Abstract
Background
Systemic autoimmune disorders are associated with an increased risk of hypercoagulability. The hypercoagulable state in people with systemic autoimmune disorders has lately gained attention.
Presentation of case
We presented a 44-year-old male with a chief complaint of progressive difficulty concentrating, memory impairment, and weakness in all limbs. Seven months before admission to our Memory Clinic, the patient began to have infrequent short-term memory loss and sometimes got lost when he went for a drive. Three months later, he complained of feeling dizzy when in a crowd, being unable to watch television for a long time, and easily forgetting. Computed tomography (CT) scan showed brain infarction. After receiving the first dose of COVID-19 vaccine (Sinovac), the patient had difficulty communicating verbally and could only point at objects, as well as tetraparesis. These conditions severely intervened in his daily activities. The patient was then referred to an immunologist and diagnosed with autoimmune disease. In our Memory Clinic, his performances of attention, memory, language, visuospatial, and executive function were very poor. We diagnosed him with autoimmune dementia. The administration of methylprednisolone, mycophenolate mofetil, vitamin D3, donepezil, and memantine could improve his condition.
Discussion
Autoimmune disease can cause microvascular thrombosis and microembolism at the central nervous system level, which would cause vascular damage and cognitive impairment leading to brain infarction and dementia.
Conclusion
There seems to be a link between autoimmune disease, hypercoagulable state, and dementia, although the magnitude of this link and the underlying processes are not fully understood.
Keywords: Autoimmune disease, Vaccination, Autoimmune dementia, Hypercoagulable state
Highlights
-
•
Systemic autoimmune disorders are associated with an increased risk of hypercoagulability.
-
•
Autoimmune diseases may develop cognitive impairment and vascular damage.
-
•
Autoimmune disease can lead to brain infarction and dementia.
1. Introduction
Recent large-scale epidemiological research has shown that systemic autoimmune disorders are associated with an increased risk of hypercoagulability [[1], [2], [3]]. Thromboembolism in people with systemic autoimmune disorders has lately gained attention. In addition, systemic autoimmune disorders are characterized by inflammation. Much research has been conducted to determine the relationship between inflammation and the hypercoagulable condition, as well as the relationship between inflammation and endothelial dysfunction [4].
Furthermore, patients with autoimmune diseases may develop cognitive impairment, this condition is sometimes referred to as autoimmune dementia [5]. This immune-mediated cognitive dysfunction is a complex disorder, involving the interplay between increased levels of inflammatory cytokines, oxidative stress, and reduced levels of brain-derived neurotrophic factor (BDNF) [6]. The symptoms range from acute limbic encephalitis to subacute or chronic cognitive impairments that resemble neurodegenerative dementia [5], making the diagnosis be challenging.
Here, we report a case of a male who had memory impairment with autoimmune disease and a hypercoagulable state worsened by COVID19 vaccination.
2. Presentation of case
In August 2021, a 44-year-old male was brought to our Memory Clinic with chief complaints of progressive difficulty concentrating and weakness in all four limbs. Seven months ago in January 2021, the patient began to have forgetfulness particularly related to short-term memory loss and sometimes got lost when he went for a drive. However, there was no interference with daily living activities. In April 2021, the patient complained of feeling dizzy when in a crowd, being unable to watch television for a long time, and persistent forgetfulness. He was then taken to a private hospital, a head computed tomography (CT) scan was performed, and he was diagnosed with a stroke infarct. Subsequently, he received routine therapy of clopidogrel 75 mg/day and donepezil 5 mg/day from the neurologist in the private hospital.
In May 2021, the patient received the first dose of the COVID-19 vaccine using Sinovac vaccine. On the same day after vaccination, the patient had difficulty communicating verbally and could only point at objects. The next day he began to have limitations in physical abilities such as walking, having to hold on to objects, and he also felt tired easily when performing light activities. On the following day, the patient was brought to another public hospital and diagnosed as having a recurrent stroke.
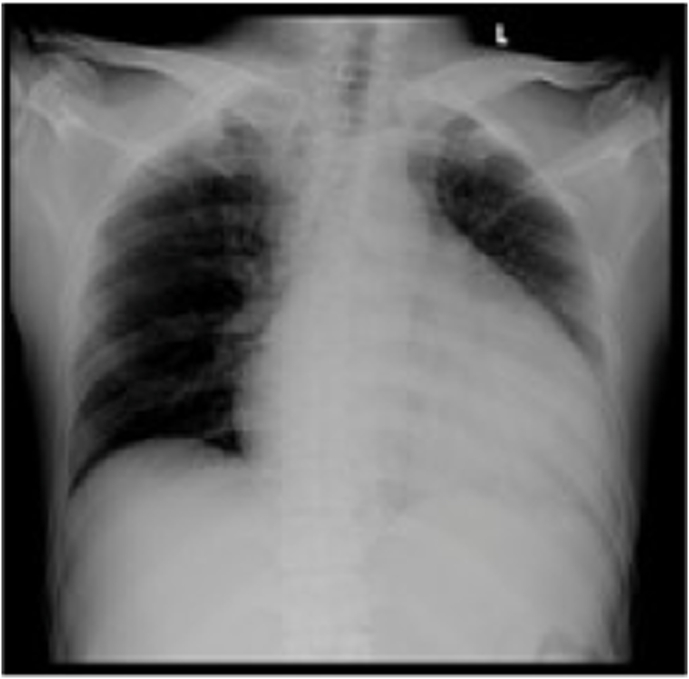
In June 2021, the patient started to have progressive weakness in all four extremities but was still able to walk with assistance. He then went to the previous public hospital and was advised to undergo physiotherapy for 2 weeks, but there was no improvement. The complaints of difficulty concentrating and forgetting were getting worse, accompanied by incoherent speech. These complaints were very disturbing activities in his daily life. A few days later, the patient felt shortness of breath. He was subsequently referred to the emergency unit of our hospital. On examination, a massive pericardial effusion was found (Fig. 1). The patient was then treated for a week with thoracocentesis to treat the pleural effusion. No malignant cells were found in the pericardial fluid. At that time, he was suspected to have a secondary anti-phospholipid syndrome and began to have a routine check-up in the department of internal medicine.
Fig. 1.
Chest X-ray showed early pulmonary edema and cardiomegaly with massive pericardial effusion.
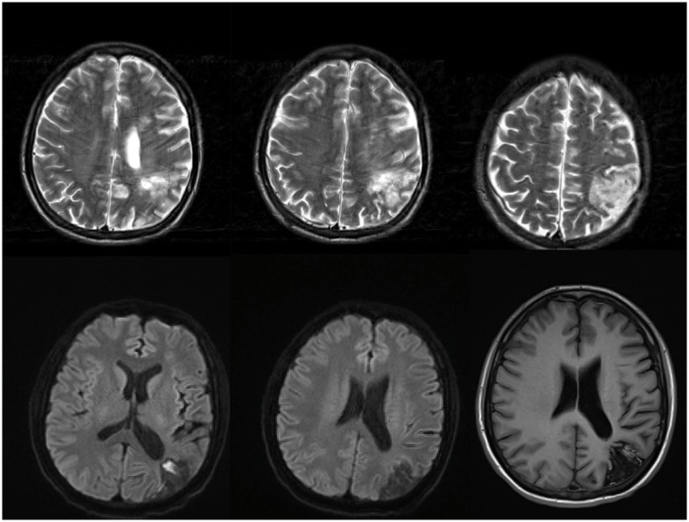
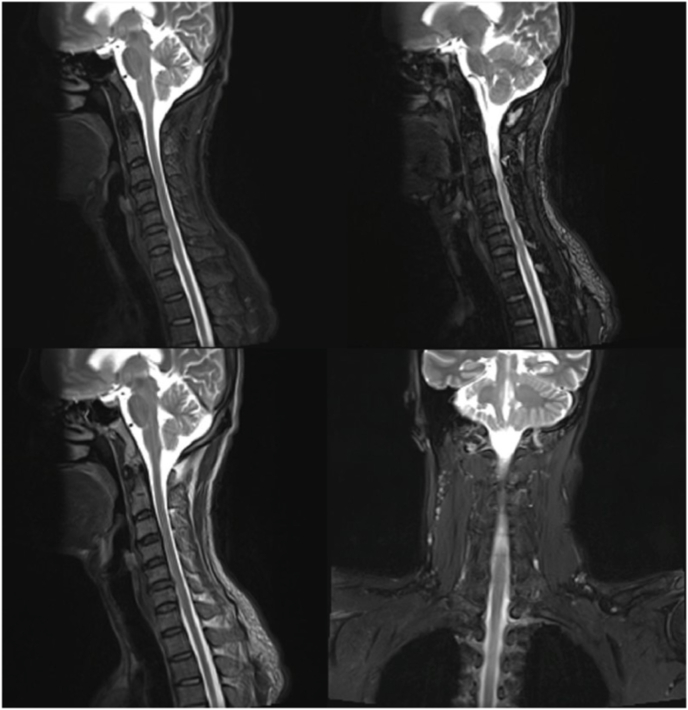
In July 2021, the patient was referred to our neurology department for further examination of the complaints of tetraparesis. Because he also had cognitive deficits, he was suspected to have multiple sclerosis. For that reason, brain and cervical Magnetic resonance imaging (MRI) was performed. Brain MRI showed chronic cortical infarct in left temporal lobe and multiple subacute lacunar infarcts in the left corona radiata, left basal ganglia and left frontal lobe (Fig. 2), and cervical MRI revealed mild to moderate canal stenosis in C3-4, C4-5, C5-6, C6-7 (Fig. 3). Subsequently, he was planned to have electroneuromyography (ENMG). The ENMG results later showed motor and sensory polyradiculoneuropathy on the upper and lower extremities, leading to the diagnosis of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). In addition, he was sent to our Memory Clinic for further evaluation of his cognitive deficits.
Fig. 2.
Head MRI showed multiple subacute lacunar infarcts in the left corona radiata, left basal ganglia, and left frontal lobe, chronic cortical infarction in the left temporal lobe, and chronic watershed infarction in the left parietoccipital lobe with encephalomalacia and cortical laminar necrosis features.
Fig. 3.
Cervical MRI showed mild canal stenosis in DIV C 3–4, 4–5, and moderate canal stenosis in DIV C 5–6, 6–7 without foraminal stenosis.
In August 2021 when he came to our Memory Clinic, the patient complained of difficulty concentrating, incoherent speech, and easy forgetting, which were getting worse. Daily activities such as eating, bathing, and dressing required the help of others. At that time, there was no history of headache, facial drop, sleep disorder, loss of consciousness, fever, head trauma, urinating disorder, defecation disorder, tingling, electrical shock, and burns. The patient had no prior history of stroke, hypertension, diabetes mellitus, heart disease, head trauma, seizures, tumors, chemotherapy and radiotherapy, or alcohol and substance abuse. Neither had his family.
On general physical examination and cranial nerves, no abnormalities were found. Then we examined muscle strength. We found weaknesses in all four extremities, with muscles strength of 4 on proximal muscles, and 3 on distal muscles. The patient's neurobehavioral status showed short-term memory impairment and impaired concentration. We did the executive function test. We found that the trail-making test B was impaired. The frontal lobe function, perseveration, and Luria test were also impaired. The forward and backward digit span of the patient were also impaired, with a forward digit span score of 4, and a backward digit span score of 3. A Consortium to Establish a Registry for Alzheimer's Disease (CERAD) test was performed, and the results showed a fluency score of 11, a modified Boston Naming Test score of 11, word list learning task of 3/6/7, the constructional praxis score of 8, word list delayed recall score of 4, and word list recognition score of 6 making the total score of CERAD was 56. The patient also had problems with calculation tests. The summary of cognitive function scores by other measurement tools were listed in Table 1.
Table 1.
Patient scoring results of the cognitive functioning.
| Scoring | |
|---|---|
| CAM ICU | Negative |
| MMSE | 17/25 (domain memory, calculation, visuospatial) |
| MoCA-INA TMT-B CERAD |
8/20 (deficit in attention, language, visuospatial/executive, memory) 3 minutes 40 seconds (wrong track) 56 (dementia) |
| CDR Morris | 3 (severe dementia) |
| CDR SOB | 16.0 (severe dementia) |
| SBT | 12 (consistent cognitive impairment with dementia) |
| FAQ | 30 (dependent) |
| HDRS | 2 (no depression) |
| HARS | 2 (no anxiety) |
| ADL | 14/18 |
| IADL | 12/14 |
| Barthel Index | 60 |
| DSM-V | 5 (major cognitive impairment) |
| Short IQ Code | 3.625 (cognitive impairment) |
| Ina AD-8 | 6 (cognitive impairment) |
CAM ICU, Confusion Assessment Method for the ICU; MMSE, Mini mental state examination; MoCA-INA, Montreal cognitive assessment Indonesia version; TMT-B, trail making test-B; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; CDR, Clinical Dementia Rating; SBT, Short Blessed Test; FAQ, Functional Activities Questionnaire; HDRS, Hamilton Depression; HARS, Hamilton Rating Scale for Anxiety; ADL, Activity of daily living; IADL, Instrumental Activities of Daily Living; DSM, Diagnostic and Statistical Manual of Mental Disorders; Ina AD-8, Alzheimer's Disease-8 Indonesia version.
Laboratory examinations were also carried out on him with the following results (Table 2).
Table 2.
Laboratory test results.
| Laboratory parameter | Value | Normal Limits | |
|---|---|---|---|
| WBC | 8.75 | 4.5–11.5 × 103/uL | |
| RBC | 6.51 | 4.4–6.1 × 106/uL | |
| Hb | 17.4 | 13.2–18.0 g/dL | |
| HCT | 54.1 | 37–52% | |
| PLT | 409 | 150–450 × 103/uL | |
| Neutrophil | 74.8 | 50–70% | |
| Lymphocyte | 16.9 | 18.0–42.0% | |
| Monocyte | 7.3 | 2–11% | |
| Eosinophil | 0.9 | 1–3% | |
| Basophil | 0.1 | 0–1% | |
| MCV | 83.1 | 80-99 fl | |
| MCH | 26.7 | 27–31 pg | |
| MCHC | 32.2 | 33–37 g/dL | |
| Random blood glucose | 128 | 74–140 | |
| Physiological hemostasis | |||
| PPT | 19.3 | 9.4–12.5 second | |
| INR | 1.56 | 0.90–1.1 | |
| Control PPT | 15.6 | second | |
| APTT | 45.3 | 25.1–36.5 second | |
| Control APTT | 32.3 | second | |
| D-Dimer | 156 | <250 ng/ml | |
| Electrolyte | |||
| Na+ | 135 | 137–145 mmol/L | |
| K+ | 5.13 | 3.4–5.4 mmol/L | |
| Cl- | 102 | 95–108 mmol/L | |
| Renal Function | |||
| BUN | 10.8 | 6.0–20.0 | |
| Creatinine | 0.70 | 0.80–1.30 | |
| Liver Function | |||
| Albumin | 3.48 | 3.40–5.00 g/dl | |
| SGOT | 17 | 15–37 U/L | |
| SGPT | 20 | 12–78 U/L | |
| Immunology | |||
| Anti-ds DNA | 10.3 U/mL | Normal: < 25.0, Increase: > = 25.0 | |
| ANA IF | Negative | Negative | |
| Ratio (LA1:LA2) | 1.36 (LA positive Mild) | Negative: < 1.2–1.5 Moderate: 1.5–2.0 Severe: > 2.0 |
|
| LA 2 Control | 29.6 second | ||
| LA 1 Control | 41.0 second | ||
| LA 1 Patient | 44.8 second | 31.0–44.0 | |
| LA 2 confirmations | 32.9 second | 30.0–38.0 | |
| ACA - IgM | 1.3 MPL | Normal: <7; Elevated: >7 | |
| IgM Beta2 Glycoprotein | Normal: 0.9 | Normal: <5; Borderline: 5–8; Elevated: >8 | |
| IgG Beta2 Glycoprotein | Normal: 1.1 | Normal: <5; Borderline: 5–8; Elevated: >8 | |
| ACA - IgG | 2.0 GPL | Normal: <10; Elevated: >10 | |
| Adenosine Deaminase (ADA) | 3 U/L | 0–50 | |
At first, we thought that the patient suffered from secondary-antiphospholipid syndrome. Although the lupus anticoagulant was detected, the level was too small which is further considered negative. Marker for systemic lupus erythematous was also negative, leading to the conclusion that the patient is not suffered from SLE. Current biomarkers available in our institution cannot detect the etiological factor and the diagnosis remains inconclusive.
The patient was then finally diagnosed with a hypercoagulable state by the internal medicine department. He was treated with oral Cavit-D3 (calcium hydrogen phosphate dihydrate 500 mg and cholecalciferol 133 IU) once daily, oral methylprednisolone 16 mg twice daily, and oral mycophenolate mofetil 180 mg twice daily. To prevent the side effect of long-term high dose steroid administration, the dose was then tapered off to 8 mg twice a day since November 2021, and 8 mg once a day since February 2022.
Elaborating all these data, we came to the conclusion that the patient has had autoimmune dementia. We have prescribed him oral memantine 10 mg once daily, oral donepezil 10 mg once daily and oral clopidogrel 75 mg once daily. Until now, the patient still regularly visits our Memory Clinic and the internal medicine outpatient department to do health check-ups. The complaints of memory impairment and difficulty of concentration were improved, with the latest cognitive test scores of MMSE and MoCA on March 2022 were 22/30 and 18/30, respectively.
During the follow up, no adverse nor unanticipated events (such as the worsening of neuro-immunological condition or systemic multi-organ failure, etc.) occurred. The patient has good adherence to the medication and shows good tolerability with having no side effects of the prescribed drugs. The patient and his spouse shared their perspective regarding the treatments they received and stated that they felt grateful for the significant clinical improvement they made after receiving the treatments. They hope that there will be more achievement until the patient could become functionally independent as before while still being monitored closely by the doctors.
3. Discussion
Autoimmune dementia is a terminology that represents cognitive deterioration caused by an autoimmune condition. In some clinical conditions, the probability of experiencing immune-mediated cognitive impairment is increased [7]. Autoimmune etiologies of dementia, for example, account for a greater percentage of cases in young-onset (<45 years) and early-onset (<65 years) dementia. Autoimmune dementia may arise without an accompanying autoantibody, and negative autoantibody testing does not rule out autoimmune dementia [7]. Autoimmune dementia in young patients accounts for 20.3% of cases, a second most common cause after neurodegenerative dementia [8].
Many autoimmune and immune-mediated diseases are characterized by inflammation [9]. According to comparative research, coagulation and innate immunity have an evolutionary basis [10]. It is not unexpected, therefore, that the immune system and the coagulation system are intertwined, with numerous molecular components crucial for both systems [11,12]. Inflammatory and coagulation pathways are linked by significant interaction and a proclivity to work together [11,12]. They are made up of a vast number of cellular and molecular components that interact in a complicated way [11,12]. Some of the key hallmarks of inflammation-induced hypercoagulability include cytokine activation of tissue factor (TF) expression, endothelial dysfunction, inhibition of the protein C system, and inhibition of fibrinolysis (increased plasminogen activator inhibitor 1 levels) [9].
Several pathways have been hypothesized in this respect to trigger the beginning of cognitive decline and dementia. These processes include not just thrombosis-related pathways, but also immune-mediated pathways [13].
The first mechanism would be direct brain injury caused by autoimmune disease [14]. Endothelial dysfunction at the blood-brain barrier (BBB) allows these APS to get into the brain, which can cause damage to neuronal cells. These neurodegenerative mechanisms, as demonstrated by a mouse model study [15], are associated with an abnormal inflammatory response that increases tumor necrosis factor (TNF) and prostaglandin E and a decrease in intrinsic brain thrombin inhibitors, resulting in increased inflammation and thrombosis.
All of these things would cause microvascular thrombosis and microembolism at the central nervous system level, which would cause vascular damage and cognitive impairment leading to brain infarction and dementia, just as found in our patient. Although the diagnosis of secondary APS was not conclusive based on the laboratory examination, our patient indeed had a tendency to have an autoimmune disease, and his symptoms were worsened by COVID-19 vaccination.
With regard to COVID-19 vaccination, previous studies have reviewed the association of nervous system demyelination in post-vaccinated patients. In most instances, neurological problems emerged during the first 1–2 weeks [16]. In our case, the symptoms emerged within 24 hours after the immunization. Females made up the vast majority of cases, accounting for almost 85% of all immune-mediated illnesses [16]. This assertion contrasted with our situation with the male gender. Furthermore, more than half of the patients had a history of probable or confirmed autoimmune disorders [16], which might put them at a higher risk of acquiring other immune-mediated diseases like ours.
da Silva et al. reported neuroimmunology symptoms of acute transverse myelitis related to COVID-19 vaccination (Oxford/AstraZeneca) in Brazil with onset of symptoms appeared after 3 weeks, while Khan et al. reported a case with the same condition with onset of five days following vaccination using Moderna. In addition, a case from Khan et al. responded well to plasmapheresis [17,18].Guillain-Barre syndrome associated with COVID-19 vaccination has been systematically reviewed from 17 publications, reporting 39 cases of GBS following COVID-19 vaccination. The GBS symptoms has occurred within 2 weeks following vaccine administration [19,20]. Vaccination may cause an immunological reaction in our bodies. When our immune system is unable to differentiate between foreign antigens and host antigens, autoimmunity develops, resulting in the destruction of host cells [18].
Autoimmunity is caused by the following processes in a person: 1) the most common mechanism is molecular mimicry between infectious antigens and self-antigens, 2) the acceleration of an ongoing autoimmune process by local activation of antigen-presenting cells and over-processing of antigens induced by foreign antigens, and 3) polyclonal activation of B lymphocytes or bystander activation, which increases cytokine production and further induces autoreactive T-cell expansion [18].
The type of antibodies related with autoimmune neurological illnesses can be used to categorize the disorders. In terms of disease pathophysiology, it is widely believed that antibodies directed against antigens expressed on the neural cell surface are more likely to be harmful, causing cell damage/dysfunction or receptor internalization by direct binding to their accessible target [21]. In contrast, antibodies targeting intracellular antigens (which are not accessible for direct binding in normal settings) are unlikely to be harmful and instead reflect disease indicators of a cytotoxic T cell activity [21].
Since the pathological foundation of dementia is complicated and varied, and no precisely targeted therapy has yet been discovered, novel therapeutic strategies that influence all processes of cognitive impairments should be developed. Moreover, as the neurological effect of the COVID-19 pandemic grows, it seems that vaccine-related illnesses may need to be included. Certainly, additional data are needed to provide a more accurate assessment of the real significance and possible danger. The rarity of this incident should not preclude vaccinations from being used, since worldwide immunization is one of the most critical measures in battling this pandemic.
4. Conclusions
To conclude, there seems to be a link between autoimmune disease, hypercoagulable state, and dementia, although the magnitude of this link and the underlying processes are not fully understood. Inflammatory reaction after vaccination may worsen the symptoms of patients with autoimmune disease. The features of people who will acquire dementia, in particular, remain unknown, and no preventative therapy is available.
Declarations of competing interest
No potential conflict of interest relevant to this article was reported.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Sources of funding for your research
The authors declare that this study had no funding source.
Ethical approval
Not applicable.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contribution
ANV and MTNMA conceived the study. ANV and ARF drafted the manuscript, DPM and ISKH critically revised the manuscript for important intellectual content. ANV, MTNMA, and ARF facilitated all project-related tasks. All authors have approved the submitted version and have agreed on both to be personally accountable for the author's contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Registration of research studies
The manuscript is a case report, not considered a formal-research involving participants.
Guarantor
Amelia Nur Vidyanti.
Acknowledgment
We want to thank all collaborators and medical staff in the patient care.
Contributor Information
Amelia Nur Vidyanti, Email: amelia.nur.v@ugm.ac.id.
Mira Tamila Nurul Maulida Awaliyah, Email: miratamila92@mail.ugm.ac.id.
Aditya Rifqi Fauzi, Email: aditya.rifqi.f@mail.ugm.ac.id.
Indra Sari Kusuma Harahap, Email: indra.sari.kusuma@ugm.ac.id.
Deshinta Putri Mulya, Email: deshintamulya@gmail.com.
References
- 1.Yusuf H.R., Hooper W.C., Beckman M.G., Zhang Q.C., Tsai J., Ortel T.L. Risk of venous thromboembolism among hospitalizations of adults with selected autoimmune diseases. J. Thromb. Thrombolysis. 2014;38(3):306–313. doi: 10.1007/s11239-014-1050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zöller B., Li X., Sundquist J., Sundquist K. Risk of pulmonary embolism in patients with autoimmune disorders: a nationwide follow-up study from Sweden. Lancet. 2012;379(9812):244–249. doi: 10.1016/S0140-6736(11)61306-8. [DOI] [PubMed] [Google Scholar]
- 3.Ramagopalan S.V., Wotton C.J., Handel A.E., Yeates D., Goldacre M.J. Risk of venous thromboembolism in people admitted to hospital with selected immune-mediated diseases: record-linkage study. BMC Med. 2011;9(1):1–8. doi: 10.1186/1741-7015-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamaki H., Khasnis A. Venous thromboembolism in systemic autoimmune diseases: a narrative review with emphasis on primary systemic vasculitides. Vasc. Med. 2015;20(4):369–376. doi: 10.1177/1358863X15573838. [DOI] [PubMed] [Google Scholar]
- 5.Flanagan E.P., Drubach D.A., Boeve B.F. Autoimmune dementia and encephalopathy. Handb. Clin. Neurol. 2016;133:247–267. doi: 10.1016/B978-0-444-63432-0.00014-1. [DOI] [PubMed] [Google Scholar]
- 6.Lim L., Lippe S., Silverman E. Effect of autoimmune diseases on cognitive function. Handb. Clin. Neurol. 2013;112:1275–1283. doi: 10.1016/B978-0-444-52910-7.00050-7. [DOI] [PubMed] [Google Scholar]
- 7.Banks S.A., Sechi E., Flanagan E.P. Autoimmune encephalopathies presenting as dementia of subacute onset and rapid progression. Therapeutic Advances in Neurological Disorders. 2021;14 doi: 10.1177/1756286421998906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelley B.J., Boeve B.F., Josephs K.A. Young-onset dementia: demographic and etiologic characteristics of 235 patients. Arch. Neurol. 2008;65(11):1502–1508. doi: 10.1001/archneur.65.11.1502. [DOI] [PubMed] [Google Scholar]
- 9.Zöller B., Li X., Sundquist J., Sundquist K. Autoimmune diseases and venous thromboembolism: a review of the literature. American journal of cardiovascular disease. 2012;2(3):171. [PMC free article] [PubMed] [Google Scholar]
- 10.Loof T.G., Schmidt O., Herwald H., Theopold U. Coagulation systems of invertebrates and vertebrates and their roles in innate immunity: the same side of two coins? Journal of innate immunity. 2011;3(1):34–40. doi: 10.1159/000321641. [DOI] [PubMed] [Google Scholar]
- 11.Dahlbäck B., editor. Seminars in Immunopathology. Springer; 2012. Coagulation and inflammation—close allies in health and disease. [DOI] [PubMed] [Google Scholar]
- 12.Esmon C.T., Esmon N.L. The link between vascular features and thrombosis. Annu. Rev. Physiol. 2011;73:503–514. doi: 10.1146/annurev-physiol-012110-142300. [DOI] [PubMed] [Google Scholar]
- 13.Ricarte I., Dutra L., Abrantes F., Toso F., Barsottini O., Silva G., et al. Neurologic manifestations of antiphospholipid syndrome. Lupus. 2018;27(9):1404–1414. doi: 10.1177/0961203318776110. [DOI] [PubMed] [Google Scholar]
- 14.Fleetwood T., Cantello R., Comi C. Antiphospholipid syndrome and the neurologist: from pathogenesis to therapy. Front. Neurol. 2018;9:1001. doi: 10.3389/fneur.2018.01001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanne D., Katzav A., Beilin O., Grigoriadis N.C., Blank M., Pick C.G., et al. Interaction of inflammation, thrombosis, aspirin and enoxaparin in CNS experimental antiphospholipid syndrome. Neurobiol. Dis. 2008;30(1):56–64. doi: 10.1016/j.nbd.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Ismail, Salama S. A systematic review of cases of CNS demyelination following COVID-19 vaccination. J. Neuroimmunol. 2022;362 doi: 10.1016/j.jneuroim.2021.577765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gustavo Figueiredo da Silva CFdS., Raddib Eduardo Noleto da Nobrega Oliveira F.W.D.S., João Pedro Ribeiro Baptista SHL, Carla Heloisa Cabral Moro. Alexandre Luiz Longo GdCR. Transverse myelitis and coronavirus disease 2019 vaccine: a temporal association. Clin Exp Neuroimmunol. 2022:1–5. doi: 10.1111/cen3.12678. 00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan E., Shrestha A.K., Colantonio M.A., Liberio R.N., Sriwastava S. Acute transverse myelitis following SARS-CoV-2 vaccination: a case report and review of literature. J. Neurol. 2021:1–12. doi: 10.1007/s00415-021-10785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao S.-C., Wang C.-H., Chang K.-C., Hung M.-J., Chen H.-Y., Liao S.-C. Guillain-barré syndrome associated with COVID-19 vaccination. Emerg. Infect. Dis. 2021;27(12):3175–3178. doi: 10.3201/eid2712.211634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo E.J., Dimova R.B., Mba-Jonas A. Presumptive guillain-Barré syndrome associated with receipt of the Ad26.COV2.2.S COVID-19 vaccine—reply. JAMA. 2022;327(4):393–394. doi: 10.1001/jama.2021.23006. [DOI] [PubMed] [Google Scholar]
- 21.Sechi E., Flanagan E.P. Diagnosis and management of autoimmune dementia. Curr. Treat. Options Neurol. 2019;21(3):1–16. doi: 10.1007/s11940-019-0550-9. [DOI] [PubMed] [Google Scholar]