Abstract
Endocrine disrupting chemicals (EDCs) include non-persistent exogenous substances such as parabens, bisphenols and phthalates which have been associated with a range of metabolic disorders and disease. It is unclear if exposure remains consistent over time. We investigated change in indicators of EDC exposure between 2009 and 2016 and assessed its consistency between and within individuals over a median follow-up time of 47 months in a sample of Dutch individuals. Of 500 Dutch individuals, two 24 hour urine samples were analysed for 5 parabens, 3 bisphenols and 13 metabolites of in total 8 different phthalates. We calculated per-year differences using meta-analysis and assessed temporal correlations between and within individuals using Spearman correlation coefficients, intra-class correlation coefficients (ICC) and kappa-statistics. We found a secular decrease in concentrations of methyl, ethyl, propyl and n-butyl paraben, bisphenol A, and metabolites of di-ethyl phthalate (DEP), di-butyl phthalate (DBP), di-(2-ethyl-hexyl) phthalate (DEHP), and butylbenzyl phthalate (DBzP) which varied from 8 to 96% (ethyl paraben, propyl paraben) between 2009 and 2016. Within-person temporal correlations were highest for parabens (ICC: 0.34 to 0.40) and poorest for bisphenols (ICC: 0.15 to 0.23). For phthalate metabolites, correlations decreased most between time periods (ICC < 48 months: 0.22 to 0.39; ≥ 48 months: −0.05 to 0.32). When categorizing EDC concentrations, 33 to 54% of individuals remained in the lowest or highest category and temporal correlations were similar to continuous measurements. Exposure to most EDCs decreased between 2009 and 2016 in a sample of individuals with impaired fasting glucose from the Dutch population. Temporal consistency was generally poor. The inconsistency in disease associations may be influenced by individual-level or temporal variation exhibited by EDCs. Our findings call for the need for repeated measurements of EDCs in observational studies before and during at-risk temporal windows for the disease.
Keywords: Bisphenols, parabens, phthalates, temporal stability, reproducibility
Introduction
Parabens, bisphenols and phthalates are man-made substances that can be found in a wide variety of everyday products (e.g. food, personal care products and plastics), leading to ubiquitous exposure in humans (Frederiksen et al., 2020; Koch et al., 2017; Moos et al., 2015; van der Meer et al., 2020). Because of their estrogenic and/or anti-androgenic activity, these chemicals are hypothesized to disrupt the endocrine system and therefore have been labelled as “Endocrine Disrupting Chemicals” (EDCs). Exposure to these EDCs has been associated with chronic diseases such as obesity, type 2 diabetes, infertility and cancer (Kahn et al., 2020; Trasande et al., 2016). Most of these studies rely on a single measurement to indicate chronic exposure and do not take the stability of these EDCs into account. Lack of consideration of time-related phenomena may lead to inconsistencies in associations between EDCs and disease.
Regulatory limits have been introduced for EDCs over the past decades (“COMMISSION REGULATION (EU) No 358/2014,” 2014; “DIRECTIVE 2005/84/EC,” n.d.). These regulations, together with a rise in consumer awareness has led to a general decrease in EDC exposures in several European countries and the US (Frederiksen et al., 2020; Koch et al., 2017; Moos et al., 2015). However, there is limited information available on the temporal change in the body burden of EDC concentrations over recent years. Moreover, biomonitoring studies as described above use cross-sectional designs which make it impossible to evaluate changes in exposure within individuals.
Further, parabens, bisphenols and phthalates are all quickly metabolized and excreted from the human body. Because of half-life times of less than 24 hour (24h) (Anderson et al., 2001; Janjua et al., 2008; Völkel et al., 2002), these EDCs are non-persistent. Due to their non-persistent nature, the consistency of these chemicals has been a subject of debate with a recent review showing a wide range of within-person variability (LaKind et al., 2019a). Inconsistencies may be caused by high variability over time, but also the medium (e.g. serum, spot urine) and/or small sample sizes. More important, studies investigating EDC consistency often focus on a follow-up period of weeks to months, while association studies require assessment of disease development over years. Furthermore, studies often investigate associations between categorized EDCs and disease (e.g. first quartile versus fourth quartile) in an attempt to “reduce” noise or investigate non-linear associations. Very little is known about the potential benefits (and pitfalls) of EDC categorization in the face of temporal inconsistency.
In short, it is unclear (a) how ubiquitous exposure levels change over time and (b) how consistent exposures remain over the course of multiple years. Here, we aimed to assess the prospective changes in adult exposure to a wide range of a mixture of parabens, bisphenols and phthalate metabolites in the Netherlands between 2009 and 2016 as measured in 24h urine samples. Using repeated measurements, we investigated temporal correlations of EDCs both within and between individuals over a time period relevant for clinical studies and assessed the change in correlations between different lengths of follow-up. Finally, we assessed the potential benefits of exposure categorization.
Materials and Methods
Study population
This study consisted of 500 native Dutch subjects with impaired fasting glucose (i.e. fasted glucose 6.1 to 7.0 mmol/l) from the Lifelines study. Individuals were selected based on their glycaemic status (i.e. fasting glucose: 6.1 to 7.0 mmol/l) and the availability of urine samples. Baseline urine samples were collected between January 2009 and December 2013. A second urine sample was collected between January 2014 and December 2015. Lifelines is a multi-disciplinary prospective population-based cohort study conducted in and representative for the north of the Netherlands (Klijs et al., 2015; Scholtens et al., 2015). The LifeLines Cohort Study is conducted in accordance with the Declaration of Helsinki and the research code of the University Medical Center Groningen (UMCG). Before study entrance, participants signed an informed consent. The study was approved by the UMCG medical ethics review committee.
Biochemical measurements
Containers for 24h urine collection were accompanied by oral and written instructions and samples. The total 24h urine volume was measured from the collected containers. Next, urine was homogenized after which a sample was taken and stored at −80°C. Methyl paraben (MeP), ethyl paraben (EtP), propyl paraben (PrP), n-butyl paraben (n-BuP) and benzyl paraben (BzP), BPA, BPF and BPS and phthalate metabolites mono-methyl phthalate (MMP), mono-ethyl phthalate (MEP), mono-iso-butyl phthalate (MiBP), mono-n-butyl phthalate (MnBP), mono-(2-ethylhexyl) phthalate (MEHP), mono-n-hexyl phthalate (MnHP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono-benzyl phthalate (MBzP), mono-iso-nonyl phthalate (MiNP), mono-hydroxy-iso-nonyl phthalate (MHiNP), and mono-iso-decyl phthalate (MiDP) were analysed in 24h urine samples using offline isotope dilution liquid chromatography tandem mass spectrometry (LC-MS/MS) technology, for which the full analytical methods have been described elsewhere (van der Meer et al., 2019). The limit of detection (LOD) was calculated as 3.3*S0 / b, where S0 is the standard deviation of the response and b the slope of the calibration curve (Ich, 2005). The limit of quantification (LOQ) was determined by analysing six different samples with progressively lower concentrations on six different days. The LOQ was set where the imprecision was ≤ 20% and the signal to noise ratio was > 10 on all days. Urinary concentrations of total excreted EDCs per 24h (ng/24h) were calculated by multiplying the measured EDCs (ng/mL) by the total urinary 24h volume (mL). This way, we corrected for dilution as a result of differences in urine volume.
Statistical analysis
We assessed the consistency of exposure to EDCs by I) examining trends in per-year exposure, II) assessing the robustness of correlations between EDCs and III) investigating inter- and intra-person correlations of EDCs between baseline and follow-up.
To investigate the temporal stability of EDCs, we calculated the proportion of samples detected above the LOD, median concentrations and distribution for each EDC at baseline and at follow-up. Solely EDCs which were detected above LOD in at least 33% of the samples were selected for subsequent analysis. Samples which were detected below the LOD were imputed with the LOD divided by the square root of 2 (“LOD/√2”) (Hornung and Reed, 1990), after which we grouped concentrations per year of urine collection. To account for a right-skewed distribution, we transformed EDC concentrations with a log10 for statistical analysis. We tested differences in exposure within individuals using linear mixed effect models. In R, our model is specified:
| (1) |
Differences in exposure over time were modelled using meta-regression approach. Again, in R:
| (2) |
In which sem stands for standard error of the mean. Second, we assessed how EDC concentrations were correlated with each other by calculating Spearman correlation coefficients between EDCs at both baseline and follow-up and examined whether the correlations remained consistent over time by comparing correlations found between baseline EDCs to those found at follow-up. Third, we investigated the temporal correlations of EDC exposure between individuals independent of temporal trends by calculating the Spearman correlation coefficients between baseline and follow-up. In addition, we assessed the within-person temporal correlation of EDCs over time by calculating intraclass coefficients (ICC) with a random effect for individuals.
As many studies which focus on disease associations categorize EDC exposure (e.g. highest concentration quartile versus lowest concentration quartile), we categorized all EDCs which were detected above LOD in at least 75% of the samples at both timepoints into quartiles. First, we assessed agreement and cross-over from one category to another over time while sticking to the cut-offs based on baseline measurements. Next, we repeated the analysis after recategorizing follow-up measurements independent of baseline categories, and tested cross-over by calculating kappa statistics. To examine the effect of time interval between baseline and follow-up measurements on temporal correlations, we calculated Spearman correlation coefficients, ICCs and Kappa statistics while stratifying for time interval (i.e. <48 months; ≥48 months).
This study was conducted in a population at-risk for metabolic diseases, which may impact the representativeness of the results. Therefore, we investigated how two levels of potential confounding variables affected our results: a) age and sex, and b) age, sex, body mass index (BMI; categorized as: normal weight [<25 kg/m2], overweight [25–30 kg/m2] or obese [>30kg/m2]) and smoking status (categorized as: never smoker, ex-smoker, and current smoker). For both categories, the healthiest level was set as reference (i.e. normal weight, never smoker). We added these variables as fixed effects in our model that assessed differences in exposure within individuals. Also, we calculated partial Spearman correlation adjusting for the variables mentioned above. Further, we conducted a sensitivity analysis in which we excluded all individuals with a body mass index (BMI) of 30 kg/m2 or higher.
We performed all analyses using the R project software (version: 3.5.2) (Team, 2017).
Results
The study population consisted of slightly more males (60%) with a mean age of 53 years at baseline. The largest proportion was overweight (48%) and ex-smoker (46%) (Supplementary table 1). The median time difference between baseline and follow-up was 47 months (interquartile range: 39 to 55 months; maximum: 85 months).
Temporal trends of endocrine disrupting chemical exposure
Urinary excretions of EDCs are reported in Table 1. The phenols MeP and EtP, and BPA were detected in at least 75% of the samples at both baseline and follow-up measurements, as were the phthalates MEP, MiBP, MnBP, MEHP, MEHHP, MEOHP, MECPP and MBzP (≥ 99%). BzP, BPS, MiNP and MiDP were detected in at most 18% of the samples and were therefore excluded from subsequent analysis. Within-person excretions decreased over time for all EDCs (p ≤ 0.006) but BPF, MMP and MnHP (p > 0.05). When analysing excretions per year (Figure 1), we observed a similar decline in all EDC excretions except for BPF, MMP, and MnHP. Between 2009 and 2016, median concentrations decreased with 8 to 96% for parabens (EtP, PrP, respectively), 50% for BPA, and 34 to 66% for phthalates (MEP, MEHP, respectively; Supplementary Table 2). For some parabens (i.e. MeP, PrP, n-BuP) a large decrease in excretions was detected between 2010 and 2011, whereas BPA and phthalate metabolites appeared to decline more gradually over time. Excretions per year are presented in Supplementary Table 3, and log10-transformed excretions used for the meta-regression analyses are depicted in Supplementary Figure 1.
Table 1.
Number of detections, concentration and distribution of endocrine disrupting chemicals.
| Abbreviation | LOD | Baseline (μg/24h) | Follow-up (μg/24h) | p-value | |||
|---|---|---|---|---|---|---|---|
| >LOD (n (%)) | Median [IQR] | >LOD (n (%)) | Median [IQR] | ||||
| Parabens | |||||||
| methyl paraben | MeP | 0.14 | 500 (100) | 12.25 (2.74; 44.84) | 500 (100) | 4.81 (1.71; 22.81) | <0.0001 |
| ethyl paraben | EtP | 0.09 | 470 (94) | 1.2 (0.35; 5.54) | 449 (90) | 0.76 (0.24; 2.75) | 0.0014 |
| propyl paraben | PrP | 0.07 | 377 (75) | 1.18 (0.08; 9.36) | 286 (57) | 0.21 (<LOD; 2.27) | <0.0001 |
| n-butyl paraben | n-BuP | 0.06 | 278 (56) | 0.07 (<LOD; 0.41) | 178 (36) | <LOD (<LOD; 0.09) | <0.0001 |
| benzyl paraben | BzP | 0.07 | 15 (3) | <LOD (<LOD; <LOD) | 8 (2) | <LOD (<LOD; <LOD) | |
| Bisphenols | |||||||
| bisphenol A | BPA | 0.22 | 399 (80) | 1.1 (0.31; 2.41) | 379 (76) | 0.77 (0.22; 1.72) | 0.0055 |
| bisphenol F | BPF | 0.23 | 276 (55) | 0.29 (<LOD; 0.81) | 263 (53) | 0.25 (<LOD; 0.77) | 0.8860 |
| bisphenol S | BPS | 0.06 | 65 (13) | <LOD (<LOD; <LOD) | 88 (18) | <LOD (<LOD; <LOD) | |
| Phthalates | |||||||
| mono-methyl phthalate | MMP | 0.43 | 351 (70) | 0.96 (<LOD; 2.02) | 311 (62) | 0.78 (<LOD; 1.83) | 0.9193 |
| mono-ethyl phthalate | MEP | 0.35 | 500 (100) | 50.46 (19.76; 148.62) | 500 (100) | 31.46 (14; 81.08) | <0.0001 |
| mono-iso-butyl phthalate | MiBP | 0.33 | 500 (100) | 19.93 (12.2; 34.55) | 500 (100) | 13.77 (8.59; 22.41) | <0.0001 |
| mono-n-butyl phthalate | MnBP | 0.22 | 500 (100) | 16.42 (10.46; 25.54) | 500 (100) | 12.53 (7.83; 18.95) | <0.0001 |
| mono-n-hexyl phthalate | MnHP | 0.12 | 288 (58) | 0.08 (<LOD; 0.14) | 230 (46) | <LOD (<LOD; 0.11) | 0.0620 |
| mono-(2-ethylhexyl) phthalate | MEHP | 0.07 | 500 (100) | 2.19 (1.34; 3.72) | 493 (99) | 1.28 (0.75; 2.23) | <0.0001 |
| mono-(2-ethyl-5-hydroxyhexyl) phthalate | MEHHP | 0.11 | 500 (100) | 8.81 (6.14; 13.67) | 500 (100) | 5.83 (3.95; 8.62) | <0.0001 |
| mono-(2-ethyl-5-oxohexyl) phthalate | MEOHP | 0.09 | 500 (100) | 5.19 (3.61; 8.29) | 500 (100) | 3.52 (2.42; 5.41) | <0.0001 |
| mono-(2-ethyl-5-carboxypentyl) phthalate | MECPP | 0.25 | 500 (100) | 9.03 (6.31; 13.99) | 500 (100) | 5.75 (3.88; 9.07) | <0.0001 |
| mono-benzyl phthalate | MBzP | 0.22 | 500 (100) | 3.73 (2.28; 6.48) | 496 (99) | 2.02 (1.22; 4.09) | <0.0001 |
| mono-iso-nonyl phthalate | MiNP | 0.10 | 3 (1) | <LOD (<LOD; <LOD) | 1 (0) | <LOD (<LOD; <LOD) | |
| mono-iso-decyl phthalate | MiDP | 0.31 | 5 (1) | <LOD (<LOD; <LOD) | 4 (1) | <LOD (<LOD; <LOD) | |
Abbreviations: LOD, limit of detection; IQR, inter-quartile range. LODs are expressed as ng/mL. Urinary concentrations of total excreted EDCs per 24h (μg/24h) were calculated by multiplying the measured EDCs (ng/mL) by the total urinary 24h volume (mL) and dividing the result by a factor 1000. Within-person differences were calculated using linear mixed effect models: “lme(EDC ~ time + (1|ID), data = data)”.
Figure 1.
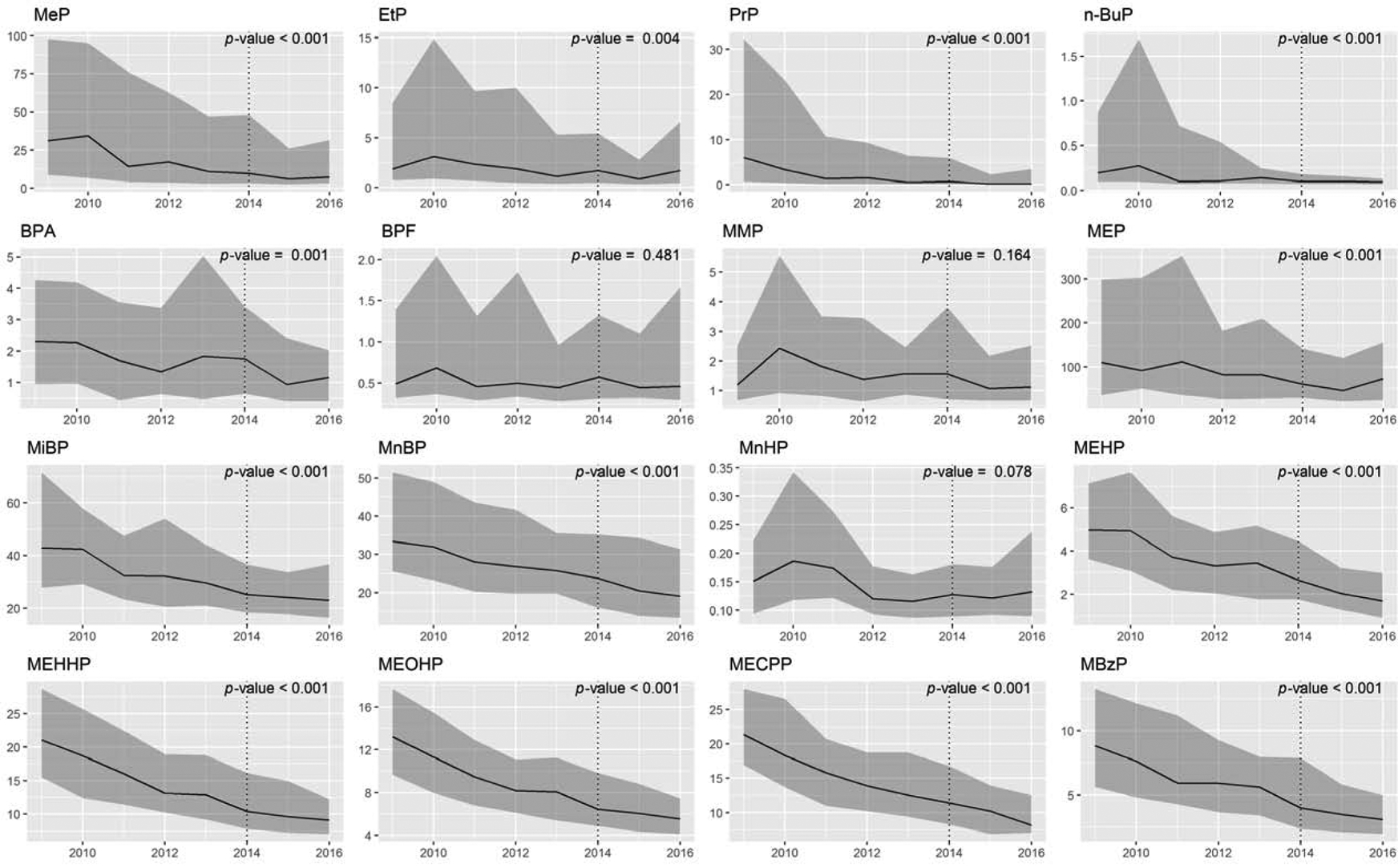
Yearly excretions of urinary endocrine disrupting chemicals.
Yearly phenol and phthalate excretions (μg/24 hour) are expressed as median [interquartile range]. The dotted line depicts the end of baseline sample collection (2009 – 2014), and the beginning of follow-up sample collection (2014 – 2016). The p-values show whether the change in EDC excretion changes significantly over time. Full names of abbreviations are shown in Table 1.
Correlations between exposure to endocrine disrupting chemicals
Figure 2a shows Spearman correlations between EDC excretions at both baseline (lower triangle) and follow-up (upper triangle). Correlations between phenol excretions in general remained consistent over time and were strong for MeP with EtP and PrP (r: 0.50 to 0.71). n-BuP was correlated stronger with MeP, EtP and PrP at follow-up (r: 0.48 to 0.59) compared to baseline (0.36 to 0.38). For phthalates, we found the strongest consistent correlations between MEHHP, MEOHP and MECPP (r: 0.86 to 0.96), which were all to a lesser extent correlated with MEHP (r: 0.60 to 0.68). MinBP and MnBP were moderately correlated at both timepoints (r: 0.44, 0.40, baseline, follow-up). When comparing correlations of EDCs between baseline and follow-up (Figure 2b), we found the phenol MeP to consistently correlate with EtP and PrP (r: 0.31 to 0.34) and PrP to be consistently correlated with n-BuP (r: 0.31, 0.32). All Spearman correlation coefficients are described in Supplementary Table 4.
Figure 2.
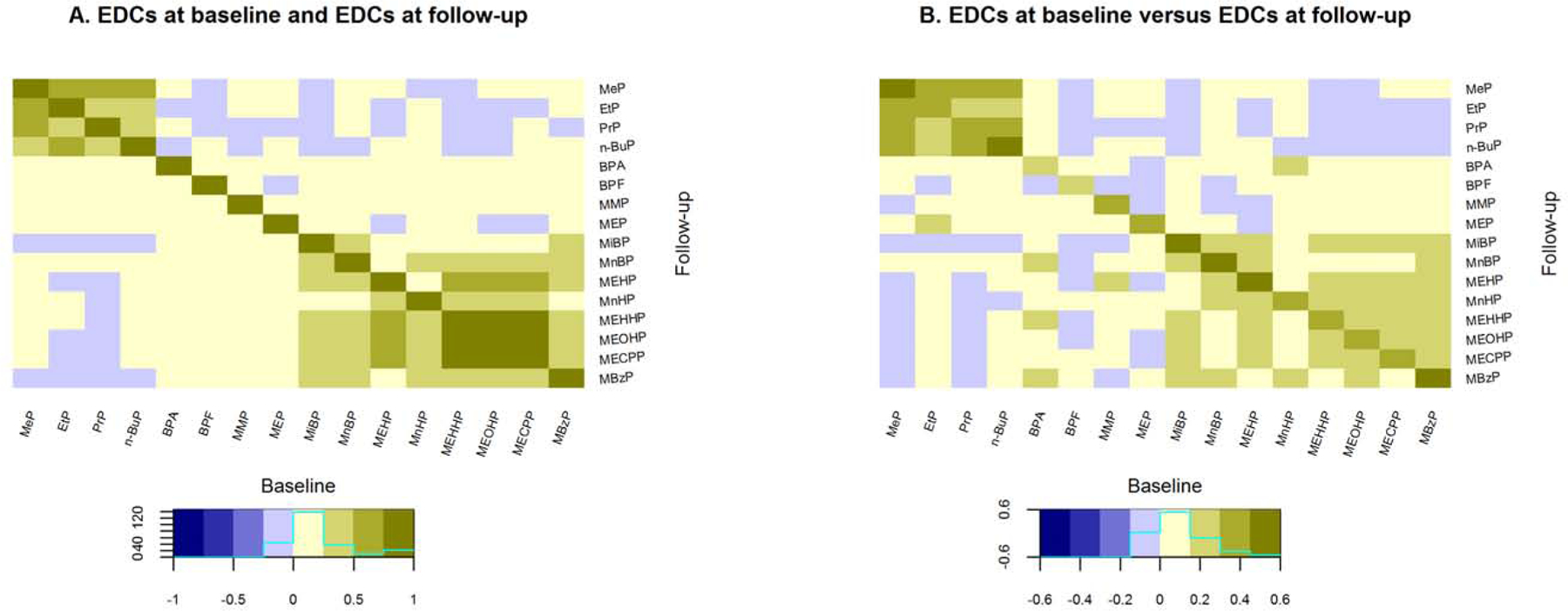
Correlations between endocrine disrupting chemicals at different timepoints.
Abbreviations: EDCs, endocrine disrupting chemicals. A.) Spearman correlation coefficients of EDCs at baseline (lower triangle) and follow-up (upper triangle). At places where the colouring is symmetrical across the diagonal, correlations are robust across time. B.) Spearman correlation coefficients of EDCs at baseline versus those at follow-up. The diagonal depicts coefficients between the baseline and follow-up of the same chemicals. Full names of EDC abbreviations are shown in Table 1.
Temporal correlations of endocrine disrupting chemical exposure
Next, we assessed the consistency of parabens, bisphenols and phthalates over time. In the total population, we detected moderate between-person temporal correlations (Figure 2b, Figure 3a) for MeP, EtP, PrP and n-BuP (Spearman’s rho: 0.38 to 0.49), and BPA and BPF (Spearman’s rho: 0.19, 0.21). This was reflected by the within-person temporal correlations (Figure 3b), which we found to be significant for all parabens (ICC: 0.34 to 0.40) but not for BPA and BPF (0.15, 0.23, respectively). For phthalates, between-person temporal correlations were moderate for MiBP, MnBP, MEHP, and MBzP (Spearman’s rho: 0.43 to 0.55). Of these phthalates, within-person temporal correlations were in agreement for MiBP, MEHP and MnBP (ICC: 0.32 to 0.37), and lower for MBzP (ICC: 0.25). We observed weak between-person (Spearman’s rho: 0.29 to 0.37) and weak within-person temporal correlations (0.09 to 0.24) for MEP, MnHP, MEHHP, MEOHP and MECPP. Though being weakly correlated between persons (r: 0.33), MMP showed a consistent within-person correlation (ICC: 0.35). All temporal correlation coefficients are reported in Supplementary table 5.
Figure 3.
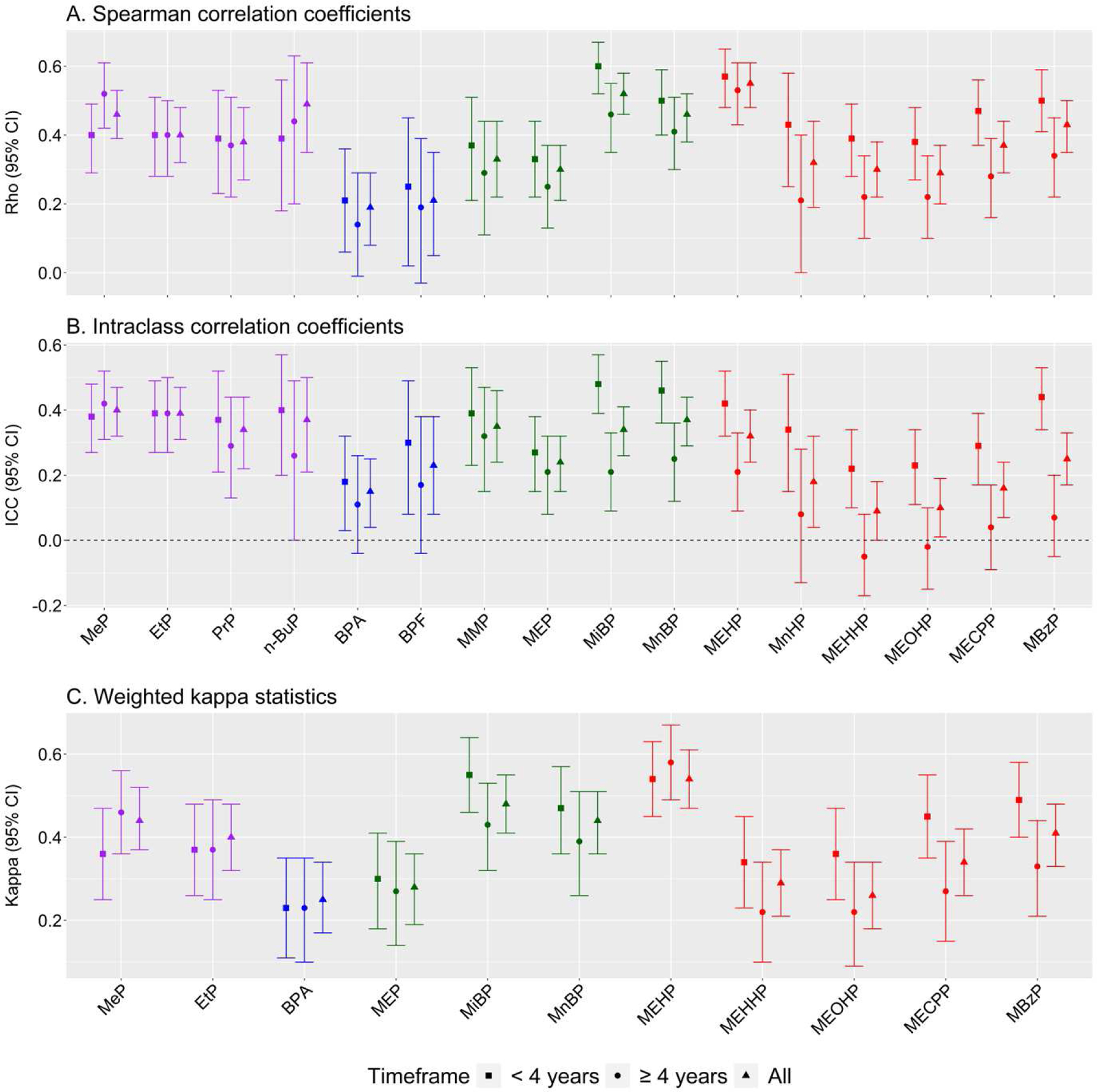
Consistency of endocrine disrupting chemicals over different timeframes.
A.) Spearman rank-order correlation coefficients to show between-person temporal correlation, which is independent of the general decrease of exposure to endocrine disrupting chemicals as depicted in Figure 1. B.) Intraclass correlation coefficients of endocrine disrupting chemicals to show temporal correlation within individuals of absolute exposure values. C.) Kappa of endocrine disrupting chemicals to show temporal correlation after categorization of the data into quartiles. Parabens are coloured purple, bisphenols blue, low molecular weight phthalates green and high molecular weight phthalates red. Full names of EDC abbreviations are shown in Table 1.
Temporal correlations of categorized endocrine disrupting chemical exposure
When categorizing EDC exposure into quartiles based on baseline cut-off points, at follow-up we observed a 1.2 to 2.5-fold increase of the lowest quartile (BPA, MEOHP) and a 1.5 to 3.1-fold decrease of the highest quartile (BPA, MEHHP; Figure 4a). When categorizing baseline and follow-up EDC excretions independent of each other, we observed a large proportion of cross-over between exposure quartiles (Figure 4b): Specifically, a total of 32 to 43% of the individuals remained in their respective category (MEHHP, MEOHP; MiBP, respectively), with 44 to 54% (MEHHP, MEOHP; MiBP, MnBP) and 33 to 54% (MEHHP, MEOHP, BPA; MEHP) remaining in respectively lowest and highest quartile. Information on changes in quartile composition are documented in Supplementary table 6.
Figure 4.
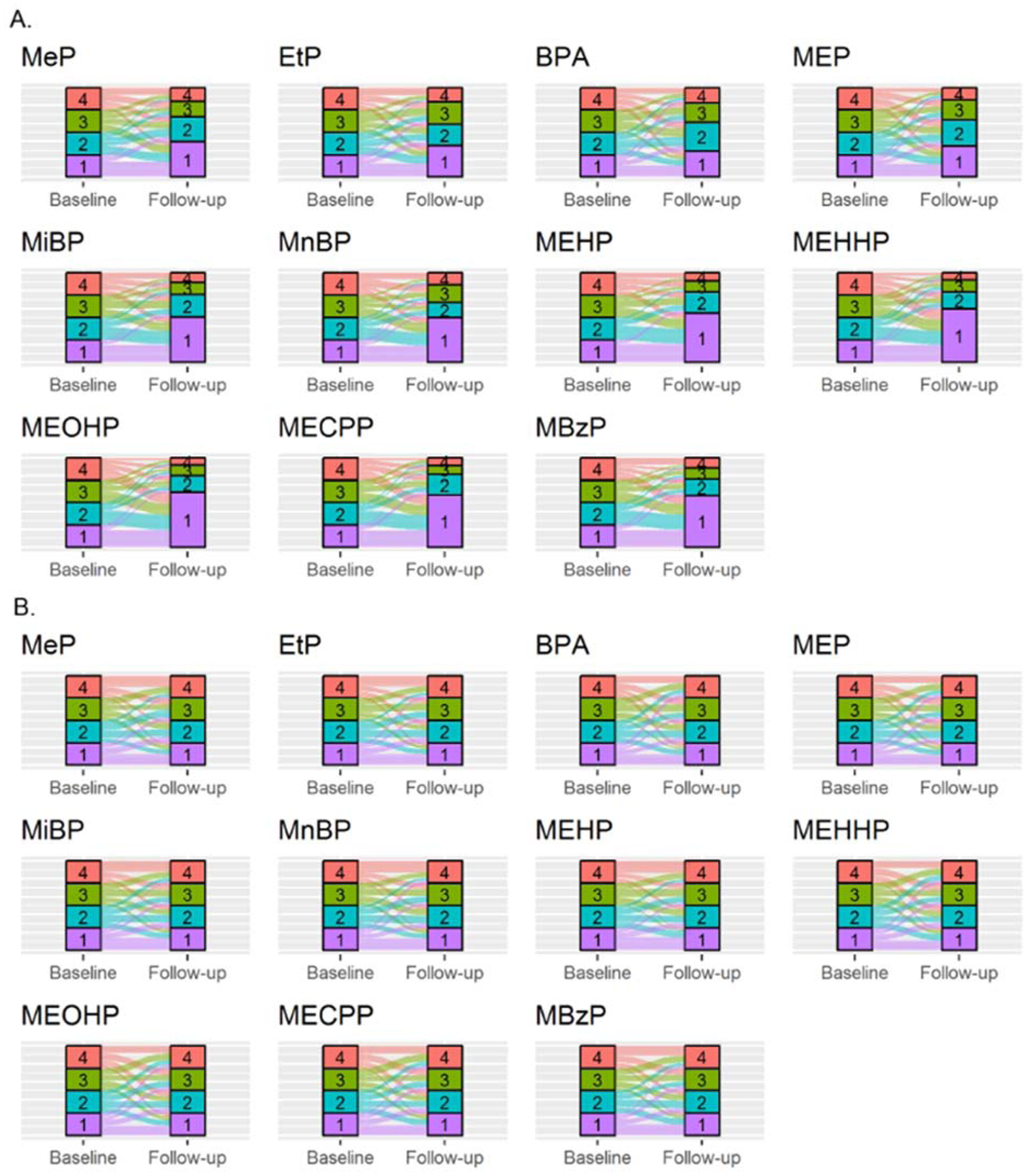
Consistency of categorized endocrine disrupting chemicals.
A.) Endocrine disrupting chemical exposure groups categorized in quartiles based on baseline cut-off values. The sizes of the categories at follow-up show the proportion in which concentrations at follow-up would be categorized based on baseline exposure measurements. The observed size increase of the lowest category is in line with the decreasing trend in EDC exposures as depicted in Figure 1. B.) Endocrine disrupting chemical exposure groups categorized in quartiles based on the cut-off values at their respective timepoint, leading to an equal distribution of individuals over the quartiles. The coloured bands express the proportion of individuals which are categorized in that respective quartile at baseline that relocate to a category at follow-up. The category labelled “1” (purple) represents the lowest exposure category, whereas the “4” (red) represents the highest exposure quartile. Full names of EDC abbreviations are shown in Table 1.
Difference in temporal correlations between less and greater than four year follow-up
As consistency has been shown to decrease over time, we compared correlation coefficients for repeated measurements with an interval of less than 48 months (n = 260) with those which had an interval of at least 48 months (n = 240). For parabens, we observed correlations to increase over time for MeP (Spearman’s rho: +30%, ICC: +11%, kappa: +28%), and to remain stable for EtP. Between-person correlations remained similar for PrP and n-BuP (Spearman’s rho: −5%, +13%), but ICCs decreased over time (−22%, −35%). Bisphenol correlations decreased when continuous (Spearman’s rho: −33%, −24%; ICC: −39%, −43%, BPA, BPS, respectively), but BPA correlations remained similar when categorized. Regarding phthalates, a longer interval between measurements impacted all correlation coefficients for MMP and MEP between −10% and −24%. Though a similar decrease was observed for the between-person and categorized correlation coefficients of MiBP, MnBP and MEHP, the ICC showed a larger decline (−46% to −50%). Coefficients for MnHP, MEHHP, MEOHP, MECPP, and MBzP varied between −32% and −51% for between-person and categorized correlation coefficients, and between −76% and −123% for within-person correlations.
Sensitivity analysis
We found within-person changes in exposure to remain similar when we adjusted for potential confounding variables (Supplementary table 2). Further, we observed that adjusting for age, sex, BMI and smoking status did not affect the Spearman correlation with more than 0.10 points between the EDCs measured at baseline (Supplementary figure 2). When we adjusted the model focusing on EDCs measured at follow-up we found that correlation coefficients became less strong between n-BuP and MeP (Rho: 0.51 to 0.39), and PrP (0.33 to 0.17). Correlation coefficients changed direction between BPF and MeP, PrP and n-BuP and remained minor (−0.01 to 0.04). We next looked at the impact of age, sex, BMI and smoking status on the Spearman correlations between the same EDCs measured at different timepoints (Supplementary figure 3). All adjusted correlation coefficients remained within 0.10 point of their unadjusted coefficient. Changes were largest for MeP (0.46 to 0.37) and PrP (0.38 to 0.29).
In total, 181 individuals had a BMI of at least 30 kg/m2 and were therefore excluded from the sensitivity analysis. Within-person changes in exposure remained similar for all EDCs but BPA (1.85 [0.62; 3.69] to 1.44 [0.50; 3.08], p = 0.076). On the other hand, when assessing exposure per year BPA showed significant decrease over time (p = 0.037), as did MnHP (p = 0.031). Correlation patterns between EDCs remained similar compared to the full study population, as did the temporal correlation coefficients (data not shown).
Discussion
Here, we used 24h urine samples to assess the exposure of a wide mixture of non-persistent EDCs in Dutch adults between 2009 and 2016. We investigated the consistency between EDCs over different time intervals leveraging individual samples taken at baseline and follow-up. Further, we investigated the effect of categorization on the stability of EDC exposure values.
Secular decrease in endocrine disrupting chemical exposure from 2009 to 2016
Over the past decades, exposure to parabens, bisphenols and phthalates have been monitored through surveillance programs such as the National Health and Nutrition Examination Survey (NHANES, US), and the German Environmental Specimen Bank (ESB). Though some studies have reported exposures without the help of biomonitoring programs (e.g. Denmark), this data is unavailable for most countries including the Netherlands. In the current study, we observed a decrease in exposure for all parabens between 2009 and 2016. Between 2010 and 2011, we observed an especially steep decline in exposure for MeP, PrP and n-BuP. The ESB did not report data on parabens for 2011, but they did observe a reduction in exposure gradient in median concentrations for EtP and PrP (1.8- and 2.2-fold decrease), but not MeP (1.3-fold increase) between 2009 and 2012 (Moos et al., 2015). In 2010, Denmark banned the use of PrP and BuP in cosmetic products for children under three years (“COMMISSION REGULATION (EU) No 358/2014,” 2014). Though this regulation did not include MeP and did not apply to the Netherlands, it might have led to changes in the production of Western-European based products. We observed another decline in paraben exposure between 2014 and 2015 that has, to the best of our knowledge, not been reported by other studies before. This decrease could be another result of legislation: As of April 2014, the European Parliament banned the use of iso-PrP, iso-BuP, and BzP in cosmetic products, next to allowing maximum concentrations of 0.19% for BuP and PrP, and 0.8% for combined parabens (“COMMISSION REGULATION (EU) No 358/2014,” 2014). In contrast to Europe, parabens are not regulated in the USA (Center for Food Safety and Nutrition, 2020), potentially limiting the representativeness of this data to other countries.
We observed a declining gradient in BPA exposure over time. Several studies showed a similar trend in the USA, in which median concentrations decreased 1.5- to 5.8-fold (LaKind et al., 2019b; Lin et al., 2020; Ye et al., 2015). For Swedish mothers, an annual decrease of 9.8% (±4.3, p = 0.029) was observed (Gyllenhammar et al., 2017), which was similar to Danish young men that showed an average decrease of 10% per year, (Frederiksen et al., 2020). BPA concentrations remained similar over time in a Canadian population (1.1 to 1.2 ng/ml) and increased from 0.7 to 1.1 ng/ml in Korea (LaKind et al., 2019b). We found the strongest decline in exposure between 2014 and 2015, in which the European Union reduced the tolerable daily intake (TDI) for BPA from 50 to 4 μg/kg body weight/day (EFSA Panel on Food Contact Materials et al., 2015). In contrast, we observed BPF concentrations to remain stable over time, in line with studies from the USA and Denmark (Frederiksen et al., 2020; Ye et al., 2015), while concentrations showed an increasing gradient of 20% change per year (±7.6, p = 0.003) in Sweden (Gyllenhammar et al., 2017).
Regarding phthalates, we observed a decreasing gradient in exposure to the DEHP metabolites MEHP, MEHHP, MEOHP and MECPP, the DBP metabolites MiBP and MnBP, and the BBP metabolite MBzP. Other studies showed a similar trend in Danish (average yearly decreases of 7.2 to 16%, all p < 0.0001) and Swedish study populations (14 to 17% decrease per year, all p < 0.001) (Frederiksen et al., 2020; Gyllenhammar et al., 2017). Moreover, median concentrations of MEHP, MiBP, MnBP and MBzP decreased 1.7- to 2.8-fold between 2009 and 2015 in a German study performed by the ESB (Koch et al., 2017). In contrast to parabens and bisphenols, this gradient was more constant. This might be due to the implementation of multiple different regulations over time focussed on different products such as children’s toys (“DIRECTIVE 2005/84/EC,” n.d.), foodstuffs (“DIRECTIVE 2007/19/EC,” n.d.), cosmetics (“REGULATION (EC) No 1223/2009,” n.d.), and electronic devices (“Directive 2011/65/EU,” n.d.), which have been gradually become active over the years and will continue to until 2021. In addition, concentrations of the metabolite for DEP showed a declining gradient over time, in line with Danish and Swedish studies which showed a yearly decrease of 16 and 9.8%, respectively (Frederiksen et al., 2020; Gyllenhammar et al., 2017). The DMP metabolite MMP remained stable. Though some studies reported decreases over time, absolute concentrations were in the same range (i.e. 1.4 μg/L versus 1.9 to 2.8 μg/L) (Frederiksen et al., 2020; Koch et al., 2017).
Parabens, bisphenols, and phthalates are mainly used as plasticizers or preservatives, and sources of exposure described in literature include food- and personal care products. Therefore, it would be of interest to investigate whether the use or consumption of specific products is associated with exposure. However, in current study data on the use of personal care products is unavailable. Though we did have data on the consumption of a large variety of food products, the packaging and way of preparation of these products was unknown. For example, we do have an indication of how much carrots an individual ate over the past month, but not whether these were freshly picked and paper-wrapped at a farm or bought canned from the supermarket. As shown previously, the packaging of products and use of cooking utensils can heavily impact exposure to EDCs (Rudel et al., 2011). Therefore, specific sources of exposure should be investigated in studies designed for that purpose.
Co-exposure and correlation patterns between different endocrine disrupting chemicals
We observed strong correlation patterns between several parabens at baseline that remained consistent at follow-up. As parabens have been shown to be more effective as antimicrobial agents when combined, they are often used as a mixture of several different parabens (Soni et al., 2005). Most phthalates showed correlations to some extent, with strong correlations between DEHP metabolites. MEHP showed a less strong correlation compared to other DEHP metabolites, which may be explained by abiotic hydrolysis of DEHP which naturally occurs in the environment (Silva et al., 2006).
The EDCs of interest have been associated with a wide range of demographic variables such as age, sex, obesity, and smoking status, and association analysis therefore often adjust for these variables (Hatch et al., 2008; Huang et al., 2014; Sun et al., 2014). Here, we found that these variables had a small impact on our findings. This may be due to the use of repeated measurements in the same individual. Further, our study population consisted of individuals with impaired fasting glucose. Although obesity and smoking status varied between individuals, this may have led to uniformity within the population.
Lack of consistency of endocrine disrupting chemical exposure over time
We investigated a mixture of EDCs that have half-lives of less than 24h (Anderson et al., 2001; Janjua et al., 2008; Völkel et al., 2002). Combined with the wide applications of these chemicals, exposure and thus urinary concentrations are susceptible for variation. A recent review showed a wide range of within-person temporal correlations, also known as reproducibility, in spot urine samples, which were in mostly poor (i.e. ICC <0.40) (LaKind et al., 2019a). In general, paraben ICCs were reported to be higher than other EDCs (LaKind et al., 2019a). To the best of our knowledge, this is the first study to assess within-person temporal correlations of parabens over the course of more than one year. Compared to studies investigating shorter time intervals, we found similar correlation coefficients. This is in line with the stability we observed between samples with a short (i.e. < 48 months) and a longer time interval (i.e. ≥ 48 months) and implies that paraben exposure remains relatively consistent over longer time periods. For BPA, studies investigating spot urine samples over an interval up to several months reported very poor within-person temporal correlations (LaKind et al., 2019a). This results was confirmed in another study investigating BPA in spot urine over a period up to three years (ICC 12 to 25 months: 0.23 [0.06; 0.60]; 25 to 36 months: 0.06 [0.00; 0.94]) (Townsend et al., 2013). The within-person temporal correlations we found for BPA was similar to that of the first interval of Townsend and colleagues, but remained stable over a much longer period of time (i.e. 48 months) potentially due to our use of 24h urine samples; The use of 24h urine samples appeared to improve within-person temporal correlation for BPA (ICC: 0.39) over two seasons (Sun et al., 2017). Yet, after a time period of more than 48 months correlation coefficients also deteriorated in 24h urine, stressing the importance of repeated measurements in BPA-focussed research. To the best of our knowledge, we are the first to report the temporal correlation of BPF, which appeared to be in the same range as BPA. So far, few studies have investigated the temporal correlations of phthalates over a time period of longer than one year (Starling et al., 2015; Townsend et al., 2013). Townsend and colleagues included spot- and morning urine samples of 40 individuals from the US with an one to three year interval (Townsend et al., 2013). We observed similar ICCs for most phthalates (i.e. MEP, MiBP, MnBP, MBzP, MECPP), whereas DEHP metabolites differed. Startling and colleagues included spot urine samples of 100 individuals from Shanghai with a two to eight year interval, and reported lower ICCs for all phthalates (Starling et al., 2015). As we observed a decrease in within-person correlations over time, this may explain differences between studies. In this study, we observed higher between-person compared to the within-person correlation coefficients, which can be explained by the general decrease of phthalate concentrations over time. This is in line with the drop in within-person correlations between time intervals, and further stresses the need for repeated measurements.
Consistency of categorized or grouped endocrine disrupting chemical exposure
It is common to categorize continuous EDC concentrations and compare EDC exposure groups in association studies. The similarity between the Spearman correlation coefficients and the weighted kappa statistics indicates that categorizing EDCs does not lead to better temporal correlation. Further, we found that about half of the individuals categorized in the highest or lowest quartile did not remain in their respective category after categorization based on a second measurement. Therefore, categorization severely reduces the dimension of the data (i.e. four groups instead of continuous values) while not increasing consistency and should therefore be avoided.
Conclusion
We found that exposure to BPA and most parabens and phthalates have decreased between 2009 and 2016, of which some may be attributed to the introduction of European legislation. Further, the consistency of 24h urine samples deteriorated over the course of years that reflects both the short half-life of these indicators of exposure and lifestyle changes over a 7-year span. Therefore, repeated measurements of non-persistent EDCs are strongly recommended in clinical studies. Last, categorization of EDC measurements does not improve consistency and should be avoided.
Supplementary Material
Highlights.
Exposure to phenols and phthalates decreased between 2009 and 2016 in the Netherlands
Some decreases coincided with the introduction of European legislation
Temporal consistency of EDCs over 7 years was generally poor
Categorization of EDCs did not improve consistency and should be avoided
We call for the need for repeated measurements in disease association investigations
Acknowledgments
The authors wish to acknowledge the services of the Lifelines Cohort Study, the contributing research centres delivering data to Lifelines, and all the study participants. We thank Irene van der Kooi-Wijbenga for her contribution to the technical measurements. JVvVO was supported by a Diabetes Funds Junior Fellowship from the Dutch Diabetes Research Foundation (project no. 2013.81.1673). CJP had financial support from the National Institutes of Health (grant R01AI127250) for the submitted work. The authors declare no conflict of interest.
Competing Financial Interests and study approval
JVvVO was supported by a Diabetes Funds Junior Fellowship from the Dutch Diabetes Research Foundation (project no. 2013.81.1673). CJP had financial support from the National Institutes of Health (grant R01AI127250) for the submitted work. All other authors declare they have no actual or potential competing financial interests. The authors declare no conflict of interest. The Medical Ethics committee of the University Medical Center Groningen approved the study, and all participants gave written informed consent.
Declaration of interests
JVvVO was supported by a Diabetes Funds Junior Fellowship from the Dutch Diabetes Research Foundation (project no. 2013.81.1673).
CJP had financial support from the National Institutes of Health (grant R01AI127250) for the submitted work. CJP is a co-founder, consultant, and equity holder of XY.health, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson WA, Castle L, Scotter MJ, Massey RC, Springall C, 2001. A biomarker approach to measuring human dietary exposure to certain phthalate diesters. Food Addit. Contam 18, 1068–1074. [DOI] [PubMed] [Google Scholar]
- Center for Food Safety, Nutrition, A., 2020. Parabens in Cosmetics [WWW Document]. U.S. Food and Drug Administration. URL https://www.fda.gov/cosmetics/cosmetic-ingredients/parabens-cosmetics (accessed 6.29.20). [Google Scholar]
- COMMISSION REGULATION (EU) No 358/2014 [WWW Document], 2014. . Official Journal of the European Union. URL https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32014R0358&from=EN (accessed 6.18.20). [Google Scholar]
- DIRECTIVE 2005/84/EC [WWW Document], n.d. URL https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32005L0084&from=EN (accessed 6.23.20).
- DIRECTIVE 2007/19/EC [WWW Document], n.d. URL https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32007L0019&from=FR (accessed 6.23.20).
- Directive 2011/65/EU [WWW Document], n.d. URL https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015L0863&from=FR
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings And Processing, 2015. Scientific Opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA Journal 13, 3978. [Google Scholar]
- Frederiksen H, Nielsen O, Koch HM, Skakkebaek NE, Juul A, Jørgensen N, Andersson A-M, 2020. Changes in urinary excretion of phthalates, phthalate substitutes, bisphenols and other polychlorinated and phenolic substances in young Danish men; 2009–2017. Int. J. Hyg. Environ. Health 223, 93–105. [DOI] [PubMed] [Google Scholar]
- Gyllenhammar I, Glynn A, Jönsson BAG, Lindh CH, Darnerud PO, Svensson K, Lignell S, 2017. Diverging temporal trends of human exposure to bisphenols and plastizisers, such as phthalates, caused by substitution of legacy EDCs?. Environ. Res 153, 48–54. [DOI] [PubMed] [Google Scholar]
- Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, Webster TF, 2008. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999–2002. Environ. Health 7, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl. Occup. Environ. Hyg 5, 46–51. [Google Scholar]
- Huang T, Saxena AR, Isganaitis E, James-Todd T, 2014. Gender and racial/ethnic differences in the associations of urinary phthalate metabolites with markers of diabetes risk: National Health and Nutrition Examination Survey 2001–2008. Environ. Health 13, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ich, 2005. Validation of Analytical Procedures: Text and Methodology Q2(R1).
- Janjua NR, Frederiksen H, Skakkebaek NE, Wulf HC, Andersson A-M, 2008. Urinary excretion of phthalates and paraben after repeated whole-body topical application in humans. Int. J. Androl 31, 118–130. [DOI] [PubMed] [Google Scholar]
- Kahn LG, Philippat C, Nakayama SF, Slama R, Trasande L, 2020. Endocrine-disrupting chemicals: implications for human health. Lancet Diabetes Endocrinol 8, 703–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klijs B, Scholtens S, Mandemakers JJ, Snieder H, Stolk RP, Smidt N, 2015. Representativeness of the LifeLines Cohort Study. PLoS One 10, e0137203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HM, Rüther M, Schütze A, Conrad A, Pälmke C, Apel P, Brüning T, Kolossa-Gehring M, 2017. Phthalate metabolites in 24-h urine samples of the German Environmental Specimen Bank (ESB) from 1988 to 2015 and a comparison with US NHANES data from 1999 to 2012. Int. J. Hyg. Environ. Health 220, 130–141. [DOI] [PubMed] [Google Scholar]
- LaKind JS, Idri F, Naiman DQ, Verner M-A, 2019a. Biomonitoring and Nonpersistent Chemicals-Understanding and Addressing Variability and Exposure Misclassification. Curr Environ Health Rep 6, 16–21. [DOI] [PubMed] [Google Scholar]
- LaKind JS, Pollock T, Naiman DQ, Kim S, Nagasawa A, Clarke J, 2019b. Factors affecting interpretation of national biomonitoring data from multiple countries: BPA as a case study. Environ. Res 173, 318–329. [DOI] [PubMed] [Google Scholar]
- Lin Y, Qiu X, Liu J, Tseng C-H, Allard P, Araujo JA, Zhu Y, 2020. Different temporal trends of exposure to Bisphenol A among international travelers between Los Angeles and Beijing. Environ. Int 141, 105758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RK, Koch HM, Angerer J, Apel P, Schröter-Kermani C, Brüning T, Kolossa-Gehring M, 2015. Parabens in 24h urine samples of the German Environmental Specimen Bank from 1995 to 2012. International Journal of Hygiene and Environmental Health. 10.1016/j.ijheh.2015.07.005 [DOI] [PubMed] [Google Scholar]
- REGULATION (EC) No 1223/2009 [WWW Document], n.d. URL https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32009R1223&from=FR (accessed 6.23.20).
- Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, Rizzo J, Nudelman JL, Brody JG, 2011. Food packaging and bisphenol A and bis(2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ. Health Perspect 119, 914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtens S, Smidt N, Swertz MA, Bakker SJ, Dotinga A, Vonk JM, van Dijk F, van Zon SK, Wijmenga C, Wolffenbuttel BH, Stolk RP, 2015. Cohort Profile: LifeLines, a three-generation cohort study and biobank. Int. J. Epidemiol 44, 1172–1180. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Reidy JA, Preau JL, Samandar E, Needham LL, Calafat AM, 2006. Measurement of eight urinary metabolites of di(2-ethylhexyl) phthalate as biomarkers for human exposure assessment. Biomarkers 11, 1–13. [DOI] [PubMed] [Google Scholar]
- Soni MG, Carabin IG, Burdock GA, 2005. Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem. Toxicol 43, 985–1015. [DOI] [PubMed] [Google Scholar]
- Starling AP, Engel LS, Calafat AM, Koutros S, Satagopan JM, Yang G, Matthews CE, Cai Q, Buckley JP, Ji B-T, Cai H, Chow W-H, Zheng W, Gao Y-T, Rothman N, Xiang Y-B, Shu X-O, 2015. Predictors and long-term reproducibility of urinary phthalate metabolites in middle-aged men and women living in urban Shanghai. Environ. Int 84, 94–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Bertrand KA, Franke AA, Rosner B, Curhan GC, Willett WC, 2017. Reproducibility of urinary biomarkers in multiple 24-h urine samples. Am. J. Clin. Nutr 105, 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Cornelis MC, Townsend MK, Tobias DK, Eliassen AH, Franke AA, Hauser R, Hu FB, 2014. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses’ Health Study (NHS) and NHSII cohorts. Environ. Health Perspect 122, 616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RDC, 2017. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Townsend MK, Franke AA, Li X, Hu FB, Eliassen AH, 2013. Within-person reproducibility of urinary bisphenol A and phthalate metabolites over a 1 to 3 year period among women in the Nurses’ Health Studies: a prospective cohort study. Environ. Health 12, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Zoeller RT, Hass U, Kortenkamp A, Grandjean P, Myers JP, DiGangi J, Hunt PM, Rudel R, Sathyanarayana S, Bellanger M, Hauser R, Legler J, Skakkebaek NE, Heindel JJ, 2016. Burden of disease and costs of exposure to endocrine disrupting chemicals in the European Union: an updated analysis. Andrology 4, 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer TP, van Faassen M, Frederiksen H, van Beek AP, Wolffenbuttel BHR, Kema IP, van Vliet-Ostaptchouk JV, 2019. Development and Interlaboratory Validation of Two Fast UPLC–MS-MS Methods Determining Urinary Bisphenols, Parabens and Phthalates. J. Anal. Toxicol 10.1093/jat/bkz027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer TP, van Faassen M, van Beek AP, Snieder H, Kema IP, Wolffenbuttel BHR, van Vliet-Ostaptchouk JV, 2020. Exposure to Endocrine Disrupting Chemicals in the Dutch general population is associated with adiposity-related traits. Sci. Rep 10, 9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völkel W, Colnot T, Csanády GA, Filser JG, Dekant W, 2002. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem. Res. Toxicol 15, 1281–1287. [DOI] [PubMed] [Google Scholar]
- Ye X, Wong L-Y, Kramer J, Zhou X, Jia T, Calafat AM, 2015. Urinary Concentrations of Bisphenol A and Three Other Bisphenols in Convenience Samples of U.S. Adults during 2000–2014. Environ. Sci. Technol 49, 11834–11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


