Abstract
In this study, we propose a new process of adding a genetically modified killer yeast to improve the aerobic stability of silage. Previously constructed Kluyveromyces lactis killer strain PCK27, defective in growth on lactic acid due to disruption of the gene coding for phosphoenolpyruvate carboxykinase, a key enzyme for gluconeogenesis, inhibited the growth of Pichia anomala inoculated as an aerobic spoilage yeast and prevented a rise in pH in a model of silage fermentation. This suppressive effect of PCK27 was not only due to growth competition but also due to the killer protein produced. From these results, we concluded that strain PCK27 can be used as an additive to prolong the aerobic stability of maize silage. In the laboratory-scale experiment of maize silage, the addition of a killer yeast changed the yeast flora and significantly reduced aerobic spoilage.
Silage fermentation involves the action of lactic acid bacteria on crops with high moisture content under anaerobic conditions. However, as silage is exposed to air during storage or at feeding time, aerobic spoilage occurs promptly due to lactic acid degradation by the mainly lactic acid-utilizing yeasts of the genera Pichia and Candida (6, 17). This consequently leads to an increase in pH and high losses of nutrient and dry matter (DM) in silage (21). Feeding of spoiled material has to be avoided because it can lead to animal disease (12). The addition of high levels of propionic acid is effective against aerobic spoilage, but its use has been restricted because of its corrosive nature, relative expensiveness, involvement in the depression of animal food intake, and the differing sensitivities of yeast (10, 12). Control of silage fermentation by microorganisms would be a safe and inexpensive alternative. Inoculation of lactic acid bacteria has been recommended to improve the aerobic stability of silage but is not effective at high concentrations of oxygen (12).
Prompted by the application of killer strains of Saccharomyces in sake (14) or wine (4, 16) making, we investigated whether killer yeasts inoculated into silage could prolong its aerobic stability. Killer yeasts are known to secrete a killer protein that is lethal to specific yeasts, and several killer yeast toxins with different actions have been reported (15, 23). In a previous paper, we selected the killer yeast Kluyveromyces lactis IFO1267 because of its wide killer spectrum against silage spoilage yeasts (7). Both the yeast and crude killer protein effectively prevented aerobic growth of Saccharomyces cerevisiae in a model of silage fermentation. Furthermore, since the K. lactis killer strain is able to use lactose as a sole carbon source, a strong killer effect was observed when lactose was used as a supplemental carbon source in the model system. Hence, dried whey could be used as a lactose source in silage additives, thereby reducing the pollution load in the environment in dairy regions. However, the killer yeast can also utilize lactic acid (7). To overcome this bottleneck, a killer strain having no ability to grow on lactic acid was constructed by disruption of the phosphoenolpyruvate carboxykinase (PEPCK) gene (KlPCK1) by site-directed mutagenesis. The killer activity of the resulting strain and its growth rate on lactose medium were the same as in the host strain (8, 9).
Here we report the use of this strain to improve the aerobic stability of maize silage, in which spoilage by lactic acid-utilizing yeasts is a major problem (10, 12).
Two killer strains, K. lactis IFO1267 (KlPCK1) and PCK27 (klpck1) (9), were used throughout. K. lactis m8, a killer defective yeast which was constructed in our laboratory from K. lactis IFO1267 by UV irradiation, was also used. UV irradiation frequently cures the two linear plasmids, pGKL1 and pGKL2 (13), which confer the killer phenotype in K. lactis (3). Pichia anomala AHU 3936, 3937, and 3938 and Lactobacillus plantarum MAFF 516001, isolated from silage, were used as target yeast strains (lactic acid-utilizing yeasts) and as lactic acid bacteria in the model of silage fermentation, respectively. For the latter and the laboratory-scale silage preparations, powdered (Zea mays; total sugar of 105 g kg of DM−1) and fresh (Z. mays; total sugar of 278 g kg DM−1 and 65% water content) maize was used, respectively.
K-nylon-layered polyethylene bags (Hiryu, Asahi Kasei, Japan) were used as silo bags for both the model of silage fermentation (model system) and the laboratory-scale silage. To create anaerobic conditions, the bags were sealed with a vacuum sealer (BH950; National, Osaka, Japan). Aerobic conditions were established by opening the silo bag. A previously reported silage model system (19) was used with some modifications. Seed cultures of strains were prepared in yeast extract-peptone-dextrose medium (7) for yeasts and in glucose-peptone-yeast extract (GPY) medium (19) for L. plantarum on a rotary shaker (48 h) at 30°C and at 37°C, respectively. Powdered dried maize was sterilized with ethylene oxide gas (37°C, 3 h). L. plantarum (106 CFU g−1 [wt/wet weight]) was introduced into sterilized crops with 1% lactose and 70% water content (final weight, 1.25 g). Depending on the objective of the experiment, the killer strain (106 CFU g−1) and the target strain (102 CFU g−1) were inoculated separately or in combination. The silo bag was incubated at 28°C for 3 weeks under anaerobic conditions and then for 2, 5, and 8 days under aerobic conditions. Laboratory-scale silage was prepared as previously reported (2, 19). In this system, the killer yeast strain (106 CFU g−1) and 1% lactose were introduced into 50-g portions of fresh maize (20-mm lengths) and packed into the bags. Treatment of bags and incubation conditions were the same as in the model system.
Viable microbial cells were counted as CFU by using selective agar media. Suspensions of microorganisms were prepared as previously reported (2, 7). Total yeasts (glucose-assimilating, lactic acid-assimilating, and lactose-assimilating yeasts) were counted on selective agar plates with yeast nitrogen base (Difco Laboratories, Detroit, Mich.), while P. anomala was counted on yeast carbon base as described previously (22). K. lactis was detected as distinct pink colonies on the medium due to the production of the red pigment pulcherrimin (20). Lactic acid bacteria and aerobic bacteria were counted on selective agar plates as previously reported (2).
The lactic acid and sugar contents of each sample were measured by a colorimetric assay on a 96-well plate by using a combination of the enzymatic reaction (1) by F-kit (Roch Diagnostics, Rotkreuz, Switzerland) and the redox reaction of tetrazolium salt, WST-1 (5) (Dojindo Laboratories, Kumamoto, Japan).
Data are presented as the means and standard deviations of results from triplicate assays for each experiment. All data were subjected to analysis of variance using the General Linear Model procedures of the Statistical Analysis System (18). Growth, pH levels, and lactic acid and residual sugar concentrations were analyzed by Duncan’s multiple range test. A value of P < 0.05 was considered to be significant.
Comparison of growth of K. lactis wild-type strain and its transformant in the model of silage fermentation.
We studied the differences between the growths of the wild-type killer strain and the PEPCK-defective killer strain in the model system. The growth was similar in both strains until the 5th day after opening the silo bag, at which time the total sugar content decreased to below 0.02%. Between the 5th and 8th days, the growth of strain PCK27 decreased while that of strain IFO1267 significantly increased. Under these conditions, the lactic acid content and pH level of the PCK27-inoculated sample did not change. In contrast, a significant decline in lactic acid content and an increase in pH were observed in the IFO1267-inoculated sample. These data indicate that the PEPCK-defective K. lactis killer strain did not grow in the silage by utilizing lactic acid as a carbon source.
Killer effect on different target yeast strains in the model of silage fermentation.
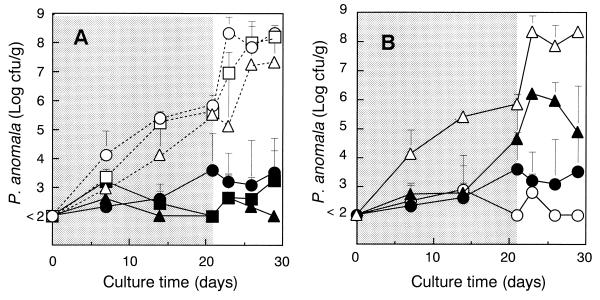
We investigated the killer effect of K. lactis PCK27 against each of the target yeast strains P. anomala AHU 3936, 3937, and 3938 in the model system. P. anomala, which has a wide fermentative ability for sugars and a high respiration capacity for organic acids (11), plays an important role in aerobic spoilage of silage (6, 17). Under both anaerobic and aerobic conditions, the addition of killer yeast significantly suppressed the growth of all target strains (Fig. 1A). After opening the silo bag, significant increases in pH levels and decreases in residual sugar concentrations were observed only in the samples without added killer yeast. Under these conditions, levels of lactic acid content had a tendency (P = 0.06) to be different between the samples with and without killer yeast. The growth curve of killer yeast was almost the same in all samples. This observation clearly indicates that PEPCK-defective killer yeast inhibited the growth of target P. anomala strains. Consequently, the degradation of sugar and lactic acid and the increase in pH levels were suppressed.
FIG. 1.
Growth curve of P. anomala strains in mixed culture with K. lactis strains in a maize silage fermentation model system. The shaded area indicates anaerobic conditions, and the blank area indicates aerobic conditions. (A) Target strain P. anomala AHU 3936 (○, ●), AHU 3937 (▵, ▴), and AHU3938 (□, ■). Open symbols refer to the single-yeast culture, and closed symbols refer to mixed cultures of killer yeast, PCK27, and the target strain. (B) Mixed culture with K. lactis m8 (▴), with K. lactis IFO1267 (○), with K. lactis PCK27 (●), and without K. lactis (▵).
Growth of P. anomala in the model of silage fermentation with coinoculation of K. lactis killer or killer-defective strains.
To evaluate the production of the killer protein and its killer effect in silage, we compared the differences in growth of P. anomala AHU 3936 among samples coinoculated with either killer strain IFO1267 or PCK27, or with killer-defective strain m8 (Fig. 1B). The growth of P. anomala AHU 3936 was found to be markedly suppressed by the addition of any of the K. lactis strains under both anaerobic and aerobic conditions. After opening the silo bag, the growth inhibition of P. anomala shows that the killer-defective strain was less effective than the wild-type killer strain. No differences in the inhibition of the target strain were observed between the two killer strains. The growths of all K. lactis strains were similar. Thus, the inhibition was not only due to growth competition but also due to the killer protein produced by the killer yeasts.
The effect of killer yeast addition on maize silage.
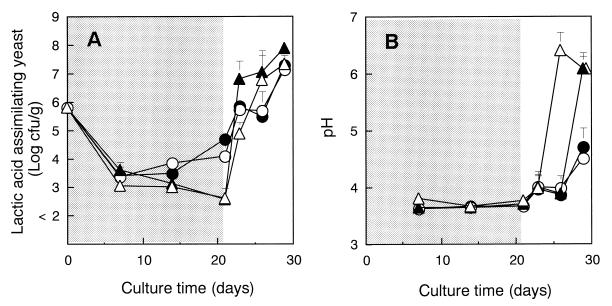
Finally, we evaluated the effects of both killer strains in laboratory silage made from freshly harvested maize and added with 1% lactose. Silages with and without 1% lactose addition were used as controls. Both K. lactis strains showed similarly significant increases in growth after 3 days of anaerobic incubation and similar decreases after further incubation. Furthermore, they failed to produce any colonies on selective medium, even after 14 days of anaerobic incubation. Because of this, we were not able to make a complete comparison between the growth of these two killer strains in the laboratory-scale silage. However, both killer yeast-inoculated samples showed significant improvement in aerobic stability compared to control samples. Figure 2 shows the growth curve of lactic acid-assimilating yeasts, excluding K. lactis, and the time course of pH levels. The population of lactic acid-assimilating yeasts significantly increased during anaerobic incubation of samples with killer yeast added, whereas no difference in yeast population was observed in the control samples between 1 and 3 weeks of anaerobic incubation. The ratio of lactic acid-assimilating yeasts to total yeasts was significantly higher in samples with killer yeast added than in control samples after incubation under anaerobic conditions, but this ratio was observed to be completely reversed after further incubation under aerobic conditions (data not shown). Under aerobic conditions, yeasts which did not assimilate lactic acid composed the biggest portion of the total yeast population in samples with killer yeast added, and the growth rate of lactic acid-assimilating yeasts was significantly lower than that in the control samples between days 2 and 5. From these data, we concluded that the inoculation of killer yeast changed the yeast flora in silage, possibly affecting the results obtained. Consequently, the pH level of silage rose significantly, from 3.7 to more than 6.0 on days 5 and 8 in the control samples with and without lactose, respectively, while the pH levels of samples inoculated with killer yeasts did not change until the 5th day, and finally displayed a pH of 4.5 on the 8th day. There were no differences in the sizes of populations of lactic acid bacteria and aerobic bacteria among samples. We conclude that the addition of PEPCK-defective killer K. lactis strain together with lactose delayed the spoilage of silage under aerobic conditions.
FIG. 2.
Growth curve of lactic acid-assimilating yeasts excluding K. lactis (A) and time course of pH (B) in laboratory-scale silage with killer yeast added. The shaded area indicates anaerobic conditions, and the blank area indicates aerobic conditions. K. lactis PCK27 (●) and K. lactis IFO1267 (○) were added in 1% lactose-enriched silage, 1% lactose only (▴), and without any additive (▵).
The K. lactis killer strain could not prevent aerobic spoilage of every silage, because the populations and species of yeasts and other microorganisms that cause aerobic spoilage, as well as the chemical and physical conditions of forage crops for silage, are always varied. Further study of the effect of killer yeast on fresh silage is essential to clarify the effect of the killer protein. There is also a need to study combinations of various killer yeasts in the construction of a killer strain that produces various killer proteins and in the construction of a killer strain that disappears immediately from the environment after playing its role. These methods and strains may be practically and efficiently used in all forage crops for silage.
Acknowledgments
We thank H. Sasaki, F. Tomita, O. Tanaka, and N. Akiyama for providing materials and K. Kikuchi for statistical analysis.
This work was supported in part by a grant in aid from the Ministry of Agriculture, Forestry, and Fisheries, Japan.
REFERENCES
- 1.Bergmeyer H U, Graßl M, Walter H-E. Biochemical reagents for general use. In: Bergmeyer H U, editor. Methods of enzymatic analysis. 3rd ed. Vol. 2. Weinheim, Germany: Verlag Chemie GmbH; 1983. pp. 126–327. [Google Scholar]
- 2.Cai Y, Benno Y, Ogawa M, Ohmomo S, Kumai S, Nakase T. Influence of Lactobacillus spp. from an inoculant of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl Environ Microbiol. 1998;64:2982–2987. doi: 10.1128/aem.64.8.2982-2987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunge N, Tamaru A, Ozawa F, Sakaguchi K. Isolation and characterization of linear deoxyribonucleic acid plasmids from Kluyveromyces lactis and the plasmid-associated killer character. J Bacteriol. 1981;145:382–390. doi: 10.1128/jb.145.1.382-390.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hara S, Iimura Y, Otsuka K. Breeding of useful killer wine yeasts. Am J Enol Vitic. 1980;31:28–33. [Google Scholar]
- 5.Ishiyama M, Miyazono Y, Sasamoto K, Ohkura Y, Ueno K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta. 1997;44:1299–1305. doi: 10.1016/s0039-9140(97)00017-9. [DOI] [PubMed] [Google Scholar]
- 6.Jonsson A, Pahlow G. Systematic classification and biochemical characterization of yeasts growing in grass silage inoculated with Lactobacillus cultures. Anim Res Develop. 1984;20:7–22. [Google Scholar]
- 7.Kitamoto H K, Ohmomo S, Nakahara T. Selection of killer yeasts (Kluyveromyces lactis) to prevent aerobic deterioration in silage making. J Dairy Sci. 1993;76:803–811. doi: 10.3168/jds.S0022-0302(93)77404-4. [DOI] [PubMed] [Google Scholar]
- 8.Kitamoto H K, Ohmomo S, Iimura Y. Isolation and nucleotide sequence of the gene encoding phosphoenolpyruvate carboxykinase from Kluyveromyces lactis. Yeast. 1998;14:963–967. doi: 10.1002/(SICI)1097-0061(199807)14:10<963::AID-YEA282>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 9.Kitamoto H K, Ohmomo S, Amaha K, Nishikawa T, Iimura Y. Construction of Kluyveromyces lactis killer strains defective in growth on lactic acid as a silage additive. Biotechnol Lett. 1998;20:725–728. [Google Scholar]
- 10.Kung L J, Sheperd A C, Smagala A M, Endres K M, Bessett C A, Ranjit N K, Glancey J L. The effect of preservatives based on propionic acid on the fermentation and aerobic stability of corn silage and total mixed ration. J Dairy Sci. 1998;81:1322–1330. doi: 10.3168/jds.S0022-0302(98)75695-4. [DOI] [PubMed] [Google Scholar]
- 11.Kurtzman C P. Description of teleomorphic ascomycetous genera and species. In: Kurtzman C P, Fell J W, editors. The yeasts: a taxonomic study. Amsterdam, The Netherlands: Elsevier Science B. V.; 1998. pp. 273–352. [Google Scholar]
- 12.McDonald P, Henderson N, Heron S. The biochemistry of silage. Aberystwyth, Wales: Chalcombe Publications; 1991. [Google Scholar]
- 13.Niwa O, Sakaguchi K, Gunge N. Curing of the killer deoxyribonucleic acid plasmids of Kluyveromyces lactis. J Bacteriol. 1981;148:988–990. doi: 10.1128/jb.148.3.988-990.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ouchi K, Akiyama H. Breeding of useful killer sake yeasts by repeated back-crossing. J Ferment Technol. 1976;54:615–623. [Google Scholar]
- 15.Ouchi K, Yamamoto T. Killer phenomenon in yeast: biosynthesis, mode of action, and practical use of killer toxin. Biseibutsu (Microorganisms) 1986;2:27–41. [Google Scholar]
- 16.Ramon-Portugal F, Delia M L, Strehaiano P, Riba J P. Mixed culture of killer and sensitive Saccharomyces cerevisiae strains in batch and continuous fermentations. World J Microbiol Biotechnol. 1998;14:83–87. [Google Scholar]
- 17.Sasaki H. Microbiological studies on grass silage fermentation. Mem Fac Agric Hokkaido University. 1972;8:189–251. . [Reprint.] [Google Scholar]
- 18.Statistical Analysis System Institute. SAS/STAT user’s guide, version 6.11. Cary, N.C: Statistical Analysis System Institute, Inc.; 1988. [Google Scholar]
- 19.Tanaka O, Kimura H, Takahashi E, Ogata S, Ohmomo S. Screening of lactic acid bacteria for silage inoculants by using a model system of silage fermentation. Biosci Biotechnol Biochem. 1994;58:1412–1415. [Google Scholar]
- 20.Wésolowski-Louvel M, Breunig K D, Fukuhara H. Kluyveromyces lactis. In: Wolf K, editor. Nonconventional yeasts in biotechnology. Berlin, Germany: Springer-Verlag; 1996. pp. 140–201. [Google Scholar]
- 21.Woolford M K. A review: the detrimental effects of air on silage. J Appl Bacteriol. 1990;68:101–116. doi: 10.1111/j.1365-2672.1990.tb02554.x. [DOI] [PubMed] [Google Scholar]
- 22.Yarrow D. Methods for the isolation, maintenance and identification of yeasts. In: Kurtzman C P, Fell J W, editors. The yeasts: a taxonomic study. Amsterdam, The Netherlands: Elsevier Science B. V.; 1998. [Google Scholar]
- 23.Young T W, Yagiu M. A comparison of the killer character in different yeasts and its classification. Antonie Leeuwenhoek. 1978;44:59–77. doi: 10.1007/BF00400077. [DOI] [PubMed] [Google Scholar]