Abstract
A versatile ligninolytic peroxidase has been cloned from Pleurotus eryngii and its allelic variant MnPL2 expressed in Aspergillus nidulans, with properties similar to those of the mature enzyme from P. eryngii. These include the ability to oxidize Mn2+ and aromatic substrates, confirming that this is a new peroxidase type sharing catalytic properties of lignin peroxidase and manganese peroxidase.
The ligninolytic enzymes lignin peroxidase (LiP) and manganese peroxidase (or manganese-dependent peroxidase) (MnP) were described in 1983 and 1984 in the fungus Phanerochaete chrysosporium (6, 9, 24). Their corresponding cDNA were cloned, and recombinant enzymes were obtained in 1987 to 1989 (11, 15, 18, 25), and the molecular structure of both proteins was reported in 1993 and 1994 (16, 17, 23). A third type of ligninolytic peroxidase has been described in several species of the genera Pleurotus and Bjerkandera, characterized by sharing catalytic properties of MnP (efficient oxidation of Mn2+ to Mn3+) and LiP (oxidation of veratryl alcohol, p-dimethoxybenzene, and non-phenolic lignin model dimers) and a high affinity for substituted hydroquinones (2, 7, 8, 12, 13, 20). Recently, two peroxidases produced in solid-state fermentation and liquid cultures of Pleurotus eryngii have been cloned, the latter appearing as two 99% identical variants (MnPL1 and MnPL2) encoded by two alleles of the same gene (3, 19). These versatile enzymes have higher sequence and structural homology with P. chrysosporium LiP than with MnP, but their molecular models showed a putative Mn2+ interaction site near the internal propionate of heme, accounting for their ability to oxidize low concentrations of this cation. Expression of the gene encoding the peroxidase isolated from P. eryngii liquid cultures, allelic variant MnPL2 (19), in Escherichia coli (by using the T7 lac promoter and terminator) resulted in a recombinant protein detected by immunoblotting (unpublished results). However, all attempts to obtain active peroxidase by protocols described previously (21) for refolding denatured proteins were unsuccessful. In order to obtain an active recombinant enzyme, the expression of P. eryngii peroxidase in Aspergillus nidulans, as a model organism for fungal genetics studies, was attempted.
A cDNA fragment encoding the whole protein with its signal peptide was amplified, cloned, and used to transform an A. nidulans arg mutant strain. For this purpose, two PCR primers (5′-CGggatccCCCATGTCTTTCAAGACGC-3′ and 5′-GgaattcTTACGATCCAGGGACGGG-3′) were synthesized (restriction sites shown in lowercase), and a BamHI-EcoRI fragment corresponding to MnPL2 cDNA (GenBank accession no. AF007222) was amplified by using MnPL2 cDNA cloned into pBSK+/− as a template (19). The PCR products were separated on 0.8% agarose and purified, and the BamHI-EcoRI fragment was cloned into palcA1 (5) (containing the alcA promoter inducible by ethanol or threonine and repressed by glucose [10], the trpC terminator, and the argB gene encoding ornithine carbamoyltransferase as a selection marker), yielding pALMP2. Automatic sequencing of the PCR fragment, with synthetic oligonucleotides as primers, confirmed that the sequence matched that of MnPL2 cDNA exactly. Mycelia of A. nidulans (biA1 methG1 argB2) (IJFM A729, derived from FGSC A89 and A219) were grown at 28°C and 180 rpm in a minimal medium (4) containing 10 μg of d-biotin per liter, 74.5 mg of l-methionine per liter, and 0.53 g of l-arginine per liter. Protoplasts were obtained by incubating washed mycelia (1 g [wet weight]) with Novozym 234 (20 mg) in 1.2 M MgSO4 (buffered at pH 5.8) containing serum albumin (24 mg) and separated by centrifugation at 4,000 × g with an overlay of 0.6 M sorbitol in 0.1 M Tris-HCl (pH 7.5). They were transformed (1 to 2 μg of pALMP2) in 10 mM Tris-HCl (pH 7.5) containing polyethylene glycol 6000, sorbitol, and CaCl2 (26), inoculated in a selective minimal medium with 10 μg of d-biotin per liter, 74.5 mg of l-methionine per liter, and 1 M sucrose, in a soft agar overlay, and incubated at 37°C. Southern blot analysis after DNA digestion (with the argB gene as a probe) showed that the plasmid containing mnpl2 cDNA was integrated in the genome of A. nidulans, occupying a position different from that of argB of the host fungus, and no evidence for multiple integration was obtained.
Different growth conditions for the recombinant A. nidulans strain containing mnpl2 DNA were investigated for peroxidase production. Since peroxidase expression was under the alcA promoter, it was induced by substituting threonine for glucose. The fungus was grown in minimal or complete media (4) containing 10 μg of d-biotin per liter and 74.5 mg of l-methionine per liter at 28°C and 180 rpm for 24 h. Then, washed mycelia were transferred to induction media with the same composition but containing 0.05% glucose and 100 mM threonine, with or without 0.5 g of hemin per liter. Peroxidase activity in culture samples collected during incubation (96 h) was estimated by the formation of Mn3+-tartrate complex (12). The addition of hemin to the medium was required for peroxidase production in both minimal and complete media, the latter yielding the highest peroxidase activity (up to 150 U/liter). It had already been reported that the addition of hemin to media increased the activities of other peroxidases expressed in ascomycetes (1, 22).
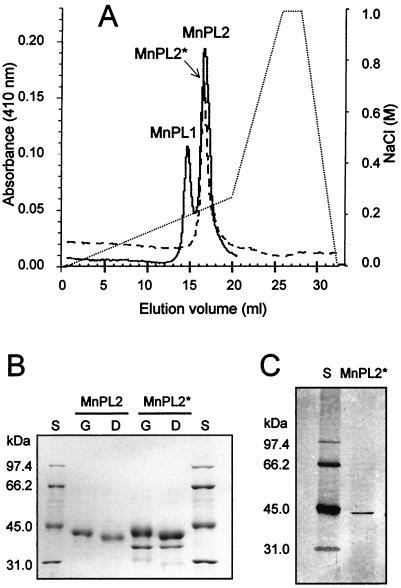
The recombinant peroxidase MnPL2* from A. nidulans was purified from cultures in a hemin-containing complete medium. Maximal peroxidase activity was observed 43 h after induction, and protein MnPL2* was purified from concentrated and dialyzed (against 10 mM sodium tartrate, pH 4.5) culture liquid by using the protocol described for P. eryngii MnPL2 (12) with an additional step. As shown in Fig. 1A, Mono-Q chromatography yielded a single peak with high absorbance at 410 nm and peroxidase activity corresponding to protein MnPL2*. This peak showed the same elution volume as allelic variant MnPL2 from P. eryngii CBS 613.91 (IJFM A169) grown in glucose-peptone medium (12) (Fig. 1A). Proteins MnPL2 and MnPL2* were N deglycosylated with endo-β-N-acetylglucosaminidase, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of native and deglycosylated proteins was performed in 12% polyacrylamide gels, which were stained with AgNO3. As shown in Fig. 1B, both proteins showed the same molecular mass before (43 kDa) and after (41 kDa) deglycosylation. In the case of protein MnPL2*, a contaminant protein representing 13% of the peak collected from Mono-Q was detected. Since this protein did not seem to be N glycosylated, a purification step was performed based on affinity for concanavalin A (ConA). The peroxidase peak from Mono-Q chromatography was concentrated, dialyzed against 10 mM sodium tartrate (pH 5) containing 0.5 M NaCl, 1 mM MnCl2, and 1 mM CaCl2, and applied to a ConA-Sepharose column. The retained protein MnPL2* was eluted with 0.2 M α-d-methylglucoside, dialyzed, and stored at −20°C. In this way, electrophoretically homogeneous MnPL2* protein was obtained, as shown in Fig. 1C. N-terminal sequencing of the recombinant protein by automated Edman degradation revealed the same sequence obtained for mature protein MnPL2 from P. eryngii. The whole process used to purify peroxidase MnPL2* is summarized in Table 1. The purification yield and factor are relative to peroxidase activity after ultrafiltration, but they should be higher if referred to activity in the culture liquid, where an unknown reaction seemed to partially interfere with the activity assay. Despite the fact that recombinant peroxidase activity in cultures of A. nidulans was at a lower level than peroxidase activity in cultures of P. eryngii (12), the specific activity of purified protein MnPL2* was similar to that of protein MnPL2 from P. eryngii. Expression in other fungal systems, such as Aspergillus oryzae or Pichia pastoris (14, 22), will be attempted in the future when high peroxidase levels are required.
FIG. 1.
Purification of peroxidase MnPL2* from recombinant A. nidulans. (A) Superimposition of Mono-Q profiles (410 nm) of protein MnPL2* from A. nidulans (dashed line) and proteins MnPL1 and MnPL2 from P. eryngii (solid line) showing the same elution volume for wild-type and recombinant MnPL2 (NaCl gradient shown as a dotted line). (B) SDS-PAGE of proteins in peaks MnPL2* and MnPL2, showing similar molecular masses before (G) and after (D) deglycosylation; lane S, low-molecular-mass standards from Bio-Rad. (C) SDS-PAGE of glycosylated protein MnPL2* after a final purification step with ConA, which removed any contaminating protein.
TABLE 1.
Purification of peroxidase MnPL2* from recombinant A. nidulans
| Purification step | Recombinant peroxidase
|
||||
|---|---|---|---|---|---|
| Amt of protein (mg) | Activity
|
Purification
|
|||
| Total (U) | Specific (U/mg) | Yield (%) | Factor (fold) | ||
| Culture liquid | 294.3 | 157 | 0.5 | 83 | |
| Ultrafiltered | 147.1 | 190 | 1.3 | 100 | 1 |
| Q-cartridge | 66.1 | 150 | 2.3 | 79 | 2 |
| Sephacryl S-200 | 28.1 | 134 | 4.8 | 71 | 4 |
| Mono-Q | 0.5 | 79 | 161.8 | 41 | 126 |
| ConA-Sepharose | 0.4 | 62 | 148.5 | 33 | 115 |
Finally, steady-state kinetic constants for oxidation of Mn2+, veratryl alcohol, and methoxyhydroquinone by peroxidases MnPL2* and MnPL2 were compared, under the conditions described for P. eryngii peroxidase (8). The apparent Km and turnover numbers (t) are shown in Table 2, revealing that both peroxidases have similar enzymatic activities oxidizing Mn2+ to Mn3+ as well as phenolic and nonphenolic aromatic substrates with high affinity on both Mn2+ and substituted hydroquinones. The recombinant peroxidase also oxidized 2,6-dimethoxyphenol, although no kinetic constants were calculated. The Km for H2O2 (around 10 μm) was similar to that of the P. eryngii enzyme.
TABLE 2.
Steady-state kinetic constants of recombinant peroxidase expressed in A. nidulans and the corresponding protein produced by P. eryngii
| Substrate |
Km (μM)
|
t (s−1)
|
||
|---|---|---|---|---|
| MnPL2* (A. nidulans) | MnPL2 (P. eryngii) | MnPL2* (A. nidulans) | MnPL2 (P. eryngii) | |
| Mn2+ | 20 | 12 | 99 | 110 |
| Veratryl alcohol | 1,780 | 2,170 | 12 | 13 |
| Methoxyhydroquinone | 23 | 19 | 10 | 17 |
In summary, the P. eryngii peroxidase (allelic variant MnPL2) could be successfully expressed and secreted in A. nidulans under the alcA promoter with the mnpl2 cDNA including its signal sequence, and electrophoretically homogeneous peroxidase MnPL2* was isolated with a final purification factor of 115. This recombinant protein exhibited the same molecular mass (determined by SDS-PAGE) of peroxidase MnPL2 before and after deglycosylation, as well as the same catalytic properties of the P. eryngii enzyme. The latter finding confirms that the enzyme produced by P. eryngii, the biochemical and molecular characteristics of which have been recently reported (19), represents a new type of versatile peroxidase sharing catalytic properties of both LiP and MnP of P. chrysosporium. Site-directed mutagenesis studies are in progress, to confirm the involvement of several key residues in the catalytic properties of this enzyme, by using the heterologous expression system described here.
Acknowledgments
We thank M. A. Peñalva (CIB, CSIC, Madrid, Spain) for valuable suggestions and for providing the A. nidulans strain and the plasmid palcA1. J. Varela and A. Díaz contributed to protein and DNA sequencing, respectively.
This work was partially supported by the Agro-Industry programme of the EU and the Spanish Biotechnology programme.
REFERENCES
- 1.Andersen, H. D., E. B. Jensen, and K. G. Welinder. 1992. A process for producing heme proteins. Patent internal publication number WO 92/16634.
- 2.Camarero S, Böckle B, Martínez M J, Martínez A T. Manganese-mediated lignin degradation by Pleurotus pulmonarius. Appl Environ Microbiol. 1996;62:1070–1072. doi: 10.1128/aem.62.3.1070-1072.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camarero S, Sarkar S, Ruiz-Dueñas F J, Martínez M J, Martínez A T. Description of a versatile peroxidase involved in natural degradation of lignin that has both Mn-peroxidase and lignin-peroxidase substrate binding sites. J Biol Chem. 1999;274:10324–10330. doi: 10.1074/jbc.274.15.10324. [DOI] [PubMed] [Google Scholar]
- 4.Cove D J. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim Biophys Acta. 1966;113:51–56. doi: 10.1016/s0926-6593(66)80120-0. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-Cañón J M, Peñalva M A. Overexpression of two penicillin structural genes in Aspergillus nidulans. Mol Gen Genet. 1995;246:110–118. doi: 10.1007/BF00290139. [DOI] [PubMed] [Google Scholar]
- 6.Glenn J K, Morgan M A, Mayfield M B, Kuwahara M, Gold M H. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983;114:1077–1083. doi: 10.1016/0006-291x(83)90672-1. [DOI] [PubMed] [Google Scholar]
- 7.Heinfling A, Martínez M J, Martínez A T, Bergbauer M, Szewzyk U. Purification and characterization of peroxidases from the dye-decolorizing fungus Bjerkandera adusta. FEMS Microbiol Lett. 1998;165:43–50. doi: 10.1111/j.1574-6968.1998.tb13125.x. [DOI] [PubMed] [Google Scholar]
- 8.Heinfling A, Ruiz-Dueñas F J, Martínez M J, Bergbauer M, Szewzyk U, Martínez A T. A study on reducing substrates of manganese-oxidizing peroxidases from Pleurotus eryngii and Bjerkandera adusta. FEBS Lett. 1998;428:141–146. doi: 10.1016/s0014-5793(98)00512-2. [DOI] [PubMed] [Google Scholar]
- 9.Kuwahara M, Glenn J K, Morgan M A, Gold M H. Separation and characterization of two extracellular H2O2-dependent oxidases from ligninolytic cultures of Phanerochaete chrysosporium. FEBS Lett. 1984;169:247–250. [Google Scholar]
- 10.Lockington R A, Sealy-Lewis H M, Scazzocchio C, Davies R W. Cloning and characterization of the ethanol utilization regulon in Aspergillus nidulans. Gene. 1985;33:137–149. doi: 10.1016/0378-1119(85)90088-5. [DOI] [PubMed] [Google Scholar]
- 11.Maione T E, Javahaerian K, Belew M A, Gómez L E, Farrell R L. Activities of recombinant ligninase. Colloq INRA. 1987;40:177–183. [Google Scholar]
- 12.Martínez M J, Ruiz-Dueñas F J, Guillén F, Martínez A T. Purification and catalytic properties of two manganese-peroxidase isoenzymes from Pleurotus eryngii. Eur J Biochem. 1996;237:424–432. doi: 10.1111/j.1432-1033.1996.0424k.x. [DOI] [PubMed] [Google Scholar]
- 13.Mester T, Field J A. Characterization of a novel manganese peroxidase-lignin peroxidase hybrid isozyme produced by Bjerkandera species strain BOS55 in the absence of manganese. J Biol Chem. 1998;273:15412–15417. doi: 10.1074/jbc.273.25.15412. [DOI] [PubMed] [Google Scholar]
- 14.Paifer E, Margolles E, Cremata J, Montesino R, Herrera L, Delgado J M. Efficient expression and secretion of recombinant α-amylase in Pichia pastoris using two different signal sequences. Yeast. 1994;10:1415–1419. doi: 10.1002/yea.320101104. [DOI] [PubMed] [Google Scholar]
- 15.Pease E A, Andrawis A, Tien M. Manganese-dependent peroxidase from Phanerochaete chrysosporium. Primary structure deduced from cDNA sequence. J Biol Chem. 1989;264:13531–13535. [PubMed] [Google Scholar]
- 16.Piontek K, Glumoff T, Winterhalter K. Low pH crystal structure of glycosylated lignin peroxidase from Phanerochaete chrysosporium at 2.5 Å resolution. FEBS Lett. 1993;315:119–124. doi: 10.1016/0014-5793(93)81146-q. [DOI] [PubMed] [Google Scholar]
- 17.Poulos T L, Edwards S L, Wariishi H, Gold M H. Crystallographic refinement of lignin peroxidase at 2 Å. J Biol Chem. 1993;268:4429–4440. doi: 10.2210/pdb1lga/pdb. [DOI] [PubMed] [Google Scholar]
- 18.Pribnow D, Mayfield M B, Nipper V J, Brown J A, Gold M H. Characterization of a cDNA encoding a manganese peroxidase, from the lignin-degrading basidiomycete Phanerochaete chrysosporium. J Biol Chem. 1989;264:5036–5040. [PubMed] [Google Scholar]
- 19.Ruiz-Dueñas F J, Martínez M J, Martínez A T. Molecular characterization of a novel peroxidase isolated from the ligninolytic fungus Pleurotus eryngii. Mol Microbiol. 1999;31:223–236. doi: 10.1046/j.1365-2958.1999.01164.x. [DOI] [PubMed] [Google Scholar]
- 20.Sarkar S, Martínez A T, Martínez M J. Biochemical and molecular characterization of a manganese peroxidase isoenzyme from Pleurotus ostreatus. Biochim Biophys Acta. 1997;1339:23–30. doi: 10.1016/s0167-4838(96)00201-4. [DOI] [PubMed] [Google Scholar]
- 21.Smith A T, Santama N, Dacey S, Edwards M, Bray R C, Thorneley R N F, Burke J F. Expression of a synthetic gene for horseradish peroxidase C in Escherichia coli and folding and activation of the recombinant enzyme with Ca2+ and heme. J Biol Chem. 1990;265:13335–13343. [PubMed] [Google Scholar]
- 22.Stewart P, Whitwam R E, Kersten P J, Cullen D, Tien M. Efficient expression of a Phanerochaete chrysosporium manganese peroxidase gene in Aspergillus oryzae. Appl Environ Microbiol. 1996;62:860–864. doi: 10.1128/aem.62.3.860-864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundaramoorthy M, Kishi K, Gold M H, Poulos T L. The crystal structure of manganese peroxidase from Phanerochaete chrysosporium at 2.06-Å resolution. J Biol Chem. 1994;269:32759–32767. [PubMed] [Google Scholar]
- 24.Tien M, Kirk T K. Lignin-degrading enzyme from the hymenomycete Phanerochaete chrysosporium Burds. Science. 1983;221:661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- 25.Tien M, Tu C-P D. Cloning and sequencing of a cDNA for a ligninase from Phanerochaete chrysosporium. Nature. 1987;326:520–523. doi: 10.1038/326520a0. [DOI] [PubMed] [Google Scholar]
- 26.Tilburn J, Scazzocchio C, Taylor G G, Zabicky-Zissman J H, Lockington R A, Davies R W. Transformation by integration in Aspergillus nidulans. Gene. 1983;26:205–221. doi: 10.1016/0378-1119(83)90191-9. [DOI] [PubMed] [Google Scholar]