Supplemental Digital Content is Available in the Text.
Key Words: CI-AKI, dapagliflozin, HIF-1α/HE4/NF-κB pathway, diabetic, apoptosis
Abstract:
Contrast-induced acute kidney injury (CI-AKI) causes clinically acquired nephropathy in patients who undergo coronary interventions. Hypoxic injury to proximal tubular epithelial cells is a pathological mechanism of CI-AKI. Previous studies have shown that hypoxia activates HIF-1α/HE4/NF-κB to enhance renal fibrosis, and the SGLT-2 inhibitor luseogliflozin inhibits hypoxia-inducible factor (HIF)-1α expression to reduce the progression of diabetic nephropathy. However, the therapeutic effects and mechanisms of SGLT-2 inhibitors on CI-AKI are unclear. We explored the role of the HIF-1α/HE4/NF-κB pathway in CI-AKI and how dapagliflozin effectively treats CI-AKI by inhibiting this pathway. In vitro, cells were divided into the control, hypoxia, hypoxia + dapagliflozin, and hypoxia + pSilencer-HIF-1α groups. Cellular hypoxia, apoptosis, and related protein expression were evaluated by immunofluorescence, western blotting, and flow cytometry, respectively. Dapagliflozin significantly decreased oxygen consumption, HIF-1α, human epididymis protein 4 (HE4), NF-κB expression, and apoptotic cells compared with the control (P < 0.01). In vivo, rats were divided into the control (C), diabetes (D), diabetes + contrast media, and diabetes + contrast media + dapagliflozin groups. Rats in the latter 2 groups were treated with dapagliflozin for 2 days. CI-AKI was induced by intravenously injecting indomethacin, N-nitro-l-arginine methyl ester, and iohexol. The effects of dapagliflozin on CI-AKI rats were elucidated by assessing renal function, H&E staining, and immunohistochemistry. Serum creatinine, urea nitrogen, TUNEL-positive tubular cells, HIF-1α, HE4, NF-κB expression, and histopathological scores were increased in diabetes + contrast media rats compared with C, D, and diabetes + dapagliflozin + contrast media rats (P < 0.01). Thus, dapagliflozin may ameliorate CI-AKI through suppression of HIF-1α/HE4/NF-κB signaling in vitro and in vivo.
INTRODUCTION
With the rapid development of interventional cardiology and the wide application of contrast agents, contrast-induced acute kidney injury (CI-AKI) has become the third leading cause of acquired kidney injury (AKI) in hospitals.1 Studies have shown that CI-AKI can increase the risk of renal replacement therapy, prolong hospitalization, increase medical expenses, and increase the mortality rate.2 CI-AKI is significantly increased in patients with diabetes-related kidney dysfunction.3 Mehran reported that the incidence of CI-AKI in nondiabetic patients is approximately 13%, while in diabetic patients, the incidence is approximately 29.4%.4 The pathophysiology associated with CI-AKI is complex and has not yet been fully elucidated. Previous studies based on animal models of CI-AKI have demonstrated that medullary hypoxic injury postcontrast accumulation in kidney may be a cause of the disease, and pathological studies may manifest because renal tubular epithelial cells seem to contain vacuoles and undergo apoptosis.5,6
Human epididymis protein 4 (HE4) is a 2-domain member belonging to the family of “4-disulfide core” proteins that can be used to screen for endometrial cancer, lung malignancies, and transitional cell carcinoma of the urinary system.7 Recent research has shown that when the glomerular filtration rate decreases, the serum HE4 level increases significantly in patients.8 Animal research has shown that the hypoxia-inducible factor (HIF)-1α/HE4/NF-κB signaling pathway involved in hypoxia-induced renal fibrosis.9 Although HE4 is considered a new biomarker with clinical applications in predicting kidney injury, the mechanisms regulating HE4 expression and its role in CI-AKI remain unclear.10 NF-κB activation may lead to an increase in the mRNA expression of the proapoptotic gene Bax and a decrease in the expression of the Bcl-2 gene, which inhibits apoptosis in HK-2 cells.11 HIF-1α, a key molecule that plays an important role in hypoxic conditions, can be quickly degraded through the ubiquitin–proteasome pathway under normoxic conditions and can directly bind to the HRE2 region of the HE4 promoter; hence, the knockdown or overexpression of HIF-1α can modulate HE4 levels.9
Inhibitors of SGLT2 represent a new generation of hypoglycemic drugs that can reduce glucose reabsorption in the renal tubules, thereby reducing blood sugar levels and prevent diabetic nephropathy at least partly by reducing hypoxia-induced HIF-1α expression and mitochondrial oxygen consumption.12,13 We hypothesized that dapagliflozin could prevent CI-AKI induced by contrast media because of its antihypoxia effects.
Because specific treatments for CI-AKI are still needed in the clinic, it is necessary to find a new therapeutic strategy that is an important complement to the existing clinical management protocol. In this study, we generated experimental models of CI-AKI in rats and hypoxic HK-2 cells. The aim of this study was to investigate the protective effect of dapagliflozin against hypoxic injury in CI-AKI and to elucidate the underlying molecular mechanisms of its action.
MATERIALS AND METHODS
Animals and Groups
Male Sprague–Dawley rats (180–200 g) were purchased from SPF Biotechnology Co, Ltd (Beijing, China), and all procedures were approved by the People's Liberation Army General Hospital Animal Care and Use Committee. The rats were placed in suitable cages, fed a standard diet and water, and adapted to this condition for 1 week. The rats were divided into the following 4 groups (n = 6/group): (1) control (C) group, (2) diabetes (D) group, (3) diabetes + contrast media (DCM) group, and (4) diabetes + dapagliflozin + contrast media (DDCM) group. Diabetic model rats were fasted for 2 hours before being intraperitoneally injected with STZ (60 mg/kg) (Sigma-Aldrich, St. Louis, MO), and after 3 days, animals with blood sugar levels over 300 mg/dL were considered diabetic.14 According to previous reports, the rat CI-AKI model was successfully established.15 In brief, after the rats were anesthetized, indomethacin (Solarbio Biological, Ltd, Beijing, China) (10 mg/kg) was injected into the tail vein and then l-NAME (10 mg/kg) (Leader Oriental Technology, Ltd, Beijing, China) and iohexol (3500 mg/kg) (Sigma-Aldrich) were injected 20 minutes later. Rats in the DDCM group were treated with dapagliflozin (5 mg/kg, dissolved in 2 mL of saline) 1 day and 30 minutes before the administration of contrast media, while rats in the control group received saline.13 One day after the establishment of the CI-AKI model, blood was collected to measure the levels of serum creatinine (Scr) and blood urea nitrogen (BUN), and the kidney was removed for histological examination after the rats were anesthetized and euthanized.
Measurement of Rat Scr and BUN
Approximately 0.5 mL of blood was collected from the intraocular canthal venous plexus. Serum was collected by centrifugation at 3000g at 4°C for 15 minutes. Scr and BUN concentrations were determined using an automatic biochemical analyzer (Chemray 800; Rayto, Shenzhen, China).
Hematoxylin & Eosin Staining and Immunohistological Staining
Kidneys were removed, fixed in 4% formalin for 24 hours, and embedded in paraffin. Sections of 3 μm thick tissues were cut and stained with hematoxylin & eosin. Morphological changes in the kidney were evaluated using a light microscope (Nikon Eclipse TE2000-U, Japan) at ×200 magnification. The degree of foaming and the detachment of renal tubular cells were scored on a scale of 0–4 as follows: no injury (0); mild: 0%–25% (1); moderate: 25%–50% (2); severe: 50%–75% (3); and very severe: 75%–100% (4).16 For immunohistochemical staining, the sections were incubated with primary rabbit polyclonal antibodies, including anti-HIF-α (bs-20399R; Bioss Biological, Ltd, Beijing, China), anti-HE4 (bs-4626R; Bioss Biological, Ltd), and anti-phospho-NF-κB (p65) (bs-3485R; Bioss Biological, Ltd), at a 1:100 dilution and incubated at 4°C overnight. The corresponding secondary antibodies (biotinylated goat anti-rabbit antibody from Bioss Biological, Ltd) were added and incubated at 37°C for 30 minutes, and diaminobenzidine was added for color development. Negative controls were prepared by omitting the primary antibody. After the sections were counterstained with hematoxylin and dehydrated, we observed the specimens using a light microscope (Nikon Eclipse TE2000-U, Nikon, Japan), and a brown–yellow color in the field of view represented a positive reaction.17
TUNEL Assay
A TUNEL detection kit was used to examine apoptotic cells and according to the manufacturer's instructions (Sigma-Aldrich, Merck KGaA). In brief, 3 μm sections of fixed kidney samples were incubated with 15 μg/mL proteinase K for 10 minutes at room temperature and then washed with phosphate-buffered saline (PBS). Endogenous peroxidase was blocked by hydrogen peroxide for 5 minutes at room temperature, and the sections were washed with PBS. The sections were immersed in terminal deoxynucleotidyl transferase and biotinylated dUTP in TdT buffer for 90 minutes. Cells that stained brown were positive for apoptosis. Five fields of view were randomly selected from each section, and the number of positive cells and the total number of cells were calculated in each field. These values were used to calculate the percentage of renal tubular cells that were apoptotic.18,19
Culture and Treatment of HK-2 Cells In Vitro
The human proximal tubule epithelial cell line (HK-2) was cultured in Dulbecco's modified Eagle's medium/F12 supplemented with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific Inc, MA) and 1% penicillin/streptomycin at 37°C in a humidified atmosphere containing 5% CO2. Cells were exposed to reagents under normoxic (21% O2) or hypoxic (1% O2) conditions for 24 hours and were then harvested for experiments as previously described.9 The cells in the treatment group were incubated with dapagliflozin at a final concentration of 100 µmol/L. These cells were used to perform a series of experiments, including examining the impact on the HIF-1α/HE4/NF-κB pathway and determining cell apoptosis. We also used siRNA expression vectors for HIF-1α and HE4 to knock out the corresponding genes. The selection of the siRNA-specific targeting sequences of HIF-1α and HE4 has been described previously, and we used Lipofectamine 2000 (Invitrogen AB, Lidinggo, Sweden) to transfect pSilencer-HIF-1α and pSilencer-HE4 (Shanghai Genechem Co, China) into HK-2 cells for 48 hours as directed by the manufacturer.9,20
Oxygen Flux Measurement
Oxygen consumption was measured according to the self-referencing method by using the noninvasive micro-test technology (NMT Physiolyzer, YoungerUSA LLC, MA, Xuyue Company, Beijing, China).21 In brief, after we replaced the cell culture medium of each group with the corresponding test solution (1.8 mM CaCl2, 5.0 mM KCl, 25 mM NaHCO3, 100 mM NaCl, 0.8 mM MgSO4, 5 mM Glucose, pH 6.8), they were kept at 36°C for testing. Then, we find the target cells under the microscope and moved the oxygen flux microsensor probe to 5–10 μm away from the HK-2 cells (Fig. 1B) and continued to detect for 5 minutes. The polarization voltage of the oxygen flux microsensor probe was −0.6 V. At this voltage, the reduction in oxygen at the probe tip was diffusion-limited, so the amount of oxygen in the solution could be evaluated. Because the oxygen concentration near the cell was much smaller than that farther away, the current difference between the proximal and distal ends of the platinum electrode represents the gradient of oxygen around the cell, which reflects oxygen consumption. Then, we directly obtained the oxygen flow rate through imFluxes V2.0 (xuyue.net), and its unit is pmol·cm−2·second−1. The positive and negative values represent the direction of oxygen flow rate across the membrane. The oxygen flux level was measured every 6 seconds. Figure 1C shows the average oxygen flux of cells in the different treatment groups after the measurements.
FIGURE 1.
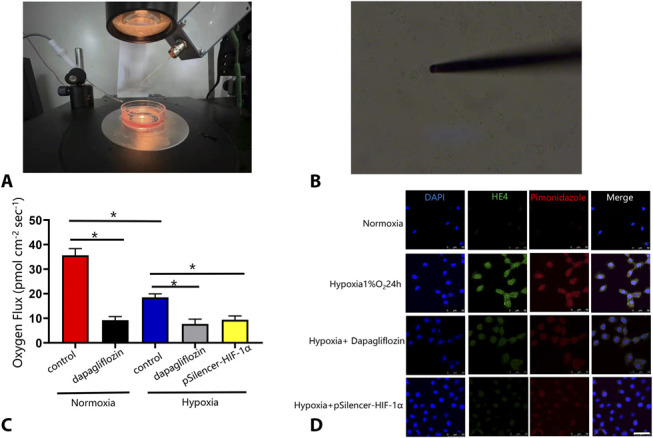
Dapagliflozin decreased oxygen consumption and suppressed intracellular hypoxia in HK-2 cells. A, The self-reference electrode. The system is set up on top of a spinning disk confocal microscope. B, Self-referencing electrode positioned next to a single HK-2 cell. C, Group data for basal respiration levels of single HK-2 cells in hypoxic and normoxia conditions (control) and HK-2 cells treated with 100 μmol/L dapagliflozin 24 hours in the same conditions. D, Immunofluorescence analysis of human HE4 and pimonidazole in HK-2 cells. HK-2 cells were grown on cover slides and then treated for 24 hours. Hypoxia induced the nuclear expression of HE4 in HK-2 cells, and dapagliflozin (100 μmol/L) inhibited the expression. Hypoxia in HK-2 cells was detected with pimonidazole hydrochloride. Dapagliflozin increased cellular oxygen levels in these cells under hypoxic conditions by decreasing oxygen consumption rate. The nuclei were stained with 4,6-diamino-2-phenyl indole (DAPI). Scale bars, 50 μm for all groups. Values are means ± SD. *P < 0.05. n = 5 in each group.
Measurement of Cellular Hypoxia and Immunocytofluorescence
Cellular hypoxia was detected by adding pimonidazole hydrochloride (100 μM Hypoxyprobe-1; Hydroxyprobe, Leader Oriental Technology, Ltd, Beijing, China), which binds to cells or tissues with pO2 levels below 10 mm Hg, to HK-2 cells that were treated with 100 µmol/L dapagliflozin and exposed to hypoxia (1% O2) for 24 hours.22 The cells were then harvested by cytospin, fixed, and immunostained with anti-HE4 and MAb1 overnight at 4°C. Then, an Alexa Fluor 594 goat anti-rabbit secondary antibody was added and incubated for 1 hour at room temperature. Finally, the samples were analyzed using a confocal microscope (LSM510; Carl Zeiss, Germany).
Mitochondrial Membrane Potential (ΔΨm) Analysis
The ΔΨm of HK-2 cells was determined using JC-1 staining. In brief, HK-2 cells in each group were incubated in 10 μg/mL JC-1 staining solution (Kang Chen Biotechnology, Guangzhou, China) at 37°C for 20 minutes and then washed with PBS to remove excess reagent. The ΔΨm was analyzed by using confocal microscopy (LSM510; Carl Zeiss; Germany). The parameters used for image acquisition were similar for all examined HK-2 cells.23,24
Western Blot Analysis
RIPA buffer (Solarbio Biological, Ltd) and phenylmethanesulfonyl fluoride (PMSF) (Beyotime Institute of Biotechnology, Shanghai, China) were added to the treated HK-2 cells to extract total proteins. Protein concentration was determined using a bicinchoninic acid assay (BCA) protein assay kit (Beyotime Institute of Biotechnology). Equal amounts of protein were separated by electrophoresis and transferred to polyvinylidene fluoride (PVDF) membranes. The membranes were then washed 3 times with Tris-buffered saline-Tween (TBST) and blocked with 5% bovine serum albumin (BSA) for 1 hour at room temperature. Next, the membranes were incubated with primary antibodies (anti-HIF-1α, 1:100, bs-20399R; Bioss Biological, Ltd; anti-HE4, 1:100, bs-4626R; Bioss Biological, Ltd; anti-NF-κB, 1:100, bs-3485R, Bioss Biological, Ltd; anti-Bax, 1:100, bsm-33283M; Bioss Biological, Ltd; anti-cleaved caspase-3, 1:500, AC033; Beyotime Biotechnology, Ltd, Shanghai, China; and anti-Bcl-2, 1:1000, ab32124; Abcam, MA). The membranes were then washed and incubated with horseradish peroxidase-conjugated secondary antibodies (SE134; Bioss Biological, Ltd diluted 1:5000) for 1 hour at room temperature. Target bands were observed using enhanced chemiluminescent reagents (Thermo Fisher Scientific Inc, MA) and a chemiluminescent imaging system (Tanon Science & Technology, Co, Ltd, Shanghai, China). All experiments were performed in triplicate.25
Annexin V/Propidium Iodide Apoptosis Analysis
We used an annexin V-fluorescein isothiocyanate/propidium iodide (PI) apoptosis detection kit (BD Biosciences) to assess apoptosis in each group of HK-2 cells. In brief, after the cells were collected and washed twice in cold binding buffer, annexin V-fluorescein isothiocyanate/binding buffer and PI were added and incubated for 30 minutes and then 10 minutes in the dark, respectively. Finally, cell apoptosis was determined by flow cytometry according to the manufacturer's instructions.26,27
Statistical Analysis
The data are expressed as the mean ± SD. Student's t test or one-way analysis of variance was used to analyze the data between 2 groups with SPSS 19.0. Statistical significance was set at P < 0.05.28
RESULTS
Dapagliflozin Decreases Oxygen Consumption and Intracellular Hypoxia Levels
According to previous studies, SGLT2 inhibitors restore the intracellular hypoxic conditions by reducing oxygen consumption rate of tubular cells in the diabetic kidney,29,30 and oxygen consumption can be indirectly measured by the translational movement of an oxygen-selective microelectrode at a known frequency through the gradient of the oxygen concentration around a cell.21 We found that dapagliflozin reduced the oxygen flux of cells to 26% compared with that in the control group under normoxic conditions (P < 0.05) (Fig. 1C). See Supplemental Digital Content 1 (Supplemental Data, http://links.lww.com/JCVP/A805) for the specific results. Compared with normoxia, hypoxia significantly reduced the oxygen flux of cells to 49% (P < 0.05), and under hypoxic conditions, dapagliflozin further inhibited the oxygen flux of cells compared with that in the control group (P < 0.05) (Fig. 1C). In addition, we used the Hypoxyprobe kits to detect the intracellular hypoxia; our immunofluorescence results showed that hypoxia apparently increased the expression of HE4 in HK-2 cells and that dapagliflozin inhibited HE4 its expression and reversed the hypoxic state. When HE4 was silenced in HK-2 cells, it could ameliorate the hypoxia conditions (Fig. 1D).
Dapagliflozin Protects the ΔΨm
Hypoxia depletes ΔΨm, and this decrease is closely related to apoptosis in cells. JC-1 is present in different forms, such as aggregates or monomers, according to the ΔΨm. Therefore, JC-1 staining can be used to determine changes in ΔΨm. When mitochondria have a higher ΔΨm, JC-1 forms red fluorescent polymers, whereas at a lower ΔΨm, JC-1 disperses into green fluorescent monomers.31 Figure 2 shows that dapagliflozin inhibited the hypoxia-induced decrease in ΔΨm in HK-2 cells. Using a fluorescence microscope, we showed that the number of JC-1 polymers emitting red fluorescence was significantly increased in cells treated with dapagliflozin.
FIGURE 2.
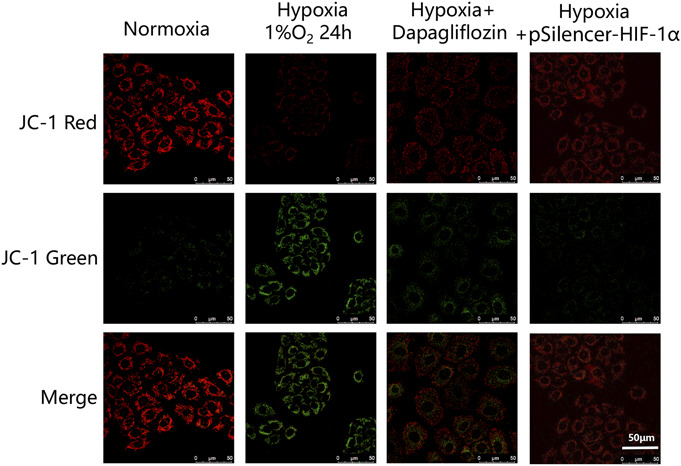
Immunofluorescence analysis of the mitochondrial membrane potential shows that the ratio of JC-1 green increased in the hypoxia group and decreased with dapagliflozin pretreatment (50 μm for all groups). n = 5 in each group.
Dapagliflozin Alleviates Hypoxia-induced Apoptosis in HK-2 Cells
Based on previous studies, a key factor in the mechanism of hypoxic kidney injury is the overexpression of HIF-1α, which can be reduced by SGLT2 inhibitors and can effectively reduce the high expression of HIF in hypoxic HK-2 cells. Therefore, we used annexin V/PI double staining and flow cytometry to measure apoptosis. The flow cytometry results showed that the total apoptosis rate of the dapagliflozin pretreatment group was significantly lower than that of the simple hypoxia group (11.84 ± 1.87 vs. 29.24 ± 3.20; P < 0.05) (Fig. 3A). Figure 3B shows the quantitative analysis of the data in Figure 3A. These results suggest that dapagliflozin plays a protective role in renal tubule cells by attenuating apoptosis during severe hypoxia.32
FIGURE 3.
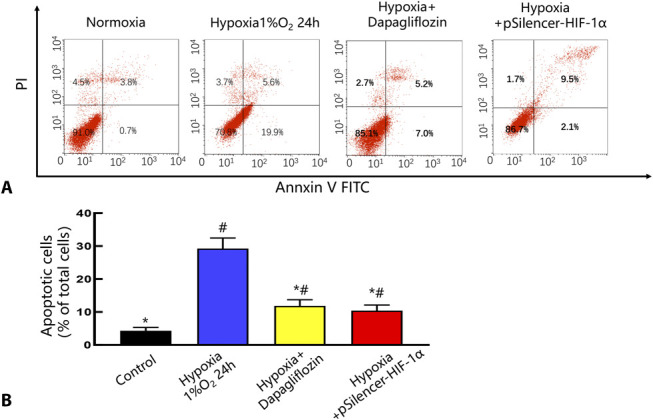
Dapagliflozin protect HK-2 cells from hypoxia injury. A, Flow cytometric detection of annexin-V/PI staining. PI+ cells are necrotic and late apoptotic, PI−/AV+ cells are early apoptotic, and PI−/AV− cells are normal. B, Quantitative analysis of apoptotic and necrotic cells by flow cytometry. *P< 0.05 versus the hypoxia 1% O2 24 hours group. #P< 0.05 versus the control group. n = 5 in each group. There are 3 replicates in each group for cellular experiments.
Protective Effects of Dapagliflozin Against Hypoxia-induced Apoptosis Through HIF-1α/HE4/NF-κB Signaling in HK-2 Cells
Previous studies have shown that inflammation and oxidative damage caused by the activation of NF-κB aggravate damage in contrast-induced nephropathy.33 In addition, hypoxia can activate the HIF-1α/HE4/NF-κB signaling pathway and regulate apoptosis-related proteins such as Bax and Bcl-2 in HK-2 cells, and SGLT2 inhibitors can regulate these proteins through previously introduced signaling pathways.9,13 To confirm the effect of HE4 on the expression of HIF-1α and NF-κB in HK-2 cells under hypoxic conditions, we transfected HK-2 cells with pSilencer-HE4 for 48 hours to downregulate HE4 expression and compared these cells with control cells under hypoxic conditions to determine expression changes. A decrease in the expression of NF-κB was observed in pSilencer-HE4-transfected HK-2 cells after 48 hours relative to control-transfected cells under hypoxic conditions. However, there was no significant difference in HIF-1α expression between the 2 groups (Fig. 4).
FIGURE 4.
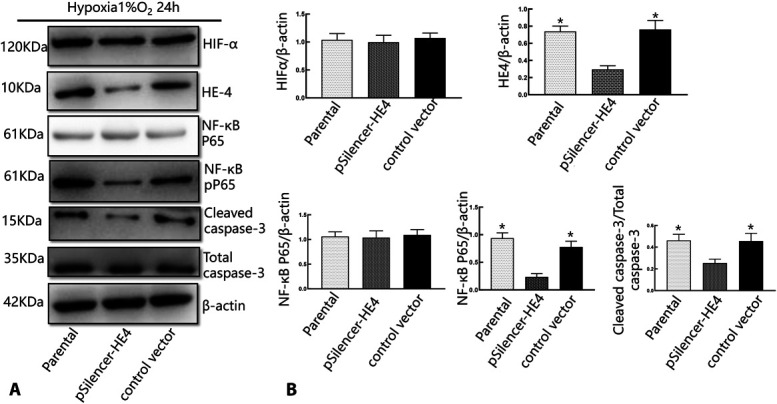
The effect HE4 on the expression of HIF-1α, NF-κB, and cleaved caspase-3 in HK-2 cells under hypoxic conditions. A, Western blot analysis of the expression of HIF-1α, HE4, and NF-κB after HK-2 cells were transfected with either pSilencer vector containing a HE4-targeting sequence or control vector. B, Representative quantitative analysis (n = 5 in each group). *P < 0.05 compared with the pSilencer-HE4 group. There are 3 replicates in each group for cellular experiments.
To investigate whether dapagliflozin exerted its protective effects by regulating HIF-1α/HE4/NF-κB signaling, we transfected HIF-1α siRNA into HK-2 cells and measured its expression by western blotting. Quantitative western blot analysis revealed that the protein expression levels of HIF-α, HE4, and NF-κB (Fig. 5) were significantly increased in the hypoxia group (P < 0.05) and were decreased in the group that was pretreated with dapagliflozin and hypoxia + pSilencer-HIF-1α (P < 0.05).
FIGURE 5.
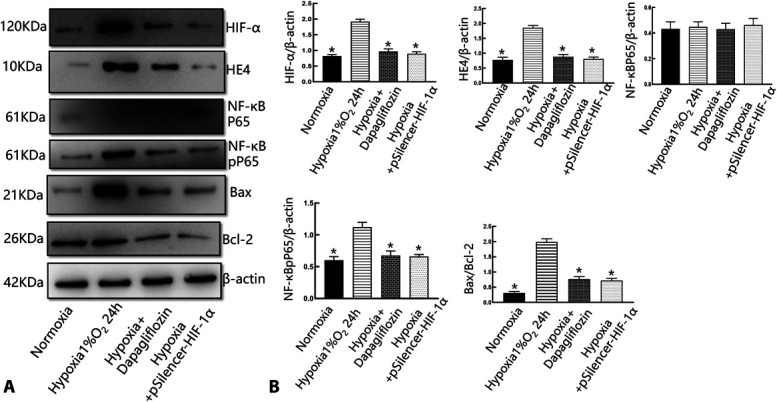
Dapagliflozin protected tubular epithelial cells through the HIF-1α/HE4/NF-κB signaling pathway. A, Western blot analysis of the expression of HIF-1α, HE4, P65, and pP65 in HK-2 cells treated with dapagliflozin and incubated hypoxia for 24 hours. Hypoxia increased the expression of Bax, whereas dapagliflozin decreased those of Bax and increased those of Bcl-2, respectively, in HK-2 cells. B, Representative quantitative analysis. *P< 0.01 versus the normoxia group; & P < 0.05 versus the dapagliflozin + hypoxia group. #P < 0.05 versus the hypoxia + pSilencer-HIF-1α group. There are 3 replicates in each group for cellular experiments.
Dapagliflozin Ameliorates CI-AKI–Induced Renal Dysfunction and Renal Histological Damage in CI-AKI Rats
As shown in Figures 6A, B, Scr and BUN levels were significantly increased in animals treated with iohexol compared with those in the DCM group (P < 0.05). However, the levels were significantly reduced by treatment with dapagliflozin (P < 0.05). The DDCM group had a 37.4% recovery in the level of Scr (Fig. 6A) and a 63.5% recovery in the level of BUN (Fig. 6B). These results were verified by pathological changes in the kidneys. Animals that were treated with iohexol showed severe pathological alterations in the kidneys, including luminal congestion, renal tubular necrosis, and vacuolar degeneration. However, the lesions improved significantly after treatment with dapagliflozin. The extent of kidney injury was further graded in the 4 groups (Fig. 6C). The differences in injury were semiquantitatively assessed. The scores ranged from 0 to 20, and a higher number represented more severe injury. The scores were 3.56 ± 0.13 in the DCM group, 0.42 ± 0.20 in the control group, and 1.62 ± 0.26 in the DDCM group (Fig. 6C). These results indicate that dapagliflozin may play a renoprotective role.
FIGURE 6.
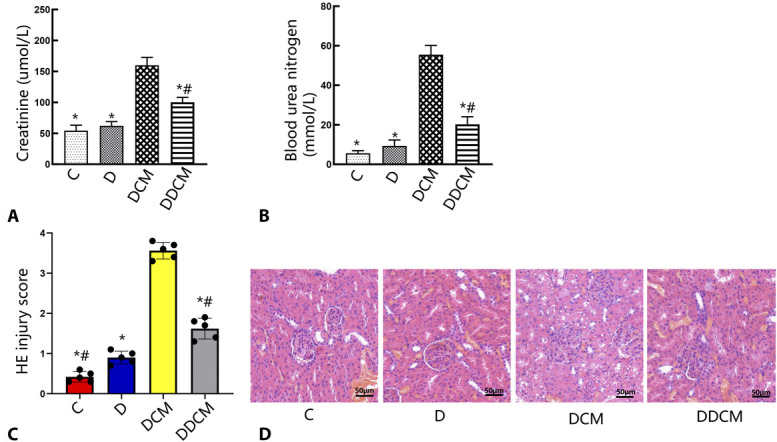
Protective effects of dapagliflozin against CI-AKI. Dapagliflozin decreased (A) serum levels and (B) blood urea nitrogen in CI-AKI rats. C, Histological injury scoring at 24 hours after the intravenous injection of iohexol. D, Representative histological image of kidney from representative photomicrographs of tubular cell injury in rat kidney tissue sections. Dapagliflozin significantly attenuated the renal tubular injury at 24 hours after the injection of iohexol. Rats in the DCM and DDCM groups were sequentially injected in the tail vein: indomethacin (10 mg/kg), followed by L-NAME (100 mg/kg) and iohexol (3.5 g/kg) 15 and 30 minutes later. Dapagliflozin was administered to rats in the DDCM group. Original magnification: ×200. H&E staining. *P < 0.05 versus the DCM group; #P < 0.05 versus the D group, n = 5 in each group. The values shown are the mean ± SD. C, control group; D, diabetic rats.
Effects of Dapagliflozin on Apoptosis in the CI-AKI Rat Model
TUNEL staining was used to assess apoptosis in renal cells. As shown in Figure 7, TUNEL staining showed that apoptosis in the DCM group was more severe than that in the D or C group (P < 0.05). The number of TUNEL-positive cells was examined and revealed that pretreatment with dapagliflozin significantly decreased the number of apoptotic cells by 57.6% in the DCM group. In addition, the percentage of apoptotic cells was slightly higher in the D group than in the C group (P < 0.05).
FIGURE 7.
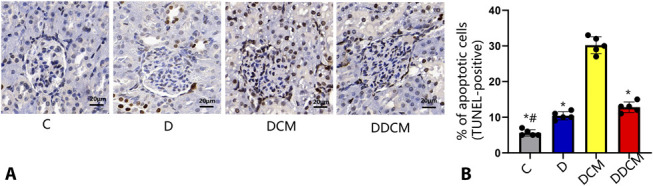
A, Representative photomicrographs of immunohistochemical staining for TUNEL in renal sections and (B) quantitative analysis of TUNEL-positive cells. The TUNEL-positive staining was localized to nuclei. Contrast media increases TUNEL-positive renal tubular cell number, whereas dapagliflozin significantly decreased them in rat kidney tissue sections 24 hours after the injection of iohexol. Magnification: ×400 *P < 0.05 versus the DCM group; #P < 0.05 versus the D group, n = 5 in each group. The values shown are the mean ± SD. C, control group; D, diabetic rats.
Effects of Dapagliflozin on HIF-1α/HE4/NF-κB Expression in the CI-AKI Model
Immunohistochemistry showed that compared with those in the untreated groups, the levels of HIF-1α, HE4, and NF-κB were significantly elevated in animals subjected to iohexol treatment, whereas dapagliflozin significantly decreased positive staining for these factors (Fig. 8) in CI-AKI rats, which was consistent with the western blot results in vitro.
FIGURE 8.
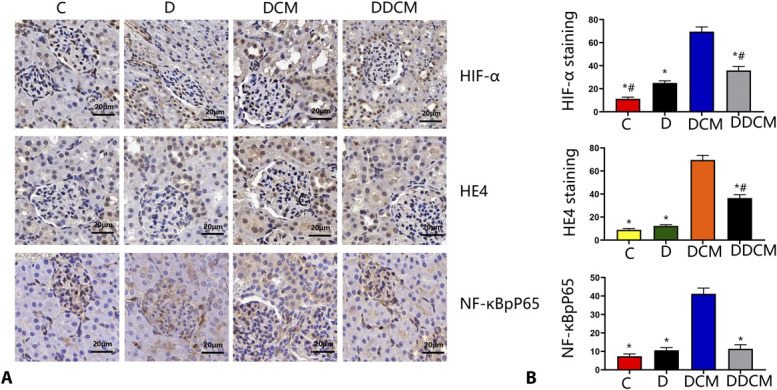
A, Immunohistochemical staining and semiquantitative analysis of HIF-1α, HE4, and NF-κBpP65 in the kidneys of different groups. B, Quantitative analysis of HIF-1α, HE4, and NF-κBpP65. SD rats were treated with or without dapagliflozin for 3 days. Immunohistochemical staining for these proteins (original magnification ×400. Compared with control, proteins significantly increased in the CIN but decreased in the dapagliflozin groups, *P < 0:05 vs. the DCM group; #P < 0:05 vs. the D group. n = 5 in each group). C, control group; D, diabetic rats.
DISCUSSION
With the pervasive use of radiocontrast agents, CI-AKI has become an increasingly common iatrogenic AKI.34,35 Besides conventional hydration methods have been clinically used to prevent CI-AKI, exploring new and effective strategies is still very important. Although the pathophysiological mechanisms of CI-AKI are not completely understood, studies have shown that contrast agents can promote the development and progression of CI-AKI by inducing hypoxic injury, inflammation, and direct toxicity in renal tubular epithelial cells, which significantly increases the levels of renal injury biomarkers, including Scr and BUN.36,37 Therefore, preventing hypoxic injury may play an important role in the prevention of CI-AKI. Dapagliflozin, one of the SGLT2 inhibitors, is widely applied in the treatment of various cardiovascular, renal, and endocrine diseases by improving oxygen metabolism, inhibiting apoptosis, and preventing fibrosis and other effects.38 However, little is known about its renoprotective effects against CI-AKI. In this study, we investigated the renoprotective effect of dapagliflozin in a CI-AKI rat model. Murine animals are resistant to CI-AKI, so the incidence is low. To increase the probability of CI-AKI animal modeling, various interventions such as a low sodium diet and pretreatment with prostaglandin synthesis inhibitors were applied. In this study, we constructed a rat model of CI-AKI using a protocol modified from Agmon et al and found that contrast agents could aggravate pathological renal damage and deteriorate renal function in rats pretreated with indomethacin and l-NAME.39 Similar to the findings of a previously published study, the pathological changes observed under the microscope showed proximal tubulopathy, including cytoplasmic vacuolar changes, tubular necrosis, cell casts, and other pathological features of CI-AKI.40
We further explored the underlying mechanisms of dapagliflozin's renoprotective effects against CI-AKI. A recent study showed that HIF-1α increases oxidative stress damage in a mouse model of CI-AKI by inducing heme oxygenase-1 expression and phosphorylation of the transcription factor forkhead box O 1 and forkhead box O 3a.41 HE4 participates in a variety of acute and chronic kidney diseases with varying degrees of activation, and a model of HE4-knockout mice was protected from AKI.42,43 However, there have been few reports on the role of HE4 in CI-AKI, and the molecular mechanism of its activation remains unknown. Recent studies have suggested that hypoxia induces renal fibrosis by activating the HIF-1α/HE4/NF-κB signaling pathway.9 Previous studies have shown that the activation of NF-κB can promote a kidney inflammatory response and activate apoptotic proteins such as caspase-1 and caspase-3, causing cell apoptosis in rats subjected to contrast-induced nephropathy.43 Conversely, silencing NF-κB inhibits apoptotic protein expression in vitro.44 The data presented herein suggest that the phosphorylation of P65 is increased and that the activation of p65 transcriptional activity after translocation into the nucleus is enhanced in HK-2 cells induced by hypoxic environments. However, the phosphorylation of P65 in HE4-silenced HK-2 cells was downregulated under hypoxic conditions. We also showed that the expression of HIF-1α, HE4, and NF-κB and the number of apoptotic cells dramatically increased in HK-2 cells after hypoxia induction in vitro, which was consistent with previously published findings.9 Furthermore, we found similar results in vivo using CI-AKI rats, and this is the first study to reveal that the expression of HIF-1α, HE4, and NF-κB was higher in iohexol-treated animals than in controls.
Previous research has demonstrated that HIF-1α protein expression can be inhibited by SGLT2 inhibitors in HK-2 cells in the presence of high glucose or hypoxia.13,45,46 SGLT2 inhibitors can reduce renal tubular sodium and glucose reabsorption by inhibiting the activity of SGLT2, thereby reducing sodium-potassium-ATPase activity and resulting in decreased oxygen consumption and increased intracellular oxygen.47,48 In our study, dapagliflozin had an inhibitory effect on the upstream protein HIF-1α and decreased the expression of HE4 in the DDCM group, reducing the expression of NF-κB and caspase-3, which is a marker of cellular apoptosis. We also found that dapagliflozin protected against these harmful effects, as evidenced by a significant 57.6% decrease in the number of apoptotic cells in the DDCM group compared with that in the DCM group. We demonstrated in vitro and in vivo that dapagliflozin alleviated HK-2 cell apoptosis and pathological changes in the kidney at least partially by suppressing the HIF-1α/HE4/NF-κB pathway in renal proximal tubular cells. Therefore, our data confirm the protective role of dapagliflozin and provide novel evidence that dapagliflozin plays a crucial role in the suppression of the HIF-1α/HE4/NF-κB signaling pathway by suppressing mitochondrial oxygen consumption, which leads to the restoration of intracellular hypoxia in HK-2 cells and that dapagliflozin can treat CI-AKI in diabetic rats. In the hypoxic environment, HIF-1α-deficient HK-2 cells had increased intracellular oxygen. According to previous studies, HIF-1α may increase glucose uptake and glycolysis, thereby increasing oxygen consumption when the generation of ATP through oxidative phosphorylation is not possible because of a lack of oxygen. However, the lack of HIF-1α leads to a decline in this function in cells, and oxygen consumption is relatively reduced; thus, the oxygen content in the cell is relatively increased (Fig. 9).49,50 For the first time, we investigated the effect of dapagliflozin on the HIF-1α/HE4/NF-κB pathway and as a treatment for diabetic rats with CI-AKI. Our study provides a new strategy for the treatment of CI-AKI. It is well-known that diabetes is an important risk factor for CI-AKI, so the incidence of CI-AKI is significantly increased in diabetic patients. The SGLT2 inhibitors, as a representative of the new generation of hypoglycemic drugs, can not only contribute to controlling blood sugar levels but also can effectively prevent the occurrence of CI-AKI after coronary angiography, especially for patients with diabetes. However, there are still some limitations to our current research. We used the HK-2 cell line to confirm that hypoxia induced HIF-1α expression and the activation of downstream HE4, which led to apoptosis. The relationship between upstream and downstream regulators of these proteins has not been clearly demonstrated in vivo in CI-AKI rats. Therefore, the next step will be to validate the effect of the HE4 protein on CI-AKI in gene knockdown rats and explore the effect of dapagliflozin on CI-AKI in high-risk patients who will undergo coronary angiography.
FIGURE 9.
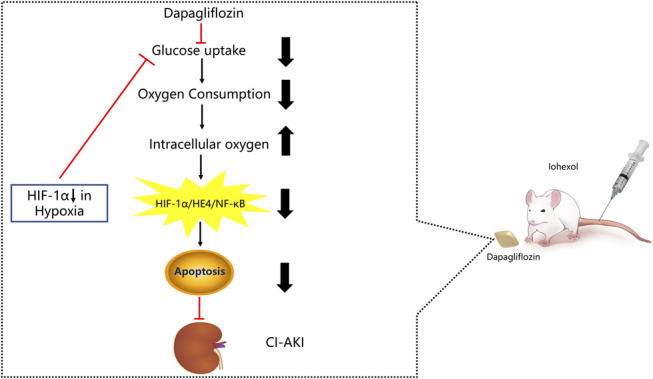
Schematic diagram of the protective mechanism of the SGLT2 inhibitors in CI-AKI. Because dapagliflozin inhibits glucose uptake, which leads to decreased oxygen consumption in renal proximal tubular cells. Subsequently, dapagliflozin induced the increase of intracellular oxygen, which could regulate the HIF-1α/HE4/NF-κB pathway and inhibit the cells apop.
In conclusion, our results suggest that hypoxia injury is one of the pathological mechanisms leading to CI-AKI and that the protective effect of dapagliflozin could be, at least partly, due to the suppression of the HIF-1α/HE4/NF-κB signaling pathway. Therefore, dapagliflozin holds promise as an adjuvant therapeutic agent for the clinical management of CI-AKI.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the following Science Foundations: the National Key R&D Program of China (2018YFC0116305). The National Natural Science Foundation of China (81970237, 81530058, 81970443, 81671731, and 91939303).
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jcvp.org).
Contributor Information
Xiaoxu Guo, Email: huangxu9336@163.com.
Weiwei Zhang, Email: yan_seu@hotmail.com.
REFERENCES
- 1.Mamoulakis C, Tsarouhas K, Fragkiadoulaki I, et al. Contrast-induced nephropathy: basic concepts, pathophysiological implications and prevention strategies. Pharmacol Ther. 2017;180:99–112. [DOI] [PubMed] [Google Scholar]
- 2.Fähling M, Seeliger E, Patzak A, et al. Understanding and preventing contrast-induced acute kidney injury. Nat Rev Nephrol. 2017;13:169–180. [DOI] [PubMed] [Google Scholar]
- 3.Hossain MA, Costanzo E, Cosentino J, et al. Contrast-induced nephropathy: pathophysiology, risk factors, and prevention. Saudi J Kidney Dis Transpl. 2018;29:1–9. [DOI] [PubMed] [Google Scholar]
- 4.Chandiramani R, Cao D, Nicolas J, et al. Contrast-induced acute kidney injury. Cardiovasc Interv Ther. 2020;35:209–217. [DOI] [PubMed] [Google Scholar]
- 5.McCullough PA, Choi JP, Feghali GA, et al. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2016;68:1465–1473. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Q, Wang X, Shao X, et al. tert-Butylhydroquinone treatment alleviates contrast-induced nephropathy in rats by activating the Nrf2/Sirt3/SOD2 signaling pathway. Oxid Med Cell Longev. 2019;2019:4657651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saarelainen SK, Peltonen N, Lehtimaki T, et al. Predictive value of serum human epididymis protein 4 andcancer antigen 125 concentrations in endometrial carcinoma. Am J Obstet Gynecol. 2013;209:141–146. [DOI] [PubMed] [Google Scholar]
- 8.Gizzo S, Ancona E, Saccardi C, et al. Could kidney glomerularfiltration impairment represent the “Achilles heel” of HE4 serum marker? A possible further implication. Clin Chem Lab Med. 2014;52:45–46. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Liu L, Bai M, et al. Hypoxia-induced HE4 in tubular epithelial cells promotes extracellular matrix accumulation and renal fibrosis via NF-κB. FASEB J. 2020;34:2554–2567. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Sun Y, Cai X, et al. The diagnostic value of human epididymis protein 4 as a novel biomarker in patients with renal dysfunction. Int Urol Nephrol. 2018;50:2043–2048. [DOI] [PubMed] [Google Scholar]
- 11.Park JS, Choi HI, Bae EH, et al. Paricalcitol attenuates indoxyl sulfate-induced apoptosis through the inhibition of MAPK, Akt, and NF-kB activation in HK-2 cells. Korean J Intern Med. 2019;34:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han E, Shin E, Kim G, et al. Combining SGLT2 inhibition with a thiazolidinedione additively attenuate the very early phase of diabetic nephropathy progression in type 2 diabetes mellitus. Front Endocrinol. 2018;9:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bessho R, Takiyama Y, Takiyama T, et al. Hypoxia-inducible factor-1α is the therapeutic target of the SGLT2 inhibitor for diabetic nephropathy. Sci Rep. 2019;9:14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng J, Wu G, Yang C, et al. Rosuvastatin attenuates contrast-induced nephropathy through modulation of nitric oxide, inflammatory responses, oxidative stress and apoptosis in diabetic male rats. J Transl Med. 2015;13:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inci MF, Salk I, Solak O, et al. Use of N-acetylcysteine for the prevention of contrast-induced nephropathy in rats. Actas Urol Esp. 2012;36:210–215. [DOI] [PubMed] [Google Scholar]
- 16.Liu T, Fang Y, Liu S, et al. Limb ischemic preconditioning protects against contrast-induced acute kidney injury in rats via phosphorylation of GSK-3β. Free Radic Biol Med. 2015;81:170–182. [DOI] [PubMed] [Google Scholar]
- 17.Xiao L, Zhu X, Yang S, et al. Rap1 ameliorates renal tubular injury in diabetic nephropathy. Diabetes. 2014;63:1366–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad A, Mondello S, Di Paola R, et al. Protective effect of apocynin, a NADPH-oxidase inhibitor, against contrast-induced nephropathy in the diabetic rats: a comparison with n-acetylcysteine. Eur J Pharmacol. 2012;674:397–406. [DOI] [PubMed] [Google Scholar]
- 19.Yuan S, Liu X, Zhu X, et al. The role of TLR4 on PGC-1α-mediated oxidative stress in tubular cell in diabetic kidney disease. Oxid Med Cell Longev. 2018;2018:6296802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun S, Ning X, Zhang Y, et al. Hypoxia-inducible factor-1alpha induces Twist expression in tubular epithelial cells subjected to hypoxia, leading to epithelial-to-mesenchymal transition. Kidney Int. 2009;75:1278–1287. [DOI] [PubMed] [Google Scholar]
- 21.Ma X, Zhang LH, Wang LR, et al. Single-walled carbon nanotubes alter cytochrome c electron transfer and modulate mitochondrial function. ACS Nano. 2012;6:10486–10496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takiyama Y, Harumi T, Watanabe J, et al. Tubular injury in a rat model of type 2 diabetes is prevented by metformin: a possible role of HIF-1α expression and oxygen metabolism. Diabetes. 2011;60:981–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu YJ, Ji DM, Liu ZB, et al. Melatonin maintains mitochondrial membrane potential and decreases excessive intracellular Ca2+ levels in immature human oocytes. Life Sci. 2019;235:116810. [DOI] [PubMed] [Google Scholar]
- 24.Tan K, Chen Y, Ma K, et al. Spatiotemporally tracking the programmable mitochondrial membrane potential evolutions by a robust molecular rotor. Small. 2019;15:e1903266. [DOI] [PubMed] [Google Scholar]
- 25.Kabei K, Tateishi Y, Shiota M, et al. Effects of orally active hypoxia inducible factor alpha prolyl hydroxylase inhibitor, FG4592 on renal fibrogenic potential in mouse unilateral ureteral obstruction model. J Pharmacol Sci. 2020;142:93–100. [DOI] [PubMed] [Google Scholar]
- 26.Park JW, Yook JM, Ryu HM, et al. Phosphate-induced apoptosis in human peritoneal mesothelial cells in vitro. Am J Nephrol. 2011;34:77–86. [DOI] [PubMed] [Google Scholar]
- 27.Heun Y, Grundler Groterhorst K, Pogoda K, et al. The phosphatase SHP-2 activates HIF-1α in wounds in vivo by inhibition of 26S proteasome activity. Int J Mol Sci. 2019;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Shen W, Zhang JY, et al. Stevioside attenuates isoproterenol-induced mouse myocardial fibrosis through inhibition of the myocardial NF-κB/TGF-β1/Smad signaling pathway. Food Funct. 2019;10:1179–1190. [DOI] [PubMed] [Google Scholar]
- 29.Takiyama Y, Haneda M. Hypoxia in diabetic kidneys. Biomed Res Int. 2014;2014:837421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blantz RC. Phenotypic characteristics of diabetic kidney involvement. Kidney Int. 2014;86:7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao X, Liu L, Li R, et al. Hypoxia-inducible factor 1-α (HIF-1α) induces apoptosis of human uterosacral ligament fibroblasts through the death receptor and mitochondrial pathways. Med Sci Monit. 2018;24:8722–8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu LL, Li D, He YL, et al. miR-210 protects renal cell against hypoxia-induced apoptosis by targeting HIF-1 alpha. Mol Med. 2017;23:258–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machado RA, Constantino Lde S, Tomasi CD, et al. Sodium butyrate decreases the activation of NF-κB reducing inflammation and oxidative damage in the kidney of rats subjected to contrast-induced nephropathy. Nephrol Dial Transpl. 2012;27:3136–3140. [DOI] [PubMed] [Google Scholar]
- 34.Crimi G, Leonardi S, Costa F, et al. Incidence, prognostic impact, and optimal definition of contrast-induced acute kidney injury in consecutive patients with stable or unstable coronary artery disease undergoing percutaneous coronary intervention. insights from the all-comer PRODIGY trial. Catheter Cardiovasc Interv. 2015;86:E19–E27. [DOI] [PubMed] [Google Scholar]
- 35.Gucun M, Kahyaoglu M, Celik M, et al. Predictive value of post-procedural hyponatremia on contrast-induced nephropathy in patients who underwent coronary angiography or percutaneous coronary intervention. Acta Cardiol. 2021;77(3):215–221. [DOI] [PubMed] [Google Scholar]
- 36.Lin Q, Li S, Jiang N, et al. Inhibiting NLRP3 inflammasome attenuates apoptosis in contrast-induced acute kidney injury through the upregulation of HIF1A and BNIP3-mediated mitophagy. Autophagy. 2020:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heyman SN, Rosenberger C, Rosen S. Regional alterations in renal haemodynamics and oxygenation: a role in contrast medium-induced nephropathy. Nephrol Dial Transpl. 2005;20(suppl 1):i6–11. [DOI] [PubMed] [Google Scholar]
- 38.Vallon V, Verma S. Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu Rev Physiol. 2021;83:503–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khaleel SA, Alzokaky AA, Raslan NA, et al. Lansoprazole halts contrast induced nephropathy through activation of Nrf2 pathway in rats. Chem Biol Interact. 2017;270:33–40. [DOI] [PubMed] [Google Scholar]
- 40.Chen YH, Fu YC, Wu MJ. Does resveratrol play a role in decreasing the inflammation associated with contrast induced nephropathy in rat model. J Clin Med. 2019;8:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang KJ, Kim JH, Chang YK, et al. Inhibition of xanthine oxidoreductase protects against contrast-induced renal tubular injury by activating adenosine monophosphate-activated protein kinase. Free Radic Biol Med. 2019;145:209–220. [DOI] [PubMed] [Google Scholar]
- 42.Tajima S, Fu R, Shigematsu T, et al. Urinary human epididymis secretory protein 4 as a useful biomarker for subclinical acute rejection three months after kidney transplantation. Int J Mol Sci. 2019;20:4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wan J, Wang Y, Cai G, et al. Elevated serum concentrations of HE4 as a novel biomarker of disease severity and renal fibrosis in kidney disease. Oncotarget. 2016;7:67748–67759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kunak CS, Ugan RA, Cadirci E, et al. Nephroprotective potential of carnitine against glycerol and contrast-induced kidney injury in rats through modulation of oxidative stress, proinflammatory cytokines, and apoptosis. Br J Radiol. 2016;89:20140724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ndibalema AR, Kabuye D, Wen S, et al. Empagliflozin protects against proximal renal tubular cell injury induced by high glucose via regulation of hypoxia-inducible factor 1-alpha. Diabetes Metab Syndr Obes. 2020;13:1953–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zapata-Morales JR, Galicia-Cruz OG, Franco M, et al. Hypoxia-inducible factor-1α (HIF-1α) protein diminishes sodium glucose transport 1 (SGLT1) and SGLT2 protein expression in renal epithelial tubular cells (LLC-PK1) under hypoxia. J Biol Chem. 2014;289:346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomita I, Kume S, Sugahara S, et al. SGLT2 inhibition mediates protection from diabetic kidney disease by promoting ketone body-induced mTORC1 inhibition. Cell Metab. 2020;32:404–419.e6. [DOI] [PubMed] [Google Scholar]
- 48.Layton AT, Vallon V, Edwards A. Modeling oxygen consumption in the proximal tubule: effects of NHE and SGLT2 inhibition. Am J Physiol Ren Physiol. 2015;308:F1343–F1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Biju MP, Akai Y, Shrimanker N, et al. Protection of HIF-1-deficient primary renal tubular epithelial cells from hypoxia-induced cell death is glucose dependent. Am J Physiol Ren Physiol. 2005;289:F1217–F1226. [DOI] [PubMed] [Google Scholar]
- 50.Iyer NV, Kotch LE, Agani F, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12:149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


