Abstract
The use of non-pharmacological alternatives to pharmacological interventions, e.g., nutritional therapy, to improve or maintain bone mineral density (BMD) in postmenopausal women has gained traction over the past decade, but limited data exist regarding its efficacy. This paper describes the design of the Prune Study, a randomized controlled trial (RCT) that explored the effectiveness of a 12-month intervention of daily prune consumption on bone density, bone structure and strength estimates, bone turnover, various biomarkers of immune function, inflammation, and cardiovascular health, as well as phenolic and gut microbiota analyses. Postmenopausal women between the ages of 55–75 years were randomized into either control group (no prune consumption; n = 78), 50g prune (50g prune/day; n = 79), or 100g prune (100g prune/day; n = 78). All participants received 1200 mg calcium +800 IU vitamin D3 daily as standard of care. The Prune Study is the largest and most comprehensive investigation of a dose response of prune consumption on bone health, biomarkers of immune function, inflammation, and cardiovascular health, as well as detailed phenolic and gut microbiota analyses in postmenopausal women. 235 women were randomized and 183 women completed the entire study. The findings of this study will help expand our current understanding of clinical implications and mechanisms underlying the resultant health effects of prune as a functional food therapy.
Keywords: Prunes, Prune, Osteoporosis, Osteopenia, Menopause, Bone
1. Introduction
By 2030, the prevalence of osteoporosis for women 50 years and older is projected to reach 13.6 million and the prevalence of low bone mass is expected to reach 57.8 million [1]. These estimates underscore the urgency for continued development and improvement of preventative strategies. Pharmacological therapies, such as estrogen therapy and bisphosphonates, are effective in the treatment of bone loss and osteoporosis, but have been declining in popularity due to undesirable side effects [2]. Alternatively, consumer interest in non-pharmacological options for preventing and treating bone loss, particularly with dietary whole food supplementation, is on the rise. While calcium and vitamin D is recommended and has been shown to have modest benefits to bone health [3], alternative natural plant-derived compounds have demonstrated promising results in animal and human preclinical and clinical studies [[4], [5], [6], [7]].
Research testing the effects of dietary supplementation with prune (i.e., dried plums) in rodent models has demonstrated decreases in bone resorption, increases in bone formation, and improvements in bone microarchitecture and bone trophic hormones [4,5,8,9]. The mechanism underlying these effects appears to be attributable to the phenolic metabolites absorbed following consumption of prune. Specifically, prunes are an abundant source of chlorogenic acids, phenolic acids, and select flavonoids (anthocyanin, flavonols and flavan-3-ols) [10,11]. These compounds are reported to mitigate the negative effects of reactive oxygen species (ROS) and oxidative stress on bone; further, phenolic compounds can target inflammatory pathways that are upregulated in a hypoestrogenic environment, helping to prevent estrogen deficiency-induced bone loss [12].
In humans, data on the effects of dietary prune intake on bone health are limited to two randomized controlled trials (RCT) [[13], [14], [15], [16]] and one RCT in older men [17]. During shorter duration investigations, three months of 75–100g/day of prune consumption resulted in an increase in bone formation and IGF-1 [13], while 6 months of either 50g or 100g/day of prune decreased markers of resorption [16]. In the 12 months of supplementation produced increases in bone mineral density (BMD) of the ulna and lumbar spine and a decrease in bone formation and resorption [15], compared to the group who consumed 75g/day of dried apple. While these studies are intriguing, limited reporting of the bone findings complicate the interpretation and generalizability of the data. Additionally, it is currently unknown whether various prune dosages yield comparable or improved effects on bone health parameters during a 12-month investigation.
2. Specific aims
The purpose of the current RCT was to test two doses of prunes (50g/day and 100g/day) consumed for 12 months compared to an unsupplemented control group that builds upon prior work in postmenopausal women [[13], [14], [15], [16]] to determine the effects on bone outcomes using dual x-ray absorptiometry (DXA) and 3-dimensional bone imaging technology to quantify bone density, geometry and estimated strength. This study also included detailed assessment of the phenolics in the prunes and effects on bone formation, resorption, and IGF-1.
2.1. Specific aims
The primary objectives of this study were to evaluate the effectiveness of two dosages of prunes as a whole food dietary supplement on percent change from baseline in areal BMD, volumetric cortical and trabecular bone density, and estimated measures of bone strength during the 12-month dietary intervention at the lumbar spine, total hip, and femoral neck in postmenopausal women. Secondary outcomes include DXA hip structural analysis, and effects on bone formation, bone resorption, and IGF-1. An additional objective of the study was to evaluate the mechanisms underlying the effects of prune as a dietary supplement for improving BMD, bone geometry, and estimated bone strength as it relates to levels of polyphenols and their bioavailable conjugated metabolites, immune and inflammatory biomarkers, and gut microbiota diversity. Specifically, the additional outcomes included: (1) Percent change from baseline in DXA hip structural analysis; (2) Percent change from baseline in DXA spine trabecular bone score; (3) Percent change from baseline in peripheral quantitative computed tomography (pQCT) volumetric BMD of the tibia and radius; (4) Percent change from baseline in pQCT geometry measurements of the tibia and radius; (5) Percent change from baseline in pQCT strength measurements of the tibia and radius; (6) Change from baseline in bone turnover markers; (7) Change from baseline in cellular bone metabolism; (8) Change from baseline in urine phenolic concentrations; (9) Change from baseline in serum phenolic concentrations; (10) Change from baseline in expression of inflammatory markers; (11) Change from baseline in gut microbiota.
3. Material and methods
3.1. Study design
The Prune Study (Clinical Trials NCT02822378, “Randomized Controlled Trial of Dietary Supplementation with Prune on Bone Density, Geometry, and Estimated Bone Strength in Postmenopausal Women”) is a RCT designed to determine the effect of a 12-month intervention of prune consumption on bone health and related outcomes in postmenopausal women. This was a single center, multi-arm parallel-group 12-month dose ranging RCT conducted in the Women's Health and Exercise Laboratory at Pennsylvania State University (PSU). This RCT was designed to compare dietary supplementation with 50g (i.e., 4–6 prunes) and 100g of prunes (i.e., 10–12 prunes) vs. a no-prune control group (Control) in postmenopausal women aged 55–75 yrs with areal BMD at the lumbar spine, total hip or femoral neck between T-score of <0.0 and >-3.0 at any site.
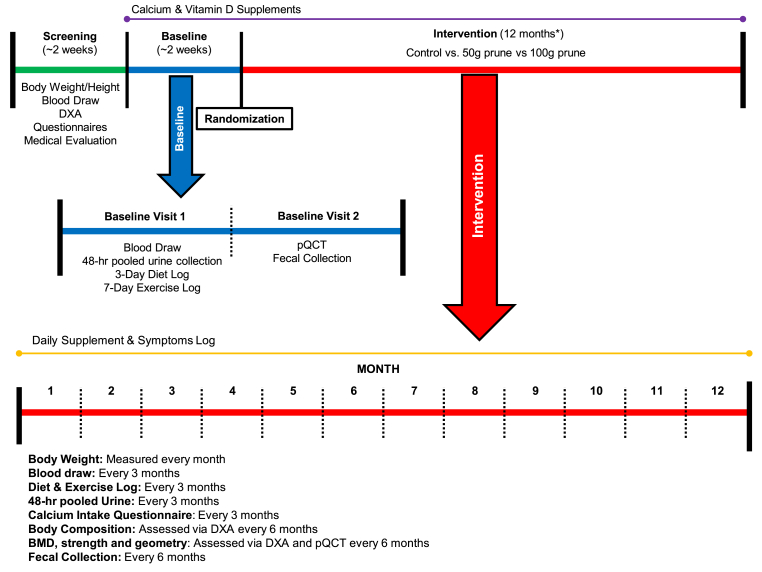
Repeated measurements for bone health and other health-related outcomes were obtained throughout the study, which included a 2–4 week screening/baseline period and 12-month intervention. A detailed depiction of study procedures and timing is outlined in Fig. 1. During the intervention, participants came into the Women's Health and Exercise Laboratory monthly for 12 consecutive months to assess body weight and review of symptoms. Every three months, bone health was assessed through 2- and 3-dimensional bone imaging, hormones were assessed in fasted blood draw, phenolics were assessed through 48-h pooled urine collection, diet and exercise were recorded over 7-days, and calcium intake was assessed through questionnaire. The study was approved by the PSU Institutional Review Board and participants signed an approved informed consent prior to study participation.
Fig. 1.
An overview of the type and timing of measurements collected during this randomized controlled trial. DXA, dual-energy X-ray absorptiometry; pQCT, peripheral quantitative computed tomography. *Due to COVID-19 university closure impacting the timing of 12-month visit, 23 women (10 Control, 10 50g, 3 100g) completed a post visit upon university opening and IRB approval beyond the 12-month intended study duration (mean measurement timing of 14 months).
3.2. Recruitment
All study procedures were performed at the Women's Health and Exercise Laboratory at PSU and utilized the service of the PSU Clinical Research Center, from June 2016–February 2021. Recruitment for the intervention was done on a rolling basis and occurred from June 2016 to February 2020, with data collection ending in February 2021. Recruitment occurred through fliers (posted on the PSU campus and surrounding community), e-mail announcements, and in advertisements posted in the university and community newspapers. We also recruited via information sessions, on campus and at local retirement communities, particularly for women seeking medical advice for menopause and osteoporosis.
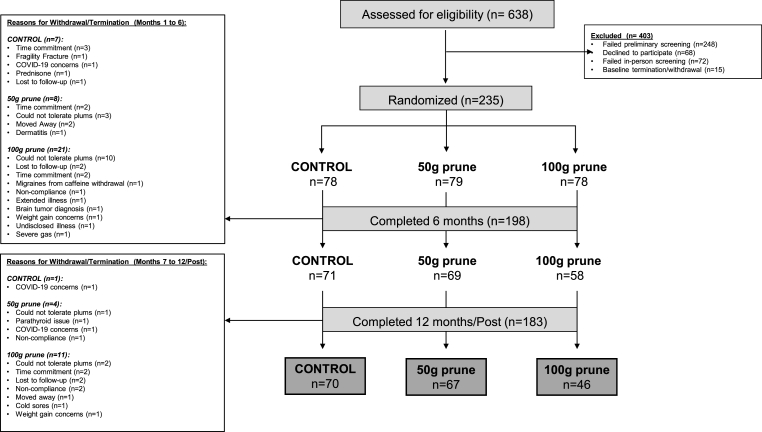
The progression of participants enrollment and dropout thought the study is displayed in Fig. 2. After screening 638 women by phone, 322 women in total were screened in person for eligibility. Seventy-two women were excluded or withdrew during screening and 250 women entered the baseline period. In total, 235 women were randomized into 1 of 3 groups: 1) Control (n = 78), 2) 50g (n = 79), or 3) 100g (n = 78).
Fig. 2.
CONSORT diagram depicting number of participants enrolled at each study phase and the reasons for dropout.
3.3. Screening and eligibility
When volunteers expressed interest in the study, a screening questionnaire was completed via phone to determine the volunteer's initial eligibility. If initial criteria were met, the volunteer was scheduled for in-person informed consent visit, to which the volunteer was provided a detailed description of study purpose, procedures, risks and benefits prior to signing an IRB-approved informed consent document. Once consent was attained, the participant was scheduled for an in-person screening visit to include, measurements of height (cm), weight (kg), BMD and body composition (DXA and pQCT), questionnaires for medical and health history, and fasted blood draw for basic metabolic panel and hormones were conducted. Participants then met with PSU Clinical Research Center clinician for a physical and evaluation of medical health history, BMD, and results from the fasted blood draw to determine eligibility.
Eligible participants met the following criteria: 1) postmenopausal women aged 55–75 yrs, determined by self-report questionnaire as having 12 or more consecutive months without a menstrual period, 2) people without severe obesity (BMI <40 kg/m2), 3) healthy, indicated by normal values for cholesterol, blood glucose, kidney function, liver function as indicated by a complete metabolic panel, 4) willing to include prunes in their daily diet, 5) not taking any natural dietary supplement containing phenolics, < 1 cup/day of blueberries or apples for at least 2 months prior to study entry, 6) non-smoking, 7) ambulatory, and 8) had eligible BMD as measured by DXA. Eligible BMD values (T-scores) for DXA measures of the lumbar spine, total hip and/or femoral neck corresponded to T-scores between 0.0 and −3.0.
Exclusion criteria included: 1) Women who regularly consume prunes, dried apples, prune juice, or heavy consumers of blueberries (1 cup or more/day), 2) history of vertebral fracture or fragility fracture of the wrist, humerus, hip or pelvis after age 50 yr, 3) untreated hyper- or hypothyroidism; current hyper- or hypoparathyroidism, 4) significantly impaired renal function, 5) current hypo- or hypercalcemia, 6) history of spinal stenosis, 7) history of heart attack, stroke, thromboembolism, kidney disease, malabsorption syndrome, seizure disorders, 8) positive for HIV, Hepatitis-C or Hepatitis-B surface antigen and malignancy, 9) use of the following agents affecting bone metabolism: intravenous bisphosphonates at any time; fluoride (for osteoporosis) within the past 24 months; denosumab at any time; bisphosphonates, parathyroid hormone or strontium within the past 12 months; calcitonin or selective estrogen receptor modulators within the past 12 months; systemic oral or transdermal estrogen within the past 3 months; systemic glucocorticosteroids (≥5 mg prednisone equivalent per day for more than 10 days); or tibolone within the past 3 months, 10) BMD values (T-scores) for DXA measures of the lumbar spine, total hip and/or femoral neck T-scores >0.0 and <-3.0.
Demographics of the subjects in this study were targeted for several reasons: 1) postmenopausal women with low bone mass aged 55–75 years represent an age range during which low bone mass is often observed. Women with previous fracture and severe osteoporosis were excluded from this study, 2) targeting this age range allowed us to generalize our results to postmenopausal women with low bone mass who may experience a high risk for fracture. Furthermore, these women represent a primary age group targeted for dietary supplementation with prunes, and lastly, this RCT was designed to improve upon the limitations of the only 12-month RCT data published to date that utilized dietary supplementation with prunes in postmenopausal women [14,15]. The primary limitations of the Hooshmand et al. [14,15] study design include the inclusion of a dried apple control group, the limited details of the bone data findings published, unclear statistical analysis, the absence of phenolics measurements, and the failure to include 3-dimensional imaging measurement of volumetric BMD, bone geometry, and estimated bone strength.
3.4. Baseline and randomization
Participants who met study criteria entered baseline, to which procedures included fasted blood draw for bone and inflammation markers, 48-h pooled urine collection for phenolic content, fecal collection, diet and exercise log, and pQCT for bone strength and bone quality measurements.
For randomized allocation of the participants, a computer-generated list of random numbers was used. Participants were randomly assigned with a 1:1:1 allocation using fixed random block sizes of 3. Only one senior research member had access to the password protected file of random numbers, to make sure the assignment sequence was concealed prior allocation. Once a participant had successfully completed screening and baseline procedures, the senior researcher would inform the study staff who was responsible for enrolling and assigning participants which treatment the participant was to receive for the duration of the year. Due to the nature of the intervention, it was not possible to blind participants and study staff to the allocated treatment arm they would receive; however, outcome assessors and data analysts were kept blinded to the allocation.
After completing in-person screening and all baseline procedures, participants were randomized into 1 of 3 treatment groups: 1) Control (n = 78; no prunes), 2) 50g (n = 79; 4–6 prunes daily), or 3) 100g (n = 78; 10–12 prunes daily). Prunes were provided by the California Prune Board, every three months. All participants met the required intake of 1200 mg of calcium and 800 IU vitamin D3 daily from diet plus supplements (Nature Made Pharmavite LLC, West Hills, CA).
For participants randomized to the 50g and 100g prune groups, a “run-in plan” was administered to slowly increase the prune dosage to help minimize gastrointestinal side effects and to achieve a desired dose. The 50g prune run-in plan was as follows: 2 prunes/day for 3 days in a divided dose of 1 prune after breakfast and 1 prune after dinner, followed by 4 prunes/day for 4 days in a divided dose of 1 prune after breakfast, 1 prune after lunch and 2 prunes after dinner, followed by 5 prunes/day for 4 days in a divided dose of 2 prunes after breakfast, 1 prune after lunch and 2 prunes after dinner, followed by the desired dose of 6 prunes/day in a divided dose of 2 prunes after breakfast, 2 prunes after lunch and 2 prunes after dinner for the remainder of the 12-month study duration. The 100g prune run-in plan was as follows: 2 prunes/day for 3 days in a divided dose of 1 prune after breakfast and 1 prune after dinner, followed by 4 prunes/day for 4 days in a divided dose of 1prune after breakfast, 1 prune after lunch and 2 prunes after dinner, followed by 6 prunes/day for 4 days in a divided dose of 2 prunes after breakfast, 2 prunes after lunch and 2 prunes after dinner, followed by 9 prunes/day for 4 days in a divided dose of 3 prunes after breakfast, 3prunes after lunch and 3 prunes after dinner, and lastly, an increase to the desired dose of 12 prunes/day for the remainder of the 12-month study duration in a divided dose of 4 prunes after breakfast, 4 prunes after lunch and 4 prunes after dinner. Additionally, participants who were randomized into either prune group met with registered dietitian to discuss various strategies to incorporate prunes into their diet.
After the “run-in” period, participants were instructed to eat the daily amount of prunes based on their assigned group, and record when prunes were consumed throughout the day. Participants could choose when to consume prunes, as long as the prescribed number of prunes was consumed each day. If some (or all) of prunes were missed for a given day, this was recorded on self-reported logs maintained for the entirety of the intervention.
3.5. Detailed assessments
3.5.1. Anthropometric assessment
Height was measured in centimeters using a stadiometer. Total body weight was measured to the nearest 0.5 kg on a physician's scale (Seca, Model 770, Hamburg, Germany) during screening and during each monthly visit during the intervention. BMI was calculated as the body mass divided by height squared (kg/m2).
3.5.2. Medical and health history assessment
Participants completed questionnaires to detail demographic, reproductive, and medical history questionnaires, which included questions regarding use of supplements, prescription medications, and NSAIDs, history of weight, purposeful physical activity, and dietary practices. Physical exams were conducted by clinician prior to study entry (screening) to ensure that subjects were in good health. During the examination, the clinician reviewed medical history, DXA scan, and blood screening tests to ensure that subjects had no contraindications to the study.
3.5.3. Diet and exercise assessment
Every 3 months (baseline, months 3, 6, 9 and 12), diet and exercise assessments were performed. Participants completed a 3-day diet diary (1 weekend day and 2 weekdays) to assess energy intake and macronutrient dietary composition. Participants were instructed to measure (using standard measuring cups/tools) and record all food and beverages consumed in detail. The nutrient data from the 3-day diet logs were coded and analyzed for total kilocalories using Nutritionist Pro software (Axxya Systems, Redmond, WA). Daily kilocalories consumed over the 3-day recording period were averaged.
A brief validated, calcium assessment tool [18] was utilized to determine the dietary intake of calcium during one laboratory visit every 3 months. Based on the quarterly calcium assessment responses, participants were provided calcium and vitamin D3 supplements in order to meet the RDA of 1200 mg of calcium and 800 IU vitamin D3 daily from diet plus supplements. If participants consumed enough calcium-rich foods to meet the RDA of 1200 mg of calcium daily, they were not provided with additional calcium supplementation. All participants were also supplemented with 800 IU vitamin D3 per day. Participants completed a food frequency questionnaire to determine how often high phenolic foods were consumed [19].
During the same week of diet assessment, participants completed a 7-day exercise log, in which they recorded type and duration of daily purposeful exercise. The metabolic equivalent (MET) level for the specific activity was determined using the Ainsworth et al. [20,21] compendium of physical activities. The MET level of the activity was multiplied by the duration (minutes) of the exercise session. Exercise type was also classified by bone loading type according to Nikander et al. [22,23], such that activities were classified base on apparent loading at the hip: high-impact, odd-impact, high-magnitude, low-impact, and nonimpact loading.
3.5.4. Blood serum and plasma assessment
Blood samples were collected once between 0730 and 1000 h during the screening, baseline, months 3, 6, 9, 12 visits for all participants. Volunteers were instructed not to exercise or consume food within 12 h prior to blood sampling. Antecubital blood samples were drawn by a registered phlebotomist using a blood collection needle (21-gauge, 19 mm), and blood collection tubes (Vacutainer, Franklin Lakes, NJ). For basic metabolic panel, blood (10 mL) was collected at screening in sterile clot activator/separator gel-coated blood tube (BD Biosciences, San Jose, CA) were allowed to clot for 30 min at room temperature (20-24 °C) and then centrifuged (Eppendorf 5804 R, Hamburg, Germany) for 15 min at 4 °C. Samples were then analyzed by Quest Diagnostics (Pittsburgh, PA facility). For bone biomarkers, blood (10 mL) was collected in each of three sterile non-coated blood tubes (BD Biosciences, San Jose, CA) and were allowed to clot for 30 min at room temperature (20-24 °C) and then centrifuged (Eppendorf 5804 R, Hamburg, Germany) for 15 min at 4 °C. The serum was aliquoted into 2-mL polyethylene storage tubes and frozen at −80 °C until analysis. For immune and inflammatory endpoints (at baseline, 6 and 12 months only), blood (10 mL) was collected in 1 sterile EDTA (K2)-coated blood tubes (BD Biosciences, San Jose, CA), centrifuged at 1800×g for 15 min at room temperature. The plasma was dispensed into 2-ml polyethylene centrifuge tubes and frozen at −80 °C until analysis. The buffy coat was collected and used for subsequent immune analyses.
Bone Biomarker Assays: At baseline, month 6, and month 12, blood was collected for measurement of serum procollagen type I N-terminal propeptide (P1NP; bone formation), serum C-terminal telopeptide (CTX; bone resorption), serum insulin-like growth factor 1 (IGF-1), and serum 25-hydroxyvitamin D (25(OH)D3).
3.5.5. Body composition assessment
Every 6 months (baseline, months 6 and 12), a total body DXA scan was performed to assess body composition to include percent body fat, fat mass, lean mass and fat-free mass, as well as that of specific regions, such as the android, gynoid, trunk, legs, and arms. Participants were scanned on a Hologic QDR4500 system (Hologic, Bedford, MA). Our lab has a precision of ≤1.1% coefficient of variation (CV) for body composition.
3.5.6. DXA assessment
DXA scans of the total body, lumbar spine, and dual hip were performed to assess areal BMD at screening, month 6, and month 12. Participants were scanned and analyzed on a Hologic QDR4500 system (Hologic, Bedford, MA) by an International Society for Clinical Densitometry certified bone densitometry technologist. Our lab has a precision of <0.8% CV at all three BMD sites (total body, spine and hip). In addition, femoral neck geometry and strength were estimated from dual hip scans by Hip Strength Analysis (HSA) [24,25]. HSA is a feature of the Hologic APEX software that is used to estimate the structural properties of the hip and the measurements were obtained from the automatic analysis. Specific measurements obtained include femoral neck cross-sectional moment of inertia (CSMI), cross-sectional area (CSA), section modulus (Z), and diameter. Fracture risk assessment was determined using the trabecular bone score (TBS)-adjusted FRAX® tool (www.shef.ac.uk/FRAX) [26,27], in which the algorithm computes 10-year probability of major osteoporotic fracture or hip fracture.
3.5.7. pQCT assessment
pQCT measurements were conducted at baseline, month 6, and month 12 visits to assess volumetric BMD (vBMD), bone geometry and strength estimates at the non-dominant radius and opposite tibia [Stratec XCT3000 (Orthometrix, White Plains, NY, USA)]. The unit is a 12-detector unit, voxel size 0.4 mm, slice thickness 2.2 mm, and scan speed of 20–50 mm/s. Scans were analyzed with Stratec software v6.00B. A scout view was first obtained to place the reference line at the distal endplate. For radius, measurements were obtained at 4% and 33% from the reference line. For tibia, measurements were obtained at 4%, 14%, 38%, and 66% from the reference line. The 4% and 14% (metaphyseal) sites were assessed for trabecular vBMD (mg/cm3) and BSI, and the 33%, 38%, and 66% (diaphyseal) sites were assessed for cortical vBMD (mg/cm3), periosteal/endosteal circumference (mm), cortical cross-sectional area (CSA, mm2), and strength estimates such as cross sectional moment of inertia (CSMI), polar section modulus (Zp, mm3), and SSI [28,29]. CSMI estimates resistance to bending, and Zp, a function of cortical periosteal and endosteal dimensions, estimates torsional strength and is associated with failure load [30]. SSI is a bending-strength estimator more strongly correlated with experimentally determined breaking force than DXA measures of aBMD or pQCT measures of CSMI and cortical vBMD. Muscle CSA (mm2) were evaluated at the 66% site of the tibia. Quality control was done, by scanning the manufacturer's hydroxyapatite phantom daily for quality assurance. The CV for short-term precision ranges from 0.7 to 7.7% at the radius and tibia for pQCT outcomes in adults [31].
3.5.8. Compliance assessment
Compliance to prune consumption use was monitored with both self-report logs and laboratory-verified measures of 48-h pooled urine total phenolic concentration every 3 months. Compliance was first assessed based on self-reported daily prune consumption on the self-reported logs maintained for the entirety of the intervention. Prune and/or calcium + vitamin D3 consumption log each day to document consumption and any adverse symptoms (bloating, cramping, gas, diarrhea, etc.). Compliance was determined by daily self-report logs, and supported by urinary assessments of total phenolics and phenolic metabolites in 48-h pooled urinary samples every 3 months. To aid in the measure of total phenolic concentration being attributable to the prunes and the relatively short half-life of phenolics (7–10 h), all participants were asked to refrain from consuming coffee, fruits, and vegetables that are also rich in similar phenolics to prunes for 12 h prior to the urine collection and fasting blood draw.
All phenolic analyses were conducted at the North Carolina State University Plants for Human Health Institute (Kannapolis, NC). Upon arrival samples were stored at −80 °C. Quantitative measurement for individual sample was then performed to determine urinary contents of total phenolics (Folin–Ciocalteu assay), creatinine (colorimetric assay kit 500701, Cayman Chemical, Ann Arbor, MI) and phenolic metabolites by LC-MS/MS as described below.
Solid phase extraction: Solid phase extraction (SPE) of phenolics and their metabolites was performed using the established method by Medina-Remón and colleagues with small modifications. Briefly, Oasis® MAX 96-well plates (Waters Co, MA, U.S.A.; 186000373) were activated by 1 mL of methanol, following with 1 mL water. Urine was thawed on ice for 3 h, centrifuged at room temperature for 5 min (21,000×g), and then diluted at a 1:1 ratio (v/v) with 0.4 M HCl. The resulting sample (1.25 mL) was spiked with 50 μL of 100 μM taxifolin (internal standard for extraction recovery) and then loaded to the activated extraction plate. The loaded plate was rinsed with 1 mL of water, followed by 1 mL of water/methanol solution (v/v, 95:5), and the eluate was discarded. Phenolic metabolites were eluted with a solution containing 50 μL of 100 μM ethyl gallate (volume control internal standard) and 450 μL of 2% formic acid in methanol. Notably, taxifolin and ethyl gallate were dissolved in water/methanol solution (v/v, 50:50) containing 2% formic acid.
Total Phenolic Assessment in Urine: Total urinary phenolics were assessed in all subjects at baseline, month 6 and month 12. A random sample of 25% of the subjects' collected urine was assessed every three months throughout the study duration. Total urinary phenolics were determined from a 48-h pooled urine sample after SPE extraction by the Folin-Ciocalteu microplate method as described by Medina-Remón et al. (Medina-Remon, Barrionuevo-Gonzalez et al., 2009) and corrected for creatinine content as described by Zamora-Ros et al. (Zamora-Ros, Rabassa et al., 2011). This method has been shown to be well correlated to phenolic intake and was used as a general marker of prune consumption and as an additional measure of compliance.
Analysis of individual prune phenolic metabolites by ultra performance liquid chromatography-tandem mass spectrometer (UPLC-MS/MS): Phenolic metabolite levels in 48-h pooled urine samples were determined by UPLC-MS/MS using a Waters UPLC Acquity H Class system equipped with a Xevo TQD Mass Spectrometric detector. Separation of a targeted set of individual phenolic metabolites was achieved using a BEH C18 column (2.1 μm, 1.7 mm id x 50 mm). Samples were eluted at a flow rate of 0.5 mL/min with a gradient of 0.2% formic acid in acetonitrile (solvent A) and 0.2% formic acid in water (solvent B) as follows: 0 min, 100% B; 0.5 min, 94% B; 2.0 min, 91% B; 3.0 min, 87% B; 4.5 min, 65% B; 5.3 min, 100% B; 6 min, 100% B. Phenolic metabolites were identified by comparing retention time, molecular mass and fragmentation pattern of sample peaks with those of authentic standards. Contents of targeted phenolic metabolites were determined according to calibration curves covering 0.1–20 μg/mL. MS conditions were as follows: ionization mode: electrospray ionization (ESI-); capillary voltage: 0.5 kV; probe temp: 150 °C; source temp: 600 °C; desolvation gas flow: 1200 L/h; cone gas flow: 5 L/h. Multiple reaction monitoring parameters for phenolic compounds are shown in Table 1.
Table 1.
Multiple Reaction Monitoring (MRM) parameters for phenolic compounds. MW, molecular weight; [M − H]-, negative ion mode.
| Compound Class | Phenolic Metabolite | MW (g mol−1) | [M − H]- (m/z) | MRM |
|---|---|---|---|---|
| Phenolic Acids | Ferulic Acid | 194.18 | 193.0 | 193.0 → 134.0 |
| Caffeic Acid | 180.16 | 179.1 | 179.1 → 131.1 | |
| p-Coumeric Acid | 164.05 | 163.0 | 163.0 → 119.1 | |
| Benzoic Acid Derivatives | 4-hydroxy-benzoic acid | 138.12 | 137.0 | 137.0 → 93.1 |
| 3,4-dihydroxy-benzoic acid | 154.12 | 153.0 | 153.0 → 109.1 | |
| 4-Hydroxy-3-(3,5)-dimethoxybenzoic acid | 198.17 | 197.1 | 197.1 → 182.0 | |
| Propionic Acid Derivatives | 3-hydroxyphenyl-propionic acid | 166.16 | 165.0 | 165.1 → 121.6 |
| 3-hydroxy-4-methoxyphenyl-propionic acid | 182.17 | 181.1 | 195.0 → 136.0 | |
| Phenyl Acetic Acid Derivatives | 3,4-Dihydroxyphenyl acetic acid | 168.15 | 167.0 | 167.0 → 123.1 |
| 3-Hydroxyphenyl acetic acid | 152.14 | 151.1 | 151.1 → 107.4 | |
| 4-Hydroxyphenyl acetic acid | 152.14 | 151.1 | 151.1 → 107.4 | |
| Homovanillic acid | 182.18 | 181.1 | 181.1 → 137.1 | |
| Hippuric Acid Derivatives | Hippuric acid | 179.17 | 178.0 | 178.0 → 77.0 |
| 3-Hydroxy hippuric acid | 195.17 | 194.0 | 194.0 → 2.9 |
3.5.9. Immune and inflammatory marker assessment
Inflammatory cytokine and C-reactive protein (CRP) measurement in sera: At baseline and after 12 months of prune consumption, serum was collected for measurement of circulating inflammatory cytokines and chemokines (interleukin-1 beta [IL-1β], IL-6, IL-8, tumor necrosis factor-alpha [TNF-α], and monocyte chemoattractant protein [MCP-1]) and C-reactive protein (CRP) as has been done to assess inflammatory responses in other clinical trials [16,32]. IL-1β, IL-6, IL-8, TNF-α, and MCP-1 were measured using the V-PLEX Proinflammatory Panel 1 Human Kit and V-PLEX Human MCP-1 kit (Meso Scale Diagnostics, LLC, Rockville, MD) as per the manufacturer's instructions. CRP in serum was quantified using an Immulite (Siemens Healthcare, Munich, Germany) high-sensitivity CRP kit as per manufacturer's instructions. Each assay was performed in duplicate.
Isolation of peripheral blood mononuclear cells (PBMCs): Packed red and white blood cells (approximately 5 mL) were diluted 1:2 with phosphate-buffered saline (PBS; Mediatech, Manassas, VA, USA), gently layered on top of lymphocyte separation media (LSM; Corning, Manassas, VA, USA), and centrifuged at 1600 rpm for 30 min at room temperature. PBMCs were collected at the plasma/LSM interface; washed twice with complete media RPMI 1640 (Mediatech) containing 10 mM HEPES (Mediatech), 10% heat-inactivated fetal bovine serum (Gemini, West Sacramento, CA, USA), 2 mM l-glutamine (Mediatech), 0.1 mM non-essential amino acids (Mediatech), 1 mM sodium pyruvate (Mediatech), 100 U/ml penicillin/streptomycin (Mediatech), and 55 μM 2-mercaptoethanol (Life Technologies, Grand Island, NY, USA) at room temperature; and counted on a hemocytometer for use in functional and phenotypic analyses as previously described [33].
Ex vivo inflammatory cytokine secretion assay: The assessment of ex vivo cytokine production from LPS-stimulated PBMCs was done to assess functional capacity of monocytes following prune consumption as previously reported [34]. PBMCs (2 × 105/mL) were stimulated with 0.625 μg/mL lipopolysaccharide (LPS) (Sigma-Aldrich, St. Louis, MO) [34] and supernatants (70 μL per replicate) were harvested after 4 h incubation at 37 °C and frozen at −80 °C until analysis. IL-1β, IL-6, IL-8, TNF-α, and MCP-1 from LPS-stimulated PBMCs were measured using the V-PLEX Proinflammatory Panel 1 Human Kit and V-PLEX Human MCP-1 kit (Meso Scale Diagnostics, LLC, Rockville, MD) as per the manufacturer's instructions.
Flow cytometric analysis: Phenolic compounds in prunes are reported to have numerous anti-inflammatory and anti-oxidative effects [35]. We chose to assess the effect of prunes on the number and activation status of monocytes as they are one of the main producers of inflammatory cytokines. If the number or activation status of monocytes was altered by prune consumption, these changes would potentially influence the level of circulating inflammatory cytokines. PBMCs were washed twice in PBS at 4 °C. Fc receptors on PBMCs were blocked by incubation with mouse anti-human CD16 (Fc block) (Human TruStain FcX, Biolegend, San Diego, CA) at 5 μL per 1 × 106 cells. PBMCs were stained with fluorescence-labeled mouse anti-human antibodies (1 μg/1 × 106 cells) to the following cell surface markers: CD3, CD14, CD282 (TLR-2) and Human Leukocyte Antigen-DR isotype (HLA-DR). Antibody isotype controls included: mouse IgG2a and mouse IgM. CD282 and CD14 were purchased from Biolegend, and all remaining antibodies were purchased from BD Biosciences (Franklin Lakes, NJ). Following incubation with the conjugated antibodies for 30 min at 4 °C, cells were washed twice in PBS and then fixed in cytofix (BD Biosciences) for flow cytometric analyses as previously described [36]. A total of 50,000 events were acquired with BD LSR-Fortessa (BD Biosciences). Data were analyzed and plotted using FlowJo software v10 (FlowJo, LLC).
3.5.10. Fecal assessment
Gut microbiota analysis: The fecal microbiome was analyzed pre- and post-treatment. Homogenized fecal samples were kept at −80 °C until DNA extraction. DNA was extracted using the FastDNA SPIN Kit for Soil (MP Biomedicals) and bead-beating according to the manufacturer's instructions. Extracted DNA quality was assessed using 0.8% agarose gel electrophoresis and a NanoDrop One spectrometer (Thermo Scientific). DNA was quantified using a NanoDrop 3300 spectrofluorometer (Thermo Scientific). The V3–V4 region of the 16S rRNA gene was amplified by PCR with primers 343F: TACGGRAGGCAGCAG and 804R: CTACCRGGGTATCTAATCC. PCR was conducted using the Q5 High-Fidelity Master Mix (New England Biolabs), with 10 ng of DNA template per 50 μl reaction, and the following cycling conditions: 5 min at 95 °C; 15 cycles of 30 s at 94 °C, 20 s at 58 °C, and 20 s at 72 °C; 10 min at 72 °C. PCR products were purified using the AMPure XP clean-up kit (Beckman-Coulter) and subjected to a second PCR phase, in which amplicons were barcoded using forward and reverse 8-base pair primer tags. The second PCR phase used the following cycling conditions: 5 min at 95 °C; 5 cycles of 30 s at 94 °C, 20 s at 64 °C, and 20 s at 72 °C; and final extension for 10 min at 72 °C. PCR products were again purified using the AMPure XP clean-up kit and quantified using the QuantiFluor dsDNA System (Promega) and a NanoDrop 3300 spectrofluorometer. Purified PCR products were then pooled in equal molar quantities and sequenced using Illumina MiSeq 2x 250 paired end sequencing at the Purdue Genomics Facility.
Sequences were trimmed to remove adapters and barcodes, and then analyzed using QIIME2 [37]. After demultiplexing, DADA2 [38] was used to trim bases for quality, merge paired-end reads and group sequences into amplicon sequence variants (ASVs). For all diversity analyses, sequences were rarified to the same depth, chosen to maximize retained features while minimizing excluded samples. Differences in alpha and beta diversity among treatment groups were analyzed using various metrics available in QIIME2. Alpha diversity (metrics used: observed OTUs, Pielou's Evenness, Faith's phylogenetic diversity [39]) measures the richness and/or evenness of taxa within a sample, while beta diversity (metrics used: Jaccard, Bray-Curtis, Unweighted Unifrac [40], and Weighted Unifrac) measures the difference in taxa distribution among samples. Beta diversity was visualized using Principal Coordinates Analysis (PCoA) plots. Significant differences in beta diversity were determined using permutational multivariate analysis of variance (PERMANOVA) with 999 permutations. Permutational analysis of multivariate dispersions (PERMDISP) was used to calculate whether significant differences in beta diversity could be due to differences in the variation of treatment groups instead of true differences in the means of groups. Pairwise differences in alpha and beta diversity before and after treatment for each participant were calculated using the pairwise functions of QIIME2 longitudinal, a plugin for analyzing microbiome changes over time. A taxonomy classifier, trained using the SILVA database (version 138) [41], was used to assign taxonomies to representative 16S rRNA gene sequences. Analysis of Composition of Microbes (ANCOM) [42] was used to find differences in the log-transformed counts of taxa between treatment groups, and linear discriminant analysis effect size (LEfSe) [43] was used to find differences in the relative abundance of taxa among treatment groups. Correspondence between bacterial taxa and other health measures was determined using Canonical Correspondence Analysis (CCA). Correlations between the relative abundance of individual taxa and urinary phenolics were determined using Spearman's rank correlation coefficient in R with the package corrplot.
Bristol Stool Scale Chart: The Bristol Stool Scale Chart will be completed by each participant at baseline, month 6 and month 12. The completion of this scale requires visual inspection of the stool sample to match to 7 images portrayed in the pictures provided on the scale images.
3.5.11. Sample size
We were not able to utilize the data from the published papers of Hooshmand et al. [14,15] to help guide the BMD sample size determination, as the necessary means and standard deviations are not available in the published work. Therefore, the sample size estimates were adapted from estimates reported in a pharmacological study of bone health in postmenopausal women [44] designed to detect a significant percent change in BMD at the lumbar spine and hip at month 12 of the intervention (study completion). We hypothesized that the prune intervention would yield a 1.4% (standard deviation 3.3) increase in lumbar spine BMD, representing 60% effectiveness of risedronate, while the control group would decrease lumbar spine BMD by 0.4% (standard deviation 3.3) [44]. We also hypothesized that the prune interventions would yield a 0.8% (standard deviation 2.7) increase in total hip BMD, representing 60% effectiveness of risedronate, while the control group would decrease total hip BMD by 0.7% (standard deviation 2.7) [44]. Allowing for a 20% drop-out rate during the 12-month intervention, two-sided type 1 error rate of 5%, a sample size of 55 completed participants per group would yield 80% power at the lumbar spine to detect a 1.4% increase in BMD with the prune intervention compared to the control group. For total hip BMD, 50 completed participants per group would yield 80% power to detect a 0.8% change in BMD with the prune intervention compared to the control group. Given the difficulty in enrolling postmenopausal women with low BMD, as reported in Sestak et al. [44], we over-enrolled each group in order to maximize the number of completers necessary to be adequately powered. No interim analyses were determined a priori, and therefore data analysis for primary outcome measures did not commence until final participants completed the intervention.
4. Results
The progression of participants enrollment and dropout throughout the study is displayed in Fig. 2. 209 women completed 3 months (74 Control, 73 50g, 62 100g). 199 women completed 6 months (71 Control, 70 50g, 58 100g), 187 women completed 9 months (71 Control, 69 50g, 47 100g), and 160 women completed 12 months (60 Control, 57 50g, 43 100g). The COVID-19 university closure impacted the timing of 12-month visits; thus, 23 women (10 Control, 10 50g, 3 100g) completed a post visit upon university opening and IRB approval to restart the study resulting in some participants enrollment beyond the 12-month intended study duration (mean measurement timing of 14 months). A total of 183 completed 12 month/post visits (70 control, 67 50g/d prune, 46 100g/d prune).
The screening dropout rate was 22%, with primary reasons for participant withdrawal/exclusion including BMD T-score ≤ −3.0, BMD T-score above 0, time commitment, and possible diagnosis of lumbar compression fracture. Overall dropout/early termination was 22%, however, there were drop-out rate group differences (Control group, 10%; 50g group, 15%; 100g group, 41%). During baseline and intervention, the primary reasons for dropout were as follows: In the Control group, the drop-out rate was lowest and time commitment was the primary reason; in the 50g group, the primary reason for drop-out was poor tolerance consuming the prunes and time commitment; and lastly, in the 100g group, the drop-out rate was highest, and the primary reason for drop-out was poor tolerance consuming the prunes. Detailed reasons for participant dropout are provided in Fig. 2.
5. Discussion
Extending beyond previous human trials [[13], [14], [15]], the Prune Study is the first RCT to evaluate a dose response effect of daily prune consumption for 12 months on bone density, structural and strength characteristics in postmenopausal women. To our knowledge, this is the largest and most comprehensive investigation of a dose response of prune consumption on BMD and bone turnover to also examine biomarkers of immune function, inflammation, and cardiovascular health, as well as detailed phenolic and gut microbiota analyses. Prior RCT investigations demonstrate favorable bone health outcomes (i.e., bone turnover) during shorter duration investigations [13,16], and site specific BMD improvements [15] compared with other dried fruit treatment. However, these studies do not address potential dose-dependent differences in bone outcomes over extended duration (12 months) and present limited data (i.e., no absolute changes in BMD) to help understand the extent to which prune improves bone health parameters.
The Prune Study is strengthened by the repeated comprehensive biological (serum, urine, fecal) and behavioral assessments (diet and exercise), and BMD and bone strength estimates. Additionally, intervention adherence monitored by self-report of prune consumption and confirmed by urinary total phenolic and targeted metabolite concentrations provide a more accurate depiction of compliance, compared to previous interventions. However, limitations of the current study do exist. The dropout rate exceeded the anticipated 20%, driven by an unanticipated high drop out in the 100g prune group (41%). While this may reduce the power to detect BMD changes in this group compared to the control, we believe this group provides important insight into the feasibility of maintaining the higher dosage of prune consumption over an extended period of time. Additionally, due to COVID-19 university closure, a subset of our participants underwent a longer than anticipated intervention duration (i.e., average 14 months). Despite this, we retained a majority of individuals (n = 3 dropped during this 8-month time frame), and can account for study duration as a covariate in generalized linear mixed model approach. Finally, the study was conducted in primarily Caucasian postmenopausal women (96%), limiting the generalizability of findings to this racial group.
6. Conclusions
The Prune Study provides the first RCT data on the impact of two dosages of daily prune consumption on bone density, structure and strength estimates, bone turnover, various biomarkers of immune function, inflammation, and cardiovascular health, as well as phenolic and gut microbiota analyses, to encompass the varied physiological effects prunes may have on health. Despite the limitations, we believe the results of this RCT will expand our current understanding of clinical implications and mechanisms underlying the resultant health effects of prune as a functional food therapy.
Funding
The California Prune Board provided funding support and prunes for this study. The funders had no role in the data collection and analysis, decision to publish, or preparation of manuscript. To avoid a conflict of interest, board members CJR and CW did not participate in any meetings whatsoever on the Nutrition Section of the California Prune Board regarding this study for the full duration of this study, to include all discussions regarding whether to fund the study, and all info on the outcome of California Prune Board meetings.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: CW and CJR are members of the Nutrition Advisory Panel for the California Prune Board. MJD, NCAS, NIW, MGF, CHN, AMRS have no competing financial interests/personal relationships to disclose.
References
- 1.Wright N.C., et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. Journal of bone and mineral research. Off. J. Am. Soc. Bone Miner. Res. 2014;29(11):2520–2526. doi: 10.1002/jbmr.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burger, H., Hormone replacement therapy in the post-Women's Health Initiative era. Report a a meeting held in Funchal, Madeira, February 24-25, 2003. Climacteric : J. Int. Menopause Soc. , 2003. 6 Suppl 1: p. 11–36. [PubMed]
- 3.Weaver C.M., et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos. Int. 2016;27(1):367–376. doi: 10.1007/s00198-015-3386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arjmandi B.H. The role of phytoestrogens in the prevention and treatment of osteoporosis in ovarian hormone deficiency. J. Am. Coll. Nutr. 2001;20(5 Suppl):398S–402S. doi: 10.1080/07315724.2001.10719175. discussion 417S-420S.discussion. [DOI] [PubMed] [Google Scholar]
- 5.Muhlbauer R.C., et al. Various selected vegetables, fruits, mushrooms and red wine residue inhibit bone resorption in rats. J. Nutr. 2003;133(11):3592–3597. doi: 10.1093/jn/133.11.3592. [DOI] [PubMed] [Google Scholar]
- 6.Pawlowski J.W., et al. Plum and soy aglycon extracts superior at increasing bone calcium retention in ovariectomized Sprague Dawley rats. J. Agric. Food Chem. 2014;62(26):6108–6117. doi: 10.1021/jf403310q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devareddy L., et al. Blueberry prevents bone loss in ovariectomized rat model of postmenopausal osteoporosis. J. Nutr. Biochem. 2008;19(10):694–699. doi: 10.1016/j.jnutbio.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Deyhim F., et al. Dried plum reverses bone loss in an osteopenic rat model of osteoporosis. Menopause. 2005;12(6):755–762. doi: 10.1097/01.gme.0000185486.55758.5b. [DOI] [PubMed] [Google Scholar]
- 9.Franklin M., et al. Dried plum prevents bone loss in a male osteoporosis model via IGF-I and the RANK pathway. Bone. 2006;39(6):1331–1342. doi: 10.1016/j.bone.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Treutter D., et al. Diversity of phenolic profiles in the fruit skin of Prunus domestica plums and related species. J. Agric. Food Chem. 2012;60(48):12011–12019. doi: 10.1021/jf303644f. [DOI] [PubMed] [Google Scholar]
- 11.Mubarak A., et al. Polyphenol composition of plum selections in relation to total antioxidant capacity. J. Agric. Food Chem. 2012;60(41):10256–10262. doi: 10.1021/jf302903k. [DOI] [PubMed] [Google Scholar]
- 12.Rendina E., et al. Dried plum's unique capacity to reverse bone loss and alter bone metabolism in postmenopausal osteoporosis model. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0060569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arjmandi B.H., et al. Dried plums improve indices of bone formation in postmenopausal women. J. Wom. Health Gend. Base Med. 2002;11(1):61–68. doi: 10.1089/152460902753473471. [DOI] [PubMed] [Google Scholar]
- 14.Hooshmand S., Brisco J.R., Arjmandi B.H. The effect of dried plum on serum levels of receptor activator of NF-kappaB ligand, osteoprotegerin and sclerostin in osteopenic postmenopausal women: a randomised controlled trial. Br. J. Nutr. 2014;112(1):55–60. doi: 10.1017/S0007114514000671. [DOI] [PubMed] [Google Scholar]
- 15.Hooshmand S., et al. Comparative effects of dried plum and dried apple on bone in postmenopausal women. Br. J. Nutr. 2011;106(6):923–930. doi: 10.1017/S000711451100119X. [DOI] [PubMed] [Google Scholar]
- 16.Hooshmand S., et al. The effect of two doses of dried plum on bone density and bone biomarkers in osteopenic postmenopausal women: a randomized, controlled trial. Osteoporos. Int. 2016;27(7):2271–2279. doi: 10.1007/s00198-016-3524-8. [DOI] [PubMed] [Google Scholar]
- 17.Hooshmand S., et al. Effect of 12 months consumption of 100g Dried plum (prunes) on bone biomarkers, density, and strength in men. J. Med. Food. 2022;25(1):40–47. doi: 10.1089/jmf.2021.0080. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y.J., Martin B.R., Boushey C.J. Development and evaluation of a brief calcium assessment tool for adolescents. J. Am. Diet Assoc. 2010;110(1):111–115. doi: 10.1016/j.jada.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Neveu, V. et al. Phenol-Explorer: an online comprehensive database on polyphenol content in food. Database, doi:10.1093/database/bap024. [DOI] [PMC free article] [PubMed]
- 20.Ainsworth B.E., et al. Compendium of Physical Activities: a second update of codes and MET values. Med. Sci. Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. 2011. [DOI] [PubMed] [Google Scholar]
- 21.Ainsworth B.E., et al. Compendium of physical activities: an update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000;32(9 Suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 22.Nikander R., et al. Femoral neck structure in adult female athletes subjected to different loading modalities. J. Bone Miner. Res. 2005;20(3):520–528. doi: 10.1359/JBMR.041119. [DOI] [PubMed] [Google Scholar]
- 23.Nikander R., et al. Loading modalities and bone structures at nonweight-bearing upper extremity and weight-bearing lower extremity: a pQCT study of adult female athletes. Bone. 2006;39(4):886–894. doi: 10.1016/j.bone.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Yoshikawa T., et al. Geometric structure of the femoral neck measured using dual-energy x-ray absorptiometry. J. Bone Miner. Res. 1994;9(7):1053–1064. doi: 10.1002/jbmr.5650090713. [DOI] [PubMed] [Google Scholar]
- 25.Beck T.J., et al. Predicting femoral neck strength from bone mineral data. A structural approach. Invest. Radiol. 1990;25(1):6–18. doi: 10.1097/00004424-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Kanis J.A., et al. Development and use of FRAX (R) in osteoporosis. Osteoporos. Int. 2010;21:407–413. [Google Scholar]
- 27.McCloskey E.V., et al. A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J. Bone Miner. Res. 2016;31(5):940–948. doi: 10.1002/jbmr.2734. [DOI] [PubMed] [Google Scholar]
- 28.Sheu Y., et al. Bone strength measured by peripheral quantitative computed tomography and the risk of nonvertebral fractures: the osteoporotic fractures in men (MrOS) study. J. Bone Miner. Res. 2011;26(1):63–71. doi: 10.1002/jbmr.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferretti J.L., Capozza R.F., Zanchetta J.R. Mechanical validation of a tomographic (pQCT) index for noninvasive estimation of rat femur bending strength. Bone. 1996;18(2):97–102. doi: 10.1016/8756-3282(95)00438-6. [DOI] [PubMed] [Google Scholar]
- 30.Liu D., et al. Tibial geometry is associated with failure load ex vivo: a MRI, pQCT and DXA study. Osteoporos. Int. 2007;18(7):991–997. doi: 10.1007/s00198-007-0325-0. [DOI] [PubMed] [Google Scholar]
- 31.Sievanen H., et al. Peripheral quantitative computed tomography in human long bones: evaluation of in vitro and in vivo precision. J. Bone Miner. Res. 1998;13(5):871–882. doi: 10.1359/jbmr.1998.13.5.871. [DOI] [PubMed] [Google Scholar]
- 32.Hong M.Y., et al. Dried plum consumption improves total cholesterol and antioxidant capacity and reduces inflammation in healthy postmenopausal woemn. J. Med. Food. 2021:1–8. doi: 10.1089/jmf.2020.0142. [DOI] [PubMed] [Google Scholar]
- 33.Meng H., et al. Consumption of Bifidobacterium animalis subsp. lactis BB-12 in yogurt reduced expression of TLR-2 on peripheral blood-derived monocytes and pro-inflammatory cytokine secretion in young adults. Eur. J. Nutr. 2017;56(2):649–661. doi: 10.1007/s00394-015-1109-5. [DOI] [PubMed] [Google Scholar]
- 34.Oh E.S., et al. Spices in a high-saturated-fat, high-carbohydrate meal reduce postprandial proinflammatory cytokine secretion in men with overweight or obesity: a 3-period, crossover, randomized controlled trial. J. Nutr. 2020;150(6):1600–1609. doi: 10.1093/jn/nxaa063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Damani J., et al. The role of prunes in modulating inflammatory pathways to improve bone health in postmenopausal women. Adv. Nutr. 2022:nmab162. doi: 10.1093/advances/nmab162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng H., et al. In vitro production of IL-6 and IFN-gamma is influenced by dietary variables and predicts upper respiratory tract infection incidence and severity respectively in young adults. Front. Immunol. 2015;6:94. doi: 10.3389/fimmu.2015.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolyen E., et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019;37(8):852–857. doi: 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Callahan B.J., et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13(7):581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faith D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992;61(1):1–10. [Google Scholar]
- 40.Lozupone C., Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005;71(12):8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quast C., et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41(Database issue):D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mandal S., et al. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb. Ecol. Health Dis. 2015;26 doi: 10.3402/mehd.v26.27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Segata N., et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sestak I., et al. Changes in bone mineral density at 3 years in postmenopausal women receiving anastrozole and risedronate in the IBIS-II bone substudy: an international, double-blind, randomised, placebo-controlled trial. Lancet Oncol. 2014;15(13):1460–1468. doi: 10.1016/S1470-2045(14)71035-6. [DOI] [PubMed] [Google Scholar]