Abstract
Background
Today, approximately 10% of participants in assisted reproductive technology (ART) are defined as having recurrent implantation failure (RIF). Recent studies show that endometrial receptivity array can improve pregnancy and implantation rates by nearly 20% in women with RIF. However, these studies are limited, with little published data in the Chinese population. Recently, we have established a transcriptome-based endometrial receptivity assessment (Tb-ERA) method of predicting the endometrial window of implantation (WOI) using transcriptome-profiling data of different phases of the menstrual cycle from healthy fertile Chinese women by RNA-Seq. It is meaningful to conduct a randomized controlled trial (RCT) to assess the clinical efficiency of Tb-ERA in Chinese patients with RIF.
Methods
In this RCT, a total of 200 RIF patients will be recruited and randomized into 2 groups. Patients in the Tb-ERA group will undergo a Tb-ERA test, after which embryo transfer time will be adjusted according to Tb-ERA results and embryo transfer will be performed again in the next cycle. Patients in the control group will not receive any interventions until the next transfer cycle. We will perform statistical analysis on both groups at the primary endpoint (clinical-pregnancy rate) and at secondary endpoints (rate of WOI displacement, embryo implantation, biochemical pregnancy, early abortion, and ectopic pregnancy). Implications: This study aims to evaluate the effectiveness of our Tb-ERA test in Chinese RIF patients and to determine that whether Tb-ERA could improve the clinical-pregnancy rate in these RIF patients.
Trial registration
NCT04497558, registered August 4, 2020.
Keywords: Endometrial receptivity assessment, Recurrent implantation failure, Endometrial receptivity, Window of implantation
1. Introduction
Assisted reproductive technology (ART) is an important procedure to treat infertility. However, 10% of patients still fail to implant more than four high-quality embryos at least three times, which is defined as recurrent implantation failure (RIF) [[1], [2], [3]]. RIF brings tremendous economic and mental pressure to patients, so it is very important to study the etiology of this condition and potential method for intervention [4,5]. Although embryonic aneuploidy is the major factor causing RIF [[6], [7], [8], [9]], previous studies have revealed the importance of endometrial receptivity in RIF patients [10,11]. However, the relationship between the embryo and endometrial receptivity (ER) has not yet been sufficiently addressed [[12], [13], [14], [15]].
Endometrial receptivity refers to the endometrium's ability to accept embryos, indicating that it is in a state that allows embryonic localization, adhesion, and invasion [[16], [17], [18]]. This period is also known as the window of implantation (WOI) [19,20]. In 2011, Spanish scholars developed a genetic diagnostic tool for assessing endometrial receptivity based on gene expression microarray technology, called endometrial receptivity array [21]. Endometrial receptivity array is a customized deoxyribonucleic acid (DNA) microarray containing 238 genes that are differentially expressed at different stages of the endometrial cycle [22,23]. This combined transcriptomic signature can help pinpoint the personalized WOI [24,25].
In 2013, the Spanish group applied endometrial receptivity array to RIF patients [26]. That randomized control trial (RCT) study found that about 25.9% of RIF patients had WOI displacement, which is significantly higher than the control group. After subsequent personalized embryo transfer (pET) guided by endometrial receptivity array, the pregnancy rate in nonreceptive RIF patients became similar to that of receptive RIF patients. In 2017, a retrospective study by Japanese scholars yielded the similar results as the Spanish group [27]. Indian scholars also retrospectively compared clinical outcomes in 248 RIF patients. The results showed that pET guided by endometrial receptivity array in patients of RIF with displaced WOI improved the embryo implantation rate (IR) and ongoing pregnancy rate (OPR) [28]. A retrospective review conducted by Tan et al. was performed for 88 patients with a history of euploid blastocyst implantation failure, who underwent endometrial receptivity array testing between 2014 and 2017. Their results showed that IR and OPR after pET were higher (73.7 vs. 54.2% and 63.2 vs. 41.7%, respectively) compared to patients without pET [29].
Combined together, limited studies with small sample sizes have been published on endometrial receptivity array in RIF patients [[26], [27], [28], [29]]. Nowadays, 15 million infertile women undergo ART in China. Unfortunately, there are limited studies on the clinical efficiency of endometrial receptivity array in the Chinese population [30]. It is largely unknown whether the endometrial receptivity analysis based genes expression test would be suitable for this population. Recently, our group and Unimed Biotech (Shanghai) company developed a transcriptome-based endometrial receptivity assessment method (Tb-ERA) based on RNA-seq technology that can be used to predict WOI in Chinese patients. The accuracy of endometrial assessment on days luteinizing hormone (LH)+3, LH+5, LH+7, and LH+9 was 100% in the training set and 85.19% in the validation set [31]. Our Tb-ERA is accurate and sensitive in identifying gene expression signatures of the endometrium to pinpoint embryo transfer timing. Of the 238 genes in the Spanish endometrial receptivity array, only 133 (55.88%) are shared in common with our test, likely due to differences in ethnic backgrounds, or in profiling methodologies and data analyses.
The aim of this RCT is to evaluate the efficiency of the Tb-ERA developed by our group in Chinese RIF patients and to determine whether our Tb-ERA could improve the clinical-pregnancy rate in these RIF patients.
2. Hypothesis
Our hypothesis is that our Tb-ERA could pinpoint the accurate WOI and can systematically survey the prevalence of WOI displacement in Chinese RIF patients. Guided by Tb-ERA result, pET will be performed and it could improve the clinical-pregnancy rate in these RIF patients.
3. Methods
3.1. Trial design
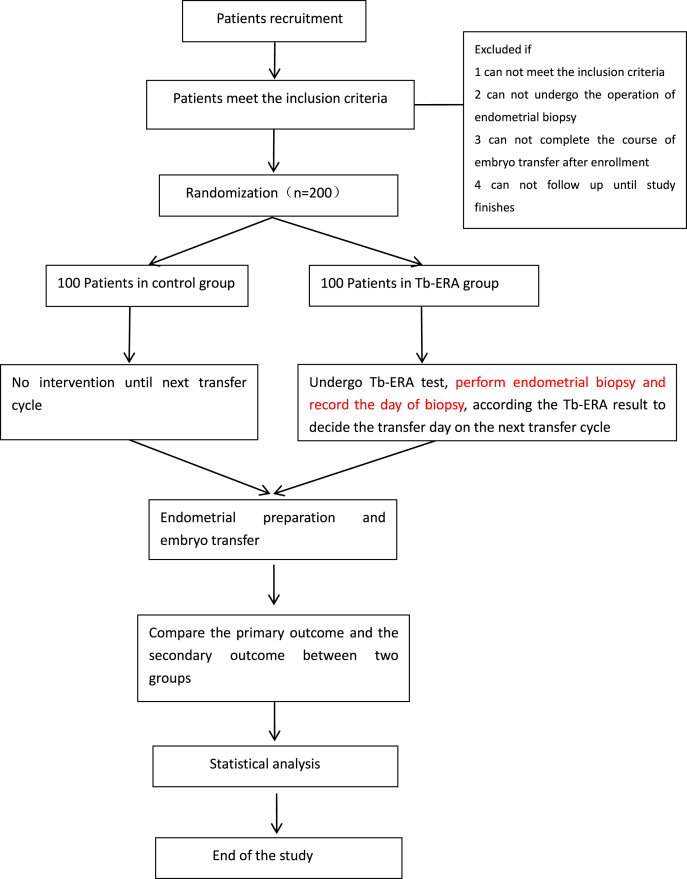
This will be a two-arm, parallel-control RCT. RIF patients will be divided randomly into the Tb-ERA group or the control group at a 1:1 ratio. Patients in the Tb-ERA group (n = 100) will undergo Tb-ERA, while those in the control group (n = 100) will not receive any treatments until the next transfer cycle. In the Tb-ERA group, embryo transfer time point will be adjusted according to Tb-ERA results, and embryo transfer will be performed again. The flowchart of this protocol is shown in Fig. 1. The schedule of patient enrollment, intervention and assessment is shown in Table 1. This study was approved by the Ethics Committee of Shanghai Ji Ai Genetics & In Vitro Fertilization (IVF) Institute, Obstetrics and Gynecology Hospital of Fudan University, Shanghai, China (approval number: JIAI E2020-015) and has been registered at Clinicaltrials.gov (Identifier NCT04497558).
Fig. 1.
Flow chart of the trial design Tb-ERA, transciptome based endometrial receptivity assessment.
Table 1.
SPIRIT table of enrolment, interventions, and assessments Tb-ERA, transcriptome based endometrial receptivity assessment.
| Enrolment Allocation Post allocation Close-out |
|---|
| ENROLMENT: |
| Eligibility screen × |
| Inclusion criteria × |
| Exclusion criteria × |
| Informed consent × |
| INTERVENTION: |
| Contro group |
| Tb-ERA group × |
| ASSESSMENTS: |
| Clinical pregnancy rate × |
| WOI displacement rate × |
| Embryo Implantation rate × |
| Biochemical pregnancy × |
| Early miscarriage rate × |
| Ectopic pregnancy rate × |
| Embryo transfer pregnancy rate × |
| Adverse events × |
Patients who have been diagnosed with RIF while undergoing in vitro fertilization (IVF) treatments at Shanghai Ji Ai Genetics & IVF Institute (Shanghai, China) will be informed of this trial. Before recruitment, we will provide them with the details and an explanation of this trial and give them enough time to decide whether to enroll in it. If they intend to enroll, and if they meet all of the inclusion criteria and none of the exclusion criteria, they will be recruited. After eligible participants sign the informed consent (IC) form, they will be randomly allocated to the Tb-ERA group or the control group.
3.2. Study population: inclusion and exclusion criteria
3.2.1. Inclusion criteria
-
●
Patients with unexplained RIF (≥3 attempts at embryo transfer, with ≥4 high-quality embryos failing to implant) [2]. (High-quality embryos were defined as either day-5 blastocysts of at least 4BB according to Gardner's classification or day-3 embryos of at least 7 cells grade B according to the Istanbul consensus criteria for cleavage stage embryos [32]).
-
●
Age: 20–40 years
-
●
Body mass index (BMI): 19–24 kg/m2.
-
●
Endometrial thickness: ≥7 mm.
3.2.2. Exclusion criteria
-
●
Known causes of embryonic-implantation failure, such as infection, reproductive-tract malformation, uterine-cavity factors, immune factors, or hydrosalpinx.
-
●
Decreased ovarian function meeting at least two of the following criteria: ① basal follicle-stimulating hormone (FSH) 10–25 IU/L, and/or estradiol (E2) > 292.8 pmoL/l, and/or FSH/luteinizing hormone (LH) > 3; ② < 5–7 antral follicles on the second to third day of menstruation; and/or antimullerian hormone (AMH) < 0.5–1.1 ng/ml [33].
-
●
People without history of genetic disorders.
-
●
Patients who have experienced abortions with positive histogenetic analysis.
3.3. Sample size estimation
This is a two-arm study. According to our previous studies, the clinical-pregnancy rate in RIF was elevated by nearly 20% according to the endometrial receptivity array test [[26], [27], [28], [29], [30]]. The sample size required for a test of equivalence would be 90 in each arm to give a power of 0.8 and type I error of 0.05. The maximum dropout rate during intervention is expected to be approximately 10%. Therefore, we will need a total of 200 randomized patients (100 per group) in this study.
3.4. Recruitment, consent and randomization
Patients at our IVF center who meet the inclusion criteria of this study will be informed of overall procedures and given enough time to decide whether to enroll. If the patients agree to enroll in the study, they will sign a consent form after detailed counseling. During the study, they can withdraw at any time.
Patients who are recruited into this study will be randomly divided into two groups by random assignment number. A randomization chart will be obtained by a web-based randomization program using random blocks (randomization.com). Patients will be assigned into the Tb-ERA or control group at a ratio of 1:1.
-
1.
Patients in the Tb-ERA group will undergo Tb-ERA tests. Embryo transfer time will be adjusted and embryo transfer will be performed again according to Tb-ERA results.
-
2.
Patients in the control group will not receive any treatment before the next cycle of transfer.
3.5. Interventions
3.5.1. Arm 1: Tb-ERA group
Patients in this group will undergo a Tb-ERA test. From the second day of menstruation, estradiol valerate (Progynova; Bayer, Leverkusen, Germany) will be administered at 4 mg daily until endometrial thickness is ≥ 7 mm. We will test levels of serum hormones, including estradiol and progesterone, on that same day. If the progesterone level is ≤ 1.5 ng/ml, 90 mg progesterone sustained-release vaginal gel (Crinone; Merck-Serono, Darmstadt, Germany) will be applied transvaginally per day. The day of first Crinone use is referred to as P+0. Endometrial biopsy will be collected on day P+5 by a dedicated physician. Endometrial-biopsy samples will be obtained using a disposable uterine-cavity tissue suction tube (Yudu medical apparatus and instruments Co., Ltd., Suzhou City, China). All collected samples will be transported to the laboratory on ice packs and stored in Allprotect Tissue Reagent (76405; QIAGEN, Hilden, Germany) at −80 °C before processing.
We will extract total RNA from 10 to 20 mg endometrial tissue using an RNAprep Pure Tissue Kit (DP341; TIANGEN Biotech Co., Ltd., Shanghai, China) per manufacturer's instructions. RNAs with an RNA Integrity Number ≥8.0 will be used for sequencing. In general, poly (A) messenger RNA will be enriched from 1 μg total RNA by using VAHTS mRNA Capture Beads (N401-02; Vazyme Biotech Co., Ltd., Nanjing, China). All poly (A) mRNA will then be used to generate sequencing libraries by using a Kapa Stranded RNA-Seq Library Preparation Kit (KR0934; Kapa Biosystems Inc., Wilmington, MA, USA) per manufacturer's instructions. Finally, complementary-DNA libraries will be subjected to paired-end 150 bp sequencing on an Illumina NovaSeq 6000 System (Illumina, Inc., San Diego, CA, USA). Each sample will generate approximately 8 GB raw data for data analysis. Transcripts per million of gene expression will be calculated based on GENCODE version 19 (https://gencodegenes.org) annotations [34,35]. Group-level differential-expression analysis will be performed by using the R software (Ihaka and Gentleman, 1996) package edgeR. Thresholds for differentially expressed genes will be 0.05 for false-discovery rate and 2 for fold change.
According to the Tb-ERA results, embryo transfer time point will be adjusted and will be carried out in the following cycle. The protocol of the endometrial-preparation method will be artificial replacement, the same as in the Tb-ERA test cycle.
3.5.2. Arm 2: control group
Patients in the control group will not receive any treatments or other interventions before the next cycle of transfer. The protocol of the endometrial-preparation method will be artificial replacement, the same as for the Tb-ERA group.
A maximum of 1–2 day-3 embryos or day-5 blastocysts will be transferred in each group. We will test serum β-human chorionic gonadotropin (HCG) levels 14 days after transfer. If the patient is HCG negative, she will stop hormone treatment; if she is HCG positive, she will continue hormone treatment until 11 weeks of gestation.
3.6. Follow-up
We will collect participants’ demographic and medical information during recruitment, including age, BMI (body mass index), time of implantation failure, endometrial thickness during transfer, baseline hormone levels, causes of infertility, duration of infertility, and reproductive history. During intervention in the Tb-ERA group, we will collect information including levels of serum estradiol and progesterone when Crinone is used, endometrial thickness, day of endometrial biopsy, and WOI evaluation. After transfer, we will collect information from pregnant participants in both groups that will include serum levels at 14, 21, and 28 days after embryo transfer, as well as ultrasound results at the 6th, 8th, 10th, and 12th gestational weeks. All adverse events (AEs) and treatments during the study should be collected. All of this information should be recorded on the common reporting format (CRF) form.
3.7. Data collection
Researchers will collect patient information and record it in detail on the CRF form. One staff member not involved in data collection will check the data and input it into the computer for electronic filing. During the study, the data supervisor will check the data weekly. All of the data must be saved in paper and electronic format and be kept for 5 years after the study is completed. Data will be encrypted and stored on a server that can be accessed only through a local-area network, and the access process is password protected.
3.8. Outcome measurements
3.8.1. Primary outcome
The clinical-pregnancy rate (CPR) will be used as the primary outcome. This rate will be calculated after the first transfer cycle of recruitment. Clinical pregnancy will be defined as ultrasonographic evidence of an intrauterine sac with or without a fetal heart at the 6th gestational week.
3.8.2. Secondary outcomes
-
●
WOI will be diagnosed by the Tb-ERA computational predictor. This will provide us with a recommendation for an estimated personalized WOI in a particular patient. The WOI displacement rate will be calculated as the number of WOI displacement/total Tb-ERA test number.
-
●
The embryo implantation rate (IR) will be calculated as the number of gestational sacs per embryo transferred.
-
●
Biochemical pregnancy will be defined by a serum HCG level ≥5.0 mIU/ml approximately 14 days after embryo transfer.
-
●
Early miscarriage will be considered pregnancy loss occurring after detection of a clinical pregnancy up to the 12th week of pregnancy.
-
●
The rate of embryo transfer pregnancy (pregnancy/transfer) in both groups will be calculated.
Rates of biochemical pregnancy (BPR), early miscarriage (MR), and ectopic pregnancy (EPR) will be measured at 3 months after transfer.
3.9. Statistical analyses
Intention-to-treat (ITT) and per-protocol (PP) analyses principles will be applied in this study. ITT will include all subjects who participate in the treatment, including those who drop out. The PP principle will only include all subjects who conform to the study protocol.
Professional statisticians will analyze the data using SPSS version 21.0 (IBM Corp., Armonk, NY, USA). Continuous variables will be described as mean ± standard deviation (SD). If they conform to normal distribution, they will be analyzed using Student's t-test; if not, they will be analyzed using the rank-sum test. Categorical variables will be presented as the composition ratio and rate and will be analyzed using the chi-square (χ2) test or Fisher's exact test. The confidence interval (CI) will be estimated at 95%. P < 0.05 will be considered statistically significant.
4. Discussion
Nowadays, there are still 10% of IVF patients experiencing RIF, which brings emotional, physical, and financial distress to these patients. Displacement of the WOI and aneuploid embryos are two major factors causing RIF [[6], [7], [8], [9], [10], [11]]. Endometrial receptivity array based on genes expression profiling is a step forward in improving IVF results through identification of the accurate WOI and personalizing embryo transfer. The clinical application of the test has been applied in some Chinese clinics; however larger studies are still required to validate its efficacy.
Some studies showed improved results, however most of the studies are retrospective and the numbers in these studies are not high enough to draw definite conclusions [[26], [27], [28], [29], [30]]. Recently, a retrospective multicenter cohort study was conducted in 2110 RIF patients. Their conclusion showed that endometrial receptivity array does not appear to be clinically useful for RIF patients [36]. An RCT study including 86 women with a history of RIF and 37 women starting their first fertility treatment demonstrated that there were no differences between the groups in the overall incidence of displaced ERA test result [37]. The result different from previous studies might be related with the different inclusion criteria or the limited participants.
The number of infertile women undergoing IVF is still large in China, but there is no data on ERA in Chinese RIF patients. So it is of value to develop an endometrial receptivity analysis method suitable for the Chinese population. Therefore, it is vital to identify possible WOI displacements in Chinese RIF patients with our Tb-ERA test and to determine whether Tb-ERA could improve the clinical-pregnancy rate in these RIF patients. Although it is possible that endometrial biopsy may affect the results, we could not investigate this further due to ethical concerns, which is a limitation in our study. This study aims to evaluate the efficacy of our Tb-ERA test in Chinese RIF patients, which may significantly improve the success rates in RIF patients undergoing IVF. If positive results are obtained, we will expand the study to a multicenter study with larger sample size in the near future.
Funding
The study was founded by Central Level Major Expenditure Increase and Decrease Projects (Grant number: 2060302).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Weijian Chen, Shanshan Song for their help in designing the clinical study and basic experiment part.
Data availability
No data was used for the research described in the article.
References
- 1.Simon A., Laufer N. Repeated implantation failure: clinical approach. Fertil. Steril. 2012;97:1039–1043. doi: 10.1016/j.fertnstert.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Coughlan C., Ledger W., Wang Q., Liu F.H., Demirol A., Gurgan T., Cutting R., Ong K., Sallam H., Li T.C. Recurrent implantation failure: definition and management. Reprod. Biomed. Online. 2014;28:14–38. doi: 10.1016/j.rbmo.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Zohni K.M., Gat I., Librach C. Recurrent implantation failure: a comprehensive review. Minerva Ginecol. 2016;68:653–667. [PubMed] [Google Scholar]
- 4.Zeyneloglu H.B., Onalan G. Remedies for recurrent implantation failure. Semin. Reprod. Med. 2014;32:297–305. doi: 10.1055/s-0034-1375182. [DOI] [PubMed] [Google Scholar]
- 5.Penzias A.S. Recurrent IVF failure: other factors. Fertil. Steril. 2012;97:1033–1038. doi: 10.1016/j.fertnstert.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Scott R.T., Jr., Upham K.M., Forman E.J., Hong K.H., Scott K.L., Taylor D., Tao X., Treff N.R. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates: a randomized controlled trial. Fertil. Steril. 2013;100:697–703. doi: 10.1016/j.fertnstert.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 7.Fiorentino F., Bono S., Biricik A., Nuccitelli A., Cotroneo E., Cottone G., Kokocinski F., Michel C.E., Minasi M.G., Greco E. Application of next-generation sequencing technology for comprehensive aneuploidy screening of blastocysts in clinical preimplantation genetic screening cycles. Hum. Reprod. 2014;29(12):2802–2813. doi: 10.1093/humrep/deu277. [DOI] [PubMed] [Google Scholar]
- 8.Pehlivan T., Rubio C., Rodrigo L., Romero J., Remohi J., Simón C., Pellicer A. Impact of preimplantation genetic diagnosis on IVF outcome in implantation failure patients. Reprod. Biomed. Online. 2003;6:232–237. doi: 10.1016/s1472-6483(10)61715-4. [DOI] [PubMed] [Google Scholar]
- 9.Kung A., Munne S., Bankowski B., Coates A., Wells D. Validation of next-generation sequencing for comprehensive chromosome screening of embryos. Reprod. Biomed. Online. 2015;31:760–769. doi: 10.1016/j.rbmo.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Noyes R.W., Hertig A.I., Rock J. Dating the endometrial biopsy. Fertil. Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 11.Rashid N.A., Lalitkumar S., Lalitkumar P.G., Danielsson K.G. Endometrial receptivity and human embryo implantation. Am. J. Reprod. Immunol. 2011;66(Suppl 1):23–30. doi: 10.1111/j.1600-0897.2011.01048.x. [DOI] [PubMed] [Google Scholar]
- 12.Revel A. Defective endometrial receptivity. Fertil. Steril. 2012;97(5):1028–1032. doi: 10.1016/j.fertnstert.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 13.Haouzi D., Dechaud H., Assou S., Vos J.D., Hamamah S. Insights into human endometrial receptivity from transcriptomic and proteomic data. Reprod. Biomed. Online. 2012;24:23–34. doi: 10.1016/j.rbmo.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Sebastian-Leon P., Garrido N., Remohi J., Pellicer A., Diaz-Gimeno P. Asynchronous and pathological windows of implantation: two causes of recurrent implantation failure. Hum. Reprod. 2018;33:626–635. doi: 10.1093/humrep/dey023. [DOI] [PubMed] [Google Scholar]
- 15.Coughlan C. What to do when good-quality embryos repeatedly fail to implant. Best Pract. Res. Clin. Obstet. Gynaecol. 2018;53:48–59. doi: 10.1016/j.bpobgyn.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Altmae S., Koel M., Vosa U., Adler P., Suhorutšenko M., Laisk-Podar T., Kukushkina V., Saare M., Velthut-Meikas A., Krjutškov K., Aghajanova L., Lalitkumar P.G., Gemzell-Danielsson K., Giudice L., Simón C., Salumets A. Meta-signature of human endometrial receptivity: a meta-analysis and validation study of transcriptomic biomarkers. Sci. Rep. 2017;7:10077. doi: 10.1038/s41598-017-10098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrido-Gómez T., Ruiz-Alonso M., Blesa D., Diaz-Gimeno P., Vilella F., Simón C. Profiling the gene signature of endometrial receptivity: cinical results. Fertil. Steril. 2013;99:1078–1085. doi: 10.1016/j.fertnstert.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Kliman H.J., Frankfurter D. Clinical approach to recurrent implantation failure: evidence-based evaluation of the endometrium. Fertil. Steril. 2019;111:618–628. doi: 10.1016/j.fertnstert.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Harper M.J. The implantation window. Baillieres Clin Obstet Gynaecol. 1992;6:351–371. doi: 10.1016/s0950-3552(05)80092-6. [DOI] [PubMed] [Google Scholar]
- 20.Wilcox A.J., Baird D.D., Weinberg C.R. Time of implantation of the conceptus and loss of pregnancy. N. Engl. J. Med. 1999;340:1796–1799. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- 21.Diaz-Gimeno P., Horcajadas J.A., Martinez-Conejero J.A., Esteban F.J., Alamá P., Pellicer A., Simón C. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil. Steril. 2011;95:50–60. doi: 10.1016/j.fertnstert.2010.04.063. e1-15. [DOI] [PubMed] [Google Scholar]
- 22.Diaz-Gimeno P., Ruiz-Alonso M., Blesa D., Bosch N., Martínez-Conejero J.A., Alamá P., Garrido N., Pellicer A., Simón C. The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil. Steril. 2013;99:508–517. doi: 10.1016/j.fertnstert.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz-Alonso M., Galindo N., Pellicer A., Simon C. What a difference two days make: “personalized” embryo transfer (pET) paradigm: a case report and pilot study. Hum. Reprod. 2014;29:1244–1247. doi: 10.1093/humrep/deu070. [DOI] [PubMed] [Google Scholar]
- 24.Mahajan N. Endometrial receptivity array: clinical application. J. Hum. Reprod. Sci. 2015;8:121–129. doi: 10.4103/0974-1208.165153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simón C., Gómez C., Cabanillas S., Vladimirov I., Castillón G., Giles J., Boynukalin K., Findikli N., Bahçeci M., Ortega I., Vidal C., Funabiki M., Izquierdo A., López L., Portela S., Frantz N., Kulmann M., Taguchi S., Labarta E., Colucci F., Mackens S., Santamaría X., Muñoz E., Barrera S., García-Velasco J.A., Fernández M., Ferrando M., Ruiz M., Mol B.W., Valbuena D., ERA-RCT Study Consortium Group A 5-year multicentre randomized controlled trial comparing personalized, frozen and fresh blastocyst transfer in IVF. Reprod. Biomed. Online. 2020;41(3):402–415. doi: 10.1016/j.rbmo.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz-Alonso M., Blesa D., Diaz-Gimeno P., Gómez E., Fernández-Sánchez M., Carranza F., Carrera J., Vilella F., Pellicer A., Simon C. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure. Fertil. Steril. 2013;100:818–824. doi: 10.1016/j.fertnstert.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto T., Koizumi M., Doshida M., Toya M., Sagara E., Oka N., Nakajo Y., Aono N., Lgarashi H., Kyono K. Efficacy of the endometrial receptivity array for repeated implantation failure in Japan: a retrospective, two-centers study. Reprod. Med. Biol. 2017;16:290–296. doi: 10.1002/rmb2.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel J.A., Patel A., Banker J.M., Shah S.I., Banker M.R. Personalized embryo transfer helps in improving in vitro fertilization/ICSI outcomes in patients with recurrent implantation failure. J. Hum. Reprod. Sci. 2019;12(1):59–66. doi: 10.4103/jhrs.JHRS_74_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan J., Kan A., Hitkari J., Taylor B., Tallon N., Warraich G., Yuzpe A., Nakhuda G. The role of the endometrial receptivity array (ERA) in patients who have failed euploid embryo transfers. J. Assist. Reprod. Genet. 2018;35(4):683–692. doi: 10.1007/s10815-017-1112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He A., Zou Y., Wan C., Zhao J., Zhang Q., Yao Z., Tian F., Wu H., Huang X., Fu J., Hu C., Sun Y., Xiao L., Yang T., Hou Z., Dong X., Lu S., Li Y. The role of transcriptomic biomarkers of endometrial receptivity in personalized embryo transfer for patients with repeated implantation failure. J. Transl. Med. 2021;19(1):176. doi: 10.1186/s12967-021-02837-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W.B., Li Q., Liu H., Chen W.J., Li H., Lu X., Chen J.L., Li L., Wu H., Sun X.X. Transcriptomic analysis of endometrial receptivity for a genomic diagnostics model of Chinese women. Fertil. Steril. 2021;116(1):157–164. doi: 10.1016/j.fertnstert.2020.11.010. A. [DOI] [PubMed] [Google Scholar]
- 32.Alpha scientists in reproductive medicine and ESHRE special interest group of embryology The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum. Reprod. 2011;26(6):1270–1283. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 33.Cohen J., Chabbert-Buffet N., Darai E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder- a plea for universal definitions. J. Assist. Reprod. Genet. 2015;32(12):1709–1712. doi: 10.1007/s10815-015-0595-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wagner G.P., Kin K., Lynch V.J. Measurement of mRNA abundance using RNA-seq data: RPKM measure is inconsistent among samples. Theor. Biosci. 2012;131(4):281–285. doi: 10.1007/s12064-012-0162-3. [DOI] [PubMed] [Google Scholar]
- 35.Harrow J., Frankish A., Gonzalez J.M., Tapanari E., Diekhans M., Kokocinski F. GENCODE: the reference human genome annotation for the ENCODE Project. Genome Res. 2012;22(9):1760–1774. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mauro C., Patricia D.G., Antonio P., Nicolas G. Evaluation of the endometrial receptivity assay and the preimplantation genetic test for aneuploidy in overcoming recurrent implantation failure. J. Assist. Reprod. Genet. 2020;37(12):2989–2997. doi: 10.1007/s10815-020-01948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malene H.S., Trine H., Gry P., Kathrine B.P., Jens O.E., Lise G.L., Thomas V.H., Nick M. Assessing endometrial receptivity after recurrent implantation failure: a prospective controlled cohort study. Reprod. Biomed. Online. 2020;41(6):998–1006. doi: 10.1016/j.rbmo.2020.08.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.