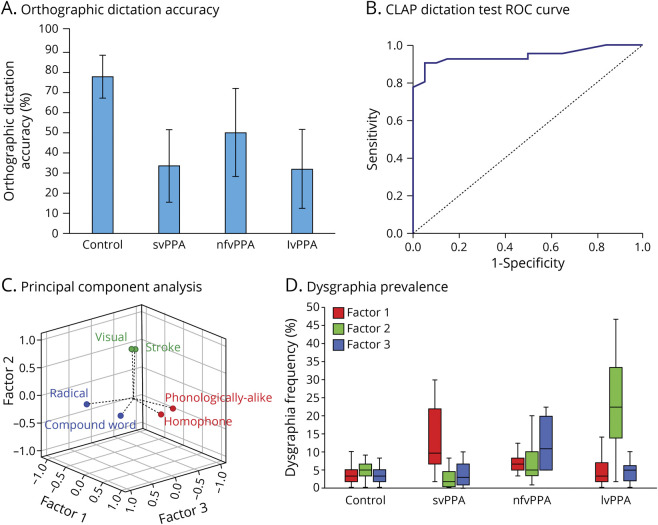
In terms of overall dictation accuracy, ANOVA with Bonferroni post hoc analysis revealed that all 3 of the PPA groups scored significantly lower than the control group (p < 0.0001), but no significant differences were noted among the PPA variants (see Figure 2A). Among the individual lexical variables examined, character frequency significantly predicted accuracy scores across all study groups (control: p < 0.0001; svPPA: p < 0.0001; nfvPPA: p < 0.0001; lvPPA: p < 0.0001). Homophone density significantly affected dictation accuracy in the participants with svPPA or lvPPA (svPPA: p = 0.007; lvPPA: p = 0.008); stroke number influenced the dictation accuracy in the lvPPA group (p < 0.0001). Accuracy in the orthographic dictation test was not found to differ between regular and irregular characters. Figure 2B shows the ROC curve of the orthographic dictation task in identifying PPA cases from the control participants. Using a cutoff value of 36 out of 60, the test reached a sensitivity level of 90% (95% CI 0.807–0.993) and a specificity level of 95% (95% CI 0.854–1.046), with an area under the curve value of 0.941 (95% CI 0.881–1.000).
Figure 2. Orthographic Dictation Performance in Chinese Cognitively Normal Individuals and Patients With PPA.
(A) Orthographic dictation accuracy (mean and SD) across cognitively normal (n = 20) and primary progressive aphasia (PPA) groups (semantic variant PPA [svPPA], n = 10; nonfluent/agrammatic variant PPA [nfvPPA], n = 9; logopenic variant PPA [lvPPA], n = 21). (B) Receiver operating characteristic (ROC) curve of the orthographic dictation task in identifying PPA cases (n = 40) from the cognitively normal participants (n = 20). Using 36 out of 60 as the cutoff value, the sensitivity and specificity of the test reached 90% and 95%, respectively, with area under the curve value of 0.941 (p < 0.0001). (C) The vector projections of 8 dysgraphia types from principal component analysis with 3 factors derived. The dysgraphia types in factors 1, 2, and 3 are represented in red, green, and blue circles and fonts, respectively. (D) The prevalence of factors 1, 2, and 3 across cognitively normal (n = 20) and PPA groups (svPPA, n = 10; nfvPPA, n = 9; lvPPA, n = 21) presented in box plot format.
Dysgraphia Phenotypes Across PPA Variants
Figure 1 lists the 12 different types of dysgraphia that were identified by the 3 raters, with definitions and examples provided. Six out of the 12 dysgraphia types were found to differ significantly in the number of occurrences among the 4 study groups (Table 2). PCA using the occurrence of these 6 dysgraphia types identified 3 factors with eigenvalues of 1.902, 1.390, and 1.107, accounting for 73.31% of the variance of the orthographic dictation scores (factor 1 = 31.70%, factor 2 = 23.16%, and factor 3 = 18.45%). The factor loadings are available in eTable 1 (links.lww.com/WNL/B962). Factor 1 is heavily weighted by homophone and phonologically alike writing errors. Conversely, factor 2 is heavily loaded with visual and stroke errors, and factor 3 is mainly dependent on compound word and radical-type writing errors (Figure 2C). The occurrence of factor 1, 2, or 3 dysgraphia types are significantly higher in svPPA, lvPPA, and nfvPPA groups, respectively (factor 1: F3,49 = 9.73, p < 0.0001; factor 2: F3,49 = 22.82, p < 0.0001; factor 3: F3,49 = 12.02, p < 0.0001) (Figure 2D).
Table 2.
Distribution of Various Writing Responses Across Study Groups (n = 60)
Correlation Between Dysgraphia Phenotypes and Speech and Language Measures
Pearson correlations between the 3 factors representing dysgraphia phenotypes and the speech and language measures are included in eTable 2 (links.lww.com/WNL/B962). Factor 1 writing errors were found to correlate with language tests heavily dependent on semantic knowledge, including the picture naming (r = −0.527, p < 0.0001), single-word comprehension (r = −0.576, p < 0.0001), and semantic associations tasks (picture: r = −0.612, p < 0.0001; word: r = −0.526, p < 0.0001). The occurrence of factor 3 dysgraphia types was correlated with motor speech (place of articulation: r = −0.584, p < 0.0001; manner of articulation: r = −0.506, p < 0.0001; tone of articulation: r = −0.659, p < 0.0001) and syntax comprehension tests (r = −0.395, p = 0.003). The frequency of factor 2 writing errors correlated with the repetition test (r = −0.293, p = 0.025).
Neuroanatomical Correlation of the Dictation Accuracy and Writing Errors
Accuracy of written dictation was found to be positively correlated with GM volume over the left fusiform, left middle, and inferior temporal gyri. As for writing errors, the prevalence of homophone errors showed negative correlations with GM volumetric changes over the bilateral temporal poles, inferior temporal gyri, left hippocampal, parahippocampal, and right orbitofrontal regions. Conversely, the frequency of homophone errors was positively correlated with left superior parietal regions. Phonologic dysgraphia correlated negatively with volumetric changes over the left temporal pole and middle temporal gyrus and were positively correlated with the right cerebellum. Visual dysgraphia was negatively correlated with GM volume over the left lingual gyrus. The frequency of compound word dysgraphia was correlated with volumetric changes over the bilateral orbitofrontal gyri and left insula regions (Figure 3 and Table 3). The correlations of GM volume and other dysgraphia types did not reach the statistical threshold.
Figure 3. Neuroanatomical Correlations of Orthographic Dictation Accuracy and Dysgraphia Types.
Neuroanatomical correlation analysis of orthographic dictation accuracy and the prevalence of dysgraphia types with the gray matter volumetric changes. We adopted the voxel-based morphometry method with multiple linear regression models adjusted for age at examination, education, total gray matter volume, diagnosis, or dictation accuracy. The cluster threshold was corrected for family-wise error with p value set at <0.05 and the voxel was set at a threshold of k > 80. No suprathreshold clusters were noted for perseveration, motor, stroke, or radical writing errors.
Table 3.
Neuroanatomical Correlates of the Writing Dictation Accuracy and Dysgraphia Types
Discussion
This study characterized the dysgraphia phenotypes in patients with PPA who use logographic script. Consistent with our hypothesis, the PPA dysgraphia phenotypes observed in Chinese language users differed from those reported in English language users. In our Chinese cohort, patients with svPPA commonly exhibited phonologically plausible writing errors at the lexical level, individuals with lvPPA frequently presented with orthographically similar writing errors, and patients with nfvPPA typically showed orthographic selection writing errors. These findings suggest that further adaptations of the 2011 PPA consensus criteria may be essential for Chinese language users. The lexical variables dictating the writing accuracy are shown to vary in Chinese language users as they likely emphasize different cognitive processes to execute the writing tasks. Consequently, the writing errors produced and their corresponding neural correlates differ from those reported in English language users.
Similar to English-speaking patients with PPA, Chinese patients with PPA also presented with prominent writing impairments. Dictation accuracy was correlated with volumetric changes over the left ventral temporal regions, which are neuroanatomical regions critical for orthographic long-term memory function. Previous studies on English language users have shown that word frequency affects orthographic long-term memory function.19,35 The dictation performance of our participants is dependent on Chinese character frequency.
Surface dysgraphia is commonly noted in English-speaking patients with svPPA.3-5 Because the sublexical phonologic representations of Chinese characters are phonetic radicals, Chinese phonetic radical writing errors (i.e., substituting the target character with its phonetic radical; e.g.,  instead of “把”) are generally considered surface dysgraphic errors.36 However, phonetic radical writing errors were uncommon in our PPA cohort and lacked predilection to svPPA cases. Because phonetic radicals seldom accurately predict the pronunciation of Chinese characters, when lacking lexical–semantic knowledge inputs, Chinese language users instead rely on homophones (e.g.,
instead of “把”) are generally considered surface dysgraphic errors.36 However, phonetic radical writing errors were uncommon in our PPA cohort and lacked predilection to svPPA cases. Because phonetic radicals seldom accurately predict the pronunciation of Chinese characters, when lacking lexical–semantic knowledge inputs, Chinese language users instead rely on homophones (e.g.,  /jia4/ instead of “架”/jia4/) and phonologically alike characters (e.g.,
/jia4/ instead of “架”/jia4/) and phonologically alike characters (e.g.,  /rong2/ instead of “龍”/long2/) as phonograms. Therefore, these lexical-level phonologically plausible errors (i.e., homophone and phonologically alike writing errors) are the more archetypical dysgraphia phenotypes for Chinese individuals with svPPA. This is in contrast with English language users who rely on phonograms at the sublexical level to produce phonologically plausible errors. No phonologic regularity effect was found in the writing accuracy of our Chinese cohort as word regularity is defined according to graphene–phoneme correspondences at the sublexical level. Instead, the writing accuracy in Chinese individuals with svPPA was associated with homophone density, which supports the speculation that Chinese language users rely on lexical-level phonograms.
/rong2/ instead of “龍”/long2/) as phonograms. Therefore, these lexical-level phonologically plausible errors (i.e., homophone and phonologically alike writing errors) are the more archetypical dysgraphia phenotypes for Chinese individuals with svPPA. This is in contrast with English language users who rely on phonograms at the sublexical level to produce phonologically plausible errors. No phonologic regularity effect was found in the writing accuracy of our Chinese cohort as word regularity is defined according to graphene–phoneme correspondences at the sublexical level. Instead, the writing accuracy in Chinese individuals with svPPA was associated with homophone density, which supports the speculation that Chinese language users rely on lexical-level phonograms.
Homophones or phonologically alike writing errors have also been described in Chinese patients with left frontal and temporal lobectomy or stroke lesions over left perisylvian and temporal regions or the right middle cerebral artery territory.30,37,38 Similar lexical-level phonologically plausible errors have previously been reported in Japanese individuals with svPPA who inaccurately used logographic Kanji homophones as phonograms when completing Kanji dictation tasks.39-41 Voxel-based morphometry (VBM) analysis revealed that the homophone and phonologically alike paragraphias were negatively correlated with bilateral anterior temporal gyri, which are brain regions known to be critical for semantic processing and are typically affected in individuals with svPPA.42-44 This is consistent with our finding that the frequency of factor 1 errors is strongly correlated with the language tasks targeting semantic knowledge. The prevalence of homophone writing errors was also positively correlated with the left parietal region. We speculate that relatively preserved ability in phonologic processing is critical for generating homophones to produce homophone paragraphia. This is in line with magnetoencephalographic imaging findings that revealed the overrecruitment of the dorsal route in individuals with svPPA.45
Our findings showed that Chinese patients with lvPPA frequently present with orthographically similar writing errors, such as visual (e.g.,  instead of 解/jie2/) and stroke errors (e.g.,
instead of 解/jie2/) and stroke errors (e.g., 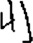 instead of 山/shan1/). These errors have also been described in 2 case reports of Chinese-speaking patients with PPA.17,18 We speculate that these orthographically similar errors were products of the inability to retain the spatial details of Chinese characters. Given the visuospatial complexity of Chinese orthography, Chinese characters place a higher demand on orthographic working memory than do English words, and thus have a higher likelihood of being affected by a graphemic buffer disorder. Graphemic buffer disorder is also known to be dependent on word length, and the dictation accuracy of Chinese individuals with lvPPA decreased with increasing number of strokes.3,19 Previous studies have also shown that orthographic working memory was associated with bilateral parieto-occipital and left posterior temporal regions, which largely overlap with the atrophic regions of lvPPA.19,46-48 VBM analysis revealed that the prevalence of visual dysgraphia was negatively correlated with volumetric changes over left lingual gyrus. Hence, one can reasonably speculate that impairment in visuospatial processing coupled with partially preserved orthographic long-term memory could frequently lead to the production of some but not all the visuospatial details of Chinese characters.
instead of 山/shan1/). These errors have also been described in 2 case reports of Chinese-speaking patients with PPA.17,18 We speculate that these orthographically similar errors were products of the inability to retain the spatial details of Chinese characters. Given the visuospatial complexity of Chinese orthography, Chinese characters place a higher demand on orthographic working memory than do English words, and thus have a higher likelihood of being affected by a graphemic buffer disorder. Graphemic buffer disorder is also known to be dependent on word length, and the dictation accuracy of Chinese individuals with lvPPA decreased with increasing number of strokes.3,19 Previous studies have also shown that orthographic working memory was associated with bilateral parieto-occipital and left posterior temporal regions, which largely overlap with the atrophic regions of lvPPA.19,46-48 VBM analysis revealed that the prevalence of visual dysgraphia was negatively correlated with volumetric changes over left lingual gyrus. Hence, one can reasonably speculate that impairment in visuospatial processing coupled with partially preserved orthographic long-term memory could frequently lead to the production of some but not all the visuospatial details of Chinese characters.
Chinese individuals with nfvPPA showed a higher prevalence for compound word (e.g., wrote 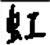 when asked to write 霓 in 霓虹) and radical dysgraphia (e.g.,
when asked to write 霓 in 霓虹) and radical dysgraphia (e.g.,  instead of 趣/qu4/). Both errors are more exclusive to Chinese language users due to the abundance of compound words in modern Chinese and the radical-based structure of the Chinese characters. As the frequency of compound word errors is correlated with bilateral orbitofrontal GM volumes, this orthographic selection error is likely secondary to a failure to inhibit the other character of the 2-character compound word. Radical writing errors have been reported in Chinese language users with stroke, amnestic mild cognitive impairment, and Alzheimer disease, albeit at varying frequencies.31,37,49,50 One interpretation of radical dysgraphia is attributed to visuoconstructional impairment that is amplified by the radical flexibility of Chinese characters.31 Another hypothesis is that radical dysgraphia is a reflection of orthographic retrieval impairment at the radical level.49-52 Although not statistically significant, motor dysgraphia (for example,
instead of 趣/qu4/). Both errors are more exclusive to Chinese language users due to the abundance of compound words in modern Chinese and the radical-based structure of the Chinese characters. As the frequency of compound word errors is correlated with bilateral orbitofrontal GM volumes, this orthographic selection error is likely secondary to a failure to inhibit the other character of the 2-character compound word. Radical writing errors have been reported in Chinese language users with stroke, amnestic mild cognitive impairment, and Alzheimer disease, albeit at varying frequencies.31,37,49,50 One interpretation of radical dysgraphia is attributed to visuoconstructional impairment that is amplified by the radical flexibility of Chinese characters.31 Another hypothesis is that radical dysgraphia is a reflection of orthographic retrieval impairment at the radical level.49-52 Although not statistically significant, motor dysgraphia (for example,  for 人/ren2/) was relatively frequent among patients with nfvPPA. This is potentially related to the parkinsonian motor features more commonly found in nfvPPA with tau pathologies.53
for 人/ren2/) was relatively frequent among patients with nfvPPA. This is potentially related to the parkinsonian motor features more commonly found in nfvPPA with tau pathologies.53
Our findings suggest that the 2011 consensus criteria could potentially benefit from further adaptations for Chinese language users. Specifically, surface dysgraphia is tailored for patients with svPPA who write in alphabetic scripts that possess opaque orthographies. Surface dysgraphia, however, does not accurately portray the dysgraphia phenotype of Chinese and Japanese individuals with svPPA.39-41 A broader depiction that encompasses the various phonologically plausible writing errors formed by both lexical and sublexical phonograms will increase the generalizability of the svPPA diagnostic criteria. Similarly, incorporating writing system–specific dysgraphia phenotypes, such as orthographically similar and orthographic selection writing errors, can potentially enhance the classification of PPA variants in the respective languages.
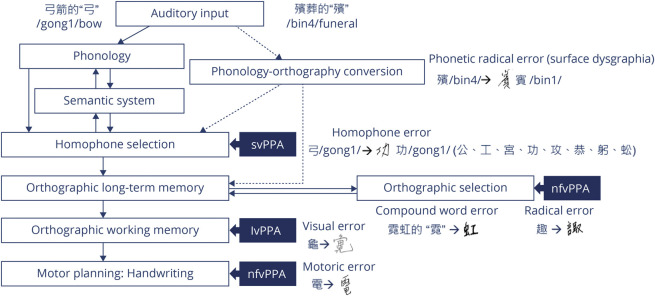
Based on our results and previous work, we propose a modified orthographic model for Chinese language users (Figure 4). We speculate that Chinese language users rely on lexical retrieval ability to generate a sufficient pool of homophones and semantic knowledge to identify the target character based on semantic context. These language abilities are impaired in lvPPA and svPPA; thus their dictation accuracies are dependent on homophone density. As most modern Chinese characters are presented in the format of compound words, we deduced that the associations between frequently paired characters are stronger in Chinese, and an inability to suppress the other frequently paired character would result in compound word writing errors. If partially impaired orthographic long-term memory is coupled with inability to select accurate radicals, the Chinese language users would exhibit radical writing errors. Given the high visuospatial complexity of Chinese characters, individuals with lvPPA and nfvPPA who are unable to retain the spatial details of the Chinese characters or execute the motoric demands to write them would exhibit orthographically similar errors and motor dysgraphia, respectively. Given the weak graphene–phoneme correspondence in the Chinese language, a phonology–orthography conversion at the sublexical level is less reliable and hence surface dysgraphia is uncommon.
Figure 4. Cognitive Architectural Model of Orthographic Dictation in Chinese Language Users.
This is a schematic cognitive architecture model of orthographic dictation for Chinese language users with additional homophone and orthographic selection processes included. For a pictographic character such as “弓” (/gong1/bow), there are 8 other common homophones “公、工、宮、功、攻、恭、躬、蚣.” As the auditory stimuli specified that target character to be “弓箭的弓” (the character “bow” in the word “bow and arrow”), Chinese language users are thus able to select the target character. This process is critical for the homophone-rich Chinese language and reliant on semantic knowledge and lexical retrieval functions, which are impaired in semantic variant primary progressive aphasia (svPPA) and logopenic variant primary progressive aphasia (lvPPA). Chinese language is also rich in compound words, with most of its characters constructed by assorted radicals. In individuals with nonfluent/agrammatic variant primary progressive aphasia (nfvPPA), the orthographic long-term memory impairment coupled with the inability to suppress the other frequently paired characters or to select the accurate radicals would result in compound word (e.g.,  for “霓虹的”/“霓”) or radical (e.g.,
for “霓虹的”/“霓”) or radical (e.g.,  for “趣”) writing errors. The visuospatial complexity of Chinese characters requires a higher demand for orthographic working memory and motor functions. When impaired, Chinese language users produce orthographically similar writing errors (e.g.,
for “趣”) writing errors. The visuospatial complexity of Chinese characters requires a higher demand for orthographic working memory and motor functions. When impaired, Chinese language users produce orthographically similar writing errors (e.g.,  for “龜”) or motor dysgraphia (e.g.,
for “龜”) or motor dysgraphia (e.g., 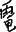 for “電”), as noted in participants with lvPPA and participants with nfvPPA. Contrary to English language users, sublexical phonology–orthography conversion such as surface dysgraphia (e.g.,
for “電”), as noted in participants with lvPPA and participants with nfvPPA. Contrary to English language users, sublexical phonology–orthography conversion such as surface dysgraphia (e.g., 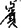 for “殯”) is uncommon.
for “殯”) is uncommon.
A limitation of this study is that we primarily focused on collecting speech and language data due to limited resources. Therefore, the cognitive mechanism underlying the reported dysgraphia phenotypes is speculative and further investigation is required with harmonizable executive and visuospatial data.
To compensate for the low prevalence of early diagnosed PPA cases in Chinese language users, this study pooled patients with PPA from Taiwan, Hong Kong, and the United States. The diverse sources of recruitment may contribute to heterogeneity in demographic and environmental profiles. However, one advantage of such an enrollment approach is better generalizability of our findings across the various populations. Given that the study participants are evaluated in traditional Chinese scripts, further studies are needed to confirm that these findings are applicable in simplified Chinese users.
Not all reported writing errors showed corresponding neural correlates on the VBM analysis. This may be due to the limited number of participants who received MRI using a similar acquisition protocol, thereby reducing the availability of MRI for analysis.
This study describes in detail the dysgraphia performance of Chinese individuals with PPA and its diagnostic utility in screening and classifying the PPA variants. Overall, our findings support the presence of language-specific symptomatology in the dysgraphia phenotypes of Chinese individuals with PPA, underlining the importance of establishing PPA diagnostic criteria and evaluation strategies in a language-specific manner.
Acknowledgment
The authors thank the participants and their families for their contributions to this work.
Glossary
- ANOVA
analysis of variance
- CLAP
Chinese Language Assessment for PPA
- GM
gray matter
- lvPPA
logopenic variant primary progressive aphasia
- MMSE
Mini-Mental State Examination
- nfvPPA
nonfluent/agrammatic variant primary progressive aphasia
- PCA
principal component analysis
- PPA
primary progressive aphasia
- ROC
receiver operating characteristic
- svPPA
semantic variant primary progressive aphasia
- VBM
voxel-based morphometry
Appendix. Authors

Footnotes
Editorial, page 915
CME Course: NPub.org/cmelist
Study Funding
The work is supported by the Global Brain Health Institute (GBHI ALZ UK-19-589,585), University of California, San Francisco; the NIH (NIA R21AG068757, NINDS R01 NS050915, NIDCD K24 DC015544, NIA P01 AG019724, NIA U01 AG052943, NIA P50 AG023501, UG3 NS105557, R01 AG038791, U01 AG045390, and U54 NS092089); and the Alzheimer’s Disease Research Center of California (P30 AG062422).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faria AV, Crinion J, Tsapkini K, et al. Patterns of dysgraphia in primary progressive aphasia compared to post-stroke aphasia. Behav Neurol. 2013;26(1-2):21-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham NL. Dysgraphia in primary progressive aphasia: characterisation of impairments and therapy options. Aphasiology. 2014;28(8-9):1092-1111. [Google Scholar]
- 4.Shim H, Hurley RS, Rogalski E, Mesulam MM. Anatomic, clinical, and neuropsychological correlates of spelling errors in primary progressive aphasia. Neuropsychologia. 2012;50(8):1929-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sepelyak K, Crinion J, Molitoris J, et al. Patterns of breakdown in spelling in primary progressive aphasia. Cortex. 2011;47(3):342-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham NL, Patterson K, Hodges JR. The impact of semantic memory impairment on spelling: evidence from semantic dementia. Neuropsychologia. 2000;38(2):143-163. [DOI] [PubMed] [Google Scholar]
- 7.Graham NL, Patterson K, Hodges JR. When more yields less: speaking and writing deficits in nonfluent progressive aphasia. Neurocase. 2004;10(2):141-155. [DOI] [PubMed] [Google Scholar]
- 8.Weekes BSH. Aphasia in Alzheimer's disease and other dementias (ADOD): evidence from Chinese. Am J Alzheimers Dis Other Demen. 2020;35:1533317520949708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsiao JH, Shillcock R. Analysis of a Chinese phonetic compound database: implications for orthographic processing. J Psycholinguist Res. 2006;35(5):405-426. [DOI] [PubMed] [Google Scholar]
- 10.Liu I-m, Chen S-C, Sue IR. Regularity and consistency effects in Chinese character naming. Chin J Psychol. 2003;45:29-46. [Google Scholar]
- 11.Zhou Y. To what degree are the “phonetics” of present-day Chinese characters still phonetic? Zhongguo Yuwen. 1978;146(3):172-177. [Google Scholar]
- 12.ISO/IEC. Universal Multiple-Octet Coded Character Set (UCS): Part 1: Architecture and Basic Multilingual Plane. ISO/IEC; 1994. evertype.com/standards/iso10646/pdf/fdam22-keyboard.pdf [Google Scholar]
- 13.Arcodia GF. Chinese: A Language of Compound Words? 2007/01/01. researchgate.net/publication/238547625_Chinese_A_Language_of_Compound_Words [Google Scholar]
- 14.Williams C, Bever T. Chinese character decoding: a semantic bias? Reading Writing. 2010;23(5):589-605. [Google Scholar]
- 15.Taylor I, Taylor MM. Writing and literacy in Chinese, Korean and Japanese: Revised edition, vol 14. John Benjamins Publishing Company; 2014. [Google Scholar]
- 16.Liu X, He F, Chen Z, Liu P, Peng G. A longitudinal study of a Chinese man presenting with non-fluent/agrammatic variant of primary progressive aphasia. Front Neurol. 2018;9:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu XQ, Liu XJ, Sun ZC, Chromik L, Zhang YW. Characteristics of dyslexia and dysgraphia in a Chinese patient with semantic dementia. Neurocase. 2015;21(3):279-288. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J, Wang JA, Jiang B, Qiu WJ, Yan B, Wang YH. A clinical, neurolinguistic, and radiological study of a Chinese follow-up case with primary progressive aphasia. Neurocase. 2013;19(5):427-433. [DOI] [PubMed] [Google Scholar]
- 19.Rapp B, Purcell J, Hillis AE, Capasso R, Miceli G. Neural bases of orthographic long-term memory and working memory in dysgraphia. Brain. 2016;139(Pt 2):588-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han Z, Song L, Bi Y. Cognitive mechanism of writing to dictation of logographic characters. Appl Psycholinguistics. 2012;33(3):517-537. [Google Scholar]
- 21.Watson CL, Possin K, Allen IE, et al. Visuospatial functioning in the primary progressive aphasias. J Int Neuropsychol. Soc. 2018;24(3):259-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. [DOI] [PubMed] [Google Scholar]
- 23.Tee BL, Deleon J, Chen Li Ying LKLK, et al. Tonal and orthographic analysis in a Cantonese-speaking individual with nonfluent/agrammatic variant primary progressive aphasia. Neurocase. 2021:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard D, Patterson K. The Pyramids and Palm Trees Test: A Test of Semantic Access from Words and Pictures. Pearson Assessment; 1992. [Google Scholar]
- 25.Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The Northwestern anagram test: measuring sentence production in primary progressive aphasia. Am J Alzheimers Dis Other Demen. 2009;24(5):408-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Human Cognition Project. Chinese character frequency: a trans-regional, diachronic survey. humanum.arts.cuhk.edu.hk/Lexis/chifreq/
- 27.Taiwan Academia Sinica. Chinese character database. words.sinica.edu.tw/sou/sou.html
- 28.Chinese University of Hong Kong. Chinese character database: with word formations: phonologically disambiguated according to the Cantonese dialect. humanum.arts.cuhk.edu.hk/Lexis/lexi-can/
- 29.Zipf GK. Human Behavior and the Principle of Least Effort: An Introduction to Human Ecology. Addison-Wesley; 1949. [Google Scholar]
- 30.Yin W, He S, Weekes BS. Acquired dyslexia and dysgraphia in Chinese. Behav Neurol. 2005;16(2-33):159-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou J, Jiang B, Huang XH, Kong LL, Li HL. Characteristics of agraphia in Chinese patients with Alzheimer's disease and amnestic mild cognitive impairment. Chin Med J. 2016;129(13):1553-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Law SP, Or B. A case study of acquired dyslexia and dysgraphia in Cantonese: evidence for nonsemantic pathways for reading and writing Chinese. Cogn Neuropsychol. 2001;18(8):729-748. [DOI] [PubMed] [Google Scholar]
- 33.Jolliffe I. Rotation and Interpretation of Principal Components. In: Principal Component Analysis. Springer; 2002:269-298. [Google Scholar]
- 34.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95-113. [DOI] [PubMed] [Google Scholar]
- 35.Rapp B, Dufor O. The neurotopography of written word production: an fMRI investigation of the distribution of sensitivity to length and frequency. J Cogn Neurosci. 2011;23:4067-4081. [DOI] [PubMed] [Google Scholar]
- 36.Yin W, Butterworth B. Deep and surface dyslexia in Chinese. In: Chen H-C, Tzeng OJL, eds. Advances in Psychology. North-Holland; 1992:349-366. [Google Scholar]
- 37.Law SP. Writing errors of a Cantonese dysgraphic patient and their theoretical implications. Neurocase. 2004;10:132-140. [DOI] [PubMed] [Google Scholar]
- 38.Reich S, Chou T-L, Patterson K. Acquired dysgraphia in Chinese: further evidence on the links between phonology and orthography. Aphasiology 2003;17(6-7):585-604. [Google Scholar]
- 39.Imura T. Aphasie, ihre eigenartigen ersheinungen in der Japanischen Sprache. Folia Psychiatrica Neurol Japonica. 1943;47:196-218. [Google Scholar]
- 40.Jibiki I, Yamaguchi N. The Gogi (word-meaning) syndrome with impaired kanji processing: alexia with agraphia. Brain Lang. 1993;45(1):61-69. [DOI] [PubMed] [Google Scholar]
- 41.Nagai C, Iwata M. Writing disorders in primary progressive aphasia. Rinsho Shinkeigaku. 2003;43:84-92. [PubMed] [Google Scholar]
- 42.Snowden JS, Harris JM, Thompson JC, et al. Semantic dementia and the left and right temporal lobes. Cortex. 2018;107:188-203. [DOI] [PubMed] [Google Scholar]
- 43.Bonner MF, Price AR. Where is the anterior temporal lobe and what does it do? J Neurosci. 2013;33(10):4213-4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joyal M, Brambati SM, Laforce RJ, et al. The role of the left anterior temporal lobe for unpredictable and complex mappings in word reading: original research. Front Psychol. 2017;8:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borghesani V, Hinkley LBN, Ranasinghe KG, et al. Taking the sublexical route: brain dynamics of reading in the semantic variant of primary progressive aphasia. Brain. 2020;143(8):2545-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hillis AE, Kane A, Tuffiash E, et al. Neural substrates of the cognitive processes underlying spelling: evidence from MR diffusion and perfusion imaging. Aphasiology. 2002;16(4-6):425-438. [Google Scholar]
- 47.Kan IP, Biran I, Thompson-Schill SL, Chatterjee A. Letter selection and letter assembly in acquired dysgraphia. Cogn Behav Neurol. 2006;19(4):225-236. [DOI] [PubMed] [Google Scholar]
- 48.Partz M-Pd. Deficit of the graphemic buffer: effects of a written lexical segmentation strategy. Neuropsychol Rehabil. 1995;5(1-2):129-147. [Google Scholar]
- 49.Law SP, Yeung O, Wong W, Chiu KM. Processing of semantic radicals in writing Chinese characters: data from a Chinese dysgraphic patient. Cogn Neuropsychol. 2005;22(7):885-903. [DOI] [PubMed] [Google Scholar]
- 50.Han Z, Zhang Y, Shu H, Bi Y. The orthographic buffer in writing Chinese characters: evidence from a dysgraphic patient. Cogn Neuropsychol. 2007;24(4):431-450. [DOI] [PubMed] [Google Scholar]
- 51.Han Z, Bi Y. Oral spelling and writing in a logographic language: insights from a Chinese dysgraphic individual. Brain Lang. 2009;110(1):23-28. [DOI] [PubMed] [Google Scholar]
- 52.Law SP. Writing errors of a Cantonese dysgraphic patient and their theoretical implications. Neurocase. 2004;10(2):132-140. [DOI] [PubMed] [Google Scholar]
- 53.Graff-Radford J, Duffy JR, Strand EA, Josephs KA. Parkinsonian motor features distinguish the agrammatic from logopenic variant of primary progressive aphasia. Parkinsonism Relat Disord. 2012;18(7):890-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data are available from the corresponding author upon reasonable request.