Summary
Background
Faecal microbiota transplantation (FMT) has previously been explored as a treatment for ulcerative colitis (UC) however, biomarkers that predict and / or are associated with clinical response are poorly defined. The aim of this systematic review was to identify donor and recipient clinical, microbial and metabolomic predictive biomarkers of response to FMT in UC.
Methods
A systematic search of the relevant literature of studies exploring FMT in UC was conducted. Data on microbial diversity, taxonomic changes, metabolic changes, donor and recipient microbiota relationship and baseline predictors was examined.
Findings
2852 studies were screened, and 25 papers were included in this systematic review. Following FMT, alpha diversity was seen to increase in responders along with increases in the abundance of Clostridiales clusters (order) and Bacteroides genus. Metabolomic analysis revealed short chain fatty acid (SCFA) production as a marker of FMT success. Donors or FMT batches with higher microbial alpha diversity and a greater abundance of taxa belonging to certain Bacteroides and Clostridia clusters were associated with clinical response to FMT. Baseline clinical predictors of response in patients with UC included younger age, less severe disease and possibly shorter disease duration. Baseline recipient microbial predictors at response consisted of higher faecal species richness, greater abundance of Candida and donor microbial profile similarity.
Interpretation
Distinct changes in gut microbiota profiles post-FMT indicate that certain baseline characteristics along with specific microbial and metabolomic alterations may predispose patients towards a successful therapeutic outcome. Opportunities towards a biomarker led precision medicine approach with FMT should be explored in future clinical studies.
Funding
There no specific funding to declare.
Key words: Ulcerative colitis, faecal microbiota transplantation, biomarkers
Research in context.
Evidence before this study
To date, eight double-blind randomised placebo-controlled trials on the use of FMT to treat UC have been published, 6 of which have reported positive findings. Whilst these studies highlight the capability of FMT to ameliorate UC, very little is known about the underpinning mechanisms. The lack of well-defined biomarkers and treatment targets makes it pragmatically challenging to determine the frequency and interval at which treatment with FMT should be administered.
Added value of this study
Through a systematic review of the current evidence base, we describe clinical, microbial and metabolomic biomarkers that are predictive of response at baseline (pre-FMT), and are associated with response following FMT treatment in patients with active UC.
Implications of all the available evidence
The findings of this systematic review highlight the possibility of enhancing a sustained response to FMT through biomarker-based selection and optimisation of donors and patients before and during the treatment with FMT. Utilising precision medicine in this field deserves further exploration as it has the potential to facilitate an individualised, biomarker driven ‘treat to microbiome/metabolome’ target approach with FMT in patients with UC.
Alt-text: Unlabelled box
Introduction
Ulcerative colitis (UC) is a subtype of inflammatory bowel disease (IBD) that is characterised by chronic inflammation of the colonic mucosa with patients typically presenting with bloody diarrhoea.1 Whilst the precise aetiology of UC remains unclear, it is considered to be triggered by dysregulated and sustained immune responses to gut microbiota in genetically susceptible individuals.2,3 Patients with UC possess an altered gut microbiota composition, known as dysbiosis, characterised by reduced microbiota diversity, decrease in the phylum Bacteroidetes and Firmicutes along with a corresponding increase in Proteobacteria.4,5 This has led to a focus on the modulation of gut bacteria as a treatment method for UC, primarily through faecal microbiota transplantation (FMT). FMT is the procedure of transferring processed faecal matter of a healthy individual into another individual with a microbiota mediated disease.6
To date, eight double-blind randomised placebo-controlled trials (RCTs) on the use of FMT to treat UC have been published, 6 of which have reported positive findings.7, 8, 9, 10, 11, 12, 13, 14 Whilst these studies highlight the capability of FMT to ameliorate UC, very little is known about the underpinning mechanisms. The heterogeneity in study designs both with regards to FMT preparation and administration protocols as well as patient selection makes it challenging to draw solid conclusions for its adoption into clinical practice. Furthermore, it remains unclear if specific donor or recipient characteristics may predict response to FMT or denote successful response following FMT.15,16 This lack of well-defined biomarkers and treatment targets makes it pragmatically challenging to determine the frequency and interval at which treatment with FMT should be administered.
Currently, there are no published systematic reviews that explore predictive biomarkers of FMT in patients with UC. This systematic review aims to answer whether clinical, microbial and metabolomic predictive biomarkers exist and if so, which of these are predictive of response at baseline (pre-FMT), and are associated with response following FMT treatment in patients with active UC.
Methods
Search strategy and study selection
The systematic review was conducted in accordance with preferred reporting items for systematic reviews and meta-analyses (PRISMA) criteria. The databases MEDLINE, EMBASE, CINAHL and Cochrane Library, were searched for suitable articles from commencement to January 2022 using search terms outlined (Supplement Table 1). In addition, references included in earlier review articles were searched to identify any additional studies. Results from the searches were imported into a bibliography manager (EndNote X9) and duplicate studies were removed.
Table 1.
Randomised control studies of FMT in ulcerative colitis
| Reference | No of subjects | Control/ Comparator | Treatment | Median / Mean Age(years) | Gender(% Male) | Average disease severity indices at baseline | Treatment Duration | Relevant study characteristics |
|---|---|---|---|---|---|---|---|---|
| Paramsothy et al* (2019)33 | 81 | Placebo group (n = 40) |
Treatment protocol Initial colonoscopic infusion followed by intensive FMT infusion enemas (n = 41) FMT preparation Pooled from multiple donors |
FMT arm - 35.6 (27.8-48.9) Placebo arm - 35.4 (27.7-45.6) |
FMT arm – 54% Placebo arm – 63% |
FMT arm – 8 (average Total Mayo score) Placebo arm – 8 (average Total Mayo score) |
FMT treatment 5 days/week for 8 weeks | Patients in the placebo group were eligible to receive open-label FMT after the double-blind study period 314 faecal samples collected from the patients at screening, every 4 weeks during treatment, and 8 weeks after the blinded or open-label FMT therapy |
| Moayyedi et al (2015)8 | 75 | Placebo group (n = 37) |
Treatment protocol Examined by flexible sigmoidoscopy followed by FMT infusion via enema (n = 38) FMT preparation Single donor per patient |
FMT arm – 42.2 (±15.0) Placebo arm – 35.8 (±12.1) |
FMT arm – 47% Placebo arm – 70% |
FMT arm – 8.24 (±2.61) Total Mayo Clinic score Placebo arm – 7.86 (±2.28) Total Mayo Clinic score |
FMT treatment 1 day/week for 6 weeks | Patients provided stool samples when the study began and during each week of FMT for microbiome analysis |
| Costello et al (2019)9 | 73 | Autologous FMT control group (n = 35) |
Treatment protocol Anaerobically prepared pooled donor FMT via colonoscopy followed by 2 enemas over 7 days (n = 38) FMT preparation Pooled from multiple donors |
Donor FMT arm – 38.528, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52 Autologous FMT arm – 3525, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 |
dFMT– 53% aFMT – 57% |
dFMT arm – 7.2 (±1.7) Mean Total Mayo score aFMT – 7.4 (±1.9) Mean Total Mayo score |
FMT treatment per week with patients monitored at 8 weeks and 12 months post-FMT | Open-label therapy was offered to autologous FMT participants at 8 weeks and they were followed up for 12 months Recipient stool samples were collected at baseline (week 0) and weeks 4, 8, and 52 for microbiome, metabolome, and faecal calprotectin assessment |
| Rossen et al (2015) †10 | 48 | Autologous FMT control group (n = 25) |
Treatment protocol Pre-treatment with bowel lavage followed by 2 duodenal infusions of a suspension of donor faeces via nasoduodenal tube (n = 23) FMT preparation Single donor per patient |
Donor FMT arm – 4033-56 Autologous FMT arm – 4130, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 |
dFMT arm – 47.8% aFMT arm – 44% |
dFMT arm – 105, 6, 7, 8, 9, 10, 11 Median SCCAI score aFMT arm – 84, 5, 6, 7, 8, 9, 10, 11 Median SCCAI score |
FMT treatment at the start of the study (week 0) and 3 weeks later (week 3) | Faecal samples were collected at baseline before bowel lavage and 6 and 12 weeks after FMT |
| Crothers et al (2021)13 | 12 | Placebo group (n = 6) |
Treatment protocol FMT induction by colonoscopy, followed by oral administration of frozen encapsulated cFMT (n = 6) FMT preparation Single donor for induction Multiple (2 pre-defined) donors during maintanence |
FMT arm – 41 (±15) Placebo arm – 52 ±15) |
FMT arm - 67% Placebo arm - 50% |
FMT arm – 6.3 (±2.0) Mean Total Mayo score Placebo arm – 6.7 (±1.2) Mean Total Mayo score |
Daily cFMT treatment for 12 weeks | Subjects were followed for 36 weeks and longitudinal clinical assessments Subjects in both arms of the study were pre-treated with antibiotics for 7 days prior to FMT (or placebo) procedure Subject stool samples were obtained weekly throughout the study period, beginning prior to antibiotic pre-treatment, and ending at 18-weeks follow-up |
| Pai et al (2021)12 | 25 | Placebo group (n = 12) |
Treatment protocol FMT administered by rectal enema (n=13) FMT preparation Multiple donors per patient (not pooled) |
Overall 10.54, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 Individual arms not specified |
Not specified | Not specified | Total 12 enemas (given biweekly) | Seven patients randomized to the placebo arm crossed over to the open-label arm after 30 weeks of placebo treatment |
| Haifer et al (2021)11 | 35 | Placebo group (n=20) |
Treatment protocol Six FMT capsules four times a day for 1 week, then six capsules twice daily for 1 week, followed by six capsules daily for the remaining 6 weeks. Each capsule contains 0.35g lyophilised stool. (n=15) FMT preparation Two donors, unclear if pooled |
FMT arm - 37.1 (31.8–46.8) Placebo arm - 36.7 (25.1–42.0) |
FMT arm – 60% Placebo arm – 45% |
FMT arm - 55, 6, 7, 8, 9 median total Mayo score Placebo arm - 75, 6, 7, 8 median total Mayo core |
8 weeks of capsules during induction, followed by 2 capsules daily for remaining 58 weeks for maintenance. | Antibiotic pre-treatment in both groups. 10 patients randomised to FMT arm with clinical response entered maintenance phase of the study - 4 assigned to FMT and 6 assigned to FMT withdrawal |
Further post hoc microbiota analysis reported separately31
FMT-faecal microbiota transplantation, cFMT-capsulised faecal microbiota transplantation, dFMT-donor FMT, aFMT-autologous FMT, SCCAI-simple clinical colitis activity index.
Randomised control trials (RCTs) and non-randomised studies were included with exclusion of case reports and conference abstracts. Double blind RCTs were further split based on comparators (placebo and non-placebo controlled studies). Studies consisting of patients of all ages with active UC examining any of the following: clinical, microbial (diversity and taxonomic changes) and metabolomic biomarkers at baseline and post FMT treatment predictive of induction and maintenance of clinical remission in patients with active UC were included. Studies were excluded if they had under 10 patients in the FMT treatment arm or only included patients with concurrent infections. No restriction on language or the comparator type for comparative study designs was implemented. Abstracts of the papers identified by the initial search were evaluated by the lead and senior authors for appropriateness to the study question. All relevant papers were obtained and analysed in detail. Articles were independently assessed by two reviewers using pre-defined eligibility criteria and any disagreements were resolved by consensus.
Data extraction
Data was extracted independently by the two reviewers onto a Microsoft Excel spreadsheet (Microsoft, Washington, USA) from the eligible studies. Data relating to donor and patient demographics, treatment groups/comparator(s) and outcome measures were collected. Exploratory data on changes in alpha and beta diversity, microbial taxa, metabolome and donor-patient microbiota similarities following FMT were collected. No unclear or missing data was noticed which would have required approaching the study authors for clarification. Risk of bias of the included RCTs was assessed with the Cochrane Collaboration's risk of bias tool and non-randomised/cohort studies was with the Newcastle-Ottawa quality assessment scale (NOS).17,18 If there were any discrepancies a third reviewer was consulted.
Role of funders
No specific funding has been received for this systematic review. This is independent work conducted by the authors of the review.
Results
Study characteristics
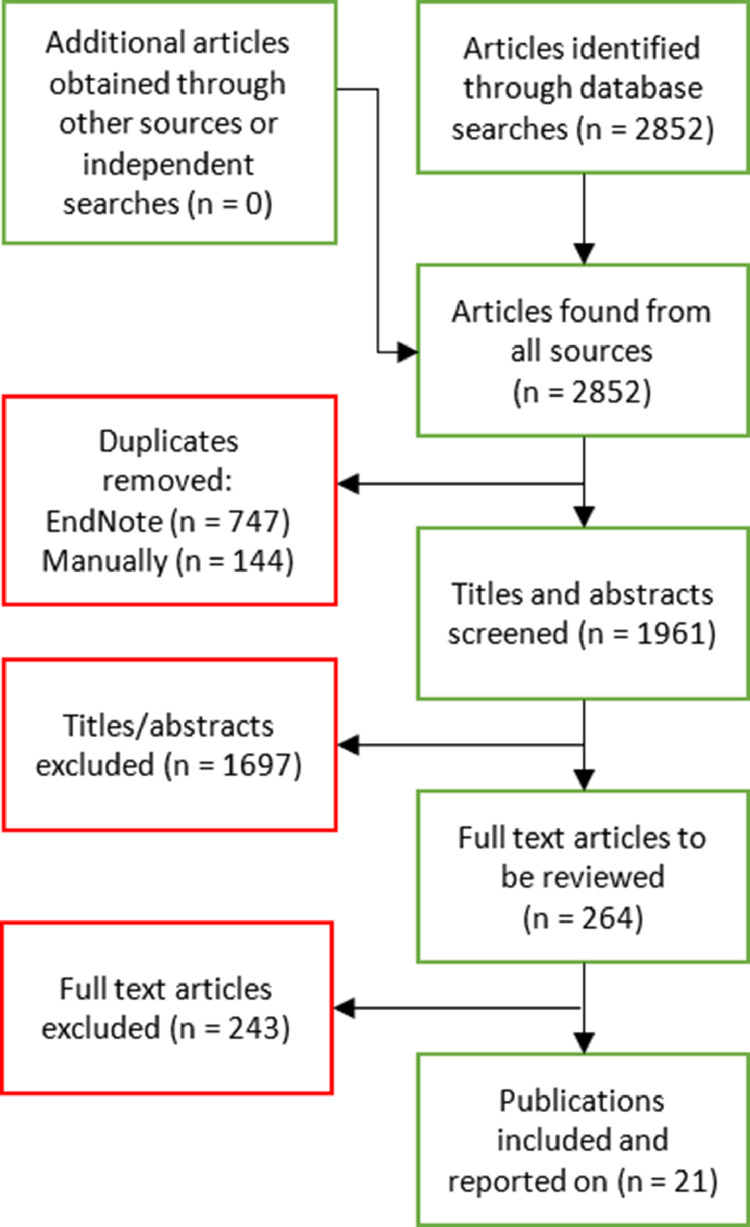
The search strategy generated 2852 citations from which 25 articles investigating the use of FMT in UC patients satisfied the study selection criteria for further assessment (Figure 1). Of these, 7 were placebo controlled double blind RCTs7, 8, 9, 10, 11, 12, 13 (Table 1; total of 8 RCTs but one did not report predictive associations and failed to meet inclusion criteria for this systematic review), 2 were non-placebo controlled blinded randomised studies19,20 and 14 were non-randomised or observational studies21, 22, 23, 24, 25, 26, 27, 28, 29, 30 (Table 2). In addition, 2 studies performed post-hoc microbiota analysis from their placebo controlled double blind RCTs.31,32 All the RCTs received a low bias ranking overall (Supplementary Table 2). None of the non-randomised / cohort studies scored at the highest end of the NOS scale, with a mean score of 5 (range 4 to 6) out of 9 (Supplementary Table 3).
Figure 1.
PRISMA flow diagram summarising the screening process for the systematic review
Table 2.
Non-placebo controlled blinded randomised trials and non-randomised studies of FMT in ulcerative colitis*
| Reference | No. of Subjects | Control/Comparator | Treatment | Median / Mean Age (years) | Gender (% Male) | Average disease severity indices at baseline | Treatment duration | Relevant study characteristics |
|---|---|---|---|---|---|---|---|---|
| Brezina et al (2021) †20 |
45 | 5-ASA treatment group (n = 22) |
Treatment protocol Multi-session FMT enemas (n = 23) FMT preparation Single donor per patient |
FMT arm – 3925-63 5-ASA arm – 39.527-70 |
FMT arm – 52% 5-ASA arm – 50% |
FMT arm – 64, 5, 6, 7, 8, 9, 10 Total Mayo score 5-ASA arm – 64, 5, 6, 7, 8, 9, 10 Total Mayo score |
FMT treatment 5 times in the first week and then once weekly for 5 weeks |
Faecal samples were collected at baseline and each study visit at weeks 2, 4, 6, and 12 in the FMT group and at the 1 year follow-up in all patients |
| Shabat et al (2021) †19 |
51 | Single donor FMT by colonoscopy without dietary conditioning of donors (group 1 (n = 17)), UC Exclusion Diet (UCED) alone for patients (group 3 (n = 15)) |
Treatment protocol FMT by colonoscopy with dietary pre-conditioning of the donor for 14 days and a UCED for the patients (group 2 (n = 19)) FMT preparation Single donor per patient |
Group 1 – 43.1 (±14.7) Group 2 – 43.5 (±10.5) Group 3 – 33.3 (±9.8) |
Group 1 – 70.6% Group 2 – 73.7% Group 3 – 73.3% |
Group 1 – 76, 7, 8, 9 Median SSCAI score Group 2 – 86, 7, 8, 9, 10 Median SCCAI score Group 3 – 65, 6, 7, 8 Median SCCAI score |
FMT by colonoscopy on day 1 and rectal enemas on days 2 and 14 |
Three arm study exploring role of donor and recipient dietary conditioning in optimisation of response to FMT |
| Tian et al (2019)21 | 20 | Before vs after FMT treatment (+ comparison against the healthy donor profile) |
Treatment protocol Pre-treatment with bowel lavage followed by FMT via gastroscopy FMT preparation Single donor per patient |
62.50 (±77.14) | 55% | 5.00 (±2.75) Mayo score | FMT treatment 5 times, once every 3 weeks | 16S ribosomal RNA sequencing analysis performed on the bacterial rRNA from stool of healthy donors and patients with UC before treatment and after the first and second treatment (groups d0, d1, and d2) |
| Li et al (2020)22 | 202 | Before vs after FMT treatment (+ comparison against the healthy donor profile) |
Treatment protocol Single FMT via gastroscopy FMT preparation Single donor per patient |
36 (29-49.25) | 58.2% | 75, 6, 7, 8, 9 Median Partial Mayo score | Patients received 1 infusion (2nd and 3rd courses were given to patients who relapsed) | 42 UC stool samples were included: 22 samples at baseline and 20 at 5 days after the first course of FMT |
| Kump et al (2018)23 | 27 | Antibiotic pre-treatment only group (n = 10) |
Treatment protocol Multi-session FMT via colonoscopy / flexible sigmoidoscopy (n = 17) FMT preparation Single donor per patient |
FMT arm – 44 (±18) Antibiotic arm – 36 (±13) |
FMT arm - 82% Antibiotic arm – 30% |
FMT arm – 8.9 (±1.6) Mean Total Mayo score Antibiotic arm – 8.1 (±3.1) Mean Total Mayo score |
FMT treatment 5 times, at 14-day intervals (1st treatment given via ileocolonoscopy, the 4 subsequent sessions at days 14, 28, 42 and 56 were via flexible sigmoidoscopy) | Faecal samples for microbiota analyses were collected at each study visit |
| Jacob et al (2017)24 | 20 | Before vs after FMT treatment (+ comparison against the healthy donor profile) |
Treatment protocol Single FMT delivery via colonoscopy FMT preparation Pooled from multiple donors |
38.4 (±12.6) | 60% | 8.1 (±2,4) Mean Total Mayo score | Patients received 1 infusion | 16S rRNA gene sequencing was performed on recipient faecal DNA samples pre- and 2 and 4 weeks post- transplant |
| Fang et al (2021)25 | 20 | FMT routine therapy control group (n = 10) |
Treatment protocol Monotherapy with a single fresh FMT via colonoscopy (n = 10) FMT preparation Single donor per patient |
FMT arm – 51.5 (±12.7) Control arm – 44.6 (±14.9) |
Unclear | FMT arm – 9.5 (±2.5) Total Mayo score Control arm – 8.6 (±2.9) Total Mayo score |
Patients received 1 infusion | Fresh faecal samples from the donors and pre-FMT and post-FMT samples from patients were collected |
| Cui et al (2015)26 | 15 | Before vs after FMT treatment (+ comparison against the healthy donor profile) |
Treatment protocol Step-up FMT treatment via endoscopic infusion tube FMT preparation Unclear if multiple / pooled donors or single donor per patient |
31.711, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48 | 73.3% | 86.7% Severe disease (S3) 13.3% Moderate disease (S2) |
Initial FMT given followed by a 2nd FMT after 1 week, followed by 1 short course of steroid therapy and monitoring for 3 months after the 2nd FMT |
Faecal samples from patients and donors pre-FMT, 1 week post-FMT and 4 months post-FMT were collected and stored for microbiota analysis by 16S rRNA sequencing |
| Chen et al (2020)27 | 47 | Before vs after FMT treatment (+ comparison against the healthy donor profile) |
Treatment protocol Single-donor FMT via colonic transendoscopic enteral tubing FMT preparation Single donor per patient |
44.4 (±15.5) | 57% | 5.9 (±2.0) Total Mayo score | Total of 3 FMT treatments given every other day for 1 week | Molecular microbiological analyses were performed using faecal samples obtained from patients 1 day prior to FMT, and 4 and 12 weeks after FMT |
| Sood et al (2020)28 | 140 | No active comparator |
Treatment protocol Multi-session FMT via colonoscopy FMT preparation Single donor per patient |
35 (±11) | 62.36% | 8.07 (±2.00) Mean Mayo Clinic score | FMT treatment at weeks 0, 2, 6, 10, 14, 18 and 22 | Single-centre prospectively analysis patients with active UC treated with FMT. Predictive clinical biomarkers of response explored |
| Okahara et al (2020)29 | 92 | Antibiotic treatment alone (n = 37) |
Treatment protocol Antibiotic pre-treatment followed by FMT (n = 55) FMT preparation Single donor per patient |
Mono-AFM arm – 42.5 (±14.7) A-FMT arm – 40.1 (±13.3) |
Mono-AFM arm – 48.7% A-FMT arm – 69.1% |
Mono-AFM arm – 1.8 (±0.8) Mayo Endoscopic score A-FMT arm – 1.9 (±0.7) Mayo Endoscopic score |
Patients received antibiotic pre-treatment for 2 weeks prior to fresh FMT by colonoscopy | Clinical response was observed at 4 weeks post-treatment and maintenance response observed at 24 weeks post-treatment |
| Zhao et al (2021)30 | 116 | No active comparator |
Treatment protocol Variable infusions / treatment sets of FMT delivered via various routes – upper GI, lower GI or capsule. FMT preparation Unclear |
Not reported | Not reported | Not reported | Unclear | Retrospective review of UC patients treated with FMT. Explored early recurrence – defined as an increase in Mayo score by ≥ 2 within one week of FMT. Predictive clinical biomarkers of response explored |
| Goyal et al (2018)34 | 21 | Before vs after FMT treatment (+ comparison against the healthy donor profile) |
Treatment protocol FMT delivery via faecal suspension into the distal duodenum or proximal jejnum, followed by a flush of normal saline and then delivery of faecal suspension into the terminal ileum and right colon FMT preparation Single donor per patient |
128, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 | 57.1% | Mayo Endoscopic score 1 – 33.3% Mayo Endoscopic score 2 – 41.7% Mayo Endoscopic score 3 – 16.7% |
Patients received 1 infusion | Patients treated with antibiotics (metronidazole/vancomycin) for 5 days starting 7 days before FMT Patients also took omeprazole (or equivalent) for 7 days starting 5 days prior to FMT All participants received 2-4mg of loperamide 2 hours prior to FMT Clinical response and adverse events were assessed at I week, 1 month, and 6 months after FMT |
| Uygun et al (2017)38 | 30 | Before vs after FMT treatment |
Treatment protocol Pre-treatment with bowel lavage followed by FMT via endoscopic infusion catheter FMT preparation Single donor per patient |
34.6 (±10.3) | 46.7% | Severe disease – 66.7% Moderate disease – 33.3% |
Patients received 1 infusion | Fresh stool samples from the donors were collected Clinical remission and response rates were calculated for participants at week 12 post-FMT |
| Nishida et al (2016)36 | 41 | Before vs after FMT treatment (+ comparison against the healthy donor profile) |
Treatment protocol Single FMT infusion via colonoscopy FMT preparation Single donor per patient |
39.6 (±16.9) | 68.3% | 5.6 (±2.4) Full Mayo score | Patients received 1 infusion | Primary end point – clinical response at 8 weeks |
| Gogokhia et al (2019)39 | 20 | Before vs after FMT treatment (+ comparison against the healthy donor profile) |
Treatment protocol Two-donor FMT via colonoscopy to the terminal ileum FMT preparation Unclear if multiple / pooled donors or single donor per patient |
Patients received 1 infusion | Faecal samples collected pre-FMT, week 2 and week 4 post-FMT |
consisting of ≥ 10 patients in FMT treated arms
non-placebo controlled blinded randomised trials
FMT-faecal microbiota transplantation, cFMT-capsulised faecal microbiota transplantation, dFMT-donor FMT, aFMT-autologous FMT, SCCAI-simple clinical colitis activity index.
Changes in microbial diversity
Five RCTs reported on changes in alpha diversity following FMT as presented in Table 3.7,9, 10, 11,13 Three observed a significant increase in alpha diversity relative to baseline following FMT in all patients regardless of clinical response.7,9,11 The FOCUS study observed this change being more pronounced in patients who entered clinical remission compared to those who did not.7,33 In contrast the LOTUS study and the RCT by Costello and colleagues observed that the increase in alpha diversity following FMT was no longer significant when stratified by clinical response.9,11 In comparison, the TURN trial observed a significant increase in alpha diversity in both donor FMT and autologous FMT responders but not in non-responders.10,31 Amongst the non-randomised studies, only one study consisting demonstrated a significant increase in alpha diversity at post-FMT compared with pre-FMT, with this effect disappearing at 6 months.34 Non-significant trends reported including in increase in alpha diversity following FMT were observed in three non-randomised studies22,24,27 and one study showed a decrease in diversity with each sequential FMT treatment.21
Table 3.
Studies characterising changes in microbial diversity and profiles following FMT for UC
| Reference | Bioinformatic methodology | α + β Diversity (after FMT) |
Taxonomic Changes (after FMT) |
|||
|---|---|---|---|---|---|---|
| Responders | Non-responders | Responders | Non-responders | |||
| Paramsothy et al (2019)33 |
16S rRNA analysis MOTHUR pipeline Shotgun metagenomics Filtering - DeconSeq, FastQC Analysis - SolexaQA, MetaPhlAn2, HUMAnN2. |
In all patients α-diversity ↑ (Phylogenetic, richness and Shannon's diversity; P < 0.0001). β-diversity (multivariate dispersion) changed (P = 0.0001) following FMT, however these were more pronounced in patients entering remission |
↑ faecal + mucosal species richness ↑ Eubacterium hallii (Firmicutes), Roseburia inulinivorans (Firmicutes Lachnospiraceae), Eggerthella species and Ruminococcus bromii (Firmicutes Ruminococcus) ↑ Firmicutes (Oscillibacter and Clostridium XVIII) |
↓ faecal + mucosal species richness ↑ Fusobacterium (Fusobacterium gonidiaformans) (most consistent association), Sutterella (Sutterella wadsworthensis), Haemophilus, Escherichia, Megamonas, Clostridium XIVa, Prevotella (Prevotella copri) Dialister, Veillonella and Bilophila |
||
| Moayyedi et al (2015)8 |
16S rRNA analysis Analysis – Phyloseq R package and QIIME. |
α-diversity not reported Significant change in β-diversity (Bray-Curtis dissimilarity) following FMT with no association with clinical response. (P = 0.02) |
↑ Lachnospiraceae family and Ruminococcus in donor B (associated with successful FMT) | ↑ Escherichia and Streptococcus in donor A | ||
| Costello et al (2019)9 |
16S rRNA analysis Unspecified in-house and open source software. Differential abundance analysis lme4, mice, and glmmTMB R packages |
↑ α-diversity (OTU analysis) in all patients following FMT with no association with clinical response. β-diversity not reported |
↑ Methanobrevibacter smithii, Peptococcus niger (Firmicutes), Faecalicoccus pleomorphus (Firmicutes), Olsenella sp. (Actinobacteria), Acidaminococcus intestini (Firmicutes), Senegalimassilia anaerobia (Actinobacteria), Prevotella copri (Bacteroidetes), Clostridium methylpentosum (Firmicutes), Alistipes indistinctus (Bacteroidetes), Slackia isoflavoniconvertens (Actinobacteria) and Odoribacter splanchnicus strain (Bacteroidetes) ↓ Anaerostipes caccae, Gordonibacter pamelaeae and Clostridium aldenense Abundance change in Anaerofilum pentosovorans (Firmicutes), Bacteroides coprophilus (Bacteroidetes), Clostridium methylpentosum (Firmicutes), Acidaminococcus intestini (Firmicutes), Senegalimassilia anaerobia (Actinobacteria) |
Abundance change in Fusicatenibacter saccharivorans (Firmicutes) and Paraprevotella xylaniphila (Bacteroidetes) | ||
| Rossen et al (2015)10 |
16S rRNA analysis USEARCH algorithms and unspecified independent classification techniques. Differential abundance analysis using Canoco5 |
↑ α-diversity (Shannon's index, P = 0.06 (FMT-D), P = 0.01 (FMT-A)) β-diversity shift (redundancy) |
No change in diversity | ↑ Clostridium IV, XIVa and XVIII (Firmicutes)(FMT-D responders) ↓ Bacteroidetes (FMT-D responders) ↑ Bacilli, Proteobacteria and Bacteroidetes (FMT-A responders) |
None presented | |
| Crothers et al (2021)13 |
16s rRNA analysis QIIME2 pipeline |
No change in α-diversity (Shannon Index) | Taxonomic data not presented | |||
| Pai et al (2021)12 |
16S rRNA analysis Custom Perl scripts, Phyloseq R package and QIIME. |
α-diversity not reported β-diversity (unspecified measure) changed in FMT arm (not significant) – no association with clinical response |
Alistipes spp. and Escherichia spp. associated with achieving composite clinical outcome | None presented | ||
| Haifer et al (2021)11 |
16S rRNA analysis MOTHUR pipeline |
α diversity (richness) ↑ in all patients with changes seen in β-diversity (ANOSIM) following FMT. However, no change in α or β in relation to response or non-response. | Increase in Bacteroides OTU19 (100% similarity to Bacteroides ovatus and Bacteroides xylanisolvens) | Increase in Bacteroides OTU14 (100% similarity to Bacteroides caccae increase | ||
| Tian et al (2019)21 |
16S rRNA analysis Full pipeline not described. Differential analysis using LEfSe. |
α-diversity (Shannon index and Chao I index) and β-diversity (ANOVA) unchanged following FMT in all patients with no association with clinical response. | ↑ Bacteroidetes, Proteus, Prevotella, Phascolarctobacterium and Lactobacillus (d1), Clostridiaceae (d2) ↓ Firmicutes, Streptococcus |
↑ Bacteroidetes, Proteus ↓ Firmicutes, Streptococcus |
||
| Li et al (2020)22 |
16S rRNA analysis Combination of MOTHUR, UPARSE and R |
α-diversity ↑ (Shannon index and Chao I index) β-diversity (MDS) shift (trend) (both analogous to the donors). No separate data in responders |
↑ Holdemania Anaerostipes, Bifidobacterium, Clostridium IV and Odoribacter (analogous to donors) Eubacterium and Ruminococcus (close to donors) Differences in relative abundance of Eggerthella, Lactobacillus and Ruminococcus positively correlated to efficacy (P < 0.05) |
Notable difference in Eubacterium and Ruminococcus abundance compared with donors (P < 0.001) | ||
| Leonardi et al (2020)32 |
ITS1 analysis BLAST with ITS1 database fllowed by QIIME v1.6 Bacterial analysis as per Paramsothy et al. (2019)33 |
↑ bacterial α-diversity (↑ Candida pre-FMT had ↑ α-diversity 8 weeks post-FMT) No change to mycobiota diversity. No association with clinical response |
Reduction in abundance of Candida positively associated with clinical response |
No change in relative abundance of Candida | ||
| Kump et al (2018)23 |
16S rRNA analysis Combination of UCHIME, MOTHUR and QIIME v1.8 |
No change in α-diversity (richness) Significant change in β-diversity (unweighted UniFrac distance) |
↑ Akkermansia muciniphila ↓ Dialister |
No increase in A. muciniphila | ||
| Jacob et al (2017)24 |
16S rRNA analysis USEARCH and UPARSE algorithms / pipelines |
α-diversity ↑(OTUs P = 0.0049, Shannon index P = 0.069) Difference in β-diversity (Bray-Curtis dissimilarity) post-FMT (P < 0.034). No association with clinical response. |
No taxonomic data presented | |||
| Fang et al (2021)25 |
16S rRNA analysis Full pipeline not described. Differential analysis using LEfSe. |
No difference in α-diversity (Kruskal–Wallis rank sum). | ↑ Bacteroidetes and Prevotella and ↓ Proteobacteria and Escherichia post FMT. Association with clinical response data not presented. |
|||
| Cui et al (2015)26 |
16S rRNA analysis Not described. |
Microbial analysis only performed on a subset of patients (n=4). ↑ α-diversity seen in 3 patients post FMT (Pearson correlation coefficient) |
No taxonomic data presented | |||
| Chen et al (2020)27 |
16S rRNA analysis UPARSE and QIIME v1.7 |
↑ α-diversity (Shannon index) week 4 but then ↓ at week 12 – no association with clinical response | ↑ F. Prausnitzii (P < 0.05) – no association with clinical response | |||
| Brezina et al (2021)20 |
16S rRNA analysis QIIME2 pipeline. Differential analysis using LEfSe. |
α-diversity ↑ (Shannon entropy index) | ↑ Bacteroidales, Prevotellaceae, Veilllonellaceae and Desulfobacteria | ↑ Staphylococcaceae, Lactobacillaceae and Bifidobacteriaceae | ||
| Fuentes et al (2017)31 |
16S rRNA USEARCH algorithms and unspecified independent classification techniques. Differential abundance analysis using Canoco5 |
Analysis of TURN patients | ↑ Clostridium XIVa (Anaerostipes caccae, Coprococcus eutactus or Eubacterium rectale (similar levels to healthy donors)) ↓ Enterococcus, Proteobacteria Positive association to Clostridium IV (F. prausnitzii) and XIVa (Eubacterium hallii, Roseburia intestinalis and Butyrivibria crossotus) |
↓ Clostridium XIVa (Anaerostipes caccae, Coprococcus eutactus or Eubacterium rectale) ↑ Enterococcus, Proteobacteria and R. gnavus (P = 0.014) Positive association with Bacteroidetes groups (B. vulgatus and B. fragilis) |
||
| Goyal et al (2018)34 |
16S rRNA analysis QIIME pipeline. Differential analysis using LEfSe. |
↑ α-diversity (OTU) Change in β-diversity (weighted UniFrac) - both seen 1-month post-FMT. No statistically significant difference in α-diversity seen at 6 months post-FMT |
No significant increase in α-diversity (OTU) at 1- and 6-months post-FMT No change in β-diversity (weighted UniFrac) 1-month post-FMT |
↑ Lachnospiraceae and ↓ Enterobacteriaceae at 1 week, 1 month and 6 months post-FMT | ||
| Nishida et al (2016)36 |
16S rRNA analysis Full pipeline not described. Phyloseq R package for diversity analysis |
No difference in α- and β-diversity (Bray-Curtis dissimilarity index) at week 8 | No taxonomic data presented | |||
| Gogokhia et al (2020)39 |
Virome analysis Filtering using BBMAP following by analysis usin VirMAP pipeline |
Not reported | No change in relative abundance of Caudovirales bacteriophages 4 weeks post FMT | Increase in relative abundance of Caudovirales bacteriophages 4 weeks post FMT | ||
FMT-faecal microbiota transplantation, OUT-Operational taxonomic units, QIIME-Quantitative Insights Into Microbial Ecology, LEfSe-Linear discriminant analysis Effect Size, MDS-Multidimensional scaling
Five RCTs reported on changes in beta diversity following FMT.7,8,10, 11, 12,31,33 Four observed a significant change in beta diversity following FMT in comparison to the placebo/inactive arm and relative to pre-FMT baseline.7,8,10,11,31,33 Both the FOCUS trial and the RCT by Moayyedi and colleagues demonstrated a significant difference in the gut microbial composition following FMT. Furthermore, they demonstrated the gut microbial profiles following FMT were more similar to donors regardless of clinical response with Moayyedi demonstrating that this similarity was only seen between FMT treated recipient and their respective donor. Similarly, the TURN trial demonstrated that the microbiota composition of responders in the donor FMT group shifted from overlap with non-responders at baseline to healthy donors following FMT.31 These microbial compositional shifts were not however observed in the patients treated with autologous FMT.
Five non-randomised studies measured changes to beta diversity in UC patients receiving FMT.21, 22, 23, 24,35 Of these three studies demonstrated a change in beta diversity following FMT relative to baseline community profiles.23,24 The study by Jacob et al and Goyal et al demonstrated that this shift in the beta diversity resulted in a greater similarity with the donor faecal microbiota.24,34 A similar donor-recipient similarly trend in beta diversity was observed by Li et al however no clear difference between responders and non-responders following FMT was seen.22
Taxonomic changes
Six of the seven eligible placebo controlled RCTs reported on microbial taxonomic changes following FMT through analysis of stool 16S rRNA profiles as presented in Table 3.7, 8, 9, 10, 11, 12 In addition, the FOCUS trial performed stool metagenomic analysis and 16S rRNA on colonic mucosal biopsies collected at baseline and at the end of the FMT treatment period (week 8).33
Changes associated with response to FMT
A significant increase in taxa belong to the Clostridia class (specifically XVIII) in responders to FMT were observed in four RCTs.8,9,31,33 Notably within this class and increase in taxa belonging to the families Oscillospiraceae (Ruminococcus bromii, Anaerofilum pentosovorans, Clostridium methylpentosum), Lachnospiraceae (Roseburia inulinivorans, Eubacterium hallii) and Clostridiaceae was observed in responders. Increases in taxa belonging to the Clostridia class were also reported in several of the non-randomised FMT studies. Faecalibacterium prausnitzii was reported to significantly increase in responders 4 weeks post-FMT relative to baseline.27 A significantly lower relative abundance of Ruminococcus and Eubacterium compared to healthy donors was reported in non-responders to FMT in a study by Li and colleagues with a non-significant increase in Ruminococcus in responders.22
Four studies reported a significant increase in taxa belonging to phylum Bacterioidetes following FMT in responders.9,11,12,31 Specifically, these included Bacteroides coprophilus, Bacteroides OTU19 (100% similarity to Bacteroides ovatus and Bacteroides xylanisolvens) and Alistipes spp.
In addition to Clostridia and Bacteroidetes, a significant increase was reported in Eggerthella (Actinobacteria), Senegalimassilia anaerobia (Actinobacteria), Acidaminococcus intestine (Negativicutes) and Escherichia (Proteobacteria). Within the non-placebo controlled or non-randomised studies Brezina and colleagues demonstrated a significant increase in Bacteroidales, Prevotellaceae, Veillonellaceae and Desulfobacteria in responders.20 A significant increase in taxa belonging to the order Bacteroidales and Verrucomicrobiales and class Coriobacteriia was noted in responders compared to non-responders in another study.22 The non-randomised paediatric FMT study by Goyal et al demonstrated a significant decrease in Enterobacteriaceae and an increase in Lachnospiraceae following FMT, however this difference was not significant when sub-grouped by response.34
Analysis of the gut mycobiome, as part of a post-hoc analysis of the FOCUS trial noted that decreased Candida abundance post-FMT was indicative of clinical response.32 The LOTUS study in contrast did not report any changes in alpha or beta diversity metrics of the mycobiome upon disease flare.11
Changes associated with lack of response to FMT
Changes in microbial taxa associated with lack of response to FMT were reported by four RCTs.8,9,11,33 These included a significant increase in species belonging to phylum Fusobacteria (Fusobacterium gonidiaformans), phylum Proteobacteria (Bilophila, Haemophilus, Escherichia, Sutterella wadsworthensis) and family Prevotellaceae (Paraprevotella xylaniphila, Prevotella copri). In addition, a significant increase in Dialister, Veillonella, Megamonas, Fusicatenibacter saccharivorans, Clostridium XIVa and Bacteroides OTU14 (100% similarity to Bacteroides caccae) was observed in non-responders. Responders in the LOTUS trial who developed a disease flare on FMT withdrawal had an enrichment of Streptococcus OTU45 (100% similarity to Streptococcus parasanguinis and other phylogenetically related species) along with depletion of Blautia OTU35 (100% similarity to Blautia faecis).11 No clear alpha diversity change was however noted. Within the non-placebo controlled or non-randomised studies Brezina and colleagues demonstrated that Staphylococcaceae, Lactobacillaceae and Bifidobacteriaceae were significantly higher in non-responders.20
Metabolomic analysis
Two RCTs9,33 analysed changes in microbial metabolites following FMT treatment. The FOCUS trial identified 97 metabolites that were different between baseline and following FMT treatment regardless of clinical response.33 Of these metabolites, N-acetylmuramate, xanthine, 2-deoxyinosine, ribothymidine and X- 17009 (unnamed biochemical) were significantly increase post-FMT but were not altered by placebo. The trial reported significant differences in global metabolomic profiles following FMT in clinical responders in comparison to baseline, after placebo and after FMT in clinical non-responders. Specifically, 228 metabolites differentiated between positive and negative outcomes following FMT of which 33 of these were different in patients achieving clinical response. Metabolites such belonging to benzoate degradation, glycerophospholipid metabolism, secondary bile acid biosynthesis, ppGpp biosynthesis and biosynthesis of ansamycins pathways were associated with positive outcomes following FMT. In contrast metabolites associated with heme and lysine metabolic pathways were associated with a negative outcome after FMT. Faecal metabolome analysis in the Costello study that was specifically targeted to short chain fatty acid levels reported no significant differences from baseline in stool concentrations of butyrate, acetate, propionate, iso-butyrate, valerate, iso-valerate and caproate following FMT regardless of clinical response or treatment arm (donor versus autologous).9
Whilst TURN trial did not report changes in faecal metabolic profiles they performed functional predictive analysis using PICRUSt and qPCR.31 Microbiota of non-responders in this study had a significantly lower butyrate production capacity, reflected by the butyrate-acetoacetate CoA transferase and ButCoA gene copies, compared with donors and responders. ButCoA levels were increased by 6.7-fold in responders, especially those who remained in remission at ⩾1-year FU. A non-randomised study that used similar predictive functional analysis gut microbiota reported on significant differences in pathways of pyruvate metabolism, sulfur metabolism, pantothenate and CoA biosynthesis, glyoxylate and dicarboxylate metabolism, synthesis and degradation of ketone bodies and other transporters were between donor, pre- and post-FMT groups.25
Donor characteristics association with clinical response
Two RCTs that explored donor recipient association demonstrated that microbial profiles of recipients were significantly more similar to their respective donors following FMT compared to controls as presented in Table 4.8,31 Notably the study by Moayyedi and colleagues noted that one particular donor, ‘Donor B’, was associated with greater success rate) in their respective recipients with a non-significant trend for faecal microbiota from responders having greater similarly to donor B than non-responders.8
Table 4.
Data summary table of the relationship between patients’ and donors’ microbiota post-FMT
| Reference | Donor Relationship (after FMT) |
|
|---|---|---|
| Responders | Non-responders | |
| Paramsothy et al (2019)33 | ↑ homogeneity in taxonomic profiles to a level seen in donors Donor batches with ↑ Bacteroides OTU187 (Bacteroides fragilis and Bacteroides finegoldii) |
Donor batches with ↑ Clostridium XIVA and association with Bacteroides uniformis, Bacteroides coprocola and Streptococcus OTU56 |
| Moayyedi et al (2015)8 | ↑ microbiota similarity to donor B (enrichment of Lachnospiraceae and Ruminococcus) | ↓ microbiota similarity to donor B |
| Rossen et al (2015)10 | • Microbiota composition overlap with healthy donors (FMT-D) characterised by ↑ Clostridium clusters IV, XIVa, XVIII and ↓ Bacteroidetes • Microbiota composition shift away from non-responders (FMT-A (different direction to FMT-D responders)) characterised by ↑ Bacilli, Proteobacteria and Bacteroidetes ↑ similarity index to corresponding donors ↑ similarity to donors which they received faeces from (P = 0.02) |
↓ similarity index to corresponding donors ↓ similarity to donors which they received faeces from (P = 0.02) |
| Haifer et al (2021)11 | Donor 1 (favourable donor) had a significantly higher bacterial diversity driven by higher species evenness with compositional differences largely related to differences in relative abundances of Bacteroidetes taxa | Not reported |
| Jacob et al (2017)24 | ↑ Similarity with donor FMT samples Donors achieving clinical remission clustered together |
Not reported |
| Chen et al (2020)27 | Abundance of F. prausnitzii ↑ towards levels similar to those of donors | Not reported |
| Li et al (2020)22 | ↓ Dissimilarity between patients and donors (α + β diversities analogous to donors) | ↓ Dissimilarity between patients and donors (α + β diversities analogous to donors) |
| Fuentes et al (2017)31 | ↑ Similarity to donors (FMT-D) (P = 0.02) Trend of ↑ similarity to donors (patients with sustained remission) (P = 0.1) No significant differences in similarity values of FMT-A patients |
↓ Similarity to donors (FMT-D) (P = 0.02) Trend of ↓ similarity to donors (relapsers) (P = 0.1) Donor batches associated with Proteobacteria (E. coli and Aeromonas) and ↑ abundance of Ruminococcus gnavus No significant differences in similarity values of FMT-A patients |
| Kump et al (2018)23 | All recipients’ microbiotas, regardless of response, shifted towards the respective donor microbiota | All recipients’ microbiotas, regardless of response, shifted towards the respective donor microbiota |
| Shabat et al (2021)19 | UCED preconditioning of donors led to reduction of alpha diversity of donor stool with numerically higher remission rates compared with FMT alone (or UCED and FMT). | |
| Okahara et al (2020)29 | ↑ Cumulative non-relapse rate in sibling FMT than parent-child FMT Donor Bacteroidetes species (Bacteroides uniformis and Parabacteroides distasonis and Bacteroides dorei) persisted in patients with no UC recurrence after 24 months ↑ Similarity of 10 Bacteroidetes species to donor levels |
↓ Cumulative non-relapse rate in ≥11-year difference group that 0-10-year difference group |
FMT-faecal microbiota transplantation, FMT-D-donor faecal microbiota transplantation, FMT-A-autologous faecal microbiota transplantation, UCED-Ulcerative colitis exclusion diet
Four RCTS reported on the association of clinical response with taxonomic characteristics in donor stool with inconsistent findings.8,11,31,33 Abundance of specific taxa belonging to Bacteroidetes phylum within donor stool and correlation with a favourable clinical response have been observed in both the FOCUS and LOTUS clinical trials. As the FOCUS trial used pooled FMT, specific donor-recipient relationships could not be explored. Effective donor batches leading to >50% remission in patients contained a higher abundance of Bacteroides OTU187, specifically Bacteroides fragilis and Bacteroides finegoldii, whilst ineffective batches were associated with Clostridium XIVA. There was also a non-significant trend towards an association between ineffective batches and the taxa Bacteroides uniformis, Bacteroides coprocola, Sutterella Wadsworthenesis and Streptococcus OTU56. The LOTUS study manufactured oral lyophilised FMT capsules from two separate donors. They demonstrated that the donor with a significantly higher bacterial diversity (greater species evenness) with significant differences in relative abundances of Bacteroidetes taxa was associated with a favourable clinical response. Higher taxonomic classification was however not provided in the study. An open label non-randomised study demonstrated that clinical response was significantly greater donors with a higher abundance of faecal Bifidobacterium, Lactobacillales and Clostridium clusters IV and XI.36 No significant difference in donor-recipient gut microbial similarity was observed between responders and non-responders.
Moayyedi and collegues noted that Donor B had enrichment of Lachnospiraceae and the genera Ruminococcus. In contrast, the TURN study observed a greater abundance of Ruminococcus gnavus in donors of patients who relapsed compared with donors of patients who achieved sustained remission. However, post-hoc analysis of the TURN study with at least one year follow up of patients in this trial observed that donor faecal samples consisting of E. coli and Aeromonas were positively associated with patients who relapsed.31
Donor (and recipient) faecal microbiome optimisation prior to stool collection and FMT administration was explored in the CRAFT UC study.19 A specific diet named UC exclusion diet (UCED) was administered as part of this study and comprised mandatory foods such as certain fruits and vegetables, prescribed amounts of chicken and eggs and certain foods that were restricted with the aim of decreasing exposure to sulphated amino acids, total protein, heme, saturated fat and food additives. Donor and recipient dietary conditioning UCED was attempted with patients randomised to either Group 1 - standard low intensity FMT followed by standard diet, Group 2 – low intensity FMT from donors pre-conditioned with UCED and post FMT recipient conditioning with UCED or Group 3 - UCED alone. Numerically higher, but not statistically significant clinical remission rates and mucosal healing in Group 3 (UCED alone) compared to the FMT arms (Groups 1 and 2). The authors showed that the UCED diet preconditioning of donors reduced the alpha diversity of donor stool microbiota rather than an anticipated increase. Recipient microbiome data or donor-recipient response was not presented as part of the study.
Baseline predictors of response
Clinical predictors
Baseline clinical predictors were reported in three RCTs and two non-randomised studies. Using demographic information obtained from baseline questionnaires, Moayyedi et al reported a trend towards patients receiving immunosuppressant therapy at baseline acquiring a greater benefit from FMT.8 Additionally, the authors found that patients were statistically significantly far more likely to respond to FMT if they had received a recent diagnosis of UC (defined as ≤1 year). In contrast the FOCUS trial observed an inverse relationship between endoscopic severity and the primary outcome however this was no longer seen when controlled for other factors.7 Correlation with clinical response was also noted with age but directionality was not reported. No relation was however observed between the primary outcome and anatomical disease extent, smoking status, disease duration, any concomitant immunosuppressive (steroids, biologics, immunomodulatory) use. Similarly, the RCT by Costello et al did not observe any interactions between age at diagnosis/randomisation, disease duration/distribution, gender, non-steroid based medication use or macronutrient intake with a change in total Mayo score following donor FMT.9 Use of oral steroids at randomisation was however associated with a greater reduction in total Mayo score.
Amongst the non-randomised studies, in a single-centre prospectively study of open label FMT statistically significant association between moderate disease severity (Mayo score 6-9) and remission in UC patients, along with endoscopic Mayo score 2.28 In addition, the authors noted that severe disease (Mayo score ≥10) and endoscopic Mayo score 3 were both significantly correlated with FMT failure. A previous study by the same authors reported that patients treated earlier on in the disease course or those with mild disease had higher rates of clinical remission.37 They noticed that in biologic-experienced patients, endoscopic Mayo score 2 was a predictor of response whereas in biologic-naïve patients younger age, moderate disease severity, shorter disease duration and endoscopic Mayo score 2 were all significantly predictive of a positive outcome. They described young age as a baseline factor which determined participants’ response with patients under 40 years demonstrating greater rates of remission. In a univariate analysis performed in an uncontrolled study that consisted 11 disease recurrences in 116 UC patients with active disease reported significant associations with a baseline high Mayo score, recent use of steroids to induce remission, low serum albumin, and peripheral blood lymphocyte deficiency were associated with a higher recurrence rate following FMT.30 These are however recognised factors associated with unfavourable disease outcomes irrespective of treatment. No association with disease extent was observed and disease duration was not explored. Two other non-randomised studies did not demonstrate any differences in clinical characteristics between responders and non-responders.36,38
Microbial predictors
Analysis of potential baseline microbial predictors of response in the FOCUS trial found that patients who achieved the primary outcome tended to have higher faecal species richness at baseline compared with patients not achieving the primary outcome.33 They also observed a similar non-significant trend in the mucosal microbiome in which a higher baseline species richness as well as an increased abundance of specific species of Bacteroides (B. fragilis and B. finegoldii) with was associated with a positive therapeutic outcome(33). Gut mycobiome analysis of the FOCUS trial observed that a greater abundance of Candida pre-FMT was associated with a clinical response (and increased bacterial diversity post-FMT).32 An open label study of 20 patients with active UC observed that FMT responders had a lower relative abundance of Caudovirales bacteriophages at baseline compared to non-responders. The relative abundance of Caudovirales in non-responders appeared to increase after FMT while no change was observed in responders.39
Patients receiving autologous FMT in the TURN trial had a greater likelihood of response to treatment if they possessed baseline microbiota profiles more similar to donor samples or to patients in sustained remission following donor FMT. Differences in baseline microbiota profiles between responders and non-responders was however not found to be a predictor of response for patients receiving donor FMT.31 Higher levels of Bacteroidetes, particularly B. vulgatus, and Prevotella in non-responders at baseline were associated with relapse at the 1 year follow up. A non-randomised study of FMT in paediatric patients with UC observed that the abundance of Fusobacterium was significantly greater at baseline in non-responders compared to responders.34
Metabolomic predictors
Potential baseline metabolomic predictors of response was only reported as part of the FOCUS study.33 Fifteen metabolites were identified-N-methylphenylalanine, N-acetylarginine, caproate, lignoceroyl ethanolamide, biotin were associated with an increased positive clinical outcome whilst the metabolites 5-aminovalerate, oleoyl-arachidonoyl-glycerol, linoleoyl-arachidonoyl-glycerol, linoleoyl-arachidonoyl-glycerol, sphingomyelin, sphingomyelin, gulonate and heme were identified as being associated with increased negative outcome.
Discussion
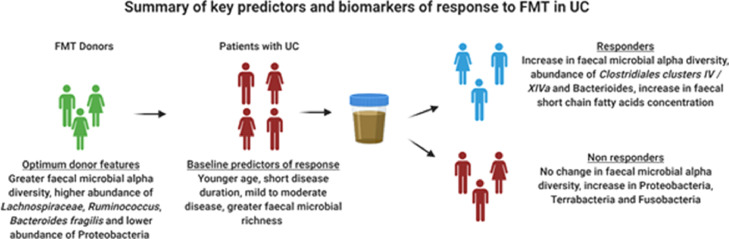
This systematic review outlines potential donor and recipient clinical and microbial biomarkers that predict and denote clinical response to FMT in patients with UC. Examination of 7 double blind placebo controlled RCTs and 12 non-randomised studies in FMT in UC identified specific consistent findings in gut microbial profiles that correlate with a favourable clinical response along with clinical and microbial profiles that have the potential of predicting response to FMT (summarised in Figure 2).
Figure 2.
Summary of key predictors and biomarkers of response to FMT in UC. FMT, faecal microbiota transplantation, UC, ulcerative colitis.
Following FMT, the overall trends of biomarkers discovered in responding patients’ microbiota communities were (a) an increase in bacterial diversity (alpha and beta), (b) increases in Firmicutes and Bacteroidetes along with key taxa belonging to these phyla, and (c) recipient microbial profiles with increased similarity to donor profiles. Responders of FMT had microbial profiles more similar to that of their donors, possibly suggesting that the donor microbiota composition profile may be used as a potential microbial treatment target for individualisation of FMT treatment regimens. At a taxonomic level, studies have consistently demonstrated an increase in abundance of Clostridium clusters IV and XIVa (members of the Firmicutes phylum), post-FMT this is associated with a favourable clinical response. These include the Lachnospiraceae and Ruminococcaceae families and are likely to induce this response through immune regulation of colonic inflammatory pathways.40,41 SCFAs are the product of bacterial fermentation of polysaccharide, oligosaccharide and particular amino acids which are non-digestable by the host.42 Producers of SCFAs specifically Clostridium clusters have a crucial role in maintaining intestinal function.43 SCFAs have been shown to induce the differentiation of naïve CD4 T cells into immunosuppressive, anti-inflammatory IL-10-producing regulatory T cells.41,44 Consistently SCFA synthesis, and the presence of components contributing to this synthesis, appears to be a metabolomic biomarker of response post-FMT.25,31,33,45 For instance, observed gene copy levels of ButCoA were increased in those patients who received successful FMT therapy whilst the microbial capacity for butyrate production of the microbiota decreased in patients lacking a response to FMT.31 The FOCUS study also identified increased levels of heme and lipopolysaccharide biosynthesis at both baseline and post-FMT as potential biomarkers associated with a negative outcome.33 Not only do various bacterial pathogens produce heme, but it is also a vital source of iron required for their survival with murine studies suggesting its role in colonic inflammation.46
Certain baseline recipient characteristics were found to be important factors in determining a favourable outcome. FMT recipients with younger age, less severe and less extensive disease and potentially shorter duration of UC (< 1 year) have been shown to associated with a greater likelihood of response. These predictive baseline factors are not too different to that of biological/small molecule therapies in UC. Whilst other biomarkers were identified in this systematic review such as the prior or concurrent immunosuppressant use in predicting response, further research is needed to corroborate these findings.8 There is some evidence to suggest that patients with higher faecal microbial richness at baseline, greater abundance of Candida, lower abundance of Caudovirales and a microbial composition closer to donors at baseline are more likely to have a favourable response.31, 32, 33,47 It is plausible that the dysbiosis seen in patients with a recent UC diagnosis as well as a relatively lower degree of microbial aberrancy is more easily manipulated with FMT resulting in a greater likelihood of successful and sustained donor microbiome engraftment and clinical response.
Only the FOCUS study reported on baseline metabolomic predictors of response, with the findings of the RCT provide significant insight into the bacterial metabolites which give a higher likelihood of achieving a positive FMT outcome.33 One of the most notable metabolites was biotin (vitamin B7), with diet and synthesis by commensal microbiota in the gut being its primary source in humans.48 Biotin results in the downregulation of the NF-κB gene thereby restricting release of various pro-inflammatory cytokines in the gut epithelium.49
Greater microbial richness in donor stool was associated with an increased rate of clinical response in patients with active UC.11,23,47 Engraftment of donor-derived microbiota ameliorates UC symptoms through either replenishing bacterial species whose abundance is decreased prior to treatment or, providing bacteria which create an unfavourable environment for disease-associated bacteria so as to repress their growth.50 Having a high bacterial species richness, therefore, may increase the chances that certain bacterial strains engraft in the gut of the recipient and become permanent members of their microbiota community.51 Along with increased bacterial richness, specific taxa were identified in donor stool associated with remission, whilst others were found in those associated with treatment failure. Donor stool which included high abundances of Bacteroides OTU187 in addition to the families Lachnospiraceae and Ruminococcaceae were more likely to induce a response in recipients, whereas the presence of Clostridium XIVA was seen in ineffective batches.8,11,33 The TURN study in contrast observed a greater abundance of Ruminococcus gnavus in donors of patients who relapse. However, it is important to note that the microbial profiles of donors were similar to the baseline profiles of the UC patients in this study. Preselecting donors based on a richer microbial diversity and greater abundances of SCFA producing bacteria or pooling FMT from donors to control for variability in donor microbial diversity. Pooling FMT is, however, no longer practical as it presents major challenges with ‘look back’ exercises and root cause analysis in cases of FMT related adverse events. One option would be to pre-condition donors with a diet that is associated with increasing microbial diversity. The CRAFT UC study attempted this with preconditioning donors with a designer diet (UCED) that consisted of dietary exclusion of specific components such as saturated fat and food additives that are thought to contribute to an immune mediated inflammatory response.19 Paradoxically the UCED diet resulted in a reduction in donor microbial richness and may have potentially contributed to the unfavourable outcomes seen with donor pre-conditioned FMT. Nevertheless, optimum microbiome-based donor selection as well as pre-conditioning with a diet that is associated with increasing gut microbial diversity are likely to play an important role enhancing response with FMT.52,53
The findings of this systematic review highlight the possibility of enhancing a sustained response to FMT through biomarker-based selection and optimisation of donors and patients before and during the treatment with FMT. Utilising precision medicine, would facilitate an individualised, biomarker driven ‘treat to microbiome/metabolome’ target approach with FMT in UC early in the disease. After the pre-defined clinical target is reached, the need for further FMT is tracked based on loss of this specified microbiome target. Studies are now needed to help define these targets with leading candidates that include alpha diversity, specific faecal SCFA producing strains such as Clostridiales and faecal butyrate levels. There are a few limitations of this systematic review. The heterogeneity of the study designs that include mode and frequency of FMT administration, the use of a single or pooled donor approaches, variable placebo and active comparators and differences in microbial analytical strategies may make interpretation in the context of a systematic review challenging. However, the reproducibility and consistency of several of the findings reported in this review, in addition to biological plausibility, does bring a level of confidence. We excluded studies with less than ten (FMT treated) participants for quality control. None of these excluded studies had detailed exploratory mechanistic data that would have significantly influenced the findings in the review.
To conclude, there is evidence of existing predictive biomarkers for the treatment of UC with FMT, the most well-defined of these being microbial indicators. Despite the exponential growth in research into FMT over recent years, the mechanistic understanding on the basis of this treatment is poor. It also remains unclear if alterations to the microbiota occur to certain pre-existing immunomodulatory bacterial strains that are enriched post-FMT, or if they are solely donor derived and engrafted after treatment. It is clear however, that the gut microbiota is fast becoming a pivotal therapeutic target which holds considerable potential.
Contributors
NPR and MNQ performed the search and data extraction. NPR wrote the first draft with critical feedback and edits from MNQ. All authors (NPR, WS, CQ, CT, RDH, NS, ADB, THI, MNQ) provided feedback and approved the final version of the draft.
Declaration of interests
All authors declare no relevant conflict of interests.
Acknowledgments
Acknowledgements
ADB is currently supported by Cancer Research UK Advanced Clinician Scientist Award (ref C31641/A23923). There no specific funding to declare.
Data sharing statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Footnotes
PROSPERO registration ID: 331957
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104088.
Appendix. Supplementary materials
References
- 1.Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. The Lancet. 2012;380(9853):1606–1619. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 2.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134(2):577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 3.de Souza HS, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13(1):13–27. doi: 10.1038/nrgastro.2015.186. [DOI] [PubMed] [Google Scholar]
- 4.Zakerska-Banaszak O, Tomczak H, Gabryel M, et al. Dysbiosis of gut microbiota in Polish patients with ulcerative colitis: a pilot study. Sci Rep. 2021;11(1):2166. doi: 10.1038/s41598-021-81628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pittayanon R, Lau JT, Leontiadis GI, et al. Differences in Gut Microbiota in Patients With vs Without Inflammatory BowelDiseases: A Systematic Review. Gastroenterology. 2020;158(4):930-46 e1. doi: 10.1053/j.gastro.2019.11.294. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Allen-Vercoe E, Petrof EO. Fecal microbiota transplantation: in perspective. Therap Adv Gastroenterol. 2016;9(2):229–239. doi: 10.1177/1756283X15607414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389(10075):1218–1228. doi: 10.1016/S0140-6736(17)30182-4. [DOI] [PubMed] [Google Scholar]
- 8.Moayyedi P, Surette MG, Kim PT, et al. Fecal Microbiota Transplantation Induces Remission in Patients With Active Ulcerative Colitis in a Randomized Controlled Trial. Gastroenterology. 2015;149(1) doi: 10.1053/j.gastro.2015.04.001. 102-9.e6. [DOI] [PubMed] [Google Scholar]
- 9.Costello SP. Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA: Journal of the American Medical Association.321(2):156-65. [DOI] [PMC free article] [PubMed]
- 10.Rossen NG, Fuentes S, van der Spek MJ, et al. Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology. 2015;149(1) doi: 10.1053/j.gastro.2015.03.045. 110-8.e4. [DOI] [PubMed] [Google Scholar]
- 11.Craig Haifer 1 SP, Kaakoush 3 Nadeem O, Saikal 4 Aiasha, Ghaly 4 Simon, Yang 5 Tao, Wai Luu 3 Laurence Don, Borody 6 Thomas J, Leong 7 Rupert W. Lancet Gastroenterol Hepatol; 2021. Lyophilised oral faecal microbiota transplantation for ulcerative colitis (LOTUS): a randomised, double-blind, placebo-controlled trial. [DOI] [PubMed] [Google Scholar]
- 12.Pai N, Popov J, Hill L, et al. Results of the First Pilot Randomized Controlled Trial of Fecal Microbiota Transplant In Pediatric Ulcerative Colitis: Lessons, Limitations, and Future Prospects. Gastroenterology. 2021;161(2):388-93 e3. doi: 10.1053/j.gastro.2021.04.067. [DOI] [PubMed] [Google Scholar]
- 13.Crothers JW, Chu ND, Nguyen LTT, et al. Daily, oral FMT for long-term maintenance therapy in ulcerative colitis: results of a single-center, prospective, randomized pilot study. BMC Gastroenterol. 2021;21(1):281. doi: 10.1186/s12876-021-01856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sood A, Mahajan R, Singh A, et al. Role of Faecal Microbiota Transplantation for Maintenance of Remission in Patients With Ulcerative Colitis: A Pilot Study. J Crohns Colitis. 2019;13(10):1311–1317. doi: 10.1093/ecco-jcc/jjz060. [DOI] [PubMed] [Google Scholar]
- 15.Olesen SW, Gerardin Y. Re-Evaluating the Evidence for Faecal Microbiota Transplantation 'Super-Donors' in Inflammatory Bowel Disease. J Crohns Colitis. 2021;15(3):453–461. doi: 10.1093/ecco-jcc/jjaa170. [DOI] [PubMed] [Google Scholar]
- 16.Yalchin M, Segal JP, Mullish BH, et al. Gaps in knowledge and future directions for the use of faecal microbiota transplant in the treatment of inflammatory bowel disease. Therap Adv Gastroenterol. 2019;12 doi: 10.1177/1756284819891038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarbagili Shabat C, Scaldaferri F, Zittan E, et al. Use of Fecal transplantation with a novel diet for mild to moderate active ulcerative colitis: The CRAFT UC randomized controlled trial. J Crohns Colitis. 2021 doi: 10.1093/ecco-jcc/jjab165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brezina J, Bajer L, Wohl P, et al. Fecal Microbial Transplantation versus Mesalamine Enema for Treatment of Active Left-Sided Ulcerative Colitis-Results of a Randomized Controlled Trial. J Clin Med. 2021;10(13) doi: 10.3390/jcm10132753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian Y. Fecal microbiota transplantation for ulcerative colitis: a prospective clinical study. BMC Gastroenterology.19(1). [DOI] [PMC free article] [PubMed]
- 22.Li Q, Ding X, Liu K, et al. Fecal Microbiota Transplantation for Ulcerative Colitis: The Optimum Timing and Gut Microbiota as Predictors for Long-Term Clinical Outcomes. Clin Transl Gastroenterol. 2020;11(8) doi: 10.14309/ctg.0000000000000224. e00224-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kump P, Wurm P, Gröchenig HP, et al. The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis. Aliment Pharmacol Ther. 2018;47(1):67–77. doi: 10.1111/apt.14387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacob V, Crawford C, Cohen-Mekelburg S, et al. Single Delivery of High-Diversity Fecal Microbiota Preparation by Colonoscopy Is Safe and Effective in Increasing Microbial Diversity in Active Ulcerative Colitis. Inflamm Bowel Dis. 2017;23(6):903–911. doi: 10.1097/MIB.0000000000001132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang H, Fu L, Li X, et al. Long-term efficacy and safety of monotherapy with a single fresh fecal microbiota transplant for recurrent active ulcerative colitis: a prospective randomized pilot study. Microb Cell Fact. 2021;20(1):18-. doi: 10.1186/s12934-021-01513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui B, Li P, Xu L, et al. Step-up fecal microbiota transplantation strategy: a pilot study for steroid-dependent ulcerative colitis. J Transl Med. 2015;13:298. doi: 10.1186/s12967-015-0646-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen HT, Huang HL, Xu HM, et al. Fecal microbiota transplantation ameliorates active ulcerative colitis. Exp Ther Med. 2020;19(4):2650–2660. doi: 10.3892/etm.2020.8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sood A, Singh A, Mahajan R, et al. Clinical Predictors of response to Faecal Microbiota Transplantation in patients with active ulcerative colitis. J Crohns Colitis. 2020 doi: 10.1093/ecco-jcc/jjaa163. [DOI] [PubMed] [Google Scholar]
- 29.Okahara K, Ishikawa D, Nomura K, et al. Matching between Donors and Ulcerative Colitis Patients Is Important for Long-Term Maintenance after Fecal Microbiota Transplantation. J Clin Med. 2020;9(6) doi: 10.3390/jcm9061650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao D, Ye C, Zhang S, Lv X, Yang B. Analysis of risk factors for early clinical recurrence of inflammatory bowel disease after fecal microbiota transplantation. Am J Transl Res. 2021;13(11):12875–12886. [PMC free article] [PubMed] [Google Scholar]
- 31.Fuentes S, Rossen NG, van der Spek MJ, et al. Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. ISME J. 2017;11(8):1877–1889. doi: 10.1038/ismej.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leonardi I, Paramsothy S, Doron I, et al. Fungal Trans-kingdom Dynamics Linked to Responsiveness to Fecal Microbiota Transplantation (FMT) Therapy in Ulcerative Colitis. Cell Host Microbe. 2020;27(5) doi: 10.1016/j.chom.2020.03.006. 823-9 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paramsothy S, Nielsen S, Kamm MA, et al. Specific Bacteria and Metabolites Associated With Response to Fecal Microbiota Transplantation in Patients With Ulcerative Colitis. Gastroenterology. 2019;156(5) doi: 10.1053/j.gastro.2018.12.001. 1440-54.e2. [DOI] [PubMed] [Google Scholar]
- 34.Goyal A, Yeh A, Bush BR, et al. Safety, Clinical Response, and Microbiome Findings Following Fecal Microbiota Transplant in Children With Inflammatory Bowel Disease. Inflamm Bowel Dis. 2018;24(2):410–421. doi: 10.1093/ibd/izx035. [DOI] [PubMed] [Google Scholar]
- 35.Schierová D, Březina J, Mrázek J, et al. Gut Microbiome Changes in Patients with Active Left-Sided Ulcerative Colitis after Fecal Microbiome Transplantation and Topical 5-aminosalicylic Acid Therapy. Cells. 2020;9(10):1. doi: 10.3390/cells9102283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishida A, Imaeda H, Ohno M, et al. Efficacy and safety of single fecal microbiota transplantation for Japanese patients with mild to moderately active ulcerative colitis. J Gastroenterol. 2017;52(4):476–482. doi: 10.1007/s00535-016-1271-4. [DOI] [PubMed] [Google Scholar]
- 37.Sood A, Mahajan R, Juyal G, et al. Efficacy of fecal microbiota therapy in steroid dependent ulcerative colitis: a real world intention-to-treat analysis. Intest Res. 2019;17(1):78–86. doi: 10.5217/ir.2018.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uygun A, Ozturk K, Demirci H, et al. Fecal microbiota transplantation is a rescue treatment modality for refractory ulcerative colitis. Medicine (Baltimore) 2017;96(16):e6479. doi: 10.1097/MD.0000000000006479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gogokhia L, Buhrke K, Bell R, et al. Expansion of Bacteriophages Is Linked to Aggravated Intestinal Inflammation and Colitis. Cell Host Microbe. 2019;25(2):285-99 e8. doi: 10.1016/j.chom.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang SL, Wang SN, Miao CY. Influence of Microbiota on Intestinal Immune System in Ulcerative Colitis and Its Intervention. Front Immunol. 2017;8:1674. doi: 10.3389/fimmu.2017.01674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quraishi MN, Shaheen W, Oo YH, Iqbal TH. Immunological mechanisms underpinning faecal microbiota transplantation for the treatment of inflammatory bowel disease. Clin Exp Immunol. 2020;199(1):24–38. doi: 10.1111/cei.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao C, Dong H, Zhang Y, Li Y. Discovery of potential genes contributing to the biosynthesis of short-chain fatty acids and lactate in gut microbiota from systematic investigation in E. coli. NPJ Biofilms Microbiomes. 2019;5(1):19. doi: 10.1038/s41522-019-0092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294(1):1–8. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- 44.Pandiyan P, Bhaskaran N, Zou M, Schneider E, Jayaraman S, Huehn J. Microbiome Dependent Regulation of Tregs and Th17 Cells in Mucosa. Front Immunol. 2019;10:426. doi: 10.3389/fimmu.2019.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nusbaum DJ, Sun F, Ren J, et al. Gut microbial and metabolomic profiles after fecal microbiota transplantation in pediatric ulcerative colitis patients. FEMS Microbiol Ecol. 2018;94(9) doi: 10.1093/femsec/fiy133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Constante M, Fragoso G, Calve A, Samba-Mondonga M, Santos MM. Dietary Heme Induces Gut Dysbiosis, Aggravates Colitis, and Potentiates the Development of Adenomas in Mice. Front Microbiol. 2017;8:1809. doi: 10.3389/fmicb.2017.01809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vermeire S, Joossens M, Verbeke K, et al. Donor Species Richness Determines Faecal Microbiota Transplantation Success in Inflammatory Bowel Disease. J Crohns Colitis. 2016;10(4):387–394. doi: 10.1093/ecco-jcc/jjv203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uebanso T, Shimohata T, Mawatari K, Takahashi A. Functional Roles of B-Vitamins in the Gut and Gut Microbiome. Mol Nutr Food Res. 2020;64(18) doi: 10.1002/mnfr.202000426. [DOI] [PubMed] [Google Scholar]
- 49.Yoshii K, Hosomi K, Sawane K, Kunisawa J. Metabolism of Dietary and Microbial Vitamin B Family in the Regulation of Host Immunity. Front Nutr. 2019;6:48. doi: 10.3389/fnut.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson BC, Vatanen T, Cutfield WS, O'Sullivan JM. The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front Cell Infect Microbiol. 2019;9:2. doi: 10.3389/fcimb.2019.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng SC, Kamm MA, Yeoh YK, et al. Scientific frontiers in faecal microbiota transplantation: joint document of Asia-Pacific Association of Gastroenterology (APAGE) and Asia-Pacific Society for Digestive Endoscopy (APSDE) Gut. 2020;69(1):83–91. doi: 10.1136/gutjnl-2019-319407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Svolos V, Hansen R, Nichols B, et al. Treatment of Active Crohn's Disease With an Ordinary Food-based Diet That Replicates Exclusive Enteral Nutrition. Gastroenterology. 2019;156(5):1354-67 e6. doi: 10.1053/j.gastro.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Merra G, Noce A, Marrone G, et al. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients. 2020;13(1) doi: 10.3390/nu13010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.