Abstract
A phytase (EC 3.1.3.8) with a high affinity for phytic acid was found in Aspergillus niger SK-57 and purified to homogeneity in four steps by using ion-exchange chromatography (two types), gel filtration, and chromatofocusing. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the purified enzyme gave a single stained band at a molecular mass of approximately 60 kDa. The Michaelis constant of the enzyme for phytic acid (18.7 ± 4.6 μM) was statistically analyzed. In regard to the orthophosphate released from phytic acid, a significant difference between a low Km phytase from A. niger SK-57 and a high Km phytase from Aspergillus ficuum was recognized.
Phytic acid (myo-inositol hexakis dihydrogen phosphate) is the major storage form of phosphate in cereals, pollen, legumes, and oilseed. Phytic acid is considered to be an antinutritional factor since it chelates minerals such as magnesium, zinc, and calcium and may also react with proteins, therefore decreasing the bioavailability of protein and nutritionally important minerals.
The use of phytase as a feed additive has been examined several times over the last 20 years, resulting in improved phosphorus availability from poultry and swine feed. However, the high cost of the enzyme, compared to the cost of inorganic phosphate, has prevented its universal use. Recently, there has been renewed interest in phytase due to the low-cost production of this enzyme by recombinant DNA technology and an increased concern for the environment. Phytase has the potential to reduce the amount of phosphate in poultry and swine wastes by enhancing phosphorus retention by the animal.
Phytase, myo-inositol hexakisphosphate phosphohydrolase (EC. 3.1.3.8), catalyzes the hydrolysis of phytic acid to inositol polyphosphates and free orthophosphoric acid. Phytase-producing microorganisms comprise bacteria such as Bacillus subtilis (8), Pseudomonas sp. (3), and Escherichia coli (1); yeasts such as Schwanniomyces castellii (9) and Saccharomyces cerevisiae (6); and fungi such as Aspergillus ficuum (2) and Aspergillus terreus (14). The phytase produced by A. ficuum NRRL 3135 has been isolated and well characterized by Ullah and Gibson (10, 11). In addition, the cloning and expression of the phyA gene have been reported for A. ficuum (13), Aspergillus awamori (7), and A. terreus (5). Recently, Kostrewa et al. reported the crystal structure of phytase from A. ficuum (4).
In this paper, we describe the purification and characterization of a phytase with a high affinity for phytate. The amount of orthophosphate released by this enzyme is compared with that released by the A. ficuum phytase.
Aspergillus niger SK-57 was inoculated on solid media with wheat bran and cultivated at 30°C for 5 days. Proteins were extracted from solid-state fermentation (koji mold grown on sterilized wheat bran) by using cones with warm water. After filtration with filter paper (no. 2; ADVANTEC, Tokyo, Japan), the crude extract was desalted by using a Sartcon mini system (Sartorius) equipped with an ultrafilter (molecular weight cutoff, 10,000) and used for enzyme purification. Purification of phytase from A. niger SK-57 was done at 4°C. In step 1, the crude enzyme was applied to an anion-exchange DIAION HPA-75 column (5.6 by 30 cm; Mitsubishi Chemical, Tokyo, Japan) that had previously been equilibrated with 50 mM acetate buffer (pH 5.5). The column was washed with equilibration buffer, and the proteins were eluted with 0.3 M NaCl in 50 mM acetate buffer (pH 4.8). The peak fractions of phytase activity were pooled and concentrated by ultrafiltration through a UK-10 membrane having a molecular weight cutoff of 10,000 (ADVANTEC) and were dialyzed overnight against 50 mM acetate buffer (pH 4.9) at 4°C. In step 2, the concentrate obtained from step 1 was applied to an S Sepharose Fast Flow column (2.5 by 30 cm; Pharmacia Biotech, Uppsala, Sweden) that had previously been equilibrated with 50 mM acetate buffer (pH 4.9). The column was washed with equilibration buffer, and the proteins were eluted with 50 mM acetate buffer (pH 5.2). Fractions containing phytase activity were pooled and concentrated by ultrafiltration, as described for step 1. In step 3, the concentrate obtained from step 2 was applied to a TOYO-PEARL HW-55F column (2.0 by 60 cm; Tosoh, Tokyo, Japan) and equilibrated with 50 mM acetate buffer (pH 4.5). Fractions with high phytase activity were pooled and dialyzed overnight against 50 mM acetate buffer (pH 6.0) at 4°C. In step 4, the enzyme solution was applied to a Mono-P HR 5/20 column (Pharmacia) that had previously been equilibrated with 25 mM histidine-HCl buffer (pH 5.8), and the phytase was eluted with 10% polybuffer 74-HCl (pH 4.2). A purification profile of the phytase from A. niger SK-57 with solid-state fermentation is shown in Table 1. The enzyme was purified 11-fold with a 2.5% yield from the crude extract. The specific activity of the purified enzyme was 158 U/mg of protein. The purified enzyme was shown as a single protein band on a sodium dodecyl sulfate (SDS)-polyacrylamide gel. The molecular masses of the native protein and the protein deglycosylated by endoglycosidase H were estimated to be approximately 60 and 55 kDa, respectively (Fig. 1), suggesting that the protein contains a small amount of carbohydrate. The isoelectric point determined by chromatofocusing was 4.7.
TABLE 1.
Summary of purification of A. niger SK57 phytase
| Step | Total protein (mg) | Total activity (U, 103)a | Sp act (U/mg) | Yield (%) | Fold |
|---|---|---|---|---|---|
| Crude enzyme | 180,000 | 2,700 | 15 | 100 | 1 |
| HPA-75 | 54,000 | 1,300 | 24 | 48 | 1.6 |
| S Sepharose | 2,200 | 320 | 145 | 12 | 9.7 |
| TOYO-PEARL HW-55F | 460 | 73 | 158 | 2.7 | 11 |
| Chromatofocusing | 430 | 68 | 158 | 2.5 | 11 |
One unit of phytase activity is defined as the amount of enzyme which liberates 1 μmol of orthophosphate from Na phytate in 1 min at 37°C and pH 5.5.
FIG. 1.
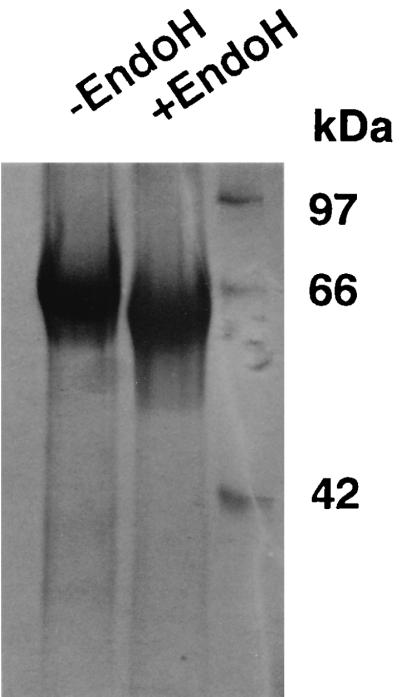
SDS-polyacrylamide gel of the purified enzyme. Electrophoresis was performed on a slab of 12% polyacrylamide gel in 25 mM Tris-HCl–0.192 M glycine containing 0.1% SDS. The gel was stained for proteins with Coomassie brilliant blue R-250. Lane −EndoH, the purified native phytase; lane +EndoH, the native phytase treated with endoglycosidase H. The following molecular masses of standard proteins are located on the right: ovalbumin (42 kDa), serum albumin (66 kDa), and phosphorylase (97 kDa).
To study the enzyme substrate affinity, the kinetic parameter of phytic acid was determined at pH 5.5 and 37°C. The apparent Michaelis constant (Km) of the phytase for phytic acid calculated from the Lineweaver-Burk plot was 18.7 ± 4.6 μM. This low Km shows a remarkably high affinity of the protein for phytic acid, higher than the Km values of 40 μM and 250 μM reported for A. ficuum by Ullah (11) and van Gorcom et al. (12), respectively. van Gorcom et al. demonstrated that the phytase from A. ficuum NRRL 3135 purified by Ullah contained two proteins, a phytase having a molecular mass of 85 kDa and an acid phosphatase having a molecular mass of 100 kDa (12). When we purified the phytase from A. ficuum NRRL 3135, we found the molecular mass and the Km of the phytase to be 85 kDa and 184.2 ± 12.5 μM, respectively (data not shown).
The action of the purified enzyme in 0.1 M sodium acetate, pH 5.5, on several phosphate compounds was tested. The tested substrates were as follows: phytic acid, p-nitrophenylphosphate, d-glucose 6-phosphate, fructose 6-phosphate, d-myo-inositol 1,4,5-triskisphosphate, glycerophosphate, and ATP. Phytic acid was hydrolyzed at the fastest rate; the other phosphorylated compounds reached a maximum of about only 2% of the phytic acid hydrolysis rate. To investigate the pH optimum and pH stability, the phytase assay was performed at a pH range of 2 to 9 with a variety of buffers by standard assay. The phytase had a double pH optimum of pH 5.5 and pH 2.5 and was virtually inactive above pH 7.0. The activity at pH 2.5 was 60% less than that at pH 5.5. When the enzyme was incubated at various pH values at 37°C for 60 min in the absence of substrate and the residual activity was measured, the phytase was found to be stable at the pH range of 5 to 7. The temperature profile of purified phytase was determined from 4 to 60°C by standard assay at the given temperature. The optimum temperature was found to be 50°C. To investigate thermal stability, the phytase was incubated at 0 to 60°C for 60 min in 0.1 M acetate buffer, pH 5.5, and its activity was determined by standard assay. No less activity was observed from 0 to 30°C, while at 50°C only 30% of the activity remained. The N-terminal amino acid sequence analysis of the enzyme was determined to be Ser-Arg-Asn-Gln-Ser-Thr-Cys-Asp-Thr-Val-Asp-Gln-Gly-Tyr-Gln with a gas-phase sequencer (Applied Biosystems).
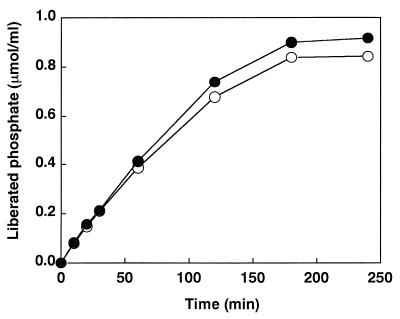
To investigate the hydrolysis of Na phytate by the A. niger SK-57 and A. ficuum phytases, an enzymatic reaction was started by the addition of enzyme (0.01 U) to the assay mixture. The final concentration of phytate was 0.2 mM in 0.1 M acetate buffer, pH 5.5. From the incubation mixture, samples (0.4 ml) were removed periodically, and the reaction was stopped by adding 0.8 ml of freshly prepared acetone–5 N H2SO4–10 mM ammonium molybdate (2:1:1 [vol/vol/vol]). After mixing, 40 μl of 1.0 M citric acid was added to each tube. The orthophosphates released from phytate were determined (Fig. 2). The A. niger SK-57 phytase with a low Km released more orthophosphate, even at a lower substrate concentration, than the A. ficuum phytase with a high Km. The difference was about 8%. To demonstrate the performance of the enzyme, the concentration of a substrate necessarily has to be higher than its Km, and if an enzyme with a low Km and an enzyme with a high Km have the same maximum reaction rate (Vmax), the enzyme with the low Km, unlike the enzyme with the high Km, does not decrease the reaction rate, even at a lower substrate concentration. That is, when compared with the enzyme with the high Km, the enzyme with the low Km has an advantage in that it can maintain a sufficient degradation rate, even at a lower substrate concentration, thereby minimizing the amount of the remaining substrate. Accordingly, there is a demand for an inexpensive A. niger phytase with a low Km for phytic acid because phytase degrades phytic acid, an antitrophic factor contained in feed, thereby improving the nutritive value of the feed and simultaneously achieving an efficient utilization of phosphoric acid released by the degradation.
FIG. 2.
Time course of the release of phosphate from Na phytate by phytases. The enzyme activity was fixed at 0.01 U. Symbols: ○, A. ficuum phytase; ●, A. niger SK-57 phytase.
Acknowledgments
We are grateful to S. Sugimoto and K. Yano for the N-terminal amino acid sequencing. We also thank K. Koide, K. Miyoshi, and T. Yamazumi for helpful discussions.
REFERENCES
- 1.Greiner R, Konietzny U, Jany K-D. Purification and characterization of two phytases from Escherichia coli. Arch Biochem Biophys. 1993;303:107–113. doi: 10.1006/abbi.1993.1261. [DOI] [PubMed] [Google Scholar]
- 2.Howson S J, Davis R P. Production of phytate-hydrolyzing enzyme by some fungi. Enzyme Microb Technol. 1983;5:377–382. [Google Scholar]
- 3.Irving G C J, Cosgrove D J. Inositol phosphate phosphatases of microbiological origin. Some properties of a partially purified bacterial phytase. Aust J Biol Sci. 1971;24:547–557. doi: 10.1071/bi9710547. [DOI] [PubMed] [Google Scholar]
- 4.Kostrewa D, Grüninger-Leitch F, D’Arcy A, Broger C, Mitchell D, van Loon A P G M. Crystal structure of phytase from Aspergillus ficuum at 2.5 Å resolution. Nat Struct Biol. 1997;4:185–190. doi: 10.1038/nsb0397-185. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell D B, Vogel K, Weimann B J, Pasamontes L, van Loon A P G M. The phytase subfamily of histidine acid phosphatases: isolation of genes for two novel phytases from the fungi Aspergillus terreus and Myceliophthora thermophila. Microbiology. 1997;143:245–252. doi: 10.1099/00221287-143-1-245. [DOI] [PubMed] [Google Scholar]
- 6.Nayini N R, Markakis P. The phytase of yeast. Lebensm Wiss Technol. 1984;17:24–26. [Google Scholar]
- 7.Piddington C S, Houston C S, Paloheimo M, Cantrell M, Miettinen-Oinonen A, Nevalainen H, Rambosek J. The cloning and sequencing of the genes encoding phytase (phy) and pH 2.5-optimum acid phosphatase (aph) from Aspergillus niger var. awamori. Gene. 1993;133:55–62. doi: 10.1016/0378-1119(93)90224-q. [DOI] [PubMed] [Google Scholar]
- 8.Power V K, Jagannathan V. Purification and properties of phytate-specific phosphatase from Bacillus subtilis. J Bacteriol. 1982;151:1102–1108. doi: 10.1128/jb.151.3.1102-1108.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Segueilha L, Lamgrechts C, Boze H, Mourin G, Galzy P. Purification and properties of the phytase from Schwanniomyces castellii. J Ferment Bioeng. 1992;74:7–11. [Google Scholar]
- 10.Ullah A H J, Gibson D M. Extracellular phytase (EC. 3.1.3.8) from Aspergillus ficuum NRRL 3135: purification and characterization. Prep Biochem. 1987;17:63–91. doi: 10.1080/00327488708062477. [DOI] [PubMed] [Google Scholar]
- 11.Ullah A H J. Aspergillus ficuum phytase: partial primary structure, substrate selectivity and kinetic characterization. Prep Biochem. 1988;18:459–471. doi: 10.1080/00327488808062544. [DOI] [PubMed] [Google Scholar]
- 12.van Gorcom, R. F. M., W. van Hartingsveldt, P. A. van Paridon, A. E. Veenstra, R. G. M. Luiten, and G. C. M. Selten. July 1995. U.S. patent 5,436,156.
- 13.van Hartingsveldt W, van Zeijl C M J, Hartevelt M, Gouka R J, Suykerbuyk M E G, Luiten R G M, van Paridon P A, Selten G C M, Veenstra A E, van Gorcom R F M, van Hondel C A M J J. Cloning, characterization and overexpression of the phytase-encoding gene (phyA) of Aspergillus niger. Gene. 1993;127:87–94. doi: 10.1016/0378-1119(93)90620-i. [DOI] [PubMed] [Google Scholar]
- 14.Yamada K, Minoda Y, Yamamoto S. Phytase from Aspergillus terreus. Agric Biol Chem. 1968;32:1275–1282. [Google Scholar]