Abstract
Severe coronavirus (COVID-19) infection has been reportedly associated with a high risk of thromboembolism. Developing macrovascular thrombotic complications, including myocardial injury/infarction, venous thromboembolism, and stroke have been observed in one-third of severe COVID-19 hospitalized patients, leading to an increase in mortality and morbidity. The diagnosis of COVID-19 associated coagulopathy may be challenging because there are close similarities between pulmonary embolism and severe COVID-19 disease. Therefore, a critical step in improving the clinical outcome of patients with hospitalized COVID-19 is the recognition of coagulation abnormalities and the identification of patients with poor prognoses, prophylactic guidance, or antithrombotic therapy. Prescribing anticoagulants in all patients hospitalized with COVID-19 and 2–6 weeks post-hospital discharge in the absence of contraindications is recommended by most consensus documents published on behalf of professional societies. However, a decision on some variable factors such as intensity and duration of anticoagulation may be made based on an individual case and needs future randomized trial studies. Regarding little information on this subject, this study aims to review how inflammation and thrombosis are related to COVID-19 patients, discuss the types of thrombosis in these patients, and summarize the diagnosis and treatment of thrombosis in COVID19 patients.
Keywords: COVID-19, Thrombosis, Anticoagulation, Inflammation
1. Introduction
The coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that can lead to the thromboinflammatory disease and plays a critical role in the pathophysiology. This virus is a new ribonucleic acid (RNA) virus strain [1] which was declared a pandemic by the World Health Organization (WHO) in March 2020. The strains of this virus (sequence identity of 99.98%) were classified into two haplogroups (A and B), one of which is predominant worldwide, and the second is reported in America and Asia more than in Africa and Europe [2].
The spike-like protein (S protein) of SARS-CoV-2 can enter cells through angiotensin-converting enzyme 2 (ACE-2). This enzyme plays a vital role in viral proliferation and is mainly found on the alveolar epithelium and endothelium [3]. The expression of ACE2 in different organs is related to the panoply of clinical systemic symptoms of COVID-19, such as respiratory, hepatic, renal, gastrointestinal, and cardiac damage [4]. The down- and upregulation of ACE2 can play a critical role in hypertension, heart failure, overall cardiovascular disease (CVD), and myocardial infarction [5].
There are clinical spectra of COVID-19 ranging from mild to critically ill cases. Patients who have chronic respiratory disease, old age, and coexisting with medical conditions can be the principal target of the virus [6], [7]. Moreover, other factors such as infection and bedridden can increase the risk of venous thromboembolism (VTE) [8], [9]. Deep vein thrombosis (DVT) or fatal pulmonary thromboembolism (PTE) in COVID-19 patients causes the treatment to be challenging and patients with VTE may have a worse clinical outcome. Therefore, understanding the mechanism taking part in the severity of COVID-19 and mortality is important to identify and treat patients with the highest thromboembolic risk and mortality. This study aims to investigate and explain the relationship between inflammation and thrombosis and summarize the prophylaxis, diagnosis, and treatment of thromboembolism in patients affected by COVID-19.
2. Research method
This study involved articles and relevant data investigating the thrombosis and blooding in patients affected by COVID-19 and the prophylaxis, diagnosis, and treatment of thromboembolism in these patients. Information that was published until March 1, 2022, was collected by searching in PubMed/MEDLINE, Google Scholar, Scopus, Clinical trials. gov website, Cochrane Library, and valid encyclopedias. The keywords including “deep vein thrombosis”, OR “Coagulopathy”, “Disseminated intravascular coagulation,” ”venous thromboembolism“, and ”hemostasis“ were combined with the keywords including “SARS-CoV-2” OR “COVID-19” to be used as search terms. All kinds of the research project,comments, editorials, reviews, letters, and eBooks that have been published were included. In addition, preprint databases (Preprints.org, biorxiv.org) were also searched for papers accepted but not yet published. Google Chrome's built-in translation tool was used to review from other languages to English language studies.
3. Results
3.1. Thrombosis and inflammation
3.1.1. How does thrombosis occur?
The vascular injury that causes clotting has several steps, including activation of the coagulation cascade and formation of a platelet plug. Components such as polymorphonuclear cells, endothelial cells, microparticles, and complement systems are involved in this complex process [10]. The coagulation cascade is divided into two pathways which converge to form fibrin as the final common pathway product. To form and expand the clot, the fibrin can entangle platelets and other cellular elements [10]. Several agonists play a role in platelet activation, including thrombin via protease-activated receptors (PAR) 1 and 4 and collagen via glycoprotein (GP) VI receptors. The first coagulation cascade, the contact activation pathway, caused intrinsic activation of the coagulation cascade via initial activation of FXII, followed by sequential activation and amplification of FXI, followed by FIX activation [11]. Typically, trauma to tissue and endothelial cell activation can induce the extrinsic coagulation cascade, known as the tissue factor (TF) pathway [12]. The expression of TF in vascular cells is upregulated after pathologic conditions, such as endothelial cell damage or endothelial cell activation [13]. Both two coagulation cascade pathways converge to thrombin that can convert fibrinogen to stabilize the fibrin clot, which rapidly entangles platelets leading to clot propagation [14]. Activation of platelet is the most prominent mechanism [15], whereas activation of the coagulation cascade is predominate in the venous circulation [16].
3.1.2. Pathophysiology of thrombosis and inflammation in COVID19
After SARS-CoV-2 infection, viral-dependent mechanisms such as invasion of endothelial and alveolar epithelial cells and viral-independent mechanisms including immunological damage such as perivascular inflammation can lead to the breakdown of the endothelial–epithelial barrier [17]. Then, the monocytes and neutrophils are invasive and extravasation of a protein-rich exudate into the alveolar space, consistent with other forms of survivors of acute respiratory distress syndrome [18]. A previous study demonstrated that increasing levels of cytokines such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, and IL-2R were observed in severe COVID-19-associated systemic inflammation [19]. The outcomes of severe COVID-19 were associated with increased levels of IL-6 [20]. The virus infection can induce acute inflammation that is associated with prothrombotic changes in fibrin clot dynamics, including increased clot turbidity of fibrin and strand density [21]. Therefore, levels of markers such as fibrinogen and D-dimer may be elevated [22]. During inflammation, not only the promotion of leukocyte recruitment occurs but also coagulation and platelet activation, leading to increasing thrombin [23], [24]. Severe inflammation can also increase prothrombotic tendency, leading to disseminated intravascular coagulation. These results can decrease detectable clotting factors as these are consumed rapidly, therefore reducing hemostatic function and increasing bleeding risk [25]. Inflammation impacting local hemodynamics, arterial circulation, and plaque stability can probably trigger thrombosis [26]. Inflammation in the arterial circulation can drive atheromatous plaque progression, which can trigger thrombosis via impacting local hemodynamics, plaque stability, and plaque rupture or erosion events [27]. Also, inflammation can induce an adverse effect on the function of endothelial such as decreasing in the activity of the antithrombotic factors, tissue factor pathway inhibitor, and protein C, as well as increasing in the release of von Willebrand factor (vWF), which facilitates platelet-endothelium and platelet-platelet binding [27].
4. Thrombosis in COVID19 patients
4.1. Microvascular complications
Pathological occlusion of arterioles, capillaries, and venules (microvessels) by platelet- and/or fibrin-rich thrombi is known as microvascular thrombosis [28]. Diagnostically, the development of microvascular thrombosis is a challenge because the microthrombi have a small size (often ≤ 10 μm) that is difficult to be visualized; there is no specific biomarker to detect microthrombi, and occurring microthrombi is only transiently [28]. Clinically, microvessel occlusion can occur in ischemia with the effects ranging from alterations in plasma coagulation markers to severe multiorgan failure [29].
Given the possible relationship between microvascular thrombosis and organ failure, it seems that microvascular thrombosis plays an important role in the course of COVID-19 [30]. Proximally, one-third of hospitalized severe COVID-19 patients develop macrovascular thrombotic complications, which are associated with an increased risk of hospital mortality [31]. In many severe COVID-19 patients, coagulation abnormalities that mimic thrombotic microangiopathy or DIC were observed; whereas coagulopathy associated-COVID-19 has distinct features [32]. The autopsy series of patients who died due to severe COVID-19 showed the presence of diffuse microthrombosis and hemorrhage along with abundant intravascular megakaryocytes in all major organs, including the heart, lungs, liver, and kidneys [33]. The lung and skin damage consistent with complement-mediated microvascular injury has also been reported [34].
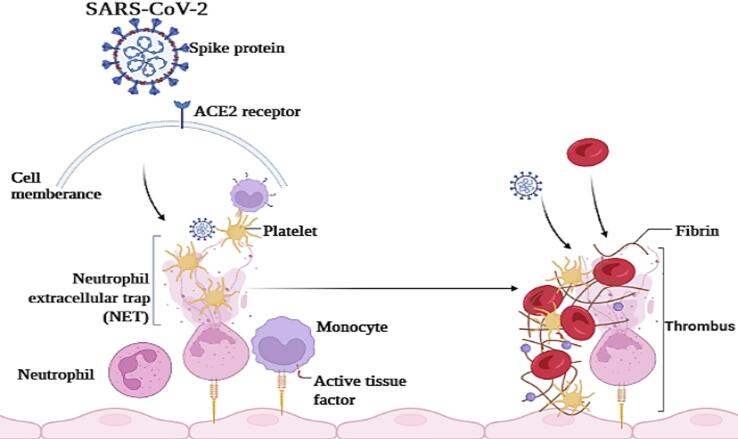
Fig. 1 shows the COVID- 19-associated coagulopathy mechanisms. SARS-CoV-2 enters the target cells via activation of ACE2 receptors on the surface of the lung, arterial, and venous epithelial cells [35]. The viral infection can induce prothrombotic gene expression injury and causes direct dysfunction and endothelial injury [36]. This viral infection has a direct effect on host innate immune responses, such as aggravation of endothelial injury, microvascular thrombosis, massive local release of proinflammatory cytokines, activation of monocytes and complements cascade, and aggravation of endothelial injury [37]. The neutrophils and tissue factor (TF) are major mediators of immunothrombosis. After activation of monocytes and monocyte-derived extracellular vesicles via TF, the process of thrombosis begins [38]. Then, the release of neutrophil extracellular traps (NETs) and neutrophil elastase can stabilize microthrombi, which immobilize inflammatory cells and promote intravascular fibrin formation via degradation of TF antagonist, a tissue factor pathway inhibitor [39]. The virus infection and NETs can directly activate platelet via toll-like receptors on platelets and other cells. Therefore, it integrates inflammation, pulmonary infection, and thrombosis [40], [41]. Some evidence demonstrated that platelets and megakaryocytes may have viral receptors that could be infected by earlier SARS-CoV [42], [43]; however, more accurate future studies are needed for more accurate conclusions.
Fig. 1.
View of the pathophysiological mechanism of coagulopathy in COVID-19 patients. First, the viral infections can injure host endothelial cells, then abnormal blood flow dynamics and uncontrolled platelet activation occurs.
4.2. Arterial thrombosis
In patients with COVID-19, increased thrombotic events such as stroke and acute myocardial infarction have been reported. It seems that 2.7 to 3.8% of COVID-19 patients have a stroke [44], [45], [46]. Acute infection of SARS-CoV-1 may induce a hypercoagulable state, which along with cardiac dysfunction and hypotension give rise to cerebral arterial thromboembolism [14]. After the ischemic and hemorrhagic stroke, the course of COVID-19 in patients can get more complicated [47]. Pre-existing cardiovascular risk in patients with COVID-19 is often associated with an acute stroke which is a risk factor and a negative prognostic factor [48], [49]. The presence of a prothrombotic state or a vasculitis-like mechanism may explain how SARS-CoV-2 infection can trigger stroke in the host [47]. Myocardial damage associated with increased troponin levels may be observed in 7–17% of COVID-19 patients admitted to the general ward and in 22–31% of those admitted to the ICU [19], [31], [50]. Also, the COVID-19 patients with myocardial injury showed higher in-hospital mortality than patients with cardiovascular disease (CVD) but without myocardial injury. In addition, the mortality increased even if the myocardial injury was present in COVID-19 patients with pre-existing CVD [51]. It seems that some factors such as cytokine storm, viral myocarditis, stress-induced cardiomyopathy, classic myocardial infarction due to infection-induced atherosclerotic plaque instability, and microangiopathy can explain the relationship between myocardial injury and underlying CVD with COVID-19 [52], [53]. Therefore, urgent reperfusion and frequent lack of standard procedures to perform reperfusion are essential to ensure the best care for COVID-19 patients with acute ischemic stroke and acute myocardial infarction.
4.3. Venous thromboembolism
Many risk factors for venous thromboembolism (VTE) such as older age, obesity, the level of ICU care, and immobility are existed for hospitalized patients with COVID-19. However, it is confirmed that severe SARS-CoV-2 infection is associated with an inherently increased risk of thromboembolic complications and coagulopathy [54]. The relative lack of consumptive coagulopathy in patients with COVID-19 may explain why these patients are more prothrombotic rather than disease evolution into a bleeding propensity due to hyperfibrinolysis [55]. Also, after SARS-CoV- 2 infections, a predilection for thrombotic microangiopathy to affect the lung vasculature rather than widespread systemic organ damage from microthrombosis was observed [33], [56].
In COVID-19 patients, the elevation of D-dimer as a fibrin degradation product may one of the earlier manifestations of the perturbed coagulation cascade [57]. The D-dimer is a marker of coagulation cascade activation in the microvascular beds and is elevated in 46% of SARS-CoV-2-infected patients and 56% of those with severe disease [58]. Increasing levels of D-dimer can be directly related to COVID-19 in-hospital mortality [31], [59]. In addition, in the setting of COVID-19 infection, elevated D-dimer levels as the surrogate marker for the thrombotic burden were reported in nearly all cases of VTE [60], [61], [62], [63]. Elevated D-dimer concentration and thrombotic microangiopathy in pulmonary vessels of patients with COVID-19 can raise the concern of pulmonary embolism (PE) as a reason for acute respiratory failure. Acute PE has the highest rate in severely COVID-19 patients admitted to the intensive care unit (ICU) [44], [64]. However, regarding the high heterogeneity observed between the results of studies, further high-quality prospective research is necessary to obtain a more accurate conclusion.
5. Diagnosis of thrombosis in COVID19 patients
For the correct and timely diagnosis of thrombosis and thromboembolic complications in patients hospitalized with COVID-19, a combination of patient history, results of laboratory tests reflecting hemostasis, physical examination findings, and/or results of focused imaging investigations must be considered [65]. Imaging studies such as bilateral compression ultrasonography of the legs and/or bedside point of care cardiac ultrasonography can be used to detect proximal DVT, myocardial thrombus, clot-in-transit to the pulmonary trunk, or right ventricular dilatation in COVID-19 patients with high suspicion of thrombosis can use [66], [67]. However, to fully exclude thrombosis, it is not enough to just trust the negative results of these tests [67]. A normal D-dimer test with high-sensitivity assays and a CTPA may be reasonable tools for the diagnosis of thrombosis in COVID-19 patients [65]. In patients with COVID-19 and critical patients with DIC and consumptive coagulopathy, the initial prothrombotic cascade is similar. However, the clear differences between COVID-19-associated coagulopathy and patients that have classical DIC are elevations in D-dimer and fibrin/fibrinogen degradation product levels in COVID-19 patients while thrombocytopenia and prolongations of activated partial thromboplastin time (aPTT) and/or prothrombin time (PT) are less common or usually mild [68].
6. Some methods of prevention and treatment of thrombosis in COVID19 patients
In hospitalized COVID-19 patients, early vigilant monitoring of coagulation abnormalities is a critical measure because it can help to identify patients at a high risk of poor prognosis, improve patients’ clinical outcomes, and guide antithrombotic prophylaxis or treatment to prevent thromboembolic complications. The elevated D-dimer in COVID-19 patients is a prognostic value for the severe course of COVID-19 infection which can predict thrombotic events, acute kidney injury, and death [69]. Generally, in severe COVID-19 patients (ICU care), a worsening trend of coagulation parameters can indicate the development of cytokine storm, impending clinical deterioration, and progression to multiorgan failure [70]. In severe COVID-19 patients, standard laboratory and viscoelastic tests, increased platelet and fibrinogen contribution to CS, elevated D-dimer levels revealed increased clot strength (CS), and hyperfibrinogenemia can be a good procoagulant pattern [71].
Since cross-link between viral infection-induced inflammatory response and thrombosis, known as infection-induced thromboinflammation, levels of C-reactive protein (CRP), IL-6, factor VIII, vWF, and plasma viscosity are increased in COVID-19 patients, while antithrombin is modestly decreased [71], [72].
To identify hospitalized COVID-19 patients at a high risk of poor in-hospital outcomes, or to triage patients that would require hospital admission and close monitoring, measuring of D-dimer levels, PT/aPTT, thrombocyte count, and fibrinogen levels (if assays are available) seem to be necessary [73], [74]. In the absence of contraindications for controlling thrombosis, the use of prophylactic doses of anticoagulants in all patients hospitalized with COVID-19 is recommended by most of the guidelines and consensus documents published on behalf of professional societies, focusing on hemostasis and thrombosis advocates [73], [75]. Also, in hospitalized COVID-19 patients especially.
those with no bleeding risk factors and with high VTE risk for 2 to 6 weeks post-hospital discharge, administration of thromboprophylaxis with low molecular weight heparin (LMWH) or a non-vitamin K antagonist oral anticoagulants is recommended [44], [46], [76]. Likewise, some available documents support that the use of intermediate-dose LMWH in selected severe COVID-19 patients may balance thrombotic and bleeding risk [77], [78].
7. Conclusion and perspective
Recent studies have provided a growing understanding of the pattern of COVID-19 in immunology, epidemiology, and subsequent coagulopathy and coagulopathy treatment strategies. Severe COVID-19 patients can lead to thromboinflammatory disease and play a critical role in the pathophysiology and clinical deterioration of these patients [79]. Severe COVID-19 can lead to thromboinflammatory disease and play a critical role in the pathophysiology and clinical deterioration of these patients [80]. Most evidence is limited by small retrospective studies. Therefore, it is necessary to evaluate the true prevalence of thrombosis in COVID-19 in more studies. Given that, a consecutive increase in D-dimer may indicate the presence of thrombosis in patients with severe COVID-19. In addition, designation of the exact role of biomarkers such as.
D-dimer and/or scoring systems to stratify patients' risk, establishing the optimal thromboprophylaxis strategy and designing adequate and feasible diagnostic protocols for venous thrombosis (DVT)/pulmonary embolism (PE) can be added to standard reporting criteria in order to facilitate future research. There is a need for markers to identify the increased risk of thrombotic events at an early stage of COVID-19 and to prevent thrombotic events and organ damage as far as possible. In severe COVID-19 patients, it is better to use prophylactic anticoagulation with LMWHs to prevent thrombotic events and counteract the pro-inflammatory influence of cytokines and other factors. The future randomized trial regarding the appropriate antithrombotic prophylaxis and treatment results may help to individualize the treatment of thrombotic in COVID-19 patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X.i., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gómez-Carballa A., Bello X., Pardo-Seco J., Martinón-Torres F., Salas A. Mapping genome variation of SARS-CoV-2 worldwide highlights the impact of COVID-19 super-spreaders. Genome Res. 2020;30(10):1434–1448. doi: 10.1101/gr.266221.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mori J., Oudit G.Y., Lopaschuk G.D. SARS-CoV-2 perturbs the renin-angiotensin system and energy metabolism. Am. J. Physiol.-Endocrinol. Metab. 2020;319(1):E43–E47. doi: 10.1152/ajpendo.00219.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.-C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceriello A., Standl E., Catrinoiu D., Itzhak B., Lalic N.M., Rahelic D., Schnell O., Škrha J., Valensi P. Issues of cardiovascular risk management in people with diabetes in the COVID-19 era. Diabetes Care. 2020;43(7):1427–1432. doi: 10.2337/dc20-0941. [DOI] [PubMed] [Google Scholar]
- 6.Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. The Lancet Respiratory Medicine. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adao R., Guzik T.J. Inside the heart of COVID-19. Cardiovasc. Research. 2020 doi: 10.1093/cvr/cvaa086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Streiff M.B., Agnelli G., Connors J.M., Crowther M., Eichinger S., Lopes R., McBane R.D., Moll S., Ansell J. Guidance for the treatment of deep vein thrombosis and pulmonary embolism. J. Thromb. Thrombolysis. 2016;41(1):32–67. doi: 10.1007/s11239-015-1317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson F.A., Jr, Spencer F.A. Risk factors for venous thromboembolism. Circulation. 2003;107(23_suppl_1):I-9-I-16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 10.Tubaro M., Vranckx P., Price S., Vrints C., Bonnefoy E., editors. The ESC Textbook of Intensive and Acute Cardiovascular CareThe ESC Textbook of Intensive and Acute Cardiovascular Care. Oxford University Press; 2021. [Google Scholar]
- 11.Neubauer K., Zieger B. Endothelial cells and coagulation. Cell Tissue Res. 2021:1–8. doi: 10.1007/s00441-021-03471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackman N., Tilley R.E., Key N.S. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2007;27(8):1687–1693. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 13.Butenas S., Orfeo T., Mann K.G. Tissue factor activity and function in blood coagulation. Thromb. Res. 2008;122:S42–S46. doi: 10.1016/S0049-3848(08)70018-5. [DOI] [PubMed] [Google Scholar]
- 14.Kamel M.H., Yin W., Zavaro C., Francis J.M., Chitalia V.C. Hyperthrombotic milieu in COVID-19 patients. Cells. 2020;9(11):2392. doi: 10.3390/cells9112392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson S.P. Arterial thrombosis—insidious, unpredictable and deadly. Nat. Med. 2011;17(11):1423–1436. doi: 10.1038/nm.2515. [DOI] [PubMed] [Google Scholar]
- 16.Koupenova M., Kehrel B.E., Corkrey H.A., Freedman J.E. Thrombosis and platelets: an update. Eur. Heart J. 2017;38(11):785–791. doi: 10.1093/eurheartj/ehw550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G.F., Bernstein E.J., Mohan S., Beckley A.A., Seres D.S., Choueiri T.K., Uriel N., Ausiello J.C., Accili D., Freedberg D.E., Baldwin M., Schwartz A., Brodie D., Garcia C.K., Elkind M.S.V., Connors J.M., Bilezikian J.P., Landry D.W., Wan E.Y. Post-acute COVID-19 syndrome. Nat. Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huppert L., Matthay M., Ware L. Pathogenesis of acute respiratory distress syndrome, Seminars in respiratory and critical care medicine. Thieme Medical Publishers. 2019;40(01):031–039. doi: 10.1055/s-0039-1683996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.G. Chen, D. Wu, W. Guo, Y. Cao, D. Huang, H. Wang, T. Wang, X. Zhang, H. Chen, H. Yu, Clinical and immunological features of severe and moderate coronavirus disease 2019, The Journal of clinical investigation 130(5) (2020) 2620-2629. [DOI] [PMC free article] [PubMed]
- 20.Herold T., Jurinovic V., Arnreich C., Lipworth B.J., Hellmuth J.C., von Bergwelt-Baildon M., Klein M., Weinberger T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. Journal of Allergy and Clinical Immunology. 2020;146(1):128–136.e4. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas M.R., Outteridge S.N., Ajjan R.A., Phoenix F., Sangha G.K., Faulkner R.E., Ecob R., Judge H.M., Khan H., West L.E., Dockrell D.H., Sabroe I., Storey R.F. Platelet P2Y12 inhibitors reduce systemic inflammation and its prothrombotic effects in an experimental human model. Arterioscler. Thromb. Vasc. Biol. 2015;35(12):2562–2570. doi: 10.1161/ATVBAHA.115.306528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiers D., van der Heijden W.A., van Ede L., Gerretsen J., de Mast Q., van der Ven A.J., el Messaoudi S., Rongen G.A., Gomes M., Kox M., Pickkers P., Riksen N.P. A randomised trial on the effect of anti-platelet therapy on the systemic inflammatory response in human endotoxaemia. Thromb. Haemost. 2017;117(09):1798–1807. doi: 10.1160/TH16-10-0799. [DOI] [PubMed] [Google Scholar]
- 23.Petros S., Kliem P., Siegemund T., Siegemund R. Thrombin generation in severe sepsis. Thromb. Res. 2012;129(6):797–800. doi: 10.1016/j.thromres.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Chen D., Dorling A. Critical roles for thrombin in acute and chronic inflammation. J. Thromb. Haemost. 2009;7:122–126. doi: 10.1111/j.1538-7836.2009.03413.x. [DOI] [PubMed] [Google Scholar]
- 25.Levi M. Disseminated intravascular coagulation. Crit. Care Med. 2007;35(9):2191–2195. doi: 10.1097/01.ccm.0000281468.94108.4b. [DOI] [PubMed] [Google Scholar]
- 26.Zuriaga M.A., Fuster J.J. Clonal hematopoiesis and atherosclerotic cardiovascular disease: a primer. Clínica e Investigación en Arteriosclerosis. 2021 doi: 10.1016/j.arteri.2021.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Wolf D., Ley K. Immunity and inflammation in atherosclerosis. Circ. Res. 2019;124(2):315–327. doi: 10.1161/CIRCRESAHA.118.313591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfeiler S., Massberg S., Engelmann B. Biological basis and pathological relevance of microvascular thrombosis. Thromb. Res. 2014;133:S35–S37. doi: 10.1016/j.thromres.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Levi M. Disseminated intravascular coagulation: a disease-specific approach, Seminars in thrombosis and hemostasis. © Thieme Medical Publishers. 2010;36(04):363–365. doi: 10.1055/s-0030-1254045. [DOI] [PubMed] [Google Scholar]
- 30.Becker R.C. COVID-19 update: Covid-19-associated coagulopathy. J. Thromb. Thrombolysis. 2020;50(1):54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. The Lancet Haematol. 2020;7(6):e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Brown J.Q., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir. Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Translational Research. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamming I., Timens W., Bulthuis M., Lely A., Navis G.V., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol.: A J. Pathol. Soc. Great Britain Ireland. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang D.e., Han Z., Oppenheim J.J. Alarmins and immunity. Immunol. Rev. 2017;280(1):41–56. doi: 10.1111/imr.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciceri F., Beretta L., Scandroglio A.M., Colombo S., Landoni G., Ruggeri A., Peccatori J., D'Angelo A., De Cobelli F., Rovere-Querini P. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Critical Care Resuscitation. 2020;22(2):95–97. doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Müller I., Klocke A., Alex M., Kotzsch M., Luther T., Morgenstern E., Zieseniss S., Zahler S., Preissner K., Engelmann B. Intravascular tissue factor initiates coagulation via circulating microvesicles and platelets. FASEB J. 2003;17(3):1–20. doi: 10.1096/fj.02-0574fje. [DOI] [PubMed] [Google Scholar]
- 39.Zuo Y.u., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., Blair C.N., Weber A., Barnes B.J., Egeblad M., Woods R.J., Kanthi Y., Knight J.S. Neutrophil extracellular traps in COVID-19. JCI insight. 2020 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li B., Liu Y., Hu T., Zhang Y., Zhang C., Li T., Wang C., Dong Z., Novakovic V.A., Hu T., Shi J. Neutrophil extracellular traps enhance procoagulant activity in patients with oral squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2019;145(7):1695–1707. doi: 10.1007/s00432-019-02922-2. [DOI] [PubMed] [Google Scholar]
- 41.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Daßler-Plenker J., Guerci P., Huynh C., Knight J.S., Loda M., Looney M.R., McAllister F., Rayes R., Renaud S., Rousseau S., Salvatore S., Schwartz R.E., Spicer J.D., Yost C.C., Weber A., Zuo Y.u., Egeblad M. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boukour S., Masse J.-M., Benit L., Dubart-kupperschmitt A., Cramer E.M. Lentivirus degradation and DC-SIGN expression by human platelets and megakaryocytes. J. Thromb. Haemost. 2006;4(2):426–435. doi: 10.1111/j.1538-7836.2006.01749.x. [DOI] [PubMed] [Google Scholar]
- 43.Rondina M.T., Brewster B., Grissom C.K., Zimmerman G.A., Kastendieck D.H., Harris E.S., Weyrich A.S. In vivo platelet activation in critically ill patients with primary 2009 influenza A (H1N1) Chest. 2012;141(6):1490–1495. doi: 10.1378/chest.11-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., Kucher N., Studt J.-D., Sacco C., Bertuzzi A., Sandri M.T., Barco S. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas W., Varley J., Johnston A., Symington E., Robinson M., Sheares K., Lavinio A., Besser M. Thrombotic complications of patients admitted to intensive care with COVID-19 at a teaching hospital in the United Kingdom. Thromb. Res. 2020;191:76. doi: 10.1016/j.thromres.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morassi M., Bagatto D., Cobelli M., D’Agostini S., Gigli G.L., Bnà C., Vogrig A. Stroke in patients with SARS-CoV-2 infection: case series. J. Neurol. 2020;267(8):2185–2192. doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y., Li M., Wang M., Zhou Y., Chang J., Xian Y., Wang D., Mao L., Jin H., Hu B. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vascular Neurol. 2020;5(3) doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nia A.M., Srinivasan V.M., Hayworth M.K., Lall R.R., Kan P. A history of cerebrovascular disease is independently associated with increased morbidity and mortality in patients with COVID-19: a cohort study of 369,563 COVID-19 cases in the USA. Cerebrovasc. Dis. 2022;51(1):18–26. doi: 10.1159/000517499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang D., Hu B.o., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan. China, Jama. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi S., Qin M.u., Shen B.o., Cai Y., Liu T., Yang F., Gong W., Liu X.u., Liang J., Zhao Q., Huang H.e., Yang B.o., Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tersalvi G., Vicenzi M., Calabretta D., Biasco L., Pedrazzini G., Winterton D. Elevated troponin in patients with coronavirus disease 2019: possible mechanisms. J. Cardiac Fail. 2020;26(6):470–475. doi: 10.1016/j.cardfail.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sala S., Peretto G., Gramegna M., Palmisano A., Villatore A., Vignale D., De Cobelli F., Tresoldi M., Cappelletti A.M., Basso C. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur. Heart J. 2020;41(19):1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bertoletti L., Couturaud F., Montani D., Parent F., Sanchez O. Venous thromboembolism and COVID-19. Venous thromboembolism and COVID-19, Elsevier. 2020;78:100759. doi: 10.1016/j.resmer.2020.100759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Angelini D.E., Kaatz S., Rosovsky R., Zon R.L., Pillai S., Robertson W.E., Elavalakanar P., Patell R., Khorana A. COVID-19 and venous thromboembolism: a narrative review. Research Pract. Thrombosis Haemostasis. 2022;6(2) doi: 10.1002/rth2.v6.210.1002/rth2.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sathe P.M., Patwa U.D. D Dimer in acute care, International Journal of Critical Illness and Injury. Science. 2014;4(3):229. doi: 10.4103/2229-5151.141435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guan W.-J., Ni Z.-y., Hu Y.u., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S.C., Du B., Li L.-J., Zeng G., Yuen K.-Y., Chen R.-C., Tang C.-l., Wang T., Chen P.-Y., Xiang J., Li S.-Y., Wang J.-L., Liang Z.-J., Peng Y.-X., Wei L.i., Liu Y., Hu Y.-H., Peng P., Wang J.-M., Liu J.-Y., Chen Z., Li G., Zheng Z.-J., Qiu S.-Q., Luo J., Ye C.-J., Zhu S.-Y., Zhong N.-S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xie Y., Wang X., Yang P., Zhang S. COVID-19 complicated by acute pulmonary embolism, Radiology: Cardiothoracic. Imaging. 2020;2(2):e200067. doi: 10.1148/ryct.2020200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marone E.M., Rinaldi L.F. Upsurge of deep venous thrombosis in patients affected by COVID-19: preliminary data and possible explanations. Journal of Vascular Surgery: Venous and Lymphatic Disorders. 2020;8(4):694–695. doi: 10.1016/j.jvsv.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poggiali E., Bastoni D., Ioannilli E., Vercelli A., Magnacavallo A. Deep vein thrombosis and pulmonary embolism: two complications of COVID-19 pneumonia? Eur. J. Case Rep. Internal Med. 2020;7(5) doi: 10.12890/2020_001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Linkins L.-A., Takach Lapner S. Review of D-dimer testing: good, Bad, and Ugly. Int. J. Lab. Hematol. 2017;39:98–103. doi: 10.1111/ijlh.12665. [DOI] [PubMed] [Google Scholar]
- 64.Cattaneo M., Bertinato E.M., Birocchi S., Brizio C., Malavolta D., Manzoni M., Muscarella G., Orlandi M. Pulmonary embolism or pulmonary thrombosis in COVID-19? Is the recommendation to use high-dose heparin for thromboprophylaxis justified? Thromb. Haemost. 2020;120(08):1230–1232. doi: 10.1055/s-0040-1712097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gąsecka A., Borovac J.A., Guerreiro R.A., Giustozzi M., Parker W., Caldeira D., Chiva-Blanch G. Thrombotic complications in patients with COVID-19: pathophysiological mechanisms, diagnosis, and treatment. Cardiovasc. Drugs Ther. 2021;35(2):215–229. doi: 10.1007/s10557-020-07084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Parry A.H., Wani A.H. Pulmonary embolism in coronavirus disease-19 (COVID-19) and use of compression ultrasonography in its optimal management. Thromb. Res. 2020;192:36. doi: 10.1016/j.thromres.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Argulian E., Sud K., Vogel B., Bohra C., Garg V.P., Talebi S., Lerakis S., Narula J. Right ventricular dilation in hospitalized patients with COVID-19 infection. Cardiovasc. Imaging. 2020;13(11):2459–2461. doi: 10.1016/j.jcmg.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Connors J.M., Levy J.H. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. 2020;18(7):1559–1561. doi: 10.1111/jth.14849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J., Berger J.S. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA. 2020;324(8):799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M., Psaltopoulou T., Gerotziafas G., Dimopoulos M.A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ranucci M., Ballotta A., Di Dedda U., Baryshnikova E., Dei Poli M., Resta M., Falco M., Albano G., Menicanti L. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. 2020;18(7):1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Groß R., Conzelmann C., Müller J.A., Stenger S., Steinhart K., Kirchhoff F., Münch J. Detection of SARS-CoV-2 in human breastmilk. Lancet. 2020;395(10239):1757–1758. doi: 10.1016/S0140-6736(20)31181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marietta M., Ageno W., Artoni A., De Candia E., Gresele P., Marchetti M., Marcucci R., Tripodi A. COVID-19 and haemostasis: a position paper from Italian Society on Thrombosis and Haemostasis (SISET) Blood Transfusion. 2020;18(3):167. doi: 10.2450/2020.0083-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M., Clark C., Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J. Thromb. Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., Nigoghossian C.D., Ageno W., Madjid M., Guo Y. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paranjpe I., Fuster V., Lala A., Russak A.J., Glicksberg B.S., Levin M.A., Charney A.W., Narula J., Fayad Z.A., Bagiella E., Zhao S., Nadkarni G.N. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J. Am. Coll. Cardiol. 2020;76(1):122–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Desborough M.J.R., Doyle A.J., Griffiths A., Retter A., Breen K.A., Hunt B.J. Image-proven thromboembolism in patients with severe COVID-19 in a tertiary critical care unit in the United Kingdom. Thromb. Res. 2020;193:1–4. doi: 10.1016/j.thromres.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.J.-f. Xu, L. Wang, L. Zhao, F. Li, J. Liu, L. Zhang, Q. Li, J. Gu, S. Liang, Q. Zhao, Risk assessment of venous thromboembolism and bleeding in COVID-19 patients, (2020). [DOI] [PMC free article] [PubMed]