Abstract
In mouse studies, the results of behavioural experiments are greatly affected by differences in the experimental environment and handling methods. The Porsolt forced swim test and tail suspension test are widely used to evaluate predictive models of depression-like behaviour in mice. It has not been clarified how the results of these tests are affected by testing single or multiple mice simultaneously. Therefore, this study evaluated the differences between testing two mice simultaneously or separately. To investigate the effect of testing multiple mice simultaneously, the Porsolt forced swim test and tail suspension test were performed in three patterns: (1) testing with an opaque partition between two mice, (2) testing without a partition between two mice, and (3) testing a single mouse. In the Porsolt forced swim test, the mice tested simultaneously without a partition demonstrated increased immobility time as compared to mice tested alone. No difference in immobility time was observed between the three groups in the tail suspension test. Our results showed that the environment of behavioural experiments investigating depression-like behaviour in mice can cause a difference in depression-like behaviour. The results of this experiment indicated that it is necessary to describe the method used for behavioural testing in detail.
Subject terms: Neuroscience, Neurology
Mice have been the most widely used animals for experiments on disease, behaviour, and pharmacology studies over the past century1. To transfer the results obtained from mouse experiments to humans successfully, it is necessary to determine the appropriate treatment, and handling and experimental methods to be used in mice.
In general, depression-like behaviour, aggression, activity, anxiety-like behaviour, and other types of behaviour, are measured in mice through a series of behavioural tests in order to analyse the effects of drugs and other stimuli2. Behavioural test batteries have been used in cohorts of inbred and mutant mouse strains to assess a variety of behavioural characteristics, such as motor activity, sensory and motor function, anxiety-like behaviour, learning, and memory3,4. Therefore, behavioural tests are generally designed to minimise the potential effects of various confounding factors. The use of a set of standardised behavioural tests is required to ensure a more accurate interpretation of behavioural phenotypes.
The tail suspension and forced swim tests are among the tests used in behavioural test batteries. These tests have been used for decades to assess depressive-like behaviour in mice and rats5. The tail suspension test is a method in which a mouse or rat is hung by attaching its tail to a box or rod using adhesive tape or similar material6. The behaviour obtained is recorded, and depressive-like behaviour is estimated by the time the suspended animal remains immobile. The equipment used in the forced swim test consists of a transparent cylinder. The cylinder is filled with water to the extent that the legs of the mouse or rat cannot touch the bottom surface of the cylinder, and the mouse or rat is placed in the water to record the behaviour7. Depression-like behaviour is again estimated by the total time during which the animal remains immobile.
It is generally accepted that the tail suspension test and the forced swim test are well established. However, some issues remain to be addressed. For instance, it is not clear how the results for individual experimental animals are affected by conducting the tests at different times and places, or how they are influenced by testing several experimental animals simultaneously. Currently, some commercially available behavioural test devices can be used with multiple mice simultaneously, while others can only be used to test a single mouse at a time. It is therefore important to determine the effect that this difference in the experimental method and in the experimental environment has on the results of these tests.
Mice use visual and olfactory information to show interest in the abnormal behaviour of conspecifics8–10. Mice visually recognise and show interest in cage mates that demonstrate abnormal behaviours11. Furthermore, it has been reported that mice can discriminate between photographs12. In addition, recent studies have shown that they can recognise virtual reality spaces13,14. Other studies have shown that mice are social animals with a strong tendency to follow allogeneic individuals15. These reports show that mice change their behaviour based on visual, olfactory, and auditory information, suggesting that there is a high possibility that the experimental animals’ behaviour could change in response to the presence of other individuals when multiple individuals simultaneously undergo the tail suspension test or the forced swim test. In fact, some reports have recommended taking steps to prevent individual animals from observing conspecifics being tested, although the rationale for this was not explained6.
In this study we tested C57BL/6 mice, an inbred strain widely used for knockout and transgenic models16. Behavioural phenotypes in genetically engineered mice are characterized through a series of behavioural experiments.
The purpose of this study was to determine the proper environment for performing the forced swim and tail suspension tests in the analysis of depressive-like behaviour. We sought to determine potential differences between testing two individual mice simultaneously or independently from each other.
Materials and methods
Animals
All animal experiments were performed in accordance with the ARRIVE guidelines (http://www.nc3rs.org.uk/arrive-guidelines) and the U.S. National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23, revised in 1996) and were approved by the Committee for Animal Experiments at Kawasaki Medical School Advanced Research Centre. All efforts were made to minimise the number of animals used and their suffering. The required sample size was calculated by a power analysis before the start of the experiment.
C57BL/6 N male mice (age: 10 weeks) were purchased from Charles River Laboratories (Kanagawa, Japan) and were housed in cages (four to five animals per cage) with food and water provided ad libitum under a 12 h light/dark cycle at 23–26 °C. Considering that behavioural diversity is partially sex-dependent, and comparing the behaviour of males versus females was not the purpose of this experiment, only male mice were included in the study.
Behavioural tests
All behavioural tests were conducted in behavioural testing rooms, between 09:00 and 16:00 during the light phase of the light/dark cycle. After the tests, the equipment and toys were cleaned with 70% ethanol and super-hypochlorous water to prevent artefacts caused by lingering olfactory cues. Behavioural tests were performed in naïve mice in accordance with the test order described below. Mice were randomly divided (http://www.randomizer.org) into two groups: a demonstrator and an observer (test mice). Cage mates were used as demonstrator mice. At the end of the study, the animals were sacrificed by CO2 inhalation.
Tail suspension test
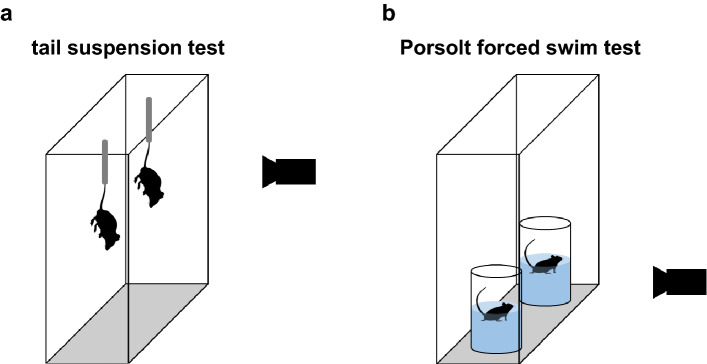
Depression-like behaviour was examined using the tail suspension test, as previously described17–19. The test apparatus consisted of white acrylic walls (20 × 40 × 60 cm), with one open wall through which the animals could be video recorded. The centre of the apparatus was illuminated with 250 lx. Two animals could be tested at the same time (Fig. 1a), and they could be separated using an opaque partition placed in the centre of the apparatus. Each mouse was suspended by the tail 60 cm above the floor of the chamber using adhesive tape placed < 1 cm from the tip of the tail. The resultant behaviour was recorded by a video camera for 6 min. The behaviour was later analysed to determine the following parameters: number of immobility episodes and total duration of immobility. The total amount of time during which each mouse remained immobile was recorded in seconds and then expressed as a percentage of total time or as a percentage per minute. In this test, the ‘immobile period’ was defined as the period when the animals stopped struggling for ≥ 1 s. Data acquisition and analysis were performed using a video tracking software (ANY-MAZE, Stoelting Co., Wood Dale, IL, USA).
Figure 1.
Schematic illustration of the experimental apparatus. (a) Tail suspension test. (b) Porsolt forced swim test.
Three patterns were used to assess whether the behaviour of the test subjects changed depending on the experimental conditions: (1) only a single mouse tested at a time (individual group), (2) presence of an opaque partition plate separating two cage mates being tested simultaneously (opaque partition group), and (3) two cage mates tested simultaneously, without the partition plate (no-partition group) (Fig. 2a).
Figure 2.
Simultaneous tail suspension testing of two mice. (a) Schematic diagram of the experiment. Graphs showing the total number of immobility episodes (b) and the immobility episodes during each 1-min period (c). Graphs showing the total time spent immobile (d) and the proportion of time spent immobile during each 1-min period (e). All data are presented as box plots (b, d) or means ± standard errors (c, e). Statistical significance is represented by asterisks: *p < 0.05, +p < 0.1. The p values were calculated using one-way analysis of variance (analysis of variance [ANOVA] in (b) and (d) and two-way repeated-measures ANOVA in (c) and (e). SEM: standard error of the mean. individual: n = 10, opaque partition: n = 10, no partition: n = 10. Respective mean and SEM are listed in Table 1.
Porsolt forced swim test
The Porsolt forced swim test was also used to examine depression-like behaviour. The apparatus consisted of four Plexiglas cylinders (20 cm [height] × 10 cm [diameter]). Each cylinder was placed in the centre of the apparatus, consisting of a square area surrounded by white acrylic walls (20 × 40 × 60 cm), with one wall open through which the behaviour was recorded (Fig. 1b). The centre of the apparatus was illuminated with 250 lx. This apparatus could also be used with an opaque partition at the centre. Only one wall of the square area surrounded by the white acrylic wall was open and we took a video of the mouse from here. The cylinders were filled with water (23 °C) to a depth of 7.5 cm, based on previous studies20,21. The mice were placed in the cylinders for 6 min and video recorded. The behaviour of the mice was analysed to determine the following parameters: the number of immobility episodes and the total duration of immobility. The amount of time that each mouse remained immobile was recorded in seconds and then expressed as a percentage of the total time or as a percentage per minute. In this test, the ‘immobile period’ was defined as the period when the animals stopped struggling for ≥ 1 s. Data acquisition and analysis were performed using the ANY-MAZE software (Stoelting Co., Ltd.).
Three patterns were used to assess whether the behaviour of the test mice was influenced by the experimental conditions, in the same way as for the tail suspension test (Fig. 3a).
Figure 3.
Simultaneous Porsolt forced swim test of two mice on day 1. (a) Schematic diagram of the experiment. Graphs showing the total number of immobility episodes (b) and the immobility episodes during each 1-min period (c). Graphs showing the total time spent immobile (d) and the proportion of time spent immobile during each 1-min period (e). All data are presented as box plots (b, d) or means ± standard errors (c, e). Statistical significance is represented by asterisks: *p < 0.05, +p < 0.1. The p values were calculated using one-way analysis of variance (ANOVA) in (b) and (d) and two-way repeated-measures ANOVA in (c) and (e). SEM: standard error of the mean. individual: n = 10, opaque partition: n = 10, no partition: n = 10. Respective mean and SEM are listed in Table 2.
Effect of the demonstrator in the Porsolt forced swim test
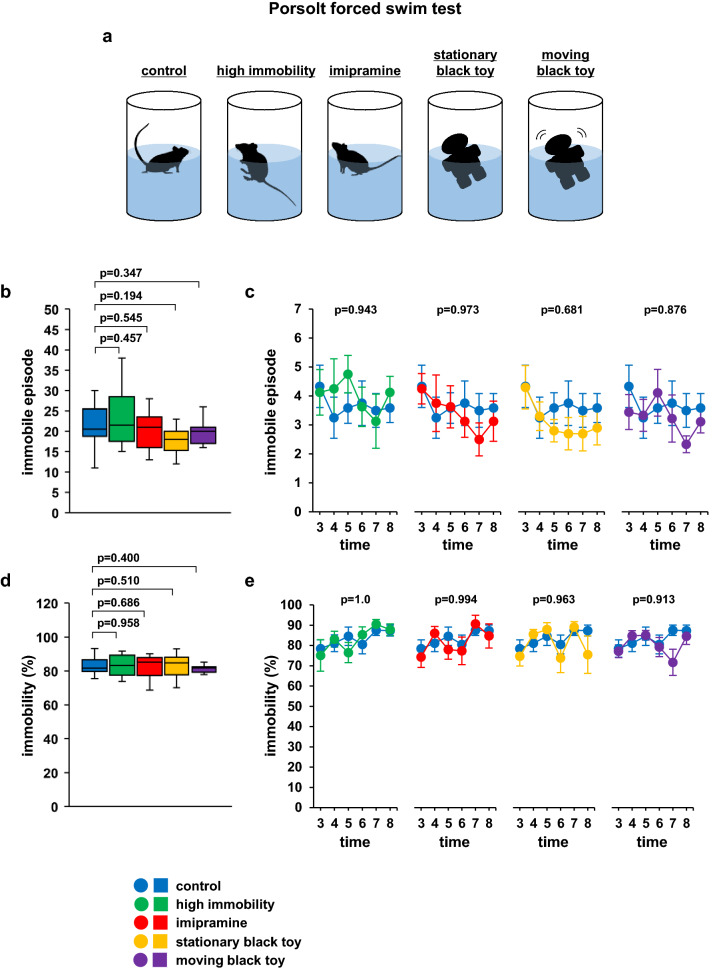
Five patterns were used to assess whether the behaviour of the test mice changed depending on the experimental conditions. We examined whether the test mice exhibited increased immobility time when allowed to observe cage mates displaying high immobility (high immobility group) or low immobility (imipramine group), and compared the results to those resulting from observing control cage mates (Fig. 5a). We also examined whether the test mice exhibited increased immobility when observing an stationary black toy (3 × 3 × 7 cm) visually similar to a mouse displaying depressive-like behaviour (stationary black toy group) or a moving black toy visually similar to an hyperactive mouse (moving black toy group) (Fig. 5a). To elicit depression-like behaviour, mice were placed in a cylinder for 10 min, and those showing depressive-like behaviour were used as demonstrators. For the imipramine group, imipramine (FUJIFILM Wako, Tokyo, Japan) was dissolved in saline to a final concentration of 5 mg/mL. Mice were injected intraperitoneally (i.p.) with 5 mg/kg imipramine 30 min before the test22. Vehicle control mice were injected with saline. We confirmed during the test that the high immobility group had an immobility time rate of 80% and the imipramine group had an immobility time rate lower than 60%. No dividers were installed between the demonstrators and the test subjects. Five patterns were used: (1) the cage mate was placed adjacent to the test mouse (control), (2) the cage mate with depression-like behaviour was placed adjacent to the test mouse (high immobility group), (3) the imipramine-treated hyperactive cage mate was placed adjacent to the test mouse (imipramine group), (4) the stationary black toy was placed adjacent to the test mouse (stationary black toy group), and (5) the moving black toy was placed adjacent to the test mouse (moving black toy group) (Fig. 5a).
Figure 5.
Behaviour due to the nature of the demonstrator inside the cylinder in the Porsolt forced swim test. (a) Schematic diagram of the experiment. Graphs showing the total number of immobility episodes (b) and the immobility episodes during each 1-min period (c). Graphs showing the total time spent immobile (d) and the proportion of time spent immobile during each 1-min period (e). All data are presented as box plots (b, d) or means ± standard errors (c, e). Statistical significance is represented by asterisks: *p < 0.05, +p < 0.1. The p values were calculated using one-way analysis of variance (ANOVA) in (b) and (d) and two-way repeated-measures ANOVA in (c) and (e). SEM: standard error of the mean. control: n = 10, high immobility: n = 10, imipramine: n = 10, stationary black toy: n = 10, moving black toy: n = 10. Respective mean and SEM are listed in Table 4.
Statistical analyses
Statistical analyses were performed using SPSS software (IBM Corp, Tokyo, Japan). Data was analysed using one-way analysis of variance (ANOVA) followed by Tukey’s test, two-way repeated-measures ANOVA followed by Fisher’s least significant difference (LSD) test, or Student’s t-test. Differences were considered statistically significant at a p value < 0.05. All data is presented as box plots or mean ± standard error.
Ethics approval and consent to participate
All animal experiments were performed in accordance with the U.S. National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23, revised in 1996) and approved by the Committee for Animal Experiments at the Kawasaki Medical School Advanced Research Centre.
Results
Behaviour of two mice simultaneously undergoing the tail suspension test
There were no significant differences in the number of immobility episodes or the immobile episode at 1 min between the three experimental groups (Fig. 2b; F2,33 = 0.461, p = 0.635, c; F10,165 = 1.23, p = 0.277, Table 1). There was no significant difference between the three groups in the percentage of time spent immobile (Fig. 2d; F2,33 = 0.478.0, p = 0.624, e; F10,165 = 0.659, p = 0.761, Table 1).
Table 1.
Mean and SEM for Fig. 2.
| mean | SEM | |||
|---|---|---|---|---|
| Figure 2 | ||||
| b | Individual | 16.2 | 1.2 | |
| Opaque partition | 16.7 | 1.4 | ||
| No partition | 17.8 | 1.1 | ||
| c | Individual | 3 min | 4.1 | 0.4 |
| 4 min | 3.3 | 0.4 | ||
| 5 min | 2.7 | 0.3 | ||
| 6 min | 1.8 | 0.3 | ||
| 7 min | 1.9 | 0.3 | ||
| 8 min | 2.4 | 0.4 | ||
| Opaque partition | 3 min | 3.6 | 0.3 | |
| 4 min | 3.3 | 0.6 | ||
| 5 min | 2.6 | 0.5 | ||
| 6 min | 2.5 | 0.3 | ||
| 7 min | 2.8 | 0.5 | ||
| 8 min | 1.9 | 0.3 | ||
| No partition | 3 min | 3.8 | 0.5 | |
| 4 min | 3 | 0.2 | ||
| 5 min | 2.8 | 0.3 | ||
| 6 min | 2.8 | 0.5 | ||
| 7 min | 3.3 | 0.4 | ||
| 8 min | 2.2 | 0.3 | ||
| d | Individual | 80.4 | 2.5 | |
| Opaque partition | 83.2 | 2 | ||
| No partition | 81.6 | 1.3 | ||
| e | Individual | 3 min | 69.7 | 4.8 |
| 4 min | 78.9 | 4.5 | ||
| 5 min | 85.9 | 2.3 | ||
| 6 min | 78.4 | 8.2 | ||
| 7 min | 87.9 | 2.8 | ||
| 8 min | 81.7 | 3.4 | ||
| Opaque partition | 3 min | 71.8 | 5.4 | |
| 4 min | 78.9 | 5.3 | ||
| 5 min | 87.6 | 2.5 | ||
| 6 min | 87 | 2.3 | ||
| 7 min | 85.1 | 2.4 | ||
| 8 min | 88.5 | 2.1 | ||
| No partition | 3 min | 74.6 | 4.6 | |
| 4 min | 79 | 3.7 | ||
| 5 min | 85 | 1.8 | ||
| 6 min | 77.6 | 3.3 | ||
| 7 min | 84.2 | 2.3 | ||
| 8 min | 89.1 | 1.8 | ||
Behaviour of two mice simultaneously undergoing the Porsolt forced swim test
The total number of immobility episodes was significantly lower in the opaque partition group than in the individual group (Fig. 3b; F2,33 = 2.518, p = 0.960, Table 2), and tended to be lower in the no-partition group than in the individual group (Fig. 3b). The total immobility time was significantly longer in the no-partition group than in the individual group (Fig. 3d; F2,33 = 2.878, p = 0.071, Table. 2), and tended to be longer in the opaque group than in the individual group (Fig. 3d). We found no significant differences in the immobile episodes in each 1-min period among the three groups (Fig. 3c; F10,165 = 1.208, p = 0.289, Table. 2), although it tended to be longer in the no-partition group than in the individual group (Fig. 3e; F10,165 = 0.726, p = 0.491, Table. 2).
Table 2.
Mean and SEM for Fig. 3.
| mean | SEM | |||
|---|---|---|---|---|
| Figure 3 | ||||
| b | Individual | 29.6 | 1.6 | |
| Opaque partition | 24.6 | 1.8 | ||
| No partition | 25.2 | 1.8 | ||
| c | Individual | 3 min | 4.3 | 0.6 |
| 4 min | 5 | 0.6 | ||
| 5 min | 5.3 | 0.5 | ||
| 6 min | 4.8 | 0.7 | ||
| 7 min | 4.5 | 0.4 | ||
| 8 min | 5.8 | 0.4 | ||
| Opaque partition | 3 min | 4.5 | 0.4 | |
| 4 min | 3.8 | 0.5 | ||
| 5 min | 4.3 | 0.5 | ||
| 6 min | 4.6 | 0.6 | ||
| 7 min | 3.8 | 0.5 | ||
| 8 min | 3.7 | 0.6 | ||
| No partition | 3 min | 4.1 | 0.4 | |
| 4 min | 3.4 | 0.5 | ||
| 5 min | 3.8 | 0.4 | ||
| 6 min | 4.3 | 0.6 | ||
| 7 min | 5.1 | 0.6 | ||
| 8 min | 4.5 | 0.7 | ||
| d | Individual | 70.1 | 2.6 | |
| Opaque partition | 76.4 | 2.6 | ||
| No partition | 77.4 | 1.7 | ||
| e | Individual | 3 min | 58.9 | 6.5 |
| 4 min | 67.9 | 6 | ||
| 5 min | 73.6 | 4 | ||
| 6 min | 73.8 | 3.6 | ||
| 7 min | 73.7 | 5.7 | ||
| 8 min | 72.8 | 4.7 | ||
| Opaque partition | 3 min | 67.1 | 5 | |
| 4 min | 69.1 | 6.1 | ||
| 5 min | 71.2 | 6.2 | ||
| 6 min | 84.2 | 2.3 | ||
| 7 min | 80.6 | 3.9 | ||
| 8 min | 85.9 | 3.9 | ||
| No partition | 3 min | 64.5 | 5.3 | |
| 4 min | 79 | 3 | ||
| 5 min | 81.7 | 2.8 | ||
| 6 min | 81.8 | 3.4 | ||
| 7 min | 78.8 | 3.4 | ||
| 8 min | 78.7 | 5.4 | ||
On day 2, there were no significant differences in the total immobile episodes and immobile episodes in each 1 min between the three experimental groups (Fig. 4a; F2,33 = 1.381, p = 0.266, b; F10,165 = 0.740, p = 0.686, Table. 3). There was no significant difference between the three groups in the percentage of time spent immobile (Fig. 4c; F2,33 = 0.592, p = 0.559, d; F10,165 = 0.827, p = 0.603, Table 3).
Figure 4.

Simultaneous Porsolt forced swim test of two mice on day 2. Graphs showing the total number of immobility episodes (a) and the immobility episodes during each 1-min period (b). Graphs showing the total time spent immobile (c) and the proportion of time spent immobile during each 1-min period (d). All data are presented as box plots (a, c) or means ± standard errors (b, d). Statistical significance is represented by asterisks: *p < 0.05, +p < 0.1. The p values were calculated using one-way analysis of variance (ANOVA) in (a) and (c) and two-way repeated-measures ANOVA in (b) and (d). SEM: standard error of the mean. individual: n = 10, opaque partition: n = 10, no partition: n = 10. Respective mean and SEM are listed in Table 3.
Table 3.
Mean and SEM for Fig. 4.
| mean | SEM | |||
|---|---|---|---|---|
| Figure 4 | ||||
| a | Individual | 25.8 | 2.3 | |
| Opaque partition | 24.6 | 1.7 | ||
| No partition | 28.8 | 1.6 | ||
| b | Individual | 3 min | 4.5 | 0.7 |
| 4 min | 5.3 | 0.6 | ||
| 5 min | 3.7 | 0.4 | ||
| 6 min | 4.6 | 0.7 | ||
| 7 min | 3.8 | 0.8 | ||
| 8 min | 3.8 | 0.5 | ||
| Opaque partition | 3 min | 4.5 | 0.6 | |
| 4 min | 4.4 | 0.6 | ||
| 5 min | 3.8 | 0.5 | ||
| 6 min | 4 | 0.3 | ||
| 7 min | 4.5 | 0.8 | ||
| 8 min | 3.3 | 0.7 | ||
| No partition | 3 min | 4.6 | 0.5 | |
| 4 min | 4.8 | 0.6 | ||
| 5 min | 3.9 | 0.4 | ||
| 6 min | 5.4 | 0.6 | ||
| 7 min | 5 | 0.6 | ||
| 8 min | 5.2 | 0.5 | ||
| c | Individual | 79.9 | 2.5 | |
| Opaque partition | 78.9 | 2.2 | ||
| No partition | 76.8 | 1.2 | ||
| d | Individual | 3 min | 84.1 | 2.1 |
| 4 min | 80.2 | 3 | ||
| 5 min | 80.1 | 3.4 | ||
| 6 min | 74.5 | 4.8 | ||
| 7 min | 79.3 | 7.8 | ||
| 8 min | 80.9 | 4 | ||
| Opaque partition | 3 min | 73.5 | 5.1 | |
| 4 min | 75.5 | 4.9 | ||
| 5 min | 78.5 | 4.2 | ||
| 6 min | 78.9 | 6.3 | ||
| 7 min | 84.6 | 3.2 | ||
| 8 min | 82.6 | 2.8 | ||
| No partition | 3 min | 78 | 4.2 | |
| 4 min | 80.3 | 3.1 | ||
| 5 min | 70.5 | 5 | ||
| 6 min | 75.3 | 4 | ||
| 7 min | 79.6 | 4.2 | ||
| 8 min | 77.3 | 4.1 | ||
Effect of the demonstrator inside the cylinder in the Porsolt forced swim test on behaviour
In the Porsolt forced swim test, we found no significant differences in the total number of immobility episodes (Fig. 5b; F4,42 = 1.160, p = 0.342, Table 4) and the immobility episode in each 1-min period among the five groups (Fig. 5c; F20,210 = 0.375, p = 0.991, Table 4). There were no significant differences in the total immobility time and immobility time in each 1-min period between the five experimental groups (Fig. 5d; F4,42 = 0.257, p = 0.904, e; F20,210 = 1.313, p = 0.173, Table 4).
Table 4.
Mean and SEM for Fig. 5.
| mean | SEM | |||
|---|---|---|---|---|
| Figure 5 | ||||
| b | Control | 22 | 1.6 | |
| High immobility | 24 | 3.1 | ||
| Imipramine | 20.4 | 1.8 | ||
| Stationary black toy | 18.7 | 1.7 | ||
| Moving black toy | 19.6 | 1.1 | ||
| c | Control | 3 min | 4.3 | 0.7 |
| 4 min | 3.3 | 0.7 | ||
| 5 min | 3.6 | 0.5 | ||
| 6 min | 3.8 | 0.8 | ||
| 7 min | 3.5 | 0.6 | ||
| 8 min | 3.6 | 0.5 | ||
| High immobility | 3 min | 4.1 | 0.8 | |
| 4 min | 4.3 | 1 | ||
| 5 min | 4.8 | 0.6 | ||
| 6 min | 3.6 | 0.7 | ||
| 7 min | 3.1 | 0.9 | ||
| 8 min | 4.1 | 0.5 | ||
| Imipramine | 3 min | 4.3 | 0.5 | |
| 4 min | 3.8 | 1 | ||
| 5 min | 3.6 | 0.7 | ||
| 6 min | 3.1 | 0.5 | ||
| 7 min | 2.5 | 0.6 | ||
| 8 min | 3.1 | 0.7 | ||
| Stationary black toy | 3 min | 4.3 | 0.7 | |
| 4 min | 3.3 | 0.5 | ||
| 5 min | 2.8 | 0.4 | ||
| 6 min | 2.7 | 0.6 | ||
| 7 min | 2.7 | 0.6 | ||
| 8 min | 2.9 | 0.6 | ||
| Moving black toy | 3 min | 3.4 | 0.6 | |
| 4 min | 3.3 | 0.6 | ||
| 5 min | 4.1 | 0.8 | ||
| 6 min | 3.2 | 0.8 | ||
| 7 min | 2.3 | 0.3 | ||
| 8 min | 3.1 | 0.4 | ||
| d | Control | 83.2 | 1.6 | |
| High immobility | 83.1 | 2.6 | ||
| Imipramine | 81.8 | 3 | ||
| Stationary black toy | 81.1 | 3.4 | ||
| Moving black toy | 80.3 | 1.4 | ||
| e | Control | 3 min | 78.5 | 4.3 |
| 4 min | 81.1 | 4.1 | ||
| 5 min | 84.5 | 4.5 | ||
| 6 min | 80.5 | 4.6 | ||
| 7 min | 87.5 | 2.6 | ||
| 8 min | 87.3 | 2.7 | ||
| High immobility | 3 min | 75.1 | 7.8 | |
| 4 min | 83.1 | 3.9 | ||
| 5 min | 76.5 | 5 | ||
| 6 min | 85.3 | 3.8 | ||
| 7 min | 90.5 | 2.4 | ||
| 8 min | 87.8 | 2.8 | ||
| Imipramine | 3 min | 74.3 | 5.2 | |
| 4 min | 86 | 3.3 | ||
| 5 min | 78 | 4.8 | ||
| 6 min | 77.3 | 6.8 | ||
| 7 min | 90.6 | 4.3 | ||
| 8 min | 84.7 | 6 | ||
| Stationary black toy | 3 min | 74.7 | 4.8 | |
| 4 min | 85.5 | 2.4 | ||
| 5 min | 87.9 | 3.4 | ||
| 6 min | 73.8 | 7.2 | ||
| 7 min | 89.1 | 2.6 | ||
| 8 min | 75.5 | 9.3 | ||
| Moving black toy | 3 min | 77.1 | 3.2 | |
| 4 min | 84.7 | 2.6 | ||
| 5 min | 85.1 | 2.4 | ||
| 6 min | 79.2 | 4.8 | ||
| 7 min | 71.7 | 6.4 | ||
| 8 min | 84.6 | 4.1 | ||
Discussion
In this study, we investigated the effect of simultaneous testing of two mice on the immobility time displayed during the forced swim and tail suspension tests. In the tail suspension test, simultaneous testing, either with or without an opaque screen between the test subjects, showed no effect on immobility time when compared with the results of testing a single mouse at a time. In the forced swim test, the immobility time of the test mice increased when they were adjacent to a cage mate. These results indicate that the test environment in the forced swim test influences immobility time.
In the tail suspension test, there was no significant difference in the number of immobility episodes or in the immobility time between the three groups. Although mice are thought to change their behaviour in response to visual information8–10, they failed to change their behaviour in this experiment despite the presence of nearby cage mates. Mice experience stress and anxiety when handled by their tails23–25. Studies of mouse behavioural phenotypes have identified handling stress as one of the most probable causes of failure in replicating phenotypes within and between experiments26,27. It is possible that mice could not appropriately grasp the surrounding environment during this test due to handling-related distress. Furthermore, unlike the case in the forced swim test, mice cannot freely turn around and explore the entire visual field during the tail suspension test because the tail is fixed and cannot rotate6, implying that they cannot grasp the surrounding environment. In this study, the results of the tail suspension test were not affected by whether one or two mice were tested simultaneously.
In the forced swim test, the proportion of immobility time was increased in the mice tested simultaneously without a partition plate, compared with that displayed by the mice that were tested alone. The proportion of immobility time tended to increase when two mice were tested simultaneously but were separated by a partition, again compared with the mice tested alone. Mice tend to avoid water28, and quickly become immobile when they are placed in this medium.
Behavioural experiments in mice using water include forced swim tests, water T-maze tests, and the Morris water maze (MWM) test29. The MWM is a widely used model for studying learning and memory behaviour. This test specifically evaluates spatial learning and memory30,31. In the MWM, the experimental subject relies on distal cues to find a submerged escape platform from the starting position around the swimming pool. Thus, it is assumed that the subject can grasp the surrounding environment while floating on water. In the forced swim test used in the study, it was suggested that mice grasped the surrounding environment from visual and auditory information while floating in the water and changed their behaviour accordingly. Alternatively, mice may cease to display active swimming behaviour to understand the environment in which they are placed and spend time collecting visual and auditory information. Mice are capable of visually identifying the behaviour of cage mates and simultaneously using olfactory and auditory cues as additional sensory information32. In this study, in the opaque group as well as in the no-partition group, they could also use olfactory and auditory information. The results of this study suggest that it is necessary to pay attention to the interpretation of the results when performing a forced swim test with two mice simultaneously, with or without a partition plate.
There were no significant differences between the groups in the results of the forced swim test performed on the second day, when it was considered that the mouse already had a good understanding of the test environment. It has been shown that immobility time increases 7 and 14 days after the initial test33. Further research is needed to investigate this phenomenon and its association with memory processes.
In an experimental environment without dividers, we investigated whether the nature of the demonstrator in the adjacent transparent cylinder changed the depressive-like behaviour of the test subjects, since it has been shown that mice change their behaviour by imitation of other individuals34–39. However, in this experiment, the behaviour of the cage mate in the adjacent cylinder, irrespective of whether they were hyperactive or immobile, did not change the behaviour of the observer. It is likely that the mice did not have the time or mental leeway to imitate the cage mate due to anxiety and fear. It is possible that changes in mouse behaviour would have occurred if the experiment duration had been extended. Further research is required to clarify these details.
Behavioural tests in mice have long been used in research worldwide40,41.Most behavioural traits are sensitive to genetic, environmental, and experimental factors such as genetic background, laboratory conditions, and previous testing experience26.The experimenter must conduct the behavioural tests appropriately; however, in many cases, it is not possible to determine from the methodological description in the research paper in what type of environment and how each researcher actually conducted the behavioural tests. Therefore, the experimental results differ across laboratories. The results we report here may be one of the causes lead to differing experimental results from different laboratories.
Studying rodent behaviour contributes to the understanding of the pathophysiology of neuropsychiatric disorders and opens new avenues for treatment. Over time, rodent behavioural tests have continued to evolve, with over 100 tests currently in use42. Unfortunately, laboratory behavioural testing of rodents has proven difficult, and test results may vary depending on the person conducting the experiment, the laboratory in which the experiment was conducted, and the environment26,43. As there is no perfect behavioural test, the best option should be chosen according to the specific project goals. To understand the behaviour of animals better, it is important to recognise that their worldview may differ significantly from that of humans44. As shown in this study, even in the extensively used forced swim test, mice change the number of immobility episodes and the immobility time in response to slight differences in the environment. Importantly, a high immobility rate is interpreted as an increase in depressive-like behaviour. As discussed above, the increased immobility rate in forced swim tests may reflect observation-like and adaptive-like behaviours. Care must therefore be taken when interpreting the results of behavioural tests.
Conclusion
We found that the number of immobility episodes and the total immobility time, which are indicators of depressive-like behaviour, changed when two mice were tested at the same time during the forced swim test instead of being tested alone. The results of this study emphasise the need to consider test conditions carefully when interpreting the behavioural test results of the forced swim test.
Acknowledgements
We thank the Kawasaki Medical School Central Research Institute for providing the instruments for this study. The authors would like to thank Editage (www.editage.jp) for English language editing.
Abbreviations
- ANOVA
Analyses of variance
- MWM
Morris water maze
- PS
Porsolt forced swim test
- TS
Tail suspension test
Author contributions
All authors had full access to all study data and take full responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: H.U., M.O., and T.I. Acquisition of data: H.U., and Y.T. Analysis and interpretation of data: H.U. and Y.T. Drafting of the manuscript: H.U. and M.O. Critical revision of the manuscript for important intellectual content: S.M., K.W., Y.T., Y.M.., and T.I. Statistical analysis: H.U. and Y.T. Study supervision: M.O. and T.I.
Funding
This research did not receive any specific grant from funding agencies in the public commercial or not-for-profit sectors.
Data availability
The dataset is available on reasonable request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Taylor K, Gordon N, Langley G, Higgins W. Estimates for worldwide laboratory animal use in 2005. Altern. Lab. Anim. 2008;36:327–342. doi: 10.1177/026119290803600310. [DOI] [PubMed] [Google Scholar]
- 2.Wahlste D. Mouse behavioral testing. Academic; 2010. [Google Scholar]
- 3.Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809–818. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Crawley JN, Paylor R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm. Behav. 1997;31:197–211. doi: 10.1006/hbeh.1997.1382. [DOI] [PubMed] [Google Scholar]
- 5.Castagné, V., Moser, P., Roux, S. & Porsolt, R. D. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr. Protoc. Neurosci. (2011). Chapter 8. [DOI] [PubMed]
- 6.Can A, et al. The tail suspension test. J. Vis. Exp. 2012;59:e3769. doi: 10.3791/3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yankelevitch-Yahav, R., Franko, M., Huly, A. & Doron, R. The forced swim test as a model of depressive-like behavior. J. Vis. Exp. 52587 (2015). [DOI] [PMC free article] [PubMed]
- 8.Ueno H, et al. Empathic behavior according to the state of others in mice. Brain Behav. 2018;8:e00986. doi: 10.1002/brb3.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith ML, Hostetler CM, Heinricher MM, Ryabinin AE. Social transfer of pain in mice. Sci. Adv. 2016;2:e1600855. doi: 10.1126/sciadv.1600855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langford DJ, et al. Social modulation of pain as evidence for empathy in mice. Science. 2006;312:1967–1970. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, et al. Altered behavior and neural activity in conspecific cagemates co-housed with mouse models of brain disorders. Physiol. Behav. 2016;163:167–176. doi: 10.1016/j.physbeh.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe S. Preference for and discrimination of paintings by mice. PLoS ONE. 2013;8:e65335. doi: 10.1371/journal.pone.0065335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drew L. The ethics of brain-computer interfaces. Nature. 2019;571:S19–S21. doi: 10.1038/d41586-019-02214-2. [DOI] [PubMed] [Google Scholar]
- 14.Sato, M. et al. Hippocampus-dependent goal localization by head-fixed mice in virtual reality. eNeuro 4: ENEURO., 0369–16.2017 (2017). [DOI] [PMC free article] [PubMed]
- 15.Hedrich HJ, Bullock GR. The laboratory mouse. Elsevier Academic; 2004. [Google Scholar]
- 16.Bryant CD, et al. Behavioral differences among C57BL/6 substrains: implications for transgenic and knockout studies. J Neurogenet. 2008;22:315–331. doi: 10.1080/01677060802357388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueno H, et al. Anti-depressive-like effect of 2-phenylethanol inhalation in mice. Biomed. Pharmacother. 2019;111:1499–1506. doi: 10.1016/j.biopha.2018.10.073. [DOI] [PubMed] [Google Scholar]
- 18.Umemura M, et al. Comprehensive behavioral analysis of activating transcription factor 5-deficient mice. Front. Behav. Neurosci. 2017;11:125. doi: 10.3389/fnbeh.2017.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onouchi T, et al. Targeted deletion of the C-terminus of the mouse adenomatous polyposis coli tumor suppressor results in neurologic phenotypes related to schizophrenia. Mol. Brain. 2014;7:21. doi: 10.1186/1756-6606-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuda I, Shoji H, Yamasaki N, Miyakawa T, Aiba A. Comprehensive behavioral phenotyping of a new semaphorin 3 F mutant mouse. Mol. Brain. 2016;9:15. doi: 10.1186/s13041-016-0196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbas MG, et al. Comprehensive behavioral analysis of male Ox1r (-/-) mice showed implication of orexin Receptor-1 in mood, anxiety, and social behavior. Front. Behav. Neurosci. 2015;9:324. doi: 10.3389/fnbeh.2015.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poleszak E, et al. Antidepressant-like activity of typical antidepressant drugs in the forced swim test and tail suspension test in mice is augmented by DMPX, an adenosine A2a receptor antagonist. Neurotox. Res. 2019;35:344–352. doi: 10.1007/s12640-018-9959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leach MC, Main DCJ. An assessment of laboratory mouse welfare in UK animal units. Anim. Welf. 2008;17:171–187. [Google Scholar]
- 24.Deacon RM. Housing, husbandry and handling of rodents for behavioral experiments. Nat. Protoc. 2006;1:936–946. doi: 10.1038/nprot.2006.120. [DOI] [PubMed] [Google Scholar]
- 25.Wahlsten D, et al. Different data from different labs: lessons from studies of gene-environment interaction. J. Neurobiol. 2003;54:283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- 26.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 27.Mandillo S, et al. Reliability, robustness, and reproducibility in mouse behavioral phenotyping: a cross-laboratory study. Physiol. Genomics. 2008;34:243–255. doi: 10.1152/physiolgenomics.90207.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellenbroek B, Youn J. Rodent models in neuroscience research: is it a rat race? Dis. Model. Mech. 2016;9:1079–1087. doi: 10.1242/dmm.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wahlsten, D. Mouse behavioral testing 1st edition, (2010).
- 30.D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res. Brain Res. Rev. 2001;36:60–90. doi: 10.1016/S0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 31.Morris RGM. Spatial localization does not require the presence of local cues. Learn. Motiv. 1981;12:239–260. doi: 10.1016/0023-9690(81)90020-5. [DOI] [Google Scholar]
- 32.Corridi P, Chiarotti F, Bigi S, Alleva E. Familiarity with conspecific odor and isolation-induced aggressive behavior in male mice (Mus domesticus) J. Comp. Psychol. 1993;107:328–335. doi: 10.1037/0735-7036.107.3.328. [DOI] [PubMed] [Google Scholar]
- 33.Suman PR, Zerbinatti N, Theindl LC, Domingues K, Lino de Oliveira C. Failure to detect the action of antidepressants in the forced swim test in Swiss mice. Acta Neuropsychiatr. 2018;30:158–167. doi: 10.1017/neu.2017.33. [DOI] [PubMed] [Google Scholar]
- 34.Ueno H, et al. Conformity-like behaviour in mice observing the freezing of other mice: a model of empathy. BMC Neurosci. 2020;21:19. doi: 10.1186/s12868-020-00566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Debiec J, Olsson A. Social fear learning: from animal models to human function. Trends Cogn. Sci. 2017;21:546–555. doi: 10.1016/j.tics.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyza KZ, Bartal IB, Monfils MH, Panksepp JB, Knapska E. The roots of empathy: Through the lens of rodent models. Neurosci. Biobehav. Rev. 2017;76:216–234. doi: 10.1016/j.neubiorev.2016.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keum S, et al. Variability in empathic fear response among 11 inbred strains of mice. Genes Brain Behav. 2016;15:231–242. doi: 10.1111/gbb.12278. [DOI] [PubMed] [Google Scholar]
- 38.Sivaselvachandran S, Acland EL, Abdallah S, Martin LJ. Behavioral and mechanistic insight into rodent empathy. Neurosci. Biobehav. Rev. 2018;91:130–137. doi: 10.1016/j.neubiorev.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Panksepp JB, Lahvis GP. Rodent empathy and affective neuroscience. Neurosci. Biobehav. Rev. 2011;35:1864–1875. doi: 10.1016/j.neubiorev.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf A, Bauer B, Abner EL, Ashkenazy-Frolinger T, Hartz AM. A comprehensive behavioral test battery to assess learning and memory in 129S6/Tg2576 mice. PLoS ONE. 2016;11:e0147733. doi: 10.1371/journal.pone.0147733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brigman JL, Graybeal C, Holmes A. Predictably irrational: assaying cognitive inflexibility in mouse models of schizophrenia. Front. Neurosci. 2010;4:13. doi: 10.3389/neuro.01.013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hånell A, Marklund N. Structured evaluation of rodent behavioral tests used in drug discovery research. Front. Behav. Neurosci. 2014;8:252. doi: 10.3389/fnbeh.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. Influences of laboratory environment on behavior. Nat. Neurosci. 2002;5:1101–1102. doi: 10.1038/nn1102-1101. [DOI] [PubMed] [Google Scholar]
- 44.Button KS, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset is available on reasonable request from the corresponding author.