Abstract
Decarbonisation of the transport sector is essential to mitigate anthropogenic climate change. Microbial metabolisms are already integral to the production of renewable, sustainable fuels and, building on that foundation, are being re-engineered to generate the advanced biofuels that will maintain mobility of people and goods during the energy transition. This review surveys the range of natural and engineered microbial systems for advanced biofuels production and summarises some of the techno-economic challenges associated with their implementation at industrial scales.
Keywords: biocatalysts, biofuels, metabolic engineering, microbiology, synthetic biology
Introduction
Since the industrial revolution, the use of fossilised biomass for energy and chemicals has altered the natural carbon (C) cycle, with major impacts now felt worldwide [1]. Liquid transport fuels are a convenient form of concentrated energy with established production standards, storage facilities and distribution networks. Transport consumes ∼65% of global petroleum production and accounts for approximately one third of total energy consumption, annually [2]. Biomass-derived transport fuels (Figure 1) are therefore key to the decarbonisation of the transport sector [3].
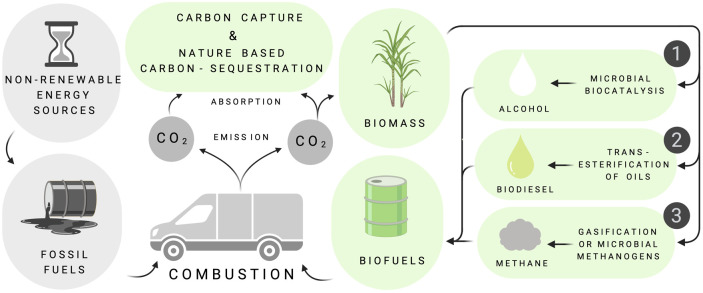
Figure 1. Routes to decarbonisation.
Decarbonisation aims to reduce the environmental impact of fossil fuel use, either by the substitution of fossil energy in the form of coal, petroleum and natural gas by fuels derived from biomass or by direct C-capture and nature-based C-sequestration strategies (termed ‘Bioenergy with Carbon Capture Solutions (BioCCS or BECCS) [4] which are currently used to offset emissions in the form of carbon credits. In the transport sector, the market penetration of emission-free electric vehicles is accelerating but is heavily reliant on C-free electrical generation capacity and accessible rapid charging infrastructures which may be difficult to implement in non-urban or more isolated settings, or in developing countries, hence the ongoing (and currently increasing) need for biofuels. Current transport biofuels include alcohols (1), lipid-derived biodiesels (2) and biomass-derived or microbially generated combustible gasses such as [bio]methane and H2 (3) that can be catalytically converted to sustainable synthetic fuels. Figure drawn using Biorender software.
Biofuels encompass solid, liquid or gaseous combustible materials that are derived from, and produced by, living organisms [5] (Figure 2). Current, commercial biofuels include microbially produced alcohols, principally ethanol (C2H5OH) from Saccharomyces cerevisiae fermentation and n-butanol (C4H9OH) from Clostridium bacteria or chemical conversion of ethanol, that are blended with gasoline. Biodiesels are produced by the catalytic conversion of tri-acyl-glycerides (TAG) with alkaline catalysts (e.g. NaOH, KOH or CH3NaO) and methanol, yielding fatty acid mono-alkyl esters (FAMEs or crude biodiesels) and glycerol. Plant oils can also be chemically hydrotreated to generate hydrogenated vegetable oils (HVOs or ‘renewable diesel fuels’ [6]) that are de-oxygenated, linear alkane hydrocarbons which can directly substitute for fossil-derived automotive gas oil [7].
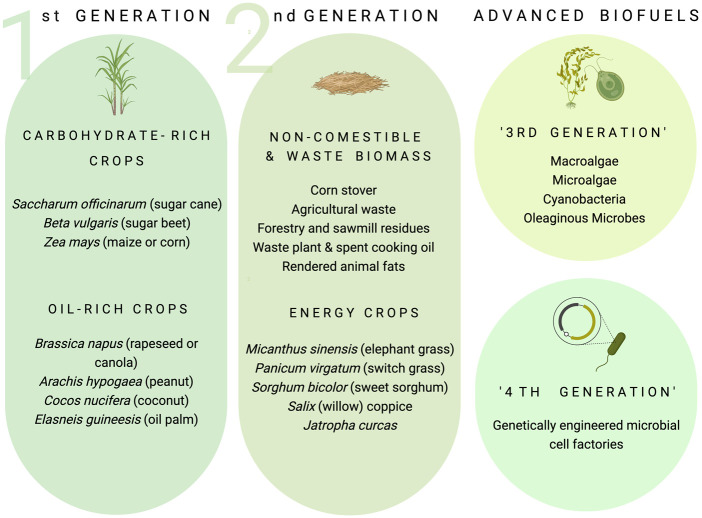
Figure 2. Classification of transport biofuels.
First (1G) and second generation (2G) biofuels describe the origin of the biomass used to manufacture the fuel. 1G biofuels are produced from carbohydrate- or oil-rich food crops whereas 2G biofuels exploit a wider variety of non-comestible and lignocellulosic energy crops, waste lignocellulosic biomass (agricultural, forestry or sawmill residues) inedible or waste plant oils, spent cooking oil or rendered animal fats. Advanced biofuels, sometimes referred to as third- or fourth- generation (3G or 4G) biofuels, encompass a range of alternative biomass sources or combustible molecules derived from microbes, notably microalgae or oleaginous yeasts, or from metabolically engineered microbial cell factories. Figure drawn using Biorender software.
‘Advanced biofuels’ encompass a range of alternative combustible molecules or biomass that are typically derived from microbes. The term ‘third generation’ (3G) is often used by researchers in academia to describe both the biomass and the fuel precursors derived from microalgae and heterotrophic microbes other than those commonly used in commercial 1G and 2G biofuel production [8,9]. Biofuels from genetically engineered microalgae, bacteria and fungi have, by extension, been dubbed ‘fourth generation’ (4G) [10–12].
Following a summary of the microbial metabolisms used for the production of current, commercialised biofuels, this review presents an overview of the variety of microbial routes to innovative biofuels and some, though by no means all, of the techno-economic challenges associated with generating advanced microbial biofuels at industrial scales.
Renewable natural gas and synthetic fuels
Renewable Natural Gas (RNG) can be produced by direct, thermochemical gasification of biomass feedstocks to generate syngas, a mix of CO, CO2 and H2, followed by chemical methanation [13], or by anaerobic fermentation (also termed ‘anaerobic digestion’; AD) of organic matter such as manure, food waste or sewerage sludge. AD generates a mix of 50–75% CH4 and 25–50% CO2, with traces of H2, siloxanes and SH2. Chemical upgrading is then performed to remove the contaminants and raise the methane content to above 90%, resulting in commercial ‘biomethane’ or ‘biogas’ [14].
Microbial methanogenesis involves 4 phases, each of which is performed by different groups of mesophilic (20–45°C) or thermophillic (50–70°C) microbes that are all present in the reactor consortium [15]. In phase 1, termed the hydrolytic phase, facultatively anaerobic bacteria degrade organic polymers from the feedstock, releasing soluble substrates. These substrates are then metabolised during the second, acidogenic phase to produce a mix of compounds including short-chain volatile fatty acids, organic acids, alcohols, ketones, and different gasses (CO2, NH3, SH2 and H2). In the third phase, acetogenesis, the products of acidogenesis are metabolised by H2-producing, acetogenic and homoacetogenic bacteria. The acetogenic bacteria, represented by Syntrophomonas, Syntrophospora, Syntrophobacter, Fusobacterium and Paleobacter, metabolise ≥ C3 organic acids, ethanol and aromatic compounds into acetate (CH3COO−), formate (CH2O2), CO2 and H2. The homoacetogenic bacteria metabolise the substrates produced in the acidogenic phase or can use H2 and CO2 via the acetyl-CoA pathway to produce acetate (CH3COO−). Finally, methanogenesis is undertaken by three groups of methanogenic archaebacteria; the hydrogenotrophs, the aceticlasts and the methylotrophs [16]. During hygenotrophic methanogenesis CO2 is reduced to CH4 and H2O, using 4 H2. In aceticlastic methanogenesis, acetate is cleaved in the presence of H+ to form CH4 and CO2 and estimates suggest that this accounts for ∼70% of the CH4 generated. In methylotropic methanogenesis, methylated C1 compounds (e.g. methanol, methylamines and di-methyl sulphide) are converted to CH4, CO2 and H2O.
Like methanated syngas produced by thermochemical biomass gasification, RNG may be used directly for energy or as precursors for catalytic re-forming to produce advanced fuel hydrocarbons [13,17,18]. Additionally, AD may be combined with other microbial processes to generate additional biofuels. For example, the fibrous material resulting from AD of cow manure can be pre-treated as a lignocellulosic feedstock and subsequently fermented to ethanol [19].
Alcohol biofuels
Ethanol
Worldwide, approximately 100 billion litres of ethanol (C2H5OH) are produced annually through the anaerobic fermentation of hexoses from 1G biomass by the yeast, S. cerevisiae. Although S. cerevisiae can also metabolise sugar aerobically via respiration, ‘Crabtree-positive’ yeast strains primarily use the fermentative pathway even when O2 is present in the medium [20].
The respiratory and fermentative (ethanogenic) pathways start with glycolysis which requires one molecule of glucose and two molecules of NAD+ to yield two molecules of ATP and two molecules of pyruvate. During respiration, the pyruvate enters the tricarboxylic acid cycle and is completely metabolised to CO2 and ATP. Under anaerobic conditions, the pyruvate is metabolised to equimolar quantities of acetaldehyde and thence to ethanol and CO2 and the recycling of NAD+. The industrial production of ethanol uses highly adapted yeast strains that can withstand the stresses imposed by large-scale bioreactor cultivation [21,22], including temperature [23], pH [24], the presence of metabolic inhibitors from treated plant biomass [25] and the accumulation of ethanol in the medium [26], such that sugar to ethanol conversion can be as high as 90% and ethanol concentrations of 20% readily achieved [27].
The production of 2G ethanol from lignocellulose first requires saccharification of the biomass to its constituent hexose and pentose monomers by various pre-treatments including chemical hydrolysis using concentrated acid, alkali, ionic liquids, or eutectic solvents; thermochemical hydrolysis that employs mechanical extrusion (milling); pyrolysis; microwaving; steam- or CO2-based explosion; and biological hydrolysis using lignin-degrading fungi (notably Trichoderma reesei), eubacteria and archaea, or purified enzyme mixes [28–35]. Lignocellulose pre-treatment yields a slurry containing high concentrations of both hexose and pentose sugars, the latter which cannot be metabolised by S. cerevisiae [36]. Pentoses, however, can be metabolised by other yeasts, including Pichia spp., Candida spp., Schizosaccharomyces spp., Pachysolen spp. and Kluyveromyces spp. [37], enbling the formulation of co-cultures capable of hexose and pentose fermentation [21,38,39]. Alternatively, hybrid yeast strains of S. cerevisiae and species capable of pentose fermentation, produced by protoplast fusion and interspecific genome shuffling, may be used to ferment both pentoses and hexoses, increasing the efficiency of the conversion from biomass to ethanol [40,41].
n-Butanol
n-Butanol is a promising alternative to ethanol because of its higher energy density, higher lubricity, lower viscosity, lower corrosiveness and lower hygroscopicity [42,43]. n-Butanol is produced by chemical conversion of ethanol using Mg or Al mixed oxides or hydroxyapatite catalysts [44], or by fermentation of 1G and 2G feedstocks via the Acetone-Butanol-Ethanol (ABE) pathway of Clostridium bacteria [45]. Clostridium are obligate anaerobes and only four species produce sufficient quantities of butanol to be industrially relevant: C. acetobutylicum (the model for ABE fermentation), C. beijerinckii (a potential candidate for lignocellulosic conversion to butanol), C. saccaroperbutylacetonicum and C. saccharoacetobutylicum [44]. ABE fermentation occurs in two stages: In stage 1, growing bacteria produce acetic and butyric acids from acetyl-CoA via a suite of enzymatic steps involving acetyl-CoA acetyltransferase (AtoB), 3-hydroxybutyryl-CoA dehydrogenase (Hbd), crotonase (Crt), butyryl-CoA dehydrogenase (Bcd), and alcohol/aldehyde dehydrogenase (AdhE2). In stage 2, the bacteria enter stationary phase during which they accumulate granulose, form endospores and re-assimilate the metabolic acids to form acetone, butanol, ethanol, CO2 and H2.
Several techno-economic challenges associated with the biocatalytic production of butanol at scale remain, including the cost of the fermentative process itself compared with production by chemical catalysis, low butanol yields due to the cytotoxicity of butanol and difficulties in improving or engineering Clostridium directly [46–48]. Consequently, the genes encoding the Clostridium butanol production pathway have been engineered in the more tractable and facultatively anaerobic E. coli [49,50] enabling pathway improvements through enzyme engineering [51]. Finally, as an alternative, ‘advanced’ n-butanol can also be generated through engineered decarboxylation and reduction in short-chain α-keto acids (Figure 3) by an α-keto acid decarboxylase/alcohol dehydrogenase combination [52]. However, these improved or alternative metabolic routes can only be commercially realised if associated with a more profound understanding of the molecular mechanisms of n-butanol tolerance and the consequent development of new host strains or biocatalysts in which that tolerance is significantly enhanced [53].
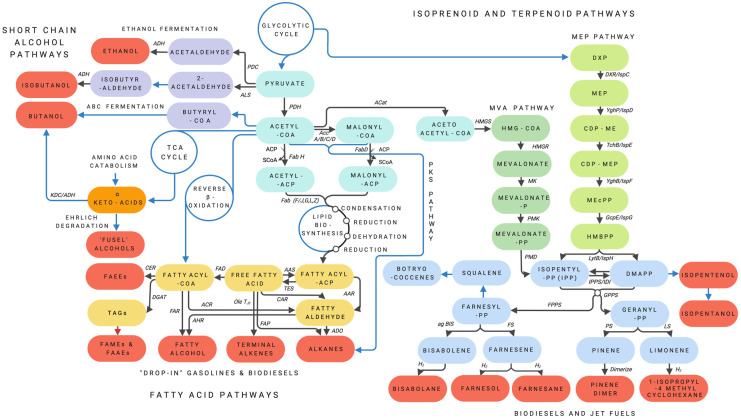
Figure 3. Natural and engineered metabolic pathways to biofuels.
Schematic diagram of natural and engineered pathways to biofuels and biofuel precursors in microbial platforms. Note, not all pathways are simultaneously present in any single microbe. Metabolic pathways are coloured thus — Core metabolism: light blue. Short chain alcohol pathways: purple. The Ehrlich degradative pathway to fusel alcohols: orange. Fatty acid metabolism: yellow. Isoprenoid MVA and MEP pathways: green. Terpenoid synthetic pathways: blue. The various biofuels are highlighted in red. Black arrows represent enzymatic transformations, with the enzymes or enzyme classes indicated. Blue arrows represent multiple enzymatic transformations in the metabolic pathways indicated. The red arrow represents abiotic, chemical conversion of TAGs to FAMEs and FAAEs. Abbreviations for enzymes are: PDC, Pyruvate dehydrogenase complex; ALS, Acetolactate (acetohydroxyacid) synthase; ADH, Alcohol dehydrogenase; KDC, α-keto acid decarboxylase; PDH, Pyruvate dehydrogenase; FabH, 3-oxoacyl-[acyl-carrier-protein] synthase; FabD, Malonyl CoA-acyl carrier protein transacylase; Acc A/B/C/D, Acetyl-CoA carboxylase; ACat, Acetyl transferase; AAS, Acyl-ACP synthase; TES, Thioesterase; AAR, Acyl-ACP reductase; FadD, acyl-CoA synthase; OleTJE, CYP152L1(cytochrome P450 fatty acid peroxygenase); FAP, fatty acid photodecarboxylase; CAR, carboxylic acid reductase; CER, Wax ester synthase; DGAT, Diglyceride acyltransferase; FAR, Fatty acid reductase; ACR, acyl-CoA reductase; AHR, Aldehyde reductase; ADO, aldehyde deformylating oxygenase; HMGS, 3-hydroxy-3-methylglutaryl-CoA synthase; HMGR, HMG-CoA reductase; MK, mevalonate kinase; PMK, phosphomevalonate kinase; PMD, phosphomevalonate decarboxylase; DXR/ispC, DXP reductoisomerase; IPPS, IPP isomerase; IDI, Isopentenyl-diphosphate delta isomerase; GPPS, geranyl diphosphate synthase; PS, pinene synthase; LS, limonene synthase; agBIS, bisabolene synthase; FS, farnesene synthase.
Engineering the production of alternative short chain and higher alcohols
The need to replace petroleum has also focussed on the microbial production of alternative short chain (≤C4) alcohols, notably n-propanol (C3H7OH) and isopropanol (CH3CHOHCH3) [54]. While n- and iso-propanol are combustible, their production cost and marginal energy gain relative to ethanol and the advantages of butanol as a fuel mean that n-propanol is probably better suited as a precursor chemical or fuel additive than as an alternative bulk biofuel.
Higher alcohols, characterised by C-chains longer than C4 are promising biofuels but, like n- or iso-propanol, do not accumulate naturally in microbes and so must be produced by metabolic engineering [55]. Higher alcohols may also be produced from amino-acid catabolism via the Ehrlich pathway (Figure 3) to yield so-called ‘fusel alcohols’. In this pathway, valine, leucine, isoleucine, methionine, and phenylalanine may be transaminated to α-keto acids and converted by alcohol dehydrogenases into fusel alcohols or, depending on the cellular redox state, to carboxylic acids. The C-chain of the fusel alcohols is equivalent to that of the amino-acid substrate minus 1 C [56].
Fatty alcohols, notably 1-octanol, are attractive targets for biofuels as they may be used to increase the physio-chemical properties of fuel blends [57]. Long-chain acyl-CoAs or acyl-ACPs are reduced to fatty alcohols via fatty-aldehyde intermediates by various acyl-CoA/ACP reductases (Figure 3). Alternatively, free fatty acids may be reduced to fatty alcohols by carboxylic acid reductases (CAR [58]) or by the Lux C/D/E complex from bioluminescent bacteria [11], also via fatty-aldehydes. The presence throughout the phylogeny of these different enzyme families provides opportunities for metabolic engineering and production of fatty-alcohols with specific C-chain lengths, in heterologous hosts including E. coli and yeasts [59–61]. However, the over-production of fatty-alcohols for biofuels is not trivial; obstacles include metabolic repression that inherently act to limit productive titres, accurate C-chain length modification and the functionalisation of the fatty acid intermediates into the desired product(s) [62] and remains an area of intense research.
Third generation microbial oils, mycodiesels and algal biofuels
Oleaginous microbes are defined as those that can accumulate over 20% of their biomass as TAG [63], usually in intracellular vesicles. Bacteria store energy principally as poly-β-hydroxy-butyrates or -alkanaoates, not TAGs, so ‘oleaginous microbes’ are primarily represented by some yeast and filamentous fungi (e.g. Candida, Cryptococcus, Debaryomyces, Lipomyces, Rhodotorula, Rhodosporidium, Saccharomycodes, Trichosporon and Yarrowia), and microalgae.
Oleaginous yeasts
Oleaginous yeasts (the model for which is Yarrowia lipolytica) are amenable to large-scale fermentation and present an attractive platform for generating natural and modified long-chain lipids and TAGs from both 1G and 2G feedstocks [64,65]. TAG accumulation in oleaginous yeasts is dependent on culture conditions such as a low C:N ratio, temperature, pH, [O2] and the concentration of trace elements and inorganic salts [64,66]. In response to nitrogen limitation, AMP-desanimase degrades intracellular adenosine monophosphate (AMP) to inosine monophosphate and NH4+ for use as an alternative source of nitrogen. The rapid decrease in [AMP] suppresses the activity of NAD- and NADP-dependent isocitrate dehydrogenase, altering the Krebs cycle and resulting in the accumulation of iso-citric acid and citrate within mitochondria. Citrate is then exported from the mitochondria in exchange for cytoplasmic malate, and cleaved by ATP citrate lyase, an enzyme exclusively found in oleaginous microorganisms, to form oxaloacetate and acetyl-CoA which is the precursor of lipid biosynthesis by reverse β-oxidation [64,66]. The fatty acids produced in this manner are subsequently stored as TAG (Figure 3) that may be extracted and converted to biodiesel.
Economically efficient production of microbial biodiesels requires economically efficient fermentation, harvesting and processing i.e. the use of cheap raw materials with minimal fermentation inhibitors [67], simple monitoring and adjustment of the culture media C : N ratio, and cost-effective processes for lipid extraction and subsequent processing [68]. For even high-producing oleaginous yeasts, separating the biomass from the culture medium and disruption of the yeast cell wall to extract the storage lipids is expensive. Consequently, microorganisms that secrete rather than store the lipids they produce can simplify downstream processing [12] and increase efficiency. To that end, lipid secretion has been achieved in modified strains of Trichosporon cutaneum, Candida lipolytica, and acetyl-CoA synthase deletion mutants of S. cerevisiae [69,70], and, although the precise mechanism of secretion remains unknown, continued research has the potential to lower processing costs and increase the potential of oleaginous yeasts as a renewable source of lipid for biodiesel.
Mycodiesels
Mycodiesels [71] describe a range of volatile organic compounds produced by some endophytic fungi, notably Gliocladeum roseum [72] and Ascoryne sarcoides [73,74], and include acetic acid esters of straight chained alkanes and higher alcohols that might readily be converted to drop-in biofuels. Currently basic research into mycodiesels appears somewhat quiescent, however the molecular pathways to these promising metabolic products are largely uncharacterised and may yet be exploited as resources for engineering innovative metabolic pathways in more tractable microbial hosts.
Advanced biodiesels from microalgae
Microalgae are a polyphyletic grouping comprising over 40 000 identified species of photoautotrophic microbes living in a range of environments, from Antarctic ice to the edges of volcanic hot-springs and from fresh to hypersaline waters. The production of advanced biofuels from microalgae has several theoretical advantages compared with terrestrial biomass: Microalgae may be cultured on marginal or non-arable land thus circumventing the food vs. fuel controversy of G1 biofuels [75] and in brackish, saline or wastewater thereby avoiding use of increasingly precious freshwater [76,77]. Microalgal cell walls are composed of cellulose, shorter polysaccharides and protein and only a few species possess lignin [78]. Consequently, microalgal cultures yield a more homogenous biomass than that produced by multicellular plants, which limits post-harvest waste and simplifies the fermentation of non-oleaginous species grown as a (3G) biomass crop or the second-stage processing of algal residues following extraction of oil or other, higher value compounds for which the algae may be exploited. Most importantly, depending on the species, location of the algal culture facility and culture conditions, microalgae have substantially higher biomass and lipid productivity compared with terrestrial feedstocks [79–81]. For example, an oleaginous microalga that produces 30% of its fresh biomass as oil can achieve biodiesel yields of ∼52 000 kg ha−1 yr−1 compared with ∼150 kg ha−1 yr−1 for Zea mays (maize; corn), ∼860 kg ha−1 yr−1 for Brassica napus (rapeseed; canola) and ∼5000 kg ha−1 yr−1 for Elaeis guineensis (oil palm), although these figures are extrapolations from laboratory experiments and may not scale as anticipated [82].
Oleaginous microalgae such as Chlorella vulgaris, Scesedesmus spp. and Nannochloropsis have attracted considerable interest as a source of TAG-derived biodiesel [83]. Under nitrogen or phosphate limitation, normal cell division is repressed resulting in excess electrons in the photosynthetic electron transport chain which increases photo-oxidative stress that may damage the photosynthetic apparatus or other cellular components. In response, oleaginous algae divert that excess energy towards fatty acid and TAG biosynthesis, consuming approximately twice the NADPH derived from the electron transport chain than is required to synthesise a comparable mass of carbohydrate or protein [84]. TAGs accumulate in cytoplasmic liposomes or chloroplastic plastioglobuli and can represent 20–50% of the dry cell mass [84]. Levels of TAG accumulation is species- or strain-specific and, despite many screening initiatives, there is little consensus regarding those most suited to TAG-derived biodiesel production [85,86]. That problem is compounded by the fact that microalgal lipid profiles are sensitive to environmental factors and the age of the culture [87] and this variability imposes severe quality control issues and costs for the biodiesel producer. Moreover, despite decades of research into cultivation, processing technologies and life-cycle assessments of microalgal culture [88,89], including the possibilities offered by polyculture to limit population crashes, integrated biorefineries in which the microalgae are used to fix waste CO2, purify waste water and produce both high-value chemicals and biomass [90], the translation of laboratory findings to large-scale culture, the upfront capital expenses of suitable land and culture installations at scales that are compatible with fuel production and the operational and downstream processing costs of algal biofuels impose extremely high barriers to investment relative to 1G or 2G biomass. Addressing and overcoming these techno-economic barriers remains an area of intense research [91,92].
Microalgal hydrocarbons
The green alga, Botryococcus braunii, has attracted considerable interest as a possible source of advanced biofuel due to its capacity to both synthesise and secrete 5–80% of its dry mass as C20–C40 hydrocarbons [93–95] that are readily converted to transport fuels by catalytic cracking [96,97]. B. braunii are grouped into four phenotypic races — A [98,99], B [100,101], L [102–104] and S [105] — depending on the hydrocarbons produced and the metabolic pathways employed. In the B race, intracellular liposomes contain ∼7% of total hydrocarbons with the rest located in the algal cell walls and extracellular matrix of the colony [95], a location that favours non-destructive extraction of the hydrocarbons [106]. Despite these considerable advantages, the production of biofuels from B. braunii is hindered by the same economic considerations of large-scale algal culture; the costs of the installation, culture and processing still vastly exceed that of the product.
A recent and exciting development in algal biology was the discovery of a light-activated enzyme from the microalga Chlorella variabilis, named fatty acid photodecarboxylase (FAP), which catalyses the decarboxylation of free fatty acids to n-alkanes or -alkenes in response to blue light [107]. Engineered into E. coli and in conjunction with a thioesterase FAP expression resulted in the production of C11–C17 hydrocarbons when illuminated by low-irradiance blue light [108].
Engineering microbial metabolisms for advanced biofuels
While short-chain alcohols, biodiesels and HVOs from 1G and 2G feedstocks are the current solution to fossil fuel mitigation in the transport sector, these are additives that, at high concentrations, compromise fuel quality [109,110]. Direct replacement of fossil-derived base-fuel with straight and cyclic hydrocarbons of varying C-chain lengths and degrees of saturation is therefore desirable [11]. Although the metabolic capacity for hydrocarbon biosynthesis is widely distributed, except for the few oleaginous microbes described above such molecules are produced only in minute quantities (<0.1% of dry mass) and serve primarily physiological rather than energy storage functions, including the regulation of membrane fluidity and permeability, cell–cell signalling, and as a defence against desiccation or environmental toxins [111]. Consequently, the production of direct fuel replacements must be engineered in microbes and would not have been possible without the discovery, characterisation and engineering of enzymes from across the phylogeny [112] such as aldehyde deformylating oxygenases (ADO) from cyanobacteria [113], the ECERIFERUMs (CER) from higher plants [114], insect cytochrome P450's (CYP) [115], the fatty acid decarboxylases P450OleTJE from Jeotgalicoccus [116] and UndA from Pseudomonas [117] and the FAP [107] that perform the final conversions of endogenous metabolic products to possible fuel molecules. In these investigations, molecules derived from the fatty-acid, terpenoid and polyketide anabolic pathways are typically targeted as substrates for engineering advanced microbial biofuels (Figure 3). These developments owe much to the increased use of Synthetic Biology [118,119] for the design, engineering and iterative development of process-tailored microbes expressing new, proof-of-principle metabolic pathways for the synthesis of potential fuel molecules.
Engineering fatty-acid advanced derived biofuels
While the enzymes involved are different, fatty acid (FA) synthesis is mechanistically conserved between the prokaryotes and eukaryotes. During FA synthesis, the intermediate metabolites are covalently bound to acyl carrier protein (ACP) by thioester linkages between the carboxyl group of the intermediates and the Ser36 of the ACP. FA synthesis occurs in two stages; initiation and cyclic elongation of the C-chain. During initiation of straight-chain FAs, malonyl Co-A is converted to malonyl-ACP which is condensed with acetyl-CoA to produce β-keto-butyryl-ACP. This compound is then reduced to generate D3-hydroxybutyryl-ACP, dehydrated to 2-enoyl-ACP and then reduced to butyryl-ACP. A cycle of condensation with malonyl-ACP and subsequent reduction-dehydration-reduction in the intermediate metabolites builds the C-chain. When the elongating fatty acyl-ACP has achieved a specific length, it is cleaved from the acyl-ACP by thioesterases (TES) to produce free FA (FFA). Overexpression of different, heterologous TES enable FFA tailoring to specific C-chain lengths [11]. Cellular FFA pools are finely balanced between synthesis and degradation (β-oxidation), which initial step is FFA conjugation with acyl-Co by acyl-CoA synthase (FadD) to produce fatty acyl-CoAs and thence acetyl-CoA by degradative thiolases producing metabolic energy. Interestingly, FFA degradation may be reversed (a process termed ‘reverse β-oxidation’) in which the thiolases function as synthetic enzymes, generating long-chain fatty acids directly from acetyl-CoA rather than first requiring malonyl-CoA, thereby increasing the C-efficiency of the overall process [120].
Early metabolic transformations focussed on engineering organisms as catalysts for existing biofuels, for example the production of biodiesel-like compounds in E. coli through conversion of free FAs to FAME by heterologous expression of a Mycobacterium marinarum fatty acid O-methyl transferase, using endogenous S-adenosylmethionione as the methyl donor [121]. FA metabolism has also been engineered to generate drop-in fuel molecules that are identical with petroleum distillates. FFA can be reduced to fatty aldehydes by the luxCED complex [11] or by CAR [58] and these are then reduced by ADO [113] to alkanes and alkenes of varying C-chain lengths. Either system has its merits, with the luxCED system allowing more refined tailoring of C-chain length and the CAR having a broader range of C-chain selectivity [122]. Alternatively, fatty acyl-ACPs may also be reduced to fatty-aldehydes by an NAD(P)H-dependent fatty acyl-ACP reductase (AAR) [59] and thence to alkanes. Fatty aldehydes and acyl-CoA's may be converted to fatty alcohols, respectively, by aldehyde reductases (AHR) and acyl-CoA reductases (ACR) [123,124]. Finally, as noted previously, a number of other enzymes (e.g. CER, CYP, OleTJE UndA and FAP) have been identified that catalyse the products of lipid biosynthesis to suitable, advanced biofuels. Some bacteria, notably Bacillus subtilis, may generate branched chain FAs, in which the initial acetyl Co-A is replaced by a different primer — e.g. isovaleryl-CoA, isobutyryl-CoA, or 2-methylbutyryl-CoA — each derived from the metabolic pathways for valine, leucine and isoleucine. Blending of branched and straight chain molecules allows tailoring and optimisation of the fuels.
While these proofs-of-principle are encouraging, given the levels of global fuel consumption, it is both necessary and possible to increase the titres of biofuels or precursors produced in this manner [125]. For example, TES overexpression has been shown to increase FFA titres [126], possibly by enhancing the ‘pull’ of the metabolic sink, and deletion of FadD, the first enzyme in FA catabolism increases further cellular FFA concentration [127].
Terpenoid-derived biofuels
Isoprenoids and terpenoids are generated via two alternative metabolisms; the mevalonate and the MEP pathways [128] (Figure 3). In the former, three molecules of acetyl-CoA are condensed via acetoacetyl-CoA to 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) to mevalonic acid which is the precursor to isopentyl diphosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). IPP and DMAPP are the basic 5-carbon isoprene building blocks for further terpenoid synthesis and undergo a head-to-tail condensation to generate the monoterpene (C10) geranyl diphosphate (GPP). Additional condensations with C5 isoprenes yield farnesyl pyrophospahate (FPP), the immediate precursor of C15 sesquiterpenes and a further isoprene addition forms geranylgeranyldiphosphate (GGPP), the precursor of C20 diterpenes. FPP and GPP may also form homodimers by head-to-tail condensation, yielding C30 triterpenes and C40 tetraterpenes. In the MEP pathway, IPP and DMAPP are formed from the glycolytic cycle.
Terpenoid hydrocarbons are suitable candidates for engineering advanced biofuels due to the branches and rings found in their hydrocarbon chains. The production of C15 bisabolane has been performed in S. cerevisiae and E. coli [129]; that of limonene and oxidosqualene in Y. lipolytica [130]; farnesane [131,132] a replacement jet-fuel in E. coli; and the tricyclic sesquiterpenes epi-isozizaene and pentalenene in E. coli, and α-isocomene in S. cerevisiae [133]. While the number of possible terpenoids is vast and represents a daunting bottleneck for further development, recent developments in AI and machine-guided genomic mining hold promise for more rapid identification and testing of possible biosynthetic pathways [134], thereby accelerating and streamlining the biosynthesis of novel biofuels.
Biofuels from the polyketide synthetic pathway
Polyketides are a structurally diverse group of metabolic products that are synthesised by multienzyme complexes, termed polyketide synthases, in a modular, iterative fashion involving three steps; initiation, elongation and functionalisation [135]. Polyketide synthesis is initiated by condensation of a starter unit (usually acetyl-CoA but can be other metabolic acids/CoA conjugates) with an extender unit, malonyl-CoA or methylmalonyl-CoA. The resulting diketide can then be extended stepwise to form intermediate polyketide chains. The extender units are arranged in a linear series of polyketide synthase modules (PKSs) scaffolded by giant polypeptide chains and have thus been likened to an assembly line. Each PKS in each module consists of an acyltransferase and a ketosynthase. The manner in which the PKS modules are aligned specifies the unique biochemistries of the products generated. The modular nature of polyketide assembly is therefore particularly attractive for re-engineering to generate new compounds [136], of which tailored, drop-in biofuels are a target [137,138].
Challenges to materiality
Despite the fact that microbes already produce the bulk of (1G) biofuel used worldwide and the increasing body of research into advanced microbial biofuels, the translation from laboratory demonstration to industrial production and commercial distribution is a substantial, multidisciplinary challenge.
While S. cerevisiae remains a chassis of choice given its general use within the biofuels industry, the potential of alternative microbial hosts that can metabolise a broader range of substrates are crucial areas for investigation. Further characterisation leading to the eventual ‘domestication’ of these (currently) unconventional microbial chassis [139–141] must therefore be performed in parallel with the development of the molecular tools for engineering robust and predictable metabolic pathways in these candidate hosts [142]. Moreover, as it is improbable that a single, engineered microbe can effectively and simultaneously catabolise complex substrates and produce desired products in large quantities, the possibilities of engineering synthetic microcosms comprising biocatalysts with complementary functions have considerable potential [143,144].
As noted for single-cell oils [68] or microalgal biofuels [81,91], the production and processing costs, not the inherent capabilities of the microorganisms, may be the main impediments to commercialisation [145]. For example, the scale, configuration and operation of the production bioreactor are critical as they dictate the upfront investment, operational costs and the bio-physical environment in which the biocatalyst must thrive, including pH, temperature, [O2], type of biomass and possible toxicity of biomass derivatives, waste products and/or of the biofuel itself, and, for non-axenic substrates, competition with the endemic microbiome. Upstream, the selection, harvesting, collection logistics and pre-processing of selected biomass determines the size of the production facility which, in turn will affect the number and geography of the assets and the subsequent distribution networks and the increasingly important impacts of overall carbon-emissions [2,3], changes in land-use or agriculture systems [75], and consequences for global biodiversity [146]. Consequently, new microbial routes to biofuels should routinely be assessed by rigorous stage-appropriate life-cycle and techno-economic assessments (LCA and TCA, respectively) in which the benefits derived from each innovation and the potential costs or diverse impacts of its production and use are clearly stated and quantified [147–149].
Conclusion
Decarbonisation of the transport sector is essential to enable the transition to net zero C-emissions by 2050 but achieving that aim against a backdrop of increasing energy demand, fragmenting global energy systems and the unpredictable adoption of different technologies is challenging. Microbes display an array of metabolisms from which innovative biofuels may be derived using biomass or other renewable substrates. The engineering of microbial metabolisms by synthetic biology has generated vast new opportunities for the production of advanced biofuels that can replace petroleum distillates. However, translation from laboratory to industry remains a challenge, including the requirement for versatile, readily engineered and industrially compatible microbial biocatalysts with the necessary molecular tools and regulatory sequences, further optimisation of engineered pathways to increase productivity and ongoing techno-economic and life-cycle assessments of these opportunities.
Perspectives
Microbes already produce the bulk of commercial biofuel but new, advanced biofuels that can directly replace petroleum distillates are urgently required to accelerate decarbonisation of the transport sector.
Bacteria, yeasts and microalgae possess extraordinarily diverse metabolisms and the design and engineering of microbial metabolisms by synthetic biology offers great potential for the production of sustainable, advanced biofuels from diverse organic substrates.
The translation from laboratory to commercial production of advanced biofuels requires a multidisciplinaty effort including the development of versatile, readily engineered and industrially compatible microbial biocatalysts with the necessary molecular tools, further innovation and optimisation of engineered pathways and systematic techno-economic and life-cycle assessments of these new opportunities.
Acknowledgements
I am extremely grateful to Anna Donnan for her assistance with the figure illustrations.
Abbreviations
- AAR
acyl-ACP reductase
- ABE
Acetone-Butanol-Ethanol
- ACP
acyl carrier protein
- ACR
acyl-CoA reductases
- ADO
aldehyde deformylating oxygenases
- AHR
aldehyde reductases
- AMP
adenosine monophosphate
- CAR
carboxylic acid reductases
- DMAPP
dimethylallyl pyrophosphate
- FA
fatty acid
- FAP
fatty acid photodecarboxylase
- IPP
isopentyl diphosphate
- RNG
renewable natural gas
- TAG
tri-acyl-glycerides
- TES
thioesterases
Competing Interests
I declare no conflict of interest and assert my academic independence regarding the research performed in my laboratory and the accurate and unbiased reporting of results, regardless of funding source or sponsor.
Funding
This work was funded by the University of Exeter and grants from Shell Research Ltd.
References
- 1.IPCC report on climate change. (2021) https://www.ipcc.ch/report/ar6/wg1/#FullReport
- 2.Solaymani, S. (2019) CO2 emissions patterns in 7 top carbon emitter economies: the case of transport sector. Energy 168, 989–1001 10.1016/j.energy.2018.11.145 [DOI] [Google Scholar]
- 3.Shears, J. (2019) Is there a role for synthetic biology in addressing the transition to a new low-carbon energy system? Microb. Biotechnol. 12, 824 10.1111/1751-7915.13462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osman, A.I., Hefny, M., Abdel Maksoud, M.I.A., Elgarahy, A.M. and Rooney, D.W. (2021) Recent advances in carbon capture storage and utilisation technologies: a review. Environ. Chem. Lett. 19, 797–849 10.1007/s10311-020-01133-3 [DOI] [Google Scholar]
- 5.Sims, R., Taylor, M., Saddler, J. and Mabee, W. (2008) From 1st-to 2nd-Generation Biofuel Technologies, International Energy Agency (IEA) and Organisation for Economic Co-Operation and Development, Paris, p. 16–20 [Google Scholar]
- 6.Wojcik, E.Z., Singleton, C., Chapman, L.N., Parker, D.A. and Love, J. (2001) Plant biomass as biofuels. eLS 1, 1–11 10.1002/9780470015902.a0023716 [DOI] [Google Scholar]
- 7.Dimitriadis, A., Natsios, I., Dimaratos, A., Katsaounis, D., Samaras, Z., Bezergianni, S.et al. (2018) Evaluation of a hydrotreated vegetable oil (HVO) and effects on emissions of a passenger car diesel engine. Front. Mech. Eng. 4, 7 10.3389/fmech.2018.00007 [DOI] [Google Scholar]
- 8.Dutta, K., Daverey, A. and Lin, J.G. (2014) Evolution retrospective for alternative fuels: first to fourth generation. Renew. Energy 69, 114–122 10.1016/j.renene.2014.02.044 [DOI] [Google Scholar]
- 9.Patel, A., Arora, N., Mehtani, J., Pruthi, V. and Pruthi, P.A. (2017) Assessment of fuel properties on the basis of fatty acid profiles of oleaginous yeast for potential biodiesel production. Renew. Sustain. Energy Rev. 77, 604–616 10.1016/j.rser.2017.04.016 [DOI] [Google Scholar]
- 10.Lü, J., Sheahan, C. and Fu, P. (2011) Metabolic engineering of algae for fourth generation biofuels production. Energy Environ. Sci. 4, 2451–2466 10.1039/c0ee00593b [DOI] [Google Scholar]
- 11.Howard, T.P., Middelhaufe, S., Moore, K., Edner, C., Kolak, D.M., Taylor, G.N.et al. (2013) Synthesis of customized petroleum-replica fuel molecules by targeted modification of free fatty acid pools in Escherichia coli. Proc. Natl Acad. Sci. U.S.A. 110, 7636–7641 10.1073/pnas.1215966110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malik, S., Shahid, A., Liu, C.G., Khan, A.Z., Nawaz, M.Z., Zhu, H.et al. (2021) Developing fourth-generation biofuels secreting microbial cell factories for enhanced productivity and efficient product recovery; a review. Fuel 298, 120858 10.1016/j.fuel.2021.120858 [DOI] [Google Scholar]
- 13.Ciliberti, C., Biundo, A., Albergo, R., Agrimi, G., Braccio, G., de Bari, I.et al. (2020) Syngas derived from lignocellulosic biomass gasification as an alternative resource for innovative bioprocesses. Processes 8, 1567 10.3390/pr8121567 [DOI] [Google Scholar]
- 14.Plugge, C.M. (2017) Biogas. Microb. Biotechnol. 10, 1128–1130 10.1111/1751-7915.12854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enzmann, F., Mayer, F., Rother, M. and Holtmann, D. (2018) Methanogens: biochemical background and biotechnological applications. Amb. Express 8, 1–22 10.1186/s13568-017-0531-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deppenmeier, U.) The unique biochemistry of methanogenesis. Prog. Nucleic Acid Res. Mol. Biol. 71, 223–283 10.1016/S0079-6603(02)71045-3 [DOI] [PubMed] [Google Scholar]
- 17.Richardson, Y., Blin, J. and Julbe, A. (2012) A short overview on purification and conditioning of syngas produced by biomass gasification: catalytic strategies, process intensification and new concepts. Prog. Energy Combust. Sci. 38, 765–781 10.1016/j.pecs.2011.12.001 [DOI] [Google Scholar]
- 18.Wilson, I.A.G. and Styring, P. (2017) Why synthetic fuels are necessary in future energy systems. Front. Energy Res. 5, 19 10.3389/fenrg.2017.00019 [DOI] [Google Scholar]
- 19.Teater, C., Yue, Z., MacLellan, J., Liu, Y. and Liao, W. (2011) Assessing solid digestate from anaerobic digestion as feedstock for ethanol production. Bioresour. Technol. 102, 1856–1862 10.1016/j.biortech.2010.09.099 [DOI] [PubMed] [Google Scholar]
- 20.Pfeiffer, T. and Morley, A. (2014) An evolutionary perspective on the Crabtree effect. Front. Mol. Biosci. 1, 17 10.3389/fmolb.2014.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azhar, S.H., Abdulla, R., Jambo, S.A., Marbawi, H., Gansau, J.A., Faik, A.A.et al. (2017) Yeasts in sustainable bioethanol production: a review. Biochem. Biophys. Rep. 10, 52–61 10.1016/j.bbrep.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang, K., Wells, P., Liang, Y., Love, J., Parker, D.A. and Botella, C. (2019) Effect of diluted hydrolysate as yeast propagation medium on ethanol production. Bioresour. Technol. 271, 1–8 10.1016/j.biortech.2018.09.080 [DOI] [PubMed] [Google Scholar]
- 23.de Melo, A.H., Lopes, A.M., Dezotti, N., Santos, I.L., Teixeira, G.S. and Goldbeck, R. (2020) Evolutionary engineering of two robust Brazilian industrial yeast strains for thermotolerance and second-generation biofuels. Ind. Biotechnol. 16, 91–98 10.1089/ind.2019.0031 [DOI] [Google Scholar]
- 24.Arroyo-López, F.N., Orlić, S., Querol, A. and Barrio, E. (2009) Effects of temperature, pH and sugar concentration on the growth parameters of Saccharomyces cerevisiae, S. kudriavzevii and their interspecific hybrid. . Int. J Food Microbiol. 131, 120–127 10.1016/j.ijfoodmicro.2009.01.035 [DOI] [PubMed] [Google Scholar]
- 25.Klinke, H.B., Thomsen, A.B. and Ahring, B.K. (2004) Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Microbiol. Biotechnol. 66, 10–26 10.1007/s00253-004-1642-2 [DOI] [PubMed] [Google Scholar]
- 26.Lam, F.H., Ghaderi, A., Fink, G.R. and Stephanopoulos, G. (2014) Engineering alcohol tolerance in yeast. Science 346, 71–75 10.1126/science.1257859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang, Q., Jin, Y.L., Fang, Y. and Zhao, H. (2019) Adaptive evolution and selection of stress-resistant Saccharomyces cerevisiae for very high-gravity bioethanol fermentation. Electron. J. Biotechnol. 41, 88–94 10.1016/j.ejbt.2019.06.003 [DOI] [Google Scholar]
- 28.Margeot, A., Hahn-Hagerdal, B., Edlund, M., Slade, R. and Monot, F. (2009) New improvements for lignocellulosic ethanol. Curr. Opin. Biotechnol. 20, 372–380 10.1016/j.copbio.2009.05.009 [DOI] [PubMed] [Google Scholar]
- 29.Agbor, V.B., Cicek, N., Sparling, R., Berlin, A. and Levin, D.B. (2011) Biomass pretreatment: fundamentals toward application. Biotechnol. Adv. 29, 675–685 10.1016/j.biotechadv.2011.05.005 [DOI] [PubMed] [Google Scholar]
- 30.Pérez, J., Munoz-Dorado, J., De la Rubia, T.D. and Martinez, J. (2002) Biodegradation and biological treatments of cellulose, hemicellulose and lignin: an overview. Int. Microbiol. 5, 53–63 10.1007/s10123-002-0062-3 [DOI] [PubMed] [Google Scholar]
- 31.Hess, J.R., Wright, C.T. and Kenney, K.L. (2007) Cellulosic biomass feedstocks and logistics for ethanol production. Biofuels Bioprod. Biorefining 1, 181–190 10.1002/bbb.26 [DOI] [Google Scholar]
- 32.Shill, K., Padmanabhan, S., Xin, Q., Prausnitz, J.M., Clark, D.S. and Blanch, H.W. (2011) Ionic liquid pretreatment of cellulosic biomass: enzymatic hydrolysis and ionic liquid recycle. Biotechnol. Bioeng. 108, 511–520 10.1002/bit.23014 [DOI] [PubMed] [Google Scholar]
- 33.Shen, D., Jin, W., Hu, J., Xiao, R. and Luo, K. (2015) An overview on fast pyrolysis of the main constituents in lignocellulosic biomass to valued-added chemicals: structures, pathways and interactions. Renew. Sustain. Energy Rev. 51, 761–774 10.1016/j.rser.2015.06.054 [DOI] [Google Scholar]
- 34.Kumar, A.K. and Sharma, S. (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour. Bioprocess. 4, 1–9 10.1186/s40643-016-0134-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu, I.K., Chen, H., Abeln, F., Auta, H., Fan, J., Budarin, V.L.et al. (2021) Chemicals from lignocellulosic biomass: a critical comparison between biochemical, microwave and thermochemical conversion methods. Crit. Rev. Environ. Sci. Technol. 51, 1479–1532 10.1080/10643389.2020.1753632 [DOI] [Google Scholar]
- 36.Hahn-Hägerdal, B., Jeppsson, H., Skoog, K. and Prior, B.A. (1994) Biochemistry and physiology of xylose fermentation by yeasts. Enzyme Microb. Technol. 16, 933–943 10.1016/0141-0229(94)90002-7 [DOI] [Google Scholar]
- 37.Mussatto, S.I., Machado, E.M., Carneiro, L.M. and Teixeira, J.A. (2012) Sugars metabolism and ethanol production by different yeast strains from coffee industry wastes hydrolysates. Appl. Energy 92, 763–768 10.1016/j.apenergy.2011.08.020 [DOI] [Google Scholar]
- 38.Karagöz, P. and Özkan, M. (2014) Ethanol production from wheat straw by Saccharomyces cerevisiae and Scheffersomyces stipitis co-culture in batch and continuous system. Bioresour. Technol. 158, 286–293 10.1016/j.biortech.2014.02.022 [DOI] [PubMed] [Google Scholar]
- 39.Singh, A., Bajar, S. and Bishnoi, N.R. (2014) Enzymatic hydrolysis of microwave alkali pretreated rice husk for ethanol production by Saccharomyces cerevisiae, Scheffersomyces stipitis and their co-culture. Fuel 116, 699–702 10.1016/j.fuel.2013.08.072 [DOI] [Google Scholar]
- 40.Kumari, R. and Pramanik, K. (2013) Bioethanol production from Ipomoea carnea biomass using a potential hybrid yeast strain. Appl. Biochem. Biotechnol. 171, 771–785 10.1007/s12010-013-0398-5 [DOI] [PubMed] [Google Scholar]
- 41.Jetti, K.D., Gns, R.R., Garlapati, D. and Nammi, S.K. (2019) Improved ethanol productivity and ethanol tolerance through genome shuffling of Saccharomyces cerevisiae and Pichia stipitis. Int. Microbiol. 22, 247–254 10.1007/s10123-018-00044-2 [DOI] [PubMed] [Google Scholar]
- 42.Hönig, V., Kotek, M. and Mařík, J. (2014) Use of butanol as a fuel for internal combustion engines. Agron. Res. 12, 333–340 https://agronomy.emu.ee/vol122/2014_2_4_b5.pdf [Google Scholar]
- 43.Lapuerta, M., Rodríguez-Fernández, J., Fernández-Rodríguez, D. and Patiño-Camino, R. (2017) Modeling viscosity of butanol and ethanol blends with diesel and biodiesel fuels. Fuel 199, 332–338 10.1016/j.fuel.2017.02.101 [DOI] [Google Scholar]
- 44.Ndaba, B. and Chiyanzu, I. (2015) Marx S. n-Butanol derived from biochemical and chemical routes: a review. Biotechnol. Rep. 8, 1–9 10.1016/j.btre.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gheshlaghi, R.E., Scharer, J.M., Moo-Young, M. and Chou, C.P. (2009) Metabolic pathways of clostridia for producing butanol. Biotechnol. Adv. 27, 764–781 10.1016/j.biotechadv.2009.06.002 [DOI] [PubMed] [Google Scholar]
- 46.Wen, Z., Li, Q., Liu, J., Jin, M. and Yang, S. (2020) Consolidated bioprocessing for butanol production of cellulolytic Clostridia: development and optimization. Microb. Biotechnol. 13, 410–422 10.1111/1751-7915.13478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qureshi, N., Lin, X., Liu, S., Saha, B.C., Mariano, A.P., Polaina, J.et al. (2020) Global view of biofuel butanol and economics of its production by fermentation from sweet sorghum bagasse, food waste, and yellow top presscake: application of novel technologies. Fermentation 6, 58 10.3390/fermentation6020058 [DOI] [Google Scholar]
- 48.Veza, I., Said, M.F. and Latiff, Z.A. (2021) Recent advances in butanol production by acetone-butanol-ethanol (ABE) fermentation. Biomass Bioenergy 144, 105919 10.1016/j.biombioe.2020.105919 [DOI] [Google Scholar]
- 49.Inui, M., Suda, M., Kimura, S., Yasuda, K., Suzuki, H., Toda, H.et al. (2008) Expression of Clostridium acetobutylicum butanol synthetic genes in Escherichia coli. Appl. Microbiol. Biotechnol. 77, 1305–1316 10.1007/s00253-007-1257-5 [DOI] [PubMed] [Google Scholar]
- 50.Atsumi, S., Cann, A.F., Connor, M.R., Shen, C.R., Smith, K.M., Brynildsen, M.P.et al. (2008) Metabolic engineering of Escherichia coli for 1-butanol production. Metab. Eng. 10, 305–311 10.1016/j.ymben.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 51.Bond-Watts, B.B., Bellerose, R.J. and Chang, M.C. (2011) Enzyme mechanism as a kinetic control element for designing synthetic biofuel pathways. Nat. Chem. Biol. 7, 222–227 10.1038/nchembio.537 [DOI] [PubMed] [Google Scholar]
- 52.Atsumi, S., Hanai, T. and Liao, J.C. (2008) Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451, 86–89 10.1038/nature06450 [DOI] [PubMed] [Google Scholar]
- 53.Peabody, G.L. and Kao, K.C. (2016) Recent progress in biobutanol tolerance in microbial systems with an emphasis on Clostridium. FEMS Microbiol. Lett. 363, fnw017 10.1093/femsle/fnw017 [DOI] [PubMed] [Google Scholar]
- 54.Walther, T. and François, J.M. (2016) Microbial production of propanol. Biotechnol. Adv. 34, 984–996 10.1016/j.biotechadv.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 55.Kremer, F., Blank, L.M., Jones, P.R. and Akhtar, M.K. (2015) A comparison of the microbial production and combustion characteristics of three alcohol biofuels: ethanol, 1-butanol, and 1-octanol. Front. Bioeng. Biotechnol. 3, 112 10.3389/fbioe.2015.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hazelwood, L.A., Daran, J.M., Van Maris, A.J., Pronk, J.T. and Dickinson, J.R. (2008) The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 74, 2259–2266 10.1128/AEM.02625-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar, B.R., Saravanan, S., Rana, D., Anish, V. and Nagendran, A. (2016) Effect of a sustainable biofuel–n-octanol–on the combustion, performance and emissions of a DI diesel engine under naturally aspirated and exhaust gas recirculation (EGR) modes. Energy Convers. Manag. 118, 275–286 10.1016/j.enconman.2016.04.001 [DOI] [Google Scholar]
- 58.Akhtar, M.K., Turner, N.J. and Jones, P.R. (2013) Carboxylic acid reductase is a versatile enzyme for the conversion of fatty acids into fuels and chemical commodities. Proc. Natl Acad. Sci. U.S.A. 110, 87–92 10.1073/pnas.1216516110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu, R., Zhu, F., Lu, L., Fu, A., Lu, J., Deng, Z.et al. (2014) Metabolic engineering of fatty acyl-ACP reductase-dependent pathway to improve fatty alcohol production in Escherichia coli. Metab. Eng. 22, 10–21 10.1016/j.ymben.2013.12.004 [DOI] [PubMed] [Google Scholar]
- 60.Rutter, C.D. and Rao, C.V. (2016) Production of 1-decanol by metabolically engineered Yarrowia lipolytica. Metab. Eng. 38, 139–147 10.1016/j.ymben.2016.07.011 [DOI] [PubMed] [Google Scholar]
- 61.Krishnan, A., McNeil, B.A. and Stuart, D.T. (2020) Biosynthesis of fatty alcohols in engineered microbial cell factories: advances and limitations. Front. Bioeng. Biotechnol. 8, 1385 10.3389/fbioe.2020.610936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marella, E.R., Holkenbrink, C., Siewers, V. and Borodina, I. (2018) Engineering microbial fatty acid metabolism for biofuels and biochemicals. Curr. Opin. Biotechnol. 50, 39–46 10.1016/j.copbio.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 63.Ratledge, C. (1993) Single cell oils—have they a biotechnological future? Trends Biotechnol. 11, 278–284 10.1016/0167-7799(93)90015-2 [DOI] [PubMed] [Google Scholar]
- 64.Ratledge, C. (2002) Regulation of lipid accumulation in oleaginous micro-organisms. Biochem. Soc. Trans. 30, 1047–1050 10.1042/bst0301047 [DOI] [PubMed] [Google Scholar]
- 65.Li, Q., Du, W. and Liu, D. (2008) Perspectives of microbial oils for biodiesel production. Appl. Microbiol. Biotechnol. 80, 749–756 10.1007/s00253-008-1625-9 [DOI] [PubMed] [Google Scholar]
- 66.Papanikolaou, S. and Aggelis, G. (2011) Lipids of oleaginous yeasts. Part I: biochemistry of single cell oil production. Eur. J. Lipid Sci. Technol. 113, 1031–1051 10.1002/ejlt.201100014 [DOI] [Google Scholar]
- 67.Longanesi, L., Bouxin, F.P., Fan, J., Auta, H., Gammons, R., Abeln, F.et al. (2020) Scaled-up microwave-assisted pretreatment and continuous fermentation to produce yeast lipids from brewery wastes. Ind. Eng. Chem. Res. 59, 19803–19816 10.1021/acs.iecr.0c03463 [DOI] [Google Scholar]
- 68.Ochsenreither, K., Glück, C., Stressler, T., Fischer, L. and Syldatk, C. (2016) Production strategies and applications of microbial single cell oils. Front. Microbiol. 7, 1539 10.3389/fmicb.2016.01539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yagi, T., Hatano, A., Nakanishi, T., Hatano, T. and Fukui, S. (1994) Extracellular production of palmitoleic triglycerides by a yeast, Trichosporon. J. Ferment. Bioeng. 77, 164–168 10.1016/0922-338X(94)90317-4 [DOI] [Google Scholar]
- 70.Scharnewski, M., Pongdontri, P., Mora, G., Hoppert, M. and Fulda, M. (2008) Mutants of Saccharomyces cerevisiae deficient in acyl-CoA synthetases secrete fatty acids due to interrupted fatty acid recycling. FEBS J. 275, 2765–2778 10.1111/j.1742-4658.2008.06417.x [DOI] [PubMed] [Google Scholar]
- 71.Strobel, G. (2014) The story of mycodiesel. Curr. Opin. Microbiol. 19, 52–58 10.1016/j.mib.2014.06.003 [DOI] [PubMed] [Google Scholar]
- 72.Strobel, G.A., Knighton, W.B., Kluck, K., Ren, Y., Livinghouse, T., Griffin, M.et al. (2010) The production of myco-diesel hydrocarbons and their derivatives by the endophytic fungus Gliocladium roseum (NRRL 50072). Microbiology 156, 3830–3833 10.1099/mic.0.30824-0 [DOI] [PubMed] [Google Scholar]
- 73.Gianoulis, T.A., Griffin, M.A., Spakowicz, D.J., Dunican, B.F., Alpha, C.J., Sboner, A.et al. (2012) Genomic analysis of the hydrocarbon-producing, cellulolytic, endophytic fungus Ascocoryne sarcoides. PLoS Genet. 8, e1002558 10.1371/journal.pgen.1002558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mallette, N.D., Pankrantz, E.M., Busse, S., Strobel, G.A., Carlson, R.P. and Peyton, B.M. (2014) Evaluation of cellulose as a substrate for hydrocarbon fuel production by Ascocoryne sarcoides (NRRL 50072). J. Sustain. Bioenergy Syst. 4, 33–49 10.4236/jsbs.2014.41004 [DOI] [Google Scholar]
- 75.Thompson, P.B. (2012) The agricultural ethics of biofuels: the food vs. fuel debate. Agriculture 2, 339–358 10.3390/agriculture2040339 [DOI] [Google Scholar]
- 76.Sheehan, J., Dunahay, T., Benemann, J. and Roessler, P. (1998) Look Back at the US Department of Energy's Aquatic Species Program: Biodiesel From Algae; Close-out Report, National Renewable Energy Laboratory, Golden, CO [Google Scholar]
- 77.Rao, A.R., Dayananda, C., Sarada, R., Shamala, T.R. and Ravishankar, G.A. (2007) Effect of salinity on growth of green alga Botryococcus braunii and its constituents. Bioresour. Technol. 98, 560–564 10.1016/j.biortech.2006.02.007 [DOI] [PubMed] [Google Scholar]
- 78.Domozych, D., Ciancia, M., Fangel, J.U., Mikkelsen, M.D., Ulvskov, P. and Willats, W.G. (2012) The cell walls of green algae: a journey through evolution and diversity. Front. Plant Sci. 3, 82 10.3389/fpls.2012.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chisti, Y. (2007) Biodiesel from microalgae. Biotechnol. Adv. 25, 294–306 10.1016/j.biotechadv.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 80.Hempel, N., Petrick, I. and Behrendt, F. (2012) Biomass productivity and productivity of fatty acids and amino acids of microalgae strains as key characteristics of suitability for biodiesel production. J. Appl. Phycol. 24, 1407–1418 10.1007/s10811-012-9795-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nascimento, I.A., Marques, S.S., Cabanelas, I.T., de Carvalho, G.C., Nascimento, M.A., de Souza, C.O.et al. (2014) Microalgae versus land crops as feedstock for biodiesel: productivity, quality, and standard compliance. Bioenergy Res. 7, 1002–1013 10.1007/s12155-014-9440-x [DOI] [Google Scholar]
- 82.Bošnjaković, M. and Sinaga, N. (2020) The perspective of large-scale production of algae biodiesel. Appl. Sci. 10, 8181 10.3390/app10228181 [DOI] [Google Scholar]
- 83.Pal, P., Chew, K.W., Yen, H.W., Lim, J.W., Lam, M.K. and Show, P.L. (2019) Cultivation of oily microalgae for the production of third-generation biofuels. Sustainability 11, 5424 10.3390/su11195424 [DOI] [Google Scholar]
- 84.Hu, Q., Sommerfeld, M., Jarvis, E., Ghirardi, M., Posewitz, M., Seibert, M.et al. (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J. 54, 621–639 10.1111/j.1365-313X.2008.03492.x [DOI] [PubMed] [Google Scholar]
- 85.Griffiths, M.J., van Hille, R.P. and Harrison, S.T. (2014) The effect of nitrogen limitation on lipid productivity and cell composition in Chlorella vulgaris. Appl. Microbiol. Biotechnol. 98, 2345–2356 10.1007/s00253-013-5442-4 [DOI] [PubMed] [Google Scholar]
- 86.Slocombe, S.P., Zhang, Q., Ross, M., Anderson, A., Thomas, N.J., Lapresa, Á.et al. (2015) Unlocking nature's treasure-chest: screening for oleaginous algae. Sci. Rep. 5, 9844 10.1038/srep09844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rodolfi, L., Chini Zittelli, G., Bassi, N., Padovani, G., Biondi, N., Bonini, G.et al. (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 102, 100–112 10.1002/bit.22033 [DOI] [PubMed] [Google Scholar]
- 88.Dutta, S., Neto, F. and Coelho, M.C. (2016) Microalgae biofuels: a comparative study on techno-economic analysis & life-cycle assessment. Algal Res. 20, 44–52 10.1016/j.algal.2016.09.018 [DOI] [Google Scholar]
- 89.Pérez-López, P., de Vree, J.H., Feijoo, G., Bosma, R., Barbosa, M.J., Moreira, M.T.et al. (2017) Comparative life cycle assessment of real pilot reactors for microalgae cultivation in different seasons. Appl. Energy 205, 1151–1164 10.1016/j.apenergy.2017.08.102 [DOI] [Google Scholar]
- 90.Kumar, L. and Bharadvaja, N. (2020) A review on microalgae biofuel and biorefinery: challenges and way forward. Energy Sources A Recovery Util. Environ. Eff. 23, 1–24 10.1080/15567036.2020.1836084 [DOI] [Google Scholar]
- 91.Quinn, J.C. and Davis, R. (2015) The potentials and challenges of algae based biofuels: a review of the techno-economic, life cycle, and resource assessment modeling. Bioresour. Technol. 84, 444–452 10.1016/j.biortech.2014.10.075 [DOI] [PubMed] [Google Scholar]
- 92.Rafa, N., Ahmed, S.F., Badruddin, I.A., Mofijur, M. and Kamangar, S. (2021) Strategies to produce cost-effective third-generation biofuel from microalgae. Front. Energy Res. 9, 1–1 10.3389/fenrg.2021.749968 [DOI] [Google Scholar]
- 93.Maxwell, J.R., Douglas, A.G., Eglinton, G. and McCormick, A. (1968) The Botryococcenes—hydrocarbons of novel structure from the alga Botryococcus braunii, Kützing. Phytochemistry 7, 2157–2171 10.1016/S0031-9422(00)85672-1 [DOI] [Google Scholar]
- 94.Banerjee, A., Sharma, R., Chisti, Y. and Banerjee, U.C. (2002) Botryococcus braunii: a renewable source of hydrocarbons and other chemicals. Crit. Rev. Biotechnol. 22, 245–279 10.1080/07388550290789513 [DOI] [PubMed] [Google Scholar]
- 95.Weiss, T.L., Roth, R., Goodson, C., Vitha, S., Black, I., Azadi, P.et al. (2012) Colony organization in the green alga Botryococcus braunii (Race B) is specified by a complex extracellular matrix. Eukaryot. Cell 11, 1424–1440 10.1128/EC.00184-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hillen, L.W., Pollard, G., Wake, L.V. and White, N. (1982) Hydrocracking of the oils of Botryococcus braunii to transport fuels. Biotechnol. Bioeng. 24, 193–205 10.1002/bit.260240116 [DOI] [PubMed] [Google Scholar]
- 97.Eroglu, E. and Melis, A. (2010) Extracellular terpenoid hydrocarbon extraction and quantitation from the green microalgae Botryococcus braunii var. Showa. Bioresour Technol. 101, 2359–2366 10.1016/j.biortech.2009.11.043 [DOI] [PubMed] [Google Scholar]
- 98.Metzger, P. and Largeau, C. (2005) Botryococcus braunii: a rich source for hydrocarbons and related ether lipids. Appl. Microbiol. Biotechnol. 66, 486–496 10.1007/s00253-004-1779-z [DOI] [PubMed] [Google Scholar]
- 99.Templier, J., Largeau, C. and Casadevall, E. (1991) Biosynthesis of n-alkatrienes in Botryococcus braunii. Phytochemistry 30, 2209–2215 10.1016/0031-9422(91)83616-S [DOI] [Google Scholar]
- 100.Metzger, P., Berkaloff, C., Casadevall, E. and Coute, A. (1985) Alkadiene-and botryococcene-producing races of wild strains of Botryococcus braunii. Phytochemistry 24, 2305–2312 10.1016/S0031-9422(00)83032-0 [DOI] [Google Scholar]
- 101.Sato, Y., Ito, Y., Okada, S., Murakami, M. and Abe, H. (2003) Biosynthesis of the triterpenoids, botryococcenes and tetramethylsqualene in the B race of Botryococcus braunii via the non-mevalonate pathway. Tetrahedron Lett. 44, 7035–7037 10.1016/S0040-4039(03)01784-2 [DOI] [Google Scholar]
- 102.Metzger, P. and Casadevall, E. (1987) Lycopadiene, a tetraterpenoid hydrocarbon from new strains of the green alga Botryococcus braunii. Tetrahedron Lett. 28, 3931–3934 10.1016/S0040-4039(00)96423-2 [DOI] [Google Scholar]
- 103.Metzger, P., Allard, B., Casadevall, E., Berkaloff, C. and Couté, A. (1990) Structure and chemistry of a new chemical race of Botryococcus braunii (Chlorophyceae) that produced lycopadiene, a tetraterpenoid hydrocarbon 1. J. Phycol. 26, 258–266 10.1111/j.0022-3646.1990.00258.x [DOI] [Google Scholar]
- 104.Thapa, H.R., Naik, M.T., Okada, S., Takada, K., Molnár, I., Xu, Y.et al. (2016) A squalene synthase-like enzyme initiates production of tetraterpenoid hydrocarbons in Botryococcus braunii Race L. Nat. Commun. 7, 11198 10.1038/ncomms11198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kawachi, M., Tanoi, T., Demura, M., Kaya, K. and Watanabe, M.M. (2012) Relationship between hydrocarbons and molecular phylogeny of Botryococcus braunii. Algal Res. 1, 114–119 10.1016/j.algal.2012.05.003 [DOI] [Google Scholar]
- 106.Kleinert, C. and Griehl, C. (2021) Identification of suitable Botryococcus braunii strains for non-destructive in situ hydrocarbon extraction. J. Appl. Phycol. 33, 785–798 10.1007/s10811-020-02342-7 [DOI] [Google Scholar]
- 107.Sorigué, D., Légeret, B., Cuiné, S., Blangy, S., Moulin, S., Billon, E.et al. (2017) An algal photoenzyme converts fatty acids to hydrocarbons. Science 357, 903–907 10.1126/science.aan6349 [DOI] [PubMed] [Google Scholar]
- 108.Moulin, S., Legeret, B., Blangy, S., Sorigué, D., Burlacot, A., Auroy, P.et al. (2019) Continuous photoproduction of hydrocarbon drop-in fuel by microbial cell factories. Sci. Rep. 9, 13713 10.1038/s41598-019-50261-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arbab, M.I., Masjuki, H.H., Varman, M., Kalam, M.A., Imtenan, S. and Sajjad, H. (2013) Fuel properties, engine performance and emission characteristic of common biodiesels as a renewable and sustainable source of fuel. Renew. Sustain. Energy Rev. 22, 133–147 10.1016/j.rser.2013.01.046 [DOI] [Google Scholar]
- 110.Gopalan, K., Raikova, S., Smith, C.R., Bannister, C.D., Savvoulidi, M., Chrysafi, S.et al. (2020) The impact of biodiesel and alternative diesel fuel components on filter blocking through accelerated testing on a novel high pressure common rail non-firing rig. Fuel 282, 118850 10.1016/j.fuel.2020.118850 [DOI] [Google Scholar]
- 111.Ladygina, N., Dedyukhina, E.G. and Vainshtein, M.B. (2006) A review on microbial synthesis of hydrocarbons. Process Biochem. 41, 1001–1014 10.1016/j.procbio.2005.12.007 [DOI] [Google Scholar]
- 112.Kang, M.K. and Nielsen, J. (2017) Biobased production of alkanes and alkenes through metabolic engineering of microorganisms. J. Ind. Microbiol. Biotechnol. 44, 613–622 10.1007/s10295-016-1814-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schirmer, A., Rude, M.A., Li, X., Popova, E. and Del Cardayre, S.B. (2010) Microbial biosynthesis of alkanes. Science 329, 559–562 10.1126/science.1187936 [DOI] [PubMed] [Google Scholar]
- 114.Bourdenx, B., Bernard, A., Domergue, F., Pascal, S., Léger, A., Roby, D.et al. (2011) Overexpression of Arabidopsis ECERIFERUM1 promotes wax very-long-chain alkane biosynthesis and influences plant response to biotic and abiotic stresses. Plant Physiol. 156, 29–45 10.1104/pp.111.172320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qiu, Y., Tittiger, C., Wicker-Thomas, C., Le Goff, G., Young, S., Wajnberg, E.et al. (2012) An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis. Proc. Natl Acad. Sci. U.S.A. 109, 14858–14863 10.1073/pnas.1208650109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Belcher, J., McLean, K.J., Matthews, S., Woodward, L.S., Fisher, K., Rigby, S.E.et al. (2014) Structure and biochemical properties of the alkene producing cytochrome P450 OleTJE (CYP152L1) from the Jeotgalicoccus sp. 8456 bacterium. J. Biol. Chem. 289, 6535–6550 10.1074/jbc.M113.527325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rui, Z., Li, X., Zhu, X., Liu, J., Domigan, B., Barr, I.et al. (2014) Microbial biosynthesis of medium-chain 1-alkenes by a nonheme iron oxidase. Proc. Natl Acad. Sci. U.S.A. 111, 18237–18242 10.1073/pnas.1419701112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Keasling, J.D. and Chou, H. (2008) Metabolic engineering delivers next-generation biofuels. Nat. Biotechnol. 26, 298–299 10.1038/nbt0308-298 [DOI] [PubMed] [Google Scholar]
- 119.Savage, D.F., Way, J. and Silver, P.A. (2008) Defossiling fuel: how synthetic biology can transform biofuel production. ACS Chem. Biol. 18, 13–16 10.1021/cb700259j [DOI] [PubMed] [Google Scholar]
- 120.Dellomonaco, C., Clomburg, J.M., Miller, E.N. and Gonzalez, R. (2011) Engineered reversal of the β-oxidation cycle for the synthesis of fuels and chemicals. Nature 476, 355–359 10.1038/nature10333 [DOI] [PubMed] [Google Scholar]
- 121.Nawabi, P., Bauer, S., Kyrpides, N. and Lykidis, A. (2011) Engineering Escherichia coli for biodiesel production utilizing a bacterial fatty acid methyltransferase. Appl. Environ. Microbiol. 77, 8052–8061 10.1128/AEM.05046-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Winkler, M. (2018) Carboxylic acid reductase enzymes (CARs). Curr. Opin. Chem. Biol. 43, 23–29 10.1016/j.cbpa.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 123.Akhtar, M.K., Dandapani, H., Thiel, K. and Jones, P.R. (2015) Microbial production of 1-octanol: a naturally excreted biofuel with diesel-like properties. Metab. Eng. Commun. 2, 1–5 10.1016/j.meteno.2014.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zheng, Y.N., Li, L.L., Liu, Q., Yang, J.M., Wang, X.W., Liu, W.et al. (2012) Optimization of fatty alcohol biosynthesis pathway for selectively enhanced production of C12/14 and C16/18 fatty alcohols in engineered Escherichia coli. Microb. Cell Fact. 11, 65 10.1186/1475-2859-11-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Das, M., Patra, P. and Ghosh, A. (2020) Metabolic engineering for enhancing microbial biosynthesis of advanced biofuels. Renew. Sustain. Energy Rev. 119, 109562 10.1016/j.rser.2019.109562 [DOI] [Google Scholar]
- 126.Tee, T.W., Chowdhury, A., Maranas, C.D. and Shanks, J.V. (2014) Systems metabolic engineering design: fatty acid production as an emerging case study. Biotechnol. Bioeng. 111, 849–857 10.1002/bit.25205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lennen, R.M. and Pfleger, B.F. (2012) Engineering Escherichia coli to synthesize free fatty acids. Trends Biotechnol. 30, 659–667 10.1016/j.tibtech.2012.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chang, W.C., Song, H., Liu, H.W. and Liu, P. (2013) Current development in isoprenoid precursor biosynthesis and regulation. Curr. Opin. Chem. Biol. 17, 571–579 10.1016/j.cbpa.2013.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Peralta-Yahya, P.P., Ouellet, M., Chan, R., Mukhopadhyay, A., Keasling, J.D. and Lee, T.S. (2011) Identification and microbial production of a terpene-based advanced biofuel. Nat. Commun. 2, 483 10.1038/ncomms1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Arnesen, J.A., Kildegaard, K.R., Cernuda Pastor, M., Jayachandran, S., Kristensen, M. and Borodina, I. (2020) Yarrowia lipolytica strains engineered for the production of terpenoids. Front. Bioeng. Biotechnol. 8, 945 10.3389/fbioe.2020.00945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Peralta-Yahya, P.P., Zhang, F., Del Cardayre, S.B. and Keasling, J.D. (2012) Microbial engineering for the production of advanced biofuels. Nature 488, 320–328 10.1038/nature11478 [DOI] [PubMed] [Google Scholar]
- 132.Walls, L.E. and Rios-Solis, L. (2020) Sustainable microbial isoprenoid derived advanced biojet fuel production: a review. Front. Bioeng. Biotechnol. 8, 1272 10.3389/fbioe.2020.599560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu, C.L., Tian, T., Alonso-Gutierrez, J., Garabedian, B., Wang, S., Baidoo, E.E.et al. (2018) Renewable production of high density jet fuel precursor sesquiterpenes from Escherichia coli. Biotechnol. Biofuels 11, 1–5 10.1186/s13068-017-1003-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ramzi, A.B., Baharum, S.N., Bunawan, H. and Scrutton, N.S. (2020) Streamlining natural products biomanufacturing with omics and machine learning driven microbial engineering. Front. Bioeng. Biotechnol. 8, 608918 10.3389/fbioe.2020.608918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Katz, L. (1997) Manipulation of modular polyketide synthases. Chem. Rev. 97, 2557–2576 10.1021/cr960025+ [DOI] [PubMed] [Google Scholar]
- 136.Maschio, L., Parnell, A.E., Lees, N.R., Willis, C.L., Schaffitzel, C., Stach, J.E.et al. (2019) Cloning, expression, and purification of intact polyketide synthase modules. Methods Enzymol. 617, 63–82 10.1016/bs.mie.2018.12.018 [DOI] [PubMed] [Google Scholar]
- 137.Yuzawa, S., Keasling, J.D. and Katz, L. (2017) Bio-based production of fuels and industrial chemicals by repurposing antibiotic-producing type I modular polyketide synthases: opportunities and challenges. J. Antibiot. 70, 378–385 10.1038/ja.2016.136 [DOI] [PubMed] [Google Scholar]
- 138.Cai, W. and Zhang, W. (2018) Engineering modular polyketide synthases for production of biofuels and industrial chemicals. Curr. Opin. Biotechnol. 50, 32–38 10.1016/j.copbio.2017.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Calero, P. and Nikel, P.I. (2019) Chasing bacterial chassis for metabolic engineering: a perspective review from classical to non-traditional microorganisms. Microb. Biotechnol. 12, 98–124 10.1111/1751-7915.13292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Adegboye, M.F., Ojuederie, O.B., Talia, P.M. and Babalola, O.O. (2021) Bioprospecting of microbial strains for biofuel production: metabolic engineering, applications, and challenges. Biotechnol. Biofuels 14, 5 10.1186/s13068-020-01853-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Singleton, C., Gilman, J., Rollit, J., Zhang, K., Parker, D.A. and Love, J. (2019) A design of experiments approach for the rapid formulation of a chemically defined medium for metabolic profiling of industrially important microbes. PLoS ONE 14, e0218208 10.1371/journal.pone.0218208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gilman, J., Singleton, C., Tennant, R.K., James, P., Howard, T.P., Lux, T.et al. (2019) Heuristic discovery and design of promoter collections in non-model microbes for industrial applications. ACS Synth. Biol. 8, 175–1186 10.1021/acssynbio.9b00061 [DOI] [PubMed] [Google Scholar]
- 143.Gavande, P.V., Basak, A., Sen, S., Lepcha, K., Murmu, N., Rai, V.et al. (2021) Functional characterization of thermotolerant microbial consortium for lignocellulolytic enzymes with central role of Firmicutes in rice straw depolymerization. Sci. Rep. 11, 3032 10.1038/s41598-021-82163-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jiang, Y., Dong, W., Xin, F. and Jiang, M. (2020) Designing synthetic microbial consortia for biofuel production. Trends Biotechnol. 38, 828–831 10.1016/j.tibtech.2020.02.002 [DOI] [PubMed] [Google Scholar]
- 145.Koutinas, A.A., Chatzifragkou, A., Kopsahelis, N., Papanikolaou, S. and Kookos, I.K. (2014) Design and techno-economic evaluation of microbial oil production as a renewable resource for biodiesel and oleochemical production. Fuel 116, 566–577 10.1016/j.fuel.2013.08.045 [DOI] [Google Scholar]
- 146.International Renewable Energy Agency (IRENA). 2019. Advanced Biofuels: What Holds Them Back?, pp. 52–56 ISBN 978-92-9260-158-4 [Google Scholar]
- 147.Scown, C.D., Baral, N.R., Yang, M., Vora, N. and Huntington, T. (2021) Technoeconomic analysis for biofuels and bioproducts. Curr. Opin. Biotechnol. 67, 58–64 10.1016/j.copbio.2021.01.002 [DOI] [PubMed] [Google Scholar]
- 148.Zimmermann, A.W., Wunderlich, J., Müller, L., Buchner, G.A., Marxen, A., Michailos, S.et al. (2020) Techno-economic assessment guidelines for CO2 utilization. Front. Energy Res. 8, 5 10.3389/fenrg.2020.00005 [DOI] [Google Scholar]
- 149.Reijnders, L. (2021) Life cycle assessment of biofuels. Methods Mol. Biol. 2290, 53–67 10.1007/978-1-0716-1323-8_4 [DOI] [PubMed] [Google Scholar]