Abstract
This study used 56 aborted and stillborn fetuses from organized swine farms in Tamil Nadu and Kerala, southern states of India. All samples were screened by using a PCR assay that targets the NS1 gene for PPV. Furthermore, the PCR positive samples were subjected to amplification of the VP2 gene of PPV1 with designed primers and sequenced for further study. The PCR screening of 56 samples found that 14.3% (n = 8) were positive for PPV genome. According to VP2 gene–based PCR for PPV1, 897 bp specific amplicons were detected in all eight of the samples. Two of the eight positive samples (L17 and T5) were sequenced and annotated randomly. The BLAST analysis of contig sequence INDTNCHN-T5 revealed 100% sequence homology with Chinese PPV1genome, whereas sequence from INDTNCHN-L17 revealed 99.43% sequence homology with Spain, Chinese, and German. PPV1 sequences and both the sequences INDTNCHN-T5 and INDTNCHN-L17 were submitted to the GenBank under the accession numbers MW822566 and MW822567 respectively. A phylogenetic analysis of the sequences in this study revealed specific grouping along with PPV1 strains in cluster E. Amino acid analysis of both isolated sequences in addition to the reference sequence from PPV1 showed variations in position 215 (I to T) in both the isolates, variation at position 228 (Q to E) in T5 isolate and variations at position 59 (L to M) and 314 (K to E) in L17 isolate. This study represents the first report of PPV1 cluster E in Tamil Nadu, southern India.
Keywords: Reproductive failure, Porcine parvovirus 1, Molecular detection, Characterization
Introduction
Porcine Parvovirus 1 (PPV1) is one of the prime causative agents associated with SMEDI (stillbirth, mummification, embryonic death, and infertility) syndrome, which causes marked loss to the swine industry worldwide (Mengeling et al. 2000). PPV was first isolated as a cell culture contaminant from a porcine primary cell culture which was used for propagation of classical swine fever virus in Germany during the early 1960s (Mayr and Mahnel 1964). The incidence of PPV associated with abortions in swine was first described by Carwright and Huck (1967). Parvoviruses are small, non-enveloped, single-stranded, and negative-sense DNA virus that belongs to the family Parvoviridae (Molitor et al. 1984). Apart from classical PPV1, six novel porcine parvoviruses (PPV2–PPV7) were described in the past two decades (Palinski et al. 2016). As per International Committee on Taxonomy of Viruses (ICTV) classification, PPV1 belongs to the genus Protoparvovirus, whereas novel parvoviruses PPV2-PPV3, PPV4–PPV6, and PPV7 belong to the genera Tetraparvovirus, Copiparvovirus, and Chapparvovirus respectively (Xing et al. 2018). Currently, PPV1 is a well-evidenced and documented pathogen associated with reproductive failure in many swine producing countries (Zhang et al. 2010). All the newer PPV genotypes were detected in China, the USA, and Poland (McKillen et al. 2007). Incidence of PPV2, PPV3, and PPV4 is well documented in Hungary, Romania, Thailand, Japan, and South Africa (Cadar et al. 2013; Cságola et al. 2012; Saekhow and Ikeda 2015; Saekhow et al. 2016; Afolabi et al. 2018). PPV6 was reported to be co-infected with PCV2, PRRS viruses, etc. and causes abortion in pregnant sows (Schirtzinger et al. 2015). PPV7 was first discovered in 2016 from adult pigs in the USA and subsequently in Poland and Korea (Ouh et al. 2018). Unlike PPV1, the importance of novel porcine parvoviruses on swine health is poorly understood and these novel viruses were detected in healthy as well as suspected swine populations (Boisvert et al. 2010). There is a paucity of information on host spectrum and pathogenic potential of novel PPV2-7 genotypes (Xiao et al. 2013).
The PPV1 genome encodes for two open reading frames (ORFs), ORF1 found at the 5′ end of genome, and codes for three non-structural (NS) proteins (NS1, 2, and 3) and plays a vital role in viral replication. The ORF2 gene which is located at the 3′end of the viral genome codes for three structural capsid viral proteins (VP1, 2, and 3). The larger VP1 and smaller VP2 are translated from a nested set of coding sequences and VP2 is produced by splicing from VP1, differing only in their amino terminus. There are 729 amino acid residues in VP1, of which 120 amino acids at amino-terminal sites are unique to VP1. VP3 is a post-translational modification product of VP2. These structural proteins help the virus to recognize and bind to the host cell nucleus and establish infection (Bergeron et al. 1996; Simpson et al. 2002). This is one of the mutating DNA viruses with 3–5 × 10−4 and 1 × 10−5 mutations/site/year were observed in VP genes and NS genes respectively. NS1 gene was highly conserved among different PPV genotypes, whereas the VP2 gene was not much conserved. VP2 region is the major protective antigen and contains major antigenic domains that elicit neutralizing antibodies (Truyen and Streck 2012; Mengeling 2006; Martínez e al. 1992). Classical porcine parvovirus 1 has been the most studied virus around the globe as an important cause of porcine fetal death. PPV1 infection during the first 70 days of gestation can lead to reproductive failure and infection in post 70 days of gestation with immune-competent fetuses, develop an antibody response, and usually survive the infection. Two weeks after the initial infection of the dam, the virus spreads vertically to the fetus and establishes infection (Mengeling et al. 1980). It is one of the sturdiest virus found in the swine community it even withstands temperature of 90 °C. The virus particles resist common disinfectants like 70% ethanol, 0.05% quaternary ammonium compounds, low concentrations of sodium hypochlorite, and 0.2% peracetic acid. Affected animal sheds viruses in the environment through their natural orifices and the virus remains intact in farm premises and tools for months together and favors natural horizontal transmission of virus (Truyen and Streck 2012). At present, abortion and stillborn conditions in swine populations are alarming in south India, but in Tamil Nadu as of date, there is no documented evidence on PPV incidence. As per literature survey in southern India, there is only one PPV molecular incidence report documented from Kerala (Aishwarya et al. 2016). PPV molecular characterization and phylogenetic studies are scarce in southern states of India; hence, this study has been undertaken.
Materials and methods
Collection and processing of samples
A total of 56 tissue samples (pool of visceral organs, lymphoid organs, and placental tissues) were aseptically collected from aborted/mummified/dead fetuses of swine (Fig. 1) from different parts of Tamil Nadu and Kerala during the period 2020–2021. Collected tissues were triturated in a pestle and mortar with sterile sand in phosphate-buffered saline (PBS). Homogenized tissues were clarified at 6000 rpm for 15 min at room temperature to sediment all tissue debris. Supernatants were subjected to DNA extraction using DNeasy Blood and Tissue Kit (Qiagen, Germany) following the standard manufacturer’s protocol and extracted nucleic acid was used as a template for screening PPV genome.
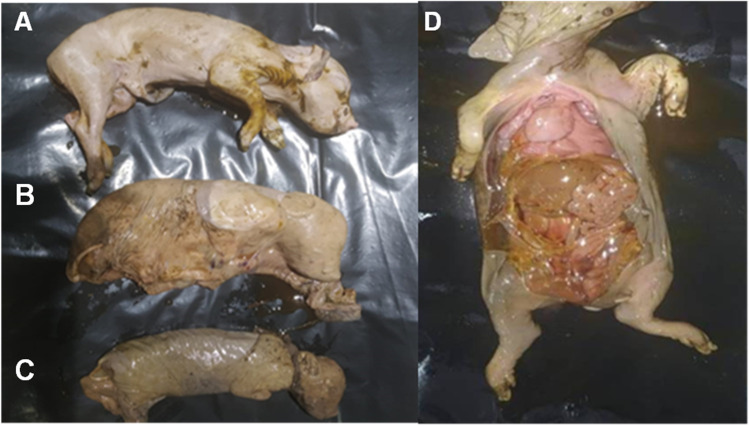
Fig. 1.
Aborted, stillborn and mummified fetuses of porcine. A. Aborted dead fetus; B. Stillborn fetus covered with fetal membrane; C. Mummified fetus; D. Postmortem examination of stillborn fetus
Molecular screening of PPV nucleic acid
Fifty six tissue samples were subjected to PPV detection using forward and reverse primer sequences 5′AGTTAGAATAGGATGCGAGGAA3′and 5′GAGTCTGTTGGTGTATTTATTGG 3′ respectively that bind to the 1761–1782 and 2002–2025 nucleotide positions of NS1 gene in PPV and generate a specific product of 265 bp (Xu et al. 2012). PCR reaction mixture includes 12.5 μl of 2 × GoTaq Green master mix (Promega, USA), 1 μl of each forward and reverse primer (with 10 pmol/μl concentration), and 4 μl of the template DNA and nuclease-free water was added to make up the 25 μl reaction volume. PCR cycling condition included initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 56 °C for 30 s, extension at 72 °C and final extension step at 72 °C for 10 min. PCR products were analyzed by gel electrophoresis and documented. Initial screenings of samples were done by NS1 gene–specific PCR and positive samples were further confirmed by amplifying the VP2 gene.
PCR amplification and sequencing of VP2 gene from PPV1 genome
To amplify the consensus region of VP2 gene in PPV1, a primer pair was designed using complete VP2 gene sequences retrieved from established PPV1 isolate (AY502115) in Primer 3 plus online tool (https://www.bioinformatics.nl/cgi-bin/primer3plus). The designed forward and reverse primer sequence are 5′GGGGTTGGTGTGTCTACAGG3′ and 5′ TCCTACCTGAGCTGGCCTAA3′ respectively and bind to the 2921–2940 and 3798–3817 nucleotide positions of VP2 gene region in PPV1 and generates a specific product of 897 bp. The specificity of the designed primers was confirmed by the primer BLAST (Basic Local Alignment Search Tool) and sequencing of amplified specific PCR product. PCR reaction conditions and annealing temperatures were optimized in a gradient thermal cycler (Eppendorf Master Cycler, Germany). The optimized PCR reaction condition includes initial denaturation at 95 °C for 5 min, followed by 30 cycles of denaturation at 95 °C for 60 s, annealing at 56 °C for 60 s, extension at 72 °C for 90 s, and the final extension at 72 °C for 10 min. PCR reaction mixture includes 12.5 ml of 2 × GoTaq Green master mix (Promega, USA), 10 pmols of each PPV1 forward and reverse primer custom synthesized (Eurofins Genomics of India Pvt. Ltd, Bangalore), and 2 µl of DNA template, and finally, nuclease-free water was added to make up the 25 μl reaction. PCR positive samples were randomly selected and sequenced at a commercial DNA sequencing facility (M/s.Eurofins Genomics of India Pvt. Ltd, Bangalore).
Molecular characterization of PPV1
The nucleotide sequences obtained from the commercial sequencing facility were aligned and annotated in SnapGene tool using PPV reference sequences. The aligned contigs were subjected to NCBI-BLAST search for similarity analysis. The contig sequences were further subjected for phylogenetic studies along with reference and established PPV1 isolates in Molecular Evolutionary Genetics analysis X (MEGA X) tool using maximum likelihood method (MLT) with Tamura-Nei model with 1000 bootstrap replicates to find evolutionary relatedness (Kumar et al. 2018). Deduced amino acid sequences of the isolates were further analyzed for amino acid variations in the VP2 gene. The isolates in this study were characterized based on BLAST homology, phylogenic studies, and deduced amino acid sequence composition.
Results
Molecular detection of PPV
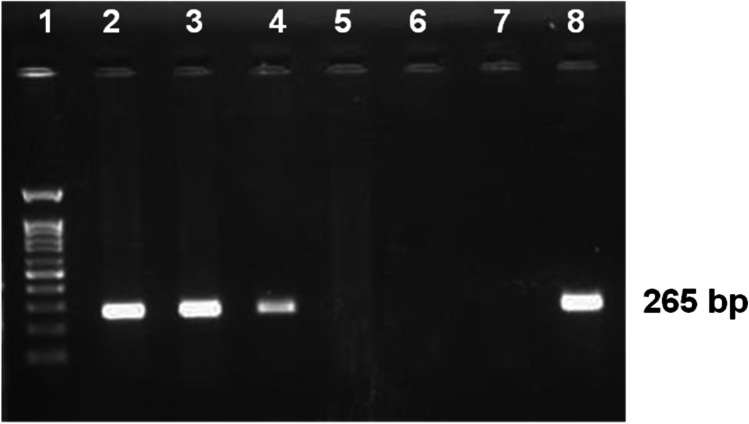
PCR-based screening for PPV genome in 56 field samples revealed, 14.3% (n = 8) positivity by producing an amplicon of 265 bp in NS1 gene–based PCR assay specific to PPV (Fig. 2). The details of PPV screening results are displayed in Table 1.
Fig. 2.
Genome based Screening of PPV infections by PCR assay targeting NS1 gene. Lane 1- 100 bp ladder, Lane 2 to 6—field samples Lane 7- Non-template control, Lane 8- known PPV positive DNA The specific PCR product 265 bp is labeled separately
Table 1.
Details of tissue samples screened for PPV genome
| S. no | Sample source (District) | Location, and type of farm | Farm size | Vaccination history | State | Year of sample | No of samples collected | No of samples positive by PCR | Percent positivity |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Chennai |
Madhavaram- -Organized |
120 |
Vaccinated only for CSFV Non vaccinated for PPV & PCV2 |
Tamil Nadu | 2021 | 17 | 3 | 17.64 |
| Guindy-unorganized | 30 | Non vaccinated for CSFV, PPV & PCV2 | 2021 | 3 | 0 | - | |||
| 2 | Chengalpattu | Kattupakkam-Organized | 320 |
Vaccinated for CSFV & PCV2 Non vaccinated for PPV |
2021 | 16 | 2 | 12.5 | |
| Chengalpattu-Unorganized | 40 |
Vaccinated for CSFV Non vaccinated for PPV& PCV2 |
2020 | 3 | 0 | - | |||
| 3 | Tirunelveli | Tiruvelveli-Organized | 40 |
Vaccinated for CSFV Non vaccinated for PPV& PCV2 |
2020 | 2 | 0 | - | |
| 4 | Vellore | Katpadi—Unorganized | 20 | Non vaccinated for CSFV, PPV & PCV2 | 2021 | 01 | 0 | - | |
| 5 | Pookod | Lakkidi, -Organized | 200 |
Vaccinated for CSFV Non vaccinated for PPV& PCV2 |
Kerala | 2020 | 14 | 3 | 21.4 |
| Total | 56 | 8 | 14.3 | ||||||
Molecular characterization of PPV1
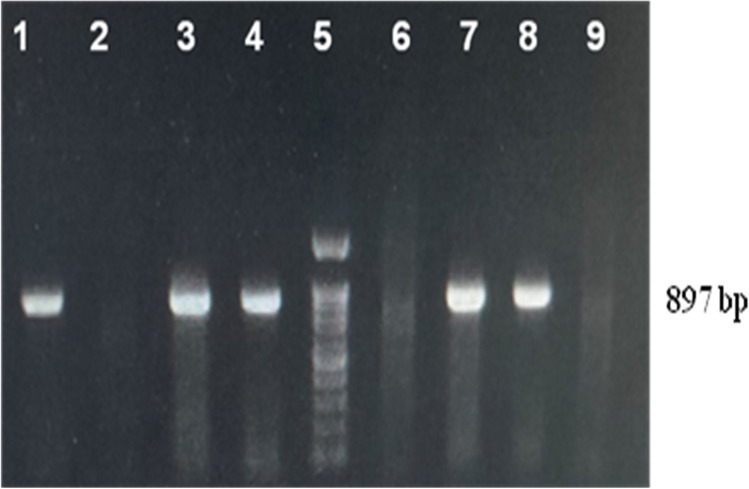
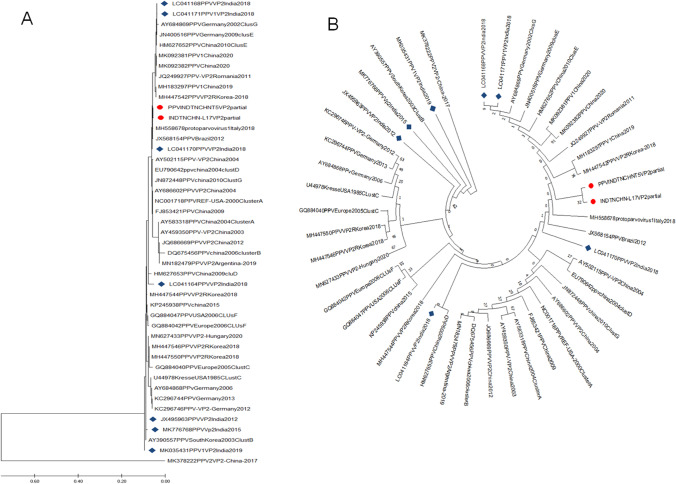
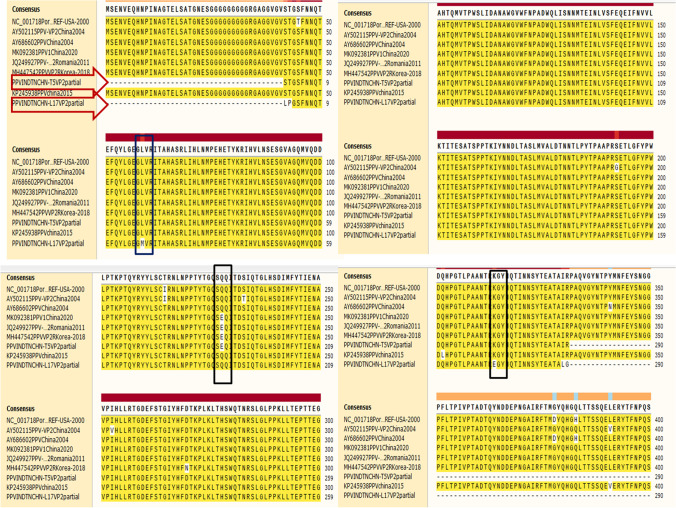
All the PPV positive samples (n = 8) yielded a specific amplicon of 897 bp in the VP2 gene–based PCR assay (Fig. 3). Two positive samples (T5 and L17) were randomly sequenced at a commercial DNA sequencing facility (M/s. Eurofins Genomics of India Pvt. Ltd, Bangalore) and assembled. Annotation of INDTNCHN-T5 and INDTNCHN-L17 of our local PPV sequences revealed 872 and 860 nts in length encoding 291 and 286 amino acids respectively. Both these sequences INDTNCHN-T5 and INDTNCHN-L17 were submitted to the GenBank under the accession numbers MW822566 and MW822567 respectively. Phylogenetic analysis of two VP2 sequences (T5 and L17) obtained in this study along with forty-two established PPV1 and one PPV2 out-group sequence (Table 2) by maximum likelihood (ML) method in Molecular Evolutionary Genetics Analysis-X (MEGA-X) tool revealed specific grouping along with cluster E strains within PPV1 sequences (Fig. 4). NCBI-BLAST analysis of contig sequence from sample INDTNCHNT5 revealed 100% sequence homology with Chinese PPV1genome (MH183297), whereas sequence from sample INDTNCHNL17 revealed 99.43% sequence homology with Spain (MH558678), Chinese (MH183297), and German (JN400516) PPV1 sequences. Deduced amino acid sequence analysis of VP2 coding region of isolates obtained in this study with reference PPV1 amino acid residues revealed variations at position 215 (I to T) in both the isolates, variation at position 228 (Q to E) in T5 isolate and variations at position 59 (L to M) and 314 (K to E) in L17 isolate (Fig. 5).
Fig. 3.
Amplification of partial VP2 gene of PPV1 using designed primer in this study. Lane 1, 2, 3, 4, 6, and 7- field samples, Lane 5 is 100 bp ladder, Lane 8—known positive DNA, Lane 9- Non-template control,. The amplified product is 897 bp and labeled separately
Table 2.
List of PPV genome genomes used in Phylogentic analysis
| S. no | Accession number | Country | Year | Virus type |
|---|---|---|---|---|
| 1 | NC001718 | REF-USA | 2000 | PPV1Cluster A |
| 2 | AY459350 | China | 2003 | PPV1 |
| 3 | AY502115 | China | 2003 | PPV1 |
| 4 | AY686602 | China | 2004 | PPV1 |
| 5 | JQ686669 | China | 2012 | PPV1 |
| 6 | MH447542 | Republic of Korea | 2018 | PPV1 |
| 7 | MH447544 | Republic of Korea | 2018 | PPV1 |
| 8 | MH447546 | Republic of Korea | 2018 | PPV1 |
| 9 | MH447550 | Republic of Korea | 2018 | PPV1 |
| 10 | MN627433 | Hungary | 2020 | PPV1 |
| 11 | KC296746 | Germany | 2012 | PPV1 |
| 12 | JQ249927 | Romania | 2011 | PPV1 |
| 13 | MK035431 | India | 2019 | PPV1VP2 |
| 14 | LC041171 | India | 2018 | PPV1VP2 |
| 15 | LC041168 | India | 2018 | PPVVP2 |
| 16 | LC041164 | India | 2018 | PPVVP2 |
| 17 | LC041170 | India | 2018 | PPVVP2 |
| 18 | MN182479 | Argentina | 2019 | PPVVP2 |
| 19 | MK776768 | India | 2019 | PPVVp2 |
| 20 | JX495963 | India | 2012 | PPVVP2 |
| 21 | MH183297 | China | 2019 | PPV1 |
| 22 | MH558678 | Italy | 2018 | PPV1 |
| 23 | MK092381 | China | 2020 | PPV1 |
| 24 | FJ853421 | China | 2009 | PPV1 |
| 25 | KC296744 | Germany | 2013 | PPV1 |
| 26 | AY684868 | Germany | 2006 | PPV1 |
| 27 | MK092382 | China | 2020 | PPV1 |
| 28 | KP245938 | China | 2015 | PPV1 |
| 29 | JX568154 | Brazil | 2012 | PPV |
| 30 | MK378222 | China | 2017 | PPV2 VP2 |
| 31 | AY583318 | China | 2004 | PPV1Cluster A |
| 32 | DQ675456 | China | 2006 | PPV1Cluster B |
| 33 | AY390557 | South Korea | 2003 | PPV1Cluster B |
| 34 | GQ884040 | Europe | 2005 | PPV1Cluster C |
| 35 | U44978 | USA | 1985 | PPV1Kresse Cluster C |
| 36 | EU790642 | China | 2004 | PPV1Cluster D |
| 37 | HM627653 | China | 2009 | PPV1Cluster D |
| 38 | HM627652 | China | 2010 | PPV1Cluster E |
| 39 | JN400516 | Germany | 2009 | PPV1Cluster E |
| 40 | GQ884042 | Europe | 2006 | PPV1Cluster F |
| 41 | GQ884047 | USA | 2006 | PPV1Cluster F |
| 42 | JN872448 | China | 2010 | PPV1Cluster G |
| 43 | AY684869 | Germany | 2002 | PPV1Cluster G |
Fig. 4.
Phylogenetic analysis PPV1 genome by Maximum Likelihood method based on p-distance model with 1000 bootstraps. A Traditional rectangular dendrogram. B Circular dendrogram. The phylogenetic tree was inferred based on alignment of VP2 protein and were midpoint rooted. The trees were drawn with two PPV1 sequences from this study (labeled by red colored circle) along with 42 established PPV1 sequences from the GenBank (including seven PPV sequences from India labeled in blue diamond) one PPV2 sequence as an out-group. The analyses were conducted in MEGA X with Bootstrap replicates of 1000
Fig. 5.
Deduced amino acid sequences of partial VP2 gene of PPV1. This alignment included deduced amino acid partial sequences of VP2 protein from two PPV1 sequences from this study (T5 and L17) and seven PPV1 sequences from different countries including one reference PPV1 sequences retrieved from GenBank. All the sequences were aligned in ClustalW and viewed in SanpGene alignment tool. The PPV1 sequences were demarcated by the red colored arrows and aminoacid variations sites were marked by rectangle shape balck box
Discussion
PPV1 is the well-documented viral pathogen associated with reproductive failure in most swine-producing countries. The detection of PPV by PCR-based molecular technique is highly specific and sensitive in comparison to hemagglutination or immunofluorescence assays (Soares et al. 1999). NS1 gene–specific PCR assay is the best suitable approach for molecular screening of PPV infections (Xu et al. 2012). NS1 gene–based PPV1 surveillance in this study revealed 14.3% positivity which is lower when compared to global prevalence status. The global prevalence of PPV1 varies from 25.8 to 71.88% (Opriessnig et al. 2014). The most popular swine-producing country, China, reported PPV positivity of 55.40% (n = 241) among 435 samples screened (Li et al. 2021). Mengeling et al. (1991) screened 302 dead fetuses in the USA and documented 35% (n = 105) positivity of PPV1 genome. At the same time, Argentina documented 13% (n = 17) PPV1 positivity out of 131 fetal tissues screened which clearly evidences variability of PPV prevalence across countries (Serena et al. 2019). But in India, during 2010, Uttar Pradesh where PPV1 was first reported had 7.14% positivity out of 70 tissue samples screened (Sharma and Saikumar 2010). Furthermore, serological testing by Kaur et al. (2016) from 90 serum samples from Punjab reported 41.1% (n = 37) positivity for PPV antibodies. A molecular detection study by Pegu et al. (2017) evidenced 14.8% (n = 8) of PPV1 positivity out of 54 porcine tissue samples screened which supports the genome-based PPV1 screening in this present study. In southern India, Aishwarya et al. (2016) reported PPV1 positivity of 5.26% (n = 2) out of 38 samples screened from Kerala, but this study included very less sample numbers and may not represent real PPV1 status in southern India. Most recent study by Bhattacharjee et al. (2021) from Northeastern states of India reported 36% (n = 18) PPV1 positivity out of 50 samples screened which clearly evidences emergence of PPV1-associated infections in recent few years in India. The present study reveals that out of 56 samples screened, 14.3% were positive for PPV1-associated infection, whereas there may be many other etiological factors like porcine circovirus 2 and 3 (PCV2 & PCV3), classical swine fever virus (CSFV), and porcine reproductive and respiratory syndrome virus (PRRSV) and Brucellosis, etc., associated with reproductive failure and their prevalence various with different geographical locations which needs to be explored. Since reproductive failure is the major concern in swine husbandry which needs to be addressed immediately with differential diagnosis and risk-based surveillance in extensive population to find true prevalence status of PPV.
Whole genome, VP2 and NS1 gene–based sequencing, and phylogenetic analysis were followed in many countries for molecular epidemiology of PPV (Oh et al. 2017). Phylogenetic analysis of PPV1 isolates in the past 50 years revealed eight phylogenic clusters which are identified by alphabets serially from A to H. Furthermore, the protective effects of commercial vaccines against these new strains are not 100% effective indicating antigenic diversity (Streck et al. 2015). Soares et al. (2003) analyzed genetic variability of PPV1 field strains in Brazil from 1994 to 2000 using partial fragments of the VP2 gene and found F and G clusters circulating in the field. Zimmermann et al. (2006) detected type C and D clusters of PPV1 associated with reproductive failure in Germany based on VP2 gene characterization. Keeping above studies as reference, the PPV1 genome in the present study was also characterized based on VP2 gene sequencing. All the eight clusters of PPV1 were associated with field infections. PPV1 clusters of A, B, and E have been reported from Austria, China, Romania, and Switzerland. Research findings from Europe and China revealed that clusters C, D, and F are the predominant clusters circulating in domestic swine (Streck et al. 2015). Each PPV1 clusters are subdivided into subgroups that include strains from both domestic and wild swine populations with varying virulence properties related to the amino acid composition of VP2 proteins (Cadar et al. 2012).
Varying amino acid substitutions have been observed in strains from several countries. Hot spots were found to be located on the capsid surface, and a surface profile distinct from the vaccine strains was observed. For PPV1, 12 linear epitopes have been proposed between amino acid positions 5–51, 85–101, 130–140, 154–167, 190–240, 260–314, 272–320, 378–458, 467–478, 502–514, 535–542, and 547–576 in the VP2 proteins numbered serially from 1 to 12, containing a huge potential role, as determined by B cell epitope prediction software. The virulent PPV1 field strains have 5 common amino acid substitutions at I-215-T, D-378-G, H-383-Q, S-436-P, and R-565-K when compared to non-virulent strains. Substitutions at 378, 383, and 436 amino acid positions of 8th B-cell epitope region determine the tissue tropism of PPV1 (Chung, et al. 2020).
Both the PPV1 (T5 and T7) sequences in this study were obtained from tissues of stillborn and mummified fetuses of swine and had amino acid substitutions at 215 positions similar to that of virulent PPV1 isolates. Additionally, one of the Tamil Nadu PPV1 sequences (T5) had host immune evasion mutations at VP2 amino acid position 228-E as like that of German 27a (AY684871) virulent field isolate (Zeeuw et al. 2007). Based on the above said evidence, the two PPV1 sequences (T5 and L17) in this study are characterized as pathogenic strains. It is hypothesized that variations in the amino acid composition of capsid could be due to viral adaptation to host and or vaccinal immune response refereed as escape mutants (Cadar et al. 2012). Commercial PPV1 (whole virus inactivated) vaccines are derived from attenuated NADL2 strains used in many countries prevent only reproductive loss and do not eliminate virus infection and dissemination (Mengeling et al. 1980). The emergence of new clusters of PPV1 in the field with vast divergence from vaccine strain necessitates the development of an alternative vaccine development approach involving suitable candidate vaccine strain circulating in the field (Streck et al. 2015). Therefore, the emergence and spread of viruses with varying amino acid profiles require close surveillance. Molecular characterization and phylogenetic analysis of PPV in India are scarce. The molecular detection of the PPV genome in the swine population of Tamil Nadu has not been reported so far. This study documents incidence of PPV1 cluster E strains for the first time in Tamil Nadu. The PPV1 isolates in this study showed homology to China and European countries isolates and these findings are supported by Cadar et al. (2012), who found that PPV1 cluster E includes the highly virulent Kresse strain along with the challenge UK and Brazilian strains of PPV. Although it could be hypothesized that these phylogenetic clustering might be due to live pig import from China and European countries to India. Additionally, investigations in contaminated commercial biological products, as porcine cell lines associated vaccines, may elucidate the origin and route of transmission of PPV across the countries. This is the foremost molecular characterization report of PPV documented from Tamil Nadu. To determine the prevalence, transmission, molecular epidemiology, and impact of PPV in commercial swine husbandry, it is necessary to extend this study to larger populations. Currently, the PPV is a very less-explored pathogen with no complete scientific data available in Indian context; hence, there is no indigenous vaccine available to control this infection; in general, the current control measures are at primitive level by adopting general hygiene.
Acknowledgements
The authors are grateful to Tamil Nadu Veterinary and Animal Sciences University (TANUVAS), Chennai -51, India, for giving the necessary funds and facilities to carry out this research work. The authors extend sincere thanks to Kerala Veterinary and Animal Sciences University (KVASU), Pookode, Wayanad, and Professor and Head, PGRIS, Kattupakkam for providing field samples to carry out this research work.
Abbreviations
- PPV
Porcine parvovirus
- ORF
Open reading frame
- MEGA
Molecular evolutionary genetics analysis
- SMEDI
Stillbirth mummification embryonic death and infertility
- NCBI
National Center for Biotechnology Information
- BLAST
Basic local alignment search tool
- PCR
Polymerase chain reaction
- MLT
Maximum likelihood method
Author contribution
PS, SRKV, PM, RP, and RA designed the study. SRKV, PS, and TPS worked and collaborated in the lab work. SK, BD, HS, BR, and RC helped in the sample collection and shipment. SRKV, PS, and RP compiled results as well as the manuscript. PM and DRG critically reviewed the manuscript. All authors read and approved the final manuscript.
Data availability
All the original data are available with corresponding author on request all the information will be shared.
Code availability
Not applicable.
Declarations
Ethical approval
Since the clinical samples were collected from aborted fetus without any invasive means hence this study doesn’t require Institutional animal ethics committee approval.
Consent to participate
Not applicable.
Consent for publication
All the authors reviewed the paper and given their consent for publication.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Afolabi KO, Iweriebor BC, Obi LC, Okoh AI. Prevalence of porcine parvoviruses in some South African swine herds with background of porcine circovirus type 2 infection. Acta Trop. 2018;190:37–44. doi: 10.1016/j.actatropica.2018.10.010. [DOI] [PubMed] [Google Scholar]
- Aishwarya J, Ravishankar C, Rajasekhar R, Sumod K, Bhaskar N, Shaji S, John K, Mini M. First report of detection and molecular characterization of porcine parvovirus in domestic and wild pigs in Kerala. India. Virusdisease. 2016;27(3):311–314. doi: 10.1007/s13337-016-0330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J, Hébert B, Tijssen P. Genome organization of the Kresse strain of porcine parvovirus: identification of the allotropic determinant and comparison with those of NADL-2 and field isolates. J Virol. 1996;70(4):2508–2515. doi: 10.1128/JVI.70.4.2508-2515.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee U, Sen A, Sharma I. Development of cost-effective quantitative PCR method for parallel detection of porcine circovirus2 and porcine parvovirus in perspective of North-eastern India. Trop Anim Health Prod. 2021;53:177. doi: 10.1007/s11250-021-02609-2. [DOI] [PubMed] [Google Scholar]
- Boisvert M, Fernandes S, Tijssen P. Multiple pathways involved in porcine parvovirus cellular entry and trafficking toward the nucleus. J Virol. 2010;84(15):7782–7792. doi: 10.1128/JVI.00479-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadar D, Dán Á, Tombácz K, Lőrincz M, Kiss T, Becskei Z, Spînu M, Tuboly T, Cságola A. Phylogeny and evolutionary genetics of porcine parvovirus in wild boars. Infect Genet Evol. 2012;12(6):1163–1171. doi: 10.1016/j.meegid.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Cadar D, Lőrincz M, Kiss T, Novosel D, Podgórska K, Becskei Z, Tuboly T, Cságola A. Emerging novel porcine parvoviruses in Europe: Origin, evolution, phylodynamics and phylogeography. J. Gen. Virol. 2013;94:2330–2337. doi: 10.1099/vir.0.055129-0. [DOI] [PubMed] [Google Scholar]
- Carwright SF, Huck RA. Viruses isolated in association with herd infertility, abortions and stillbirths in pigs. Vet. Rec. 1967;81:196–197. [Google Scholar]
- Chung HC, Nguyen VG, Huynh TM, Park YH, Park KT, Park BK. PCR-based detection and genetic characterization of porcine parvoviruses in South Korea in 2018. BMC Vet. Res. 2020;16(1):113. doi: 10.1186/s12917-020-02329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cságola A, Lorincz M, Cadar D, Tombácz K, Biksi I, Tuboly T. Detection, prevalence and analysis of emerging porcine parvovirus infections. Arch. Virol. 2012;157:1003–1010. doi: 10.1007/s00705-012-1257-3. [DOI] [PubMed] [Google Scholar]
- Kaur, A., Mahajan, V., Leishangthem, G. D., Singh, N. D., Bhat, P., Banga, H. S., Filia, G., 2016. Epidemiological and immunopathological studies on Porcine parvovirus infection in Punjab. Vet.World. 9(8), 827–831. 10.14202/vetworld.2016.827-831 [DOI] [PMC free article] [PubMed]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol Biol Evol. 2018;35(6):1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Xiao Y, Qiu M, Li X, Li S, Lin H, Li X, Zhu J, Chen N. A Systematic Investigation Unveils High Coinfection Status of Porcine Parvovirus Types 1 through 7 in China from 2016 to 2020. Microbiol. Spectr. 2021;9(3):e0129421. doi: 10.1128/Spectrum.01294-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez C, Dalsgaard K, López de Turiso JA, Cortés E, Vela C, Casal JI. Production of porcine parvovirus empty capsids with high immunogenic activity. Vaccine. 1992;10(10):684–690. doi: 10.1016/0264-410x(92)90090-7. [DOI] [PubMed] [Google Scholar]
- Mayr, A., Mahnel, H., 1964. Züchtung von Schweinepestvirus in Schweinenieren-Kulturen mit cytopathogenem Effekt [Cultivation of hog cholera virus in pig kidney cultures with cytopathogenic effect]. Zentralblatt fur Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. 1. Abt. Medizinisch-hygienische Bakteriologie, Virusforschung und Parasitologie. Originale, 195(2), 157–166. [PubMed]
- McKillen J, Hjertner B, Millar A, McNeilly F, Belák S, Adair B, Allan G. Molecular beacon real-time PCR detection of swine viruses. J. Virol. Methods. 2007;140(1–2):155–165. doi: 10.1016/j.jviromet.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Mengeling WL, Paul PS, Brown TT. Transplacental infection and embryonic death following maternal exposure to porcine parvovirus near the time of conception. Arch. Virol. 1980;65(1):55–62. doi: 10.1007/BF01340540. [DOI] [PubMed] [Google Scholar]
- Mengeling WL, Lager KM, Zimmerman JK, Samarikermani N, Beran GW. A current assessment of the role of porcine parvovirus as a cause of fetal porcine death. J. Vet. Diagn. 1991;3(1):33–35. doi: 10.1177/104063879100300107. [DOI] [PubMed] [Google Scholar]
- Mengeling WL, Lager KM, Vorwald AC. The effect of porcine parvovirus and porcine reproductive and respiratory syndrome virus on porcine reproductive performance. Anim. Reprod. Sci. 2000;60–61:199–210. doi: 10.1016/s0378-4320(00)00135-4. [DOI] [PubMed] [Google Scholar]
- Mengeling, W. L., 2006. Porcine parvovirus, p 373–385 In Straw, B.E., Zimmerman, J.J., D'Allaire, S., Taylor, D.J., (ed), Diseases of swine, 9th ed Blackwell Publishing, Ames, IA.
- Molitor TW, Joo HS, Collett MS. Porcine parvovirus DNA: characterization of the genomic and replicative form DNA of two virus isolates. Virology. 1984;137(2):241–254. doi: 10.1016/0042-6822(84)90216-2. [DOI] [PubMed] [Google Scholar]
- Oh WT, Kim RY, Nguyen VG, Chung HC, Park BK. Perspectives on the Evolution of Porcine Parvovirus. Viruses. 2017;9(8):196. doi: 10.3390/v9080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opriessnig T, Xiao CT, Gerber PF, Halbur PG. Identification of recently described porcine parvoviruses in archived North American samples from 1996 and association with porcine circovirus associated disease. Vet. Microbiol. 2014;173(1–2):9–16. doi: 10.1016/j.vetmic.2014.06.024. [DOI] [PubMed] [Google Scholar]
- Ouh IO, Park S, Lee JY, Song JY, Cho IS, Kim HR, Park CK. First detection and genetic characterization of porcine parvovirus 7 from Korean domestic pig farms. J. Vet. Sci. 2018;19(6):855–857. doi: 10.4142/jvs.2018.19.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski RM, Mitra N, Hause BM. Discovery of a novel Parvovirinae virus, porcine parvovirus 7, by metagenomic sequencing of porcine rectal swabs. Virus Genes. 2016;52(4):564–567. doi: 10.1007/s11262-016-1322-1. [DOI] [PubMed] [Google Scholar]
- Pegu, S.R., Sarma,D.K. Rajkhowa, S., Choudhury,M., Sarma, D., Das J.P., 2017. Molecular detection of porcine circo virus type 2 and porcine parvo virus in pigs having reproductive problems and histopathological studies in the tissue of aborted pig fetuses. Indian J. Anim. Res. 51 (4) 2017, 732–736.
- Saekhow P, Ikeda H. Prevalence and genomic characterization of porcine parvoviruses detected in Chiangmai area of Thailand in 2011. Microbiol. Immunol. 2015;59:82–88. doi: 10.1111/1348-0421.12218. [DOI] [PubMed] [Google Scholar]
- Saekhow P, Kishizuka S, Sano N, Mitsui H, Akasaki H, Mawatari T, Ikeda H. Coincidental detection of genomes of porcine parvoviruses and porcine circovirus type 2 infecting pigs in Japan. J. Vet. Med. Sci. 2016;77:1581–1586. doi: 10.1292/jvms.15-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirtzinger EE, Suddith AW, Hause BM, Hesse RA. First identification of porcine parvovirus 6 in North America by viral metagenomic sequencing of serum from pigs infected with porcine reproductive and respiratory syndrome virus. Virol J. 2015;12:170. doi: 10.1186/s12985-015-0401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serena MS, Cappuccio JA, Metz GE, Aspitia CG, Dibárbora M, Calderón MG, Echeverría MG. Detection and molecular characterization of porcine parvovirus in fetal tissues from sows without reproductive failure in Argentina. Heliyon. 2019;5(11):e02874. doi: 10.1016/j.heliyon.2019.e02874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Saikumar G. Porcine parvovirus- and porcine circovirus 2-associated reproductive failure and neonatal mortality in crossbred Indian pigs. Trop Anim Health Prod. 2010;42:515–522. doi: 10.1007/s11250-009-9454-0. [DOI] [PubMed] [Google Scholar]
- Simpson AA, Hébert B, Sullivan GM, Parrish CR, Zádori Z, Tijssen P, Rossmann MG. The structure of porcine parvovirus: comparison with related viruses. J. Mol. Biol. 2002;315(5):1189–1198. doi: 10.1006/jmbi.2001.5319. [DOI] [PubMed] [Google Scholar]
- Soares RM, Durigon EL, Bersano JG, Richtzenhain LJ. Detection of porcine parvovirus DNA by the polymerase chain reaction assay using primers to the highly conserved nonstructural protein gene, NS-1. J. Virol. Methods. 1999;78(1–2):191–198. doi: 10.1016/s0166-0934(98)00177-3. [DOI] [PubMed] [Google Scholar]
- Soares RM, Cortez A, Heinemann MB, Sakamoto SM, Martins VG, Bacci M, de Campos Fernandes FM, Richtzenhain LJ. Genetic variability of porcine parvovirus isolates revealed by analysis of partial sequences of the structural coding gene VP2. J Gen Virol. 2003;84(Pt 6):1505–1515. doi: 10.1099/vir.0.19011-0. [DOI] [PubMed] [Google Scholar]
- Streck AF, Canal CW, Truyen U. Molecular epidemiology and evolution of porcine parvoviruses. Infect. Genet. Evol. 2015;36:300–306. doi: 10.1016/j.meegid.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Truyen, U., Streck, A.F., 2012. Porcine parvovirus. p 447–455. In: Zimmerman, J.K., Karriker, L., Ramirez, A., Schwartz, K.J., Stevenson, G.W., editors. Diseases of Swine. 10th ed. Wiley
- Xiao CT, Halbur PG, Opriessnig T. Molecular evolutionary genetic analysis of emerging parvoviruses identified in pigs. Infect. Genet. Evol. 2013;16:369–376. doi: 10.1016/j.meegid.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Xing X, Zhou H, Tong L, Chen Y, Sun Y, Wang H, Zhang G. First identification of porcine parvovirus 7 in China. Arch. Virol. 2018;163(1):209–213. doi: 10.1007/s00705-017-3585-9. [DOI] [PubMed] [Google Scholar]
- Xu XG, Chen GD, Huang Y, Ding L, Li ZC, Chang CD, Wang CY, Tong DW, Liu HJ. Development of multiplex PCR for simultaneous detection of six swine DNA and RNA viruses. J. Virol. Methods. 2012;183(1):69–74. doi: 10.1016/j.jviromet.2012.03.034. [DOI] [PubMed] [Google Scholar]
- Zeeuw E, Leinecker N, Herwig V, Selbitz HJ, Truyen U. Study of the virulence and cross-neutralization capability of recent porcine parvovirus field isolates and vaccine viruses in experimentally infected pregnant gilts. J Gen Virol. 2007;88(Pt 2):420–427. doi: 10.1099/vir.0.82302-0. [DOI] [PubMed] [Google Scholar]
- Zhang CF, Song CP, Chen CM, Cui SJ, Miao LF. Reproductive failure in wild boars associated to porcine parvovirus infection and in vivo and in vitro characterization of the causal isolate. Trop. Anim. Health Prod. 2010;42:1611–1613. doi: 10.1007/s11250-010-9644-9. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Ritzmann M, Selbitz HJ, Heinritzi K, Truyen U. VP1 sequences of German porcine parvovirus isolates define two genetic lineages. J Gen Virol. 2006;87(Pt 2):295–301. doi: 10.1099/vir.0.81086-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the original data are available with corresponding author on request all the information will be shared.
Not applicable.