Abstract
For over 15 years the lytic cell death termed pyroptosis was defined by its dependency on the inflammatory caspase, caspase-1, which, upon pathogen sensing, is activated by innate immune cytoplasmic protein complexes known as inflammasomes. However, this definition of pyroptosis changed when the pore-forming protein gasdermin D (GSDMD) was identified as the caspase-1 (and caspase-11) substrate required to mediate pyroptotic cell death. Consequently, pyroptosis has been redefined as a gasdermin-dependent cell death. Studies now show that, upon liberation of the N-terminal domain, five gasdermin family members, GSDMA, GSDMB, GSDMC, GSDMD and GSDME can all form plasma membrane pores to induce pyroptosis. Here, we review recent research into the diverse stimuli and cell death signaling pathways involved in the activation of gasdermins; death and toll-like receptor triggered caspase-8 activation of GSDMD or GSMDC, apoptotic caspase-3 activation of GSDME, perforin-granzyme A activation of GSDMB, and bacterial protease activation of GSDMA. We highlight findings that have begun to unravel the physiological situations and disease states that result from gasdermin signaling downstream of inflammasome activation, death receptor and mitochondrial apoptosis, and necroptosis. This new era in cell death research therefore holds significant promise in identifying how distinct, yet often networked, pyroptotic cell death pathways might be manipulated for therapeutic benefit to treat a range of malignant conditions associated with inflammation, infection and cancer.
Keywords: apoptosis, gasdermin, GSDMD, inflammasome, necroptosis, pyroptosis
Introduction
Systemic, tissue and cellular homeostasis is threatened by infection and injury. Surveillance of pathogenic and sterile insults is upheld in multicellular organisms by the innate immune system. The evolutionarily conserved pattern recognition receptors (PRRs) respond to pathogenic virulence factors, environmental toxins and host-derived danger signals to initiate cytokine and chemokine production. In more recent years, the identification of new cell death effectors has highlighted the crucial role of PRRs in signaling cell death during infections, and has uncovered how cell death can act to eliminate intracellular pathogens [1–9]. Conversely, in some contexts, PRR-driven cell death can also increase pathogen infection by limiting the immune response [10–13], or can cause host-tissue damage and increase disease severity, as evidenced in cytokine shock syndromes [14–16].
Inflammasome forming PRRs represent one of the crucial cellular danger surveillance mechanisms that cause both potent inflammatory responses and cell death. These innate immune multimeric protein complexes maintain cytoplasmic homeostasis through the regulation of caspase-1 activation, proinflammatory cytokine maturation, specifically interleukin-1β (IL-1β) and IL-18, and gasdermin D (GSDMD)-driven pyroptosis, a cell death modality that, similar to necroptotic cell death, results in the release of immunogenic molecules [17]. The importance of correct inflammasome functioning in mammalian health has been documented in a multitude of studies detailing how diverse pathogens can overcome host defences when the inflammasome machinery is removed while, conversely, autoactivating mutations and excess inflammasome signaling have been shown to cause both rare hereditary and common autoinflammatory conditions, such as atherosclerosis [1,18,19].
Inflammasome signaling has long been synonymous with pyroptosis. The act of pyroptosis was first described in 1992 as a lytic form of apoptosis occurring in macrophages in response to Shigella flexneri-infection [20]. Cookson and Brennan later termed the caspase-1-dependent lytic cell death that they and others observed in Salmonella or Shigella infected macrophages ‘pyroptosis', derived from the Greek ‘pyro' meaning fire and ‘ptosis' (of piptein), meaning to fall [21–24]. Recently pyroptosis has been redefined as a gasdermin-dependent cell death, following two seminal studies showing that activated caspase-1 (and caspase-11) cleaves the pore-forming protein GSDMD to bring about the cells demise [25,26]. However, with this discovery and the classification of pyroptosis being a gasdermin-driven cell death [27,28], pyroptotic killing is no longer limited to inflammasome signaling. GSDMD and other gasdermin family members incorporating GSDMA, GSDMB, GSDMC and GSDME, can be processed independent of inflammasome complexes, by both cell death associated caspases and other proteases, such as granzymes, to form plasma membrane pores and trigger pyroptotic cell death [29]. These gasdermins are generally composed of a N-terminal pore-forming domain, though the gasdermin family member Pejvakin (PJVK) is an exception to this, an interdomain linker that is cleaved to unleash gasdermin activity, and an autoinhibitory C-terminal domain. The pore-forming ability of the N-terminal domain of gasdermin proteins is highly conserved, with mammalian pore-forming family members (GSDMA-E) having arisen from genetic duplications of a gasdermin gene present in ancient metazoans, suggesting a high degree of evolutionary pressure [30,31]. Experimental studies also show that the N-terminal domain of coral and lancelet gasdermin are both capable of causing pyroptosis, indicating that gasdermin function is conserved beyond mammals [31,32].
Unlike pyroptosis, necrotic cell death represents a passive cell lysis caused by overwhelming chemical, metabolic or physical stress that is not genetically encoded. While both types of cell death are viewed as highly inflammatory, due to membrane rupturing that allows the release of immunogenic content, gasdermin pore formation results from cellular sensing of specific host or pathogen-derived molecules and highly regulated cell signaling pathways, and as such, its activity can be finely tuned. The regulation of gasdermin pore formation, in addition to its size and charge preference for specific molecules such as IL-1β, can control the generation and release of specific inflammatory cytokines. Indeed, quantitative proteomics of cells undergoing pyroptosis has highlighted GSDMD-mediated release of smaller sized proteins, including IL-1β as well as alarmins (e.g. Galectin 3 and HMGB1) and luminal lysosomal proteins, even when cell lysis is blocked [33]. As discussed below, the modulation of the gasdermin pore can therefore act to delay, or even abrogate, pyroptotic cell death in some cell types such as neutrophils, thereby ensuring a prolonged anti-pathogen inflammatory response.
In mammals, recent insights into the molecular signals and proteases that trigger gasdermin cleavage to cause pyroptosis, and the networking of other cell death modalities with pyroptosis, including apoptosis, necroptosis and even ferroptosis-associated events, has arguably heralded in a new era of programmed cell death research. In this review we discuss the growing number of studies supporting the interplay between inflammasome activation, pyroptosis and its role in alternate cell death signaling pathways. These findings highlight how distinct programmed cell deaths can contribute to inflammasome activation to cause inflammation, how inflammasome signaling can bypass GSDMD and activate apoptosis, and conversely, how the apoptotic cell death machinery can act in a lytic manner by activating specific gasdermin family members to cause pyroptosis. We suggest that this emerging intricate web of cell death signaling underlines the importance of cell death for blocking microbial replication and exposing pathogens to immune-mediated attack, and that the repurposing of targeted anti-cancer cell death therapeutics represent a promising new class of anti-pathogen and immunomodulatory treatments.
A conventional look at pyroptosis: canonical and non-canonical inflammasome-triggering of GSDMD
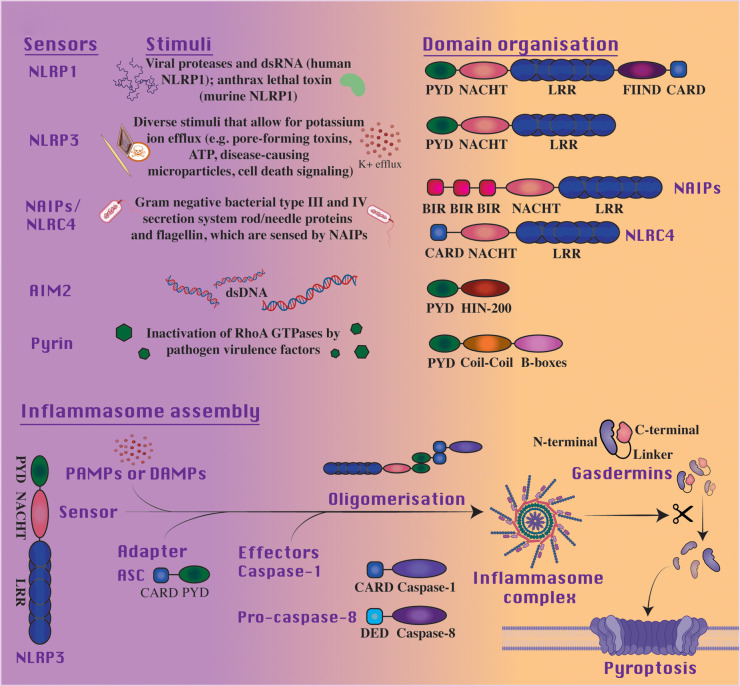
Three canonical inflammasome components exist that are required for the formation of an active inflammasome complex; an inflammasome sensor protein, an adaptor, and zymogenic caspase-1. Activation is initiated through inflammasome forming PRRs in response to cytoplasmic pathogen-associated molecular patterns (PAMPs), host-derived damage-associated molecular patterns (DAMPs) or environmental dangers [34]. Intracellular sensors of the prior stimuli are distinguished by structural features described in Figure 1; they include members of the nucleotide-binding and oligomerization domain (NOD)-like receptor (NLR) and absent in melanoma (AIM2)-like (ALR) receptor families and pyrin. While NLRP3 has been the most frequently investigated PRR in canonical inflammasome triggering due to its sensing of diverse cellular threats, NLRP1, NLRC4, AIM2 and pyrin are all capable of similar pathway activation. Further, autoactivating mutations in the inflammasome forming NLRs and pyrin cause autoinflammatory syndromes [18]. In all instances the inflammasome sensors oligomerize following PAMP or DAMP detection, which allows recruitment of the adaptor molecule apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC). The bipartite structure of ASC, containing a pyrin domain (PYD) and a caspase recruitment domain (CARD), facilitates homotypic interactions between both PYD containing receptors and the CARD containing caspase-1. Caspase-1 recruitment, and its dimerization, results in proximity-induced autocatalytic cleavage and activation. Distinctly, function-to-find domain (FIIND) cleavage and NLRP1's C-terminal CARD (which is inhibited by the protease DPP9), and not the PYD, allow for ASC and caspase-1 recruitment [35–38]. In addition, unlike PYD-containing NLRs, NLRC4 can recruit caspase-1 directly via its CARD to facilitate cleavage and subsequent cytokine maturation [39].
Figure 1. Inflammasomes share structural similarities despite being stimulated by different signals.
Inflammasome sensors are activated by differing stimuli though share similarities in domain organization. Within the NLR family, receptors are generally characterized by a tripartite structure, with an N-terminal signaling domain, a central nucleotide-binding domain (NBD) and a leucine-rich repeat (LRR)-containing C-terminal domain, involved in ligand binding or agonist sensing. Members of the NLR family can be partitioned into NLRPs or NLRCs through the variability of the N-terminal regions, typically containing a pyrin or a caspase activation and recruitment domain (PYD or CARD, respectively), in support of protein–protein interactions. Of these receptors, NLRP1, NLRP3 and NLRC4 are recognized inflammasome sensors, although NLRC4 assembly can occur with or without the ASC adaptor. Alternatively, the ALR family is defined structurally by an N-terminal PYD domain and a DNA binding C-terminal hematopoietic interferon-inducible nuclear protein with 200-amino acid repeat (HIN-200) domain. Of the ALR family, Absent in melanoma 2 (AIM2) is the best characterized. The N-terminal of the pyrin inflammasome contains a PYD to support homotypic interaction with the ASC adaptor and subsequent caspase recruitment, while a Coil–Coil domain can be identified in a central region with a B-boxes domain at the C-terminal. Broadly, with signaling by PAMPs or DAMPs these inflammasome sensors, for example NLRP3, oligomerize with effectors through adapter proteins to form inflammasome complexes. Subsequently, activated effector protein cleavage of gasdermins at their linker region releases the pore-forming N-terminal to bring about pyroptotic cell death.
Active caspase-1 mediates the cleavage of IL-1β and IL-18, in addition to the pyroptotic effector, GSDMD. A central linker region separating the pore-forming N- and autoinhibitory C-terminals of GSDMD is cleaved at the site 272FLTD|G276 in human GSDMD (hGSDMD) and 273LLSD|G277 in murine GSDMD (mGSDMD) by caspase-1 (and caspase-11 in mouse or caspase-4 and -5 in human) to cause its activation [25,26]. This cleavage allows cardiolipin, phosphatidylinositol 4-phosphate, phosphatidylinositol 4,5-biphosphate (PIP2), and/or phosphatidylserine membrane lipid binding by the N-terminal that induces conformational changes and oligomerization, resulting in the formation of 215 Å GSDMD pores in the plasma membrane, non-selective ionic fluxes and cell death [25,26,40–43]. The preferential redistribution of mature IL-1β and IL-18 to PIP2 lipids within the plasma membrane is dictated by their net positive charge imparted by a polybasic motif, which may become exposed upon caspase-1 cleavage, and the subsequent exit of IL-1β and IL-18 from the cell is preferentially engaged by a negatively charged GSDMD conduit [42,44]. Furthermore, the decoration of precursor IL-1β by ubiquitin chains acts to target it for degradation and prevent its processing by caspase-1 [45]. Recent reports indicate that bacterial effectors or viral proteases can also target GSDMD for ubiquitination and degradation, or inactivating proteolysis, respectively, to block host cell death and enable efficient infection [46–49], while other viral proteases may activate GSDMD killing activity [50,51]. The loss of GSDMD can protect mice from IL-1-driven inflammation caused by autoactivating NLRP3 inflammasome mutations, highlighting the importance of the GSDMD pore in not just eliciting cell death, but also allowing for efficient IL-1β release [52,53]. However, in other IL-1β-driven inflammatory models, such as K/BxN serum transfer-induced arthritis and gout-associated uric acid crystal-induced peritonitis, GSDMD does not drive disease severity, which may reflect inflammasome-independent IL-1β activation and cell death [54,55].
Non-canonical inflammasome signaling results upon exposure of gram-negative bacterial lipopolysaccharide (LPS) to cytosolic caspase-11 (caspase-4 and -5 in humans) [25,56,57] (Figure 2). Guanylate-binding proteins (GBPs) are not only proposed to mediate the lysis of vacuole membranes containing bacteria [58,59] and facilitate the disruption of cytosolic pathogen membranes [60,61], but have been shown to present LPS to caspase-11/4/5 by behaving as polyvalent signaling platforms [62]. Specifically, GBP1 appears to initiate platform assembly through electrostatic association with LPS or cytosolic Salmonella, with GBP2 and 4 being suggested as mediators, while GBP3 likely governs caspase activation [61,63–65]. Through GBP-mediated exposure of LPS, caspase-11 interacts with the LPS lipid A moiety resulting in caspase-11 activation and processing of GSDMD into the pore-forming N-terminal fragment although, unlike caspase-1, caspase-11 does not directly process IL-1β [66–72]. Instead, the GSDMD pore allows for cellular potassium ion efflux, which is a common determinant for NLRP3 inflammasome triggering in response to diverse stimuli and cellular stressors [73–76] (Figure 3). Thereby, the non-canonical caspase-11-GSDMD inflammasome can indirectly induce NLRP3 signaling and IL-1β and IL-18-driven inflammatory responses. This same triggering of NLRP3 via the GSDMD pore and potassium ion efflux has also recently been proposed to occur downstream of a TLR or death receptor-caspase-8-GSDMD axis [77,78] (Figure 3). Contrary to canonical inflammasome signaling, death through non-canonical signaling has not been observed in the absence of GSDMD, though as caspase-7 is a recently proposed substrate of caspase-4, further investigation into cross-talk with apoptotic pathways is warranted [79].
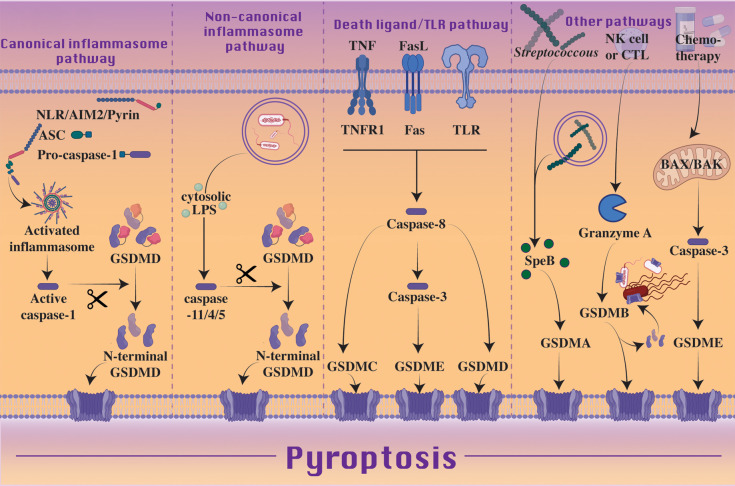
Figure 2. Pyroptosis is executed by the gasdermin pore-forming family of proteins.
Canonical inflammasome signaling can activate pyroptosis through sensing of potentially injurious agents, including bacteria, toxins, viruses, ATP, crystalline material, and DAMPs. Inflammasome sensors, NLRs, AIM2 or pyrin, assemble into large multimeric protein complexes alongside adaptor proteins such as ASC and effectors such as caspase-1. Activation of the inflammasome results in caspase-1 cleavage of GSDMD, releasing the pore-forming N-terminal effector of pyroptosis. IL-1β and IL-18 are also processed by caspase-1, and released through GSDMD pores. Caspase-11/4/5 activation downstream of cytosolic lipopolysaccharide (LPS) represents the noncanonical pathway to GSDMD cleavage and pyroptosis, though in this instance, cytokines are not processed. Alternatively, ligation of death receptors or TLRs by their cognate ligands, can signal caspase-8 activation. Caspase-8 can then trigger GSDMC/D/E pore formation, though a caspase-3 intermediate step is required for GSDME processing. Chemotherapy-induced pyroptosis has been reported to occur in a similar manner, with BAX and BAK activation followed by caspase-3/7 resulting in GSDME pore formation. Streptococcus-associated protein SpeB and Granzyme A of natural killer cells and cytotoxic T lymphocytes have been shown to trigger GSDMA and GSDMB, respectively, to initiate pyroptosis. The N-terminal of GSDMB also has the propensity to directly target and lyse bacteria, including Shigella, E. coli, Citrobacter and Salmonella.
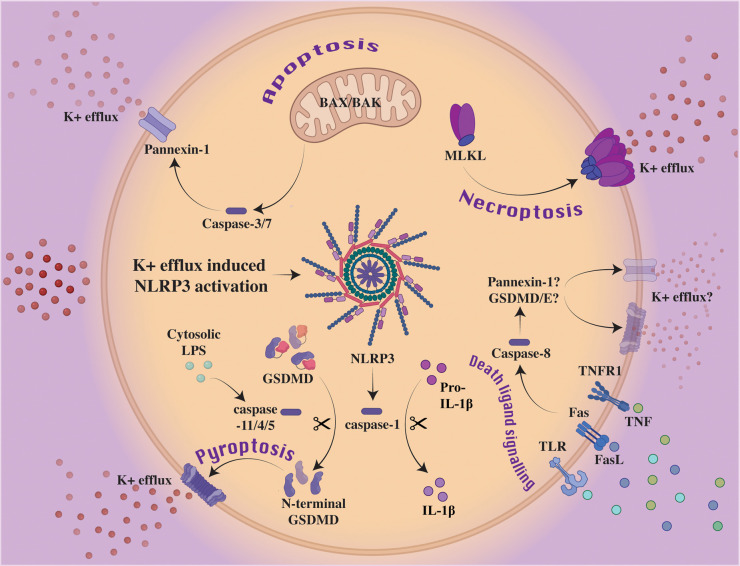
Figure 3. The NLRP3 inflammasome is activated downstream of several cell death signaling pathways via potassium ion efflux.
Classically, NLRP3 processes caspase-1 to support its subsequent cleavage of GSDMD and the cytokines IL-1β and IL-18. Potassium ion efflux is a common determinant required to activate NLRP3 in nearly all circumstances. Non-canonical inflammasome triggering allows for potassium ion efflux via GSDMD pores, which causes NLRP3 oligomerization and signaling. Similarly, downstream of necroptotic MLKL oligomerization and membrane permeabilization, potassium ion efflux has also been reported to activate NLRP3. Proapoptotic proteins, BAX and BAK, are known to trigger similar potassium ion efflux in a caspase-3/7 and pannexin-1 channel dependent manner. The potential role of pannexin-1 and/or GSDMD/E in activating NLRP3 downstream of death ligand signaling to capase-8 remains a question of interest.
GSDMD pore function: more than just cell death
A number of studies have now identified diverse cellular functions for the GSDMD pore. In neutrophils, non-canonical inflammasome activity and GSDMD targeting of the nuclear membrane results in neutrophil extracellular trap formation to control Salmonella infection [80], while in macrophages it has been reported that both non-canonical and canonical inflammasome signaling and GSDMD pore formation causes calcium ion influx to allow for IL-1α maturation [81]. GSDMD activation and potassium ion efflux downstream of dsDNA-AIM2 inflammasome activation can also act to inhibit dsDNA-induced cGAS/STING signaling, thereby limiting type I interferon (IFN) production [82,83]. This function of GSDMD was reported to occur independent of pyroptosis, and the specific increase in IFNβ production, not bacterial numbers, in AIM2 deficient animals increased their susceptibility to lethal Francisella novicida infection. Mechanistically, it was shown that GSDMD loss and maintenance of intracellular potassium ion levels allowed increased cGAS oligomerization and enzymatic activity, and this was a direct result of potassium ions promoting cGAS catalytic activity. In contrast, GSDMD mitochondrial membrane targeting following non-canonical inflammasome activation has been reported to release mitochondrial DNA (mtDNA) into the cytoplasm, to activate cGAS and suppress endothelial cell proliferation, and hence regeneration, following lung injury [84]. Interestingly, in addition to nuclear GSDMD targeting in neutrophils, the N-terminal of GSDMD has also been reported to preferentially accumulate in primary granules and autophagosomes, as opposed to the plasma membrane [85]. This distinct intracellular targeting of neutrophil GSDMD may therefore limit neutrophil pyroptosis and can induce autophagy-dependent IL-1β secretion [85,86]. The release of granule stored neutrophil elastase into the cytosol can also act on GSDMD to generate a N-terminal pore-forming fragment which, in this case, was associated with neutrophil death and, therefore, the loss of GSDMD increased neutrophil levels and the hosts ability to combat extracellular E. coli infection [13]. More recently, intestinal epithelial cell GSDMD was shown to mediate goblet cell mucin secretion independent of cell death, and as such GSDMD was important in the formation of the mucus layer [87]. Therefore, GSDMD pores may serve several purposes beyond cell death and IL-1β release.
Death receptor and caspase-8 activation of GSDMD to drive pyroptosis
Despite being classified as an apoptotic initiator caspase, caspase-8 has been described to function similarly to caspase-1 [88]. Following TLR or death receptor triggering, or fungal-derived β-glucan activation of dectin-1, activated caspase-8 can cleave precursor IL-1β into its bioactive fragment at the same site as caspase-1 [89–94]. Similarly, caspase-1 has a reported capacity to cleave Bid into its pro-apoptotic fragment, tBid, to drive BAX and BAK activation and mitochondrial apoptosis, a function typically assigned to caspase-8 [95–97] (Figure 4). Finally, recent studies have shown that caspase-8, like caspase-1 and -11, can also directly cleave GSDMD into its N-terminal pore-forming fragment to trigger pyroptosis; upon TNF-induced lethality, deletion of the caspase-8 inhibitor cFLIP or following Yersinia infection and/or the inhibition of NF-κB signaling [78,98–100] (Figure 2). On the other hand, catalytically inactive caspase-8 generated through gene editing, which cannot process GSDMD, appears to act in a scaffolding capacity to engage ASC and caspase-1 to cause spontaneous lethality in mice, and this even occurs on an MLKL deficient background (to prevent the necroptotic activity that is unleased by a loss of caspase-8 catalytic function) [101,102]. While GSDMD deficiency did not confer a survival advantage upon loss of caspase-8 catalytic activity, this may be a result from catalytically inactive caspase-8 still being able to drive caspase-1-mediated apoptotic caspase-3 and -7 activation.
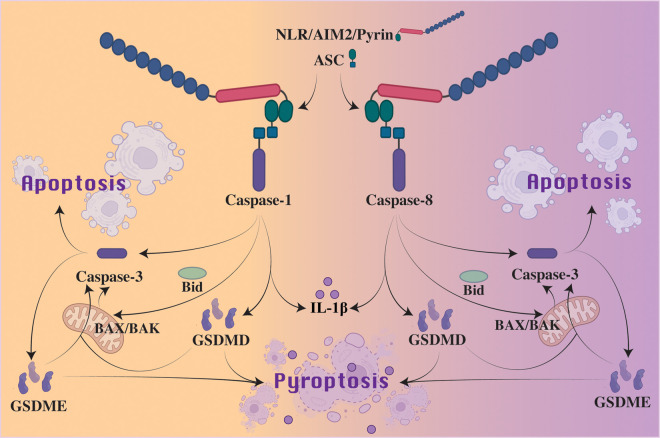
Figure 4. Cross-talk between apoptosis and pyroptosis following inflammasome activation.
Effectors recruited to the inflammasome complex, such as caspase-1 and caspase-8, prompt strikingly similar downstream signaling. Cross-talk between caspase-3 and caspase-1 or -8 following inflammasome activation can induce apoptosis. Through inflammasome-mediated caspase-1 or caspase-8 cleavage of Bid, some studies have suggested that inflammasomes can initiate BAK and BAX signaling to activate caspase-3 to bring about apoptosis. Caspase-8 has also been shown to directly cleave GSDMD, thus curtailing apoptosis to induce pyroptosis. Furthermore, when activated by caspase-1 or caspase-8, caspase-3 can cleave GSDME to release N-terminal pores and initiate pyroptosis. Activated GSDMD and GSDME may also directly target mitochondrial membranes to induce a non-canonical mitochondrial apoptosis that results in apoptosome formation, and hence initiate a feedback caspase-3 amplification loop. It should be noted that in commonly studied inflammasome-responsive cell types, such as macrophages, the primary cell death observed from inflammasome sensor engagement is caspase-1-driven GSDMD activation and pyroptosis. However, alternate cell death pathways are often engaged upon (i) prolonged inflammasome signaling, (ii) reduced levels of activating stimuli (e.g. a lower level of cytosolic DNA has been shown to engage AIM2-mediated caspase-8 activation and apoptosis), or (iii) altered levels of relevant cell death effectors, such as loss, or reduced expression, of caspase-1 or GSDMD.
Apoptotic activation of pyroptosis by caspase-3 cleavage of GSDME
Both death receptor and intrinsic mitochondrial (BAX and BAK-dependent) apoptosis converge on the activation of the apoptotic effector caspases, caspase-3, -6, and -7. While these caspases can dismantle the cell to limit its immunogenic potential, caspase-3 has recently been documented to cleave (at 267 DMPD 270) and activate GSDME (or DFNA5, deafness autosomal dominant 5), a gene implicated in hearing loss and often silenced in cancer [103,104]. Consequently, caspase-3 triggering of GSDME can cause pyroptosis downstream of viral, chemotherapy-induced mitochondrial, or death receptor-mediated, apoptotic signaling (Figure 2) [103,105]. In mammals, the expression levels of GSDME likely dictate whether this pyroptosis follows apoptotic signaling events (historically referred to as secondary necrosis), or is preferentially engaged upon caspase-3 activation [103,105]. Therefore, it will be important that future studies examining the physiological consequences of caspase-3 and -7-mediated cell death also examine the potential role of GSDME in generating the relevant phenotype, either as the primary driver of cell death, or through initiating the secondary necrosis that can succeed apoptosis.
The pyroptotic function of GSDME is evolutionarily conserved in coral, where coral infection with Vibrio coralliilyticus can trigger caspase-3 and GSDME to cause cell death [32]. A role for GSDME-induced pyroptosis and bactericidal activity in the marine teleost Cynoglossus semilaevis has also been proposed through its cleavage by caspase-1 and to a lesser extent caspase-3/7, with preservation of caspase-1 cleavage of GSDME being observed in many teleost species [106]. Furthermore, while GSDMA-GSDMD orthologs appear restricted to mammals, phylogenetic analysis of GSDME in invertebrates, including hydra, molluscs, brachiopods, corals, and sea anemones indicates a closer relation to mammalian DFNB59 (or PJVK) which, unlike other mammalian gasdermins, does not cause pyroptosis when the N-terminal fragment is expressed in cell lines [31]. Recent work reported a gasdermin N-terminal homolog, regulator of cell death 1 (RCD1), in the filamentous fungi Neurospora crassa, which like gasdermins localizes to peripheral cell margins, interacts with phospholipids including cardiolipin and when expressed in 293T kidney cells, can induce a pyroptosis-like death [107]. Although these results illustrate similarities in cell death execution proteins between the fungal and animal kingdoms, the level of RCD1 sequence identity with mammalian gasdermins has been disputed, and the lack of Rcd genes in organisms beyond fungi and bacteria have led others to suggest that RCD1 should not be considered a gasdmerin family member [31]. Regardless, it appears that, at least functionally, gasdermin-like pore-forming proteins predate the metazoan era.
Although mammalian GSDMD loss prevents non-canonical inflammasome triggered cell death, it limits, but does not abrogate, cell death induced by canonical inflammasomes, including NLRP3, NLRC4, NLRP1 and AIM2. This appears to be a result of the ability of ASC to either (i) recruit caspase-8 and thereby engage caspase-3, largely when caspase-1 or GSDMD activity is lost [108–113], or (ii) trigger caspase-1 activation of caspase-3 [96,113,114] (Figure 4). The activation of caspase-3 by canonical inflammasome activity can, in some circumstances, result in GSDME processing and pyroptotic cell death. For example, in LPS or TNF challenged NLRP3 autoactivating mice, high levels of GSDMD-independent IL-1β and IL-18 release is observed, which largely results from GSDME activity [115]. Alternatively, as observed for the NLRC4 inflammasome, caspase-8 recruitment and activation does not always cause GSDME-driven pyroptosis [109], which may reflect limited GSDME expression levels or the ability of NINJ1 to cause cell lysis in its absence [116].
Interestingly, in caspase-1 deficient cells Francisella novicida mediated AIM2-caspase-8 inflammasome-induced cell death was reduced by inhibiting mitochondrial BAX and BAK activation [117]. This finding is similar to that reported in a more recent study showing that inflammasome mediated caspase-1 and caspase-8 triggering of Bid could cause mitochondrial membrane permeabilization, with subsequent caspase-3 activation inducing cell lysis when GSDMD was genetically deleted [97]. While no defect in cell lysis was observed upon GSDME deletion (possibly reflecting NINJ1 activity), the results largely mirror a recently described caspase-1-driven pyroptosis, whereby cleavage of Bid is mediated by caspase-1, with consequent mitochondrial apoptotic triggering and downstream caspase-3 activation of GSDME [96]. Notably, this pyroptotic cell death was implicated in cell types that naturally express low levels of GSDMD, such as cortical neurons and mast cells, and speculatively, may also take place upon pathogen-mediated inhibition of GSDMD [46,47,49]. While the induction of mitochondrial apoptosis resulting from caspase-8 or caspase-1 signaling result, at least in part, from caspase-8 or caspase-1 cleavage of Bid to activate BAX and BAK, it is worth noting that caspase-8 can also promote BAX and BAK activity independent of Bid, by regulating the transcription of Bcl-2 family members [14]. Whether caspase-1 may also control gene transcription [118,119], akin to caspase-8 processing of N4BP1 to control TLR-induced transcriptional activity [120], will be of interest to determine.
Despite the above findings, the consequences of GSDME deletion to chemotherapy responses and cellular homeostasis in infection, remain to be fully explored. However, recent research highlights a potential role for caspase-3 and GSDME in causing pyroptosis in eye diseases-associated with all trans-retinal-mediated photoreceptor degeneration [121]. Emerging studies also indicate that GSDME expression in cancer cells can in some [105], but not all [122], situations enhance anti-tumor immune responses to limit tumor growth, or can drive neutrophil IL-1β release and pyroptosis downstream of RIPK1 and caspase-3 activation to protect the host from infection by the gram negative bacterial pathogen Yersinia [123]. On the other hand, GSDME-induced pyroptosis and the consequent inflammatory response contributes to H7N9 influenza-mediated lethality in mice [124]. Recent findings also correlate GSDME levels and activation in patient intestinal mucosa or synovial immune cells with Crohn's disease and rheumatoid arthritis severity, respectively, and mice deficient in GSDME were protected from chemical-induced (2,4,6-trinitrobenzenesulfonic acid) colitis or collagen-induced arthritis [125,126]. These studies highlight how an excess of GSDME-driven cell death can cause pathological inflammatory responses.
Death receptor induced pyroptosis via activation of GSDMC
Recent evidence has linked the death receptor initiator caspase, caspase-8, to the cleavage of GSDMC and pyroptosis (Figure 2). Under hypoxic conditions the immune checkpoint inhibitor PD-L1 (programmed death ligand 1) was reported to interact with phosphorylated STAT3 in cancer cells to induce the transcription of GSDMC [127]. Consequently, upon TNF-induced caspase-8 activation, caspase-8 cleaved GSDMC at 362 LELD 365 to release a pore-forming N-terminal domain causing cancer cell pyroptosis. Although cleaved by caspase-8, GSDMC was not cleaved by the highly homologous death receptor initiator caspase, caspase-10, which, while not present in mice, in humans can act like caspase-8 to cleave caspase-3 and -7 downstream of death receptor activation. Tumor associated macrophages were identified as a source of TNF in vivo able to trigger GSDMC-driven cancer cell pyroptosis although, unlike other studies implicating pyroptosis as triggering cancer cell immunogenicity [105,128], increased GSDMC-expression has been linked to promoting tumor growth and/or inhibiting anti-tumor immune responses [127,129]. The metabolite α-ketoglutarate was also suggested to cause caspase-8-mediated GSDMC processing and pyroptosis in cancer cells via death receptor-6 signaling, although in this case GSDMC expression was beneficial in decreasing tumor size and metastasis (following α-ketoglutarate treatment) in murine xenograft cancer models [130]. Whether hypoxia or α-ketoglutarate can induce innate immune cell GSDMC expression to alter death receptor signaling responses outside of the cancer setting, or how GSDMC deficient mice (four murine GSDMC orthologues exist) respond to genetically and/or chemically induced cancer development, or infection, remains to be full explored. However, the gastrointestinal roundworm Nippostrongylus brasiliensis, the tricarboxyclic acid cycle (TCA) intermediate succinate, and the type 2 cytokines, IL-4 and IL-13, can increase GSDMC expression in mouse intestinal organoids, and pore-forming GSDMC is detected in the intestine of mice infected with N. brasiliensis, suggesting GSDMC-induced pyroptosis may regulate intestinal cell death resulting from type 2 immune responses [131].
Granzyme A processing of GSDMB to cause pyroptosis
Although GSDMB has no orthologs in mice, which makes the analysis of its physiological function more difficult, GSDMB was initially shown to cause pyroptosis when its N-terminal domain was expressed in 293T cells [43]. More recently it was demonstrated that natural killer cells and cytotoxic T lymphocytes release Granzyme A into target cells via perforin, resulting in Granzyme A cleavage of GSDMB at K244 (and K229) into its pore-forming fragment to cause target cell pyroptosis [132] (Figure 2). Interestingly, IFNγ, type I IFNs and to a lesser extent TNF, could induce GSDMB expression in some cancer cell lines, and IFNγ treatment could thereby confer susceptibility to Granzyme A-driven pyroptosis in vitro, while the expression of GSDMB in tumor cells facilitated tumor clearance in vivo following immune checkpoint blockade [132]. The delivery of Granzyme A into host cells containing bacteria was also shown to activate GSDMB, but in this case GSDMB was suggested to directly target bacterial membranes enriched in the lipid cardiolipin (e.g. Shigella, E. coli, Citrobacter and Salmonella) to cause bacterial lysis independent of host cell death, and that pore-forming GSDMB was comparatively inefficient at binding mammalian liposomes [133]. The Shigella effector, IpaH7.8 was also shown to target GSDMB for proteasomal degradation [133], akin to its reported targeting of human (but not mouse) GSDMD [46], indicating that pathogens may target multiple gasdermin family members at the same time to shut down a cells pyroptotic capacity. It has been suggested that IpaH7.8 targets GSDMB but not GSDMD for degradation [133], though this conclusion may be attributed to findings demonstrating that epitope tagging of the N-terminal of GSDMD disrupts degradation by IpaH7.8 [46]. However, other incongruities in the GSDMB literature remain to be resolved, including reports that the N-terminal of GSDMB does not bind cardiolipin, but preferentially binds phosphoinositides or sulfatide [134], and that GSDMB does not directly cause pyroptosis, but associates with caspase-4 to promote its activity and non-canonical inflammasome mediated cell death [135].
GSDMB polymorphisms and/or expression levels have been associated with allergic and inflammatory diseases, such as asthma and inflammatory bowel disease (IBD) [134–141], with some disease-associated GSDMB variants linked to decreased activity [142,143]. Mice engineered to express human GSDMB develop an asthma phenotype [139], while in IBD increased GSDMB expression in colonocytes was reported to promote intestinal epithelial repair independent of its pyroptotic signaling capacity [143]. GSDMB expression can also correlate with a positive or negative prognosis depending on the cancer type [132,144]. Whether GSDMB cell death-independent activity might contribute to GSDMB-associated cancer cell growth in some contexts [145,146] remains to be fully investigated, although it has been speculated that GSDMB binding to signaling lipids may impart non-pyroptotic functions [134].
Bacterial activation of GSDMA to trigger pyroptotic death
The N-terminal of GSDMA3 (three homologs of GSDMA exist in mice) forms pores with a diameter of ∼180 Å and, similar to GSDMD, GSDMA binds phosphoinositides and cardiolipin [40,43,147]. Recent research has uncovered how GSDMA, which is highly expressed in the skin epithelia, is activated by group A Streptococcus (GAS) via the bacterial protease SpeB, which processes GSDMA after Q246 in the linker region to unleash the N-terminal pore-forming fragment [148] (Figure 2). Mice lacking GSDMA1 showed increased systemic infection and mortality upon infection with GAS, highlighting the important role of GSDMA in sensing pathogen virulence factors to protect the host. Whether GSDMA is cleaved by other microbial or host cell proteases, and if GSDMA variants linked to autoimmune diseases with skin pathologies, such as systemic sclerosis [149], alter GSDMA activity, will be of significant interest to determine.
Tuning pyroptotic activity and cell lysis via host cell factors: apoptotic caspases, free radicals, membrane shedding and NINJ1
The intricate control of inflammasome signaling and other cell death modalities is required to prevent pathogen infections and inflammatory diseases [150,151]. As such, it comes as no surprise that pyroptotic cell death and cell lysis are tightly controlled at the genetic level, and can be evaded or even overridden, thus highlighting the essential role of its regulation in responding to cellular threats while attempting to limit collateral damage to the host.
Reactive oxygen species (ROS) have long been implicated in activating the NLRP3 inflammasome, although its exact roles are still debated [152]. More recently, the loss of glutathione peroxidase 4 (GPX4), which acts to prevent free radical generation and lipid peroxidation, was shown to promote caspase-11 and GSDMD-driven pyroptosis in a murine sepsis model. Lethality was reduced by administration of the antioxidant vitamin E or the deletion of caspase-11 and GSDMD but, perhaps surprisingly, not by the inhibition of ferroptotic cell death (a caspase-independent cell death often triggered by GPX4 removal and overwhelming lipid peroxidation) [153]. GPX4 deficiency increased both canonical and non-canonical inflammasome activation of caspase-1 and caspase-11, respectively, to enhance GSDMD processing. Notably, pyroptosis caused by expression of the pore-forming N-terminal domain of GSDMD alone was also exacerbated in GPX4 deficient macrophages, and this effect was blocked by vitamin E supplementation, or the targeting of phospholipase C (PLC) and its ability to initiate calcium signaling via hydrolysis of PIP2. These findings support the notion that increased free radical production is a significant driver of GSDMD-mediated pyroptosis, and that GSDMD activity at the plasma membrane may be facilitated by phospholipid peroxidation and calcium mobilization.
Mitochondrial ROS have also been implicated in pyroptosis. It has been suggested that mitochondrial ROS oxidize four cysteine residues of GSDMD (Cys38, 56, 268 and 367) to facilitate caspase-1 cleavage of GSDMD [154], while recent work indicates that mitochondrial ROS also promote N-terminal GSDMD oligomerization at the plasma membrane [155]. In the later study, a genetic screen was conducted to identify factors that are required to prevent pyroptotic cell death upon inducible expression of the pore-forming N-terminal fragment of GSDMD. This identified the Ragulator-Rag complex, previously implicated in metabolic sensing as well as lysosome and mitochondrial homeostasis, as stimulating GSDMD-mediated killing. The deletion of the Ragulator-Rag complex components, the GTPases RagA, RagB or RagC, and Raptor, a component of the mTORC1 complex that is activated by Ragulator-Rag signaling, reduced N-terminal GSDMD-mediated cell death upon its expression, or NLRC4 inflammasome activation and pyroptosis, by ∼50%. Notably, GSDMD oligomerization, but not GSDMD cleavage nor membrane localization, was reduced upon the loss of Ragulator-Rag signaling, and this was shown to result from a protection from N-terminal GSDMD-mediated decreases in mitochondrial membrane potential and increased ROS generation. Therefore, these findings suggest that cleaved GSDMD activates the Ragulator-Rag complex and mTORC1 to induce mitochondrial ROS, which subsequently facilitates plasma membrane GSDMD oligomerization and pore formation. Intriguingly, and in contrast with these findings, in a screen for genes involved in either caspase-8- or caspase-11-dependent cell death, it was reported that the Ragulator-Rag complex was not required for either canonical or non-canonical driven pyroptosis, but was required for caspase-8-mediated macrophage killing via its assembly of the death-inducing RIPK1 and caspase-8 complex on lysosomal membranes [156]. The reasons for these opposing findings will be important to evaluate. Regardless, a significantly altered cellular metabolism resulting from GSDMD activity is consistent with a single cell analysis indicating that GSDMD may initiative several cellular events, such as ion channel opening, calcium influx, and mitochondrial depolarization, prior to a loss of plasma membrane integrity [157]. Whether other gasdermins may impart similar changes to cellular homeostasis, and are also regulated by free radical production, remains to be tested.
Rupture of the plasma membrane upon gasdermin pore formation was considered a passive process until recent work by Kayagaki et al. [116] demonstrated that a conserved extracellular α-helix of NINJ1 was a mediator of cell lysis downstream of pyroptosis- and apoptosis-induced cell death. Although NINJ1-deficient bone marrow derived macrophages display attenuated LDH release (a measure of cell lysis), NINJ1 was dispensable in the formation of GSDMD pores, cell death and the release of IL-1β and IL-18 via canonical and non-canonical inflammasome signaling. Notably, NINJ1 deficient mice were susceptible to Citrobacter-mediated lethality, thereby implicating this cell lysis in protection from bacterial infection. Similarly, Bjanes et al. [158] noted significantly reduced NINJ1 expression protected macrophages from lytic death, including forms induced by canonical and non-canonical inflammasome signaling. NINJ1 has also been documented to mediate membrane rupture and efficient LDH release following a caspase-8 and inducible nitric oxide synthase (iNOS)-mediated cell death caused by IFNγ and pathogen-ligand TLR signaling [14]. It is tempting to speculate that NINJ1 may therefore contribute to the damaging caspase-8- and iNOS-driven host response observed following SARS-CoV-2 infection [14], however how NINJ1 causes cell lysis requires further study.
Recently, Santa Cruz Garcia and colleagues provided evidence that GSDMD pores are capable of both opening and closing in a phosphoinositide-dependent manner to mediate calcium influx, potassium ion efflux and, with sustained pore opening, bring about cell death [159]. Interestingly, as highlighted by the impact of GPX4 deficiency on lipid peroxidation, PLC activity and GSDMD pore formation, these results further suggest the potential for lipid membrane dynamics to play a regulatory role in pyroptosis, as opposed to merely offering membrane support to GSDMD pores, and should stimulate further research. Phosphoinositides are also suggested recruiters of the endosomal sorting complex required for transport (ESCRT) machinery for membrane repair, which has been identified as a negative regulator of necroptosis by facilitating the shedding of MLKL damaged membranes [160]. Similarly, Rühl et al. [161] observed that upon LPS delivery into the cytosol of macrophages, calcium influx occurred in a GSDMD and caspase-11 dependent manner, which was able to recruit the ESCRT machinery to the damaged plasma membrane for shedding through bleb formation, to avoid pyroptosis. While the role of phosphoinositides in the regulation of this process was not evaluated, the potential for some cells to evade death after GSDMD activation may be attributed to phosphoinositide regulation of GSDMD pore and calcium ion dynamics. When taken with reports that phosphoinositides contribute to membrane budding and fusion [162], the ‘bubbling’ morphology that is characteristic of inflammasome-induced pyroptosis could be explained by overwhelming recruitment of the ESCRT machinery to the plasma membrane in an attempt to override cell death, although this requires further study. Other regulators capable of blocking gasdermin dependent pyroptosis have been identified, including apoptotic caspase-3 cleavage and inactivation of GSDMB and GSDMD, and succination of GSDMD by fumarate (an intermediate product of the TCA cycle) [134,163,164], while some evidence exists to suggest that GSDME phosphorylation on tyrosine six blocks its capacity to cause pyroptosis by preventing N-terminal domain oligomerization [165]. The notion that pyroptosis is a passive process with inescapable consequences is antiquated and, therefore, means of its external regulation and potential self-regulation should be explored further.
Mitochondrial apoptotic BAX and BAK triggering of inflammasome activity and pyroptosis
Mitochondrial apoptosis is executed by BAX and BAK oligomerization and permeabilization of the mitochondrial outer membrane. This allows cytochrome c release, which nucleates the formation of the caspase-9 apoptosome that enables caspase-3 and -7 cleavage and activation, thereby promoting cellular disassembly and clearance in a manner that limits the leakage of immunogenic content [166]. The activity of BAX and BAK is tightly controlled by BCL-2 family members and, in the case of macrophages, pro-survival BCL-2, BCL-xL, MCL-1 and A1 have all been documented as playing important roles in restraining BAX and BAK activation [2,14,167,168].
Despite the assumption that BAX and BAK cause an immunologically silent cell death, recent studies have delineated how BAX and BAK signaling can feed into NLRP3 inflammasome activation and pyroptosis [5,167–172]. Using a cyclic peptolide from myxobacteria, vioprolide A, BCL-2 antagonists (BH3-mimetics) and protein synthesis inhibitors, it was identified that BAX and BAK-triggering result in caspase-3 and -7-mediated potassium ion efflux and NLRP3 activation, in addition to direct caspase-8-induced IL-1β proteolysis [167,168]. Subsequently, it was shown that caspase-3 and -7 activation of the pannexin-1 channel is required for mitochondrial apoptosis-driven NLRP3 signaling [171,172] (Figure 3). The relevance of these in vitro genetic and biochemical experiments was highlighted by the discovery that bacterial outer membrane vesicles derived from pathogenic bacteria such as Neisseria gonorrhoeae can trigger mitochondrial apoptosis to activate NLRP3 and drive inflammatory IL-1β responses when injected into mice [169]. Similarly, iNOS and caspase-8 can induce a BAX and BAK dependent cell death that is likely to cause damaging immune responses following SARS-CoV-2 infection in mice [14].
Although mitochondrial cell death may cause damaging inflammation in some circumstances, mitochondrial apoptosis is a potent mechanism by which host cells can facilitate clearance of intracellular bacterial and viral infections to prevent lethality in murine models of disease [2,4]. Importantly, mitochondrial apoptotic triggering of pyroptosis is likely conserved in humans as it was recently suggested that, in human skin organoids, mitochondrial-driven caspase-3 and GSDME-mediated pyroptosis blocks the replication of viruses such as herpes simplex virus 1 and vesicular stomatitis virus [5]. Therefore, BH3-mimetics, which have been developed to trigger mitochondrial apoptosis in cancer cells, represent an interesting new approach in the therapeutic treatment of intracellular pathogens, and the above studies indicate that they may cause cell death of infected cells via both mitochondrial apoptotic and pyroptotic signaling
In an interesting twist, research has also uncovered how pore-forming GSDMD and GSMDE can directly target mitochondrial membranes [84,165] (Figure 4). Activated GSDMD and GSDME were reported to interact with and permeabilize mitochondrial membranes to induce the apoptosome downstream of both mitochondrial and death receptor-mediated apoptotic caspase-3 activation, thereby contributing to classical caspase-9 apoptotic signaling [165]. As such, like the feedback-augmentation of apoptotic caspase activity [173], a feedback amplification loop is established whereby GSDME is not only is activated by caspase-3, but also contributes to apoptotic caspase-3 activation via a non-canonical, gasdermin-driven, mitochondrial apoptotic pathway.
Necroptosis triggering of inflammasome activity
In contrast with pyroptosis and apoptosis, necroptosis is a lytic form of programmed death that is caspase independent and is strictly defined by its requirement for Mixed lineage kinase domain like pseudokinase (MLKL)-mediated membrane damage. Caspase-8 acts as a molecular switch for necroptotic cell death and is essential for inhibiting spontaneous necroptosis in mice [174–176]. As such, necroptosis is thought to have evolved as a host defence against pathogens, specifically those that have developed anti-apoptotic strategies [177]. Following ligand engagement of death receptors or TLRs, caspase-8 complexed with Fas-associated death domain (FADD) can induce extrinsic apoptosis. The TLR adaptor protein TRIF and inhibitor of apoptosis proteins (IAPs; X-linked IAP, cellular IAP1 and IAP2) contribute to this apoptotic death by regulating the ubiquitylation of Receptor interacting protein kinase 1 (RIPK1) and RIPK3 [178,179]. However, upon decreased caspase-8 functioning necroptosis ensues, whereby homotypic interactions between the RIP homotypic interaction motifs (RHIMs) of RIPK1 and the necroptotic effector, RIPK3, initiate RIPK3's phosphorylation of MLKL. Phosphorylation of MLKL drives the oligomerization of its N-terminal and plasma membrane insertion to execute a lytic cell death, being necroptosis [17]. The regulation of necroptosis is tightly controlled at the post-translational level by caspase-8, the deubiquitinases CYLD and A20, and several E3 ubiquitin ligases [180]. Regulation of this lytic death is critical given the release of DAMPs, such as High Mobility Group Box 1 (HMGB1), ATP, IL-33, IL-1α, S100a9 and self-DNA, induce inflammation in surrounding cells [181].
Inflammasome activation downstream of necroptosis is not completely understood and while roles for key necroptotic mediators, RIPK3 and MLKL, have been proposed, further research is required to clarify the different stimuli and cellular contexts that regulate activation, and elucidate its physiological significance. Some of the documented DAMPs released by necroptotic cell lysis have been proposed to induce inflammasome activation [182,183]. However, several studies have also shown that MLKL activity can mediate inflammasome activation downstream of TLR or death receptor signaling [184–186], and that this is caused by potassium ion efflux resulting from MLKL-induced membrane damage, which allows for NLRP3 activation in a cell-intrinsic manner [184] (Figure 3). MLKL triggering of NLRP3 is thereby similar to how GSDMD pores and potassium ion efflux activate NLRP3 following non-canonical inflammasome activation. However, unlike GSDMD being required for efficient IL-1β release following canonical and non-canonical inflammasome signaling, MLKL-driven membrane damage allows the efflux of activated IL-1β from cells upon necroptosis even when GSDMD is deleted [184,185].
The pathological in vivo relevance of necroptotic-driven NLRP3 activity is highlighted by the fact that dendritic cell caspase-8 deletion confers susceptibility to endotoxic shock, mediated by RIPK3 and MLKL driven NLRP3-IL-1 activation, but not TNF production [187]. Similarly, A20 deficiency, which causes inflammatory arthritis, results in increases in RIPK1-RIPK3 complexes, RIPK3 and MLKL-mediated NLRP3 inflammasome assembly and unchecked auto-inflammation associated with IL-1β activation [188–192]. Further studies are required to define if IBD and/or arthritis resulting from the loss of other inhibitors of necroptotic cell death, specifically RIPK1 and caspase-8, might drive pathological MLKL-mediated NLRP3 inflammasome responses in humans [193–195].
Concluding remarks
There is growing evidence supporting the interplay between inflammasome activation and diverse cell death modalities [17,88,196]. Recent work has highlighted the importance of gasdermin family proteins downstream of the inflammasome in mediating pyroptotic cell death, the release of inflammatory cytokines and, by extension, a range of autoinflammatory, infectious and tumorigenic disease states. The potentially interchangeable roles of caspase-1 and caspase-8 in inflammasome signaling offers insight into the complex relationship between pyroptosis and apoptosis, with both pathway intermediates such as caspase-3 and effectors such as GSDMC, GSDMD and GSDME being utilized to steer cells down the various roads to death. Further study of the plasticity of cell death downstream of inflammasome activation, as well as apoptosis and necroptosis, is thus of great importance in understanding the interconnectedness, and potential distinctions, in death signaling, and in informing therapeutic targets of innumerable diseases.
Acknowledgements
This work was supported by an Australian National Health and Medical Research Council (NHRMC) investigator grant to J.E.V. (2008692).
Abbreviations
- AIM2
Absent in melanoma 2
- CARD
caspase recruitment domain
- DAMPs
damage-associated molecular patterns
- ESCRT
endosomal sorting complex required for transport
- GAS
group A Streptococcus
- GPX4
glutathione peroxidase 4
- GSDMD
gasdermin D
- HMGB1
high mobility group box 1
- IBD
inflammatory bowel disease
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- PAMPs
pathogen-associated molecular patterns
- PIP2
phosphatidylinositol 4,5-biphosphate
- PJVK
Pejvakin
- PLC
phospholipase C
- PRRs
pattern recognition receptors
- PYD
pyrin domain
- RCD1
regulator of cell death 1
- RIPK1
receptor interacting protein kinase 1
- ROS
reactive oxygen species
- TCA
tricarboxylic acid
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Nozaki, K., Li, L. and Miao, E.A. (2022) Innate sensors trigger regulated cell death to combat intracellular infection. Annu. Rev. Immunol. 40, 469–498 10.1146/annurev-immunol-101320-011235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Speir, M., Lawlor, K.E., Glaser, S.P., Abraham, G., Chow, S., Vogrin, A.et al. (2016) Eliminating legionella by inhibiting BCL-XL to induce macrophage apoptosis. Nat. Microbiol. 1, 15034 10.1038/nmicrobiol.2015.34 [DOI] [PubMed] [Google Scholar]
- 3.Naderer, T. and Fulcher, M.C. (2018) Targeting apoptosis pathways in infections. J. Leukoc. Biol. 103, 275–285 10.1189/JLB.4MR0717-286R [DOI] [PubMed] [Google Scholar]
- 4.Suzuki, T., Okamoto, T., Katoh, H., Sugiyama, Y., Kusakabe, S., Tokunaga, M.et al. (2018) Infection with flaviviruses requires BCLXL for cell survival. PLoS Pathog. 14, e1007299 10.1371/journal.ppat.1007299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orzalli, M.H., Prochera, A., Payne, L., Smith, A., Garlick, J.A. and Kagan, J.C. (2021) Virus-mediated inactivation of anti-apoptotic Bcl-2 family members promotes Gasdermin-E-dependent pyroptosis in barrier epithelial cells. Immunity 54, 1447–62.e5 10.1016/j.immuni.2021.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doerflinger, M., Deng, Y., Whitney, P., Salvamoser, R., Engel, S., Kueh, A.J.et al. (2020) Flexible usage and interconnectivity of diverse cell death pathways protect against intracellular infection. Immunity 53, 533–47.e7 10.1016/j.immuni.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebert, G., Lopaticki, S., O'Neill, M.T., Steel, R.W.J., Doerflinger, M., Rajasekaran, P.et al. (2020) Targeting the extrinsic pathway of hepatocyte apoptosis promotes clearance of Plasmodium liver infection. Cell Rep. 30, 4343–54.e4 10.1016/j.celrep.2020.03.032 [DOI] [PubMed] [Google Scholar]
- 8.Ebert, G., Preston, S., Allison, C., Cooney, J., Toe, J.G., Stutz, M.D.et al. (2015) Cellular inhibitor of apoptosis proteins prevent clearance of hepatitis B virus. Proc. Natl Acad. Sci. U.S.A. 112, 5797–5802 10.1073/pnas.1502390112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebert, G., Allison, C., Preston, S., Cooney, J., Toe, J.G., Stutz, M.D.et al. (2015) Eliminating hepatitis B by antagonizing cellular inhibitors of apoptosis. Proc. Natl Acad. Sci. U.S.A. 112, 5803–5808 10.1073/pnas.1502400112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakkola, L., Denisova, O.V., Tynell, J., Viiliainen, J., Ysenbaert, T., Matos, R.C.et al. (2013) Anticancer compound ABT-263 accelerates apoptosis in virus-infected cells and imbalances cytokine production and lowers survival rates of infected mice. Cell Death Dis. 4, e742 10.1038/cddis.2013.267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imre, G. (2020) Cell death signalling in virus infection. Cell Signal. 76, 109772 10.1016/j.cellsig.2020.109772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doitsh, G., Galloway, N.L., Geng, X., Yang, Z., Monroe, K.M., Zepeda, O.et al. (2014) Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505, 509–514 10.1038/nature12940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kambara, H., Liu, F., Zhang, X., Liu, P., Bajrami, B., Teng, Y.et al. (2018) Gasdermin D exerts anti-inflammatory effects by promoting neutrophil death. Cell Rep. 22, 2924–2936 10.1016/j.celrep.2018.02.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson, D.S., Pang, J., Weir, A., Kong, I.Y., Fritsch, M., Rashidi, M.et al. (2022) Interferon-gamma primes macrophages for pathogen ligand-induced killing via a caspase-8 and mitochondrial cell death pathway. Immunity 55, 423–441.e9 10.1016/j.immuni.2022.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karki, R., Sharma, B.R., Tuladhar, S., Williams, E.P., Zalduondo, L., Samir, P.et al. (2021) Synergism of TNF-alpha and IFN-gamma triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell 184, 149–68.e17 10.1016/j.cell.2020.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fajgenbaum, D.C. and June, C.H. (2020) Cytokine storm. N. Engl. J. Med. 383, 2255–2273 10.1056/NEJMra2026131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank, D. and Vince, J.E. (2019) Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 26, 99–114 10.1038/s41418-018-0212-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aksentijevich, I. and Schnappauf, O. (2021) Molecular mechanisms of phenotypic variability in monogenic autoinflammatory diseases. Nat. Rev. Rheumatol. 17, 405–425 10.1038/s41584-021-00614-1 [DOI] [PubMed] [Google Scholar]
- 19.Menu, P. and Vince, J.E. (2011) The NLRP3 inflammasome in health and disease: the good, the bad and the ugly. Clin. Exp. Immunol. 166, 1–15 10.1111/j.1365-2249.2011.04440.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zychlinsky, A., Prevost, M.C. and Sansonetti, P.J. (1992) Shigella flexneri induces apoptosis in infected macrophages. Nature 358, 167–169 10.1038/358167a0 [DOI] [PubMed] [Google Scholar]
- 21.Cookson, B.T. and Brennan, M.A. (2001) Pro-inflammatory programmed cell death. Trends Microbiol. 9, 113–114 10.1016/S0966-842X(00)01936-3 [DOI] [PubMed] [Google Scholar]
- 22.Brennan, M.A. and Cookson, B.T. (2000) Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 38, 31–40 10.1046/j.1365-2958.2000.02103.x [DOI] [PubMed] [Google Scholar]
- 23.Hersh, D., Monack, D.M., Smith, M.R., Ghori, N., Falkow, S. and Zychlinsky, A. (1999) The Salmonella invasin sipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl Acad. Sci. U.S.A. 96, 2396–2401 10.1073/pnas.96.5.2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hilbi, H., Moss, J.E., Hersh, D., Chen, Y., Arondel, J., Banerjee, S.et al. (1998) Shigella-induced apoptosis is dependent on caspase-1 which binds to ipaB. J. Biol. Chem. 273, 32895–32900 10.1074/jbc.273.49.32895 [DOI] [PubMed] [Google Scholar]
- 25.Kayagaki, N., Stowe, I.B., Lee, B.L., O'Rourke, K., Anderson, K., Warming, S.et al. (2015) Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 10.1038/nature15541 [DOI] [PubMed] [Google Scholar]
- 26.Shi, J., Zhao, Y., Wang, K., Shi, X., Wang, Y., Huang, H.et al. (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 10.1038/nature15514 [DOI] [PubMed] [Google Scholar]
- 27.Shi, J., Gao, W. and Shao, F. (2017) Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 42, 245–254 10.1016/j.tibs.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 28.Galluzzi, L., Vitale, I., Aaronson, S.A., Abrams, J.M., Adam, D., Agostinis, P.et al. (2018) Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 25, 486–541 10.1038/s41418-017-0012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, Z., Busscher, B.M., Storl-Desmond, M. and Xiao, T.S. (2022) Mechanisms of gasdermin recognition by proteases. J. Mol. Biol. 434, 167274 10.1016/j.jmb.2021.167274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Schutter, E., Roelandt, R., Riquet, F.B., Van Camp, G., Wullaert, A. and Vandenabeele, P. (2021) Punching holes in cellular membranes: biology and evolution of gasdermins. Trends Cell Biol. 31, 500–513 10.1016/j.tcb.2021.03.004 [DOI] [PubMed] [Google Scholar]
- 31.Angosto-Bazarra, D., Alarcon-Vila, C., Hurtado-Navarro, L., Banos, M.C., Rivers-Auty, J. and Pelegrin, P. (2022) Evolutionary analyses of the gasdermin family suggest conserved roles in infection response despite loss of pore-forming functionality. BMC Biol. 20, 9 10.1186/s12915-021-01220-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang, S., Zhou, Z., Sun, Y., Zhang, T. and Sun, L. (2020) Coral gasdermin triggers pyroptosis. Sci. Immunol. 5, eabd2591 10.1126/sciimmunol.abd2591 [DOI] [PubMed] [Google Scholar]
- 33.Phulphagar, K., Kuhn, L.I., Ebner, S., Frauenstein, A., Swietlik, J.J., Rieckmann, J.et al. (2021) Proteomics reveals distinct mechanisms regulating the release of cytokines and alarmins during pyroptosis. Cell Rep. 34, 108826 10.1016/j.celrep.2021.108826 [DOI] [PubMed] [Google Scholar]
- 34.Broz, P. and Dixit, V.M. (2016) Inflammasomes: mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16, 407–420 10.1038/nri.2016.58 [DOI] [PubMed] [Google Scholar]
- 35.Gong, Q., Robinson, K., Xu, C., Huynh, P.T., Chong, K.H.C., Tan, E.Y.J.et al. (2021) Structural basis for distinct inflammasome complex assembly by human NLRP1 and CARD8. Nat. Commun. 12, 188 10.1038/s41467-020-20319-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finger, J.N., Lich, J.D., Dare, L.C., Cook, M.N., Brown, K.K., Duraiswami, C.et al. (2012) Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity. J. Biol. Chem. 287, 25030–7 10.1074/jbc.M112.378323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang, M., Zhang, X., Toh, G.A., Gong, Q., Wang, J., Han, Z.et al. (2021) Structural and biochemical mechanisms of NLRP1 inhibition by DPP9. Nature 592, 773–777 10.1038/s41586-021-03320-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hollingsworth, L.R., Sharif, H., Griswold, A.R., Fontana, P., Mintseris, J., Dagbay, K.B.et al. (2021) DPP9 sequesters the C terminus of NLRP1 to repress inflammasome activation. Nature 592, 778–783 10.1038/s41586-021-03350-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poyet, J.L., Srinivasula, S.M., Tnani, M., Razmara, M., Fernandes-Alnemri, T. and Alnemri, E.S. (2001) Identification of Ipaf, a human caspase-1-activating protein related to Apaf-1. J. Biol. Chem. 276, 28309–28313 10.1074/jbc.C100250200 [DOI] [PubMed] [Google Scholar]
- 40.Liu, X., Zhang, Z., Ruan, J., Pan, Y., Magupalli, V.G., Wu, H.et al. (2016) Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 10.1038/nature18629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuang, S., Zheng, J., Yang, H., Li, S., Duan, S., Shen, Y.et al. (2017) Structure insight of GSDMD reveals the basis of GSDMD autoinhibition in cell pyroptosis. Proc. Natl Acad. Sci. U.S.A. 114, 10642–7 10.1073/pnas.1708194114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xia, S., Zhang, Z., Magupalli, V.G., Pablo, J.L., Dong, Y., Vora, S.M.et al. (2021) Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 593, 607–611 10.1038/s41586-021-03478-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding, J., Wang, K., Liu, W., She, Y., Sun, Q., Shi, J.et al. (2016) Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 10.1038/nature18590 [DOI] [PubMed] [Google Scholar]
- 44.Monteleone, M., Stanley, A.C., Chen, K.W., Brown, D.L., Bezbradica, J.S., von Pein, J.B.et al. (2018) Interleukin-1beta maturation triggers its relocation to the plasma membrane for Gasdermin-D-dependent and -independent secretion. Cell Rep. 24, 1425–1433 10.1016/j.celrep.2018.07.027 [DOI] [PubMed] [Google Scholar]
- 45.Vijayaraj, S., Feltham, R., Rashidi, M., Frank, D., Liu, Z., Simpson, D.S.et al. (2021) The ubiquitylaton of IL-1beta limits its cleavage by caspase-1 and targets it for proteasomal degradation. Nat. Commun. 12, 2713 10.1038/s41467-021-22979-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luchetti, G., Roncaioli, J.L., Chavez, R.A., Schubert, A.F., Kofoed, E.M., Reja, R.et al. (2021) Shigella ubiquitin ligase IpaH7.8 targets gasdermin D for degradation to prevent pyroptosis and enable infection. Cell Host Microbe 29, 1521–30.e10 10.1016/j.chom.2021.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lei, X., Zhang, Z., Xiao, X., Qi, J., He, B. and Wang, J. (2017) Enterovirus 71 inhibits pyroptosis through cleavage of gasdermin D. J. Virol. 91, e01069-17 10.1128/JVI.01069-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao, G., Li, T., Liu, X., Zhang, T., Zhang, Z., Kang, L.et al. (2022) African swine fever virus cysteine protease pS273R inhibits pyroptosis by noncanonically cleaving gasdermin D. J. Biol. Chem. 298, 101480 10.1016/j.jbc.2021.101480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi, F., Lv, Q., Wang, T., Xu, J., Xu, W., Shi, Y.et al. (2022) Coronaviruses Nsp5 antagonizes porcine gasdermin D-mediated pyroptosis by cleaving pore-Forming p30 fragment. mBio 13, e0273921 10.1128/mbio.02739-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaoka, Y., Matsunaga, S., Jeremiah, S.S., Nishi, M., Miyakawa, K., Morita, T.et al. (2021) Zika virus protease induces caspase-independent pyroptotic cell death by directly cleaving gasdermin D. Biochem. Biophys. Res. Commun. 534, 666–671 10.1016/j.bbrc.2020.11.023 [DOI] [PubMed] [Google Scholar]
- 51.Wen, W., Li, X., Wang, H., Zhao, Q., Yin, M., Liu, W.et al. (2021) Seneca valley virus 3C protease induces pyroptosis by directly cleaving porcine gasdermin D. J. Immunol. 207, 189–199 10.4049/jimmunol.2001030 [DOI] [PubMed] [Google Scholar]
- 52.Kanneganti, A., Malireddi, R.K.S., Saavedra, P.H.V., Vande Walle, L., Van Gorp, H., Kambara, H.et al. (2018) GSDMD is critical for autoinflammatory pathology in a mouse model of familial Mediterranean fever. J. Exp. Med. 215, 1519–1529 10.1084/jem.20172060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao, J., Wang, C., Yao, J.C., Alippe, Y., Xu, C., Kress, D.et al. (2018) Gasdermin D mediates the pathogenesis of neonatal-onset multisystem inflammatory disease in mice. PLoS Biol. 16, e3000047 10.1371/journal.pbio.3000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang, T., Sun, K., Wang, C., Swarnkar, G., Quan, S., Kress, D.et al. (2021) Gasdermin D deficiency attenuates arthritis induced by traumatic injury but not autoantibody-assembled immune complexes. Arthritis Res. Ther. 23, 286 10.1186/s13075-021-02668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rashidi, M., Simpson, D.S., Hempel, A., Frank, D., Petrie, E., Vince, A.et al. (2019) The pyroptotic cell death effector gasdermin D is activated by gout-associated uric acid crystals but is dispensable for cell death and IL-1beta release. J. Immunol. 203, 736–748 10.4049/jimmunol.1900228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kayagaki, N., Warming, S., Lamkanfi, M., Vande Walle, L., Louie, S., Dong, J.et al. (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 10.1038/nature10558 [DOI] [PubMed] [Google Scholar]
- 57.Kayagaki, N., Wong, M.T., Stowe, I.B., Ramani, S.R., Gonzalez, L.C., Akashi-Takamura, S.et al. (2013) Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 10.1126/science.1240248 [DOI] [PubMed] [Google Scholar]
- 58.Meunier, E., Dick, M.S., Dreier, R.F., Schurmann, N., Kenzelmann Broz, D., Warming, S.et al. (2014) Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature 509, 366–370 10.1038/nature13157 [DOI] [PubMed] [Google Scholar]
- 59.Haldar, A.K., Foltz, C., Finethy, R., Piro, A.S., Feeley, E.M., Pilla-Moffett, D.M.et al. (2015) Ubiquitin systems mark pathogen-containing vacuoles as targets for host defense by guanylate binding proteins. Proc. Natl Acad. Sci. U.S.A. 112, E5628–E5637 10.1073/pnas.1515966112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Man, S.M., Karki, R., Sasai, M., Place, D.E., Kesavardhana, S., Temirov, J.et al. (2016) IRGB10 liberates bacterial ligands for sensing by the AIM2 and caspase-11-NLRP3 inflammasomes. Cell 167, 382–96.e17 10.1016/j.cell.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fisch, D., Bando, H., Clough, B., Hornung, V., Yamamoto, M., Shenoy, A.R.et al. (2019) Human GBP1 is a microbe-specific gatekeeper of macrophage apoptosis and pyroptosis. EMBO J. 38, e100926 10.15252/embj.2018100926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ragland, S.A. and Kagan, J.C. (2021) Cytosolic detection of phagosomal bacteria-Mechanisms underlying PAMP exodus from the phagosome into the cytosol. Mol. Microbiol. 116, 1420–1432 10.1111/mmi.14841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wandel, M.P., Kim, B.H., Park, E.S., Boyle, K.B., Nayak, K., Lagrange, B.et al. (2020) Guanylate-binding proteins convert cytosolic bacteria into caspase-4 signaling platforms. Nat. Immunol. 21, 880–891 10.1038/s41590-020-0697-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Santos, J.C., Boucher, D., Schneider, L.K., Demarco, B., Dilucca, M., Shkarina, K.et al. (2020) Human GBP1 binds LPS to initiate assembly of a caspase-4 activating platform on cytosolic bacteria. Nat. Commun. 11, 3276 10.1038/s41467-020-16889-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kutsch, M., Sistemich, L., Lesser, C.F., Goldberg, M.B., Herrmann, C. and Coers, J. (2020) Direct binding of polymeric GBP1 to LPS disrupts bacterial cell envelope functions. EMBO J. 39, e104926 10.15252/embj.2020104926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi, J., Zhao, Y., Wang, Y., Gao, W., Ding, J., Li, P.et al. (2014) Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 10.1038/nature13683 [DOI] [PubMed] [Google Scholar]
- 67.Aglietti, R.A., Estevez, A., Gupta, A., Ramirez, M.G., Liu, P.S., Kayagaki, N.et al. (2016) Gsdmd p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc. Natl Acad. Sci. U.S.A. 113, 7858–7863 10.1073/pnas.1607769113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kronheim, S.R., Mumma, A., Greenstreet, T., Glackin, P.J., Van Ness, K., March, C.J.et al. (1992) Purification of interleukin-1 beta converting enzyme, the protease that cleaves the interleukin-1 beta precursor. Arch. Biochem. Biophys. 296, 698–703 10.1016/0003-9861(92)90629-B [DOI] [PubMed] [Google Scholar]
- 69.Black, R.A., Kronheim, S.R., Merriam, J.E., March, C.J. and Hopp, T.P. (1989) A pre-aspartate-specific protease from human leukocytes that cleaves pro-interleukin-1 beta. J. Biol. Chem. 264, 5323–5326 10.1016/S0021-9258(18)83546-3 [DOI] [PubMed] [Google Scholar]
- 70.Faucheu, C., Diu, A., Chan, A.W., Blanchet, A.M., Miossec, C., Herve, F.et al. (1995) A novel human protease similar to the interleukin-1 beta converting enzyme induces apoptosis in transfected cells. EMBO J. 14, 1914–1922 10.1002/j.1460-2075.1995.tb07183.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Munday, N.A., Vaillancourt, J.P., Ali, A., Casano, F.J., Miller, D.K., Molineaux, S.M.et al. (1995) Molecular cloning and pro-apoptotic activity of ICErelII and ICErelIII, members of the ICE/CED-3 family of cysteine proteases. J. Biol. Chem. 270, 15870–6 10.1074/jbc.270.26.15870 [DOI] [PubMed] [Google Scholar]
- 72.Hagar, J.A., Powell, D.A., Aachoui, Y., Ernst, R.K. and Miao, E.A. (2013) Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 10.1126/science.1240988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ruhl, S. and Broz, P. (2015) Caspase-11 activates a canonical NLRP3 inflammasome by promoting K(+) efflux. Eur. J. Immunol. 45, 2927–2936 10.1002/eji.201545772 [DOI] [PubMed] [Google Scholar]
- 74.Baker, P.J., Boucher, D., Bierschenk, D., Tebartz, C., Whitney, P.G., D'Silva, D.B.et al. (2015) NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur. J. Immunol. 45, 2918–2926 10.1002/eji.201545655 [DOI] [PubMed] [Google Scholar]
- 75.Munoz-Planillo, R., Kuffa, P., Martinez-Colon, G., Smith, B.L., Rajendiran, T.M. and Nunez, G. (2013) K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38, 1142–1153 10.1016/j.immuni.2013.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pétrilli, V., Papin, S., Dostert, C., Mayor, A., Martinon, F. and Tschopp, J. (2007) Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14, 1583–1589 10.1038/sj.cdd.4402195 [DOI] [PubMed] [Google Scholar]
- 77.Donado, C.A., Cao, A.B., Simmons, D.P., Croker, B.A., Brennan, P.J. and Brenner, M.B. (2020) A Two-Cell model for IL-1β release mediated by death-receptor signaling. Cell Rep. 31, 107466 10.1016/j.celrep.2020.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Orning, P., Weng, D., Starheim, K., Ratner, D., Best, Z., Lee, B.et al. (2018) Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science 362, 1064–1069 10.1126/science.aau2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davies, C.W., Stowe, I., Phung, Q.T., Ho, H., Bakalarski, C.E., Gupta, A.et al. (2021) Discovery of a caspase cleavage motif antibody reveals insights into noncanonical inflammasome function. Proc. Natl Acad. Sci. U.S.A. 118, e2018024118 10.1073/pnas.2018024118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen, K.W., Monteleone, M., Boucher, D., Sollberger, G., Ramnath, D., Condon, N.D.et al. (2018) Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci. Immunol. 3, eaar6676 10.1126/sciimmunol.aar6676 [DOI] [PubMed] [Google Scholar]
- 81.Tsuchiya, K., Hosojima, S., Hara, H., Kushiyama, H., Mahib, M.R., Kinoshita, T.et al. (2021) Gasdermin D mediates the maturation and release of IL-1alpha downstream of inflammasomes. Cell Rep. 34, 108887 10.1016/j.celrep.2021.108887 [DOI] [PubMed] [Google Scholar]
- 82.Banerjee, I., Behl, B., Mendonca, M., Shrivastava, G., Russo, A.J., Menoret, A.et al. (2018) Gasdermin D restrains type I interferon response to cytosolic DNA by disrupting ionic homeostasis. Immunity 49, 413–26.e5 10.1016/j.immuni.2018.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feltham, R. and Vince, J.E. (2018) Ion Man: GSDMD punches pores to knock Out cGAS. Immunity 49, 379–381 10.1016/j.immuni.2018.08.026 [DOI] [PubMed] [Google Scholar]
- 84.Huang, L.S., Hong, Z., Wu, W., Xiong, S., Zhong, M., Gao, X.et al. (2020) mtDNA activates cGAS signaling and suppresses the YAP-mediated endothelial cell proliferation program to promote inflammatory injury. Immunity 52, 475–86.e5 10.1016/j.immuni.2020.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Karmakar, M., Minns, M., Greenberg, E.N., Diaz-Aponte, J., Pestonjamasp, K., Johnson, J.L.et al. (2020) N-GSDMD trafficking to neutrophil organelles facilitates IL-1beta release independently of plasma membrane pores and pyroptosis. Nat. Commun. 11, 2212 10.1038/s41467-020-16043-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen, K.W., Gross, C.J., Sotomayor, F.V., Stacey, K.J., Tschopp, J., Sweet, M.J.et al. (2014) The neutrophil NLRC4 inflammasome selectively promotes IL-1beta maturation without pyroptosis during acute Salmonella challenge. Cell Rep. 8, 570–582 10.1016/j.celrep.2014.06.028 [DOI] [PubMed] [Google Scholar]
- 87.Zhang, J., Yu, Q., Jiang, D., Yu, K., Yu, W., Chi, Z.et al. (2022) Epithelial Gasdermin D shapes the host-microbial interface by driving mucus layer formation. Sci. Immunol. 7, eabk2092 10.1126/sciimmunol.abk2092 [DOI] [PubMed] [Google Scholar]
- 88.Feltham, R., Vince, J.E. and Lawlor, K.E. (2017) Caspase-8: not so silently deadly. Clin. Transl. Immunol. 6, e124 10.1038/cti.2016.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maelfait, J., Vercammen, E., Janssens, S., Schotte, P., Haegman, M., Magez, S.et al. (2008) Stimulation of toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J. Exp. Med. 205, 1967–1973 10.1084/jem.20071632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vince, J.E., Wong, W.W., Gentle, I., Lawlor, K.E., Allam, R., O'Reilly, L.et al. (2012) Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity 36, 215–227 10.1016/j.immuni.2012.01.012 [DOI] [PubMed] [Google Scholar]
- 91.Moriwaki, K., Bertin, J., Gough, P.J. and Chan, F.K. (2015) A RIPK3-caspase 8 complex mediates atypical pro-IL-1beta processing. J. Immunol. 194, 1938–1944 10.4049/jimmunol.1402167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gringhuis, S.I., Kaptein, T.M., Wevers, B.A., Theelen, B., van der Vlist, M., Boekhout, T.et al. (2012) Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat. Immunol. 13, 246–254 10.1038/ni.2222 [DOI] [PubMed] [Google Scholar]
- 93.Shenderov, K., Riteau, N., Yip, R., Mayer-Barber, K.D., Oland, S., Hieny, S.et al. (2014) Cutting edge: endoplasmic reticulum stress licenses macrophages to produce mature IL-1beta in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. J. Immunol. 192, 2029–2033 10.4049/jimmunol.1302549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bossaller, L., Chiang, P.I., Schmidt-Lauber, C., Ganesan, S., Kaiser, W.J., Rathinam, V.A.et al. (2012) Cutting edge: FAS (CD95) mediates noncanonical IL-1β and IL-18 maturation via caspase-8 in an RIP3-independent manner. J. Immunol. 189, 5508–5512 10.4049/jimmunol.1202121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li, H., Zhu, H., Xu, C.J. and Yuan, J. (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94, 491–501 10.1016/S0092-8674(00)81590-1 [DOI] [PubMed] [Google Scholar]
- 96.Tsuchiya, K., Nakajima, S., Hosojima, S., Thi Nguyen, D., Hattori, T., Manh Le, T.et al. (2019) Caspase-1 initiates apoptosis in the absence of gasdermin D. Nat. Commun. 10, 2091 10.1038/s41467-019-09753-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heilig, R., Dilucca, M., Boucher, D., Chen, K.W., Hancz, D., Demarco, B.et al. (2020) Caspase-1 cleaves Bid to release mitochondrial SMAC and drive secondary necrosis in the absence of GSDMD. Life Sci. Alliance 3, e202000735 10.26508/lsa.202000735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Muendlein, H.I., Jetton, D., Connolly, W.M., Eidell, K.P., Magri, Z., Smirnova, I.et al. (2020) cFLIPL protects macrophages from LPS-induced pyroptosis via inhibition of complex II formation. Science 367, 1379–1384 10.1126/science.aay3878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sarhan, J., Liu, B.C., Muendlein, H.I., Li, P., Nilson, R., Tang, A.Y.et al. (2018) Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl Acad. Sci. U.S.A. 115, E10888–E10E97 10.1073/pnas.1809548115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Demarco, B., Grayczyk, J.P., Bjanes, E., Le Roy, D., Tonnus, W., Assenmacher, C.A.et al. (2020) Caspase-8-dependent gasdermin D cleavage promotes antimicrobial defense but confers susceptibility to TNF-induced lethality. Sci. Adv. 6, eabc3465 10.1126/sciadv.abc3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Newton, K., Wickliffe, K.E., Maltzman, A., Dugger, D.L., Reja, R., Zhang, Y.et al. (2019) Activity of caspase-8 determines plasticity between cell death pathways. Nature 575, 679–682 10.1038/s41586-019-1752-8 [DOI] [PubMed] [Google Scholar]
- 102.Fritsch, M., Gunther, S.D., Schwarzer, R., Albert, M.C., Schorn, F., Werthenbach, J.P.et al. (2019) Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575, 683–687 10.1038/s41586-019-1770-6 [DOI] [PubMed] [Google Scholar]
- 103.Rogers, C., Fernandes-Alnemri, T., Mayes, L., Alnemri, D., Cingolani, G. and Alnemri, E.S. (2017) Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 8, 14128 10.1038/ncomms14128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang, Y., Gao, W., Shi, X., Ding, J., Liu, W., He, H.et al. (2017) Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103 10.1038/nature22393 [DOI] [PubMed] [Google Scholar]
- 105.Zhang, Z., Zhang, Y., Xia, S., Kong, Q., Li, S., Liu, X.et al. (2020) Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 579, 415–420 10.1038/s41586-020-2071-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jiang, S., Gu, H., Zhao, Y. and Sun, L. (2019) Teleost gasdermin E is cleaved by caspase 1, 3, and 7 and induces pyroptosis. J. Immunol. 203, 1369–1382 10.4049/jimmunol.1900383 [DOI] [PubMed] [Google Scholar]
- 107.Daskalov, A., Mitchell, P.S., Sandstrom, A., Vance, R.E. and Glass, N.L. (2020) Molecular characterization of a fungal gasdermin-like protein. Proc. Natl Acad. Sci. U.S.A. 117, 18600–7 10.1073/pnas.2004876117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sagulenko, V., Thygesen, S.J., Sester, D.P., Idris, A., Cridland, J.A., Vajjhala, P.R.et al. (2013) AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ. 20, 1149–1160 10.1038/cdd.2013.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee, B.L., Mirrashidi, K.M., Stowe, I.B., Kummerfeld, S.K., Watanabe, C., Haley, B.et al. (2018) ASC- and caspase-8-dependent apoptotic pathway diverges from the NLRC4 inflammasome in macrophages. Sci. Rep. 8, 3788 10.1038/s41598-018-21998-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vajjhala, P.R., Lu, A., Brown, D.L., Pang, S.W., Sagulenko, V., Sester, D.P.et al. (2015) The inflammasome adaptor ASC induces procaspase-8 death effector domain filaments. J. Biol. Chem. 290, 29217–29230 10.1074/jbc.M115.687731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mascarenhas, D.P.A., Cerqueira, D.M., Pereira, M.S.F., Castanheira, F.V.S., Fernandes, T.D., Manin, G.Z.et al. (2017) Inhibition of caspase-1 or gasdermin-D enable caspase-8 activation in the Naip5/NLRC4/ASC inflammasome. PLoS Pathog. 13, e1006502 10.1371/journal.ppat.1006502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schneider, K.S., Gross, C.J., Dreier, R.F., Saller, B.S., Mishra, R., Gorka, O.et al. (2017) The inflammasome drives GSDMD-independent secondary pyroptosis and IL-1 release in the absence of caspase-1 protease activity. Cell Rep. 21, 3846–3859 10.1016/j.celrep.2017.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Vasconcelos, N.M., Van Opdenbosch, N., Van Gorp, H., Martin-Perez, R., Zecchin, A., Vandenabeele, P.et al. (2020) An apoptotic caspase network safeguards cell death induction in pyroptotic macrophages. Cell Rep. 32, 107959 10.1016/j.celrep.2020.107959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sagulenko, V., Vitak, N., Vajjhala, P.R., Vince, J.E. and Stacey, K.J. (2018) Caspase-1 is an apical caspase leading to caspase-3 cleavage in the AIM2 inflammasome response, independent of caspase-8. J. Mol. Biol. 430, 238–247 10.1016/j.jmb.2017.10.028 [DOI] [PubMed] [Google Scholar]
- 115.Wang, C., Yang, T., Xiao, J., Xu, C., Alippe, Y., Sun, K.et al. (2021) NLRP3 inflammasome activation triggers gasdermin D-independent inflammation. Sci. Immunol. 6, eabj3859 10.1126/sciimmunol.abj3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kayagaki, N., Kornfeld, O.S., Lee, B.L., Stowe, I.B., O'Rourke, K., Li, Q.et al. (2021) NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 591, 131–136 10.1038/s41586-021-03218-7 [DOI] [PubMed] [Google Scholar]
- 117.Pierini, R., Juruj, C., Perret, M., Jones, C.L., Mangeot, P., Weiss, D.S.et al. (2012) AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ. 19, 1709–1721 10.1038/cdd.2012.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li, Y.F., Nanayakkara, G., Sun, Y., Li, X., Wang, L., Cueto, R.et al. (2017) Analyses of caspase-1-regulated transcriptomes in various tissues lead to identification of novel IL-1β-, IL-18- and sirtuin-1-independent pathways. J. Hematol. Oncol. 10, 40 10.1186/s13045-017-0406-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Aries, A., Whitcomb, J., Shao, W., Komati, H., Saleh, M. and Nemer, M. (2014) Caspase-1 cleavage of transcription factor GATA4 and regulation of cardiac cell fate. Cell Death Dis. 5, e1566 10.1038/cddis.2014.524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gitlin, A.D., Heger, K., Schubert, A.F., Reja, R., Yan, D., Pham, V.C.et al. (2020) Integration of innate immune signalling by caspase-8 cleavage of N4BP1. Nature 587, 275–280 10.1038/s41586-020-2796-5 [DOI] [PubMed] [Google Scholar]
- 121.Cai, B., Liao, C., He, D., Chen, J., Han, J., Lu, J.et al. (2022) Gasdermin E mediates photoreceptor damage by all-trans-retinal in the mouse retina. J. Biol. Chem. 298, 101553 10.1016/j.jbc.2021.101553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Croes, L., Fransen, E., Hylebos, M., Buys, K., Hermans, C., Broeckx, G.et al. (2019) Determination of the potential tumor-suppressive effects of gsdme in a chemically induced and in a genetically modified intestinal cancer mouse model. Cancers 11, 1214 10.3390/cancers11081214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen, K.W., Demarco, B., Ramos, S., Heilig, R., Goris, M., Grayczyk, J.P.et al. (2021) RIPK1 activates distinct gasdermins in macrophages and neutrophils upon pathogen blockade of innate immune signaling. Proc. Natl Acad. Sci. U.S.A. 118, e2101189118 10.1073/pnas.2101189118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wan, X., Li, J., Wang, Y., Yu, X., He, X., Shi, J.et al. (2022) H7n9 virus infection triggers lethal cytokine storm by activating gasdermin E-mediated pyroptosis of lung alveolar epithelial cells. Natl Sci. Rev. 9, nwab137 10.1093/nsr/nwab137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tan, G., Huang, C., Chen, J., Chen, B. and Zhi, F. (2021) Gasdermin-E-mediated pyroptosis participates in the pathogenesis of crohn's disease by promoting intestinal inflammation. Cell Rep. 35, 109265 10.1016/j.celrep.2021.109265 [DOI] [PubMed] [Google Scholar]
- 126.Zhai, Z., Yang, F., Xu, W., Han, J., Luo, G., Li, Y.et al. (2022) Attenuation of rheumatoid arthritis through the inhibition of tumor necrosis factor-induced caspase 3/Gasdermin E-mediated pyroptosis. Arthritis Rheumatol. 74, 427–440 10.1002/art.41963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hou, J., Zhao, R., Xia, W., Chang, C.W., You, Y., Hsu, J.M.et al. (2020) PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 22, 1264–1275 10.1038/s41556-020-0575-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang, Q., Wang, Y., Ding, J., Wang, C., Zhou, X., Gao, W.et al. (2020) A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature 579, 421–426 10.1038/s41586-020-2079-1 [DOI] [PubMed] [Google Scholar]
- 129.Miguchi, M., Hinoi, T., Shimomura, M., Adachi, T., Saito, Y., Niitsu, H.et al. (2016) Gasdermin C is upregulated by inactivation of transforming growth factor beta receptor type II in the presence of mutated Apc, promoting colorectal cancer proliferation. PLoS ONE 11, e0166422 10.1371/journal.pone.0166422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang, J.Y., Zhou, B., Sun, R.Y., Ai, Y.L., Cheng, K., Li, F.N.et al. (2021) The metabolite alpha-KG induces GSDMC-dependent pyroptosis through death receptor 6-activated caspase-8. Cell Res. 31, 980–997 10.1038/s41422-021-00506-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xi, R., Montague, J., Lin, X., Lu, C., Lei, W., Tanaka, K.et al. (2021) Up-regulation of gasdermin C in mouse small intestine is associated with lytic cell death in enterocytes in worm-induced type 2 immunity. Proc. Natl Acad. Sci. U.S.A. 118, e2026307118 10.1073/pnas.2026307118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou, Z., He, H., Wang, K., Shi, X., Wang, Y., Su, Y.et al. (2020) Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 368, eaaz7548 10.1126/science.aaz7548 [DOI] [PubMed] [Google Scholar]
- 133.Hansen, J.M., de Jong, M.F., Wu, Q., Zhang, L.S., Heisler, D.B., Alto, L.T.et al. (2021) Pathogenic ubiquitination of GSDMB inhibits NK cell bactericidal functions. Cell 184, 3178–91.e18 10.1016/j.cell.2021.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chao, K.L., Kulakova, L. and Herzberg, O. (2017) Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein. Proc. Natl Acad. Sci. US.A. 114, E1128–E1137 10.1073/pnas.1616783114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen, Q., Shi, P., Wang, Y., Zou, D., Wu, X., Wang, D.et al. (2019) GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity. J. Mol. Cell Biol. 11, 496–508 10.1093/jmcb/mjy056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Acevedo, N., Reinius, L.E., Greco, D., Gref, A., Orsmark-Pietras, C., Persson, H.et al. (2015) Risk of childhood asthma is associated with CpG-site polymorphisms, regional DNA methylation and mRNA levels at the GSDMB/ORMDL3 locus. Hum. Mol. Genet. 24, 875–890 10.1093/hmg/ddu479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhao, C.N., Fan, Y., Huang, J.J., Zhang, H.X., Gao, T., Wang, C.et al. (2015) The association of GSDMB and ORMDL3 gene polymorphisms with asthma: a meta-analysis. Allergy Asthma Immunol. Res. 7, 175–185 10.4168/aair.2015.7.2.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kang, M.J., Yu, H.S., Seo, J.H., Kim, H.Y., Jung, Y.H., Kim, Y.J.et al. (2012) GSDMB/ORMDL3 variants contribute to asthma susceptibility and eosinophil-mediated bronchial hyperresponsiveness. Hum. Immunol. 73, 954–959 10.1016/j.humimm.2012.06.009 [DOI] [PubMed] [Google Scholar]
- 139.Das, S., Miller, M., Beppu, A.K., Mueller, J., McGeough, M.D., Vuong, C.et al. (2016) GSDMB induces an asthma phenotype characterized by increased airway responsiveness and remodeling without lung inflammation. Proc. Natl Acad. Sci. U.S.A. 113, 13132–7 10.1073/pnas.1610433113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li, X., Christenson, S.A., Modena, B., Li, H., Busse, W.W., Castro, M.et al. (2021) Genetic analyses identify GSDMB associated with asthma severity, exacerbations, and antiviral pathways. J. Allergy Clin. Immunol. 147, 894–909 10.1016/j.jaci.2020.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kitazawa, H., Masuko, H., Kanazawa, J., Shigemasa, R., Hyodo, K., Yamada, H.et al. (2021) ORMDL3/GSDMB genotype as a risk factor for early-onset adult asthma is linked to total serum IgE levels but not to allergic sensitization. Allergol. Int. 70, 55–60 10.1016/j.alit.2020.04.009 [DOI] [PubMed] [Google Scholar]
- 142.Panganiban, R.A., Sun, M., Dahlin, A., Park, H.R., Kan, M., Himes, B.E.et al. (2018) A functional splice variant associated with decreased asthma risk abolishes the ability of gasdermin B to induce epithelial cell pyroptosis. J. Allergy Clin. Immunol. 142, 1469–78.e2 10.1016/j.jaci.2017.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rana, N., Privitera, G., Kondolf, H.C., Bulek, K., Lechuga, S., De Salvo, C.et al. (2022) GSDMB is increased in IBD and regulates epithelial restitution/repair independent of pyroptosis. Cell 185, 283–98.e17 10.1016/j.cell.2021.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li, L., Li, Y. and Bai, Y. (2020) Role of GSDMB in pyroptosis and cancer. Cancer Manag. Res. 12, 3033–3043 10.2147/CMAR.S246948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hergueta-Redondo, M., Sarrio, D., Molina-Crespo, A., Vicario, R., Bernado-Morales, C., Martinez, L.et al. (2016) Gasdermin B expression predicts poor clinical outcome in HER2-positive breast cancer. Oncotarget 7, 56295–56308 10.18632/oncotarget.10787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Molina-Crespo, A., Cadete, A., Sarrio, D., Gamez-Chiachio, M., Martinez, L., Chao, K.et al. (2019) Intracellular delivery of an antibody targeting gasdermin-B reduces HER2 breast cancer aggressiveness. Clin. Cancer Res. 25, 4846–4858 10.1158/1078-0432.CCR-18-2381 [DOI] [PubMed] [Google Scholar]
- 147.Ruan, J., Xia, S., Liu, X., Lieberman, J. and Wu, H. (2018) Cryo-EM structure of the gasdermin A3 membrane pore. Nature 557, 62–67 10.1038/s41586-018-0058-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Deng, W., Bai, Y., Deng, F., Pan, Y., Mei, S., Zheng, Z.et al. (2022) Streptococcal pyrogenic exotoxin B cleaves GSDMA and triggers pyroptosis. Nature 602, 496–502 10.1038/s41586-021-04384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Terao, C., Kawaguchi, T., Dieude, P., Varga, J., Kuwana, M., Hudson, M.et al. (2017) Transethnic meta-analysis identifies GSDMA and PRDM1 as susceptibility genes to systemic sclerosis. Ann. Rheum. Dis. 76, 1150–1158 10.1136/annrheumdis-2016-210645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Anderton, H., Wicks, I.P. and Silke, J. (2020) Cell death in chronic inflammation: breaking the cycle to treat rheumatic disease. Nat. Rev. Rheumatol. 16, 496–513 10.1038/s41584-020-0455-8 [DOI] [PubMed] [Google Scholar]
- 151.Harapas, C.R., Idiiatullina, E., Al-Azab, M., Hrovat-Schaale, K., Reygaerts, T., Steiner, A.et al. (2022) Organellar homeostasis and innate immune sensing. Nat. Rev. Immunol. 10.1038/s41577-022-00682-8 [DOI] [PubMed] [Google Scholar]
- 152.Lawlor, K.E. and Vince, J.E. (2014) Ambiguities in NLRP3 inflammasome regulation: is there a role for mitochondria? Biochim. Biophys. Acta 1840, 1433–1440 10.1016/j.bbagen.2013.08.014 [DOI] [PubMed] [Google Scholar]
- 153.Kang, R., Zeng, L., Zhu, S., Xie, Y., Liu, J., Wen, Q.et al. (2018) Lipid peroxidation drives gasdermin D-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe 24, 97–108.e4 10.1016/j.chom.2018.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang, Y., Shi, P., Chen, Q., Huang, Z., Zou, D., Zhang, J.et al. (2019) Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J Mol. Cell. Biol. 11, 1069–1082 10.1093/jmcb/mjz020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Evavold, C.L., Hafner-Bratkovic, I., Devant, P., D'Andrea, J.M., Ngwa, E.M., Borsic, E.et al. (2021) Control of gasdermin D oligomerization and pyroptosis by the ragulator-Rag-mTORC1 pathway. Cell 184, 4495–511.e19 10.1016/j.cell.2021.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zheng, Z., Deng, W., Bai, Y., Miao, R., Mei, S., Zhang, Z.et al. (2021) The lysosomal Rag-ragulator complex licenses RIPK1 and caspase-8-mediated pyroptosis by Yersinia. Science 372, eabg0269 10.1126/science.abg0269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.de Vasconcelos, N.M., Van Opdenbosch, N., Van Gorp, H., Parthoens, E. and Lamkanfi, M. (2019) Single-cell analysis of pyroptosis dynamics reveals conserved GSDMD-mediated subcellular events that precede plasma membrane rupture. Cell Death Differ. 26, 146–161 10.1038/s41418-018-0106-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bjanes, E., Sillas, R.G., Matsuda, R., Demarco, B., Fettrelet, T., DeLaney, A.A.et al. (2021) Genetic targeting of Card19 is linked to disrupted NINJ1 expression, impaired cell lysis, and increased susceptibility to Yersinia infection. PLoS Pathog. 17, e1009967 10.1371/journal.ppat.1009967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Santa Cruz Garcia, A.B., Schnur, K.P., Malik, A.B. and Mo, G.C.H. (2022) Gasdermin D pores are dynamically regulated by local phosphoinositide circuitry. Nat. Commun. 13, 52 10.1038/s41467-021-27692-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gong, Y.N., Guy, C., Olauson, H., Becker, J.U., Yang, M., Fitzgerald, P.et al. (2017) ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell 169, 286–300.e16 10.1016/j.cell.2017.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ruhl, S., Shkarina, K., Demarco, B., Heilig, R., Santos, J.C. and Broz, P. (2018) ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362, 956–960 10.1126/science.aar7607 [DOI] [PubMed] [Google Scholar]
- 162.Schink, K.O., Tan, K.W. and Stenmark, H. (2016) Phosphoinositides in control of membrane dynamics. Annu. Rev. Cell Dev. Biol. 32, 143–171 10.1146/annurev-cellbio-111315-125349 [DOI] [PubMed] [Google Scholar]
- 163.Humphries, F., Shmuel-Galia, L., Ketelut-Carneiro, N., Li, S., Wang, B., Nemmara, V.V.et al. (2020) Succination inactivates gasdermin D and blocks pyroptosis. Science 369, 1633–1637 10.1126/science.abb9818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Taabazuing, C.Y., Okondo, M.C. and Bachovchin, D.A. (2017) Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem. Biol. 24, 507–14.e4 10.1016/j.chembiol.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rogers, C., Erkes, D.A., Nardone, A., Aplin, A.E., Fernandes-Alnemri, T. and Alnemri, E.S. (2019) Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun. 10, 1689 10.1038/s41467-019-09397-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Czabotar, P.E., Lessene, G., Strasser, A. and Adams, J.M. (2013) Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 15, 49–63 10.1038/nrm3722 [DOI] [PubMed] [Google Scholar]
- 167.Vince, J.E., De Nardo, D., Gao, W., Vince, A.J., Hall, C., McArthur, K.et al. (2018) The mitochondrial apoptotic effectors BAX/BAK activate caspase-3 and -7 to trigger NLRP3 inflammasome and caspase-8 driven IL-1β activation. Cell Rep. 25, 2339–53.e4 10.1016/j.celrep.2018.10.103 [DOI] [PubMed] [Google Scholar]
- 168.Chauhan, D., Bartok, E., Gaidt, M.M., Bock, F.J., Herrmann, J., Seeger, J.M.et al. (2018) BAX/BAK-induced apoptosis results in caspase-8-dependent IL-1β maturation in macrophages. Cell Rep. 25, 2354–68.e5 10.1016/j.celrep.2018.10.087 [DOI] [PubMed] [Google Scholar]
- 169.Deo, P., Chow, S.H., Han, M.L., Speir, M., Huang, C., Schittenhelm, R.B.et al. (2020) Mitochondrial dysfunction caused by outer membrane vesicles from Gram-negative bacteria activates intrinsic apoptosis and inflammation. Nat. Microbiol. 5, 1418–1427 10.1038/s41564-020-0773-2 [DOI] [PubMed] [Google Scholar]
- 170.Allam, R., Lawlor, K.E., Yu, E.C., Mildenhall, A.L., Moujalled, D.M., Lewis, R.S.et al. (2014) Mitochondrial apoptosis is dispensable for NLRP3 inflammasome activation but non-apoptotic caspase-8 is required for inflammasome priming. EMBO Rep. 15, 982–990 10.15252/embr.201438463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen, K.W., Demarco, B. and Broz, P. (2020) Pannexin-1 promotes NLRP3 activation during apoptosis but is dispensable for canonical or noncanonical inflammasome activation. Eur. J. Immunol. 50, 170–177 10.1002/eji.201948254 [DOI] [PubMed] [Google Scholar]
- 172.Chen, K.W., Demarco, B., Heilig, R., Shkarina, K., Boettcher, A., Farady, C.J.et al. (2019) Extrinsic and intrinsic apoptosis activate pannexin-1 to drive NLRP3 inflammasome assembly. EMBO J. 38, e101638 10.15252/embj.2019101638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.McComb, S., Chan, P.K., Guinot, A., Hartmannsdottir, H., Jenni, S., Dobay, M.P.et al. (2019) Efficient apoptosis requires feedback amplification of upstream apoptotic signals by effector caspase-3 or -7. Sci. Adv. 5, eaau9433 10.1126/sciadv.aau9433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kaiser, W.J., Upton, J.W., Long, A.B., Livingston-Rosanoff, D., Daley-Bauer, L.P., Hakem, R.et al. (2011) RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471, 368–372 10.1038/nature09857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Oberst, A., Dillon, C.P., Weinlich, R., McCormick, L.L., Fitzgerald, P., Pop, C.et al. (2011) Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 10.1038/nature09852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Alvarez-Diaz, S., Dillon, C.P., Lalaoui, N., Tanzer, M.C., Rodriguez, D.A., Lin, A.et al. (2016) The pseudokinase MLKL and the kinase RIPK3 have distinct roles in autoimmune disease caused by loss of death-receptor-induced apoptosis. Immunity 45, 513–526 10.1016/j.immuni.2016.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Eng, V.V., Wemyss, M.A. and Pearson, J.S. (2021) The diverse roles of RIP kinases in host-pathogen interactions. Semin. Cell Dev. Biol. 109, 125–143 10.1016/j.semcdb.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Simpson, D.S., Gabrielyan, A. and Feltham, R. (2021) RIPK1 ubiquitination: evidence, correlations and the undefined. Semin. Cell Dev. Biol. 109, 76–85 10.1016/j.semcdb.2020.08.008 [DOI] [PubMed] [Google Scholar]
- 179.Lawlor, K.E., Khan, N., Mildenhall, A., Gerlic, M., Croker, B.A., D'Cruz, A.A.et al. (2015) RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat. Commun. 6, 6282 10.1038/ncomms7282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Murphy, J.M. and Vince, J.E. (2015) Post-translational control of RIPK3 and MLKL mediated necroptotic cell death. F1000Res. 4, F1000 Faculty Rev-1297 10.12688/f1000research.7046.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Weir, A., Hughes, S., Rashidi, M., Hildebrand, J.M. and Vince, J.E. (2020) Necroptotic movers and shakers: cell types, inflammatory drivers and diseases. Curr. Opin. Immunol. 68, 83–97 10.1016/j.coi.2020.09.008 [DOI] [PubMed] [Google Scholar]
- 182.Chi, W., Chen, H., Li, F., Zhu, Y., Yin, W. and Zhuo, Y. (2015) HMGB1 promotes the activation of NLRP3 and caspase-8 inflammasomes via NF-kappaB pathway in acute glaucoma. J. Neuroinflammation 12, 137 10.1186/s12974-015-0360-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhao, B., Lu, R., Chen, J., Xie, M., Zhao, X. and Kong, L. (2021) S100a9 blockade prevents lipopolysaccharide-induced lung injury via suppressing the NLRP3 pathway. Respir. Res. 22, 45 10.1186/s12931-021-01641-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Conos, S.A., Chen, K.W., De Nardo, D., Hara, H., Whitehead, L., Nunez, G.et al. (2017) Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc. Natl Acad. Sci. U.S.A. 114, E961–E9E9 10.1073/pnas.1613305114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gutierrez, K.D., Davis, M.A., Daniels, B.P., Olsen, T.M., Ralli-Jain, P., Tait, S.W.et al. (2017) MLKL activation triggers NLRP3-mediated processing and release of IL-1 β independently of Gasdermin-D. J. Immunol. 198, 2156–2164 10.4049/jimmunol.1601757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kang, S., Fernandes-Alnemri, T., Rogers, C., Mayes, L., Wang, Y., Dillon, C.et al. (2015) Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat. Commun. 6, 7515 10.1038/ncomms8515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kang, T.B., Yang, S.H., Toth, B., Kovalenko, A. and Wallach, D. (2013) Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity 38, 27–40 10.1016/j.immuni.2012.09.015 [DOI] [PubMed] [Google Scholar]
- 188.Duong, B.H., Onizawa, M., Oses-Prieto, J.A., Advincula, R., Burlingame, A., Malynn, B.A.et al. (2015) A20 restricts ubiquitination of pro-interleukin-1β protein complexes and suppresses NLRP3 inflammasome activity. Immunity 42, 55–67 10.1016/j.immuni.2014.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vande Walle, L., Van Opdenbosch, N., Jacques, P., Fossoul, A., Verheugen, E., Vogel, P.et al. (2014) Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature 512, 69–73 10.1038/nature13322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Voet, S., Mc Guire, C., Hagemeyer, N., Martens, A., Schroeder, A., Wieghofer, P.et al. (2018) A20 critically controls microglia activation and inhibits inflammasome-dependent neuroinflammation. Nat. Commun. 9, 2036 10.1038/s41467-018-04376-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhou, Q., Wang, H., Schwartz, D.M., Stoffels, M., Park, Y.H., Zhang, Y.et al. (2016) Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat. Genet. 48, 67–73 10.1038/ng.3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Polykratis, A., Martens, A., Eren, R.O., Shirasaki, Y., Yamagishi, M., Yamaguchi, Y.et al. (2019) A20 prevents inflammasome-dependent arthritis by inhibiting macrophage necroptosis through its ZnF7 ubiquitin-binding domain. Nat. Cell Biol. 21, 731–742 10.1038/s41556-019-0324-3 [DOI] [PubMed] [Google Scholar]
- 193.Li, Y., Fuhrer, M., Bahrami, E., Socha, P., Klaudel-Dreszler, M., Bouzidi, A.et al. (2019) Human RIPK1 deficiency causes combined immunodeficiency and inflammatory bowel diseases. Proc. Natl Acad. Sci. U.S.A. 116, 970–975 10.1073/pnas.1813582116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cuchet-Lourenco, D., Eletto, D., Wu, C., Plagnol, V., Papapietro, O., Curtis, J.et al. (2018) Biallelic RIPK1 mutations in humans cause severe immunodeficiency, arthritis, and intestinal inflammation. Science 361, 810–813 10.1126/science.aar2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lehle, A.S., Farin, H.F., Marquardt, B., Michels, B.E., Magg, T., Li, Y.et al. (2019) Intestinal inflammation and dysregulated immunity in patients with inherited caspase-8 deficiency. Gastroenterology 156, 275–278 10.1053/j.gastro.2018.09.041 [DOI] [PubMed] [Google Scholar]
- 196.Gaidt, M.M. and Hornung, V. (2018) The NLRP3 inflammasome renders cell death pro-inflammatory. J. Mol. Biol. 430, 133–141 10.1016/j.jmb.2017.11.013 [DOI] [PubMed] [Google Scholar]