Abstract
Apolipoprotein E epsilon-4 (APOEε4 or APOE4), an allelic variation of the APOE gene, not only increases the risk of developing the late-onset form of Alzheimer’s disease (AD), but also influences the outcome of treatment. Indeed, data from clinical studies show that the beneficial effect of insulin on cognition is blunted in APOE4 carriers. To investigate how APOE impacts insulin response, we assessed the effects of an acute insulin injection in APOE3- and APOE4-targeted replacement mice that respectively express the human APOE3 or APOE4 isoform in place of the endogenous murine ApoE protein. We evaluated cognition, insulin signaling and proteins implicated in Aβ transport and tau phosphorylation in the cortex and brain capillaries. We found that a single acute insulin injection increased Akt pSer473 in APOE4 compared to APOE3 mice (+113% versus +78.5%), indicating that APOE4 carriage potentiates activation of insulin upstream signaling pathway in the brain. Insulin also led to decreased concentrations of the receptor for advanced glycation endproducts (RAGE) in brain capillaries in both groups of mice. Moreover, higher phosphorylation of tau at Ser202, one of the key markers of AD neuropathology, was observed in insulin-injected APOE4 mice (+44%), consistent with findings in human APOE4 carriers (+400% compared to non-carriers). Therefore, our data suggest that APOE4 carriage leads to an increased insulin-induced activation of cerebral Akt pathway, associated with higher AD-like tau neuropathology. Our results provide evidence of altered insulin signaling in APOE4 carriers as well as a possible mechanism to explain the absence of cognitive benefit from insulin therapy in these individuals.
Keywords: APOE4, Alzheimer’s disease, insulin
Introduction
As the prevalence of Alzheimer’s disease (AD) is rising, there is a strong worldwide need to better understand its etiopathophysiology in order to develop treatments. Although the genetics of AD are complex, numerous studies have confirmed that a common polymorphism in the apolipoprotein E (APOE) gene is the main genetic risk factor for developing sporadic and familial Alzheimer’s disease (AD) (Corder et al., 1993; Strittmatter et al., 1993). Among patients suffering from AD, the prevalence of the APOE4 allele reaches 36.7% (Farrer et al., 1997). There is a wealth of AD-relevant pathogenic processes known to be influenced by APOE alleles, and which could explain its role as a risk factor (Salem et al., 2015). Beside its major impact on AD risk, APOE genotype has been repeatedly shown to influence treatment outcomes in clinical studies performed in AD patients (Choi et al., 2008; Salem et al., 2015; Vandal et al., 2014a), making the APOEε4 allele an unescapable pharmacogenetic factor to consider in AD clinical trial design. Mounting evidence indicates that stimulating insulin pathways in the brain may have a therapeutic value on AD-related cognitive impairment (Benedict et al., 2011; De Felice, 2013; Dhuria et al., 2010; Freiherr et al., 2013; Schiöth et al., 2012). Improvement of memory function is seen following insulin administration in clinical trial with AD patients (Craft et al., 2012) and in mouse models of the disease (Vandal et al., 2014b). However, in clinical trial, the cognitive response to insulin critically depends on APOE alleles (Reger et al., 2006) (Reger et al., 2008). At the mechanistic level, these results suggest that APOE genotype influences brain insulin signaling. To directly investigate this question in vivo, we compared central and peripheral response to a single insulin injection between APOE3- and APOE4-targeted replacement mice.
Methods
Animals
APOE-targeted replacement mice, in which, the endogenous murine APOE gene was replaced by human APOE3 or APOE4 genes (Sullivan et al., 1997), were purchased from Taconic (Hudson, NY, USA). In this mouse model, the endogenous murine APOE gene was replaced by human APOE3 or APOE4 genes (Sullivan et al., 1997). Mice were fed a commercial chow (Teklad 2018; Harlan Laboratories, Indianapolis, IN, USA) and were killed at 12 months of age. Before sacrifice, mice were fasted for 6 hours and received an intravenous (i.v.) injection of insulin (3.8 U/kg of human insulin) or saline 5 minutes before death, a dose known to strongly activate muscle (White et al., 2014) and brain (Vandal et al., 2014b) insulin signaling. Mice were sacrificed by intracardiac perfusion with 40 ml of ice-cold 0.1 M phosphate buffer saline (PBS) containing inhibitors of phosphatases (sodium pyrophosphate, 1 mM and sodium fluoride 50 mM) and proteases (SigmaFast protease inhibitor tablets, Sigma-Aldrich, St-Louis, US) while deeply anesthetized (100 mg/kg ketamine and 10 mg/kg xylazine). One brain hemisphere and the gastrocnemius muscle were dissected and kept frozen at −80°C, the other brain hemisphere was kept in ice-cold 0.1 M PBS and was rapidly homogenized for brain capillary extraction. All animal experiments were approved by Laval University ethics committee.
Human brain samples
Brain parietal cortex samples were obtained from participants in the Religious Order Study (ROS), a longitudinal clinical-pathologic study of aging and dementia from which an extensive amount of clinical and neuropathological data are available (Bennett, 2006; Tremblay et al., 2007a). The cohort used here included volunteers suffering from mild cognitive impairment (MCI, n = 12), AD (n = 12), and subjects with no obvious cognitive impairment (n = 12). Cortical extracts were prepared as previously described (Tremblay et al., 2011). APOE genotype was determined using DNA extracted from peripheral blood lymphocytes as previously described (Buchman et al., 2009). When the participants had one copy or more of the ε4 allele, they were considered APOE4 positive (Buchman et al., 2009). Characteristics of participants are presented in Table 1.
Table 1 :
Selected Characteristics of Subjects From the Religious Order Study APOE4 carrier or non-carrier.
| APOE4 | |||
|---|---|---|---|
| Characteristics | non-carrier | carrier | Statistical analysis |
| n | 23 | 12 | |
| AD clinical diagnosis, % | 26 | 42 | A; p = 0.451 |
| Men, % | 22 | 42 | A; p = 0.258 |
| Mean age at death (SD) | 86.9 (5.1) | 80.4 (4.1) | B; U = 65, p = 0.009 |
| Mean education, y (SD) | 18.6 (3.4) | 21.0 (2.6) | B; U = 121, p = 0.561 |
| Mean MMSE (SD) | 24.3 (7.8) | 20.0 (7.1) | B; U = 79, p = 0.041 |
| Global cognition score (SD) | −0.7 (1.0) | −1.2 (0.9) | B; U = 113, p = 0.510 |
| Post mortem delay, hours (SD) | 5.9 (3.9) | 7.8 (6.4) | B; U = 116, p = 0.455 |
| Braak stage I/II/III/IV/V (n) | 2/0/13/6/2 | 0/0/2/5/5 | C; X2 = 6.919, df = 4, p = 0.140 |
| CERAD score, 4/3/2/1 (n) | 3/9/4/7 | 7/3/0/2 | C; X2 = 6.452, df = 3, p = 0.092 |
| Reagan score, 3/2/1 (n) | 2/9/12 | 5/5/2 | C; X2 = 6.784, df = 2, p = 0.034 |
AD : Alzheimer’s Disease, MMSE : mini-mental state examination, CERAD : Consortium to Establish a Registry for Alzheimer’s Disease. A : Fisher’s exact test. B : Mann Whitney test. C : Chi-squared test.
Insulin tolerance test
For insulin tolerance test (ITT), mice were fasted for 6 hours and were injected intraperitoneally (i.p.) with human insulin (1 U/kg). A blood drop was taken from the saphenous vein and glycemia was measured with a glucometer (ONETOUCH UltraMini, LifeScan, CA, USA). We identified two APOE4 mice that were insulin-resistant and both were excluded from statistical analysis for all experiments.
Plasma insulin
Fasting plasma insulin was evaluated with an ELISA (Ultrasensitive Insulin ELISA, Mercodia, Uppsala, Sweden) according to the instructions from the manufacturer, before the injection of insulin during the ITT.
Behavioral testing
Mice received an i.p. injection of human insulin (1 U/kg) or saline 2 hours before behavioral testing. Three parameters were evaluated, as previously described: memory with a novel object recognition (NOR) test, anxiety-like behavior with a dark-light emergence test and locomotor activity with an open field test (St-Amour et al., 2014; Vandal et al., 2014b).
Recognition memory was evaluated by subjecting the mice to the NOR test. During the familiarization phase, the mouse was introduced 5 minutes in a standard clear cage (29.2 cm × 19 cm × 12.7 cm) with two identical objects. An hour later, they were exposed to a novel and a familiar object for another five-minute period. The time exploring the object represents the time the mouse spent smelling or exploring an object while subjected to the test (St-Amour et al., 2014). Recognition index = [Time exploring the new object/(Time exploring the new object + Time exploring the familiar object)] × 100. Animals whose recognition index was below 30% were excluded from statistical analysis due to an exploration time, during the familiarization phase, considered insufficient to allow recognition.
Anxiety-like behaviour was evaluated by the dark-light box emergence test as previously described (St-Amour et al., 2014). Mice were placed in the center of the dark chamber with a free access to the illuminated chamber for 5 minutes. The time the mouse took to enter the illuminated compartment was set as the escape latency. Animals that stayed in the dark chamber without coming out within 5 minutes were excluded from the statistical analysis (1 APOE3 saline and 1 APOE3 insulin were excluded on that basis).
Voluntary locomotor activity was evaluated by subjecting the mice to a one-hour open field session, as previously described (St-Amour et al., 2014). The open field apparatus is made up of 10 plexiglas cages with translucent walls (80 cm × 80 cm). Each mouse was introduced in the center of a cage and an automated recording of photobeam breaks (San Diego Instruments) tracked movements in order to calculate the distance traveled by the mice.
Brain capillaries isolation
Murine brain microvessels were isolated by density-gradient centrifugation using the capillary depletion technique as previously described (Alata et al., 2014). The cerebellum, meninges, brain stem and large superficial blood vessels were removed from mice brain hemisphere. The resulting cortex was transferred into ice-cold 0.1 M PBS and was gently homogenized in ice-cold Dulbecco’s Modified Eagles Medium (DMEM) containing 10% fetal bovine serum (FBS) with a Potter homogenizer. The homogenate was centrifuged at 500 g for 10 minutes at 4°C and the supernatant was eliminated. The pellet was homogenized in 5 ml of DMEM, 25% bovine serum albumin (BSA) and centrifuged at 1 500 g for 20 min at 4°C. Next, this pellet was homogenized in DMEM containing 10% FBS and the homogenate passed through a 60-μm mesh nylon filter to eliminate larger vessels. This filtrate was centrifuged at 12 000 g for 45 min at 4°C. The pellet containing the microvessels was washed in ice-cold PBS and centrifuged again at 12 000 g for 20 min at 4°C. The supernatant was excluded and the pellets were stored at −80°C until protein extraction.
Protein extraction
Proteins were extracted from tissue as previously described (St-Amour et al., 2014). Proteins were extracted from gastrocnemius muscle, parieto-temporal cortex and brain capillaries. Cortices were homogenized in eight times their volume of Tris-buffered saline (0.05 mol/L Tris-Base, 0.138mol/L NaCl, 2.7 mol/L KCl) for the soluble protein fraction and a RIPA buffer (150 mmol/L NaCl, 50 mmol/L Tris-Cl, 0.5% sodium deoxycholate, 0.5% sodium dodecyl sulfate, 1% Triton X-100) for the membrane soluble protein fraction. Powderized muscles and brain capillaries were homogenized in RIPA buffer. These buffers contain a cocktail of proteases inhibitors (Complete™, Roche Diagnostics, Indianapolis, IN, USA) and phosphatases inhibitors [Phostop (Roche Diagnostics, Indianapolis, IN, USA), sodium orthovanadate, and sodium fluoride]. The samples were sonicated and centrifuged sequentially at 100 000 g for 20 minutes at 4°C. All fractions were kept frozen at −80°C. The proteins were quantified using bicinchoninic acid assays (Pierce, Rockford, IL, USA).
Western immunoblotting
20 μg of protein from the cortex and gastrocnemius or 15 μg of protein from brain capillaries were loaded and separated by sodium dodecyl sulfate-polyacrylamide electrophoresis gel and afterwards electroblotted onto a polyvinylidene difluoride PVDF membrane (Immobilon, Millipore, MA, USA). 5% milk with 0.5% bovine serum albumin (BSA) blocking agent was used to block the membranes for 1 hour. Subsequently, membranes were immunoblotted with primary and then with secondary antibodies followed by chemiluminescence reagents (LumiGLO Reserve Chemiluminescent Substrate Kit, KPL, Gaithersburg, MD, USA). Intensity of the bands obtained was evaluated with a KODAK Image Station 4000MM Digital Imaging System (Molecular Imaging Software version 4.0.5f7, KODAK, New Haven, CT). The list of primary antibodies used in our experiments and the optical densities for the Western blot results presented as ratios in Figures 1–4 are shown in the supplementary material (Table S1 and Table S2, respectively).
Figure 1 : APOE4 mice have similar peripheral insulin sensitivity but impaired memory compared to APOE3 mice.
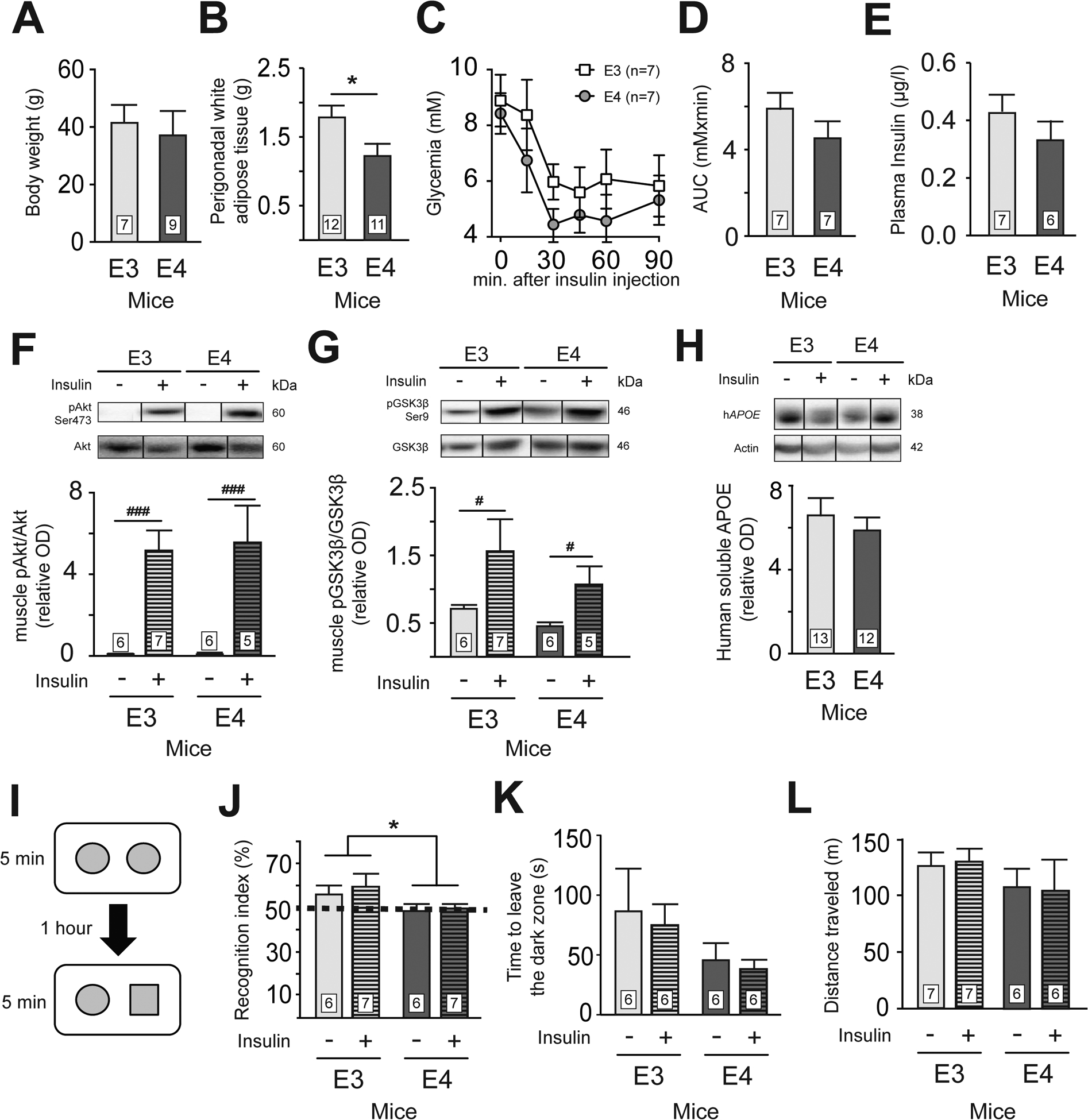
Body weight (A), visceral fat accumulation (B), glycemia (C) and area under the curve (D) during the insulin tolerance test (ITT). Fasting plasma insulin (E). Insulin sensitivity in the gastrocnemius muscle as assessed by pAkt(Ser473)/Akt (F) and pGSK3βSer9/GSK3β (G) ratio following intravenous insulin injection. Human soluble APOE concentration in parietotemporal cortex (H). Recognition memory (I and J), anxiety-like behavior (K) and locomotor activity (L) of APOE3 and APOE4 mice 2 hours after saline or insulin injection. Recognition memory is expressed as a percentage of the time spent exploring the novel object compared to the total time allocated to both objects. The dashed line represents a recognition index of 50%, where the mouse devotes as much time to both objects. Anxiety was evaluated using the latency to escape from the dark chamber in the black-and-white box test. Locomotor activity was measured using the distance traveled during a one-hour session of open field. Data are presented as mean ± SEM. The weight of perigonadal white adipose tissue and the recognition index were compared with an unpaired Student t-test. *p<0.05. pAkt/Akt and pGSK3β/GSK3β ratio were compared using two-way ANOVA. #p < 0.05; ###p < 0.0001. Akt = protein kinase B, PKB. GSK3β = glycogen synthase kinase. APOE = apolipoprotein E.
Figure 4: The APOE4 genotype is associated with increased phospho-tau (pSer202) in the human cortex.

Tau phosphorylation at Ser202 (A), Ser396/404 (B), Thr181 (C) were evaluated as well as total tau (D) and APOE content (E), using Western blots with TBS-soluble extracts from the parietal cortex of volunteers clinically classified as Controls, MCI (mild cognitive impaired) and AD patients (Bennett, 2006; Tremblay et al., 2007a; Tremblay et al., 2011) and genotyped as ApoE4− (n=23) or ApoE4+ (n=11 to 12). Individual data are presented with mean ± SEM. Data were compared using a Mann–Whitney test. &p<0.05. Correlations were evaluated using a simple regression. APOE = apolipoprotein E.
Immunofluorescence
Free floating brain sections (25 μm) from APOE3 and APOE4 mice were blocked for 1 h in a PBS solution containing 5% horse serum (Invitrogen, Carlsbad, CA) and 0.4% Triton X-100. Sections were then incubated over-night at 4°C with primary antibodies in the blocking solution: biotinylated Lectin antibody (Vector laboratories, Burlingame, CA) and rabbit anti-insulin receptor (INSR, 1:100; Fitzgerald; Acton, MA). After incubation with primary antibodies, slices were exposed to Alexa Fluor-488 conjugated streptavidin (Invitrogen, Carlsbad, CA) or Alexa Fluor-647 conjugated donkey anti-rabbit secondary antibodies (1:1000; Invitrogen, Carlsbad, CA). Then, slices were counterstained with 4’, 6-diamino-2-phenylindole (DAPI; Invitrogen) for 10 min, mounted on SuperFrost Plus slides and treated with 0.5% Sudan black (in 70% methanol) for 5 min. Finally, slides were placed under coverslips with Mowiol mounting media. Immunofluorescence was examined using an epifluorescence microscope (Olympus Provis AX70; Olympus, Melville, NY) and photographs were taken using a Spot digital camera (Diagnostic Instruments, Sterling Heights, MI). All images were prepared for illustration with Fiji/ImageJ software.
Statistical analysis
Data are presented as means ± SEM. Statistical analysis and number of mice or individuals per group are specified in each Figure. For analysis performed in mice, equality of the variances between the groups was determined using Bartlett’s test. When comparing two groups, an unpaired Student t test was performed, with a Welch correction included when variances were not equal Normality of the data was evaluated using a Shapiro-Wilk test for groups with at least 7 values and assessed using a Kolmogorov-Smirnov test for groups with less than 5 values. When normality could not be assumed and variances were unequal, a non-parametric Mann-Whitney test was performed. When more than two groups were compared, one-way or two-way ANOVA were used. For one-way ANOVA with comparable variances, Tukey’s post hoc analysis was performed. To compare means of the insulin group with the average saline (hypothetical value of 1), a one-sample Student t test was used. Data in human samples were compared using Mann-Whiney test when data were continuous. Data adjustment was performed using an ANCOVA analysis with age of death or MMSE score as covariates. For data distributed in categories, a contingency analysis was performed (Fisher’s exact test for two group or Chi-squared for more than two groups). For all data, statistical significance was set at P < 0.05. All statistical analyzes were performed with Prism 6 (GraphPad software, San Diego, CA, USA) or JMP (version 10.0.0; SAS Institute Inc., Cary, IL) softwares.
Results
Despite similar body weight (Fig. 1A), and in line with previous reports (Pendse et al., 2009), APOE4 mice had lower accumulation of white adipose tissue compared to APOE3 mice (Fig. 1B). However, the ITT (Fig. 1C and D), plasma insulin (Fig. 1E) and measures of gastrocnemius insulin signaling protein (Fig. 1F and G) revealed a peripheral response similar between both groups. Brain human ApoE concentrations were also similar between APOE3 and APOE4 mice Fig. 1H), consistent with some reports (Sullivan et al., 2011) but not all (Riddell et al., 2008; Vandal et al., 2014a). Next, in line with previous data in mice (Liraz et al., 2013; Raber et al., 1998) and humans (Caselli et al., 2009), APOE4 mice had impaired object recognition compared to APOE3 mice (Fig. 1I and J). However, we observed no significant effect of a single insulin injection on memory (Fig. 1I and J), anxiety-like behavior (Fig. 1K) or locomotor activity (Fig. 1L). Therefore, these results confirm that APOE4 mice have impaired memory function that remains uncorrected after acute insulin administration, as in clinical trials (RCT) (Craft et al., 2012; Reger et al., 2006).
We next investigated insulin signaling in the brain according to APOE genotypes by evaluating phosphorylated kinase concentrations in the cortex after a single insulin injection. No difference between genotypes was observed for levels of insulin receptor (INSR) in brain capillaries (Fig. 2A and 2B), cortical insulin receptor substrate (IRS1) (Fig. 2C) or other insulin signaling protein (Fig. S1). Cortical ratios of pAkt/Akt (pSer473) were twice higher in APOE4 compared to APOE3 mice after insulin injection (Fig. 2D).
Figure 2 : APOE4 mice have an increased cortical pAkt compared to APOE3 mice following insulin injection.
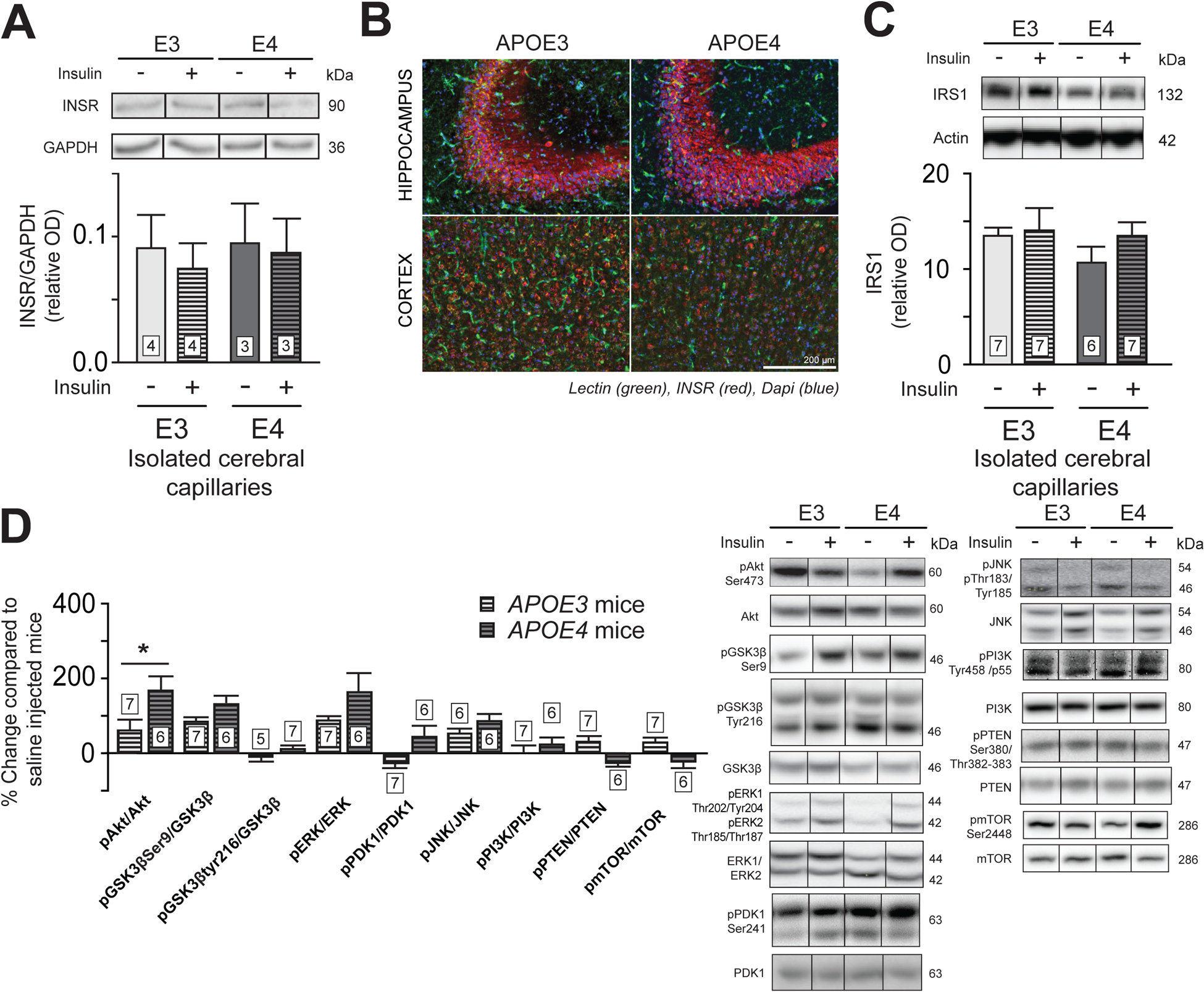
Western blot analysis in brain capillaries (A) and immunofluorescence in hippocampus and cortex (B) of INSR, and IRS1 content in brain capillaries (C). Change in concentrations of insulin signaling-related protein (D) in TBS-soluble fractions of the parietotemporal cortex of insulin injected mice compared to saline injected mice. Data are presented compared to the average of saline-injected mice. For immunofluorescence, blue = DAPI, green = blood vessels (lectin) and red = INSR. Data are presented as mean ± SEM. Data were compared with an unpaired Student t-test. *p < 0.05. INSR = insulin receptor, IRS1 = insulin receptor substrate 1, Akt = protein kinase B, PKB. GSK3β = glycogen synthase kinase-3β. ERK = extracellular signal-regulated kinases. PDK1 = Pyruvate Dehydrogenase Kinase 1. JNK = c-Jun N-terminal kinases. PI3K = Phosphoinositide 3-kinase. PTEN = Phosphatase and tensin homolog. mTOR = mammalian target of rapamycin.
Several pieces of evidence suggest that the APOE4 genotype is associated with impairments of the blood-brain barrier (BBB), which are possibly involved with AD (Alata et al., 2015; Bell et al., 2012). APOE4 genotype has previously been linked to higher Aβ cerebral accumulation (Drzezga et al., 2009) and impaired Aβ clearance (Castellano et al., 2011). Therefore, we evaluated concentrations of the receptor for advanced glycation endproducts (RAGE) and of the low density lipoprotein receptor-related protein 1 (LRP1), which are implicated in brain influx and efflux of Aβ, respectively, in brain microvessel endothelial cells. Despite no difference between genotypes, insulin acutely reduced RAGE concentrations in isolated brain capillaries from both APOE3 and APOE4 mice (Fig. 3A and B), an effect that was undetectable without separating capillaries from whole brain homogenates (Fig. S2). Interestingly, RAGE concentrations in brain capillaries were positively correlated with cortical pAkt/Akt (pSer473) (Pearson’s correlation coefficient, r2 = 0.61, p = 0.02) in APOE4 mice, suggesting that a higher insulin response in APOE4 mice might be linked to higher RAGE concentrations.
Figure 3 : Insulin increased phospho-tau (pSer202/Thr205) in the cortex of APOE4 mice.
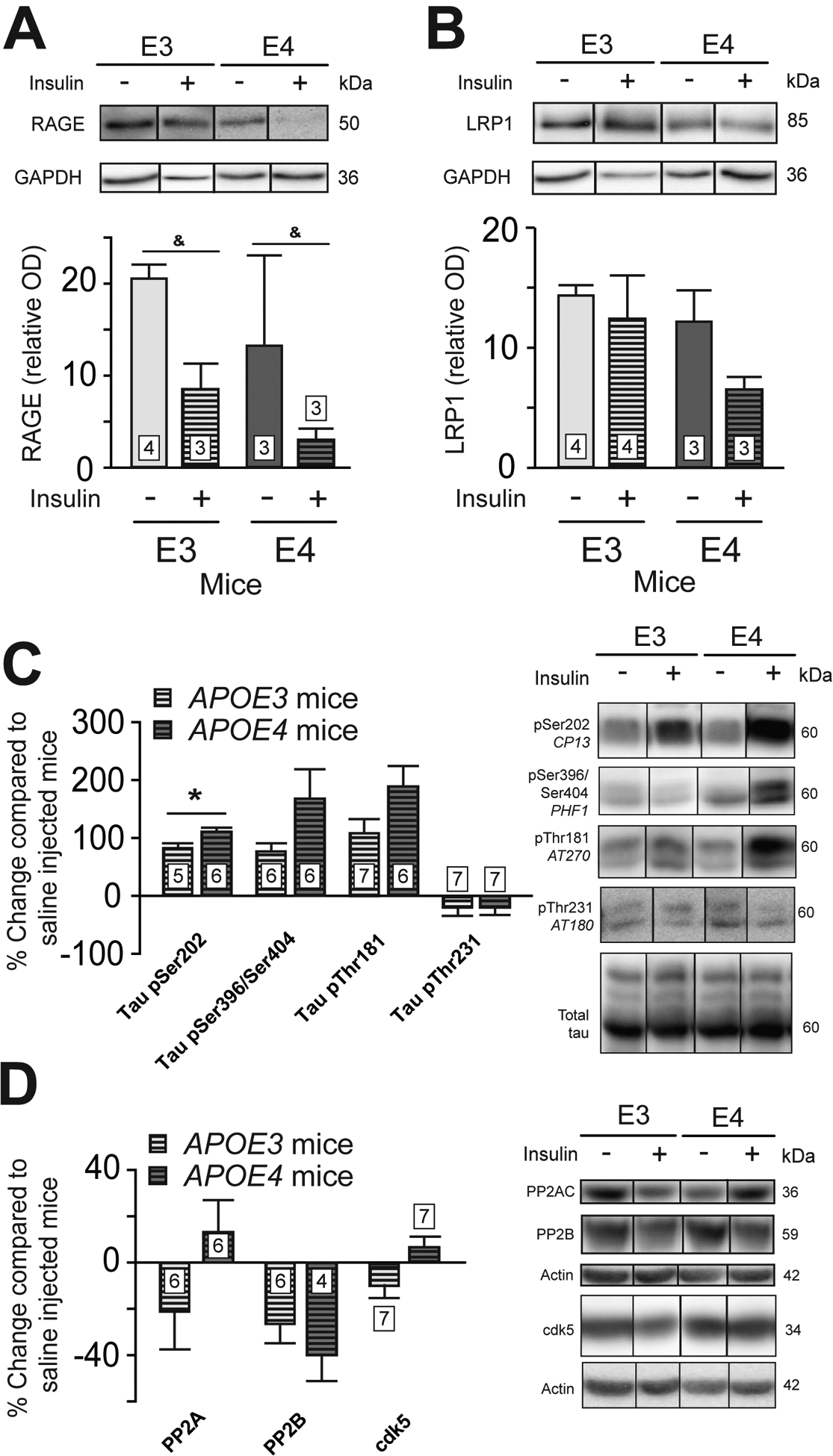
Western blots analysis of RAGE (A) and LRP1 (B) concentrations in brain capillaries 5 minutes after insulin or saline injection. Levels of soluble phosphorylated tau in response to insulin injection in cortex (C). Data for p-tau are presented compared to the average of saline-injected mice. Concentrations of phosphatases and cdk5 (D). Data are presented as mean ± SEM. Statistical analysis: Mann-Whitney test, &p<0.05 (A and B) or unpaired t-test, *p<0.05 (C and D). RAGE = receptor for advanced glycation end products. LRP1 = low density lipoprotein receptor-related protein 1. cdk5 = cyclin-dependent kinase 5. PP2AC = catalytic subunit of protein phosphatase 2A. PP2B = protein phosphatase 2B.
Next, we investigated cortical phosphorylation of tau, one of the main neuropathological hallmarks of AD in brain cortex homogenates (Querfurth and LaFerla, 2010). Following an acute insulin injection, the change of ptau Ser202/Thr205 reached 113% in APOE4 compared to 78.5% in APOE3 mice (p<0.05) in the soluble fraction (Fig. 3C). Our data are consistent with previous studies showing increased ptau Ser202 in the brain of APOE4 mice following administration of insulin (Freude et al., 2005) or pioglitazone (To et al., 2011), a peripheral insulin sensitizer used in the treatment of type 2 diabetes. Since Akt directly phosphorylates tau protein (Ksiezak-Reding et al., 2003) and regulates the activity of several kinases implicated in tau phosphorylation (Cross et al., 1995), the increase in pAkt observed here in APOE4 mice might partly explain the amplification in tau phosphorylation following insulin injection.
Finally, to confirm if the APOE4 genotype was also linked with a higher phosphorylation of tau in AD patients, we assessed different phospho-epitopes in TBS-extracts from the parietal cortex of human subjects (Fig. 4). Interestingly, we found that human APOE4 carriers had 5-fold higher levels of ptau Ser202/Thr205 and Ser396/404 (detected with AT8 and PHF1 antibodies, respectively) compared to non-carriers volunteers (Fig. 4A and B), in line with our observations in APOE4 mice. However, we need to be careful interpreting these results since the APOE4 group was enriched in AD patients with Braak Stage V or VI. Since Braak staging is based on postmortem neurofibrillary tangle counts, higher tau levels in cortex homogenates is expected as well. Therefore, we performed two-way ANOVAs using Braak scores as one independent categorical variable and APOE carriage as the other. The impact of APOE carriage on tau phosphorylation at Ser202/Thr205 became non-significant (p = 0.0960) but remained higher than the effect of Braak stages (I, II and III versus IV and V; p = 0.251). This suggests a stronger effect of APOE carriage on ptau Ser202/Thr205 compared to Braak scores. For ptau pSer396/404, the difference between APOE4 and APOE3 did not reach significance (p = 0.254) but almost did for Braak scores (p = 0.0597). This indicates that the higher proportion in individuals with Braak scores IV and V in the APOE4 group contributed to the differences detected between APOE4 and APOE3 carriers, particularly for ptau Ser396/404.
Next, although there were more AD subjects in the APOE4 group, the impact of APOE carriage on tau phosphorylation at Ser202/Thr205 was stronger (p = 0.023) than the effect of clinical diagnosis (p = 0.084), as revealed by two-way ANOVA analyses for this particular phosphoepitope. On the other hand, the opposite was found with ptau Ser396/404 (Diagnosis, p = 0.048; APOE4 carriage, p = 0.063), indicating that AD diagnosis might drive phosphorylation of tau to a slightly greater extent than APOE4 genotype. Since there was a significant difference between APOE4 carriers and non-carriers for the MMSE score and age of death, we included those parameters as covariates in our statistical analyses (Table S3). However, ptau Ser396/404 and Ser202/Thr205 remain statistically higher in APOE4 carriers after the adjustment for both age of death and MMSE score. Overall, this suggests that the higher insulin response in APOE4 mice is associated with hyperphosphorylation of tau, a key AD neuropathological marker.
Discussion
In this study, we used APOE3- and APOE4-targeted replacement mice to investigate the relationship between human APOE genotypes, cerebral insulin responses, and AD-related parameters, such as BBB transporters of Aβ and phosphorylation of tau. The brain of APOE4 mice showed a higher response to insulin, at least with respect to the Akt pathway. Furthermore, acute insulin injection increased tau phosphorylation at Ser202/Thr205, suggesting that the activation of the insulin-signaling pathway leads to tau hyperphosphorylation in APOE4 mice. Several pieces of evidence in human and animal models suggest that insulin might be a therapeutic tool in AD (De Felice, 2013). The beneficial effect of insulin on several memory-related endpoints has been consistently observed in at least 10 RCTs (Craft et al., 2012; Freiherr et al., 2013; Reger et al., 2006; Reger et al., 2008). Preclinical data also support a memory-enhancing effect of insulin. For example, systemic insulin also improved object recognition memory in the 3xTg-AD mouse model of AD fed a high-fat diet (Vandal et al., 2014b). Since memory improvement was associated with a reduction in soluble cortical Aβ and a sharp increase in plasma Aβ (Vandal et al., 2014b), clearance of Aβ through the BBB was suggested as a plausible mechanism (Vandal et al., 2015). The present observation that insulin lowers RAGE levels in brain capillaries of APOE3 and APOE4 mice provides further support for the contention that insulin potentiates Aβ clearance from the brain.
In spite of these encouraging data, clinical trials also tell us that insulin, at least when administered intranasally (Claxton et al., 2013; Craft et al., 2012; Reger et al., 2006; Reger et al., 2008) or intravenously (Craft et al., 1999), does not provide cognitive benefits to APOE4 carriers, contrary to non-carriers. Consistent results are also found in studies using a similar time frame than the present study. Rosenbloom et al. compared the effect of a single dose of intranasal rapid-acting insulin glulisine with a placebo on cognitive function of APOE4 carrier with mild to moderate AD (Rosenbloom et al., 2014). A battery of cognitive test executed 20 min following the treatment revealed no effect of the acute insulin treatment (Rosenbloom et al., 2014). In line with these results, Reger et al. in 2006 previously reported no effect of intranasal insulin on total story and total list recall, 15 min following administration, in memory-impaired APOE4 carriers (Reger et al., 2006). Since APOE4 is predominant in AD, representing over 36% of patients suffering from the disease (Farrer et al., 1997), it is critical to find out why these patients are not benefiting from insulin treatment and the underlying mechanisms (Claxton et al., 2013). APOE4-positive AD patients show increased plasma insulin compared to non-APOE4 carriers, which might indicate that they are insulin resistant (Craft et al., 1999). Furthermore, recent studies in the same mouse model reported lower insulin signaling-related proteins at the basal state in the brain of 32- and 72-week-old mice (Keeney et al., 2015; Ong et al., 2014). Indeed, Ong et al. 2014 showed that APOE4 mice display a reduction in the phosphorylation of IRS and Akt proteins, in line with a reduction of brain insulin signaling (Keeney et al., 2015; Ong et al., 2014). However, the present data do not lend direct support to that hypothesis as we found increased Akt activation following acute insulin administration in APOE4 mice, when compared to APOE3 mice. On the other hand, we found no difference in IRS1 concentrations, similarly to the results from Keeney et al., 2015, who studied insulin signaling in 6-month-old APOE3 and APOE4 female mice (Keeney et al., 2015). Interestingly, a higher phosphorylation status of IRS1 at Ser636 has been shown to impair insulin sensitivity (Li et al., 2004; Tremblay et al., 2007b). Lower level of pIRS1 Ser636 was observed in the brain of APOE4 mice (Ong et al., 2014), which may partly explain the higher insulin-induced Akt activation observed in the present study. Another possible explanation for this increased Akt activation may stem from the mTORC2 complex. The loss of mTORC2 was shown to promote severe hyperglycemia in mice (Treins et al., 2012). The RICTOR-binding subunit of mTORC2 can also phosphorylate Akt at Ser473 (Sarbassov et al., 2005). Thus, an upregulation of RICTOR-mTOR complex activity in APOE4 mice could also contribute to the higher Akt activation observed here.
The higher activation of the Akt pathway in APOE4 carriers observed here might thus have several outcomes on the function of brain cells. One of the possible consequences of the amplification of cerebral Akt signaling in APOE4 mice observed here is an increased phosphorylation of tau at site Ser202. Phospho-tau Ser202/Thr205 is detected at early stages of AD (Blazquez-Llorca et al., 2010) and is used for postmortem staging of AD (Braak and Braak, 1997). Also, it is known to promote aggregation of the protein over time and therefore might affect formation of neurofibrillary tangles (Rankin et al., 2005). Insoluble tau species are found in AD brain and are well correlated with ante-mortem cognitive deficits (Tremblay et al., 2007a). Although the rise detected here is quickly triggered by acute insulin and might wane off over time, on the long term, daily systemic peaks of insulin might still have particularly detrimental effects in APOE4 carriers.
Interestingly, in agreement with the animal data, higher phosphorylation of tau at Ser202/Thr205 and pSer396/404 was also observed in human APOE4 carriers from the ROS cohort compared to non-APOE4 carriers, whereas no such difference was detected for pThr181 or total tau. These results should be considered with caution since, as APOE4 carriage is more frequent in AD than in the general population, the APOE4 group of the present cohort was also enriched in persons with Braak stages V or VI. Although not statistically significant, this difference in proportion certainly contributed to the observed higher tau phosphorylation in APOE4 subjects.
Nevertheless, it is interesting to observe that the difference found between APOE4 and APOE3 carriers was particularly strong for pSer202/Thr205 tau (AT8) and virtually absent for total tau, as observed in the mouse models.
Our present data therefore reveal that the Akt pathway in APOE4 mice responds more strongly to insulin, translating into a rapid increase in tau phosphorylation at Ser202/Thr205 after a single injection. Since total APOE levels were unchanged between groups, this is consistent with a gain function of the APOE4 allele (Salem et al., 2015; Teter, 2007). Our data suggest that a faulty downstream mechanism such as tau hyperphosphorylation makes the action of insulin deleterious in APOE4 carriers, at least from an AD perspective. As far as these findings are applicable to humans, they put forward a possible mechanism explaining why AD patients who are APOE4 carriers do not benefit from insulin therapy as non-carriers do.
Supplementary Material
Highlights.
APOE4 carriage potentiates the activation of brain insulin signaling pathway.
Insulin induces higher phosphorylation of tau at Ser202 in APOE4 mice.
Human APOE4 carriers have higher phosphorylation of tau at Ser202.
Defects in brain response may explain insulin poor clinical effect in APOE4 carriers.
Acknowledgements
We would like to thank Arnaud François for the help with data analysis. Technicians from the animal facility (Sonia Francoeur and Stephanie Bernard) for the IV injection and animal care.
Role of funding sources
This study was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canada Foundation for Innovation (CFI). MTT is supported by a scholarship from the Fonds d’enseignement et de recherche (FER) of the Faculty of Pharmacy of Laval University. FC is supported by a salary award from the Fonds de la recherche en santé du Québec (FRQ-S). We acknowledge study participants, RADCC staff, and NIA Grant P30AG10161 for data on human brain cortex samples
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors have no conflict of interest to declare.
References
- Alata W, Paris-Robidas S, Emond V, Bourasset F, and Calon F (2014). Brain uptake of a fluorescent vector targeting the transferrin receptor: a novel application of in situ brain perfusion. Mol Pharm 11, 243–253. [DOI] [PubMed] [Google Scholar]
- Alata W, Ye Y, St-Amour I, Vandal M, and Calon F (2015). Human apolipoprotein E varepsilon4 expression impairs cerebral vascularization and blood-brain barrier function in mice. J Cereb Blood Flow Metab 35, 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C, Armulik A, Sallstrom J, Berk BC, and Zlokovic BV (2012). Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 485, 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C, Frey WH, Schiöth HB, Schultes B, Born J, and Hallschmid M (2011). Intranasal insulin as a therapeutic option in the treatment of cognitive impairments. Exp Gerontol 46, 112–115. [DOI] [PubMed] [Google Scholar]
- Bennett DA (2006). Postmortem indices linking risk factors to cognition: results from the Religious Order Study and the Memory and Aging Project. Alzheimer Dis Assoc Disord 20, S63–8. [DOI] [PubMed] [Google Scholar]
- Blazquez-Llorca L, Garcia-Marin V, and Defelipe J (2010). Pericellular innervation of neurons expressing abnormally hyperphosphorylated tau in the hippocampal formation of Alzheimer’s disease patients. Front Neuroanat 4, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, and Braak E (1997). Diagnostic criteria for neuropathologic assessment of Alzheimer’s disease. Neurobiol Aging 18, S85–8. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Boyle PA, Wilson RS, Beck TL, Kelly JF, and Bennett DA (2009). Apolipoprotein E e4 allele is associated with more rapid motor decline in older persons. Alzheimer Dis Assoc Disord 23, 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, Baxter LC, Rapcsak SZ, Shi J, Woodruff BK, Locke DE, Snyder CH, Alexander GE, Rademakers R, and Reiman EM (2009). Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med 361, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Fagan AM, Morris JC, Mawuenyega KG, Cruchaga C, Goate AM, Bales KR, Paul SM, Bateman RJ, and Holtzman DM (2011). Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med 3, 89ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Kim SY, Na HR, Kim BK, Yang DW, Kwon JC, and Park MY (2008). Effect of ApoE genotype on response to donepezil in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord 25, 445–450. [DOI] [PubMed] [Google Scholar]
- Claxton A, Baker LD, Wilkinson CW, Trittschuh EH, Chapman D, Watson GS, Cholerton B, Plymate SR, Arbuckle M, and Craft S (2013). Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer’s disease. J Alzheimers Dis 35, 789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, and Pericak-Vance MA (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923. [DOI] [PubMed] [Google Scholar]
- Craft S, Asthana S, Schellenberg G, Cherrier M, Baker LD, Newcomer J, Plymate S, Latendresse S, Petrova A, Raskind M, Peskind E, Lofgreen C, and Grimwood K (1999). Insulin metabolism in Alzheimer’s disease differs according to apolipoprotein E genotype and gender. Neuroendocrinology 70, 146–152. [DOI] [PubMed] [Google Scholar]
- Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, and Gerton B (2012). Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol 69, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, and Hemmings BA (1995). Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789. [DOI] [PubMed] [Google Scholar]
- De Felice FG (2013). Alzheimer’s disease and insulin resistance: translating basic science into clinical applications. J Clin Invest 123, 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhuria SV, Hanson LR, and Frey WH (2010). Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci 99, 1654–1673. [DOI] [PubMed] [Google Scholar]
- Drzezga A, Grimmer T, Henriksen G, Muhlau M, Perneczky R, Miederer I, Praus C, Sorg C, Wohlschlager A, Riemenschneider M, Wester HJ, Foerstl H, Schwaiger M, and Kurz A (2009). Effect of APOE genotype on amyloid plaque load and gray matter volume in Alzheimer disease. Neurology 72, 1487–1494. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, and van Duijn CM (1997). Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA 278, 1349–1356. [PubMed] [Google Scholar]
- Freiherr J, Hallschmid M, Frey WH, Brünner YF, Chapman CD, Hölscher C, Craft S, De Felice FG, and Benedict C (2013). Intranasal insulin as a treatment for Alzheimer’s disease: a review of basic research and clinical evidence. CNS Drugs 27, 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freude S, Plum L, Schnitker J, Leeser U, Udelhoven M, Krone W, Bruning JC, and Schubert M (2005). Peripheral hyperinsulinemia promotes tau phosphorylation in vivo. Diabetes 54, 3343–3348. [DOI] [PubMed] [Google Scholar]
- Keeney JT, Ibrahimi S, and Zhao L (2015). Human ApoE Isoforms Differentially Modulate Glucose and Amyloid Metabolic Pathways in Female Brain: Evidence of the Mechanism of Neuroprotection by ApoE2 and Implications for Alzheimer’s Disease Prevention and Early Intervention. J Alzheimers Dis 48, 411–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiezak-Reding H, Pyo HK, Feinstein B, and Pasinetti GM (2003). Akt/PKB kinase phosphorylates separately Thr212 and Ser214 of tau protein in vitro. Biochim Biophys Acta 1639, 159–168. [DOI] [PubMed] [Google Scholar]
- Li Y, Soos TJ, Li X, Wu J, Degennaro M, Sun X, Littman DR, Birnbaum MJ, and Polakiewicz RD (2004). Protein kinase C Theta inhibits insulin signaling by phosphorylating IRS1 at Ser(1101). J Biol Chem 279, 45304–45307. [DOI] [PubMed] [Google Scholar]
- Liraz O, Boehm-Cagan A, and Michaelson DM (2013). ApoE4 induces Abeta42, tau, and neuronal pathology in the hippocampus of young targeted replacement apoE4 mice. Mol Neurodegener 8, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong QR, Chan ES, Lim ML, Cole GM, and Wong BS (2014). Reduced phosphorylation of brain insulin receptor substrate and Akt proteins in apolipoprotein-E4 targeted replacement mice. Sci Rep 4, 3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendse AA, Arbones-Mainar JM, Johnson LA, Altenburg MK, and Maeda N (2009). Apolipoprotein E knock-out and knock-in mice: atherosclerosis, metabolic syndrome, and beyond. J Lipid Res 50 Suppl, S178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfurth HW, and LaFerla FM (2010). Alzheimer’s disease. N Engl J Med 362, 329–344. [DOI] [PubMed] [Google Scholar]
- Raber J, Wong D, Buttini M, Orth M, Bellosta S, Pitas RE, Mahley RW, and Mucke L (1998). Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: increased susceptibility of females. Proc Natl Acad Sci U S A 95, 10914–10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin CA, Sun Q, and Gamblin TC (2005). Pseudo-phosphorylation of tau at Ser202 and Thr205 affects tau filament formation. Brain Res Mol Brain Res 138, 84–93. [DOI] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Frey WH, Baker LD, Cholerton B, Keeling ML, Belongia DA, Fishel MA, Plymate SR, Schellenberg GD, Cherrier MM, and Craft S (2006). Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging 27, 451–458. [DOI] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Green PS, Baker LD, Cholerton B, Fishel MA, Plymate SR, Cherrier MM, Schellenberg GD, Frey WH, and Craft S (2008). Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis 13, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell DR, Zhou H, Atchison K, Warwick HK, Atkinson PJ, Jefferson J, Xu L, Aschmies S, Kirksey Y, Hu Y, Wagner E, Parratt A, Xu J, Li Z, Zaleska MM, Jacobsen JS, Pangalos MN, and Reinhart PH (2008). Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J Neurosci 28, 11445–11453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom MH, Barclay TR, Pyle M, Owens BL, Cagan AB, Anderson CP, Frey WH, and Hanson LR (2014). A single-dose pilot trial of intranasal rapid-acting insulin in apolipoprotein E4 carriers with mild-moderate Alzheimer’s disease. CNS Drugs 28, 1185–1189. [DOI] [PubMed] [Google Scholar]
- Salem NJ, Vandal M, and Calon F (2015). The benefit of docosahexaenoic acid for the adult brain in aging and dementia. Prostaglandins Leukot Essent Fatty Acids 92, 15–22. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, and Sabatini DM (2005). Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101. [DOI] [PubMed] [Google Scholar]
- Schiöth HB, Craft S, Brooks SJ, Frey WH, and Benedict C (2012). Brain insulin signaling and Alzheimer’s disease: current evidence and future directions. Mol Neurobiol 46, 4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Amour I, Pare I, Tremblay C, Coulombe K, Bazin R, and Calon F (2014). IVIg protects the 3xTg-AD mouse model of Alzheimer’s disease from memory deficit and Abeta pathology. J Neuroinflammation 11, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, and Roses AD (1993). Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A 90, 1977–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PM, Han B, Liu F, Mace BE, Ervin JF, Wu S, Koger D, Paul S, and Bales KR (2011). Reduced levels of human apoE4 protein in an animal model of cognitive impairment. Neurobiol Aging 32, 791–801. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, Quarfordt SH, and Maeda N (1997). Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem 272, 17972–17980. [DOI] [PubMed] [Google Scholar]
- Teter B (2007). Life-span influences of apoE4 on CNS function. Neurobiol Aging 28, 693–703; discussion 704. [DOI] [PubMed] [Google Scholar]
- To AW, Ribe EM, Chuang TT, Schroeder JE, and Lovestone S (2011). The epsilon3 and epsilon4 alleles of human APOE differentially affect tau phosphorylation in hyperinsulinemic and pioglitazone treated mice. PLoS One 6, e16991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treins C, Alliouachene S, Hassouna R, Xie Y, Birnbaum MJ, and Pende M (2012). The combined deletion of S6K1 and Akt2 deteriorates glycemic control in a high-fat diet. Mol Cell Biol 32, 4001–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay C, Pilote M, Phivilay A, Emond V, Bennett DA, and Calon F (2007a). Biochemical characterization of Abeta and tau pathologies in mild cognitive impairment and Alzheimer’s disease. J Alzheimers Dis 12, 377–390. [DOI] [PubMed] [Google Scholar]
- Tremblay C, St-Amour I, Schneider J, Bennett DA, and Calon F (2011). Accumulation of transactive response DNA binding protein 43 in mild cognitive impairment and Alzheimer disease. J Neuropathol Exp Neurol 70, 788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay F, Brûlé S, Hee Um S, Li Y, Masuda K, Roden M, Sun XJ, Krebs M, Polakiewicz RD, Thomas G, and Marette A (2007b). Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci U S A 104, 14056–14061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandal M, Alata W, Tremblay C, Rioux-Perreault C, Salem NJ, Calon F, and Plourde M (2014a). Reduction in DHA transport to the brain of mice expressing human APOE4 compared to APOE2. J Neurochem 129, 516–526. [DOI] [PubMed] [Google Scholar]
- Vandal M, Bourassa P, and Calon F (2015). Can insulin signaling pathways be targeted to transport Abeta out of the brain? Front Aging Neurosci 7, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandal M, White PJ, Tremblay C, St-Amour I, Chevrier G, Emond V, Lefrancois D, Virgili J, Planel E, Giguere Y, Marette A, and Calon F (2014b). Insulin reverses the high-fat diet-induced increase in brain Abeta and improves memory in an animal model of Alzheimer disease. Diabetes 63, 4291–4301. [DOI] [PubMed] [Google Scholar]
- White PJ, St-Pierre P, Charbonneau A, Mitchell PL, St-Amand E, Marcotte B, and Marette A (2014). Protectin DX alleviates insulin resistance by activating a myokine-liver glucoregulatory axis. Nat Med 20, 664–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


