Abstract
Background:
Maternal HIV and antiretroviral therapy exposure in-utero may influence infant weight, but the contribution of maternal y body mass index (BMI) to early life overweight and obesity is not clear.
Objective:
To estimate associations between maternal BMI at entry to antenatal care and infant weight through approximately 1 year of age and to evaluate if associations were modified by maternal HIV status, maternal HIV and viral load, breast feeding intensity through 6 months or timing of entry into antenatal care.
Methods:
We followed HIV-uninfected and -infected pregnant women initiating efavirenz-based antiretroviral therapy from first antenatal visit through 12 months postpartum. Infant weight was assessed via World Health Organization BMI and weight-for-length (WL) z-scores at 6 weeks, 3, 6, 9, and 12 months. We used multivariable linear mixed effects models to estimate associations between maternal BMI and infant z-scores over time.
Results:
In 861 HIV-uninfected infants (454 HIV-exposed; 407 HIV-unexposed), nearly 20% of infants were overweight or obese by 12 months of age, regardless of HIV-exposure status. In multivariable analyses, increasing maternal BMI category was positively associated with higher infant BMIZ and WLZ scores between 6 weeks and 12 months of age and did not differ by HIV exposure status. However, HIV-exposed infants had slightly lower BMIZ and WLZ trajectories through 12 months of age, compared to HIV-unexposed infants across all maternal BMI categories. Differences in BMIZ and WLZ scores by HIV exposure were not explained by timing of entry into antenatal or maternal viral load pre-antiretroviral therapy initiation, but z-scores were slightly higher for HIV-exposed infants who were predominantly or exclusively versus partially breast fed.
Conclusions:
These findings suggest maternal BMI influences early infant weight gain, regardless of infant HIV-exposure status. Intervention to reduce maternal BMI may help to address growing concerns about obesity among HIV-uninfected children.
Keywords: BMI, HIV, HIV-exposed, infant weight, infant growth, South Africa
Background
The successful scale-up of antiretroviral therapy (ART) for prevention of mother-to-child transmission of HIV has led to a decline in pediatric HIV incidence globally, but introduced new potential health consequences for children exposed to, but uninfected with HIV (CEUH). In particular, concerns have been raised about the impact of HIV and ART exposure on infant growth, with inconsistent findings.1–5 Recently, in a South African cohort of HIV-uninfected breast fed infants whose mothers had access to universal efavirenz-based ART in pregnancy, we reported few differences in child growth outcomes between CEUH and children unexposed to HIV (CUH), but elevated levels of overweight/obesity at 12 months of age. These findings suggest that in South Africa, disparities in infant growth by HIV exposure status5 may be declining under polities of universal ART for women living with HIV (WLHIV), longer infant ART prophylaxis and continued breast feeding, 6, 7 but that childhood obesity may be an emerging issue.
Globally, rising levels of obesity in pregnancy have the potential to increase infant weight and obesity in early life. 8–10 Of the 14.8 million CEUH globally, nearly 90% live in sub-Saharan Africa where the prevalence of obesity in pregnancy is rapidly increasing.11, 12 In South Africa, up to 50% of pregnant women are affected by obesity and obesity in pregnancy has increased nearly 20% since 2004.13, 14 Obesity in pregnancy is well-known to increase the risk of obesity in childhood for CUH 9, 10, 15, but less is known about CEUH. 16–19 In addition, maternal viral load, an indicator of HIV severity and control, 20–22and breast feeding intensity and duration 23–27 may influence the relationship between maternal obesity and infant weight gain. As the number of CEUH expands across sub-Saharan Africa, there is a need to understand how maternal body mass index (BMI) influences early postnatal weight in the context of universal ART for WLHIV.
To address this gap and extend our previous findings, we examine associations between maternal BMI and infant weight through 12 months of age in a prospective cohort of breast fed CUH and CEUH exposed to efavirenz-based ART during pregnancy in South Africa. We examined whether trajectories of infant weight differed by maternal BMI overall, and by HIV-status, HIV status and maternal viral load at entry into antenatal care (ANC), and breast feeding status. We hypothesized that maternal BMI would influence infant weight trajectories for both CEUH and CUH.
Methods
Cohort Selection
Data come from two prospective cohort studies in Cape Town, South Africa of WLHIV and HIV-unexposed pregnant women and their infants from 2013–2015. Details of both cohorts have been published previously.1, 28, 29 Briefly, using similar inclusion/exclusion criteria, parallel cohorts of pregnant WLHIV who were initiating ART and pregnant HIV-uninfected women who were ≥18 years of age and presented for ANC in Gugulethu, an urban Black South African community in Cape Town, were enrolled. All women were followed from their first ANC visit through delivery. A sub-set of women breast feeding at any intensity at 6–28 days postpartum were enrolled for postpartum follow-up through 12 months.1, 28, 29 Starting in 2013, pregnant WLHIV were eligible for lifelong ART, regardless of CD4 count or WHO clinical stage.6 WLHIV initiated efavirenz-based ART (tenofovir 300 mg + emtricitabine 200 mg/lamivudine 300 mg + efavirenz 600 mg), the preferred first line regimen at the time of the study; 73% initiated ART on the same day as their first ANC visit.30
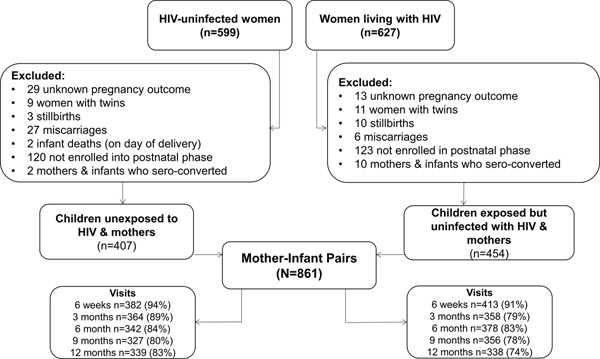
Participants were included in the present analysis if they were enrolled into postpartum follow-up, and had a live born, singleton, HIV-uninfected infant (Figure 1). All HIV-exposed infants were tested by study nurses at 6 and 48 weeks of age using HIV-PCR testing (Roche COBAS AmpliPrep/COBAS TaqMan HIV-1 qualitative assay; Roche Molecular systems, Branchburg, NJ, USA).29 Women who tested negative for HIV at their first ANC visit were re-tested for HIV in the third trimester when feasible, and approximately every 3 months while breast feeding as part of routine clinical care. If women seroconverted, infants were tested for HIV and both women and infants were referred to HIV care services for further management.
Figure 1.
Study Population
Exposure
The exposure of interest was maternal BMI at entry into ANC (range 4–39 weeks’ gestation, IQR 14, 26 weeks). Maternal BMI was categorized as underweight (<18.5 kg/m2), normal (18.50<25.0 kg/m2), overweight (25.0 < 30.0 kg/m2), or obese (≥ 30.0 kg/m2) according to WHO standards. For WLHIV, BMI was assessed prior to ART initiation. Due to the small number of underweight women (n=9), underweight and normal BMI categories were combined in statistical analyses.
Outcomes
The outcomes of interest were infant weight and overweight/obesity status through approximately 1 year of age. Infant anthropometry was measured at 6 weeks of age and every 3 months between 3 months and 12 months of age by trained study staff using standardized protocols based on WHO guidelines.31 Staff training was repeated at regular intervals, with structured, supervised competency assessments. Infant weight was measured to the nearest 10g using a calibrated digital infant scale (MTB 20 Baby Scale, Adam Equipment, Milton Keynes, UK) and length was measured to the nearest 0.25 cm using a rigid recumbent length-board (ADE MZ 1013 Baby Length Measuring Rod, ADE, Hamburg, Germany). Anthropometry measures were converted to continuous infant age-and-sex adjusted BMI (BMIZ), weight-for-length (WLZ), and weight-for-age (WAZ) z-scores, based on WHO child growth standards.32 Secondary outcomes included binary measures of overweight, defined as BMIZ or WLZ >2 SD and obesity, defined as BMIZ or WLZ >3 SD.33, 34 We descriptively evaluated rapid weight gain, defined as increase of >0.67 SD in WAZ between 6 weeks and 12 months.32
All covariate information was assessed at entry into ANC unless otherwise noted. Based on the local context, we developed35 a composite score to assess poverty level based on current employment, housing type, and access to household assets and categorized women based on tertiles as ‘most’, ‘moderate’ or ‘least’ disadvantaged. Household food security was assessed at 12 months of age using a measure adapted from the Household Food Insecurity Access Scale, Food and Nutrition Technical Assistance Project, and the Community Childhood Hunger Identification Project Index and categorized as ‘none’, ‘at risk’, or ‘food insecure’.36 Alcohol use was measured using the 3-item Alcohol Use Disorders Identification Test-Consumption (AUDIT-C; range 0–12). An AUDIT-C score ≥3 indicates hazardous drinking in the previous 12 months for women.37 Depressive symptoms were measured using the Edinburgh Postnatal Depression Scale (range 0–30); a score of ≥13 was used to indicate probable depressive symptoms.38 Blood pressure was measured at baseline as a part of routine care and categorized using standard clinical guidelines.39 To assess maternal HIV and viral load status at enrollment into ANC, we created a three-level categorical variable defined as HIV-uninfected, WLHIV <4 log10 copies/ml, or WLHIV ≥ 4 log10 copies/ml.
Following clinical practice in South Africa, gestational age was measured by ultrasound for women ≤ 28 weeks’ gestation (n=692, 80%); after 28 weeks gestation fundal height was used unless it differed from late ultrasound measurement by more than 21 days (n=169, 20% fundal height measure).41, 42 Size for gestational age at birth was defined as birthweight <10th percentile (small for gestational age; SGA), >90th percentile (large for gestational age; LGA) or otherwise appropriate for gestational age (AGA) based on the INTERGROWTH-21 standards. 40, 41 Preterm birth was defined as a live birth at <37 weeks gestation, low birthweight (LBW) as <2500 grams, and high birthweight (HBW) as >4000 grams.
Infant feeding was assessed at all postnatal visits via 24 hour maternal recall.29 Breast feeding decreased as infant age increased, therefore we evaluated a summary measure of exclusive, predominant or partial breast feeding in the first 6 months of life, when breast feeding was most common. Exclusive breast feeding (EBF) was defined as only receiving breastmilk and prescribed medication; predominant breast feeding was defined as breast feeding with any clear liquids (ex. water) but no nutritive liquids (e.g. infant formula) or food; partial breast feeding was defined as any breast feeding in addition to nutritive liquids or food, following WHO guidelines. 42 Due to few women predominately breast feeding, breast feeding status through 6 months was collapsed into a binary measure of exclusive or predominant breast feeding versus partial breast feeding in statistical analyses.
Statistical analysis
The goal of the statistical analysis was to examine the relationship between estimated maternal BMI and measures of infant weight through ~12 months of age. First, we descriptively evaluated mean BMIZ and WLZ scores, as well as indicators of overweight, obesity, and rapid weight gain between birth and 12 months overall and unadjusted differences by HIV-exposure status. Few children were obese over follow-up, thus modeling analyses focused on mean BMIZ/WLZ and overweight outcomes. Second, we used multivariable linear (continuous BMIZ/WLZ outcomes) and modified Poisson (binary overweight outcomes) mixed effects models to evaluate if maternal BMI was associated with trajectories of infant BMIZ and WLZ scores between 6 weeks and ~12 months of age. Mixed effects models were used to estimate marginal predicted means or probabilities of each outcome across maternal BMI categories. Models included random intercepts for each infant to account for variation across children and robust variance estimators to account for multiple observations per person. Confounders and predictors of the outcome were identified using directed acyclic graphs (Figure S1)43 and included baseline poverty, marital status, maternal age, gravidity, depression, alcohol use, maternal HIV status/viral load and food insecurity at 12 months postpartum.
Missing data
We used multiple imputation (n=50 imputations) from a multivariate normal distribution to address missing covariate and outcome data.44 The imputation model contained all identified confounders and predictors of the outcome, exposure and outcome information, as well as additional measured infant (ex. infant sex, duration of breast feeding), maternal (ex. planned pregnancy and duration of ANC in days), HIV if applicable (ex. CD4 cell count and viral load) and delivery characteristics (ex. birthweight, size for gestational age) hypothesized to be associated with missing data.
Sensitivity Analyses
In stratified analyses, we explored potential effect measure modification of associations between maternal BMI and continuous BMIZ and WLZ scores, to maximize statistical power. Potential modifiers of interest included maternal HIV status, maternal HIV and viral load (HIV-uninfected, WLHIV <4 log10 copies/ml, WLHIV ≥ 4 log10 copies/ml), breast feeding status through 6 months (exclusive or predominant vs partial), and timing of entry into ANC (<24 weeks, ≥ 24 weeks’ gestation) as a proxy for health seeking status, since health outcomes may differ among women presenting earlier in pregnancy compared to later in pregnancy. Since maternal BMI at entry into ANC likely over-estimates BMI, we conducted a sensitivity analysis where we estimated pre-pregnancy BMI using a previously published method to account for gestational weight gain between pre-pregnancy and entry into ANC, conditional maternal BMI category.12, 14, 45.
Ethics approval
Ethics approval was provided by the University of Cape Town’s Human Research Ethics Committee and Columbia University Institutional Review Board.
Results
Of the 861 mother-infant pairs with HIV-uninfected infants included, 454 were CEUH and 407 were CUH (Figure 1). Compared to HIV-uninfected women at enrollment into ANC, WLHIV were more likely to be in the most disadvantaged poverty category, endorse hazardous drinking, have stage 1 or 2 hypertension and to be food insecure at 12 months postpartum, but were less likely to be affected by obesity (Table 1). There were no meaningful differences between gestational age at entry into ANC, presenting to ANC before 24 weeks, or maternal age by HIV status.
Table 1.
Characteristics at enrollment into antenatal care among 861 mothers and HIV-uninfected infants pairs in Cape Town, South Africa
| HIV-uninfected women | Women living with HIV | Total | |
|---|---|---|---|
|
| |||
| N=407 | N=454 | N=861 | |
|
| |||
| Maternal characteristics | |||
| Median, IQR | |||
|
| |||
| Maternal age | 27 (23, 32) | 28 (24, 32) | 28 (24, 32) |
| Gravidity | 2 (2, 3) | 1 (1, 2) | 2 (1, 3) |
| Gestational age, weeks | 20 (13, 26) | 21 (16, 26) | 20 (14, 26) |
| BMI, kg/m2 | 30.1 (25.9, 35.5) | 28.7 (25.2, 33.9) | 29.5 (25.6, 34.8) |
| N (%) | |||
|
| |||
| BMI, category | |||
| Underweight (<18.5) | 5 (1.3) | 4 (1.0) | 9 (1.2) |
| Normal (18.5 – <25.0) | 72 (18.3) | 87 (22.5) | 159 (20.4) |
| Overweight (25.0 – <30.0) | 115 (29.3) | 128 (33.2) | 243 (31.2) |
| Obese (≥ 30.0) | 201 (51.2) | 167 (43.3) | 368 (47.2) |
| First antenatal care visit ≤ 24 weeks gestation | |||
| No | 129 (31.7) | 160 (35.2) | 289 (33.6) |
| Yes | 278 (68.3) | 294 (64.8) | 572 (66.4) |
| Education | |||
| Less than secondary | 224 (55.0) | 345 (76.0) | 569 (66.1) |
| Secondary or higher | 183 (45.0) | 109 (24.0) | 292 (33.9) |
| Poverty category | |||
| Most disadvantaged | 118 (29.0) | 156 (34.4) | 274 (31.8) |
| Moderate disadvantage | 176 (43.2) | 155 (34.1) | 331 (38.4) |
| Least disadvantaged | 113 (27.8) | 143 (31.5) | 256 (29.7) |
| Planned current pregnancy | |||
| No | 270 (66.3) | 324 (71.4) | 594 (69.0) |
| Yes | 137 (33.7) | 130 (28.6) | 267 (31.0) |
| Marital status | |||
| Not married/cohabitating | 226 (55.5) | 267 (58.8) | 493 (57.3) |
| Married/cohabitating | 181 (44.5) | 187 (41.2) | 368 (42.7) |
| Primigravida | |||
| No | 308 (75.7) | 369 (81.3) | 677 (78.6) |
| Yes | 99 (24.3) | 85 (18.7) | 184 (21.4) |
| Perinatal depression1 | |||
| No probable depression | 378 (92.9) | 408 (90.3) | 786 (91.5) |
| Probable depression | 29 (7.1) | 44 (9.7) | 73 (8.5) |
| Alcohol use2 | |||
| Below threshold | 377 (92.6) | 337 (74.6) | 714 (83.1) |
| Hazardous drinking | 30 (7.4) | 115 (25.4) | 145 (16.9) |
| Blood pressure category3 | |||
| Normal | 210 (56.8) | 177 (45.7) | 387 (51.1) |
| Elevated | 76 (20.5) | 85 (22.0) | 161 (21.3) |
| Stage 1 or 2 hypertension | 84 (22.7) | 125 (32.3) | 209 (27.6) |
| Food security4 | |||
| None | 243 (69.8) | 212 (55.8) | 455 (62.5) |
| At risk | 94 (27.0) | 100 (26.3) | 194 (26.7) |
| Food insecure | 11 (3.2) | 68 (17.9) | 79 (10.8) |
| Maternal HIV characteristics (N=454) | |||
| Median, IQR | |||
|
| |||
| Gestational age at ART initiation, weeks | -- | 21 (16, 26) | |
| Days between first ANC and ART initiation | 0 (0, 0.6) | ||
| Log 10 viral load, copies/ml | -- | 4.0 (3.3, 4.5) | -- |
| CD4 count, cells/mm3 | -- | 354 (247, 517) | -- |
| N (%) | |||
|
| |||
| HIV diagnosis | |||
| Before this pregnancy | -- | 193 (42.5) | -- |
| During this pregnancy | -- | 261 (57.5) | -- |
| Initiated ART before 24 weeks gestation | |||
| <24 weeks GA | -- | 284 (63.0) | -- |
| ≥24 weeks GA | -- | 167 (37.0) | -- |
| PMTCT prophylaxis in a previous pregnancy | |||
| No | -- | 259 (70.2) | -- |
| Yes | -- | 110 (29.8) | -- |
| Previous combination ART | |||
| No | -- | 436 (96.0) | -- |
| Yes | -- | 18 (4.0) | -- |
| Viral load, log10 copies/ml | |||
| <4.0 | 237 (52.2) | ||
| ≥4.0 | 217 (47.8) | ||
| CD4 count, cells/mm3 | |||
| ≤200 | -- | 73 (16.5) | -- |
| 201 – ≤350 | -- | 143 (32.4) | -- |
| 351– ≤500 | -- | 108 (24.4) | -- |
| >500 | -- | 118 (26.7) | -- |
BMI = body mass index; ART = antiretroviral therapy, GA= gestational age; PMTCT = prevention of mother-to-child HIV transmission.
Based on the Edinburgh Postnatal Depression Scale (range 0–30); a score of ≥13 indicates probable depression.
Based on the AUDIT-C (range 0–12); a score of ≥3 indicates hazardous drinking.
Blood pressure categorized as: normal (< 120/80 mm Hg), elevated (systolic 120–129 and diastolic <80 mm Hg), or stage 1 high (systolic 130–139 or diastolic 80–89 mm Hg) or stage 2 hypertension (systolic > 140 or diastolic > 90 mm Hg).
Household food security was assessed using adapted measures of the Household Food Insecurity Access Scale, Food and Nutrition Technical Assistance Project, and the Community Childhood Hunger Identification Project Index and categorized as ‘none’, ‘at risk’, or ‘food insecure’. Missing data: pre-pregnancy BMI n=85 (9.9%) gestational age at enrollment n=3 (0.4%), perinatal depression n=2 (0.2%), alcohol use n=2 (0.2%), blood pressure n=104 (12.1%), food insecurity n=133 (15.5%); GA at ART initiation 3 (0.7%), previous antiretroviral prophylaxis n=85 (18.7%), CD4 cell count n=12 (2.6%).
The median gestational age at delivery regardless of HIV-exposure was 39 weeks (IRQ 28, 40) and 10% of infants were born preterm. At delivery, CUH were slightly heavier and more likely to be LGA or high birth weight, compared to CEUH (Table S1). CEUH were slightly more likely to be SGA, low birth weight or to be born preterm, relative to CUH. The median duration of EBF for CUH was 5.4 weeks (IQR 0.4, 13 weeks) and 6 weeks (IQR 0.9, 15.6 weeks) for CEUH. Between birth and 6 months, nearly half of all women partially breast fed, but EBF was more common among CEUH than CUH. For women who were normal weight and above, partial breast feeding between birth and 6 months was most common (Table S2).
In descriptive analyses, both BMIZ and WLZ scores increased over time (Table 2). Compared to CUH, BMIZ and WLZ scores were lower at all time-points for CEUH. In addition, at 6 months of age more CUH were overweight and obese, relative to CEUH. By ~12 months of age, differences in infant overweight by HIV exposure status largely disappeared and few infants were obese. By 12 months, nearly half of CEUH and CUH experienced rapid weight gain (Table S3), with few differences by HIV exposure status and maternal BMI.
Table 2.
Body mass index (BMIZ) and weight for length (WLZ) z-scores and unadjusted differences in means or percentages between 6 weeks and 12+ months of age among HIV-uninfected infants, overall and by infant HIV-exposure status.
| Continuous BMIZ | Overweight: BMIZ >2SD | Obese: BMIZ >3SD | |||||
|
| |||||||
| N | Mean (SD) | Crude Mean Difference (95% CI) | N(%) | Crude Percent Difference (95% CI) | N(%) | Crude Percent Difference (95% CI) | |
|
| |||||||
| 6-weeks | |||||||
| Total | 795 | 0.08 (1.26) | -- | 44 (5.70) | -- | 6 (0.78) | -- |
| CUH | 382 | 0.34 (1.25) | 0.00 | 34 (8.99) | 0.00 | 2 (0.53) | 0.00 |
| CEUH | 413 | −0.16 (1.22) | −0.50 (−0.68, −0.33) | 10 (2.55) | −6.46 (−9.73, −3.18) | 4 (1.02) | 0.49 (−0.74, 1.72) |
| 6-months | |||||||
| Total | 720 | 0.54 (1.26) | -- | 92 (12.89) | -- | 23 (3.22) | -- |
| CUH | 342 | 0.69 (1.25) | 0.00 | 51 (15.09) | 0.00 | 17 (5.03) | 0.00 |
| CEUH | 378 | 0.41 (1.25) | −0.28 (−0.46, −0.10) | 41 (10.90) | −4.18 (−9.13, 0.76) | 6 (1.60) | −3.43 (−6.09, −0.78) |
| 12+ months | |||||||
| Total | 677 | 0.85 (1.32) | -- | 122 (18.15) | -- | 40 (5.95) | -- |
| CUH | 339 | 0.92 (1.26) | 0.00 | 63 (18.69) | 0.00 | 18 (5.34) | 0.00 |
| CEUH | 338 | 0.77 (1.38) | −0.14 (−0.34, 0.06) | 59 (17.61) | −1.08 (−6.91, 4.74) | 22 (6.57) | 1.22 (−2.35, 4.80) |
|
| |||||||
| Continuous WLZ | Overweight: WLZ >2SD | Obese: WLZ >3SD | |||||
|
| |||||||
| N | Mean (SD) | Crude Mean Difference (95% CI) | N(%) | Crude Percent Difference (95% CI) | N(%) | Crude Percent Difference (95% CI) | |
|
| |||||||
| 6-weeks | |||||||
| Total | 795 | 0.31 (1.37) | -- | 71 (9.23) | -- | 19 (2.47) | -- |
| CUH | 382 | 0.56 (1.37) | 0.00 | 51 (13.49) | 0.00 | 11 (2.91) | 0.00 |
| CEUH | 413 | 0.06 (1.33) | −0.51 (−0.70, −0.31) | 20 (5.12) | −8.38 (−12.45, −4.30) | 8 (2.05) | −0.86 (−3.06, −1.33) |
| 6-months | |||||||
| Total | 720 | 0.65 (1.24) | -- | 101 (14.15) | -- | 24 (3.36) | -- |
| CUH | 342 | 0.79 (1.23) | 0.00 | 55 (16.27) | 0.00 | 17 (5.03) | 0.00 |
| CEUH | 378 | 0.52 (1.23) | −0.27 (−0.45, −0.09) | 46 (12.23) | −4.04 (−9.18, 1.11) | 7 (1.86) | −3.17 (−5.87, −0.47) |
| 12+ months | |||||||
| Total | 677 | 0.78 (1.32) | -- | 114 (16.96) | -- | 36 (5.36) | -- |
| CUH | 339 | 0.89 (1.24) | 0.00 | 60 (17.86) | 0.00 | 20 (5.95) | 0.00 |
| CEUH | 338 | 0.68 (1.40) | −0.18 (−0.39, 0.01) | 54 (16.07) | −1.78 (−7.46, 3.89) | 16 (4.76) | −1.19 (−4.59, 2.21) |
SD = standard deviation; CUH = children unexposed to HIV; CEUH = children exposed, but uninfected with HIV. BMIZ and WLZ scores based on WHO standards and are adjusted for sex and age. Total N represents all children who attended each visit. N (%) for binary outcomes represent the proportion of infants overall and by infant HIV exposure status with an outcome.
In multivariable analyses, there were positive associations between increasing maternal BMI category with increasing infant BMIZ/WLZ scores and the probability of infants being overweight between 6 weeks and 12 months (Figure 2). In analyses stratified by infant HIV-exposure status, compared to CUH, CEUH had lower BMIZ and WLZ scores across all time points and maternal BMI. Among WLHIV, trajectories of infant BMIZ and WLZ scores did not differ meaningfully by maternal viral load at entry into ANC (Figure 3).
Figure 2. Multivariable association between maternal body mass index (BMI) category at entry into antenatal care and infant body mass index BMIZ and weight for length (WLZ) z-scores between 6 weeks and approximately 12 months of age.
Estimates from linear (continuous BMIZ and WLZ) and modified Poisson (BMIZ and WLZ >2 SD) multivariable mixed effects models with random intercepts adjusted for baseline measures of maternal poverty (categorical); marital status (categorical); age (spline); gravidity (spline); depressive symptoms (binary); alcohol use (binary); and maternal HIV/viral load status at entry into antenatal care (categorical) and food insecurity (categorical) at 12 months postpartum.
Figure 3. Multivariable association between maternal BMI category at entry into antenatal care and infant BMIZ and WLZ scores between 6 weeks and approximately 12 months of age – stratified by maternal HIV status and disease severity.
Estimates from linear multivariable mixed effects models with random intercepts adjusted for baseline measures of poverty (categorical); marital status (categorical); maternal age (spline); gravidity (spline); depressive symptoms (binary); alcohol use (binary) and postpartum food security (categorical). VL: viral load, measured by number of log10 HIV RNA copies/mL and assessed at entry into antenatal care.
In analyses stratified by a combined indicator of infant HIV-exposure status and breast feeding, breast feeding appeared to influence trajectories somewhat more for CUEH than CUH. Across maternal BMI categories, CUH had slightly higher BMIZ and WLZ scores than CEUH, regardless of breastfeeding status (Figure S2). Among CEUH, small increases in BMIZ and WLZ scores were seen for infants who were predominantly or exclusively breast fed, compared to those partially breast fed, across maternal BMI categories. Differences in BMIZ or WLZ measures by breast feeding status were not observed for CUH. There was no evidence that timing of entry into ANC influenced associations between maternal BMI and infant weight trajectories (Figure S3). Findings were similar when estimated pre-pregnancy BMI was used as the primary exposure (Figures S4–S6).
Comment
Principal Findings
In a cohort of 861 CEUH and CUH, nearly 20% of children were affected by overweight or obesity by ~12 months of age, with no differences by HIV-exposure status. For both CUH and CEUH, higher maternal BMI at entry into ANC was positively associated with higher infant BMIZ and WLZ scores between 6 weeks and 12 months of age. However, compared to CUH, CEUH had slightly lower BMIZ and WLZ trajectories through 12 months across all maternal BMI categories. Differences in weight trajectories by infant HIV-exposure status did not appear to be explained by maternal viral load prior to ART initiation or timing of entry into ANC, with small differences by breast feeding intensity among CEUH.
Strengths of the study
Strengths include repeated infant weight measures in a well-characterized cohort of HIV-uninfected infants followed through 1 year of age, rich data on maternal factors during pregnancy and delivery information, and the use of multiple imputation to address potential selection bias due to missing data and attrition during follow-up.
Limitations of the study
Limitations include the fact that our analysis included a subset of women with live born HIV-uninfected infants who were breast feeding in the early postpartum period and enrolled into postnatal follow-up. To explore the possibility of selection bias, we examined potential predicators of not being followed postpartum including HIV status, maternal BMI, timing of entry into ANC, comorbidities such as hypertension, alcohol use, and perinatal depression, and infant indicators including birthweight, preterm delivery, and length of gestation. We did not find any meaningful differences between the full cohort and the subset of women included in this analysis with respect to measured covariates potentially associated with not being followed postpartum (Table S8). In South Africa approximately 10% of women report never initiating breast feeding46; the relatively high level of breastfeeding initiation in this context may help to explain the lack of evidence of selection bias in this analysis of breast feeding women followed postpartum. Additional limitations include the fact that ultrasound-based gestational age dating was not available for a minority of women, nor was pre-pregnancy weight. However, no meaningful differences were observed in sensitivity analyses estimating pre-pregnancy BMI.
Interpretation
These findings document a high level of overweight and rapid weight gain through 1 year of age in breast fed infants in the era of universal ART, regardless of HIV-exposure status. In Southern Africa 13% of children under 5 were estimated to be overweight in 2018, up from 9% in 1990.47 Early life overweight or obesity and rapid weight gain increases the risk for obesity in childhood and type 2 diabetes and cardiovascular disease in adulthood.48, 49 For infants exposed to HIV and ART in utero, the mechanisms by which HIV and ART exposure may influence growth and metabolic health are not well understood but may related to mitochondrial toxicity or placental insufficiency. 18 Impaired growth has been reported among CEUH, relative to CUH, in other cohorts sub-Saharan African cohorts.50–52 However, in this cohort high levels of overweight and rapid weight gain were observed, and did not differ by HIV exposure status. In the South African context, high levels of maternal obesity and relatively short duration of breastfeeding may in part explain the high prevalence of overweight and rapid weight gain observed in infants. 46, 53 Future work is needed to better understand mechanisms of early infant weight gain among both CUH and CEUH in low and middle income countries with high levels of HIV and ART exposure. Overall, these findings highlight the growing burden of overweight early in life among CEUH and CUH in South Africa and the potential for obesity in pregnancy to exacerbate childhood obesity. 54, 55
Our findings also demonstrate that in some areas of South Africa, obesity, rather than under-nutrition, is a growing concern for WLHIV. Nearly half of pregnant women were affected by obesity in this cohort, regardless of HIV status. WLHIV had a slightly lower prevalence of obesity at entry into ANC compared to HIV-uninfected women, which could influence infant weight. Compared to CUH, CEUH had slightly lower BMIZ and WLZ scores and a slightly lower probability of being overweight through 12 months across maternal BMI categories. Differences in breastfeeding (discussed below) and the fact that CEUH were born at the same median gestational age as CUH, but were lighter may help to explain the remaining differences in weight by infant HIV exposure status.
Breast feeding is hypothesized to influence infant weight by protecting against rapid weight gain, but may also increase adiposity.23–27 In a cohort of CUH in South Africa, 30% were overweight by 2 years of age, but infants who were breast fed at 12 weeks of age had lower BMIZ and weight velocity z-scores, relative to infants who were not breast fed.56 Data from the pre-universal ART era on the impact of breast feeding on infant weight in CEUH is mixed, with some studies demonstrating that breast feeding mitigates deficits in early weight gain for CEUH2 and others showing no differences between breast and formula fed CEUH.57 In this cohort of breast fed infants from the universal ART era, CEUH who were predominately or EBF between birth and 6 months had slightly higher BMIZ and WLZ scores through 12 months across all maternal BMI categories, relative to CEUH who were partially breast fed; however these differences were small and may not be clinically meaningful. Breast feeding status between birth and 6 months did not have the same influence on weight for CUH. Larger differences by breast feeding status many not have been observed due to the fact that breast feeding information was only assessed at study visits (every 6 weeks to 3 months) and the limited variation in breast feeding overall in this cohort, where all women breast fed for at least some duration.
Conclusions
In a cohort of breast fed CEUH and CUH, we observed high levels of overweight and rapid weight gain among infants by 12 months of age regardless of HIV exposure status. For both CEUH and CUH, increasing maternal BMI was associated with higher infant weight and the probability of being overweight through 12 months of age. Compared to CUH, CEUH had slightly lower BMIZ and WLZ scores over time; however, BMIZ and WLZ scores remained above average WHO values for both CEUH and CUH. For CEUH differences in BMIZ and WLZ scores did not appear to differ by maternal viral load prior to ART initiation or duration of ANC, with small differences by breast feeding intensity between birth and 6 months of age. These findings highlight the growing burden of overweight among infants, including among CEUH, in South Africa and suggest high levels of obesity in pregnancy may be a driver of this relationship. Public health interventions to mitigate y obesity in pregnancy and address the growing burden of obesity in childhood are urgently needed in South Africa to support optimal infant growth and long-term cardiometabolic health for women and children.
Supplementary Material
Synopsis.
Study question:
In South Africa, a country with a high burden of HIV and obesity, how does maternal body mass index (BMI) influence infant weight through 1 year?
What’s already known:
Maternal BMI is well-known to influence early infant weight gain among HIV-unexposed infants, but its impact among infants exposed to, but uninfected with, HIV is unclear.
What this study adds:
In a cohort of HIV-exposed and HIV-unexposed South African infants, maternal BMI in pregnancy was positively associated with increasing infant BMI and weight for-length (WL) z-scores through 1 year, regardless of HIV-exposure status. HIV-exposed infants had slightly lower BMIZ and WLZ trajectories over 1 year, compared to HIV-unexposed infants. However, nearly 20% of HIV-exposed and HIV-unexposed infants were overweight by 1 year of age.
Acknowledgements:
The researchers thank the study participants, research, and clinical staff that made this study possible. We also thank the President’s Emergency Plan for AIDS Relief (PEPFAR), the National Institute of Child Health and Human Development and the National Institute of Mental Health, the Elizabeth Glaser Pediatric AIDS Foundation, South African Medical Research Council, Fogarty Foundation and the Office of AIDS Research for grant support.
Funding:
This research was supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; Grant Number 1R01HD074558) and was supported through the National Institute of Mental Health (NIMH; Grant Number R00MH112413). Additional funding comes from the Elizabeth Glaser Pediatric AIDS Foundation, South African Medical Research Council (Clinician-Researcher PhD Scholarship), the Fogarty Foundation (NIH Fogarty International Center; Grant Number 5R25TW009340) and the Office of AIDS Research.
Footnotes
Conflicts of interest: None declared
Social Media Quote: In a cohort of South African HIV-exposed and HIV-unexposed infants, nearly 20% of infants were overweight by 1 year of age. Maternal BMI was positively associated with increasing infant weight, regardless of HIV-exposure status.
References
- 1.le Roux SM, Abrams EJ, Donald KA, Brittain K, Phillips TK, Nguyen KK, et al. Growth trajectories of breastfed HIV-exposed uninfected and HIV-unexposed children under conditions of universal maternal antiretroviral therapy: a prospective study. Lancet Child Adolesc Health. 2019; 3:234–244. [DOI] [PubMed] [Google Scholar]
- 2.Arpadi S, Fawzy A, Aldrovandi GM, Kankasa C, Sinkala M, Mwiya M, et al. Growth faltering due to breastfeeding cessation in uninfected children born to HIV-infected mothers in Zambia. Am J Clin Nutr. 2009; 90:344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neri D, Somarriba GA, Schaefer NN, Chaparro AI, Scott GB, Lopez Mitnik G, et al. Growth and body composition of uninfected children exposed to human immunodeficiency virus: comparison with a contemporary cohort and United States National Standards. J Pediatr. 2013; 163:249–254 e241–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omoni AO, Ntozini R, Evans C, Prendergast AJ, Moulton LH, Christian PS, et al. Child Growth According to Maternal and Child HIV Status in Zimbabwe. Pediatr Infect Dis J. 2017; 36:869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel D, Bland R, Coovadia H, Rollins N, Coutsoudis A, Newell ML. Breastfeeding, HIV status and weights in South African children: a comparison of HIV-exposed and unexposed children. AIDS. 2010; 24:437–445. [DOI] [PubMed] [Google Scholar]
- 6.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva, Switzerland: World Health Organization; 2013. [PubMed] [Google Scholar]
- 7.WHO, United Nations Children’s Fund. Guideline: updates on HIV and infant feeding: the duration of breastfeeding, and support from health services to improve feeding practices among mothers living with HIV. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- 8.Collaborators GBDO, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017; 377:13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voerman E, Santos S, Patro Golab B, Amiano P, Ballester F, Barros H, et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: An individual participant data meta-analysis. PLoS Med. 2019; 16:e1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patro Golab B, Santos S, Voerman E, Lawlor DA, Jaddoe VWV, Gaillard R, et al. Influence of maternal obesity on the association between common pregnancy complications and risk of childhood obesity: an individual participant data meta-analysis. Lancet Child Adolesc Health. 2018; 2:812–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slogrove AL, Powis KM, Johnson LF, Stover J, Mahy M. Estimates of the global population of children who are HIV-exposed and uninfected, 2000–18: a modelling study. Lancet Glob Health. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bengtson AM, Phillips TK, le Roux SM, Brittain K, Buba A, Abrams EJ, et al. Postpartum obesity and weight gain among human immunodeficiency virus-infected and human immunodeficiency virus-uninfected women in South Africa. Matern Child Nutr. 2020:e12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C, Xu X, Yan Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PloS one. 2018; 13:e0202183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bengtson AM, Phillips TK, le Roux SM, Brittain K, Zerbe A, Madlala H, et al. Does HIV infection modify the relationship between pre-pregnancy body mass index and adverse birth outcomes? Paediatr Perinat Epidemiol. 2020. [DOI] [PubMed] [Google Scholar]
- 15.Gaillard R, Durmuş B, Hofman A, Mackenbach JP, Steegers EA, Jaddoe VW. Risk factors and outcomes of maternal obesity and excessive weight gain during pregnancy. Obesity (Silver Spring). 2013; 21:1046–1055. [DOI] [PubMed] [Google Scholar]
- 16.Jao J, Kirmse B, Yu C, Qiu Y, Powis K, Nshom E, et al. Lower Preprandial Insulin and Altered Fuel Use in HIV/Antiretroviral-Exposed Infants in Cameroon. J Clin Endocrinol Metab. 2015; 100:3260–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jao J, Jacobson DL, Yu W, Borkowsky W, Geffner ME, McFarland EJ, et al. A comparison of metabolic outcomes between obese HIV-exposed uninfected youth from the PHACS SMARTT Study and HIV-unexposed youth from the NHANES Study in the U.S. J Acquir Immune Defic Syndr. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jao J, Abrams EJ. Metabolic complications of in utero maternal HIV and antiretroviral exposure in HIV-exposed infants. Pediatr Infect Dis J. 2014; 33:734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geffner ME, Patel K, Jacobson DL, Wu J, Miller TL, Hazra R, et al. Changes in insulin sensitivity over time and associated factors in HIV-infected adolescents. AIDS. 2018; 32:613–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn L, Kasonde P, Sinkala M, Kankasa C, Semrau K, Scott N, et al. Does severity of HIV disease in HIV-infected mothers affect mortality and morbidity among their uninfected infants? Clin Infect Dis. 2005; 41:1654–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morden E, Technau KG, Giddy J, Maxwell N, Keiser O, Davies MA. Growth of HIV-Exposed Uninfected Infants in the First 6 Months of Life in South Africa: The IeDEA-SA Collaboration. PloS one. 2016; 11:e0151762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkatesh KK, Lurie MN, Triche EW, De Bruyn G, Harwell JI, McGarvey ST, et al. Growth of infants born to HIV-infected women in South Africa according to maternal and infant characteristics. Trop Med Int Health. 2010; 15:1364–1374. [DOI] [PubMed] [Google Scholar]
- 23.Yu X, Rong SS, Sun X, Ding G, Wan W, Zou L, et al. Associations of breast milk adiponectin, leptin, insulin and ghrelin with maternal characteristics and early infant growth: a longitudinal study. Br J Nutr. 2018; 120:1380–1387. [DOI] [PubMed] [Google Scholar]
- 24.Ellsworth L, Perng W, Harman E, Das A, Pennathur S, Gregg B. Impact of maternal overweight and obesity on milk composition and infant growth. Matern Child Nutr. 2020; 16:e12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ode KL, Gray HL, Ramel SE, Georgieff MK, Demerath EW. Decelerated early growth in infants of overweight and obese mothers. J Pediatr. 2012; 161:1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horta BL, Loret de Mola C, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr. 2015; 104:30–37. [DOI] [PubMed] [Google Scholar]
- 27.Sauder KA, Kaar JL, Starling AP, Ringham BM, Glueck DH, Dabelea D. Predictors of Infant Body Composition at 5 Months of Age: The Healthy Start Study. J Pediatr. 2017; 183:94–99 e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myer L, Phillips TK, Zerbe A, Brittain K, Lesosky M, Hsiao NY, et al. Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: A randomised controlled trial. PLoS Med. 2018; 15:e1002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Myer L, Phillips TK, Zerbe A, Ronan A, Hsiao NY, Mellins CA, et al. Optimizing Antiretroviral Therapy (ART) for Maternal and Child Health (MCH): Rationale and Design of the MCH-ART Study. J Acquir Immune Defic Syndr. 2016; 72 Suppl 2:S189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langwenya N, Phillips TK, Brittain K, Zerbe A, Abrams EJ, Myer L. Same-day antiretroviral therapy (ART) initiation in pregnancy is not associated with viral suppression or engagement in care: A cohort study. J Int AIDS Soc. 2018; 21:e25133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Training Course on Child Growth Assessment. Geneva: WHO; 2008. [Google Scholar]
- 32.World Health Organization, United Nations Children’s Fund. WHO child growth standards and the identification of severe acute malnutrition in infants and children. A Joint Statement. Geneva Switzerland: World Health Organization,2009. [PubMed] [Google Scholar]
- 33.Furlong KR, Anderson LN, Kang H, Lebovic G, Parkin PC, Maguire JL, et al. BMI-for-Age and Weight-for-Length in Children 0 to 2 Years. Pediatrics. 2016; 138. [DOI] [PubMed] [Google Scholar]
- 34.Roy SM, Spivack JG, Faith MS, Chesi A, Mitchell JA, Kelly A, et al. Infant BMI or Weight-for-Length and Obesity Risk in Early Childhood. Pediatrics. 2016; 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brittain K, Mellins CA, Phillips T, Zerbe A, Abrams EJ, Myer L, et al. Social Support, Stigma and Antenatal Depression Among HIV-Infected Pregnant Women in South Africa. AIDS Behav. 2017; 21:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Labadarios D, McHiza ZJ, Steyn NP, Gericke G, Maunder EM, Davids YD, et al. Food security in South Africa: a review of national surveys. Bull World Health Organ. 2011; 89:891–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998; 158:1789–1795. [DOI] [PubMed] [Google Scholar]
- 38.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987; 150:782–786. [DOI] [PubMed] [Google Scholar]
- 39.American College of Obstetricians and Gynecologists. Preeclampsia and High Blood Pressure During Pregnancy, FAQ 034: American College of Obstetricians and Gynecologists; 2018. [Google Scholar]
- 40.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014; 384:857–868. [DOI] [PubMed] [Google Scholar]
- 41.Villar J, Giuliani F, Fenton TR, Ohuma EO, Ismail LC, Kennedy SH. INTERGROWTH-21st very preterm size at birth reference charts. Lancet. 2016; 387:844–845. [DOI] [PubMed] [Google Scholar]
- 42.WHO, UNICEF, USAID, AED, UCDAVIS, IFPRI. Indicators for assessing infant and young child feeding practices Part 1: definitions. Geneva: World Health Organization; 2008. [cited 2021 21 January]; Available from: https://apps.who.int/iris/bitstream/handle/10665/43895/9789241596664_eng.pdf;jsessionid=EF7C2AC8C14431C7BB5A8BBB310E2523?sequence=1. [Google Scholar]
- 43.VanderWeele TJ, Hernan MA, Robins JM. Causal directed acyclic graphs and the direction of unmeasured confounding bias. Epidemiology. 2008; 19:720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubin DB. Multiple imputation for nonresponse in surveys. New York, New York: Wiley; 1987. [Google Scholar]
- 45.Santos S, Eekhout I, Voerman E, Gaillard R, Barros H, Charles MA, et al. Gestational weight gain charts for different body mass index groups for women in Europe, North America, and Oceania. BMC Med. 2018; 16:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horwood C, Haskins L, Engebretsen I, Connolly C, Coutsoudis A, Spies L. Are we doing enough? Improved breastfeeding practices at 14 weeks but challenges of non-initiation and early cessation of breastfeeding remain: findings of two consecutive cross-sectional surveys in KwaZulu-Natal, South Africa. BMC public health. 2020; 20:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Cesare M, Sorić M, Bovet P, Miranda JJ, Bhutta Z, Stevens GA, et al. The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action. BMC Med. 2019; 17:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA. 2009; 301:2234–2242. [DOI] [PubMed] [Google Scholar]
- 49.Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009; 123:1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lane CE, Bobrow EA, Ndatimana D, Ndayisaba GF, Adair LS. Determinants of growth in HIV-exposed and HIV-uninfected infants in the Kabeho Study. Matern Child Nutr. 2019; 15:e12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lane CE, Widen EM, Collins SM, Young SL. HIV-Exposed, Uninfected Infants in Uganda Experience Poorer Growth and Body Composition Trajectories than HIV-Unexposed Infants. J Acquir Immune Defic Syndr. 2020; 85:138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ndiaye A, Suneson K, Njuguna I, Ambler G, Hanke T, John-Stewart G, et al. Growth patterns and their contributing factors among HIV-exposed uninfected infants. Matern Child Nutr. 2021; 17:e13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Houle B, Rochat TJ, Newell ML, Stein A, Bland RM. Breastfeeding, HIV exposure, childhood obesity, and prehypertension: A South African cohort study. PLoS Med. 2019; 16:e1002889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond). 2011; 35:891–898. [DOI] [PubMed] [Google Scholar]
- 55.Adair LS, Fall CH, Osmond C, Stein AD, Martorell R, Ramirez-Zea M, et al. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013; 382:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramokolo V, Lombard C, Chhagan M, Engebretsen IM, Doherty T, Goga AE, et al. Effects of early feeding on growth velocity and overweight/obesity in a cohort of HIV unexposed South African infants and children. Int Breastfeed J. 2015; 10:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGrath CJ, Nduati R, Richardson BA, Kristal AR, Mbori-Ngacha D, Farquhar C, et al. The prevalence of stunting is high in HIV-1-exposed uninfected infants in Kenya. J Nutr. 2012; 142:757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.