Abstract
Flagellation of a nonswimming variant of the mixed flagellated bacterium Azospirillum lipoferum 4B was characterized by electron microscopy, and polyclonal antibodies were raised against polar and lateral flagellins. The variant cells lacked a polar flagellum due to a defect in flagellin synthesis and constitutively expressed lateral flagella. The variant cells were able to respond to conditions that restricted the rotation of lateral flagella by producing more lateral flagella, suggesting that the lateral flagella, as well as the polar flagellum, are mechanosensing.
Azospirillum spp. are nitrogen-fixing rhizobacteria with the potential to increase the yield of economically important cereals and grasses (18). Motility and chemotaxis are thought to be important factors for efficient plant colonization (21). Azospirillum spp. move using flagella (for a review on flagellar motility, see reference 12). Within the genus Azospirillum, Azospirillum brasilense, A. lipoferum, and A. irakense have mixed flagellation: a single polar flagellum when grown in a liquid medium and additional lateral flagella when grown on a solid medium (10, 20). The polar flagellum of A. brasilense rotates in both clockwise and counterclockwise directions and is required for swimming in liquid media (22). The lateral flagella are thinner in diameter and have a shorter wavelength (20) and are required for movement across solid surfaces, i.e., swarming (6). Mixed flagellation is typical of several proteobacterial species which can both swim and swarm (for a review, see reference 14).
A. lipoferum 4B produces a stable variant form, 4VI, at high frequencies as a result of a phenotypic switching process similar to phase variation in other bacteria (2). Compared to the parental strain, the 4VI variant shows pleiotropic modifications of metabolic and morphologic characteristics, including changes in motility. The 4VI variant is not capable of swimming. In the present study, we analyzed the motility and the flagellation pattern of the 4VI variant of A. lipoferum in comparison with its parental strain, 4B.
A. lipoferum 4B and 4VI cells (2) were grown in tryptone-yeast extract medium at 30°C. Swimming motility was observed by using a phase-contrast microscope (Zeiss, Jena, Germany). Flagellation of bacteria was observed by using a Philips CM 120 transmission electron microscope. Grids coated with Formvar and carbon were incubated for 30 s in a drop of a bacterial suspension and then for 20 to 30 s in 1% sodium silicotungstate. Spreading of bacteria through the semisoft medium or across the surface of the solid medium was tested on plates with different agar concentrations. Cell fractionation was performed as described previously (5). Induction of lateral flagella was achieved by incubating liquid bacterial cultures (2 × 108 cells/ml) with different concentrations of antiflagellum antibodies as described previously for A. brasilense Sp7 (17). Lateral flagellin was then detected by using the As-Laf polyclonal antibody (see below) on whole-cell protein extracts. Polar and lateral flagella were obtained by the method of Alberti and Harshey (1). Protein concentration was determined by the bicinchoninic acid protein assay (Pierce, Rockford, Ill.). Polar (Fla) and lateral (Laf) flagellins were purified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (11). Gels were stained with copper (Copper Stain and Destain kit; Bio-Rad, Richmond, Calif.). Bands corresponding to polar and lateral flagellins were cut and destained. Each protein was electroeluted (Electro-eluter 422; Bio-Rad) and then lyophilized. Purified lyophilized flagellins were injected into New Zealand White rabbits. Antisera were collected 2 weeks after the final injection. The specificity of each antiserum (As-Laf and As-Fla) was checked on Western blots of whole-cell protein extracts from A. lipoferum 4B and 4VI. No cross-reaction was detected for the As-Laf antibody; the As-Fla antibody cross-reacted slightly with lateral flagellin. For immunodetection of flagellins, samples were subjected to SDS-PAGE and electroblotting (Cera Labo, Aubervilliers, France) onto a nitrocellulose membrane. The membranes were incubated in a 1:100,000 (As-Fla) or 1:50,000 (As-Laf) dilution of each flagellin-specific antiserum, followed by a goat anti-rabbit immunoglobulin G-peroxidase conjugate. Blotted proteins were sugar stained by using the periodic acid-Schiff technique as described previously for A. brasilense Sp7 (16).
Motility of A. lipoferum 4B and its variant, 4VI, in agar media.
In liquid media, A. lipoferum 4B is motile whereas the 4VI variant is not (2). In agar-containing media, two types of motility were observed in A. lipoferum: swimming (spreading through the semisoft medium) and swarming (spreading across the surface) (Table 1). Low agar concentrations (0.1 to 0.2%) were optimal for swimming of the wild type, whereas only very limited spreading was observed for the variant (Table 1). No typical swimming motility (22) was observed in variant cells recovered from 0.1 or 0.2% agar. Both wild-type and variant cells showed optimal swarming on media containing 0.4 to 0.6% agar (Table 1). Thus, the 4VI variant was capable of swarming but not of swimming.
TABLE 1.
Spreading of A. lipoferum 4B and 4VI on agar platesa
| Strain | Spreading through medium with the indicated agar concn (%)
|
Spreading across the surface of medium with the indicated agar concn (%)
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.5 | 1.2 | 1 | 0.8 | 0.6 | 0.5 | 0.4 | 0.2 | 0.1 | 1.5 | 1.2 | 1 | 0.8 | 0.6 | 0.5 | 0.4 | 0.2 | 0.1 | |
| 4B | − | − | − | − | +++ | +++ | +++ | ++++ | ++++ | + | + | + | + | ++ | ++ | ++ | − | − |
| 4VI | − | − | − | − | − | − | + | + | + | + | + | + | + | ++ | ++ | ++ | − | − |
−, no spreading from the inoculation spot. Plus signs indicate spreading. The diameters of the spreading zones are as follows: +, <0.5 cm; ++, 0.5 to 3.0 cm; +++, 3 to 5 cm; ++++, >7 cm. Bacteria from exponentially growing cultures (107 cells/ml) were applied as a small drop (5 μl) on the surface of a Bacto Nutrient Agar plate. The diameter of the spreading zone was measured after 5 days of incubation at 30°C.
Flagellation of A. lipoferum 4VI.
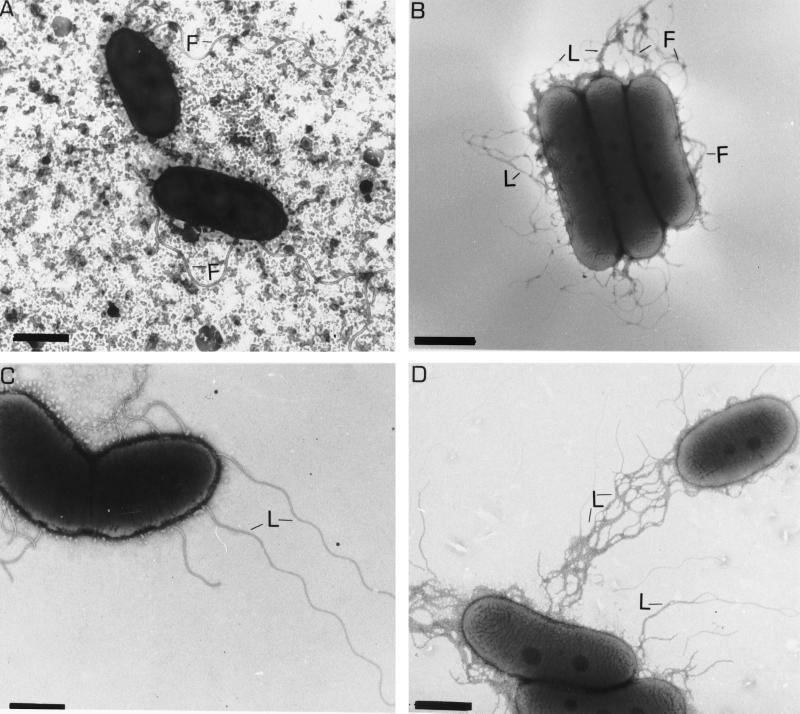
The lack of swimming motility can be due to a paralysis of the flagellar motor (Mot−) or a deficiency in flagellar synthesis or assembly (Fla−). First, electron microscopy was used in order to distinguish between these possibilities. The wild type, A. lipoferum 4B, had a single polar flagellum when grown in liquid medium and mixed flagellation when grown on solid medium (Fig. 1A and B). No polar flagellum was observed in the 4VI variant either in liquid or on solid medium, whereas lateral flagella were constitutively expressed (Fig. 1C and D). Therefore, the variant has a Fla− phenotype. The variant produced more lateral flagella when grown on solid medium than when grown in liquid medium. In contrast to the best-studied mixed flagellated bacterium, Vibrio parahaemolyticus (3, 13, 14), A. lipoferum hyperflagellated swarming cells were not elongated (Fig. 1B and D). The presence of constitutively expressed lateral flagella in the 4VI cells grown in liquid media may explain the ability of these cells to spread through the medium at low agar concentrations (Table 1).
FIG. 1.
Transmission electron micrographs of A. lipoferum 4B (A and B) and 4VI (C and D) cells. Cells were grown in liquid (A and C) and on solid (B and D) media. The lateral flagella are thinner in diameter and have a shorter wavelength. Abbreviations: F, polar flagellum; L, lateral flagella. Bars, 1 μm (A, B, and D) and 0.7 μm (C).
Polar and lateral flagellins.
The lateral flagellin, Laf, of A. lipoferum 4B had an apparent molecular size of approximately 45 kDa (Fig. 2), which is similar to that of A. brasilense Sp7 (17). However, the apparent molecular size of the polar flagellin, Fla, of A. lipoferum 4B was slightly higher; 110 kDa (Fig. 2), versus 100 kDa in A. brasilense Sp7 (16). As in A. brasilense Sp7 (16), Fla appeared to be glycosylated, as revealed by periodic acid-Schiff staining (data not shown).
FIG. 2.
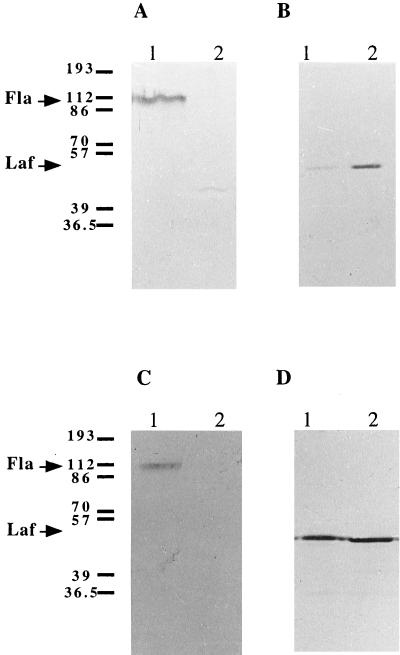
Detection of polar and lateral flagellins in intracellular protein extracts of A. lipoferum 4B (lanes 1) and 4VI (lanes 2) cells grown in liquid medium (A and B) and on solid medium (C and D) by using polyclonal As-Fla (A and C) and As-Laf (B and D) antibodies. The same number of wild-type and variant cells was recovered (108 cells/ml from liquid medium and 109 cells/ml from solid medium). Equal amounts of total protein (260 μg) were loaded in each sample on the gel. Arrows indicate positions of the 45-kDa lateral flagellin (Laf) and the 110-kDa polar flagellin (Fla). Molecular mass markers (in kilodaltons) are indicated on the left. The weak signal corresponding to Laf observed in 4B liquid-grown cells (panel B, lane 1) may be due to a small fraction of cells for which the rotation of the polar flagellum was impaired due to cell-cell aggregation or adherence to the surface of the tube (see text for details).
In Salmonella typhimurium, defects in flagellar assembly lead to switching off of flagellin biosynthesis (9). In flagellar-assembly mutants of Helicobacter spp., however, the level of flagellin production was unchanged (19). In order to differentiate between defects in flagellum biosynthesis and assembly in A. lipoferum 4VI, flagellins were detected in different cell compartments: intracellular proteins, free-flagellum and extracellular proteins, and excreted proteins from the culture medium. Expression of polar and lateral flagellins was analyzed in cells grown in liquid and on solid media by using As-Fla and As-Laf polyclonal antibodies, respectively. The results obtained in a typical experiment are shown in Fig. 2. No polar flagellin was detected in the extra- or intracellular extracts of the 4VI variant or in the culture liquid (Fig. 2A and C, lanes 2), suggesting that the lack of polar flagellum in the variant was due to a deficiency in flagellin synthesis and not in assembly. Similar results were obtained with phase variants of Xenorhabdus nematophilus (5). In the wild-type strain, 4B, Fla was detected under all experimental conditions, whereas Laf was detected only in cells grown on solid medium (Fig. 2D). In the 4VI variant, Laf was detected both in liquid media and, at a higher level, on solid media (Fig. 2B and D). The levels of lateral flagellin production on solid media were not significantly different for the variant and the wild type (Fig. 2B). These data were in a good agreement with the electron microscopy observations (Fig. 1C and D). The results indicated that the 4VI variant cells were unable to synthesize polar flagellin but constitutively expressed lateral flagellin and prompted analysis of Laf expression in A. lipoferum.
Laf expression in the wild type and the 4VI variant.
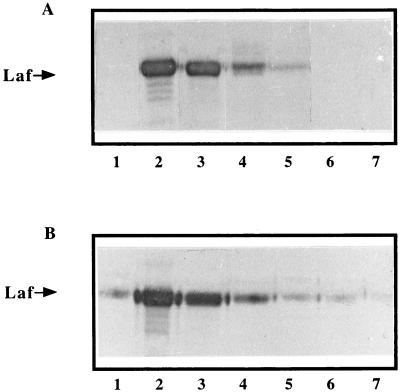
Expression of lateral flagella in mixed flagellated bacteria is controlled by a polar flagellum (3, 13, 17). V. parahaemolyticus mutants defective in the polar flagellum constitutively expressed lateral flagella, indicating that this control is negative (13). The polar flagellum senses a physical constraint of its movement, such as increased viscosity of the medium or agglutination with antiflagellum antibody (13, 14). As in other mixed flagellated bacteria, the polar flagellum of A. lipoferum 4B appeared to negatively control expression of lateral flagella. Addition of the As-Fla polyclonal antibody to the 4B cultures caused cell agglutination, several cells being bound together at one pole and rotating (data not shown). Under these conditions, Laf induction correlated with the concentration of the antiserum in the medium (Fig. 3A). Impeded movement of the polar flagellum by the specific As-Fla antibody led to lateral flagellum expression in liquid medium (data not shown), as it did on solid medium (Fig. 1B). The same results were obtained for A. brasilense (17), the most closely related species of the same genus. Since the polar flagellum may negatively control lateral-flagellum expression in A. lipoferum 4B, we propose that the lack of a polar flagellum in the 4VI variant led to a lack of the control, and thus lateral flagella were expressed constitutively.
FIG. 3.
Induction of expression of lateral flagellin in A. lipoferum 4B (A) and 4VI (B) cells by antibody agglutination of the polar or lateral flagella. Polar flagellin (As-Fla) or lateral flagellin (As-Laf) polyclonal antibodies diluted in ultrapure water were added to exponential-phase-grown cultures of A. lipoferum 4B and 4VI, respectively. Whole-cell protein extracts were used for immunodetection of lateral flagellin. Equal amounts of total protein (260 μg) were loaded in each sample on the gel. Incubation with preimmune antisera led to the same result (data not shown) as that in the control experiment, in which cells were incubated with ultrapure water (panels A and B, lanes 1). Antibodies (As-Fla [A] or As-Laf [B]) were added to final dilutions of 100 (lane 2), 10−1 (lane 3), 10−2 (lane 4), 10−3 (lane 5), 10−4 (lane 6), and 10−5 (lane 7).
Addition of the As-Laf antibody to the 4VI cultures caused cell agglutination, cells being attached to each other (data not shown). In order to reveal whether lateral flagella were rotating, most of the lateral flagella were detached from variant cells by vigorous shaking, followed by cell agglutination with As-Laf. Individual cells were bound at different points of their bodies and rotated unidirectionally around their points of attachment. Under these conditions, Laf induction correlated with the concentration of the antiserum in the medium (Fig. 3B) and led to an overexpression of lateral flagella, similar to that in cells grown on solid medium (Fig. 1D).
Altogether, the results obtained led us to conclude that the lateral flagella are able to sense and to respond to conditions that constrain their motion by inducing expression of more lateral flagella. Like the polar flagellum, the lateral flagella appear to be mechanosensing. In peritrichously flagellated bacteria, such as Proteus mirabilis, Serratia marcescens, Escherichia coli, and S. typhimurium, physical forces induce expression of more flagella of the same type, which are used for both swimming and swarming (4, 7, 8). However, this phenomenon has not previously been described for lateral flagella that are used only for swarming in bacteria with mixed flagellation. Swarming has been suggested to play an important role in tissue colonization by P. mirabilis during urinary-tract infections (15) and in surface colonization by V. parahaemolyticus (14). Similarly, emergence of the nonswimming but swarming variant (4VI) during phenotypic switching in A. lipoferum (2) may be important in colonization of the plant root by the bacteria. Swimming motility in Azospirillum is thought to play a role in the movement of the bacteria toward the plant roots, and chemotaxis to plant root exudates is presumed to be the initial stage of colonization (21). Swarming across the surfaces of the roots may be important for long-term colonization.
Acknowledgments
We are grateful to A. Givaudan and S. Moens for helpful comments and to I. B. Zhulin for editing the manuscript.
G.A. was funded by a grant from the MENESR (France).
REFERENCES
- 1.Alberti L, Harshey R M. Differentiation of Serratia marcescens 274 into swimmer and swarmer cells. J Bacteriol. 1990;172:4322–4328. doi: 10.1128/jb.172.8.4322-4328.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexandre G, Bally R. Emergence of a laccase-positive variant of Azospirillum lipoferum occurs via a two-step phenotypic switching process. FEMS Microbiol Lett. 1999;174:371–378. doi: 10.1111/j.1574-6968.1999.tb13592.x. [DOI] [PubMed] [Google Scholar]
- 3.Belas R, Simon M, Silverman M. Regulation of lateral flagella gene transcription in Vibrio parahaemolyticus. J Bacteriol. 1986;167:210–218. doi: 10.1128/jb.167.1.210-218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belas R, Erskine D, Flaherty D. Proteus mirabilis mutants defective in swarmer cell differentiation and multicellular behavior. J Bacteriol. 1991;173:6279–6288. doi: 10.1128/jb.173.19.6279-6288.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Givaudan A, Baghdiguian S, Lanois A, Boemare N. Swarming and swimming changes concomitant with phase variation in Xenorhabdus nematophilus. Appl Environ Microbiol. 1995;61:1408–1413. doi: 10.1128/aem.61.4.1408-1413.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall P G, Krieg N R. Swarming of Azospirillum brasilense on solid media. Can J Microbiol. 1983;29:1592–1594. [Google Scholar]
- 7.Harshey R M, Matsuyama T. Dimorphic transition in Escherichia coli and Salmonella typhimurium: surface-induced differentiation into hyperflagellate swarmer cells. Proc Natl Acad Sci USA. 1994;91:8631–8635. doi: 10.1073/pnas.91.18.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harshey R M. Bees aren’t the only ones: swarming in Gram-negative bacteria. Mol Microbiol. 1994;13:389–394. doi: 10.1111/j.1365-2958.1994.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 9.Hughes K T, Gillen K L, Semon M J, Karlinsey J E. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science. 1993;262:1277–1280. doi: 10.1126/science.8235660. [DOI] [PubMed] [Google Scholar]
- 10.Khammas K M, Ageron E, Grimont P A D, Kaiser P. Azospirillum irakense sp. nov., a nitrogen-fixing bacterium associated with rice roots and rhizosphere soils. Res Microbiol. 1989;140:679–693. doi: 10.1016/0923-2508(89)90199-x. [DOI] [PubMed] [Google Scholar]
- 11.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.Macnab R M. Genetics and biogenesis of bacterial flagella. Annu Rev Genet. 1992;26:131–158. doi: 10.1146/annurev.ge.26.120192.001023. [DOI] [PubMed] [Google Scholar]
- 13.McCarter L, Hilmen M, Silverman M. Flagellar dynamometer controls swarmer cell differentiation of V. parahaemolyticus. Cell. 1988;54:345–351. doi: 10.1016/0092-8674(88)90197-3. [DOI] [PubMed] [Google Scholar]
- 14.McCarter L, Silverman M. Surface-induced swarmer cell differentiation of Vibrio parahaemolyticus. Mol Microbiol. 1990;4:1057–1062. doi: 10.1111/j.1365-2958.1990.tb00678.x. [DOI] [PubMed] [Google Scholar]
- 15.Mobley H L, Belas R. Swarming and pathogenicity of Proteus mirabilis in the urinary tract. Trends Microbiol. 1995;3:280–284. doi: 10.1016/s0966-842x(00)88945-3. [DOI] [PubMed] [Google Scholar]
- 16.Moens S, Michiels K, Vanderleyden J. Glycosylation of the flagellin of the polar flagellum of Azospirillum brasilense, a Gram-negative nitrogen-fixing bacterium. Microbiology. 1995;141:2651–2657. [Google Scholar]
- 17.Moens S, Schloter M, Vanderleyden J. Expression of the structural gene, laf1, encoding the flagellin of the lateral flagella in Azospirillum brasilense Sp7. J Bacteriol. 1996;178:5017–5019. doi: 10.1128/jb.178.16.5017-5019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okon Y, Vanderleyden J. Root-associated Azospirillum species can stimulate plants. ASM News. 1997;63:366–370. [Google Scholar]
- 19.O’Toole P W, Kostrzynska M, Trust T J. Non-motile mutants of Helicobacter pylori and Helicobacter mustelae defective in flagellar hook production. Mol Microbiol. 1994;14:691–703. doi: 10.1111/j.1365-2958.1994.tb01307.x. [DOI] [PubMed] [Google Scholar]
- 20.Tarrand J J, Krieg N R, Dobereiner J. A taxonomic study of the Spirillum lipoferum group, with descriptions of a new genus, Azospirillum gen. nov. and two species, Azospirillum lipoferum (Beijerinck) comb. nov. and Azospirillum brasilense sp. nov. Can J Microbiol. 1978;24:967–980. doi: 10.1139/m78-160. [DOI] [PubMed] [Google Scholar]
- 21.Zhulin I B, Armitage J P. The role of taxis in the ecology of Azospirillum. Symbiosis. 1992;13:199–206. [Google Scholar]
- 22.Zhulin I B, Armitage J P. Motility, chemokinesis, and methylation-independent chemotaxis in Azospirillum brasilense. J Bacteriol. 1993;175:952–958. doi: 10.1128/jb.175.4.952-958.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]