Abstract
Background
Fibroids have been identified as a possible risk factor for preterm birth, however, the magnitude of this risk is unclear. Our objective was to determine the risk of total, spontaneous, and medically indicated preterm birth in women with fibroids.
Methods
A literature search was performed on 9 June 2021. We selected studies reporting on preterm birth in women with and without fibroids. Fibroids had to be diagnosed by routine ultrasound before or during pregnancy. Main outcomes were total preterm birth <37, <34, <32, and <28 weeks of gestation, and spontaneous and medically indicated preterm birth. Two authors independently performed study selection, data extraction and quality assessment. We performed quality assessment with the Newcastle-Ottawa scale. Meta-analyses were presented as Odds Ratios (ORs) with 95% Confidence Intervals (95%CIs).
Main results
The search yielded 2078 unique articles of which 11 were included. Meta-analysis for preterm birth <37 weeks of gestation included 256,650 singleton deliveries: 12,309 with fibroids and 244,341 without fibroids. Women with fibroids had a higher rate of preterm birth (11.6% versus 9.0%; OR 1.66, 95%CI 1.29–2.14). Fibroids were also associated with preterm birth <34 (OR 1.88, 95%CI 1.34–2.65), <32 (OR 2.03, 95%CI 1.40–2.95) and <28 (OR 2.24, 95%CI 1.45–3.47) weeks of gestation. Data on type of preterm birth was limited: one study showed a significant association of fibroids with spontaneous preterm birth and another with indicated preterm birth. The main limitations of the included studies were the lack of correction for confounders, the risk of ascertainment bias due to possible underreporting of fibroids, and the substantial heterogeneity between studies.
Conclusions
Our results suggest fibroids are associated with an increased risk of preterm birth, with a stronger risk at earlier gestational ages. We encourage further research to clarify the association between fibroids and preterm birth by systematic myometrial assessment in pregnancy.
Registration
Prospero database [CRD42020186976].
Introduction
Worldwide 15 million babies are born preterm each year [1]. Preterm birth is the most important cause of perinatal mortality and morbidity [2]. Approximately 65% of preterm births has a spontaneous onset and the remainder is medically indicated for maternal or fetal reasons [3]. The aetiology of preterm birth is multifactorial and complex [4]. Identifying risk factors for preterm birth is important to provide accurate counselling and it might create opportunities for improvement of antenatal care for women who are at risk.
Previous reviews have suggested that women with fibroids have an increased risk of preterm birth [5, 6]. Both associations with spontaneous and medically indicated preterm birth have been described. Several theories on the genesis of spontaneous preterm birth exist, such as a less distensible uterus and increased oxytocin levels, resulting in premature contractions and cervical change [7, 8], Studies have indeed found women with fibroids have a shorter cervix [9, 10]. Fibroids could also disturb uterine contraction patterns [11–14]. Furthermore, the hyperinflammatory state of fibroids could precipitate preterm labour. As described in women with endometriosis, this hyperinflammatory state could impair decidua/trophoblast interaction and uteroplacental development, which, in turn, could increase the risk of hypertensive disorders, placental abruption and fetal growth restriction, for which preterm birth might be medically indicated [15]. Studies have indeed shown an increased risk of these pregnancy complications in women with fibroids [5, 6, 16–19].
To quantify the risk of preterm birth in women with fibroids compared to women without fibroids, we performed a systematic review and meta-analysis. Furthermore, we aimed to differentiate spontaneous from medically indicated preterm birth, and to evaluate how fibroid characteristics (i.e. size, number, localisation) modify the risk of preterm birth.
Methods
Protocol and registration
This systematic review was reported according to the PRISMA guidelines for systematics reviews and meta-analyses (S1 Appendix) [20]. No funding was received for the conduct of this systematic review and meta-analysis. Ethical approval was not required for secondary use of data in this systematic review and meta-analysis. The protocol was registered in the PROSPERO database in July 2020 (CRD4202018697]. After registration, the following changes were made to the protocol: 1) we only included studies that performed routine ultrasound/imaging for the diagnosis of fibroids to reduce the risk of ascertainment bias; 2) we removed additional outcomes such as preeclampsia as these were not incorporated in the search terms; and 3) we changed the statistical analysis technique from a random-effects model to the generic inverse-variance method to include confounder-adjusted estimates.
Data sources and search strategies
A systematic literature search was performed by a medical information specialist (JK) and two researchers (GV and AL) on 9 June 2021 in the following databases: PubMed, Embase and Web of Science. The search terms included both keywords as well as free text terms for ‘fibroids’ and ‘preterm birth’, along with their synonyms. The search strategies are presented in S2 Appendix. No language, publication date or other restrictions were applied. All duplicates were excluded using EndNote. A cited-reference search was performed to identify potential additional relevant studies.
Eligibility and study selection
Original studies reporting on the risk of preterm birth in women with and without fibroids were eligible for the present systematic review and meta-analysis. To limit the risk of the opportunistic detection (i.e. incidental identification) of fibroids, the fibroids had to be diagnosed by routine ultrasound or other imaging before or during pregnancy, indicating we excluded studies that, for example, included women that were admitted to the hospital or selectively referred for ultrasound screening. We also excluded studies including women with multiple gestations due to their increased baseline risk of preterm birth, and studies reporting on preterm birth after surgical or non-surgical treatment of fibroids as this treatment may have influenced obstetric outcome. Furthermore, conference abstracts, case reports, case series, reviews and guidelines were excluded. For the purpose of feasibility, we excluded non-English articles. The main outcomes were preterm birth <37, <34, <32 and <28 weeks of gestation. In addition, we aimed to differentiate between the spontaneous onset of preterm birth following either the onset of contractions or rupture of membranes, and medically indicated preterm birth due to maternal or fetal complications. Other relevant outcomes were preterm prelabour rupture of membranes (PPROM) and mid-trimester fetal loss (between 16 and 22 weeks of gestation).
Two authors (GV and AL) independently performed screening of all potential articles using Rayyan [21]. First screening was based on title and abstract. In case of inconsistency between authors, the full article was screened. We screened the full text of the remaining articles based on the aforementioned predefined in- and exclusion criteria for eligibility. Disagreements between the authors were resolved by discussion or consultation of a third author (MdB).
Data extraction and quality assessment
We used the Newcastle-Ottawa Quality Assessment Scale (NOS) for nonrandomised studies to evaluate the validity of the included studies [22]. We slightly adapted the questions and answers to obtain a better fit for our studies. Each item could be given a maximum of one star. Studies were categorised as good, fair and poor quality according to the Agency for Health Research and Quality standards. Our customised NOS scale and thresholds for quality assessment are demonstrated in S3 Appendix. The final judgement of quality was summarised in a risk of bias table. No assessment of certainty was planned in the protocol.
Two authors (GV and AL) independently extracted data from the included articles and performed quality assessment. We collected data on author, year and country of publication, language, publication status, start date and end date of the study, ethical approval, type and source of funding, study design, sample size, population characteristics (e.g. age, ethnicity, parity, obstetrics history, previous cervical cerclage surgery), diagnostics method used for the identification of uterine fibroids, fibroid characteristics (size, location, number, vascularisation, FIGO classification), past treatment of fibroids (surgical or non-surgical), the outcome measures as described previously, and key conclusions. Corresponding authors of the included articles were contacted for further information if relevant outcomes or other data were not presented in the original publication. Discrepancies in data extraction and quality assessment were resolved by discussion and consulting a third author (MdB).
Statistical analysis
Outcome event rates and crude odds ratios were calculated based on the number of deliveries. If studies included miscarriages in their results, these were excluded from the sample size. If studies were unclear about in- or exclusion of miscarriages in their sample size, we used the reported sample size for our calculations. The meta-analysis included adjusted odds ratios (ORs) of those studies that corrected for confounders and crude ORs of studies that did not. We used a random-effects model with the generic inverse-variance method [23, 24]. As we included both cohort and case-control studies, the results of the meta-analyses were presented as odds ratios and 95% confidence intervals (95% CI). Heterogeneity between studies was evaluated by using X2 and I2 statistics and possible publication bias was assessed with a funnel plot. We performed a pre-planned sensitivity analysis of good-quality studies. In addition, we planned to perform subgroup analyses based on the size, number, location and vascularisation of the fibroids; parity, a history of previous miscarriages or preterm birth, and a history of dilation or curettage. Statistical analyses were performed using Review Manager (RevMan 5.3.5) and R Studio (version 4.0.3: meta, metafor and dmetar packages).
Results
Study selection
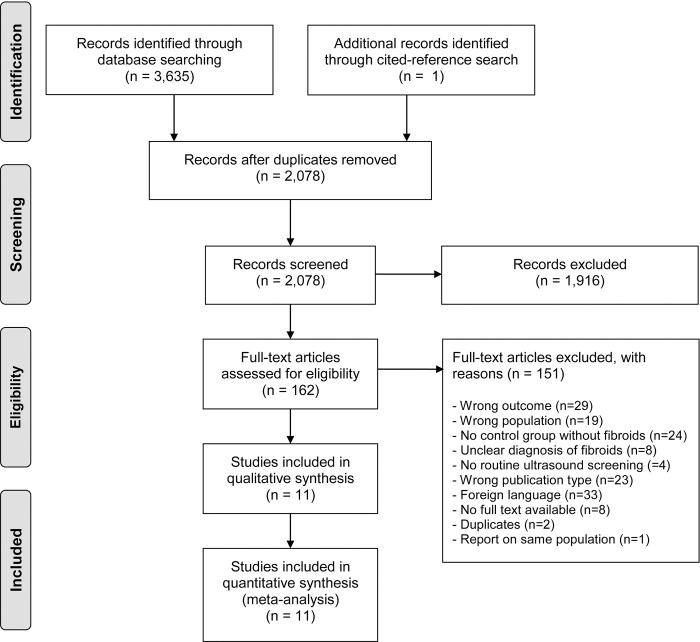
The search provided 2078 unique articles which were assessed for eligibility (Fig 1). One additional relevant article was found through cited-reference search, finally leading to the inclusion of 11 studies and 256,650 deliveries in this systematic review. Two publications reporting on an identical study population and were both used to complement the study data [25, 26]. Characteristics of excluded studies which may appear to meet the inclusion criteria are available in S1 Table [27–38].
Fig 1. Flow diagram of study selection.
Study and population characteristics
The included studies were conducted between 2009 and 2015. Five of the studies were cohort studies [9, 16, 18, 19, 25, 26, 39] and the other five were case-control studies based on the exposure of fibroids [10, 40–43]. One study was prospective [43]. Study characteristics and sample sizes are demonstrated in Table 1. A total of 256,683 singleton pregnancies with 256,650 deliveries were included in the systematic review: 12,309 in the fibroid group and 244,341 in the control group. The prevalence of fibroids within the cohort studies was 2.9% (6,185/221,813), ranging between 0.8% and 16.8%. All women received routine ultrasound screening (Table 2). Two studies performed screening in the first trimester [16, 39]. Egbe et al. included fibroids >3 cm and Arisoy et al. included intramural fibroids >3 cm [39, 40]. Other studies did not specify a minimum measurement threshold in their exposure definition. Studies did not report whether they used 2D or 3D imaging with or without colour Doppler.
Table 1. Study characteristics and sample size.
| Study | Year | Country | Study design | Singleton pregnancies | Deliveries | Fibroid group (%)a | Control group | |
|---|---|---|---|---|---|---|---|---|
| Eze et al. | 2013 | Nigeria | Case control | Prospective | 200 | 174 | 77 | 97 |
| Ratio 1:1 | ||||||||
| Zhao et al. | 2017 | China | Cohort | Retrospective | 112,403 | Unclear | 3,012 (2.7%) | 109,391 |
| Stout et al. | 2010 | USA | Cohort | Retrospective | 64,047 | Unclear | 2,058 (3.2%) | 61,989 |
| Chen et al. | 2009 | Taiwan | Case control | Retrospective | 33,762 | Unclear | 5,627 | 28,135 |
| Ratio 1:5 | ||||||||
| Girault et al. | 2018 | France | Cohort | Retrospective | 19,719 | 19,719 | 154 (0.8%)b | 19,565 |
| Lai et al., | 2012 | USA | Cohort | Retrospective | 15,104 | 15,104 | 401 (2.7%) | 14,703 |
| Qidwai et al. | ||||||||
| Blitz et al. | 2016 | USA | Cohort | Retrospective | 10,314 | 10,314 | 522 (5.1%) | 9,792 |
| Ciavattini et al. | 2015 | Italy | Case control | Retrospective | 438 | 438 | 219 | 219 |
| Ratio 1:1 | ||||||||
| Arisoy et al. | 2016 | Turkey | Case control | Retrospective | 280 | 273 | 106 | 167 |
| Egbe et al. | 2018 | Cameroon | Cohort | Retrospective | 226 | Unclear | 38 (16.8%) | 188 |
| Shavell et al. | 2012 | USA | Case control | Retrospective | 190 | 190 | 95 | 95 |
| Ratio 1:1 | ||||||||
| Total | 256,683 | 256,650 | 12,309 | 244,341 | ||||
a Prevalence of fibroids reported for non-fertility cohort studies only
b This study reported a fibroid prevalence of 1.5% including operated fibroids, which were excluded in the present analysis.
Table 2. Methodology of diagnosis of fibroids.
| Study | Sample selection | Diagnostic imaging | Timing of ultrasound | Methodology of fibroid measurements | Exposure definition fibroids | ||
| Fibroid size | Fibroid number | Fibroid location | |||||
| Eze et al. | Routine ultrasound screening | Ultrasound | Not reported | Size and location | NS | NS | all |
| Size: mean of 3 dimensions | |||||||
| Zhao et al. | All deliveries | Ultrasound | 2nd trimester | Not reported | NS | ≥ 1 | all |
| 18–22 weeks | |||||||
| Stout et al. | Routine ultrasound screening | Ultrasound | 2nd trimester | Number, size (largest mean diameter), volume (H x W x L), location, relationship to placenta. Description of 6 largest fibroids according to American Institute of Ultrasound in medicine guidelines [44]. | NS | ≥ 1 | all |
| Chen et al. | Pregnant women who accepted ultrasound screening (national birth registry) | Ultrasound | 1st trimester: 73.8% | Diagnosis of fibroids during pregnancy based on ICD-9-CM codes | NS | NS | all |
| 2nd trimester: 25.2% | |||||||
| 3d trimester: 1% | |||||||
| Girault et al. | Routine ultrasound screening | Ultrasound | 1st trimester | Number, size, and location | ≥1 measuring ≥2 cm, or multiple whatever the size | All | |
| (11+0–13+6 weeks) | |||||||
| Lai et al., | Routine ultrasound screening | Ultrasound | 2nd trimester | Number, size and location | ≥ 1 cm | ≥ 1 | all |
| Qidwai et al. | |||||||
| Blitz et al. | Routine ultrasound screening | Ultrasound | 2nd trimester | Size (3 dimensions), number and location | NS | ≥ 1 | all |
| 17–23 weeks | |||||||
| Ciavattini et al. | Routine ultrasound screening | Ultrasound | 2nd trimester | Size (largest diameter), number (≥2 irrespective of size) and location | NS | NS | all |
| Arisoy et al. | Routine ultrasound screening | Ultrasound | 2nd trimester | Size (3 dimensions) and location | > 3 cm | ≥ 1 | intramural |
| 16–24 weeks | |||||||
| Egbe et al. | All pregnancies | Ultrasound | 1st trimester | Size (3 dimensions). Mean of two measurements. Criteria by Muram et al. [45] | > 3 cm | ≥ 1 | all |
| Shavell et al. | Routine ultrasound screening | Ultrasound | 1st and 2nd trimester | Number, number of fibroids >5cm, diameter largest fibroid, location fibroids >5cm | NS | NS | all |
| Volume: H x W x L x π/6 | |||||||
| Total fibroid volume per patient | |||||||
NS not specified
Baseline characteristics of the included study populations are described in Table 3. Women with fibroids were generally older and more often black, except in the studies that matched for age and ethnicity. In most studies, women with fibroids had lower parity. Three studies corrected for a previous preterm birth [16, 18, 19]. Blitz et al. excluded women with previous cervical surgery [9]. Eze et al. and Egbe et al. did not adjust for confounders by either statistical analysis or matching of characteristics [39, 43].
Table 3. Characteristics of the study population and handling of confounders.
| Study | Maternal age (years) | Maternal ethnicity | Parity | Handling of potential confounders | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fibroid | Control | P | Fibroid | Control | p | Fibroid | Control | p | ||
| Eze et al. | 31.6 (±5.5) | 29.1 (±5.5) | <0.001 | NR | NR | NR | NR | NR | NR | No |
| Zhao et al. | 32.0 (±4.9) | 27.9 (±5.2) | <0.001 | NR | NR | NR | 1.15 (±0.4) | 1.21 (±0.5) | <0.01 | Adjusted for e.g. maternal age, parity, BMI, HDP, GDM, previous preterm birth. |
| Stout et al. | 35.1 (±4.6) | 30.0 (±6.3) | <0.001 | Black: 34.5% | Black: 20.3% | <0.001 | 0.82 (±1.1) | 1.06 (±1.2) | <0.01 | Adjusted for e.g. maternal age, ethnicity, gravidity, previous preterm birth. |
| White: 51.5% | White: 64.0% | |||||||||
| Chen et al. | 35–39: 22.6% >39: 4.2% | 35–39: 9.3% >39: 1.3% | <0.001 | NR | NR | NR | Nulliparous: 55.0% | Nulliparous: 51.7% | <0.001 | Adjusted for e.g. maternal age, parity, diabetes, hypertension. |
| Girault et al. | 35.3 (±5.1) | 32.0 (±5.4) | <0.001 | France: 29.2% Sub-Saharan Africa: 39.6% Other: 31.2% | France: 48.6% Sub-Saharan Africa: 16.1% | <0.001 | Nulliparous: 39.0% | Nulliparous: 41.0% | 0.62 | Adjusted for e.g, maternal age, ethnicity, BMI, parity, previous preterm birth, ART. |
| Other: 35.3% | ||||||||||
| Lai et al., Qidwai et al. | 33.7 | 28.6 | <0.001 | Black: 24.2% | Black: 15.7% | <0.001 | Nulliparous: 57.4% | Nulliparous 53.7% | <0.001 | Adjusted for e.g. maternal age, ethnicity, parity, previous uterine surgery. |
| White: 33.1% | White: 38.3% | |||||||||
| Hispanic: 9.2% | Hispanic: 14.4% | |||||||||
| Asian: 26.4% | Asian: 25.6% | |||||||||
| Blitz et al. | 33.3 (±3.6) | 30.9 (±4.5) | <0.001 | Black: 26.6% | Black: 6.8% | <0.001 | Nulliparous: 67.4% | Nulliparous: 53.9% | <0.001 | Excluded women with previous preterm birth. |
| White: 43% | White: 66.2% | |||||||||
| Hispanic: 13.5% | Hispanic: 15.4% | |||||||||
| Asian 16.9% | Asian: 11.5% | |||||||||
| Ciavattini et al. | 34.8 (±4.2) | 34.8 (±4.2) | 1.0 | NR | NR | NR | 2.1 (±1) | 2 (±1) | 0.30 | Controls matched for age |
| Arisoy et al. | 34.4 (±4.9) | 34.3 (±2.7) | 0.83 | NR | NR | NR | 1.2 (±1.3) | 1.3 (±1.0) | 0.47 | Controls matched for age |
| Egbe et al. | 31.4 (±3.4) | 27.4 (±4.2) | <0.001 | NR | NR | NR | Nulliparous: 50.0% | Nulliparous: 20.0% | 0.02 | No |
| Shavell et al. | Small fibroids: 31.6 (±5.7) | 31.9 (±5.6) | 0.89 | Small fibroids: | Black: 86.3% | 0.32 | Small fibroids: 2.0 (±1.5) | 2.1 (±1.7) | 0.14 | Controls matched for age. |
| Black: 90.5% | ||||||||||
| White: 4.8% | ||||||||||
| Other: 4.8% | White: 10.5% | |||||||||
| Other: 3.2% | ||||||||||
| Large fibroids: 32.3 (±5.5) | Large fibroids: | Large fibroids: 1.6 (±1.3) | ||||||||
| Black: 92.5% | ||||||||||
| White: 1.9% | ||||||||||
| Other: 5.7% | ||||||||||
BMI Body Mass Index; HDP hypertensive disease of pregnancy; GDM Gestational Diabetes Mellitus; NR not reported
Risk of bias
According to the Newcastle-Ottawa scale, seven of the included studies were of good quality [10, 16, 18, 19, 25, 26, 41, 42], and four of poor quality [9, 39, 40, 43]. See S1 Fig for the complete risk of bias assessment. The most important reasons for a fair or poor quality rating of studies were: not controlling or matching for important confounders such as maternal age and ethnicity, and high or unknown rates of loss to follow-up. The funnel plot of included studies has a fairly symmetrical pattern, indicating there is no clear evidence of publication bias (S2 Fig).
Preterm birth
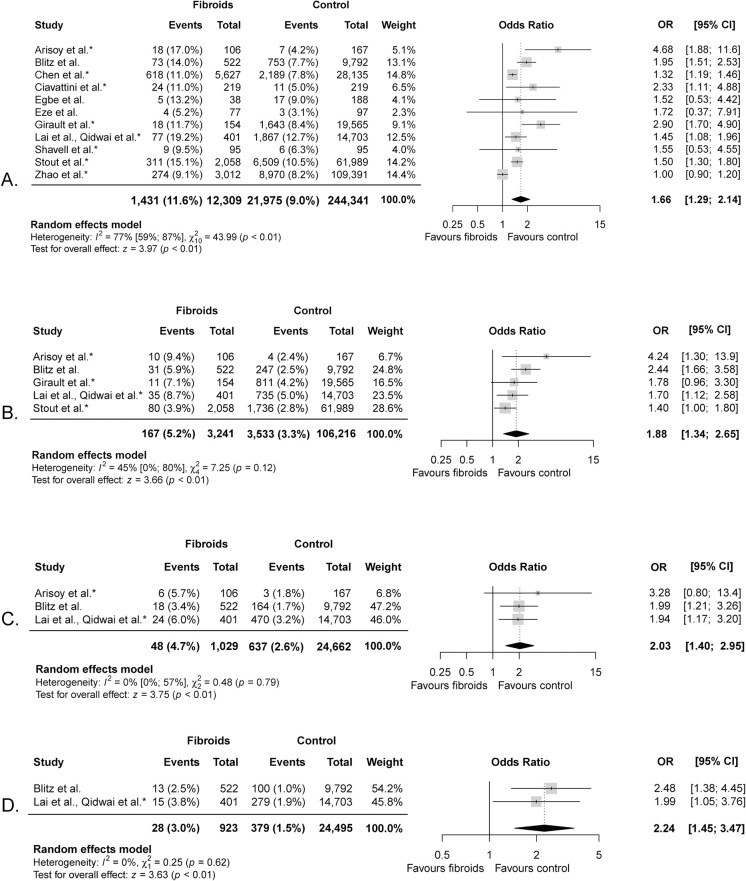
Preterm birth was defined as birth <37 weeks of gestation by all studies and several studies made subgroups at other gestational age cut-offs. The lower limit of the gestational age cut-off mostly varied between 20–24 weeks of gestation. Zhao et al. and Egbe et al. used a cut-off at 28 weeks of gestation (S2 Table) [19, 39]. Pooled preterm birth rates <37 weeks of gestation were 11.6% in the fibroid group and 9.0% in the control group (Fig 2A). The meta-analysis of 256,650 women showed women with fibroids had an increased risk of preterm birth <37 weeks compared to women without fibroids (OR 1.66, 95% CI 1.29–2.14). Heterogeneity was considerable (X2 = 43.99, p<0.01; I2 = 77%). Meta-analyses also showed a significant association of fibroids with preterm birth <34, <32, and <28 weeks of gestation and the strength of this association increased with preterm birth at earlier gestational ages (Fig 2B–2D). Meta-analyses for preterm birth <34 weeks of gestation included five studies with 109,457 women (5.2% versus 3.3%; OR 1.88, 95% CI 1.34–2.65), <32 weeks of gestation included three studies with 25,691 women (4.7% versus 2.6%; OR 2.03, 95% CI 1.40–2.95) and <28 weeks gestation included two studies with 25,418 women (3.0% versus 1.5%; OR 2.24, 95% CI 1.45–3.47). We did not identify any studies who reported on mid-trimester fetal losses between 16 and 22 gestational weeks.
Fig 2.
Meta-analysis of preterm birth <37 (A.), <34 (B.), <32 (C.) and <28 (D.) weeks of gestation in women with fibroids. * indicates studies that corrected for potential confounders.
Only Girault et al. differentiated between spontaneous and medically indicated preterm birth <37 weeks of gestation. They found that women with fibroids are at increased risk of medically indicated preterm birth compared to women without fibroids (RR 1.99, 95% CI 1.18–3.36). The medical indications leading to these preterm birth were not reported. There was no difference in spontaneous preterm birth between the fibroid group (5/154 = 3.2%) compared to the control group (820/19,565 = 4.2%); RR 0.77 (95% CI 0.33–1.84) [16]. Conversely, Ciavattini et al. did show an increased risk of spontaneous preterm birth in women with fibroids (OR 2.33, 95% CI 1.11–4.88) [42].
PPROM
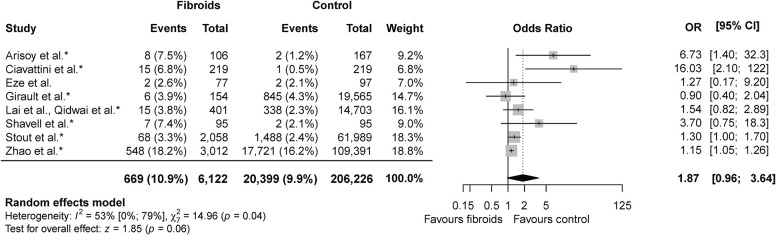
Eight studies reported on PPROM, including 212,348 women: 6,122 in the fibroid group and 206,226 in the control group [10, 16, 18, 19, 25, 26, 41, 42]. Meta-analysis showed no difference in PPROM rate between the fibroid and the control group (10.9% versus 9.9%; OR 1.87, 95% CI 0.96–3.64) (Fig 3). Statistical heterogeneity was substantial (X2 = 14.96, p = 0.04; I2 = 53%). An overview of the crude and adjusted effect sizes per study and outcome can be found in S3 Table.
Fig 3. Meta-analysis PPROM.
* indicates studies that corrected for potential confounders.
Sensitivity analyses
Good quality studies were included in sensitivity analyses (S3 Fig). All these studies adjusted for potential confounders. The meta-analyses showed a smaller, yet still significant, association between fibroids and preterm birth <37 (11.5% versus 9.1%; OR 1.50, 95% CI 1.15–1.95) and <34 (4.7% versus 3.2%; OR 1.50, 95% CI 1.16–1.94) weeks of gestation. A meta-analysis of preterm birth <32 and <28 weeks of gestation could not be performed as there was one good quality study assessing these outcomes. There was no significant association between fibroids and PPROM in a sensitivity analysis (11.3% versus 10.5%; OR 1.97, 95% CI 0.84–4.61). Girault et al. was rated as good quality but was not included in sensitivity analyses for preterm birth <34 weeks of gestation and PPROM [16]. They provided additional preterm birth rates in their unoperated fibroid group upon our request. As we did not have the adjusted ORs for these particular outcomes, this lead to the deduction of points in quality in the comparability section with regards to these outcomes.
Subgroups based on fibroid characteristics
The results of the subgroup analyses based on fibroid characteristics are shown in S4 Fig. Subgroup analysis including studies comparing women with fibroids >3 cm and >5 cm to women without fibroids showed that, overall, there was a significant association between fibroids and preterm birth <37 weeks of gestation (n = 177,521; OR 2.03, 95% CI 1.26–3.27). Heterogeneity was substantial (X2 = 13.29, p = 0.02; I2 = 62%). Meta-analyses did not show differences in preterm birth <37 weeks of gestation when comparing large (>5 cm) to small (≤5 cm) fibroids (OR 1.38, 95% CI 0.78–2.46), nor when comparing multiple to single fibroids (OR 1.89, 95% CI 0.79–4.53). No subgroup analyses could be performed on fibroid location (submucosal, intramural, subserosal), vascularisation, FIGO classification or other ultrasound features. Neither could we perform subgroup analyses based on parity, a history of previous mid-trimester fetal losses or preterm birth, or a history of dilation and curettage.
Discussion
The present meta-analysis, including 11 studies and 256,650 deliveries, suggests uterine fibroids are associated with preterm birth. This association was present for preterm birth <37 weeks of gestation and became stronger with preterm birth at earlier gestational ages <34, <32 and <28 weeks. Moreover, the association with preterm birth <37 and <34 weeks of gestation was also significant in sensitivity analyses including good-quality studies. No association was found between PPROM and the presence of fibroids. No distinction could be made between spontaneous and medically indicated preterm birth in meta-analyses. This distinction is crucial for the interpretation the study results due the disparate underlying aetiologies of these types of preterm birth. Only two included studies made this distinction: one included study showed an association between spontaneous preterm birth and the other showed an association with medically indicated preterm birth. No conclusions can be drawn from these limited data.
There is insufficient evidence to conclude whether the size or number of fibroids modifies the risk of preterm birth or not. Other potentially relevant fibroid characteristics have not been reported in the included studies. Fibroid activity, for instance, may be important in the genesis of preterm birth. Fibroid activity could be determined by assessing vascularisation, fibroid growth, signs of degeneration, signs of calcification or other MUSA criteria [46, 47].
Two past reviews also found an association between fibroids and preterm birth. Klatsky et al. found a preterm birth rate of 16.0% in the fibroid group and 10.8% in the control group (OR 1.5, 95% CI 1.3–1.7), and Perez-Roncero et al. of 11.7% versus 9.0% (OR 1.43; 95% CI 1.27–1.60) [5, 6]. These reviews also included studies that did not perform routine ultrasound screening during pregnancy, which are at risk of ascertainment bias, and they did not quantitatively address possible bias resulting from important confounders in their meta-analyses. Furthermore, the studies did not aim to distinguish between spontaneous and medically indicated preterm birth [5, 6].
We did not identify any studies that reported on mid-trimester fetal loss between 16 and 22 weeks of gestation. These mid-trimester fetal losses are often included in the group of miscarriages defined as loss <20–24 weeks of gestation. A recent systematic review and meta-analysis concluded that the risk of miscarriage, including mid-trimester fetal losses, was not increased in women with fibroids in a general obstetric population [48]. However, we presume that first-trimester fetal losses and mid-trimester losses have different aetiologies and, therefore, we propose future studies to make a distinction between these entities [49].
We performed an in-depth evaluation of preterm birth in women with fibroids, and a meta-analysis including confounder-adjusted effect sizes. We also performed sensitivity analyses including good-quality studies. Nevertheless, the results of our study should be interpreted in light of the following considerations. Not all studies controlled for or matched for all relevant confounders (e.g. cervical surgery, uterine malformations, socioeconomic status), and there still might be residual confounding present.
We minimised the risk of ascertainment bias by excluding studies with women who were selectively referred for ultrasound screening. However, even when routine prenatal ultrasound screening is performed, there is a risk of underreporting of fibroids in the absence of a prospective protocol for myometrial assessment because the primary focus lies on the fetus rather than the uterine wall. Moreover, ultrasound screening in the second and third trimester also leads to underreporting, as not all fibroids, especially those located in the posterior uterine wall, can be visualised due to the size of the fetus. It seems impossible to quantify the frequency of undiagnosed fibroids in these studies. As the risk of the opportunistic recording of fibroids is likely smaller with larger fibroids, we performed a subgroup analysis including studies that compared fibroids >3 cm and >5 cm to women without fibroids. In this analysis, there was still a significant association present between fibroids and preterm birth. This association was stronger for fibroids >3 cm than for fibroids >5 cm, but uncertainty is large due to the wide confidence interval in the subgroup of fibroids >3 cm. This is probably a consequence of a small number of studies, including one study with an outlying effect size.
Finally, there was substantial heterogeneity between studies, which might have been caused by variations in study populations and methodology. There was a wide range in the prevalence of fibroids between studies. This is probably a reflection of differences in ethnicity, as the lowest prevalence of fibroids was found in a French study and the higher prevalence in a Cameroonian study. However, selection bias resulting from opportunistic reporting could also play a role. Furthermore, variations in exposure definitions of fibroids and lower gestational age cut-offs may have caused heterogeneity by differential misclassification.
Based on our findings, systematic myometrial assessment before or during the first trimester of pregnancy is warranted. Future studies should have a protocol for prospective fibroid assessment on ultrasound including evaluation of fibroid size, number, FIGO classification, location (corporeal or cervical) growth, and ultrasound features such as vascularisation, degeneration, and calcification [46, 47]. Registration of these characteristics may provide insight into the association with preterm birth and the underlying pathophysiologic mechanism(s). This, in turn, could give more insight as to which screening- and preventative strategies could be helpful to improve antenatal care for women with fibroids.
Conclusions
This systematic review and meta-analysis suggests fibroids are associated with total preterm birth and this association is stronger with earlier gestational age of the preterm birth. However, the considerable amount of heterogeneity between studies may indicate biased results. Considering the magnitude of the disease burden of preterm birth, as well as the biological plausibility of an association between preterm birth and fibroids, we encourage further research to clarify this association through prospective and systematic myometrial assessment in early pregnancy. Finally, it is important that these studies distinguish between spontaneous and medically indicated preterm birth.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(TIF)
(TIF)
Sensitivity analyses of good quality studies for preterm birth (A) <37 and (B) <34 gestational weeks and (C) PPROM. * indicates studies that corrected for potential confounders.
(TIF)
Meta-analysis of (A) fibroids >3 and >5 cm compared to women without fibroids; (B) large versus small fibroids; and (C) multiple versus single fibroids. * indicates studies that corrected for potential confounders.
(TIF)
Data Availability
All relevant data are within the paper and Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. The Lancet Global health. 2019;7(1): e37–e46. doi: 10.1016/S2214-109X(18)30451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations Inter-Agency Group for Child Mortality Estimation. Levels and trends in child mortality: Report 2019. New York: United Nations Children’s Fund, 2019. [Google Scholar]
- 3.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606): 75–84. doi: 10.1016/S0140-6736(08)60074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG. 2006;113 Suppl 3(Suppl 3): 17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klatsky PC, Tran ND, Caughey AB, Fujimoto VY. Fibroids and reproductive outcomes: a systematic literature review from conception to delivery. Am J Obstet Gynecol. 2008;198(4): 357–366. doi: 10.1016/j.ajog.2007.12.039 [DOI] [PubMed] [Google Scholar]
- 6.Pérez-Roncero GR, López-Baena MT, Ornat L, Cuerva MJ, Garcia-Casarrubios P, Chedraui P, et al. Uterine fibroids and preterm birth risk: A systematic review and meta-analysis. The journal of obstetrics and gynaecology research. 2020;46(9): 1711–1727. doi: 10.1111/jog.14343 [DOI] [PubMed] [Google Scholar]
- 7.Rice JP, Kay HH, Mahony BS. The clinical significance of uterine leiomyomas in pregnancy. Am J Obstet Gynecol. 1989;160(5 Pt 1): 1212–1216. doi: 10.1016/0002-9378(89)90194-4 [DOI] [PubMed] [Google Scholar]
- 8.Blum M. Comparative study of serum CAP activity during pregnancy in malformed and normal uterus. Journal of perinatal medicine. 1978;6(3): 165–168. doi: 10.1515/jpme.1978.6.3.165 [DOI] [PubMed] [Google Scholar]
- 9.Blitz MJ, Rochelson B, Augustine S, Greenberg M, Sison CP, Vohra N. Uterine fibroids at routine second-trimester ultrasound survey and risk of sonographic short cervix. J Matern Fetal Neonatal Med. 2016;29(21): 3454–3460. doi: 10.3109/14767058.2015.1131261 [DOI] [PubMed] [Google Scholar]
- 10.Shavell VI, Thakur M, Sawant A, Kruger ML, Jones TB, Singh M, et al. Adverse obstetric outcomes associated with sonographically identified large uterine fibroids. Fertility and sterility. 2012;97(1): 107–110. doi: 10.1016/j.fertnstert.2011.10.009 [DOI] [PubMed] [Google Scholar]
- 11.Kido A, Ascher SM, Hahn W, Kishimoto K, Kashitani N, Jha RC, et al. 3 T MRI uterine peristalsis: comparison of symptomatic fibroid patients versus controls. Clinical radiology. 2014;69(5): 468–472. doi: 10.1016/j.crad.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 12.Nishino M, Togashi K, Nakai A, Hayakawa K, Kanao S, Iwasaku K, et al. Uterine contractions evaluated on cine MR imaging in patients with uterine leiomyomas. European journal of radiology. 2005;53(1): 142–146. doi: 10.1016/j.ejrad.2004.01.009 [DOI] [PubMed] [Google Scholar]
- 13.Orisaka M, Kurokawa T, Shukunami K, Orisaka S, Fukuda MT, Shinagawa A, et al. A comparison of uterine peristalsis in women with normal uteri and uterine leiomyoma by cine magnetic resonance imaging. Eur J Obstet Gynecol Reprod Biol. 2007;135(1): 111–115. doi: 10.1016/j.ejogrb.2006.07.040 [DOI] [PubMed] [Google Scholar]
- 14.Yoshino O, Hayashi T, Osuga Y, Orisaka M, Asada H, Okuda S, et al. Decreased pregnancy rate is linked to abnormal uterine peristalsis caused by intramural fibroids. Human reproduction (Oxford, England). 2010;25(10): 2475–2479. doi: 10.1093/humrep/deq222 [DOI] [PubMed] [Google Scholar]
- 15.Petraglia F, Arcuri F, de Ziegler D, Chapron C. Inflammation: a link between endometriosis and preterm birth. Fertility and sterility. 2012;98(1): 36–40. doi: 10.1016/j.fertnstert.2012.04.051 [DOI] [PubMed] [Google Scholar]
- 16.Girault A, Le Ray C, Chapron C, Goffinet F, Marcellin L. Leiomyomatous uterus and preterm birth: an exposed/unexposed monocentric cohort study. Am J Obstet Gynecol. 2018;219(4): 410.e411–410.e417. doi: 10.1016/j.ajog.2018.08.033 [DOI] [PubMed] [Google Scholar]
- 17.Pan L, Fu Z, Yin P, Chen D. Pre-existing medical disorders as risk factors for preeclampsia: an exploratory case-control study. Hypertension in pregnancy. 2019;38(4): 245–251. doi: 10.1080/10641955.2019.1667381 [DOI] [PubMed] [Google Scholar]
- 18.Stout MJ, Odibo AO, Graseck AS, Macones GA, Crane JP, Cahill AG. Leiomyomas at routine second-trimester ultrasound examination and adverse obstetric outcomes. Obstet Gynecol. 2010;116(5): 1056–1063. doi: 10.1097/AOG.0b013e3181f7496d [DOI] [PubMed] [Google Scholar]
- 19.Zhao R, Wang X, Zou L, Li G, Chen Y, Li C, et al. Adverse obstetric outcomes in pregnant women with uterine fibroids in China: A multicenter survey involving 112,403 deliveries. PloS one. 2017;12(11): e0187821. doi: 10.1371/journal.pone.0187821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical research ed). 2021;372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Systematic reviews. 2016;5(1): 210. doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells GA SB, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses (2013). http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 23.Harrer M CP, Furukawa TA, Ebert DD. Doing Meta-Analysis in R: A Hands-on Guide (2019). https://bookdown.org/MathiasHarrer/Doing_Meta_Analysis_in_R/ 2019. [Google Scholar]
- 24.Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
- 25.Lai J, Caughey AB, Qidwai GI, Jacoby AF. Neonatal outcomes in women with sonographically identified uterine leiomyomata. J Matern Fetal Neonatal Med. 2012;25(6): 710–713. doi: 10.3109/14767058.2011.572205 [DOI] [PubMed] [Google Scholar]
- 26.Qidwai GI, Caughey AB, Jacoby AF. Obstetric outcomes in women with sonographically identified uterine leiomyomata. Obstet Gynecol. 2006;107(2 Pt 1): 376–382. doi: 10.1097/01.AOG.0000196806.25897.7c [DOI] [PubMed] [Google Scholar]
- 27.Conti N, Tosti C, Pinzauti S, Tomaiuolo T, Cevenini G, Severi FM, et al. Uterine fibroids affect pregnancy outcome in women over 30 years old: role of other risk factors. J Matern Fetal Neonatal Med. 2013;26(6): 584–587. doi: 10.3109/14767058.2012.745504 [DOI] [PubMed] [Google Scholar]
- 28.Davis JL, Ray-Mazumder S, Hobel CJ, Baley K, Sassoon D. Uterine leiomyomas in pregnancy: a prospective study. Obstet Gynecol. 1990;75(1): 41–44. [PubMed] [Google Scholar]
- 29.Doğan S, Özyüncü Ö, Atak Z. Fibroids During Pregnancy: Effects on Pregnancy and Neonatal Outcomes. The Journal of reproductive medicine. 2016;61(1–2): 52–57. [PubMed] [Google Scholar]
- 30.Exacoustòs C, Rosati P. Ultrasound diagnosis of uterine myomas and complications in pregnancy. Obstet Gynecol. 1993;82(1): 97–101. [PubMed] [Google Scholar]
- 31.Harlev A, Wainstock T, Walfisch A, Landau D, Sheiner E. Perinatal outcome and long-term pediatric morbidity of pregnancies with a fibroid uterus. Early Hum Dev. 2019;129: 33–37. doi: 10.1016/j.earlhumdev.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 32.Karlsen K, Schiøler Kesmodel U, Mogensen O, Humaidan P, Ravn P. Relationship between a uterine fibroid diagnosis and the risk of adverse obstetrical outcomes: a cohort study. BMJ open. 2020;10(2): e032104. doi: 10.1136/bmjopen-2019-032104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SJ, Ko HS, Na S, Bae JY, Seong WJ, Kim JW, et al. Nationwide population-based cohort study of adverse obstetric outcomes in pregnancies with myoma or following myomectomy: retrospective cohort study. BMC Pregnancy Childbirth. 2020;20(1): 716. doi: 10.1186/s12884-020-03406-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majeed T, Waheed F, Sattar Y, Mobusher I, Saba K. Impact of uterine fibroids on the obstetric performance of the women; Complications and pregnancy outcome. Pakistan Journal of Medical and Health Sciences. 2011;5: 274–277. [Google Scholar]
- 35.Murata T, Kyozuka H, Endo Y, Fukuda T, Yasuda S, Yamaguchi A, et al. Preterm Deliveries in Women with Uterine Myomas: The Japan Environment and Children’s Study. Int J Environ Res Public Health. 2021;18(5). doi: 10.3390/ijerph18052246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oliveira FG, Abdelmassih VG, Diamond MP, Dozortsev D, Melo NR, Abdelmassih R. Impact of subserosal and intramural uterine fibroids that do not distort the endometrial cavity on the outcome of in vitro fertilization-intracytoplasmic sperm injection. Fertility and sterility. 2004;81(3): 582–587. doi: 10.1016/j.fertnstert.2003.08.034 [DOI] [PubMed] [Google Scholar]
- 37.Oztekin D, Sofuoglu K, Caliskan E, Kars B, Cetinkaya T, Tug N. Effects of leiomyomas less than 5 cm in diameter on the outcomes of assisted reproductive technology. Ginecoro. 2012;8: 128–130. [Google Scholar]
- 38.Sheiner E, Bashiri A, Levy A, Hershkovitz R, Katz M, Mazor M. Obstetric characteristics and perinatal outcome of pregnancies with uterine leiomyomas. The Journal of reproductive medicine. 2004;49(3): 182–186. [PubMed] [Google Scholar]
- 39.Egbe TO, Badjang TG, Tchounzou R, Egbe EN, Ngowe MN. Uterine fibroids in pregnancy: prevalence, clinical presentation, associated factors and outcomes at the Limbe and Buea Regional Hospitals, Cameroon: a cross-sectional study. BMC research notes. 2018;11(1): 889. doi: 10.1186/s13104-018-4007-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arisoy R, Erdogdu E, Bostancι E, Ergin RN, Kumru P, Demirci O, et al. Obstetric outcomes of intramural leiomyomas in pregnancy. Clinical and experimental obstetrics & gynecology. 2016;43(6): 844–848. [PubMed] [Google Scholar]
- 41.Chen YH, Lin HC, Chen SF, Lin HC. Increased risk of preterm births among women with uterine leiomyoma: a nationwide population-based study. Human reproduction (Oxford, England). 2009;24(12): 3049–3056. doi: 10.1093/humrep/dep320 [DOI] [PubMed] [Google Scholar]
- 42.Ciavattini A, Clemente N, Delli Carpini G, Di Giuseppe J, Giannubilo SR, Tranquilli AL. Number and size of uterine fibroids and obstetric outcomes. J Matern Fetal Neonatal Med. 2015;28(4): 484–488. doi: 10.3109/14767058.2014.921675 [DOI] [PubMed] [Google Scholar]
- 43.Eze CU, Odumeru EA, Ochie K, Nwadike UI, Agwuna KK. Sonographic assessment of pregnancy co-existing with uterine leiomyoma in Owerri, Nigeria. African health sciences. 2013;13(2): 453–460. doi: 10.4314/ahs.v13i2.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.American Institute of Ultrasound in Medicine. AIUM practice guideline for the performance of obstetric ultrasound. Laurel (MD): The American Institute of Ultrasound in Medicine; 2007. [DOI] [PubMed] [Google Scholar]
- 45.Muram D, Gillieson M, Walters JH. Myomas of the uterus in pregnancy: ultrasonographic follow-up. Am J Obstet Gynecol. 1980;138(1): 16–19. doi: 10.1016/0002-9378(80)90005-8 [DOI] [PubMed] [Google Scholar]
- 46.Munro MG, Critchley HO, Fraser IS. The FIGO classification of causes of abnormal uterine bleeding in the reproductive years. Fertility and sterility. 2011;95(7): 2204–2208, 2208.e2201-2203. doi: 10.1016/j.fertnstert.2011.03.079 [DOI] [PubMed] [Google Scholar]
- 47.Van den Bosch T, Dueholm M, Leone FP, Valentin L, Rasmussen CK, Votino A, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol. 2015;46(3): 284–298. doi: 10.1002/uog.14806 [DOI] [PubMed] [Google Scholar]
- 48.Sundermann AC, Velez Edwards DR, Bray MJ, Jones SH, Latham SM, Hartmann KE. Leiomyomas in Pregnancy and Spontaneous Abortion: A Systematic Review and Meta-analysis. Obstet Gynecol. 2017;130(5): 1065–1072. doi: 10.1097/AOG.0000000000002313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sneider K, Christiansen OB, Sundtoft IB, Langhoff-Roos J. Recurrence of second trimester miscarriage and extreme preterm delivery at 16–27 weeks of gestation with a focus on cervical insufficiency and prophylactic cerclage. Acta Obstet Gynecol Scand. 2016;95(12): 1383–1390. doi: 10.1111/aogs.13027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(TIF)
(TIF)
Sensitivity analyses of good quality studies for preterm birth (A) <37 and (B) <34 gestational weeks and (C) PPROM. * indicates studies that corrected for potential confounders.
(TIF)
Meta-analysis of (A) fibroids >3 and >5 cm compared to women without fibroids; (B) large versus small fibroids; and (C) multiple versus single fibroids. * indicates studies that corrected for potential confounders.
(TIF)
Data Availability Statement
All relevant data are within the paper and Supporting Information files.