Abstract
Background and purpose
The ubiquitous presence of microplastics (MPs) in aquatic environments has been studied widely. Due to toxicological impacts of MPs and associated contaminants, it is crucial to understand the performance of MPs removal in drinking water treatment plants (DWTPs). Few studies have investigated removal characteristics of MPs via coagulation/flocculation processes, yet removal characterization of polypropylene microplastics (PPMPs) in this process is poorly understood. This study aims to optimize coagulation of virgin PPMPs in conventional DWTPs.
Methods
In this study, samples were synthesized through response surface methodology (RSM), polyaluminium chloride (PACl) was applied as a conventional coagulant to remove PPMPs in the coagulation/flocculation process, which has the least density among common polymers and is one of the most abundant manufactured polymers worldwide. A particle size analyzer (PSA) was used to measure floc size at different pH levels. Additionally, a zeta potential analyzer was used to measure stability of the flocs at different pH.
Results
Base on the experimental range in Design-Expert, results revealed that the optimum removal rate was predicted to be at pH 9, PACl concentration of 200 ppm, polyacrylamide (PAM) concentration of 21 ppm, and PPMPs size of d < 0.25 mm. According to the predicted optimum condition, actual and predicted removal rates were 18.00 ± 1.43% and 19.69%, respectively.
Conclusion
According to this study, PACl is not capable of efficiently removing virgin PPMPs in DWTPs, thereby exposing humans to eco-toxicological impacts of PPMPs through tap water.
Keywords: Coagulation, Drinking water treatment plant, Microplastics (MPs), Response Surface methodology (RSM)
Introduction
Consumption of plastic products has increased dramatically in recent decades and is predicted to continue to increase without mitigation [1, 2]. Plastic production reached 359 million metric tons in 2018 [3]. Consequently, pervasive plastic pollution has become a growing global problem [4–6]. Plastics are durable, resistant to degradation and persistent in the environment for decades [7]. However, due to affordability of production, lightness, and convenience of transport, plastics are widely used [8]. The most abundant types of plastic polymers at production are polypropylene (PP; 16%), low-density polyethylene and linear low-density polyethylene (LDPE and LLDPE; 12%), polyvinylchloride (PVC; 11%), high-density polyethylene (HDPE; 10%), polyethylene terephthalate (PET; 5%) [9]. These materials can be fragmented into smaller particles which are called microplastics (MPs; < 5 mm) by photodegradation, mechanical degradation, biodegradation [10–13]. A plethora of studies investigating water resources have identified presence of MPs in lakes [14–16], rivers [17, 18], seas, and oceans [19–21], aquatic biota [22, 23] and water treatment plants (WTPs, a collective term for drinking water treatment plants and wastewater treatment plants) [24–28]
Removal of MPs in wastewater treatment plants (WWTPs) has been reported to be > 73%, to as high as 99% by various studies [26, 29–32]. However, WWTPs still are point sources of MPs that emit millions of these particles into freshwater resources daily [33–35]. Therefore, marine organisms have been observed to ingest [22, 36, 37]. Thus, the negative effect of MPs has been identified in marine biota [38–41]. For example, Qiang L. and Cheng J. [42] demonstrated that polystyrene microplastics (PSMPs) could negatively impact the reproductive organs of freshwater fish. PSMPs induce increased reactive oxygen species (ROS) levels in gonads of zebrafish (an animal model), and they identified these particles as a source of reproductive stress in freshwater fish. Conversely, high removal of MPs in drinking water treatment plants (DWTPs) has also been reported [24, 25, 27]. Since these facilities cannot remove MPs completely, humans are exposed to these particles through drinking water. For example, Tong et al. [43] investigated 38 tap water in different cities of China to measure MP pollution, in which 36 of them contained MPs, ranging from 125 to 1247 MP particles/L. They observed that MP particles smaller than 50 µm predominated in all of the samples. Hence, other studies have investigated the effects of human exposure to MPs [44, 45]. For instance, Forte et al. [46] reported that 44 and 100 nm PSMPs accumulate in gastric adenocarcinoma (AGS) cells. They also affect inflammatory gene expression, cell viability, and cell morphology.
In WTPs, the coagulation/flocculation process plays a prominent role in removing MPs [27]. Coagulation is characterized by adding some determined amount of chemicals as coagulants (mostly aluminium and iron salts) [47] to destabilize colloidal suspended particles that are stable through their mostly negative surface charges. Then, destabilized particles tend to settle by absorbing together to form flocs [48]. Few studies have investigated the coagulation performance of MPs by various coagulants [49–54]. These results suggest that polyethylene (PE), due to its low density, directly influences sedimentation. Ma et al. [53] compared AlCl3.6H2O and FeCl3.6H2O in different pHs to analyze polyethylene microplastics (PEMPs), and they observed that the sedimentation, along with the usage of PAM as a coagulant aid, reached 61.19% ± 3.67%. Multiple coagulants, both organic and inorganic, were utilized as coagulants in MP coagulation, including ferric chloride, PACl, polyamine [55], chitosan, sodium alginate [54]. Polypropylene Microplastics (PPMPs) have been reported to be one of the most abundant polymers found in water and sediment samples in the environment [24, 56] and is among the first three detected type of MPs both in raw and potable water [57]. However, removal characteristics of polypropylene microplastics is poorly understood. This study aims to synthesize samples to compare the performance of PACl and ferric chloride through a pre-experiment to identify more efficient coagulant in order to use it to optimize virgin PPMPs removal via response surface methodology (RSM). Moreover, to the knowledge of the author, this is the first study to use experimental design to optimize MP coagulation. The findings of this study improve our understanding of the characterization of PPMP removal in the coagulation/flocculation process.
Materials and Methods
Materials
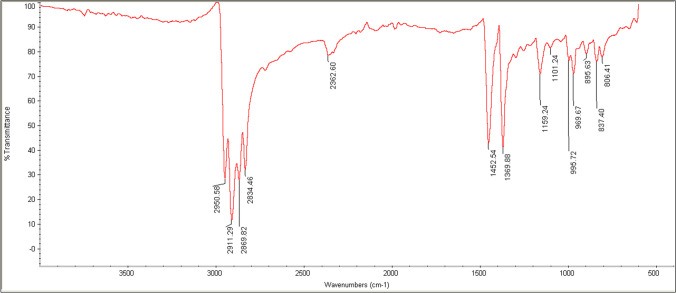
PPMPs were prepared by milling PP pellets, with a density of 0.90 g/cm3 (Z30S, Marun Petrochemical Complex, Iran). The pellets' temperature was decreased to below -196 ºC by liquid nitrogen before milling by an ultra-centrifugal mill (ZM 200, Retsch®, Germany) [58]. PP polymer composition was characterized by Fourier Transform Infrared Spectrophotometer (FT-IR) (Avatar 380, Thermo Scientific, USA). Data acquisition was conducted in the transmission mode of 2 cm−1 resolution, and a 3 s collection time, wavenumber ranging from 500 to 4000 cm−1 (Fig. 1). Spectra were compared with a database provided by Omnic software (Thermo Phisher Scientific, USA). Prepared PPMPs were sieved into five different diameter size classes (d < 0.25 mm, 0.25 < d < 0.85 mm, 0.85 < d < 1.45 mm, 1.45 < d < 2.05 mm and 2.05 < d < 2.65 mm). Other stock solutions were analytical grade, including FeCl3.6H2O, HCl, NaOH, Kaolin, and NaCl that were purchased from Merck Millipore (USA). Humic Acid (HA; Sigma Aldrich, USA), as a model for natural organic matter (NOM), was dissolved in deionized (DI) water at a concentration of 1 mg/L [59]. PACl and PAM were also purchased from Tianshi (Jiangsu) Fine Chemicals Co. (Changzhou, China). DI water was utilized for all experiments, and all stock solutions were maintained in the dark at 4ºC.
Fig. 1.
FT-IR spectrum of PPMPs particles
Coagulation experiment
To remove residuals, PPMPs were immersed with 1 M HCl and stored in an oven at 70ºC for 24 h. As described in Ma et al. [53], 500 mL beakers were prepared and filled with stock solution. 0.1 M NaCl was added to the solution as the background ionic strength [60]. Required turbidity was obtained by adding kaolin to achieve five nephelometric turbidity unit (NTU) via a turbidity meter (AL450T-IR, Aqualytic, Germany). pH was adjusted by 1 M HCl and 1 M NaOH solution by a pH meter (3510, Jenway, UK). A predetermined amount of chemicals was balanced by a precision balance with a minimum range of 1.0 × 10–3 g (LST-JM-102, CGOLDENWALL, China). The final solution was added 0.100 g of PPMPs and a predetermined amount of one of the coagulants and anionic or cationic PAM. Samples were stirred by a Jar test apparatus with blade dimensions 6.5 × 2 × 0.75 cm (Tak Azama, Iran) at 300 rpm for 1 min and a subsequent 14 min at 100 rpm. Samples were left for 30 min for sedimentation [47]. For characterization of PPMPs removal, a weighing method was chosen in this study [53]. Briefly, after sedimentation and formation of the flocs, supernatants were carefully filtered through a 0.45 µm membrane filter (membrane solutions LLC., USA). Then, PPMPs and flocs on the filters were immersed in 1 M HCL and were treated with an ultrasonic bath for 5 min, then left for 1 h to dissolve residues and flocs. Subsequently, PPMPs in HCL solution were filtered again and stored in an oven at 70ºC for 12 h prior to being weighed (Wdried). Removal percentage was determined using Eq. 1.
| 1 |
A zeta potential analyzer was used to measure the electro-kinetic potential of the solutions and its relationship with different pHs with a measurement range of ± 200 mV (SZ-100, Horiba Scientific, Japan). Dynamic sizes of flocs were measured using a static light scattering particle size analyzer (SLS-PSA) with a particle size range of 0.02–2000 µm (Mastersizer 2000, Malvern panalytical, UK), and diameter of flocs (d50) were analyzed every 30 s using Malvern 2000 software (Version 5.1, Malvern panalytical, UK). Morphology of the PPMPs and images of these particles trapped in the flocs were taken using a scanning electron microscope (SEM) (XL-30, Philips, Netherlands); the images were taken with an acceleration voltage of 10 kV. Before imaging, a double gold layer was sputtered to the samples to maintain the conductivity of the samples. To capture the particles trapped in the flocs, a hollow tube was used to move the settled flocs at the bottom of the beaker on a membrane filter. Then filters were dried in the oven for 1 h.
Experimental design and data analysis
The RSM with central composite design (CCD) was applied to achieve the optimum percentage in PPMPs removal. The pH, PPMPs size, coagulant dosage, and PAM dosage were selected as independent variables, and the reduction of PPMPs as a percentage was chosen as the result. For statistical analysis, Design-Expert® software (Version 11, Stat-Ease Inc., USA) was used. The range of variables was 5 to 9 for pH, 0.25 to 2.65 mm for PPMPs size, 200 to 1000 ppm for coagulant dosage (whether PACl or ferric chloride), and 10 to 22 ppm for PAM (whether cationic or anionic). The range of variables and the type of coagulants and PAM were selected based upon a pre-experiment designed to compare the PPMPs removal performance. In this pre-experiment, the dosage of coagulants (100 ppm), PAM (8 ppm), pH (7), PPMPs dosage (100 mg), and the size of the PPMPs (0.85 mm) were constant. The only variables in the pre-experiment are the type of coagulant and PAM (Table 1). All the tests in the pre-experiment were conducted in triplicate. The CCD full factorial design gives 30 experiments, according to Eq. 2 [61], including 16 factorial point (2 k), eight axial points (2 k), and six replicated center points (N0). Replicate center points are used to predict the pure error of the lack of fit test and data reproducibility [62].
| 2 |
where N is the total number of experimental runs that have to be performed, and k is the number of independent variables. Based on the previous studies, the concentration of coagulants and PAM for actual DWTPs is always below 20 and 1 ppm, respectively [59, 63]. Nevertheless, to characterize the factors influencing PPMPs removal, different concentrations of these chemicals were utilized. By determining the range of four variables in Design-Expert software by choosing low and high coded (± 1 levels, alpha = face-centered; Table 2), different experimental conditions were provided (Table 3). After performing 30 practical tests, the general correlation between independent variables (pH, coagulant dosage, PAM dosage, and PPMPs size) and dependent variable (percentage of PPMPs removal) was acquired using Eq. 3.
| 3 |
where Y represents response of the dependent variable, f is the function which relates the response to independent variables, and X1, X2, X3, …, Xn is the n independent variables that affect the response [62]. Subsequently, for establishing relationships between the dependent and independent variables, a form of the second-order polynomial was applied using Eq. 4 [64].
| 4 |
where Y is predicted response (removal, %), β0 is the constant regression coefficient for the intercept, βi is the linear coefficient, βii is the quadratic coefficient, βij is the interaction coefficient, xi and xj are coded values for independent variables. Analysis of variance (ANOVA) and regression coefficients were applied to analyze data at the 95% confidence level using Design-Expert® software. Multiple factors of practical data sets were evaluated to test fitness of the model, including F-value, p-value, degree of freedom (DF), mean square (MS), sum of squares (SS), correlation coefficient (R), determination coefficient (R2), adjusted determination coefficient (Radj2), standard deviation (SD) and coefficient of variance (CV).
Table 1.
Pre-experiment tests of efficiency rate of two types of coagulants and PAM in PPMPs removal. Error bars indicate one standard deviation of the mean
| Anionic PAM | Cationic PAM | |
|---|---|---|
| Ferric Chloride | 8% ± 0 | 6% ± 1 |
| PAC | 9% ± 2 | 4% ± 2 |
Table 2.
Five levels and experimental range of independent variables
| Variable | -α | Low | Middle | High | + α |
|---|---|---|---|---|---|
| A- PPMPs size (mm) | 0.25 | 0.85 | 1.45 | 2.05 | 2.65 |
| B- pH | 5 | 6 | 7 | 8 | 9 |
| C- PACl dosage (ppm) | 200 | 400 | 600 | 800 | 1000 |
| D- PAM dosage (ppm) | 10 | 13 | 16 | 19 | 22 |
Table 3.
RSM design in CCD and actual and predicted value of PPMPs removal
| Run | PPMPs size (mm) | pH | PACl dosage (ppm) | PAM dosage (ppm) | Removal (%) | |
|---|---|---|---|---|---|---|
| Predicted | Actual | |||||
| 1 | 0.85 | 6 | 400 | 13 | 6.39 | 6 |
| 2 | 0.85 | 6 | 800 | 13 | 6.39 | 6 |
| 3 | 2.05 | 6 | 800 | 13 | 1.85 | 2 |
| 4 | 2.05 | 6 | 400 | 13 | 1.85 | 3 |
| 5 | 2.65 | 7 | 600 | 16 | 0.27 | 1 |
| 6 | 1.45 | 7 | 600 | 16 | 7.19 | 7 |
| 7 | 1.45 | 7 | 600 | 16 | 7.19 | 7 |
| 8 | 2.05 | 6 | 800 | 19 | 3.60 | 3 |
| 9 | 1.45 | 7 | 600 | 16 | 7.19 | 8 |
| 10 | 1.45 | 7 | 600 | 10 | 3.47 | 4 |
| 11 | 1.45 | 7 | 1000 | 16 | 7.19 | 6 |
| 12 | 0.85 | 8 | 400 | 19 | 12.93 | 13 |
| 13 | 2.05 | 8 | 400 | 13 | 1.89 | 1 |
| 14 | 0.85 | 6 | 800 | 19 | 8.14 | 7 |
| 15 | 1.45 | 7 | 600 | 16 | 7.19 | 7 |
| 16 | 1.45 | 7 | 200 | 16 | 7.19 | 9 |
| 17 | 2.05 | 6 | 400 | 19 | 3.60 | 2 |
| 18 | 1.45 | 7 | 600 | 22 | 6.97 | 9 |
| 19 | 1.45 | 7 | 600 | 16 | 7.19 | 8 |
| 20 | 0.25 | 7 | 600 | 16 | 14.10 | 16 |
| 21 | 2.05 | 8 | 400 | 19 | 3.64 | 3 |
| 22 | 2.05 | 8 | 800 | 19 | 3.64 | 2 |
| 23 | 1.45 | 5 | 600 | 16 | 2.80 | 3 |
| 24 | 1.45 | 9 | 600 | 16 | 7.64 | 10 |
| 25 | 0.85 | 8 | 800 | 19 | 12.93 | 12 |
| 26 | 0.85 | 8 | 400 | 13 | 11.18 | 11 |
| 27 | 1.45 | 7 | 600 | 16 | 7.19 | 8 |
| 28 | 0.85 | 8 | 800 | 13 | 11.18 | 8 |
| 29 | 0.85 | 6 | 400 | 19 | 8.14 | 8 |
| 30 | 2.05 | 8 | 800 | 13 | 1.89 | 2 |
Results and discussion
Pre-experiment
Prior to conducting the thirty experiments given by the Design-Expert software, the type of coagulant (whether ferric chloride or PACl) chosen for the set of experiments needed to be selected (Table 1). PACl coupled with anionic PAM performed better at removing PPMPs with a rate of 9% ± 2. Although this removal rate was < 10%, ferric chloride with anionic or cationic PAM (8% ± 0 and 6% ± 1, respectively), was less effective at removing PPMPs, compared to PACl. Therefore, for thirty given experiments, PACl was chosen as the coagulant (dependent variable). In pre-experiment tests, the role of PAM was more significant in removing PPMPs than that of coagulants, which in this case, anionic PAM, acted better in removing these particles when it was coupled with 8% ± 0 and 9% ± 2 for ferric chloride and PACl, respectively.
PACl and ferric chloride are the two commonly used types of iron based coagulants [65]. These two coagulants have been reported to efficiently remove antimony [66], arsenate [67], blended surface water, wastewater and rainwater [68], textile industry wastewater [69], automotive wastewater [70], PSMPs [55] and PEMPs [53]. However, PACl utilization by WTPs is more expensive than ferric chloride. Rajala et al. [55] reported that PACl and ferric chloride are more efficient in removing PSMPs than cationic polyamine. They observed that 1 µm and 6.3 µm PSMPs were removed by ferric chloride and PACl at a maximum rate of 99.4% and 98.2%, respectively. But Ma et al. [52] reported that ferric chloride, without PAM, removed d < 0.5 mm PEMPs at a rate of 8.24% ± 1.22 and 12.65% ± 1.09 with 0.5 mM and 5 mM concentration, respectively. However, density of PS (1.05 g/ cm3) and PE (0.94–0.97 g/cm3) polymers are higher than that of PP polymer (0.90 g/cm3) which was investigated in this study.
Statistical analysis
Independent variables (A: PPMPs size, B: pH, and D: PAM dosage) in CCD were applied to Eq. 5 to predict the response value (Y). CCD was able to evaluate interactions among variables affecting the response [71]. AB, B2, and D2 represent interaction effect, two second-order effect for A and B, respectively. Significant coefficients using ANOVA are indicated at p > 0.05 (Table 4). F-value indicates effectiveness of the model, which was 34.45. Generally, lower p-value and higher F-values imply goodness of fit.
| 5 |
Table 4.
Table of ANOVA for the quadratic model for PPMPs removal
| Source | Sum of squares | df | Mean square | F- value | p- value |
|---|---|---|---|---|---|
| Model | 375.42 | 6 | 62.57 | 34.45 | < 0.0001 |
| A- PPMPs size | 287.04 | 1 | 287.04 | 158.03 | < 0.0001 |
| B- pH | 35.04 | 1 | 35.04 | 19.29 | 0.0002 |
| D- PAM dosage | 18.37 | 1 | 18.37 | 10.12 | 0.0042 |
| AB | 22.56 | 1 | 22.56 | 12.42 | 0.0018 |
| B2 | 6.89 | 1 | 6.89 | 3.79 | 0.0638 |
| D2 | 6.89 | 1 | 6.89 | 3.79 | 0.0638 |
| Residual | 41.78 | 23 | 1.82 | ||
| Lack of fit | 40.28 | 18 | 2.24 | 7.46 | 0.0177 |
| Pure error | 1.50 | 5 | 0.30 | ||
| Cor. total | 417.20 | 29 |
SD = 1.35, C.V. = % 21.06, R2 = 0.900, R2adj = 0.8737
Determination coefficient (R2) and the adjusted R2 for the model were 0.900 and 0.874, respectively. The higher R2 indicates that the predicted value and the actual value are in good agreement. To our knowledge, this was the first study to apply RSM on MPs coagulation.
Effect of variables on removal rate
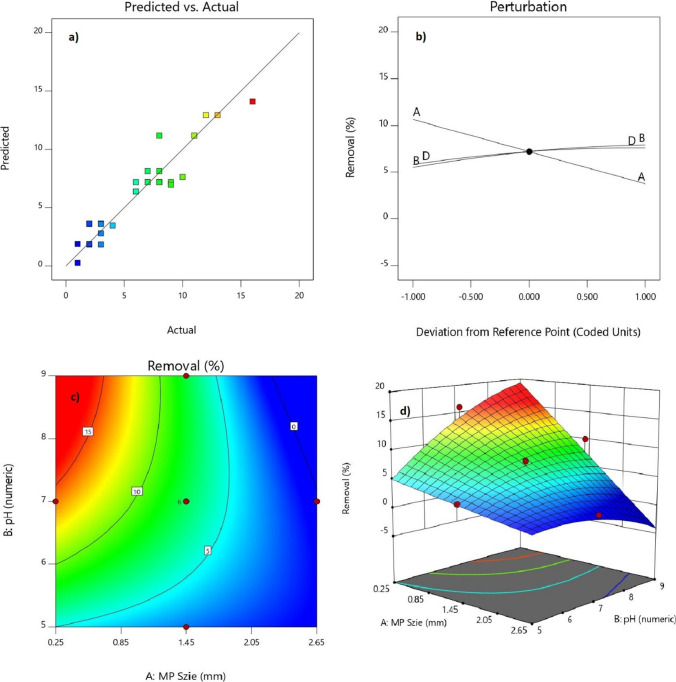
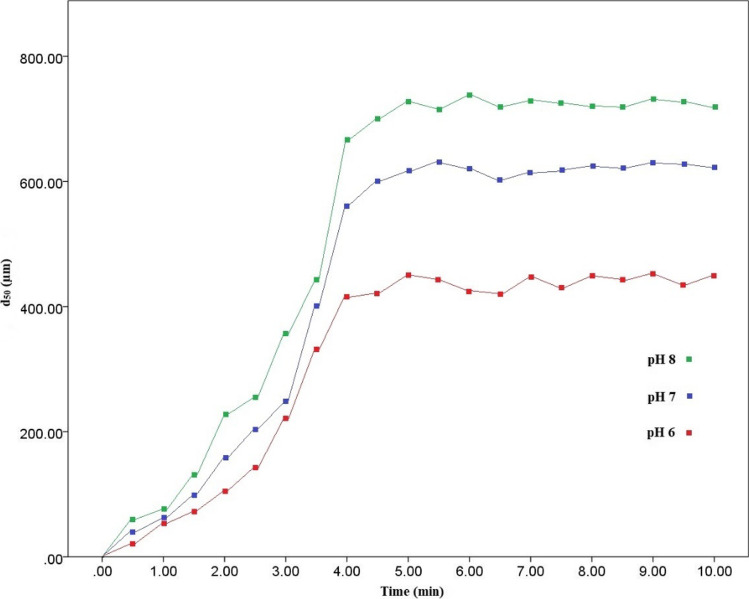
Perturbation plots illustrate that PPMPs size had the most effect on response, and by the decrease in size of particles, there was an increase in removal rate (F-value = 158.03) (Fig. 2b). This suggests that smaller MPs (d < 0.25 and 0.25 > d > 0.85 mm) can easily be trapped in formed flocs than larger ones. In other words, other variables (pH, PACl, and PAM dosage) were less significant in removal rates. However, dynamic floc sizes detected in pH 6, 7 and 8 by PSA revealed that by increase in pH value (F-value = 19.29), larger flocs were formed, which implies that more MPs can be trapped in them, leading to a rise in removal rates (Fig. 3). Conversely, lower removal rates were observed at lower pH due to the smaller size of flocs. 2D and 3D plots of pH and PPMPs size interaction affecting removal rates in which the highest degree of removal was observed to be 16% (Figs. 2c and d). In all thirty experiments conducted, almost no 1.45 < d < 2.05 and 2.05 < d < 2.65 mm PPMPs were removed, but 0.85 < d < 1.45 mm PPMPs varied in sedimentation ranging from 3 to 10% with regards to the other variables’ effect. Mean particle sizes (d50) of flocs were 331.53, 460.34, and 544.66 µm for pH 6, 7, and 8, respectively (Fig. 3). This supports the higher removal rate of d < 0.25 and 0.25 < d < 0.85 mm PPMPs; accordingly, it was observed that the removal rate at the pH 8 and 9 was higher than that of the lower pH (5, 6, and 7) and even 10% of 0.85 < d < 1.45 mm PPMPs were observed to be removed at pH 9 (run 24). Different size range of MPs for coagulation experiment have been investigated. Rajala et al. [55] investigated two different sizes of 1 µm and 6.3 µm spherical PSMPs and Skaf et al. [51] used 1–5 µm spherical MPs and three types of microfibers with a diameter of 5 and 15 µm. Rajala et al. [55] reported an almost complete removal rate and Skaf et al. [51] demonstrated a decrease in turbidity from 16 to less than 1 NTU, while Ma et al. [53] reported a 61.19% ± 3.67 and 18.34% ± 3.28 removal rate for d < 0.5 mm and 2 < d < 5 mm, respectively. This implies that findings of this study was comparable to Ma et al. [53]. This shows that, except from the polymer type, size of MPs can directly influence removal rate in coagulation process. However, water chemistry in samples preparation can influence coagulation process [72]. Rajala et al. [55] used effluent of a WWTP for sample preparation, Skaf et al. [51] used different types of non-ionic and anionic surfactants, while the sample solution in Ma et al. [53] and in this study were conducted in deionized water to simulate DWTPs water condition.
Fig. 2.
A) Actual vs. predicted removal rate of PPMPs. B) perturbation plot removal rate of PPMPs and effectiveness of independent variables. C) 2D plot of pH and PPMPs size' effect on removal rate. D) 3D plot of pH and PPMPs size' effect on removal rate
Fig. 3.
Particle size of flocs in different pH in predicted optimum condition
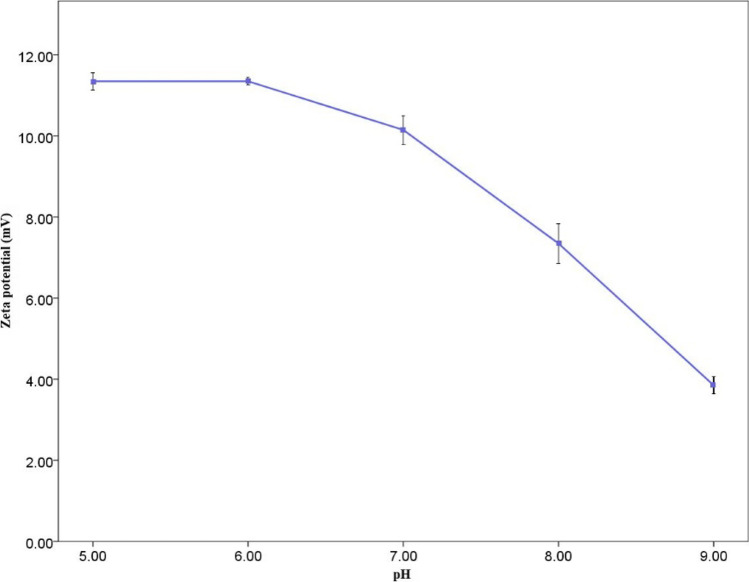
Figure 4 shows the zeta potential of different solution pH and the formed flocs in the optimum condition predicted by the RSM. Zeta potential for all the 5 pH was positive. However, by increasing the pH rate, zeta potential decreased, indicating instability of the particles at the higher pH. At pH 5 and 6, the mean zeta potential was almost the same, 11.35 ± 0.15% and 11.35 ± 0.05%, respectively. However, it dropped sharply at pH 8 and 9 to 7.35 ± 0.35% and 3.85 ± 0.15%, close to zero, which means more particles' instability. As reported by Ma et al. [53], by the increase in pH value from 6 to 8, zeta potential dropped to 1.91 ± 0.34, 0.52 ± 0.14 and -3.43 ± 1.18 mV, respectively. This is in line with the findings of this study, however, in all pH range, zeta potential was positive. Additionally, Arenas et al. [50] reported that increase in pH in chitosan solution (100 ppm), zeta potential value decreased steadily from 60 mV at pH 3 to just above zero at pH 9, remaining positive at all pH range. Likewise, in this study, higher pH (8 and 9) indicates instability of the particles and rapid coagulation [73]. However, zeta potential remained negative from pH 3 to 11 with sodium alginate solution (100 ppm), dropping from -13 to under -30 mV. Moreover, Perren et al. [54], using electrocoagulation (EC) to remove PE microbeads, demonstrated that optimum PE removal was 99.24% at pH 7.5.
Fig. 4.
Zeta potential of flocs in different pH in predicted optimum condition. Error bars indicate one standard deviation of the mean
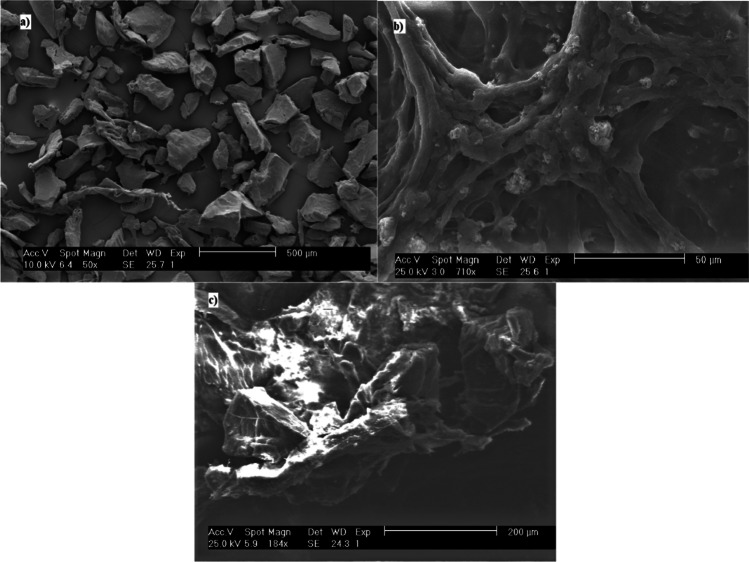
Images of the d < 0.25 PPMPs, before and after coagulation is shown in Fig. 5. The morphology of particles were detected as fragments (Fig. 5) [15]. The PAM dosage in the perturbation plot (Fig. 2a) shows that it was less effective in removal rate than variables, as mentioned earlier. However, by the increase in PAM dosage (F-value = 10.12), an increase in removal rate was observed. Addition of PAM caused the flocs to be heavier and settle faster, and the sticky characteristics of PAM had a positive effect in trapping PPMPs in the flocs. The lower amount of PAM in the solution (10 and 13 ppm) caused the maximum removal rate to be 11% (run 26), but a higher amount of PAM (19 and 22 ppm) had an influence in sedimentation of 0.25 < d < 0.85 PPMPs to reach a maximum of 13% (run 12). Additionally, PACl dosage was observed to have the least impact on the removal rate, whereas a higher amount in PACl dosage was not efficient in forming larger flocs. The size of the flocs had an indirect relation with a decrease in PACl dosage (200 and 400 ppm). PAM usage in Ma et al. [53] was significant in PEMPs removal. They observed the removal of d < 0.5 mm PEMPs with 5 mM AlCl3.6H2O to be 25.83% ± 2.91 without cationic PAM, but 45.34% ± 3.93 with 15 ppm cationic PAM. However, as of the findings of this study, they demonstrated that anionic PAM is more efficient in PEMPs removal. They reported an increase in removal rate of these particles from 25.83% ± 2.91 without anionic PAM to 61.19% ± 3.67 with 15 ppm anionic PAM with the same PPMP size. Moreover, in another study, Ma et al. [52] reported that 2 mM ferric chloride removed 13.27% ± 2.19 of d < 0.5 mm PEMPs, while this amount increased sharply to 90.91% ± 1.01 with 15 ppm anionic PAM. They also showed that ferric chloride coupled with anionic PAM is more efficient than with cationic PAM in removing PEMPs.
Fig. 5.
SEM images of a) PPMPs before coagulation b) flocs without PPMPs c) PPMPs trapped in flocs
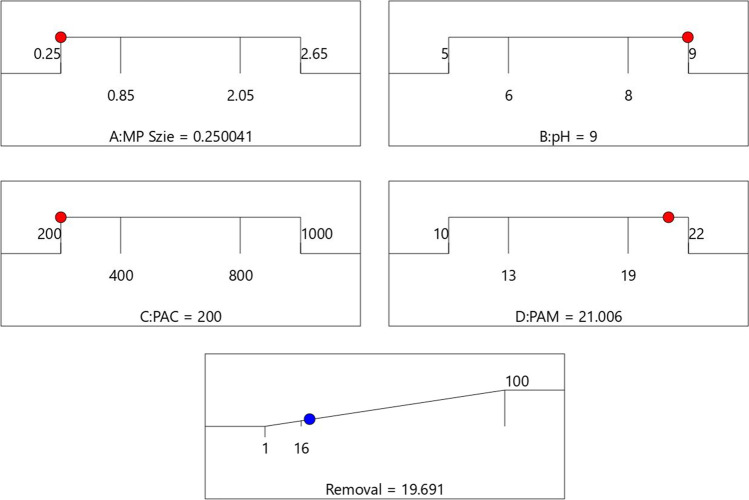
Based on response optimization criteria in Design-Expert software, the maximum rate of removal was obtained by the maximum pH rate and PAM dosage, and the minimum rate of PPMP size and PACl dosage. Maximum removal rate was predicted to be 19.69% (Table 5), which was obtained by setting independent variables to "in range" and the response to "maximize" with the upper limit of 100% (Fig. 6, Table 6). The predicted maximum removal rate was chosen among 100 different scenarios with the highest removal rate and desirability. Subsequently, four experimental tests were conducted with the offered optimum conditions to obtain the highest removal rate. Table 5 shows the mean sedimentation rate of the experimental test that was 18.75 ± 1.48%, with the SD error amount of -0.94%. This revealed that the chosen model is well fitted to the experimental coagulation results.
Table 5.
A comparison between predicted and actual rate of removal in optimum condition. Error bar indicates one standard deviation of the mean
| Condition | Removal (%) | Desirability |
|---|---|---|
| Experimental | 18.75 ± 1.48 | - |
| Predicted | 19.61 | 0.186 |
| Error | - 0.94 | - |
Fig. 6.
Optimum condition for PPMPs removal predicted by RSM
Table 6.
Experimental range and levels of independent variables
| Variables | Variables selection criteria |
|---|---|
| A- PPMPs size (mm) | In range |
| B- pH | In range |
| C- PACl dosage (ppm) | In range |
| D- PAM dosage (ppm) | In range |
| Removal (%) | maximize |
Further study
Our results indicated that ferric chloride and PACl are inefficient in removing PPMPs particles from drinking water and numerous MPs can be ingested through tap water. This supports findings of Tong et al. [43] that demonstrated humans may ingest up to 660 MP particles/L. Not only does ingestion of PPMPs cause potential health risks [74], but also these particles can potentially transfer harmful chemicals to humans via adsorption after ingestions of MPs [75–78], so removal of MPs from DWTPs is of high importance. Therefore further studies are recommended to investigate PPMPs fate in DWTPs. Since conventional coagulants fail to remove PPMPS efficiently in DWTPs, it is recommended that alternative coagulants and coagulant aids be analyzed to achieve higher rate of PPMPs removal to mitigate the negative impacts for human health. Additionally, removal characteristics of other polymers in coagulation/flocculation process of DWTPs, for example PS, PET and PVC are required to be investigated to fill the knowledge gap in MPs removal in DWTPs.
Conclusions
In this study, the characterization of PACl and anionic PAM in the removal of PPMPs in DWTPs were optimized using RSM. In the Design Expert, five levels of pH, PPMPs size, PACl dosage, and PAM dosage as independent variables and removal rate as the response were investigated. Among the chosen independent variables, PPMPs size and PACl dosage had an indirect relation with removal rate and with decrease in the size of PPMPs and PACl dosage, removal rate increased. Conversely, PAM dosage and pH had a direct relation with removal rate and with the increase in pH, larger flocs formed and removal rate increased. Therefore, optimum condition of the independent variables was the pH 9, 200 ppm of PACl, PPMPs size of d < 0.25 mm, and 21 ppm of PAM, resulting in a maximum removal rate of 18.75 ± 1.48%. According to the results of this study, conventional DWTPs that use ferric chloride or PACl as coagulants, are incapable of removing PPMPs microplastics.
Acknowledgements
This work was conducted in the laboratory of Islamic Azad University, West Tehran Branch by the authors with no funding support.
Funding
All the expenditures on the findings of this study were provided by the authors.
Data availability
Not applicable.
Declarations
Conflict of interest
This manuscript has not been submitted to, nor is under review at, another journal or other publishing venue. The following authors have affiliations with organizations with no financial support in the subject matter discussed in the manuscript.
Footnotes
Highlights
• Higher pH results in higher PPMPs coagulation.
• A higher dosage of PACl does not guarantee a higher rate of coagulation.
• Smaller PPMPs can be trapped in flocs and settle in the coagulation/flocculation process.
• DWTPs using conventional coagulants are not capable of efficiently removing PPMPs.
• Maximum rate of PPMPs removal in coagulation/flocculation in DWTPs is < 20%.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017;3:e1700782. doi: 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrelle SB, Ringma J, Law KL, Monnahan CC, Lebreton L, McGivern A, Murphy E, Jambeck J, Leonard GH, Hilleary MA, Eriksen M, Possingham HP, De Frond H, Gerber LR, Polidoro B, Tahir A, Bernard M, Mallos N, Barnes M, Rochman CM. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science. 2020;369:515–1518. doi: 10.1126/science.aba3656. [DOI] [PubMed] [Google Scholar]
- 3.PlasticsEurope, Plastics – the Facts 2019. An analysis of European plastics production, demand and waste data. 2019. www.plasticseurope.org
- 4.Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL. Plastic waste inputs from land into the ocean. Science. 2015;347:768–771. doi: 10.1126/science.1260352. [DOI] [PubMed] [Google Scholar]
- 5.Walker TR. Drowning in debris: Solutions for a global pervasive marine pollution problem. Mar Pollut Bull. 2018;126(338):338–338. doi: 10.1016/j.marpolbul.2017.11.039. [DOI] [PubMed] [Google Scholar]
- 6.Kutralam-Muniasamy G, Pérez-Guevara F, Elizalde-Martínez I, Shruti VC. Review of current trends, advances and analytical challenges for microplastics contamination in Latin America. Environ Pollut. 2020;267:115463. doi: 10.1016/j.envpol.2020.115463. [DOI] [PubMed] [Google Scholar]
- 7.Schmaltz E, Melvin EC, Diana Z, Gunady EF, Rittschof D, Somarelli JA, Virdin J, Dunphy-Daly MM. Plastic pollution solutions: emerging technologies to prevent and collect marine plastic pollution. Environ Int. 2020;144:106067. doi: 10.1016/j.envint.2020.106067. [DOI] [PubMed] [Google Scholar]
- 8.Tang Y, Liu Y, Chen Y, Zhang W, Zhao J, He S, Yang C, Zhang T, Tang C, Zhang C, Yan Z. A review: Research progress on microplastic pollutants in aquatic environments. Sci Tot Environ. 2020;42572. 10.1016/j.scitotenv.2020.142572 [DOI] [PubMed]
- 9.Morten WR, Alexis L, Michael H. Mapping of global plastics value chain and plastics losses to the environment: with a particular focus on marine environment. UN Environment Program. 2018;1–99. http://hdl.handle.net/20.500.11822/26745
- 10.Jaikumar G, Brun NR, Vijver MG, Bosker T. Reproductive toxicity of primary and secondary microplastics to three cladocerans during chronic exposure. Environ Pollut. 2019;249:638–646. doi: 10.1016/j.envpol.2019.03.085. [DOI] [PubMed] [Google Scholar]
- 11.Rochman CM. The complex mixture, fate and toxicity of chemicals associated with plastic debris in the marine environment. In: Bergmann M, Gutow L, Klages M. editors. Marine anthropogenic litter. Springer, Cham. 2015;117–140. 10.1007/978-3-319-16510-3_5
- 12.Andrady AL. Persistence of plastic litter in the oceans. In: Bergmann M, Gutow L, Klages M. editors. Marine anthropogenic litter, Springer, Cham. 2015;57–72. 10.1007/978-3-319-16510-3_3
- 13.El Hadri H, Gigault J, Maxit B, Grassl B, Reynaud S. Nanoplastic from mechanically degraded primary and secondary microplastics for environmental assessments. NanoImpact. 2020;17:100206. doi: 10.1016/j.impact.2019.100206. [DOI] [Google Scholar]
- 14.Su L, Xue Y, Li L, Yang D, Kolandhasamy P, Li D, Shi H. Microplastics in Taihu Lake, China. Environ Pollut. 2016;216:711–719. doi: 10.1016/j.envpol.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 15.Mao R, Hu Y, Zhang S, Wu R, Guo X. Microplastics in the surface water of Wuliangsuhai Lake, northern China. Sci Total Environ. 2020;723:137820. doi: 10.1016/j.scitotenv.2020.137820. [DOI] [PubMed] [Google Scholar]
- 16.Anderson PJ, Warrack S, Langen V, Challis JK, Hanson ML, Rennie MD. Microplastic contamination in Lake Winnipeg, Canada. Environ Pollut. 2017;225:223–231. doi: 10.1016/j.envpol.2017.02.072. [DOI] [PubMed] [Google Scholar]
- 17.Lin L, Zuo LZ, Peng JP, Cai LQ, Fok L, Yan Y, Li HX, Xu XR. Occurrence and distribution of microplastics in an urban river: a case study in the Pearl River along Guangzhou City, China. Sci Total Environ. 2018;644:375–381. doi: 10.1016/j.scitotenv.2018.06.327. [DOI] [PubMed] [Google Scholar]
- 18.Slootmaekers B, Catarci Carteny C, Belpaire C, Saverwyns S, Fremout W, Blust R, Bervoets L. Microplastic contamination in gudgeons (Gobio gobio) from Flemish rivers (Belgium) Environ Pollut. 2019;244:675–684. doi: 10.1016/j.envpol.2018.09.136. [DOI] [PubMed] [Google Scholar]
- 19.Wang S, Chen H, Zhou X, Tian Y, Lin C, Wang W, Zhou K, Zhang Y, Lin H. Microplastic abundance, distribution and composition in the mid-west Pacific Ocean. Environ Pollut. 2020;264:114125. doi: 10.1016/j.envpol.2020.114125. [DOI] [PubMed] [Google Scholar]
- 20.Kor K, Mehdinia A. Neustonic microplastic pollution in the Persian Gulf. Mar Pollut Bull. 2020;150:110665. doi: 10.1016/j.marpolbul.2019.110665. [DOI] [PubMed] [Google Scholar]
- 21.Nematollahi MJ, Moore F, Keshavarzi B, Vogt RD, Nasrollahzadeh Saravi H, Busquets R. Microplastic particles in sediments and waters, south of Caspian Sea: frequency, distribution, characteristics, and chemical composition. Ecotoxicol Environ Saf. 2020;206:111137. doi: 10.1016/j.ecoenv.2020.111137. [DOI] [PubMed] [Google Scholar]
- 22.Karbalaei S, Golieskardi A, Hamzah HB, Abdulwahid S, Hanachi P, Walker TR, Karami A. Abundance and characteristics of microplastics in commercial marine fish from Malaysia. Mar Pollut Bull. 2019;148:5–15. doi: 10.1016/j.marpolbul.2019.07.072. [DOI] [PubMed] [Google Scholar]
- 23.Karbalaei S, Golieskardi A, Watt DU, Bioret M, Hanachi P, Walker TR, Karami A. Analysis and inorganic composition of microplastics in commercial Malaysian fish meals. Mar Pollut Bull. 2020;150:110687. doi: 10.1016/j.marpolbul.2019.110687. [DOI] [PubMed] [Google Scholar]
- 24.Pivokonsky M, Cermakova L, Novotna K, Peer P, Cajthaml T, Janda V. Occurrence of microplastics in raw and treated drinking water. Sci Total Environ. 2018;643:1644–1651. doi: 10.1016/j.scitotenv.2018.08.102. [DOI] [PubMed] [Google Scholar]
- 25.Pivokonský M, Pivokonská L, Novotná K, Čermáková L, Klimtová M. Occurrence and fate of microplastics at two different drinking water treatment plants within a river catchment. Sci Total Environ. 2020;741:140236. doi: 10.1016/j.scitotenv.2020.140236. [DOI] [PubMed] [Google Scholar]
- 26.Gündoğdu S, Çevik C, Güzel E, Kilercioğlu S. Microplastics in municipal wastewater treatment plants in Turkey: a comparison of the influent and secondary effluent concentrations. Environ Monit Assess. 2018;190(11):626. doi: 10.1007/s10661-018-7010-y. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Lin T, Chen W. Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP) Sci Tot Environ. 2020;700:134520. doi: 10.1016/j.scitotenv.2019.134520. [DOI] [PubMed] [Google Scholar]
- 28.Adib D, Mafigholami R, Tabeshkia H. Identification of microplastics in conventional drinking water treatment plants in Tehran. Iran J Environ Health Sci Eng. 2021 doi: 10.1007/s40201-021-00737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prata JC. Microplastics in wastewater: State of the knowledge on sources, fate and solutions. Mar Pollut Bull. 2018;129(1):262–65. doi: 10.1016/j.marpolbul.2018.02.046. [DOI] [PubMed] [Google Scholar]
- 30.Gies EA, LeNoble JL, Noel L, Etemadifar A, Bishay F, Hall ER, Ross PS. Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada. Mar Pollut Bull. 2018;133:553–561. doi: 10.1016/j.marpolbul.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Mintenig SM, Int-Veen I, Löder MGJ, Primpke S, Gerdts G. Identification of microplastic in effluents of waste water treatment plants using focal plane array-based micro-Fourier-transform infrared imaging. Water Res. 2017;108:365–372. doi: 10.1016/j.watres.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Simon M, van Alst N, Vollertsen J. Quantification of microplastic mass and removal rates at wastewater treatment plants applying Focal Plane Array (FPA)-based Fourier Transform Infrared (FT-IR) imaging. Water Res. 2018;142:1–9. doi: 10.1016/j.watres.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt C, Kumar R, Yang S, Büttner O. Microplastic particle emission from wastewater treatment plant effluents into river networks in Germany: Loads, spatial patterns of concentrations and potential toxicity. Sci Tot Environ. 2020;737:139544. doi: 10.1016/j.scitotenv.2020.139544. [DOI] [PubMed] [Google Scholar]
- 34.Conley K, Clum A, Deepe J, Lane H, Beckingham B. Wastewater treatment plants as a source of microplastics to an urban estuary: removal efficiencies and loading per capita over one year. Water Res X. 2019;3:100030. doi: 10.1016/j.wroa.2019.100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kay P, Hiscoe R, Moberley I, Bajik L, McKenna M. Wastewater treatment plants as a source of microplastics in river catchments. Environ Sci Pollut Res. 2018;25:20264–20267. doi: 10.1007/s11356-018-2070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calderon EA, Hansen P, Rodríguez A, Blettler M, Syberg K, Khan FR. Microplastics in the digestive tracts of four fish species from the Ciénaga Grande de Santa fMarta Estuary in Colombia. Water Air Soil Pollut. 2019;230:257. doi: 10.1007/s11270-019-4313-8. [DOI] [Google Scholar]
- 37.Wardlaw C, Prosser RS. Investigation of microplastics in freshwater mussels (Lasmigona Costata) from the Grand River watershed in Ontario. Canada Water Air Soil Pollut. 2020;231(8):1–14. doi: 10.1007/s11270-020-04741-5. [DOI] [Google Scholar]
- 38.Naidoo T, Glassom D. Decreased growth and survival in small juvenile fish, after chronic exposure to environmentally relevant concentrations of microplastic. Mar Pollut Bull. 2019;145:254–259. doi: 10.1016/j.marpolbul.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 39.Huang JS, Koongolla JB, Li HX, Lin L, Pan YF, Liu S, He WH, Maharana D, Xu XR. Microplastic accumulation in fish from Zhanjiang mangrove wetland. South China Sci Tot Environ. 2020;708:134839. doi: 10.1016/j.scitotenv.2019.134839. [DOI] [PubMed] [Google Scholar]
- 40.Ahrendt C, Perez-Venegas DJ, Urbina M, Gonzalez C, Echeveste P, Aldana M, Pulgar J, Galbán-Malagón C. Microplastic ingestion cause intestinal lesions in the intertidal fish Girella laevifrons. Mar Pollut Bull. 2020;151:110795. doi: 10.1016/j.marpolbul.2019.110795. [DOI] [PubMed] [Google Scholar]
- 41.Wang W, Ge J, Yu X. Bioavailability and toxicity of microplastics to fish species: a review. Ecotoxicol Environ Saf. 2020;189:109913. doi: 10.1016/j.ecoenv.2019.109913. [DOI] [PubMed] [Google Scholar]
- 42.Qiang L, Cheng J. Exposure to Polystyrene microplastics impairs Gonads of Zebrafish (Danio Rerio) Chemosphere. 2021;263:128161. doi: 10.1016/j.chemosphere.2020.128161. [DOI] [PubMed] [Google Scholar]
- 43.Tong H, Jiang Q, Hu X, Zhong X. Occurrence and identification of microplastics in tap water from China. Chemosphere. 2020;252:126493. doi: 10.1016/j.chemosphere.2020.126493. [DOI] [PubMed] [Google Scholar]
- 44.Cox KD, Covernton GA, Davies HL, Dower JF, Juanes F, Dudas SE. Human consumption of microplastics. Environ Sci Technol. 2019;53:7068–7074. doi: 10.1021/acs.est.9b01517. [DOI] [PubMed] [Google Scholar]
- 45.Fiorentino I, Gualtieri R, Barbato V, Mollo V, Braun S, Angrisani A, Turano M, Furia M, Netti PA, Guarnieri D, Fusco S, Talevi R. Energy independent uptake and release of polystyrene nanoparticles in primary mammalian cell cultures. Exp Cell Res. 2015;330(2):240–247. doi: 10.1016/j.yexcr.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 46.Forte M, Iachetta G, Tussellino M, Carotenuto R, Prisco M, De Falco M, Laforgia V, Valiante S. Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells. Toxicol In Vitro. 2015;31:126–136. doi: 10.1016/j.tiv.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Sillanpää M, Ncibi MC, Matilainen A, Vepsäläinen M. Removal of natural organic matter in drinking water treatment by coagulation: a comprehensive review. Chemosphere. 2018;190:54–71. doi: 10.1016/j.chemosphere.2017.09.113. [DOI] [PubMed] [Google Scholar]
- 48.Nyström F, Nordqvist K, Herrmann I, Hedström A, Viklander M. Removal of metals and hydrocarbons from stormwater using coagulation and flocculation. Water Res. 2020;182:115919. doi: 10.1016/j.watres.2020.115919. [DOI] [PubMed] [Google Scholar]
- 49.Lapointe M, Farner JM, Hernandez LM, Tufenkji N. Understanding and improving microplastic removal during water treatment: impact of coagulation and flocculation. Environ Sci Technol. 2020;14(54):8719–8727. doi: 10.1021/acs.est.0c00712. [DOI] [PubMed] [Google Scholar]
- 50.Arenas R, Marcela L, Ramseier S, Zimmermann S, Stoll S. Comparative study of the effect of aluminum chloride, sodium alginate and chitosan on the coagulation of polystyrene micro-plastic particles. J Colloid Sci Biotechnol. 2017;5(2):190–198. doi: 10.1166/jcsb.2016.1149. [DOI] [Google Scholar]
- 51.Skaf DW, Punzi VL, Rolle JT, Kleinberg KA. Removal of micron-sized microplastic particles from simulated drinking water via alum coagulation. Chem Eng J. 2020;386:123807. doi: 10.1016/j.cej.2019.123807. [DOI] [Google Scholar]
- 52.Ma B, Xue W, Hu C, Liu H, Qu J, Li L. Characteristics of microplastic removal via coagulation and ultrafiltration during drinking water treatment. Chem Eng J. 2018;359:159–167. doi: 10.1016/j.cej.2018.11.155. [DOI] [Google Scholar]
- 53.Ma B, Xue W, Ding Y, Hu C, Liu H, Qu J. Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. J Environ Sci (China) 2019;78:267–275. doi: 10.1016/j.jes.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 54.Perren W, Wojtasik A, Cai Q. Removal of microbeads from wastewater using electrocoagulation. ACS Omega. 2018;3(3):3357–3364. doi: 10.1021/acsomega.7b02037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rajala K, Grönfors O, Hesampour M, Mikola A. Removal of microplastics from secondary wastewater treatment plant effluent by coagulation/flocculation with iron, aluminum and polyamine-based chemicals. Water Res. 2020;183:116045. doi: 10.1016/j.watres.2020.116045. [DOI] [PubMed] [Google Scholar]
- 56.Bridson JH, Patel M, Lewis A, Gaw S, Parker K. Microplastic contamination in Auckland (New Zealand) beach sediments. Mar Pollut Bull. 2020;151:110867. doi: 10.1016/j.marpolbul.2019.110867. [DOI] [PubMed] [Google Scholar]
- 57.Novotna K, Cermakova L, Pivokonska L, Cajthaml T, Pivokonsky L. Microplastics in drinking water treatment – current knowledge and research needs. Sci Tot Environ. 2020;667:730–740. doi: 10.1016/j.scitotenv.2019.02.431. [DOI] [PubMed] [Google Scholar]
- 58.Karbalaei S, Hanachi P, Rafiee G, Seifori P, Walker TR. Toxicity of polystyrene microplastics on juvenile Oncorhynchus Mykiss (Rainbow Trout) after individual and combined exposure with Chlorpyrifos. J Hazard Mater. 2021;403:123980. doi: 10.1016/j.jhazmat.2020.123980. [DOI] [PubMed] [Google Scholar]
- 59.Mamba BB, Krause RW, Malefetse TJ, Sithole SP, Nkambule TI. Humic acid as a model for Natural Organic Matter (NOM) in the removal of odorants from water by Cyclodextrin Polyurethanes. Water SA. 2009;35(1):117–120. doi: 10.4314/wsa.v35i1.76648. [DOI] [Google Scholar]
- 60.Wang HT, Ye YY, Qi J, Li FT, Tang YL. Removal of titanium dioxide nanoparticles by coagulation: effects of coagulants, typical ions, alkalinity and natural organic matters. Water Sci Technol. 2013;68(5):1137–1143. doi: 10.2166/wst.2013.356. [DOI] [PubMed] [Google Scholar]
- 61.Ait-Amir B, Pougnet P, El Hami A. Meta-Model Development, In: El Hami A, Pougnet P. editors. Embedded Mechatronic Systems, first ed. ISTE press- Elsevier Inc., 2015;151–79. 10.1016/C2014-0-04739-9
- 62.Singh B, Pradeep K. Pre-Treatment of petroleum refinery wastewater by coagulation and flocculation using mixed coagulant: optimization of process parameters using Response Surface Methodology (RSM) J Water Process Eng. 2020;36:101317. doi: 10.1016/j.jwpe.2020.101317. [DOI] [Google Scholar]
- 63.Environmental Protection Agency. Water Treatment Manuals: Coagulation, Flocculation & Clarification. 2002; http://www.epa.ie/pubs/advice/drinkingwater/EPA_water_treatment_mgt_coag_flocc_clar2.pdf
- 64.Mohammadi Parsa M, Pourfakhar H, Baghdadi M. Application of graphene oxide nanosheets in the coagulation-flocculation process for removal of Total Organic Carbon (TOC) from surface water. J Water Process Eng. 2020;37:101367. doi: 10.1016/j.jwpe.2020.101367. [DOI] [Google Scholar]
- 65.Sinha S, Yoon Y, Amy G, Yoon J. Determining the effectiveness of conventional and alternative coagulants through effective characterization schemes. Chemosphere. 2004;57:1115–1122. doi: 10.1016/j.chemosphere.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 66.Kang M, Kamei T, Magara Y. Comparing polyaluminum chloride and ferric chloride for antimony removal. Water Res. 2003;37(17):4171–4179. doi: 10.1016/S0043-1354(03)00351-8. [DOI] [PubMed] [Google Scholar]
- 67.Matsui Y, Shirasaki N, Yamaguchi T, Kondo K, Machida K, Fukuura T, Matsushita T. Characteristics and components of poly-aluminum chloride coagulants that enhance arsenate removal by coagulation: Detailed analysis of aluminum species. Water Res. 2017;118:177–186. doi: 10.1016/j.watres.2017.04.037. [DOI] [PubMed] [Google Scholar]
- 68.Park H, Lim SI, Lee H, Woo DS. Water blending effects on coagulation-flocculation using aluminum sulfate (alum), polyaluminum chloride (PAC), and ferric chloride (FeCl3) using multiple water sources. Desalination Water Treat. 2015;57(16):7511–7521. doi: 10.1080/19443994.2015.1025583. [DOI] [Google Scholar]
- 69.Rana S, Suresh S. Comparison of different Coagulants for Reduction of COD from Textile industry wastewater. Mater Today. 2017;4(2):567–574. doi: 10.1016/j.matpr.2017.01.058. [DOI] [Google Scholar]
- 70.Abu Bakar A, Abdul HA. Treatment of automotive wastewater by coagulation-flocculation using polyaluminum chloride (PAC), ferric chloride (FeCl3) and aluminum sulfate (alum) AIP Conf Proc. 2013;1571:524. doi: 10.1063/1.4858708. [DOI] [Google Scholar]
- 71.Şahan T. Application of RSM for Pb(II) and Cu(II) adsorption by bentonite enriched with SH groups and a binary system study. J Water Process Eng. 2019;31:100867. doi: 10.1016/J.JWPE.2019.100867. [DOI] [Google Scholar]
- 72.Ma S, Liu C, Yang K, Lin D. Coagulation removal of humic acid-stabilized carbon nanotubes from water by PACl: influences of hydraulic condition and water chemistry. Sci Tot Environ. 2012;439:123–128. doi: 10.1016/j.scitotenv.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 73.Kumar A, Dixit C. Methods for characterization of nanoparticles. In: Nimseh, S., Chandra, R., Gupta, N. editors. Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids. Cambridge. Woodhead Publishing. 2017. 43–58. ISBN: 978–0–08–100557–6
- 74.Hwang J, Choi D, Han S, Choi J, Hong J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci Total Environ. 2019;684:657–669. doi: 10.1016/j.scitotenv.2019.05.071. [DOI] [PubMed] [Google Scholar]
- 75.Dong Y, Gao M, Song Z, Qui W. As(III) adsorption onto different-sized polystyrene microplastic particles and its mechanism. Chemosphere. 2020;239:124792. doi: 10.1016/j.chemosphere.2019.124792. [DOI] [PubMed] [Google Scholar]
- 76.Llorca M, Schirinzi G, Martinez M, Barcelo D, Farre M. Adsorption of perfluoroalkyl substances on microplastics under environmental conditions. Environ Pollut. 2018;235:680–691. doi: 10.1016/j.envpol.2017.12.075. [DOI] [PubMed] [Google Scholar]
- 77.Hu JQ, Yang SZ, Guo L, Xu X, Yao T, Xie F. Microscopic investigation on the adsorption of lubrication oil on microplastics. J Mol Liq. 2017;227:351–355. doi: 10.1016/j.molliq.2016.12.043. [DOI] [Google Scholar]
- 78.Zhang H, Jiaqing W, Zhou B, Zhou Y, Dai Z, Zhou Q, Chriestie P, Luo Y. Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: Kinetics, isotherms and influencing factors. Environ Pollut. 2018;51(21):12254–12263. doi: 10.1016/j.envpol.2018.09.122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.