Summary
Background
Cartilage damage in inflammatory arthritis is attributed to inflammatory cytokines and pannus infiltration. Activation of the coagulation system is a well known feature of arthritis, especially in rheumatoid arthritis (RA). Here we describe mechanisms by which fibrin directly mediates cartilage degeneration.
Methods
Fibrin deposits were stained on cartilage and synovial tissue of RA and osteoarthritis (OA) patients and in murine adjuvant-induced arthritis (AIA) in wild-type or fibrinogen deficient mice. Fibrinogen expression and procoagulant activity in chondrocytes were evaluated using qRT-PCR analysis and turbidimetry. Chondro-synovial adhesion was studied in co-cultures of human RA cartilage and synoviocytes, and in the AIA model. Calcific deposits were stained in human RA and OA cartilage and in vitro in fibrinogen-stimulated chondrocytes.
Findings
Fibrin deposits on cartilage correlated with the severity of cartilage damage in human RA explants and in AIA in wild-type mice, whilst fibrinogen deficient mice were protected. Fibrin upregulated Adamts5 and Mmp13 in chondrocytes. Chondro-synovial adhesion only occurred in fibrin-rich cartilage areas and correlated with cartilage damage. In vitro, autologous human synoviocytes, cultured on RA cartilage explants, adhered exclusively to fibrin-rich areas. Fibrin co-localized with calcification in human RA cartilage and triggered chondrocyte mineralization by inducing pro-calcification genes (Anx5, Pit1, Pc1) and the IL-6 cytokine. Similar fibrin-mediated mechanisms were observed in OA models, but to a lesser extent and without pseudo-membranes formation.
Interpretation
In arthritis, fibrin plaques directly impair cartilage integrity via a triad of catabolism, adhesion, and calcification.
Funding
None.
Keywords: Fibrin, Fibrinogen, Arthritis, Cartilage degradation, Calcification, Chondro-synovial adhesion
Research in context.
Evidence before this study
Fibrin deposition in arthritic joints was evidenced previously in synovial fluid and synovial membrane, and we published in the past that deficiency of fibrinolysis could exacerbate experimental rheumatoid arthritis while coagulation inhibition could alleviate it.
Added value of this study
In our new work we showed that fibrin deposition was prominent in arthritic damaged cartilage. Moreover, we have identified mechanisms that could account for fibrin deleterious effects on cartilage: (1) Fibrin(ogen) strongly upregulated the expression of cartilage catabolic enzymes in chondrocytes in vitro and, accordingly, cartilage of fibrinogen-deficient (Fg-/-) arthritic mice was protected; (2) Fibrin(ogen) triggered chondro-synovial adhesion (CSA), likely causing stripping of superficial layers and cartilage degradation; (3) Fibrin(ogen) colocalized with calcium-containing crystal deposits on cartilage explants from arthritic patients and, in vitro, fibrinogen exacerbated chondrocytes mineralization.
Implications of all the available evidence
Altogether, our new data indicate that fibrin(ogen) deposition on cartilage has direct detrimental effects on cartilage. Therefore, targeting fibrin deposition in cartilage could represent a new therapeutic strategy to reduce cartilage degradation in inflammatory arthritis.
Alt-text: Unlabelled box
Introduction
The activation of coagulation pathways, with subsequent fibrin deposition, is a commonly encountered phenomenon in chronic inflammatory disorders, including rheumatoid arthritis (RA).1,2 Fibrinogen levels in blood of RA patients are elevated and correlate with other inflammatory markers and disease activity.3,4 Increased fibrinogen production by the liver and local tissue factor expression by inflammatory cells in the inflamed synovium lead to diffuse clotting in arthritic joints after thrombin cleaves fibrinogen into fibrin.3,5 Systemic and local dysregulation of the coagulation and fibrinolytic pathways further contribute to fibrin deposition in synovial tissue and intra-articularly,6 in form of vascularized conglomerates called rice-bodies.7 Generally, fibrin is susceptible to structural modifications by inflammatory cytokines such as C-reactive protein and serum amyloid A, with a denser fiber network and increased resistance to fibrinolysis in RA.8 As currently understood, fibrin networks are a matrix for inflammatory cells in pannus formation.9
The severity and duration of prolonged morning stiffness are associated with fibrin, derived from neutrophilic leukocytes, deposited along RA patients’ synovial membranes.10 The mechanisms of how fibrin is associated with joint stiffness and destruction remain elusive.
Both thrombin inhibition and fibrinolysis effectively reduce inflammation in experimental arthritis,11, 12, 13 but these approaches have not yet been translated into humans, likely due to the risk of intra- and extra-articular bleeding and the advent of targeted therapies. On the other hand, in many patients, disease-modifying anti-rheumatic drugs do not stop the progression of joint damage despite successfully controlling inflammation.14 As observed in chronic neuro-inflammation, persisting fibrin deposits act as para-inflammatory, pro-degenerative stimulus that can be targeted therapeutically by monoclonal antibodies.15
Different fibrin-associated immunopathological mechanisms have been revealed in RA, notably, the promotion of leukocyte recruitment via interaction with the MAC-1 (CD11b/CD18) integrin receptor,16 and the citrullination of fibrin.17 Anti-citrullinated protein antibodies have shown cross-reactivity to joint cartilage18 and, in animal models, fibrinogen has shown arthritogenic properties triggering both autoreactive T-cell and autoantibody responses.19
Until now, the two main known mechanisms involved in cartilage damage in RA remain invasion by pannus tissue (invasive vascularized connective tissue) and the catabolic effects of inflammatory cytokines and proteases.20,21 Although high amounts of intra-articular fibrin are well known in arthritis, fibrin's direct effects on cartilage damage have never been observed. We postulated that, as a consequence of chronic inflammation and activated coagulation, and analogous to atherosclerosis, fibrin deposition on cartilage is a key element of degeneration.
Methods
Mice and induction of experimental arthritis
Antigen-induced arthritis (AIA) was provoked in the right knee of 12-week old wild-type (WT) mice and fibrinogen knockout (Fg−/−), as previously described,22 while the left sham knee was used as control. Collagen-induced arthritis (CIA) was induced in 12-week old WT mice and CD11b knockout (CD11b−/−) mice, as previously decribed.23 Fg−/- mice were kindly provided by Prof. Jay Degen and Dr. Thomas Bugge (National Institute of Dental and Craniofacial Research, Bethesda, USA) while CD11b−/− were obtained by Prof. Britta Engelhardt (University of Bern, Switzerland). As OA model, meniscectomy of the medial meniscus was performed in the right knee of 12-week old WT mice, while the left knee was sham-operated as control, as previously described.24 To minimize suffering, we anesthesized mice with isofurane during the injection or surgery, we kept them on a warm pad and we applied ocular gel (Viscotears Liquid gel-Novartis). Anaesthesia was coupled with anaelgesia (Temgesic) as suggested by the official veterinarian.
We estimated sample size by power analysis: based on our previous studies in similar experimental settings, we can expect around 50% difference in cartilage damage. Considering our experimental standard deviation, analysis by post-hoc one-way Anova followed by bilateral one-sample t-test, an α-error of 0.05 and a power ≥0.8, n = 7 animals/group is the ideal sample size to adopt. For the MNX model, 3 WT mice were excluded from the analysis as the histological sections were not scorable (overlapped). Other exclusion creteria were set by the animal authorization officer, which stated that mice had to be sacrificed in case of high score suffering (humane endpoint). We have not excluded any mice for this reason. Randomization was made by simple random allocation; minimization of confunders was controlled by using sex- and age-matched mice. During AIA/CIA induction or the MNX surgery, the operator was blinded with respect to mice groups. Mice were housed in groups of 4, in ventilated cages with enrichement, bedding and water and food ad libitum.
Murine cartilage histology
After their sacrifice, mice knees were fixed in formol, decalcified in formic acid, and embedded in paraffin. Sagittal knee sections (6 µm) were stained with Safranin-O and counterstained with fast green/iron hematoxylin to evaluate cartilage degradation, synovial thickness and chondro-synovial adhesion. Cartilage degradation in the AIA and CIA models was assessed on both the tibia and femur using a scale from 0 (healthy cartilage) to 3 (severe cartilage erosion), and a total score (tibia + femur) was calculated. For the meniscectomy (MNX) model, osteoarthritis severity in tibia and femur was assessed using the Osteoarthritis Research Society International (OARSI) score from 0 (healthy cartilage) to 24 (severe cartilage erosion), and the total knee score was calculated.25 Briefly, OARSI scores (cartilage damage and Safranin-O loss) were obtained by multiplying the grade (depth progression into cartilage) by the stage (horizontal extent of cartilage involvement). Six grades were determined: 0 = intact cartilage surface; 1 = uneven cartilage surface; 2 = fibrillated cartilage surface; 3 = fissured cartilage; 4 = erosion till deep cartilage; 5 = bone surface denudation; 6 = bone remodeling and defor-mation. Four stages were also distinguished: 0 = no joint involvement; 1 ≤ 10% joint in-volvement; 2 = 10–25% involvement; 3 = 25–50% involvement; 4 ≥ 50% involvement. In all murine models, chondro-synovial adhesion (or CSA: the percentage of cartilage area with signs of synovial membrane adhesion) on the tibia and femur were scored from 0 (0% CSA) to 4 (100% CSA), and a total score was calculated. Samples were blinded and scored by two independent observers.
Murine chondrocyte cell culture
Chondrocytes were isolated from 4- to 7-day old WT C57Bl/6 mice, as described previously.26 qRT-PCR analysis experiments were performed in DMEM + 1% P/S for 4 h, whereas for chondrocyte crystal formation analysis, experiments were performed in DMEM + 1% P/S + 10% FBS for 24 h. In all the experiments, the medium was supplemented, or not, with secondary calciprotein particles27 (CPP, Cf equivalent to 100 mg/mL calcium) to induce calcification. Where reported, cells were stimulated with different concentrations of fibrinogen (from purified human plasma, EMD Millipore Corp).
Murine chondrocyte mineralization and crystal detection
Chondrocyte monolayers were washed in PBS, fixed in ice-cold methanol, and crystal deposits analysed using alizarin red staining, as previously described.28 Quantification of calcium content (QuantiChrom Calcium Assay Kit, BioAssays System) was performed following the manufacturer's protocol. Results were normalized over the sample protein amount (Pierce BCA Protein Assay Kit, ThermoFisher). For the detection of calcium-containing crystals on human cartilage, paraffin sections were stained with Alizarin red, as previously described.29 Cartilage calcification was scored from 0 (no crystals) to 3 (a deep layer of crystals).
Human cartilage and synovial membrane samples
Human cartilage and synovial membrane from 4 RA patients (mean age 57 ± 10 years) undergoing total hip or knee replacement and from 10 OA patients (mean age 72 ± 10 years) undergoing total knee replacement were obtained from the Orthopedics Department (DAL, CHUV, Lausanne, Switzerland). Dissected cartilage and synovial membranes were fixed in formol for 7 days, and paraffin sections (4 μm) were cut and analysed by immunohistochemistry for fibrin(ogen) deposition. Cartilage was additionally scored as follows for the severity of damage: preserved with uneven surface (scoring 0-1 = low ), moderately damaged with superficial fissuration (scoring 2 = medium ) and severely damaged with deep fissuration (scoring 3-4 = high). Scoring was blinded and perfrormed by two independent observers.
Ex vivo chondro-synovial adhesion (CSA) studies
For human chondro-synovial studies, knee cartilage explants from 3 RA and 3 OA patients were put into 96-well plates in DMEM + 1%P/S + 10%FBS + 50 µg/ml ascorbic acid. Synovial membrane was cut into small pieces and digested by Collagenase type II (Sigma) for 4 h at 37°C. The resulting cell suspension was plated and synoviocytes were selected after one week of culture using plastic adherence. Synoviocytes were detached, replated and incubated for 24 h with 2µg/ml BrdU in DMEM+10%FBS. For the CSA assay, BrdU-labeled synoviocytes were seeded on the top of cartilage explants and co-incubated for 3 days in DMEM + 1%P/S + 10%FBS + 50 µg/ml ascorbic acid. Samples were fixed in 10% formol and processed for histological analysis via H&E, and immunohistochemical analysis for fibrin(ogen) deposition and BrdU incorporation.
Immunohistochemical analysis of fibrin(ogen) deposition
Human and murine fibrin(ogen) expression was evaluated by immunohistochemistry on paraffin sections using an anti-fibrin(ogen) rabbit polyclonal antibody (BT-BS6607 Nordic BioSite). BT-BS6607 was validated as explained in the Reagent Validation section (Supplementary material). Fibrin(ogen) deposits in human samples were scored using an arbitrary scale from 0 (no fibrin) to 3 (fibrin deposits from surface to deep cartilage layers, > 200 µm). For murine samples, fibrin deposits were assessed on both tibia and femur cartilage using a scale from 0 (no fibrin) to 4 (fibrin deposits on 100% of cartilage), and a total score was calculated.
Immunohistochemical analysis of BrdU incorporation
BrdU incorporation in human synoviocytes was evaluated by immunohistochemistry on paraffin sections using an anti-BrdU rat monoclonal antibody (6326 Abcam). 6326 Abcam has been validated as explained in the Reagent Validation section (Supplementary material).
Real-time PCR analysis
RNA was extracted (RNA Clean & Concentrator5, Zymoresearch), reverse transcribed (Superscript II, Invitrogen), and qRT-PCR analysis was performed using gene-specific primers (Supplementary Table 1), as previously described (LightCycler480®syste, Roche Applied Science).30 Data were normalized against Tbp and Gapdh reference genes, with the fold induction of transcripts calculated against control cells (Table 1).
Table 1.
Gene specific primers for qRT-PCR.
| 5Gene | Forward primer (5′ > 3′) | Reverse primer (5′ > 3′) |
|---|---|---|
| Adamts4 | GCC CGA GTC CCA TTT CCC GC | GCC ATA ACC GTC AGC AGG TAG CG |
| Adamts5 | GAC AGA CCT ACG ATG CCA CCC AGC | ATG AGC GAG AAC ACT GAC CCC AGG |
| Agg | GCC CCC ACC CTC TCT TCT TCA G | CAC CGT CTC TCC GCA TCC ACC |
| Alpl | TTG TGC CAG AGA AAG AGA GAG | GTT TCA GGG CAT TTT TCA AGG T |
| Ank | TGT CAA CCT CTT CGT GTC CC | GAC AAA ACA GAG CGT CAG CG |
| Anx5 | CCT CAC GAC TCT ACG ATG CC | AGC CTG GAA CAA TGC CTG AG |
| Comp | TTG CGA CAG CAG GTC AAG GAG A | GCG GGA AAC AGG GGT GAG CA |
| Fbg α | AGC CAT CCC TAA ACG CAG AC | TGG CTT CGT CAA TCA ACC CT |
| Fbg β | CTA TGG CTG CTG CTG CTA TTG | GGC TCT TCC TTT CTC CTG TCA AC |
| Fbg ɣ | CAA CCC CCA AAG CCA GGT AT | GCT GGG CTA CCT TCT GTT TT |
| Gapdh | CTC ATG ACC ACA GTC CAT GC | CAC ATT GGG GGT AGG AAC AC |
| Mmp-3 | ATA CGA GGG CAC GAG GAG | AGA AGT AGA GAA ACC CAA ATG CT |
| Mmp-13 | GCA GTT CCA AAG GCT ACA AC | GCT GGG TCA CAC TTC TCT G |
| Pc-1 | CTG GTT TTG TCA GTA TGT GTG CT | CTC ACC GCA CCT GAA TTT GTT |
| Pit-1 | CTC TCC GCT GCT TTC TGG TA | AGA GGT TGA TTC CGA TTG TGC |
| Pit-2 | AAA CGC TAA TGG CTG GGG AA | AAC CAG GAG GCG ACA ATC TT |
| Tbp | CTT GAA ATC ATC CCT GCG AG | CGC TTT CAT TAA ATT CTT GAT GGT C |
Procoagulant activity in chondrocytes
Pooled platelet-free plasma was prepared by centrifuging citrated blood (3.2% sodium citrate, pH 6.5) from donors with normal coagulation times (Laboratory of Haemostasis, CHUV, Lausanne). The procoagulant activity of murine and human primary chondrocytes was evaluated via clot formation. Briefly, chondrocytes (5*104 cells/well in 96-well plates) were incubated with 200 µl platelet-free plasma (diluted ½ in DMEM + 10% FBS) at 37°C and then treated with 55 ng/ml factor IIa or factor Xa inhibitors. Clotting was initiated by adding CaCl2 (25 mM final). Clot formation was determined by turbidimetry, reading optical density at 405 nm in a SpectraMax plate reader every 5 min for 160 min.
Imaging flow cytometry
WT and CD11b KO murine primary chondrocytes were stimulated, or not, for 1 h with 10 µg/ml of FITC-labelled human fibrinogen (Molecular Innovations, LOXO, Cat. IHUFBGFITC10MG) or with 10 µg/ml of APC-labelled CD11b antibody (Abcam, ab25482) or with a combination of them. Both antibodies were validated as explained in the Reagent Validation section (Supplementary material). Chondrocytes were detached using a dissociation buffer (5 mM EDTA, 20 mM HEPES), centrifuged, and pellets were resuspended in 50 µl of FACS buffer (2% FBS, 4 mM). Data were acquired using an Amnis ImageStreamX Mark II Imaging Flow Cytometer (Merck Millipore). We collected 10,000 events for all samples. All images were captured using a 40X lens. A cell classifier (threshold) was applied to the brightfield channel to exclude doublet events. Data were analysed using the IDEAS software package with gating on single-positive cells and then on fibrinogen and CD11b double-positive cells.
Gadolinium-enhanced Magnetic Resonance Imaging (MRI)
We retrospectively extracted 111 gadolinium-enhancement MRI reports including the term ‘rheumatoid’. Of those, we selected 10 MRI reports on the peripheral joints (excluding hands and severely damaged joints) of RA patients (mean age 62.5 ± 12.5 years) (Supplementary Table 2). Our controls were 20 gadolinium-enhancement analyses from patients undergoing MRI of the peripheral joints for other reasons (mostly tumors of the lower limbs). We assessed the presence of synovitis and superficial contrast-enhancement at cartilage surfaces in areas of cartilage-to-cartilage contact. All imaging was performed using 3-T whole-body MR imagers (MAGNETOM Skyra, SkyraFit, PrismaFit, Verio; Siemens Healthcare), with no hardware adjustments, using dedicated extremity coils, and with intravenous injection of a gadolinium-based contrast agent (DOTAREM 0.5 mmol/mL, Guerbet Aulnay/Bois).
Study approval
Human samples and MRI images were obtained in compliance with the Human Research Ethics Committee of the Canton of Vaud and with patients’ general written informed consent at the time of hospitalization. All samples were anonymized.
Murine experiments were performed in accordance with Swiss Federal Regulations and approved by the Canton of Vaud's Department of Consumer and Veterinary Affairs (authorization number 1153 and 2711).
Statistics
For both the in vitro and in vivo experiments, values are presented as mean ± SD. Variation between data sets was evaluated using Student's t-test or the one-way or two-way ANOVA test, where appropriate. Correlation between datasets, particularly the coefficients of correlation (r) and coefficients of determination (R2), were evaluated using the Pearson r test. All data were analysed using GraphPad Prism software (GraphPad, San Diego, CA). Differences were considered statistically significant at *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001, with 95% confidence intervals.
Role of Funders
No specific funding was obtained for this study.
Results
Fibrin(ogen) deposition on cartilage correlates with cartilage damage in human and murine arthritis
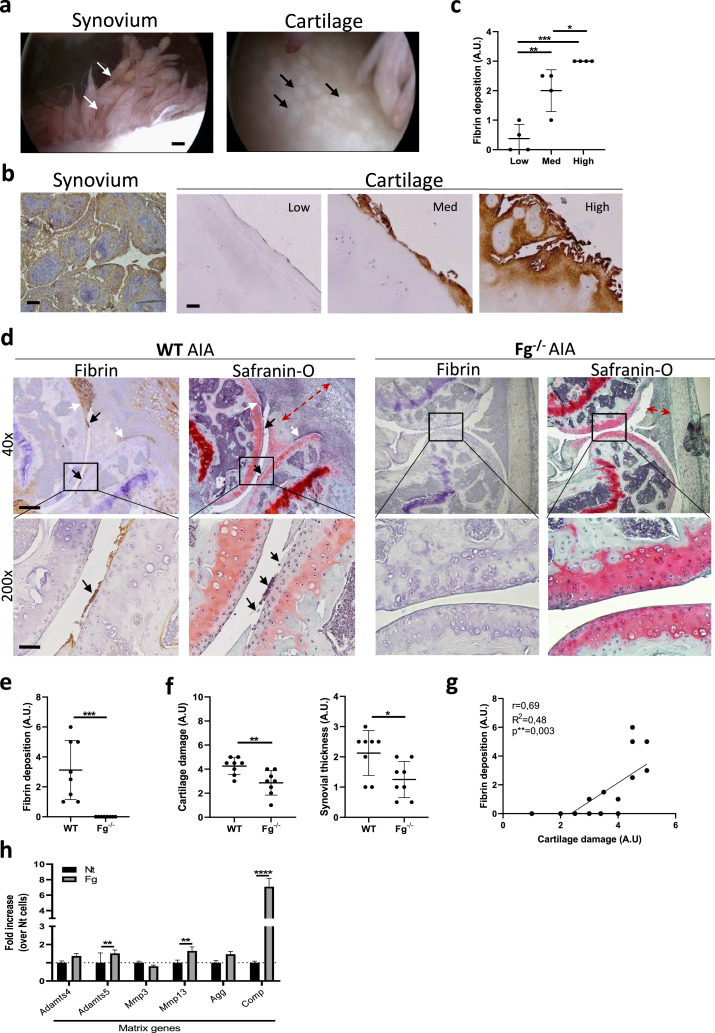
The present study was based on clinical observations made during knee arthroscopy performed on patients with RA. This revealed chronic synovitis in the suprapatellar recess, with golden shimmering fibrin deposits on synovial villi (white arrows, Figure 1a) and vis-à-vis localized, round cartilage lesions (black arrows, Figure 1a). Using immunohistochemistry, we confirmed fibrin(ogen) deposits on both synovial membrane and cartilage of patients with longstanding seropositive (RF+ and ACPA+) and destructive RA, undergoing knee or hip joint replacement. Fibrin(ogen) deposits in the synovium were found throughout the tissue analysed, while in cartilage they localized in superficial and intermediate layers (Figure 1b). Moreover, fibrin(ogen) deposits were minimal in cartilage with minor damage (low) but significantly greater in medium (med) and highly (high) damaged cartilage (Figure 1b, c).
Figure 1.
Fibrin deposition on cartilage correlates with the severity of cartilage damage in human and experimental RA. (a) Knee arthroscopy of an end-stage RA patient displays synovitis with goden shimmering deposits (white arrows), and vis-à-vis round plaques appearing on cartilage (black arrows). Scale bar = 200 μm. n = 4. (b) Immunohistochemistry showed fibrin(ogen) deposits on both synovium and cartilage. Scale bar = 30 μm. n = 4. (c) Graph shows fibrin deposit scores (arbitrary unit = AU) in low, medium, and high damaged areas of cartilage from 4 RA patients. For each level of cartilage degradation, one dot corresponds to one of the 4 patients. n = 4. One-way ANOVA post hoc Bonferroni correction. *p < 0.05, **p < 0.01, ***p < 0.001. (d) Representative immunohistochemical staining for fibrin deposits in WT and Fg−/− murine knees with AIA and representative Safranin-O histologies for cartilage damage and synovial thickness on consecutive sections. Black arrows show cartilage damage and fibrin deposits in WT knees. White arrows show recess occlusion in WT knees. Red arrows show synovial thickness. Scale bars for 40x = 300 μm and 200x = 120 μm. WT = 8, Fg−/− = 8. (e) Score for fibrin deposition and for (f) cartilage damage and synovial thinckness in WT and Fg−/− knees. WT = 8, Fg−/− = 8. (g) Correlation graph between fibrin deposit score (AU) and cartilage damage score (AU). Linear regression and Pearson correlation tests. **p < 0.01. (h) qRT-PCR of the indicated genes in murine chondrocytes stimulated for 4 h with 500 ug/ml Fg. Two-way ANOVA post hoc Bonferroni correction. n = 3 experiments. **p < 0.01, ****p < 0.0001.
Next, the association between fibrin(ogen) deposits and cartilage degradation was studied in vivo in wild-type (WT) and fibrinogen knockout (Fg−/−) mice subjected to the experimental AIA model (Figure 1d–g). WT AIA mice showed extensive fibrin(ogen) deposits on cartilage surfaces (black arrow, Figure 1d) and in the articular recess (white arrows, Figure 1d). On consecutive slides, by Safranin-O saining we observed a higher degree of cartilage damage in WT AIA mice (black arrows, Figure 1d), mainly where fibrin(ogen) deposits were present, and inflamed thickened synovial lining (red arrows, Figure 1d). Contrarily, Fg−/− AIA mice showed absence of fibrin(ogen) deposition, significantly healthier cartilage and milder synovitis (Figure 1d-f)). As in human RA, we further demonstrated a significant positive correlation between fibrin(ogen) deposits and the extent of cartilage damage (Figure 1g). No cartilage damage was found in both WT and Fg−/- AIA sham knees (Supplementary Figure 1a).
To explain a possible underlying mechanism of fibrin(ogen)-induced cartilage damage, we incubated murine primary chondrocytes with fibrinogen and found statistically significant upregulation of cartilage matrix-degrading enzymes (Adamts5 and Mmp13). We also found a strong induction of cartilage oligomeric matrix protein (Comp), a marker of cartilage turnover, also known to be a prognostic factor in RA (Figure 1h).
Using qRT-PCR analysis, we detected minimal expression of the three fibrinogen chains (Fg α (Ct = 33), Fg β (Ct = 30), and Fg γ (Ct = 36)) in primary chondrocytes, suggesting that they might not have generated the majority of the histologically observed fibrin(ogen). We therefore hypothesized that fibrinogen seen on cartilage comes from synovial fluid and then is converted into fibrin by chondrocyte-induced coagulation. To explore this possibility, we evaluated the procoagulant activity of murine chondrocytes (Supplementary Figure 2a) and human chondrocytes obtained from RA patients (Supplementary Figure 2b). Both chondrocytes were able to initiate clot formation, as evidenced by increased turbidimetry of recalcified plasma; without calcium, there was no signal. Moreover, thrombin (factor IIa) and factor Xa inhibitors reduced the procoagulant activity of murine chondrocytes (results not shown).
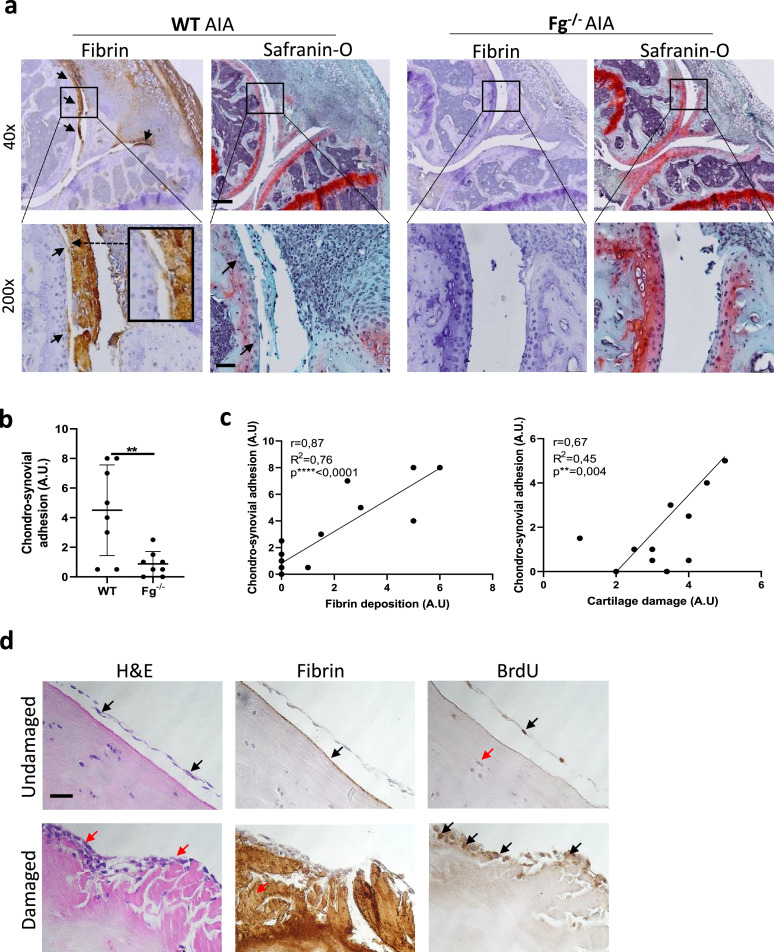
Fibrin(ogen)-mediated chondro-synovial adhesion correlates with cartilage damage in arthritis
WT AIA mice displayed adhesions between synovial membranes and cartilage, especially in fibrin-rich area in proximity of the articular recess (black arrows, Figure 2a). Concomitantly, we observed stripped off superficial cartilage layers (magnified image Figure 2a). Fibrin-mediated chondro-synovial adhesion (CSA) was accompanied by Safranin-O loss and cartilage fibrillation (black arrows, Figure 2a). Contrarily, the absence of fibrin(ogen) deposits in Fg−/− mice was associated with almost undetectable CSA (Figure 2a-b) and preserved cartilage (Figure 2a). We found a positive correlation between CSA and both fibrin(ogen) deposits and cartilage damage (Figure 2c). No CSA was found in both WT and Fg−/- AIA sham knees (Supplementary Figure 1a). To prove that fibrin(ogen) deposits on cartilage could indeed encourage synoviocyte adhesion, we co-incubated human cartilage explants with synoviocytes. On undamaged cartilage, we observed a floating synoviocyte layer, unable to adhere (H&E, black arrows, Figure 2d), where fibrin(ogen) deposition was almost absent (Fibrin, black arrows, Figure 2d). On damaged cartilage, multilayers of synoviocytes sticking to cartilage (H&E, red arrows, Figure 2d) were found in correspondence of massive fibrin(ogen) deposition (Fibrin, red arrows, Figure 2d). Selective BrdU staining in synoviocytes and not in chondrocytes (BrdU, black versus red arrows, Figure 2d) confirms that the observed adherent cells were the synoviocytes we incubated ex novo and not pre-existing cells.
Figure 2.
Fibrin-mediated chondro-synovial adhesion (CSA) positively correlates with cartilage damage in murine RA. (a) Representative immunohistochemical staining for fibrin deposits and Safranin-O staining for cartilage damage in WT and Fg−/− murine knees with AIA (RA). Black arrows show fibrin-mediated CSA and cartilage damage in WT mice. On the left, the magnified box shows a fibrin-rich pseudo-membrane detaching the superficial cartilage layer. Scale bars for 40x = 300 μm and 200x = 120 μm. WT = 8, Fg−/− = 8. (b) Graphs show CSA scores (AU). WT = 8, Fg−/− = 8. Student's t-test. **p < 0.01. (c) Correlation graphs between fibrin deposit score (AU) and CSA score (AU), and between cartilage damage score (AU) and CSA score (AU). Linear regression and Pearson correlation tests. **p < 0.01, ****p < 0.0001. (d) Representative histologies (H&E) and immunohistochemistry (Fibrin and BrdU) of damaged and undamaged cartilage explants from end-stage RA patients, co-incubated with synoviocytes. Black arrows show floating synoviocytes and almost absent fibrin on undamaged cartilage while red arrows show sticking synoviocytes and massive fibrin deposits on damaged cartilage; in BrdU images black arrows show positive synoviocytes and red arrow shows BrdU negative chondrocyte. Pictures from one representative patient per group are shown. Scale bar 30 μm. n = 4.
Finally, to study the occurrence of chondro-synovial adhesion in men, we analysed MRI on peripheral joints of 10 patients with longstanding erosive RA and 20 non-RA controls with other joint pathologies (Supplementary Figure 2a-b). None of the RA patients was anti-coagulated. We encountered a non-infiltrating linear contrast-enhancement along the cartilage surfaces of 7 out of 10 RA patients compatible with adhering pseudo-membranes rather than pannus tissue (white arrows, Supplementary Figure 3a). No such finding was observed in MRIs of 20 knees from non-RA patients (white dashed cyrcle, Supplementary Figure 3a)
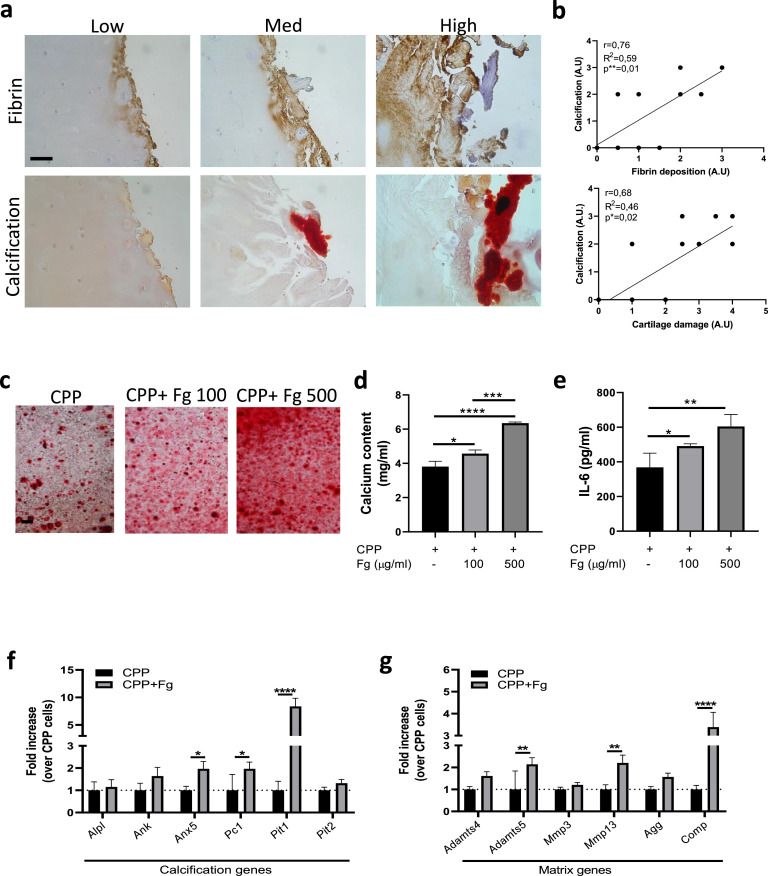
Fibrin(ogen) deposits associate with increased cartilage calcification in arthritis
We provide demonstration of calcium-containing crystal deposition in cartilage of RA patients, as shown using Alizarin red staining (Figure 3a) and Von Kossa staining (Supplementary Figure 4a-b). Increased cartilage calcification was found in fibrin(ogen)-rich cartilage area in this series of RA patients (Figure 3a). Using quantification, we confirmed a positive correlation between calcification and fibrin deposits and between calcification and the severity of cartilage damage (Figure 3b).
Figure 3.
Fibrin deposits are associated with greater cartilage calcification. (a) Representative images of fibrin immunohistochemical staining and Alizarin red staining for calcium-containing crystals on consecutive sections of cartilage explants from end-stage RA patients. Staining was analysed in the low, medium, and high degraded cartilage areas of each patient. Pictures from one representative patient per group are shown. Scale bar = 50 μm. n = 4. (b) Correlation graphs between cartilage damage scores (AU) and calcification scores (AU), and between fibrin deposit scores (AU) and calcification scores (AU). Linear regression and Pearson correlation tests. *p < 0.05, **p < 0.01. n = 4. (c) Representative Alizarin red staining of WT murine chondrocytes stimulated for 24 h with CPP and increasing concentrations of Fg. Scale bar = 100 μm. (d) Corresponding quantification of calcium content in cell monolayer and (e) IL-6 secretion in cell supernatant. Two-way ANOVA post hoc Bonferroni correction. n = 3 experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. (f) qRT-PCR of the indicated calcification genes in murine chondrocytes stimulated for 4 h with CPP and 500 ug/ml Fg. Two-way ANOVA post hoc Bonferroni correction. n = 3 experiments. *p < 0.05, ****p < 0.0001. (g) qRT-PCR of the indicated matrix genes in murine chondrocytes stimulated for 4 h with CPP and 500 ug/ml Fg. Two-way ANOVA post hoc Bonferroni correction. n = 3 experiments. **p < 0.01, ****p < 0.0001.
To study whether calcification could be a consequence of fibrin(ogen) deposition, we cultured primary murine chondrocytes in calcification medium with or without fibrinogen (100, 500 µg/ml). After 24 h, we observed that fibrinogen exacerbated chondrocyte calcification (Figure 3c-d) and secretion of the pro-calcification cytokine IL-627 in a dose-dependent manner (Figure 3e). Accordingly, fibrinogen induced between two- and five-fold upregulation of genes codifying for proteins involved in chondrocyte mineralization: Anx5, Pc1, and Pit1 (Figure 3f). However, no modulation of early- and late-stage chondrocytic differentiation genes (Agg, Coll2, Sox9, Coll10, Runx2) was seen (data not shown). Using the same calcification settings, we found that fibrinogen induced cartilage catabolic genes as well: Adamts5, Mmp13, and Comp (Figure 3g).
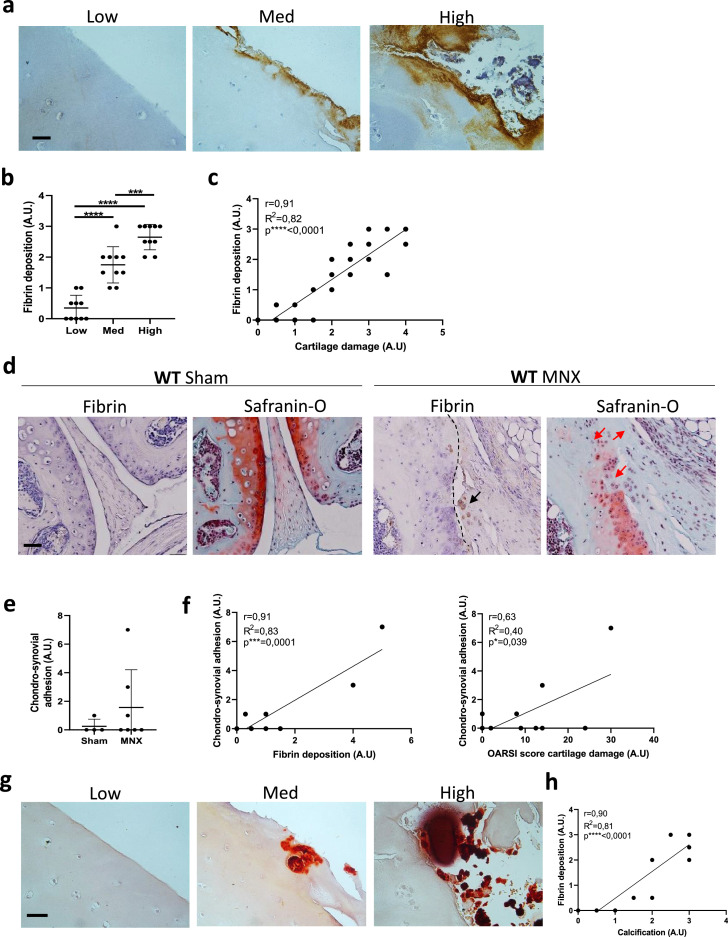
Fibrin(ogen)-induced mechanisms of cartilage damage are recapitulated in OA
We also evaluated fibrin(ogen) deposits in cartilage from OA patients undergoing knee joint replacement. As in RA patients, we found a positive gradient of fibrin(ogen) deposition as OA cartilage damage increased (Figure 4a-c). As for RA chondrocytes, we analysed the procoagulant activity of human chondrocytes obtained from OA patients and found the same ability to initiate clot formation (Supplementary Figure 2c).
Figure 4.
Fibrin deposits correlate with cartilage damage and cartilage calcification in human and murine OA. (a) Representative images of fibrin immunohistochemical staining in cartilage explants from end-stage osteoarthritis (OA) patients. Staining was analysed in the low, medium, and high degraded cartilage areas of each patient. Pictures from one representative patient are shown. Scale bar = 30 μm. n = 10. (b) Graph shows fibrin deposit scores (arbitrary unit = AU) in low, medium, and high damaged areas of cartilage from 10 OA patients. For each level of cartilage degradation, one dot corresponds to one of the 10 patients. n = 10. One-way ANOVA post hoc Bonferroni correction. ***p < 0.001, ****p < 0.0001. (c) Correlation graphs between fibrin deposit scores (AU) and cartilage damage scores (AU). Linear regression and Pearson correlation tests. ****p < 0.0001. (d) Representative immunohistochemical staining for fibrin deposits in sham-operated and meniscectomized WT murine knees, and representative Safranin-O histological staining for cartilage damage on consecutive sections. Black arrows show fibrin deposition in MNX operated knees. Red arrows show cartilage damage in MNX knees. Dashed line shows CSA in MNX knees. Scale bars 120 μm. Sham = 4, MNX = 7. (e) Score for CSA in Sham and MNX knees. Sham = 4, MNX = 7. (f) Correlation graph between CSA (AU) and fibrin deposit score (AU) and between CSA (AU) and OARSI cartilage damage score (AU). Linear regression and Pearson correlation tests. *p < 0.05, ***p < 0.001. (g) Representative images of alizarin red staining of cartilage explants from end-stage OA patients. Staining was analysed in the low, medium, and high degraded cartilage areas of each patient. Pictures from one representative patient are shown. Scale bar = 50 μm. n = 10. (h) Correlation graphs between fibrin deposit scores (AU) and calcification scores (AU). Linear regression and Pearson correlation tests. ****p < 0.0001.
We then evaluated the presence of fibrin(ogen) in the MNX model of murine OA. MNX joints showed more fibrin(ogen) deposits (black arrows) and cartilage damage (red arrows) than sham-operated joints (Figure 4d). Nevertheless, fibrin(ogen) deposition in MNX knees was less severe than in AIA knees (Figure 1d) with no formation of pseudo-membranes. Fibrin-dependent CSA was still present in the OA model (dashed line, Figure 4d, e) and correlated with fibrin deposits and the OARSI score for cartilage damage (Figure 4f).
Finally, in human OA cartilage exacerbation of cartilage calcification corresponded to greater fibrin(ogen) deposits (Figure 4g) and was confirmed by their correlation (Figure 4h).
CD11b plays a minor role in chondrosynovial adhesion
We investigated the role of the integrin CD11b, as part of the CD11b/CD18 fibrin receptor, in fibrin adherence to chondrocytes and its effect on CSA. After in vitro incubation with fibrinogen, WT chondrocytes showed 28.4% positivity for fibrinogen, whereas CD11b KO chondrocytes only showed 18.4% positivity (Supplementary Figure 5a); 11% were APC+ (CD11b+), and only 3.31% were double-positive FITC+/APC+ (Fg+/CD11b+). As expected, APC+ (CD11b+) cells were undetectable, as were FITC+/APC+ cells in CD11b KO chondrocytes. To ensure that the cell population analysed was not contaminated by cells other than chondrocytes, we confirmed that 100% of these cells expressed collagen 2, a marker exclusively expressed by chondrocytes (data not shown). Finally, we scored cartilage damage, fibrin(ogen) deposits, and CSA in the knees of WT and CD11b KO mice subjected to the CIA model of arthritis Although we detected more cartilage damage in CD11b-deficient mice than in WT mice, no differences in fibrin(ogen) deposits and CSA were found between genotypes (Supplementary Figure 5b). Finally, any correlation between the analysed parameters existed (Supplementary Figure 5c)
Discussion
The present work describes a direct and pannus-independent role of fibrin adhesions in arthritic cartilage degeneration. In RA, and to a lesser degree in OA, fibrin attaches to cartilage surfaces and subsequently triggers cartilage catabolism, mechanical damage, and calcification (Figure 5). These results show parallels with the fibrin plaques occurring in atherosclerosis, where chronic inflammation and fibrin clotting lead to calcification and impaired mechanical function of vessels.31,32 In RA, fibrin clotting in synovial tissue is well known, as is its putative role in pannus formation.9 In contrast to this, and analogous to increased atherosclerosis occurring in RA and OA, we suggest to name the current findings as chondrosclerosis. The underlying mechanisms are not driven by immune cells or inflammatory cytokines, but they do include para-inflammatory and degenerative elements. Considering the long half-life of the polymerized fibrin attached to cartilage in bioengineering experiments, we postulated that fibrin adhesion can lead to cartilage damage despite the resolution of inflammation under adequate therapy.33 It is indeed of note that increased fibrinogen expression by the liver and potentially by synovial tissue may remain high in RA patients despite their inflammation being well controlled.3 Thus, intra-articular fibrin deposits may contribute to the radiographic progression of «secondary osteoarthritis» occurring in asymptomatic RA patients.14
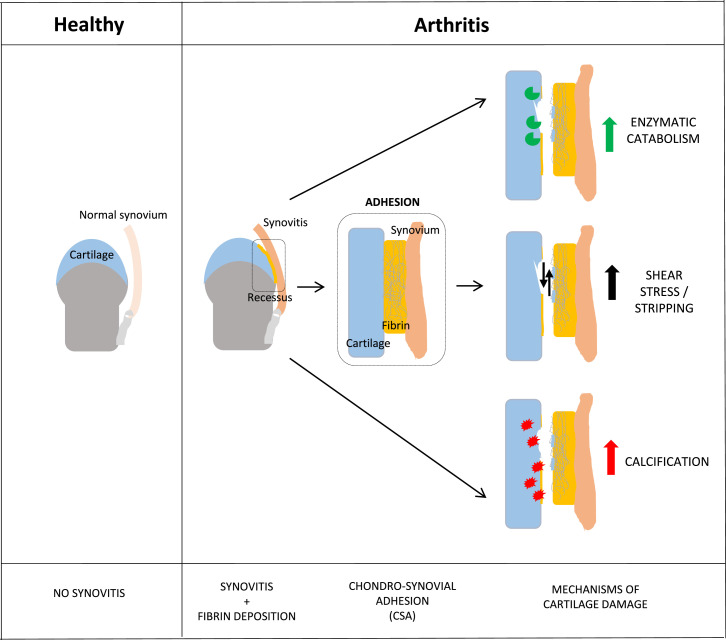
Figure 5.
Proposed mechanism based on the observed findings. No synovitis or fibrin deposition occurs in healthy joints. In RA or OA joints, there are massive fibrin deposits in synovial membrane and cartilage. This lead to cartilage damage via direct: (1) induction of catabolic enzymes; (2) induction of CSA with subsequent mechanical stress and stripping of the superficial cartilage layers; (3) induction of calcification.
Only low levels of fibrinogen expression was detected in chondrocytes, but chondrocytes possess a procoagulant activity converting fibrinogen into fibrin. Based on our histological results showing large amounts of fibrin within the synovial tissue and the fact that fibrin deposits adhered to and affected superficial cartilage structures, we assumed that fibrin mainly came from synovial tissue via diffusion or adhesion. We observed the marked fibrinolysis around chondrocytes to be a potential protective mechanism against fibrin deposition. The fibrinolytic capacity of chondrocytes is known, especially under inflammatory conditions created by increased levels of plasminogen activators.34 Although intra-articular fibrinolysis can be beneficial, e.g., after trauma, it may be harmful to patients in ongoing, chronic, hypercoagulable states such as in RA. A deficiency in plasminogen activator inhibitor (PAI)-1 might underly this observation, similar to our previous findings involving synovial tissue.35 Conversely, an excess of PAI-1 has also been associated with cartilage damage in OA because it increases the numbers of metalloproteinases.36
As a first mechanism of cartilage damage, we showed that fibrinogen up-modulated the expression of proteases known to affect cartilage extracellular matrix integrity in chondrocytes,37, 38, 39, 40 namely Adamts5 and Mmp13. Our experimental conditions did not induce Mmp9, another metalloprotease reported to be induced by fibrinogen in monocytes.41
We described fibrin-mediated CSA as a second mechanism of cartilage damage. Fibrin-rich cartilage and synovium were shown to adhere to each other due to fibrin's glue-like properties, creating a firm connection in the joint and possibly forming during phases of rest such as sleep. Indeed, morning stiffness, a clinical hallmark of RA, has been associated with a fibrin-rich synovial phenotype.10 We suppose that, following transient joint movement, this connection undergoes mechanical stress that causes peeling and tearing of the cartilage surface. In our AIA model experiments, we observed a waxing effect as fibrin pseudo-membranes attach to cartilage and stripp the superficial cartilage layer. As a proof of concept of this adhesion, AIA Fg−/− mice displayed no fibrin deposition, very limited CSA, and undamaged cartilage. Ex vivo, when synoviocytes were co-cultured on human RA cartilage explants, we detected their adherence solely on fibrin-rich degraded areas of cartilage.
With this perspective, we investigated fibrin adhesion and cartilage damage in CD11b integrin KO mice. In experimental RA, disrupted fibrinogen binding to the CD11b/CD18 integrin receptor reduced disease severity.42 In contrast, in the CIA model, we observed more severe disease development in CD11b KO mice than in WT mice. We postulate that this is explained by weaker CD11b suppression of IL‐6 and subsequent Th17‐cell differentiation.23 Here, we found similar fibrin deposits in CD11b KO and WT mice. In addition, we found elevated fibrinogen binding to WT chondrocytes, but less than 5% of this was via the CD11b integrin, and CSA was similar in both CD11b KO and WT mice. Indeed, although other fibrinogen receptors have been identified on leukocytes and glial cells, no fibrinogen receptors in chondrocytes have yet been identified.16
In patients with longstanding RA, we demonstrated structures compatible with CSA by using contrast-enhanced MRI. We observed a thin layer of vascularized T1/T2 material covering superficial cartilage, co-localizing with cartilage damage, and resembling the fibrin-rich synovial pseudo-membranes adhearing to cartilage as observed in our experimental AIA model. One limitation of this method was that the contrast medium was gadolinium and not specific for fibrin.
Fibrin's adhesive effect in RA joints is in line with its glue-like properties that are frequently exploited in orthopedic surgery as part of glue formulations that include fibrinogen, thrombin, factor XIII, and calcium, which together mediate fibrin crosslinking.43,44 Fibrinogen has calcium binding sites which influence crosslinking, and factor XIII is a clot stabilizer.45 In our model, it remains chronologically unclear whether cartilage calcification precedes synovial adhesion or vice versa, and factor XIII was not investigated.
It is also unclear whether fibrin fibers, which are thicker in RA patients than in healthy controls, are, e.g., less susceptible for fibrinolysis and thus more chondrotoxic.46
A third mechanism of cartilage damage can be linked to fibrin(ogen)’s pro-calcification effects. Using alizarin red staining, we detected calcium-containing crystal deposits in fibrin-rich cartilage explants from both RA and OA patients. These crystals have been described in microcrystalline arthropathies such as OA and chondrocalcinosis, but to the best of our knowledge, the evidence of calcium-containing crystals in RA was never been described before. The link between fibrin(ogen) and calcium crystal formation was suggested in a study where high fibrinogen levels predicted the progression of arterial calcification in type 1 diabetes.47 The present study revealed a causal link between fibrinogen and calcification, with fibrinogen triggering chondrocytes to form calcium crystals via the induction of calcification genes. Moreover, fibrinogen also increased chondrocytes’ secretion of IL-6, a pro-inflammatory cytokine known to support chondrocyte mineralization.48 The presence of phosphatidylserine could also explain the interplay between coagulation and calcification, as it displays both procoagulant49 and pro-mineralizing50 effects.
Platelets have multifunctional roles in RA. The number, distribution, size and hyperactivity of platelets are all altered in patients with RA.51,52 Platelets are also sources of multiple autocrine and paracrine inflammatory mediators. Under specific circumstances, such as apoptosis or strong platelet activation, platelet plasma membranes become enriched in exposed phosphatidylserine, concentrating blood coagulation factors. Therefore, upon activation of coagulation factors, fibrin deposition occurs in synchrony with platelet activation and aggregation, leading to the stabilization of the haemostatic clot.53 In addition to platelet pro-coagulant response, microparticles are generated from the membrane of hyper-activated platelets in RA.
Theoretically, in murine and human RA joints, hyperactive platelets could account for fibrin deposition on cartilage via at least three independent mechanisms: 1-providing fibrinogen and other factors contained in their alpha granules, 2- enhancing binding of fibrinogen following a conformational change in the GPIIbIIIa receptor 3- facilitating procoagulant activity on cartilage surface. Future experiments will be performed to identify the predominant mechanism involved.
Finally, fibrin(ogen) citrullination has been demonstrated in RA and links the formation of autoantibodies to coagulation activation in RA.46,54 Citrullinated fibrin(ogen) potentially can interact with amyloid-b protein and modify its coagulation properties.55
Fibrinolysis for the treatment of RA has been intensely investigated in the past, with only contradictory findings partly resulting from the experimental models used. Thrombin inhibition using hirudin resulted in reduced disease severity in both the AIA13 and CIA11 models. However, the treatment of CIA mice with uPA and tPA diminished fibrinogen deposits and the severity of arthritis.56 In a monoarticular model, uPA KO mice and plasminogen KO mice demonstrated aggravated disease features,22 whereas in polyarticular animal models of RA these mice were protected.57,58 Studies in tPA KO animals have indicated enhanced inflammation and disease severity.59 In OA, aberrant activation of the coagulation cascade is well-known,60 but there are no reports on how its inhibition affects disease severity.
Fibrin-inhibiting monoclonal antibodies have a promising therapeutic effect on neuroinflammation and neurodegeneration, where persisting fibrin deposits trigger matrix degeneration.15
In conclusion, we provide evidence of direct cartilage damage due to fibrin deposits in inflammatory arthritis. In the context of an hypercoagulable state, chondrosclerosis with disrupted cartilage integrity resembles atherosclerosis. Overall, more studies are needed to understand how coagulation factors are expressed and regulated in cartilage cells and whether anticoagulants, fibrinolytic drugs, or newer anti-adhesive agents61 could help to preserve cartilage integrity in arthropathies.
Contributors
T.H. conceived the study and wrote the manuscript; S.N. conceived the study, performed the experiments, and wrote the manuscript; D.E. performed FACS experiments; P.O. performed the MRI study; A.S. supervised the study and wrote the manuscript; N.B. conceived and supervised the study and wrote the manuscript. T.H. and S.N verified the underlying data. All authors had full access to data, read and approved the final version of the manuscript and accepted responsability to submit for publication
Declaration of Interests
The authors declare that they have no conflict of interest.
Acknowledgments
Data sharing statement
All data (experimental such as study protocol and statistical analysis, and clinical after de-identification) collected during the study will be made available immediately after publication to anyone who wishes to access the data. Data will be available indefinitely upon direct request to Sonia.Nasi@chuv.ch or Thomas.Hugle@chuv.ch.
Acknowledgments
We deeply thank Mrs. Véronique Chobaz (Laboratory of Rheumatology, CHUV, Lausanne) for her excellent technical support. No specific funding was obtained for this study
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104081.
Appendix. Supplementary materials
References
- 1.Gobel K., Eichler S., Wiendl H., Chavakis T., Kleinschnitz C., Meuth S.G. The coagulation factors fibrinogen, thrombin, and factor XII in inflammatory disorders-A systematic review. Front Immunol. 2018;9:1731. doi: 10.3389/fimmu.2018.01731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luyendyk J.P., Schoenecker J.G., Flick M.J. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. 2019;133(6):511–520. doi: 10.1182/blood-2018-07-818211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rooney T., Scherzer R., Shigenaga J.K., Graf J., Imboden J.B., Grunfeld C. Levels of plasma fibrinogen are elevated in well-controlled rheumatoid arthritis. Rheumatology. 2011;50(8):1458–1465. doi: 10.1093/rheumatology/ker011. (Oxford) [DOI] [PubMed] [Google Scholar]
- 4.Arvidson N.G., Larsson A., Larsen A. Disease activity in rheumatoid arthritis: fibrinogen is superior to the erythrocyte sedimentation rate. Scand J Clin Lab Invest. 2002;62(4):315–319. doi: 10.1080/003655102760145889. [DOI] [PubMed] [Google Scholar]
- 5.Busso N., Morard C., Salvi R., Péclat V., So A. Role of the tissue factor pathway in synovial inflammation. Arthritis Rheum. 2003;48(3):651–659. doi: 10.1002/art.10869. [DOI] [PubMed] [Google Scholar]
- 6.So A.K., Varisco P.A., Kemkes-Matthes B., et al. Arthritis is linked to local and systemic activation of coagulation and fibrinolysis pathways. J Thromb Haemost. 2003;1(12):2510–2515. doi: 10.1111/j.1538-7836.2003.00462.x. [DOI] [PubMed] [Google Scholar]
- 7.Berg E., Wainwright R., Barton B., Puchtler H., McDonald T. On the nature of rheumatoid rice bodies: an immunologic, histochemical, and electron microscope study. Arthritis Rheum. 1977;20(7):1343–1349. doi: 10.1002/art.1780200707. [DOI] [PubMed] [Google Scholar]
- 8.Salonen E.M., Vartio T., Hedman K., Vaheri A. Binding of fibronectin by the acute phase reactant C-reactive protein. J Biol Chem. 1984;259(3):1496–1501. [PubMed] [Google Scholar]
- 9.Sanchez-Pernaute O., Largo R., Calvo E., Alvarez-Soria M.A., Egido J., Herrero-Beaumont G. A fibrin based model for rheumatoid synovitis. Ann Rheum Dis. 2003;62(12):1135–1138. doi: 10.1136/ard.2003.011767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orange D.E., Blachere N.E., DiCarlo E.F., et al. Rheumatoid arthritis morning stiffness is associated with synovial fibrin and neutrophils. Arthritis Rheumatol. 2020;72(4):557–564. doi: 10.1002/art.41141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marty I., Peclat V., Kirdaite G., Salvi R., So A., Busso N. Amelioration of collagen-induced arthritis by thrombin inhibition. J Clin Invest. 2001;107(5):631–640. doi: 10.1172/JCI11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busso N., Hamilton J.A. Extravascular coagulation and the plasminogen activator/plasmin system in rheumatoid arthritis. Arthritis Rheum. 2002;46(9):2268–2279. doi: 10.1002/art.10498. [DOI] [PubMed] [Google Scholar]
- 13.Varisco P.A., Peclat V., van Ness K., et al. Effect of thrombin inhibition on synovial inflammation in antigen induced arthritis. Ann Rheum Dis. 2000;59(10):781–787. doi: 10.1136/ard.59.10.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ten Klooster P.M., Versteeg L.G.A., Oude Voshaar M.A.H., et al. Radiographic progression can still occur in individual patients with low or moderate disease activity in the current treat-to-target paradigm: real-world data from the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry. Arthritis Res Ther. 2019;21(1):237. doi: 10.1186/s13075-019-2030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryu J.K., Rafalski V.A., Meyer-Franke A., et al. Fibrin-targeting immunotherapy protects against neuroinflammation and neurodegeneration. Nat Immunol. 2018 doi: 10.1038/s41590-018-0232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flick M.J., LaJeunesse C.M., Talmage K.E., et al. Fibrin(ogen) exacerbates inflammatory joint disease through a mechanism linked to the integrin alphaMbeta2 binding motif. J Clin Invest. 2007;117(11):3224–3235. doi: 10.1172/JCI30134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma M., Damgaard D., Senolt L., et al. Expanding the citrullinome of synovial fibrinogen from rheumatoid arthritis patients. J Proteomics. 2019;208 doi: 10.1016/j.jprot.2019.103484. [DOI] [PubMed] [Google Scholar]
- 18.Ge C., Tong D., Liang B., et al. Anti-citrullinated protein antibodies cause arthritis by cross-reactivity to joint cartilage. JCI Insight. 2017;2(13) doi: 10.1172/jci.insight.93688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho P.P., Lee L.Y., Zhao X., et al. Autoimmunity against fibrinogen mediates inflammatory arthritis in mice. J Immunol. 2010;184(1):379–390. doi: 10.4049/jimmunol.0901639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pap T., Korb-Pap A. Cartilage damage in osteoarthritis and rheumatoid arthritis–two unequal siblings. Nat Rev Rheumatol. 2015;11(10):606–615. doi: 10.1038/nrrheum.2015.95. [DOI] [PubMed] [Google Scholar]
- 21.Bartok B., Firestein G.S. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233(1):233–255. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busso N., Peclat V., Van Ness K., et al. Exacerbation of antigen-induced arthritis in urokinase-deficient mice. J Clin Invest. 1998;102(1):41–50. doi: 10.1172/JCI2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevanin M., Busso N., Chobaz V., et al. CD11b regulates the Treg/Th17 balance in murine arthritis via IL-6. Eur J Immunol. 2017;47(4):637–645. doi: 10.1002/eji.201646565. [DOI] [PubMed] [Google Scholar]
- 24.Kamekura S., Hoshi K., Shimoaka T., et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthr Cartil. 2005;13(7):632–641. doi: 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Pritzker K.P., Gay S., Jimenez S.A., et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartil. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Gosset M., Berenbaum F., Thirion S., Jacques C. Primary culture and phenotyping of murine chondrocytes. Nat Protoc. 2008;3(8):1253–1260. doi: 10.1038/nprot.2008.95. [DOI] [PubMed] [Google Scholar]
- 27.Aghagolzadeh P., Bachtler M., Bijarnia R., et al. Calcification of vascular smooth muscle cells is induced by secondary calciprotein particles and enhanced by tumor necrosis factor-alpha. Atherosclerosis. 2016;251:404–414. doi: 10.1016/j.atherosclerosis.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 28.Gregory C.A., Gunn W.G., Peister A., Prockop D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329(1):77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Ea H.K., Chobaz V., Nguyen C., et al. Pathogenic role of basic calcium phosphate crystals in destructive arthropathies. PLoS One. 2013;8(2):e57352. doi: 10.1371/journal.pone.0057352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasi S., Ea H.K., Liote F., So A., Busso N. Sodium thiosulfate prevents chondrocyte mineralization and reduces the severity of murine osteoarthritis. PLoS One. 2016;11(7) doi: 10.1371/journal.pone.0158196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dhawan S.S., Avati Nanjundappa R.P., Branch J.R., et al. Shear stress and plaque development. Expert Rev Cardiovasc Ther. 2010;8(4):545–556. doi: 10.1586/erc.10.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolf D., Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124(2):315–327. doi: 10.1161/CIRCRESAHA.118.313591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroez M., Lang W., Dickneite G. Wound healing and degradation of the fibrin sealant Beriplast P following partial liver resection in rabbits. Wound Repair Regen. 2005;13(3):318–323. doi: 10.1111/j.1067-1927.2005.130315.x. [DOI] [PubMed] [Google Scholar]
- 34.Meats J.E., Elford P.R., Bunning R.A., Russell R.G. Retinoids and synovial factor(s) stimulate the production of plasminogen activator by cultured human chondrocytes. A possible role for plasminogen activator in the resorption of cartilage in vitro. Biochim Biophys Acta. 1985;838(1):161–169. doi: 10.1016/0304-4165(85)90262-4. [DOI] [PubMed] [Google Scholar]
- 35.Van Ness K., Chobaz-Péclat V., Castellucci M., So A., Busso N. Plasminogen activator inhibitor type-1 deficiency attenuates murine antigen-induced arthritis. Rheumatology. 2002;41(2):136–141. doi: 10.1093/rheumatology/41.2.136. (Oxford) [DOI] [PubMed] [Google Scholar]
- 36.Moritake A., Kawao N., Okada K., et al. Plasminogen activator inhibitor-1 is involved in interleukin-1β-induced matrix metalloproteinase expression in murine chondrocytes. Mod Rheumatol. 2019;29(6):959–963. doi: 10.1080/14397595.2018.1525018. [DOI] [PubMed] [Google Scholar]
- 37.Dancevic C.M., McCulloch D.R. Current and emerging therapeutic strategies for preventing inflammation and aggrecanase-mediated cartilage destruction in arthritis. Arthritis Res Ther. 2014;16(5):429. doi: 10.1186/s13075-014-0429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuzaka K., Itami Y., Takeuchi T., Shinozaki N., Morishita T. ADAMTS5 is a biomarker for prediction of response to infliximab in patients with rheumatoid arthritis. J. Rheumatol. 2010;37(7):1454–1460. doi: 10.3899/jrheum.091285. [DOI] [PubMed] [Google Scholar]
- 39.Singh A., Rajasekaran N., Hartenstein B., et al. Collagenase-3 (MMP-13) deficiency protects C57BL/6 mice from antibody-induced arthritis. Arthritis Res Ther. 2013;15(6):R222. doi: 10.1186/ar4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jungel A., Ospelt C., Lesch M., et al. Effect of the oral application of a highly selective MMP-13 inhibitor in three different animal models of rheumatoid arthritis. Ann Rheum Dis. 2010;69(5):898–902. doi: 10.1136/ard.2008.106021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaneider N.C., Mosheimer B., Gunther A., Feistritzer C., Wiedermann CJ. Enhancement of fibrinogen-triggered pro-coagulant activation of monocytes in vitro by matrix metalloproteinase-9. Thromb J. 2010;8(1):2. doi: 10.1186/1477-9560-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinbrecher K.A., Horowitz N.A., Blevins E.A., et al. Colitis-associated cancer is dependent on the interplay between the hemostatic and inflammatory systems and supported by integrin alpha(M)beta(2) engagement of fibrinogen. Cancer Res. 2010;70(7):2634–2643. doi: 10.1158/0008-5472.CAN-09-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spotnitz W.D. Fibrin sealant: the only approved hemostat, sealant, and adhesive-a laboratory and clinical perspective. ISRN Surg. 2014;2014 doi: 10.1155/2014/203943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Irwin R.M., Bonassar L.J., Cohen I., et al. The clot thickens: autologous and allogeneic fibrin sealants are mechanically equivalent in an ex vivo model of cartilage repair. PLoS One. 2019;14(11) doi: 10.1371/journal.pone.0224756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weisel J.W., Litvinov R.I. Fibrin formation, structure and properties. Subcell Biochem. 2017;82:405–456. doi: 10.1007/978-3-319-49674-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bezuidenhout J.A., Venter C., Roberts T.J., Tarr G., Kell D.B., Pretorius E. Detection of citrullinated fibrin in plasma clots of rheumatoid arthritis patients and its relation to altered structural clot properties, disease-related inflammation and prothrombotic tendency. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.577523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodrigues T.C., Snell-Bergeon J.K., Maahs D.M., Kinney G.L., Rewers M. Higher fibrinogen levels predict progression of coronary artery calcification in adults with type 1 diabetes. Atherosclerosis. 2010;210(2):671–673. doi: 10.1016/j.atherosclerosis.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nasi S., So A., Combes C., Daudon M., Busso N. Interleukin-6 and chondrocyte mineralisation act in tandem to promote experimental osteoarthritis. Ann Rheum Dis. 2016;75(7):1372–1379. doi: 10.1136/annrheumdis-2015-207487. [DOI] [PubMed] [Google Scholar]
- 49.Lentz B.R. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog Lipid Res. 2003;42(5):423–438. doi: 10.1016/s0163-7827(03)00025-0. [DOI] [PubMed] [Google Scholar]
- 50.Cui L., Houston D.A., Farquharson C., MacRae V.E. Characterisation of matrix vesicles in skeletal and soft tissue mineralisation. Bone. 2016;87:147–158. doi: 10.1016/j.bone.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Olumuyiwa-Akeredolu O.O., Page M.J., Soma P., Pretorius E. Platelets: emerging facilitators of cellular crosstalk in rheumatoid arthritis. Nat Rev Rheumatol. 2019;15(4):237–248. doi: 10.1038/s41584-019-0187-9. [DOI] [PubMed] [Google Scholar]
- 52.Mac Mullan P.A., Peace A.J., Madigan A.M., Tedesco A.F., Kenny D., McCarthy G.M. Platelet hyper-reactivity in active inflammatory arthritis is unique to the adenosine diphosphate pathway: a novel finding and potential therapeutic target. Rheumatology. 2010;49(2):240–245. doi: 10.1093/rheumatology/kep377. (Oxford) [DOI] [PubMed] [Google Scholar]
- 53.Aliotta A., Bertaggia Calderara A., Zermatten M.G., Marchetti M., Alberio L. Thrombocytopathies: not just aggregation defects-the clinical relevance of procoagulant platelets. J Clin Med. 2021;10(5) doi: 10.3390/jcm10050894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sanchez-Pernaute O., Filkova M., Gabucio A., et al. Citrullination enhances the pro-inflammatory response to fibrin in rheumatoid arthritis synovial fibroblasts. Ann Rheum Dis. 2013;72(8):1400–1406. doi: 10.1136/annrheumdis-2012-201906. [DOI] [PubMed] [Google Scholar]
- 55.Kell D.B., Pretorius E. Proteins behaving badly. Substoichiometric molecular control and amplification of the initiation and nature of amyloid fibril formation: lessons from and for blood clotting. Prog Biophys Mol Biol. 2017;123:16–41. doi: 10.1016/j.pbiomolbio.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 56.Kwiecinski J., Josefsson E., Jin T. Fibrinolysis is down-regulated in mouse collagen-induced arthritis, but its normalization does not alleviate the course of disease. Inflamm Res. 2011;60(11):1021–1029. doi: 10.1007/s00011-011-0363-0. [DOI] [PubMed] [Google Scholar]
- 57.Li J., Ny A., Leonardsson G., Nandakumar K.S., Holmdahl R., Ny T. The plasminogen activator/plasmin system is essential for development of the joint inflammatory phase of collagen type II-induced arthritis. Am J Pathol. 2005;166(3):783–792. doi: 10.1016/S0002-9440(10)62299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J., Guo Y., Holmdahl R., Ny T. Contrasting roles of plasminogen deficiency in different rheumatoid arthritis models. Arthritis Rheum. 2005;52(8):2541–2548. doi: 10.1002/art.21229. [DOI] [PubMed] [Google Scholar]
- 59.Yang Y.H., Carmeliet P., Hamilton J.A. Tissue-type plasminogen activator deficiency exacerbates arthritis. J Immunol. 2001;167(2):1047–1052. doi: 10.4049/jimmunol.167.2.1047. [DOI] [PubMed] [Google Scholar]
- 60.Chou P.Y., Su C.M., Huang C.Y., Tang C.H. The characteristics of thrombin in osteoarthritic pathogenesis and treatment. Biomed Res Int. 2014;2014 doi: 10.1155/2014/407518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Capella-Monsonis H., Kearns S., Kelly J., Zeugolis D.I. Battling adhesions: from understanding to prevention. BMC Biomed Eng. 2019;1:5. doi: 10.1186/s42490-019-0005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.