Abstract
A 23-year-old man presented with complaints of macrohematuria and hematospermia and was referred to our hospital for further examination. Magnetic resonance imaging revealed a round 30 × 25 mm tumor in the right peripheral zone; hence, a rare prostate tumor was suspected. Grayscale transrectal ultrasonography (TRUS) was performed using the Aplio-i800 PVL-715RST-transducer and revealed a well-defined round tumor. Although regular color Doppler flow imaging could not detect internal blood flow, superb microvascular imaging (SMI) identified the low-velocity blood flow in the tumor. Based on the results of a TRUS-guided targeted biopsy assisted by SMI, the patient was diagnosed with stromal sarcoma. He underwent total pelvic exenteration with construction of ileal conduit and colostomy, the tumor was finally diagnosed as prostate stromal sarcoma (PSS). Since PSS is a rare malignant prostate tumor, reports on the characteristic findings in imaging tests are scarce. To the best of our knowledge, this study reports the first case in which a poor internal blood flow was detected in PSS, but not through regular color Doppler flow imaging. SMI revealed that the blood flow signal to the PSS was relatively poor; however, its definite presence was confirmed, suggesting a malignant disease with relatively poor blood supply and the findings of SMI would assist the adequate targeted biopsy-sampling from the presence site of viable cells with the blood supply.
Keywords: Prostate stromal sarcoma, Transrectal ultrasonography, Superb microvascular imaging
Introduction
Primary prostate sarcoma is a rare malignant tumor with extremely poor prognosis; therefore, detecting and diagnosing the lesion at an early stage is crucial [1, 2]. Among the several types of prostate sarcoma (PS), prostate stromal sarcoma (PSS) is especially rare, accounting for < 0.1% of primary prostate malignant tumors. Unfortunately, PS, including PSS, do not present specific symptoms or characteristics, including tumor makers, and are often diagnosed when serious conditions such as metastasis occur. Although imaging examination using computed tomography (CT), magnetic resonance imaging (MRI), or ultrasonography (US) can be used to detect PSS, the findings are not specific [3]. Here, we report the first case of PSS examined using transrectal ultrasonography (TRUS) with SMI to detect low-velocity blood flow. The characteristics of SMI findings may guide targeted biopsy and imaging diagnosis of PSS.
Case presentation
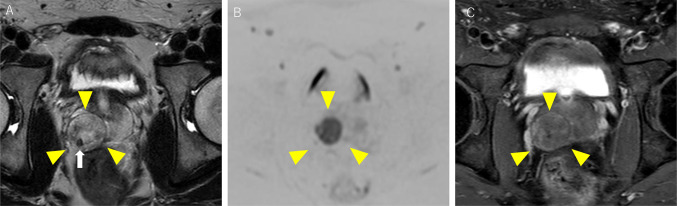
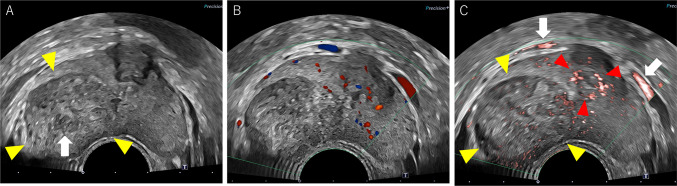
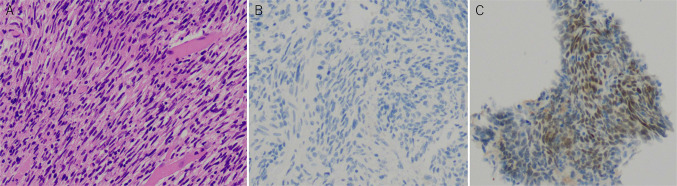
A 23-year-old man visited a local hospital with complaints of dysuria, macrohematuria, and hematospermia that began 1 month ago. He had no relevant medical or family history. Although no abnormalities were detected in the blood analysis and urinalysis, he underwent CT, which revealed a solid tumor measuring 30 mm in the right lobe of the prostate and was referred to our hospital for further examination. MRI revealed a well-defined 30 × 25 mm orbicular tumor in the right peripheral zone (Fig. 1). T2-weighted imaging showed a heterogeneous iso- to hyperintense signal of the tumor with a pseudocapsule, and diffusion-weighted imaging revealed the tumor as areas of markedly high signal intensity (Fig. 1A, B). Dynamic contrast-enhanced imaging revealed a slow enhancement within the solid part of the tumor (Fig. 1C). Digital rectal examination confirmed a smooth, palpable, and enlarged nodule in the right lobe without pain. Blood analysis revealed an increase in the neutrophil count, but all other routine indices were unremarkable. Urinalysis and cytology revealed no abnormal findings. The prostate-specific antigen level was 1.120 ng/ml, which was within the normal range (< 4.0 ng/ml). The levels of other tumor markers, including carcinoembryonic antigen, carbohydrate antigen 19–9, and neuron-specific enolase, were also within the normal range. Based on these findings, rare prostate tumors, such as phyllodes tumor, stromal tumor of uncertain malignant potential (STUMP), or stromal sarcoma (SS), were suspected. Grayscale TRUS was performed using the Aplio-i800 PVL-715RST-transducer (Canon Medical Systems, Japan) and demonstrated an orbicular well-defined tumor, consistent with the MRI findings (Fig. 2A). The internal echo of the tumor was heterogeneous, including a low-echoic area, suggesting a necrotic lesion. Regular color Doppler flow imaging could detect normal prostate blood flow but not the internal blood flow of the tumor; however, superb microvascular imaging (SMI) identified the tumor’s low-velocity blood flow (Fig. 2B, C). By comparing the negative blood flow signal in the tumor using conventional Doppler imaging (Fig. 2B), SMI clarified the relatively poor but definitive tumor blood flow (Fig. 2C). A TRUS-guided biopsy targeting the area with blood flow assisted by SMI was performed. Histopathological examination revealed that the tumor consisted of dense spindle-shaped cells with hyperchromatic nuclei and necrotic foci. Moreover, immunohistochemistry showed cytokeratin AE1/AE3(-), synaptophysin(-), Myo-D1(-), PgR(+ , partly), and CD34(+ , partly) expression, leading to a diagnosis of stromal sarcoma (Fig. 3A–C). Positron emission tomography-CT revealed neither metastatic lesions nor other tumors but showed intense accumulation of FDG at the tumor (SUVmax = 11.81); therefore, we concluded that the stromal sarcoma originated from the prostate. Based on the pathological diagnosis and imaging findings, total pelvic exenteration with construction of ileal conduit and colostomy was performed. The prostate tumor was composed of spindle-shape cells showing invasive proliferation and intricately fasciculation with extensive necrosis, corresponded with the findings of previous biopsy specimen. The tumor cells were immunohistochemically positive for vimentin and CD34 partly but negative for desmin, SMA, S-100, AE1/AE3 nor PSA. Although the morphological findings suggest synovial sarcoma (SyS), the Transducer-Like Enhancer 1 (TLE1), one of the SyS diagnostic markers, was weakly positive and more SS18-SSX, specific maker for SyS, was negative. From these histopathological results, the tumor was finally diagnosed as prostate stromal sarcoma. Unfortunately, 2 months after the surgery, distant metastatic lesions in lung and endopelvic lymph node appeared, he has received chemotherapy with doxorubicin and ifosfamide.
Fig. 1.
Magnetic resonance imaging (MRI) findings of the prostate tumor. A Axial T2-weighted image showing the tumor located in the right peripheral zone of the prostate with heterogeneous iso- to hyperintense signal and surrounding low signal intensity pseudocapsule (yellow arrowheads). In particular, the low signal intensity area in the posterior part of the tumor is indicative of fibrotic components (white arrow). B Axial diffusion-weighted image showing the tumor as areas of markedly high signal intensity (yellow arrowheads). C Axial dynamic contrast-enhanced MR image showing a weakly and gradually enhanced solid part of the tumor (yellow arrowheads)
Fig. 2.
Transrectal ultrasonography (TRUS) findings of the prostate tumor. A Grayscale TRUS image showing a well-defined round tumor (30 × 25 mm) at the right peripheral zone of the prostate with a heterogeneous internal echo (white arrowheads). The hypoechoic area in the center of the tumor indicates necrosis (white arrow). B TRUS color Doppler imaging did not reveal internal blood flow in the tumor, except for normal prostate blood flow. C TRUS with superb microvascular imaging (SMI) revealed low-velocity blood flow in the tumor (yellow arrowheads), hypervascularity in the urethra (red arrowheads), and Santorini plexus (white arrows). By comparing the negative blood flow signal in the tumor using conventional Doppler imaging (B), SMI clarified the relatively poor but definitive tumor blood supply
Fig. 3.
Histopathological findings from transrectal biopsy of the prostate tumor. Magnification: 200 × . Hematoxylin and eosin staining revealed abundant proliferating tumor cells with spindle-shaped hyperchromatic nuclei (A). Immunohistochemical examination shows that the tumor is negative for MyoD1 (B) and partly positive for PgR (C)
Discussion
Primary prostate sarcoma originates in the mesoderm of the reproductive tract, and the prognosis of this rare malignant tumor is extremely poor [1]. Among the subtypes of prostate sarcoma, rhabdomyosarcoma is the most common, accounting for approximately 40% of cases, followed by leiomyosarcoma [2]. Unclassified prostate stromal tumors are divided into two categories: PSSs and prostatic STUMPs. PSS is especially rare, accounting for < 0.1% of all primary malignant prostate tumors. The median age of patients with PSS was 38.5 years, which is less than that of patients with typical prostate adenocarcinoma [2]. Since clinical symptoms such as dysuria are not specific, the tumor is easily misdiagnosed as benign prostatic hyperplasia and is often significantly enlarged with serious conditions by the time of diagnosis. Therefore, detection of PSS at an early stage is critical. The MRI features of PSS have been reported to show a heterogeneous high signal intensity and a low signal intensity pseudocapsule on T2-weighted imaging and gradual weak enhancement on DCE-MRI, as demonstrated in the present case; however, this feature is not specific for PSS [3]. Importantly, characteristics such as large size, heterogeneity, and central necrosis are similar findings for various types of imaging modalities, including ultrasound (US), reflecting rapid growth and high malignancy of this tumor. Regarding US imaging, Wu et al. presented the usefulness of contrast-enhanced ultrasound (CEUS) for prostate sarcoma (PS) diagnosis and biopsy guidance [4]. In their study, US revealed an enlarged prostate, non-uniform internal echo, and a necrotic liquefying area in the center, whereas CEUS clearly revealed enhancement of the peritumoral parenchyma, and the physician could distinguish the solid mass of viable cells from necrotic area. Recently, SMI technology has been improved to guide prostate cancer biopsies. SMI is an innovative technique for detecting low-flow signals with minimal motion artifacts. A recent report described the usefulness of SMI for prostate biopsy, resulting from a positive correlation between microvascular quantity and the Gleason score [5, 6]. Zhu et al. described SMI detected blood flow signals in 97.3% (72/74) of the prostate cancer group which was statistically superior to color Doppler US. In addition, they reported that SMI identify prostate cancer foci as enriched blood signal zone even though the velocity is either high or low. According to their findings, typical prostate adenocarcinoma is visualized as the lesion with abundant blood flow signal area using SMI, however, the present case did not show enriched blood flow. The blood flow signals in PSS detected by SMI were relatively poorer than that surrounding normal prostate tissue but definitively some were detected, which was not detected with regular color Doppler images and was detected as the region with viable cells. To our best knowledge, this study is the first to report the ultrasound detection of the internal blood flow in PSS.
At present, TRUS-guided biopsy is the gold-standard method for the diagnosis of not only prostate adenocarcinoma but also PS. In most cases of PS, the enlarged tumor includes a large necrotic liquefying area that is inadequate for biopsy; therefore, it is crucial to detect the area comprising viable tumor cells. Although the tumor with 30 mm in diameter was easily detected through regular B-mode US in this case, SMI suggested some parts, which had relatively lower but definitive blood flow signals, where suggest non-necrotic tissue area. Importantly, advantages of the biopsy guidance of SMI in real-time TRUS include no need to use contrast medium, although such biopsy is difficult to be achieved with CT or MRI.
In conclusion, SMI revealed that the blood flow signal to the PSS was relatively poor, but its definite presence was confirmed, suggesting a malignant disease with relatively poor blood supply. Although prostate biopsy is required to confirm pathological diagnosis, decision of biopsy site assisted by SMI blood flow signals can be an instrumental method for the accurate diagnosis of PSS.
Declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical approval
All the procedures performed in studies involving human participants were in accordance with ethical standards of the institutional and national research committee and with the Helsinki Declaration of 1964 and later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from the patient for publication of this case report and any accompanying images.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bostwick DG, Egevad L. Prostatic stromal proliferations: a review. Pathology. 2021;53:12–25. doi: 10.1016/j.pathol.2020.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Yang W, Liu A, Wu J, et al. Prostatic stromal sarcoma: a case report and literature review. Medicine (Baltimore) 2018;97:e0495. doi: 10.1097/md.0000000000010495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreou A, Whitten C, MacVicar D, et al. Imaging appearance of sarcomas of the prostate. Cancer Imaging. 2013;13:228–237. doi: 10.1102/1470-7330.2013.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu G, Sun R, Hong H, et al. Contrast-enhanced ultrasound diagnosis of prostatic sarcoma: two case reports. Medicine (Baltimore) 2021;100:e24038. doi: 10.1097/md.0000000000024038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu YC, Shan J, Zhang Y, et al. Prostate cancer vascularity: superb microvascular imaging ultrasonography with histopathology correlation. Med Sci Monit. 2019;25:8571–8578. doi: 10.12659/msm.918318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C, Tao Y. Superb microvascular imaging in guiding targeted biopsy of prostate cancer: a protocol for systematic review and meta analysis. Medicine (Baltimore) 2020;99:e23604. doi: 10.1097/md.0000000000023604. [DOI] [PMC free article] [PubMed] [Google Scholar]