Abstract
β-Cell failure and loss of β-cell mass are key events in diabetes progression. Although insulin hypersecretion in early stages has been implicated in β-cell exhaustion/failure, loss of β-cell mass still occurs in KATP gain-of-function (GOF) mouse models of human neonatal diabetes in the absence of insulin secretion. Thus, we hypothesize that hyperglycemia-induced increased β-cell metabolism is responsible for β-cell failure and that reducing glucose metabolism will prevent loss of β-cell mass. To test this, KATP-GOF mice were crossed with mice carrying β-cell–specific glucokinase haploinsufficiency (GCK+/−), to genetically reduce glucose metabolism. As expected, both KATP-GOF and KATP-GOF/GCK+/− mice showed lack of glucose-stimulated insulin secretion. However, KATP-GOF/GCK+/− mice demonstrated markedly reduced blood glucose, delayed diabetes progression, and improved glucose tolerance compared with KATP-GOF mice. In addition, decreased plasma insulin and content, increased proinsulin, and augmented plasma glucagon observed in KATP-GOF mice were normalized to control levels in KATP-GOF/GCK+/− mice. Strikingly, KATP-GOF/GCK+/− mice demonstrated preserved β-cell mass and identity compared with the marked decrease in β-cell identity and increased dedifferentiation observed in KATP-GOF mice. Moreover KATP-GOF/GCK+/− mice demonstrated restoration of body weight and liver and brown/white adipose tissue mass and function and normalization of physical activity and metabolic efficiency compared with KATP-GOF mice. These results demonstrate that decreasing β-cell glucose signaling can prevent glucotoxicity-induced loss of insulin content and β-cell failure independently of compensatory insulin hypersecretion and β-cell exhaustion.
Introduction
Failure of pancreatic β-cell function and loss of β-cell mass are key events in the development and progression of multiple forms of diabetes. In type 2 diabetes, an initial increase of insulin secretion followed by loss of β-cell function are early events, while substantial loss of β-cell mass occurs closer to the clinical manifestation (1). In pancreatic β-cells, ATP-sensitive potassium (KATP) channels play a critical role in linking glucose metabolism to insulin secretion. Accordingly, KATP gain-of-function (GOF) mutations cause human neonatal diabetes (2,3), and KATP-GOF polymorphisms are associated with development of type-2 diabetes (4). Recapitulating the features of human neonatal diabetes, transgenic expression of KATP-GOF mutations in mouse β-cells causes rapid and severe diabetes as a result of suppression of insulin secretion (5–7). As diabetes progresses, islets from KATP-GOF mice show marked loss of both β-cell mass and identity (8). Decreased β-cell mass and identity has also been demonstrated in multiple animal models of monogenic, type 1, and type 2 diabetes (9) and in pancreata from individuals with diabetes (10–13), suggesting potentially common mechanisms in different forms of diabetes independent of the underlying diabetes etiology.
Glucose phosphorylation by glucokinase (GCK) is normally a rate-limiting step in glucose metabolism and a central regulator of glucose-stimulated insulin secretion (GSIS) (14), and absence of GCK is expected to reduce glucose flux in β-cells and as a consequence, decrease insulin secretion and increase blood glucose levels (15). Sustained catalytic activation of β-cell GCK has been proposed as a potential cause of the compensatory increase in glucose sensitivity in early stages of the type 2 diabetes (16,17), but the same overstimulation of β-cell metabolism is also proposed as a culprit in subsequent “exhaustion” and loss of β-cell mass (18–20). We previously demonstrated increased glucose metabolism, as reflected in increased NAD(P)H autofluorescence and mitochondrial depolarization, in islets from KATP-GOF diabetic mice (21). We hypothesize that chronic hyperglycemia increases β-cell metabolic activity, leading to glucotoxicity-induced β-cell failure, potentially in multiple forms of diabetes, and therefore could be prevented by reducing β-cell glucose metabolism. To test this hypothesis in vivo, we examined the effects of reduced GCK activity on the development of diabetes in KATP-GOF–induced neonatal diabetic mice crossed with β-cell–specific GCK haploinsufficient mice (GCK+/−). We show that double-mutant KATP-GOF/GCK+/− mice, with deceleration of β-cell glucose metabolism, exhibit markedly slower development of systemic diabetes, with preservation of insulin content and β-cell mass and identity, as well as improved insulin sensitivity and adipose tissue mass and function, compared with KATP-GOF mice with wild-type GCK. This improvement of glucose homeostasis in neonatal diabetic mice by reduction of hypermetabolism-induced β-cell glucotoxicity raises the possibility that such paradoxical protection may be found in other forms of diabetic glucotoxicity.
Research Design and Methods
Animals
All animal studies were performed in accordance with protocols approved by the institutional animal care and use committee of Washington University. Mice were maintained on a regular chow ad libitum diet and housed in a 12 h light/dark cycle. β-Cell–specific heterozygous GCK knockout (GCK+/−) mice (22,23) were crossed with β-cell–specific tamoxifen-inducible KATP-GOF (Rosa-kir6.2[K185Q,ΔN30] × tamoxifen-inducible Pdx1PBCreERTM) mice (6) to generate double-transgenic KATP-GOF/GCK+/− mice. Ten- to 12-week-old KATP-GOF or KATP-GOF/GCK+/− mice were injected with five consecutive daily doses of tamoxifen (50 μg/g body weight) to induce KATP-GOF expression (6). Littermate wild-type, Rosa-kir6.2[K185Q,ΔN30], or Pdx1PBCreERTM mice were used as controls since no significant differences were found among genotypes (6,7) and among GCK-deficient GCK+/−, Pdx1PBCreERTM/GCK+/−, or [K185Q,ΔN30]/GCK+/− mice. All controls and GCK+/− mice were injected with the same daily dose of tamoxifen. Approximately equal number of males and females were used for all experiments; males and females were averaged together since no significant sex differences were found in any of the genotypes tested.
Blood Glucose and Plasma Hormone Measurements
Nonfasted and fasted blood glucose were measured by using a Bayer Contour T5 glucometer (Mishiwaka, IN). Whole blood was collected in heparinized tubes with protease inhibitor cocktail (3.6 mg/mL benzamidine hydrochloride, 1 mg/mL aprotinin, 1 mmol/L sitagliptin, and 25 mmol/L EDTA). Plasma insulin was assayed using rat/mouse ELISA kit (Crystal Chem, Elk Grove Village, IL). Plasma hormones (insulin, glucagon, glucagon-like peptide 1 [GLP-1], and leptin) were measured using the Luminex MILLIPLEX Mouse Metabolic Hormone panel at the Immunomonitoring Laboratory, Bursky Center for Human Immunology and Immunotherapy Programs Assay Core (https://chiips.wustl.edu) (24). After a 4 h fast, blood glucose was collected for triglyceride, cholesterol, and free fatty acid (FFA) measurement at the Washington University Diabetes Models Phenotyping Core (https://diabetesresearchcenter.dom.wustl.edu/diabetes-models- phenotyping-core) (25).
Glucose Tolerance Tests and Insulin Tolerance Tests
Glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs) were performed after overnight and 5-h fast, respectively. Blood glucose was measured before (0 min) and after (15, 30, 45, 60, 90, and 120 min) intraperitoneal injection of 1.5 mg/kg dextrose (GTT) or 0.5 units/kg human insulin (ITT) (HI-210; Eli Lilly). Blood was also collected at 0 and 30 min after glucose challenge (GTT) for measurement of insulin secretion as described above. Insulinogenic index (II) at 30 min after glucose challenge was calculated as follows (26): II30min = 0.0077 × (insulin30min − insulin0min [pmol/L]) / (glucose30min − glucose0min [mmol/L]).
Islet Isolation and Assessment of Insulin Secretion
For pancreatic islet isolation, ice-cold 0.45 mg/mL collagenase type V buffer (Sigma-Aldrich, St. Louis, MO) (in Hanks’ balanced salt solution without Ca2+) was perfused into the bile duct. The pancreas was immediately dissected out and incubated at 37°C for 10–11 min in a water bath for digestion, and islets were washed twice in Hanks’ balanced salt solution and then handpicked under a stereo microscope. Islets were incubated overnight in RPMI complete media supplemented with 10% FCS, 100 units/mL penicillin, and 100 μg/mL streptomycin (Thermo Fisher Scientific). For GSIS, islets were preincubated in Krebs-Ringer buffer solution (bicarbonate HEPES) containing 2% BSA and 2.8 mmol/L glucose for 1 h. Islets were then separated into groups of 10 and incubated with either 2 or 20 mmol/L glucose for 1 h, and media were collected for GSIS. Total insulin content was extracted from 10 islets in 0.2 N/80% acid ethanol. Insulin released into the medium and total insulin content were measured using a rat/mouse insulin ELISA kit (Crystal Chem) (25). Total proinsulin content was measured using a rat/mouse proinsulin ELISA kit (Mercodia).
Body Fat and Lean Mass Analysis Using MRI
For measurement of percent body fat and lean mass in awake animals, an EchoMRI instrument (Echo Medical Systems, Houston, TX) at the Washington University Diabetes Phenotyping Core was used, as previously described (25).
Metabolic Measurements
Comprehensive metabolic, behavioral, and physiological variables were determined by using TSE PhenoMaster at the Washington University Diabetes Models Phenotyping Core, as previously described (25). The respiratory exchange rate (RER) was calculated as the ratio between the amount of CO2 produced in metabolism and the O2 used. The metabolic efficiency was calculated as the ratio between body weight and food consumption.
Hematoxylin-Eosin Staining and Immunohistochemistry
Mice were sacrificed 50 days after the first dose of tamoxifen. Pancreata, epididymal white adipose tissue (WAT), interscapular brown adipose tissue (BAT), and liver from all groups were fixed in 10% neutral buffered formalin and paraffin embedded for sectioning. Serial sections of 5 μm and hematoxylin-eosin (H-E) staining were performed at the Anatomic/Molecular Pathology Core, Washington University. Immunohistochemistry was performed, as previously described (8), by staining with rabbit anti-insulin (1:100; Cell Signaling Technology), mouse antiglucagon (1:100; Cell Signaling Technology), and aldehyde dehydrogenase 1A3 (Aldh1A3) (5 μg/mL; Abcam) antibodies; distribution was visualized using goat antibody conjugated with Alexa Fluor 488 or Alexa Fluor 594 (Molecular Probes, Eugene, OR) with an EXC-500 fluorescence microscope (Visual Dynamix, Chesterfield, MO).
Measurement of β-Cell Mass and Dedifferentiation
Four to five mice from each genotype were sampled on 5-μm-thick sections 25 μm apart (spanning the whole pancreas) and used for immunohistochemical/β-cell mass and immunohistological/dedifferentiation analysis. At least three pancreatic sections from three to five mice of each genotype were covered systematically by accumulating images from nonoverlapping fields on an inverted EXC-500 fluorescent microscope. For β-cell mass, insulin-positive area was measured using ImageJ (RRID:SCR 003070) and calculated as the product of relative β-cell area to whole pancreatic area and pancreatic weight for each mouse. Dedifferentiation was calculated as the number of ALDH1A3+ cells per islet using ImageJ as previously described (11).
Quantitative PCR Analysis
Islet RNA extraction and quantitative real-time PCR analysis were performed as previously described (27). Primers for β-cell identity markers Ins1, NKX6.1, and Pdx1 were previously described (8). Assays for uncoupling protein 1 (UCP-1), peroxisomal proliferator–activated receptor γ (PPARγ), and PPARα genes in BAT; adiponectin, CD36, PPARγ, and leptin in WAT (28); and PPARα/γ, carnitine palmitoyltransferase 1 α (CPT1α), fatty acid synthase (FAS), adipose triglyceride lipase (AGTL), and hormone-sensitive lipase (HSL) in the liver (29) were performed in duplicate and normalized to ribosomal protein mL32 mRNA.
Western Blot Analysis
Fifteen micrograms of total protein lysate were loaded per lane. Blots were incubated overnight with the following antibodies: β-actin (1:1,000; EMD Millipore, St. Louis, MO), thioredoxin-interacting protein (TXNIP) (1:1,000; MBL International, Woburn, MA), and sXBP1 (1:1,000; Santa Cruz Biotechnology, Dallas, TX). Blots were washed and probed with RDye infrared fluorescent dye-labeled red or green secondary antibody conjugates (1:10,000; LI-COR Biosciences). Fluorescence intensity was quantified by Image Studio Lite (LI-COR Biosciences).
Measurement of Liver Triglyceride Content
Total triglycerides were determined by homogenizing 20–30 mg fresh weight liver tissue in 1.5 mL of chloroform:methanol (2:1 v/v). Samples were centrifuged at 12,000 rpm for 10 min at 4°C. An aliquot of 20 μL was evaporated, and triglyceride content was determined by adding 100 μL of reagent after 30 min incubation at room temperature. Measurement of liver triglyceride content was performed at the Washington University Diabetes Models Phenotyping Core.
Statistical Methods
Data are expressed as mean ± SEM. Statistical differences between two groups were determined using Student t test and among several groups using ANOVA and post hoc Turkey test for multiple comparisons. Statistical analyses were performed using GraphPad Prism version 8.0 software (GraphPad Software, La Jolla, CA). The threshold of significance was set at P < 0.05. Nonsignificant differences are not shown. For Fig. 1, we used lowercase letters in some panels to indicate significance differences of P < 0.05 between groups, with the same letter indicating no significant difference.
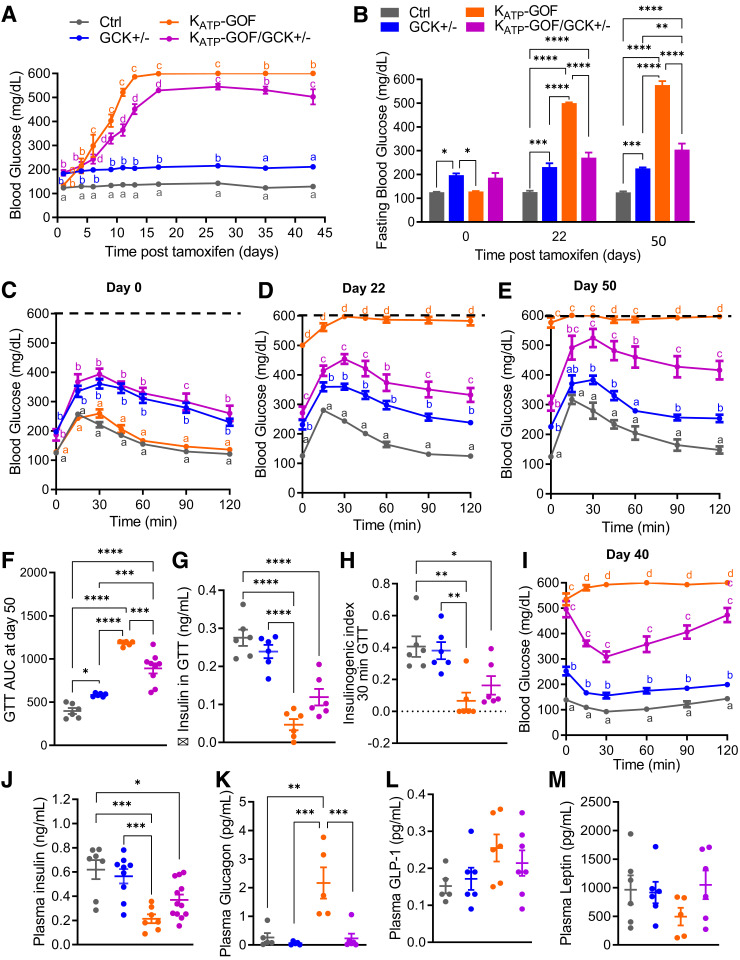
Figure 1.
Blood glucose is reduced in KATP-GOF diabetic mice with reduced β-cell glucokinase. A: Nonfasted blood glucose over time in KATP-GOF mice after tamoxifen induction of transgene expression (n = 9–18 mice/group). B: Fasting blood glucose levels at day 0, 22, and 50 after tamoxifen induction (n = 6–9 mice/group). C–F: GTT performed at day 0 and at days 22 (n = 4–9 mice/group) and 50 after tamoxifen induction (dashed lines indicate the limit of the detection of the glucose meter) and area under the curve (AUC) (n = 6–9 mice/group). G and H: Change (Δ) in insulin secretion at 30 min after glucose challenge over fasting (0 min) and II during GTT at day 50 after tamoxifen induction (n = 6 mice/group). I: ITT performed in mice 40 days after tamoxifen induction. J–M: Plasma insulin (n = 8–12 mice/group), glucagon (n = 5 mice/group), GLP-1, and leptin (n = 5–7 mice/group) at day 40 after tamoxifen induction. Data are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Nonsignificant differences are not shown. For panels A, C, D, E, and H, lowercase letters are used to indicate significant differences of P < 0.05 between groups. The same letters indicate no significant difference. Ctrl, control.
Data and Resource Availability
The data sets generated and/or analyzed during the current study are available in the figshare repository (https://figshare.com/s/1ec45fbad2f1af649e9b).
Results
Delayed Diabetes Progression and Improved Glucose Tolerance in KATP-GOF Mice Carrying GCK Haploinsufficiency in β-Cells
The β-cell–specific GCK haploinsufficient mouse (GCK+/−) models maturity-onset diabetes of the young 2, a form of monogenic diabetes caused by heterozygous inactivating mutations in the GCK gene (22). As a result of decreased β-cell glucose metabolism, individuals with maturity-onset diabetes of the young 2 and GCK+/− mice show mild, nonprogressive hyperglycemia and do not normally require antidiabetic therapy. As predicted (22,23), both GCK+/− and noninduced KATP-GOF/GCK+/− mice demonstrated mildly elevated fed and fasting blood glucose levels (Fig. 1A and B) and slightly impaired glucose tolerance (Fig. 1C), while wild-type and noninduced KATP-GOF mice showed normal blood glucose (Fig. 1A and B) and glucose tolerance (Fig. 1C). After tamoxifen induction of the KATP-GOF transgene, both KATP-GOF and KATP-GOF/GCK+/− mice became progressively diabetic because of KATP overactivity–induced suppression of GSIS, but the progression is strikingly slowed in the latter (Fig. 1A and B). At day 22 after tamoxifen induction, KATP-GOF mice showed remarkably high fasting blood glucose and severely impaired glucose tolerance, but KATP-GOF/GCK+/− mice were only minimally worse than before induction (Fig. 1D). Improved glucose tolerance persisted, with a significantly lower area under the curve (Fig. 1F), in KATP-GOF/GCK+/− compared with KATP-GOF mice at day 50 (Fig. 1E). Lack of increase in blood glucose in severely diabetic KATP-GOF mice after glucose challenge might be explained by the limit of detection of the glucose meter (values >600 mg/dL considered maximum as denoted by the dashed lines in Fig. 1C–E); therefore, glucose tolerance in KATP-GOF mice may be underestimated. Change in plasma insulin (insulin30 min – insulin0 min during GTT) and II were higher in KATP-GOF/GCK+/− mice than in KATP-GOF mice but did not reach significance (Fig. 1G and H). At day 40 after tamoxifen induction, random plasma insulin levels were also significantly reduced in both KATP-GOF and KATP-GOF/GCK+/− mice compared with controls and not significantly different between them (Fig. 1J), reflecting a markedly better insulin sensitivity in KATP-GOF/GCK+/− mice (Fig. 1I). Plasma glucagon, which was significantly increased in KATP-GOF mice, dropped to normal levels in KATP-GOF/GCK+/− mice (Fig. 1K), and although GLP-1 levels did not change (Fig. 1L), leptin levels increased (Fig. 1M) in KATP-GOF/GCK+/− mice with respect to KATP-GOF mice.
Improved Insulin Content and Reduced Proinsulin and Proinsulin-to-Insulin Ratio in KATP-GOF/GCK+/− Mice
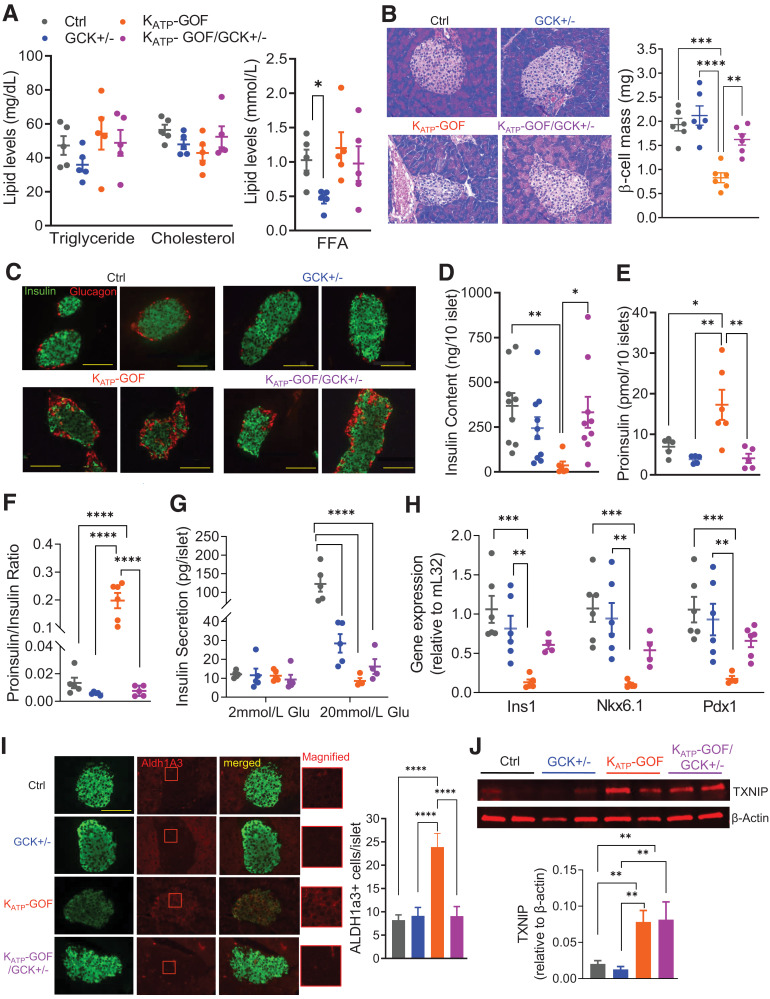
Plasma lipids, such as triglycerides, cholesterol, and FFAs, were essentially normal in all groups, except for lower FFA levels that were detected in GCK+/− mice (Fig. 2A). H-E–stained pancreatic sections revealed disruption of islet architecture only in KATP-GOF mice (Fig. 2B), and immunostaining indicated more insulin and less glucagon in KATP-GOF/GCK+/− mice compared with KATP-GOF, which showed a marked decrease in insulin, and infiltration of α-cells into the core of the islet (Fig. 2C). These results correlated with the increased plasma glucagon and decreased insulin observed in KATP-GOF mice but were normal in KATP-GOF/GCK+/− mice (Fig. 1I and J). Decreased insulin content in KATP-GOF islets (Fig. 2D) was accompanied by increased proinsulin content and proinsulin-to-insulin ratio (Fig. 2E and F). Both were normalized in KATP-GOF/GCK+/− islets (Fig. 2D–F), but as predicted, GSIS was reduced in GCK+/−, and markedly so in KATP-GOF and KATP-GOF/GCK+/− islets, with respect to control islets (Fig. 2G).
Figure 2.
Plasma hormones and lipid levels and cellular stress markers. A: Plasma triglyceride, cholesterol (left), and FFAs (right) at day 45 after tamoxifen induction (n = 5 mice/group). B and C: Representative images for H-E staining (left) and β-cell mass (right), and insulin (green) and glucagon (red) staining in pancreata at day 45 (scale bars 100 µm, n = 5 mice/group). D: Total insulin content in islets at day 45 (n = 6–10 mice/group). E and F: Total proinsulin content and proinsulin-to-insulin ratio in islets at day 45 (n = 5–6 mice/group). G: GSIS at basal (2 mmol/L) and high (20 mmol/L) glucose in islets at day 45 (n = 5 mice/group). H: Quantitative real-time PCR analysis of Ins1, NKX6.1, and Pdx1 for islets (n = 4–6 mice/group). I: Representative images for insulin (green), ALDH1A3 (red) (magnified on right), and merged staining on pancreatic sections from all genotype mice, with quantification of ALDH1A31 cells per islet (scale bar 100 µm, magnified box is 30x30 µm; n = 5 mice/group at day 45). J: Representative Western blot and quantitative analysis for islets for TXNIP at day 40 (n = 6–8 mice/group). Data are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Nonsignificant differences are not shown. Ctrl, control.
Islet β-Cell Identity Is Preserved in KATP-GOF/GCK+/− Mice
While Ins1, NKX6.1, and Pdx1 markers of mature β-cell identity were all significantly decreased in KATP-GOF islets compared with control and GCK+/− islets, they were partially restored and not significantly different from controls in KATP-GOF/GCK+/− islets (Fig. 2H). Since the progenitor cell marker ALDH1A3 is enriched in dedifferentiated islet endocrine cells from mice and humans with diabetes (11,30), we measured this in islets from the four genotypes. While KATP-GOF islets showed a significant increase in ALDH1A3+ cells (Fig. 2I), KATP-GOF/GCK+/− islets demonstrated a similar number of dedifferentiated cells to control and GCK+/− islets (Fig. 2I). TXNIP, which induces β-cell stress by inhibiting thioredoxin, was significantly increased in both KATP-GOF and KATP-GOF/GCK+/− islets, suggesting that the markedly high glucose in both models still causes oxidative stress and not mitigated by GCK+/− haploinsufficiency (Fig. 2J).
KATP-GOF/GCK+/− Mice Showed Restored Metabolic Efficiency and Fat Mass
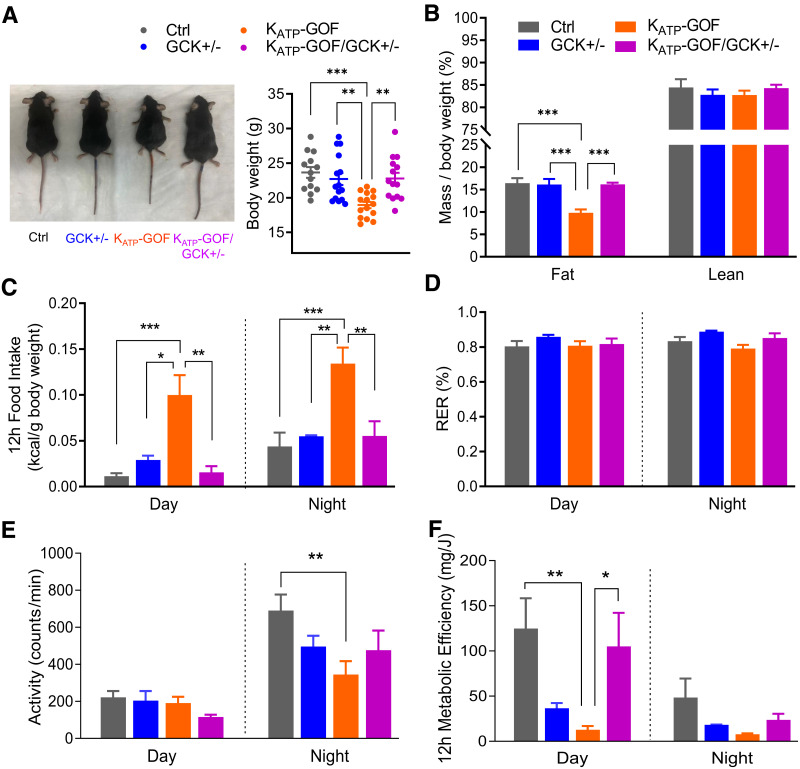
While KATP-GOF mice demonstrated a significant reduction in body weight by day 40 after tamoxifen induction (6), KATP-GOF/GCK+/− mice maintained similar weight to control and GCK+/− mice (Fig. 3A). MRI assessments of body composition showed no differences in lean mass among the four groups but a significant reduction in fat mass only in KATP-GOF mice (Fig. 3B). Metabolic cage measurements demonstrated a significant increase in food intake in KATP-GOF mice but normal food intake in KATP-GOF/GCK+/− (Fig. 3C). The RER was not significantly different among the four groups (Fig. 3D). Both physical activity and metabolic efficiency were reduced in KATP-GOF mice, but both were normalized in KATP-GOF/GCK+/− mice (Fig. 3E and F).
Figure 3.
Metabolic phenotyping is improved in KATP-GOF mice with reduced GCK. A: Representative images of mice and quantification of body weight at day 40 after tamoxifen induction (n = 13–15 mice/group). B: Fat and lean mass over body weight (n = 5–9 mice/group). C–F: Metabolic analysis of food intake in 12 h, RER, physical activity/movement, and metabolic efficiency in 12 h (n = 3–5 mice/group). Data are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001. Nonsignificant differences are not shown. Ctrl, control.
WAT and BAT Loss in KATP-GOF Mice Is Restored in KATP-GOF/GCK+/− Mice
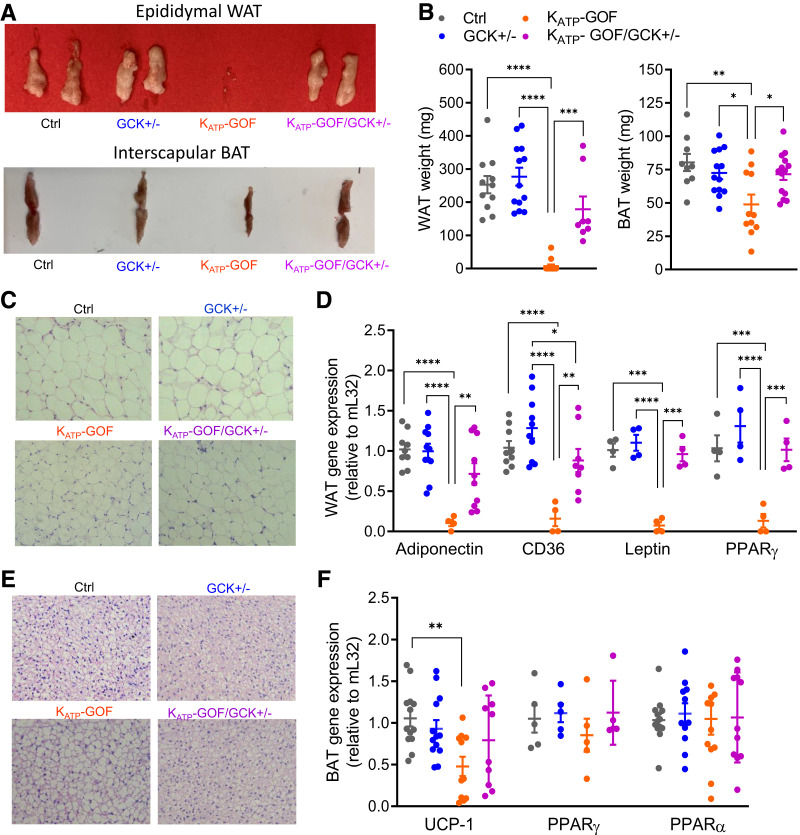
Correlating with fat mass (Fig. 3A), both epididymal WAT and interscapular BAT weights were reduced in KATP-GOF mice but were normal in KATP-GOF/GCK+/− mice (Fig. 4A and B). H-E–stained WAT sections showed a slightly reduced adipocyte size and disrupted architecture in KATP-GOF but a normal size in KATP-GOF/GCK+/− mice (Fig. 4C). BAT staining demonstrated a heterogeneous mixture of large adipocytes containing large vacuoles and small adipocytes in KATP-GOF mice but normal adipocyte size in KATP-GOF/GCK+/− mice (Fig. 4E). Adiponectin, CD36, leptin, and PPARγ were all markedly reduced in KATP-GOF WAT but restored to normal levels in KATP-GOF/GCK+/− (Fig. 4D). Because of a lack of insulin and loss of BAT mass in KATP-GOF mice, UCP-1 would be predicted to increase to maintain thermal homeostasis. However, UCP-1 message was decreased in these mice (Fig. 4F) and normalized in KATP-GOF/GCK+/− mice. PPARγ and PPARα messages were not significantly different between groups (Fig. 4F).
Figure 4.
Adipose tissue mass and function are improved in diabetic mice with reduced β-cell GCK. A: Representative images of epididymal/gonadal WAT and interscapular BAT. B: Quantification epididymal WAT and interscapular BAT (n = 8–15 mice/group). C and E: Representative images of paraffin sections of H-E–stained WAT and BAT. D and F: Quantitative real-time PCR analysis of adiponectin, CD36, leptin, and PPARγ for WAT or UCP-1, PPARγ, and PPARα for BAT (n = 4–13 mice/group). Data are mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Nonsignificant differences are not shown. Ctrl, control.
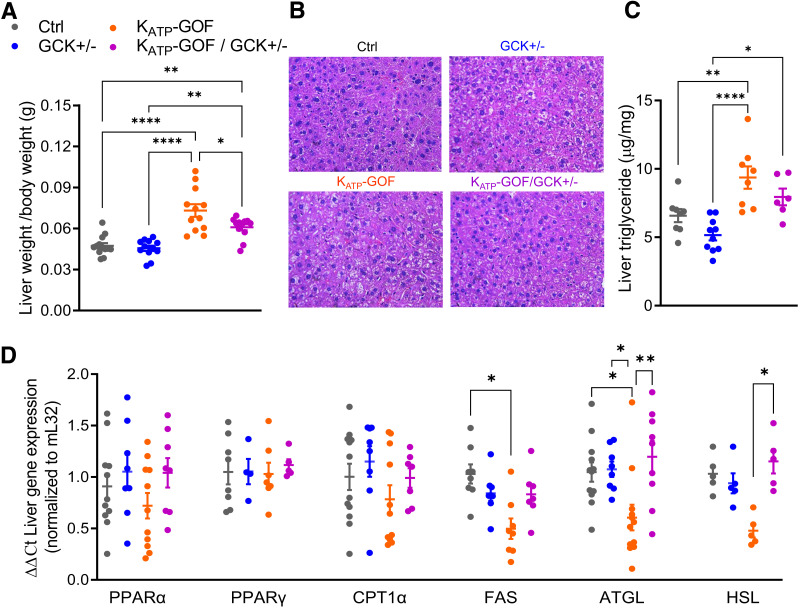
Liver Architecture and Function Are Improved in KATP-GOF/GCK+/− Mice
Both KATP-GOF and KATP-GOF/GCK+/− mice showed significantly increased liver weight with respect to control and GCK+/− mice, and KATP-GOF livers were heavier than KATP-GOF/GCK+/− livers (Fig. 5A). H-E staining demonstrated altered liver architecture with hepatocytes containing large lipid droplets only in KATP-GOF mice (Fig. 5B), with similar liver architecture and hepatocyte size in KATP-GOF/GCK+/− relative to those in control and GCK+/− mice (Fig. 5B). While triglyceride content significantly increased in livers of KATP-GOF mice, it was reduced in livers of KATP-GOF/GCK+/− mice (Fig. 5C). There were no changes in PPARα, PPARγ, and CPT1α between groups (Fig. 5D). FAS, ATGL, and HSL were all reduced only in livers of KATP-GOF mice (Fig. 5D) but normal in KATP-GOF/GCK+/− mice, suggesting impaired lipolysis in KATP-GOF but improved in KATP-GOF/GCK+/− with GCK+/− haploinsufficiency in β-cells.
Figure 5.
Liver function is improved in diabetic mice with reduced β-cell GCK. A: Liver weight/body weight (g) at day 45 after tamoxifen induction (n = 12 mice/group). B: Representative images of liver paraffin sections stained with H-E at day 45 after tamoxifen induction. C: Liver triglyceride content (n = 8–10 mice/group). D: Quantitative real-time PCR analysis for genes involved in β-oxidation (PPARα, PPARγ, and CPT1α), lipogenesis (FAS), and lipolysis (ATGL and HSL) (n = 4–12 mice/group). Data are mean ± SEM. *P < 0.05, **P < 0.01, ****P < 0.0001. Nonsignificant differences are not shown. Ctrl, control.
Discussion
Decreased Glucose-Metabolism as a β-Cell–Protective Mechanism in Glucotoxicity
Progressive deterioration in β-cell function and loss of β-cell mass are common findings in various forms of diabetes (1). We previously demonstrated that chronic hyperglycemia gradually leads to a marked loss of insulin content in KATP-GOF mice (6), changes that were due to loss of β-cell identity rather than to cell death (8), paralleling findings in other rodent models of diabetes of various etiologies (18,31,32). In KATP-GOF mice, we showed that the loss of insulin content and β-cell identity could be prevented or even reversed by normalization of blood glucose with antidiabetic sulfonylureas or with exogenous insulin treatment (7,8,32) or by feeding with a high-fat diet (25).
There has been much interest in the notion that β-cells become exhausted as a result of the excess demands of secretion induced by hyperglycemia in the diabetic state and that β-cell “rest” through exogenous insulin treatment permits restoration of β-cell function in individuals with type 2 diabetes (33–37). However, our finding that loss of insulin content and β-cell identity occurs in β-cells that are intrinsically inexcitable, and are therefore chronically low in [Ca2+]i and do not secrete insulin in response to glucose (6,21), indicates that this is not exhaustion due to the demands of excitation or insulin secretion. Instead, somewhere upstream in the pathway from glucose elevation to excitation and secretion must be affected. Increased NAD(P)H autofluorescence and mitochondrial membrane potential in KATP-GOF mice (21) suggest that augmented β-cell glucose metabolism may be the driving factor in loss of β-cell insulin content in this, and potentially other, diabetic states. This is consistent with the demonstration that metabolism increases when insulin secretion cannot take place and that both increased glycolytic flux and membrane depolarization are necessary for β-cell proliferation (38) and with loss of β-cell mass and apoptosis if proliferation cannot occur. Thus, we suggest that it is rest from hyperstimulation of metabolism, rather than rest from hyperexcitability and secretion, that permits β-cell recovery of insulin content/function in KATP-GOF mice (27,39).
In the current study we have directly tested the above hypothesis by genetic reduction of glucose metabolism in KATP-GOF mice that are also haplodeficient for GCK. Consistent with this hypothesis, KATP-GOF mice with GCK haploinsufficiency, and thus reduced glucose metabolism (KATP-GOF/GCK+/−), showed delayed diabetes progression, reduced β-cell glucotoxicity, and preserved insulin content and β-cell mass and identity, even though they were still hyperglycemic because of KATP being permanently “on” and insulin secretion therefore being chronically suppressed. Reduction of GCK activity by d-mannoheptulose (a GCK inhibitor) in newly diabetic db/db mouse islets restored glucose sensing and pulsatility of insulin secretion (40). Consistent with previous findings in KATP-GOF (6) and in GCK (38) mice, β-cell proliferation is not expected to increase in KATP-GOF/GCK+/− mice since both increased glycolytic flux and membrane depolarization are absent. Intracellular glucose flux appears to also regulate proliferation and apoptosis in human β-cells (41), as individuals bearing an activating mutation in GCK demonstrate hyperplastic islets (42,43). Together, these findings are well aligned with postprandial glucose increasing metabolic rate and insulin secretion, with an unchanged mass, and with persistently elevated glucose tilting the balance to 1) increased insulin secretion and proliferation or 2) increased metabolic rate with decompensation and reduced β-cell mass. Thus, paradoxically, reduction of glucose metabolism emerges as a novel mechanism to enhance glucose sensing, improve β-cell function, and maintain β-cell insulin content and β-cell identity in diabetes. This has critical implications for the prevention of β-cell exhaustion and glucotoxicity in diabetes progression, and preservation of β-cell mass and function by reduction of glucose metabolism in mouse models of diabetes may be key to explaining beneficial effects of insulin therapy and tight glycemic control observed (20,33,34,44–46), at least in the early stages of T2D.
Horses for Courses: Differential Consequences of Decreased Glucose Metabolism in Various Forms of Diabetes
In addition to the effects of glucose metabolism in proliferation and apoptosis, Ha et al. (47) developed a mathematical model of diabetes pathogenesis supporting the idea that metabolism-induced stress triggers reduction of β-cell mass, especially when insulin secretion is reduced. Consistent with this, haploinsufficiency of GCK in the db/db mouse model of obesity/T2D decreased β-cell stress–related gene expression and mitochondrial damage and improved insulin content and β-cell mass and identity (48). This correlates with our observation of reduced oxidative stress, improved mature β-identity genes, and reduced dedifferentiation in KATP-GOF/GCK+/− mice with respect to KATP-GOF. In contrast, genetic reduction of GCK in Akita mice (49) further increased endoplasmic reticulum (ER) stress and expression of apoptotic genes (50). Importantly, the Akita mouse is a model of ER stress–induced diabetes, and hence, the discrepancy in the outcome of reduced glucose metabolism in this model versus KATP-GOF and db/db mice could arise from the distinctive underlying causes of the insulin deficiency. Thus, impaired β-cell growth/increased apoptosis in Akita mice (51), versus failure of glucose-dependent secretion in KATP-GOF mice (6,21) and potentially in db/db mice (40), could explain the differential outcomes by decreased β-cell glucose metabolism.
A balance between food intake and energy consumption is reflected in body weight. In contrast to the significant reduction of body weight, fat mass, and energy efficiency in KATP-GOF (even with increased food intake), KATP-GOF/GCK+/− mice maintained normal body weight, fat mass, and energy efficiency and had normal food intake and physical activity. The contribution of fat mass to energy expenditure is substantially greater than predicted from the metabolic cost of adipose tissue, and it is leptin dependent. In KATP-GOF mice, increased plasma glucagon and decreased leptin, both of which were normalized in KATP-GOF/GCK+/− mice, could therefore explain the significant increase in blood glucose and food intake. Several lines of evidence implicate a role of leptin in inhibiting glucagon secretion from pancreatic α-cells (52,53). Thus, we speculate that the increase in plasma leptin levels in KATP-GOF/GCK+/− mice will reduce glucagon secretion and plasma glucagon levels, which in turn will help to maintain body weight and adiposity and to restore physical activity and energy expenditure in these mice. Similar improvements in metabolic parameters were observed in ob/ob mice exposed to a ketogenic diet (54) and in KATP-GOF mice subjected to a high-fat diet (25), both interventions inducing reduction of β-cell glucose metabolism (55).
Systemic Consequences of Improved β-Cell Identity and Function
Maintenance of normal WAT and improvement of hepatic steatosis in KATP-GOF/GCK+/− mice is consistent with the idea that reducing β-cell glucotoxicity improves peripheral tissue mass and function and correlates with the increase in PPARα and amelioration of fatty liver observed in mice fed a high-fructose diet (56,57). On the other hand, BAT is recognized as the major site of sympathetically activated nonshivering thermogenesis during cold exposure and after spontaneous hyperphagia (58). Enlarged brown adipocytes with large unilocular vacuoles in KATP-GOF mice, but not in KATP-GOF/GCK+/− mice, suggests a BAT-to-WAT transition in the former. Transcriptional regulation of the Ucp-1 gene is controlled by regulatory elements critical for both white and brown fat adipogenesis, in agreement with our findings of decreased Ucp-1 and Pparα messages in KATP-GOF mice and their partial restoration in KATP-GOF/GCK+/− mice.
Although liver PPARγ contributes to hepatic steatosis and regulation of body fat mass, we did not see changes in either KATP-GOF or KATP-GOF/GCK+/− mice. Highlighting the crucial role of liver PPARα for whole-body fatty acid homeostasis and protection against fatty liver (59), increased PPARα in KATP-GOF/GCK+/− mice correlated with hydrolysis of hepatic triglycerides and reduction in triglyceride content and with the effects observed in liver-specific PPARα knockout mice (60). Reduced FAS in livers of KATP-GOF mice suggest that fat accumulation is promoted by peripheral lipolysis rather than increased liver lipogenesis. Liver-specific FAS knockout mice showed fatty liver upon high-carbohydrate diet feeding potentially because of increased hepatic malonyl-CoA, which will inhibit fatty acid β-oxidation (61). On the other hand, increased ATGL and HSL in livers of KATP-GOF/GCK+/− is consistent with decreased liver fat and increased β-oxidation, correlating with amelioration of hepatic steatosis and improvement of liver function in ob/ob mice and in high-fat diet–induced obesity ATGL- and HSL-overexpressing mice (29).
Conclusions
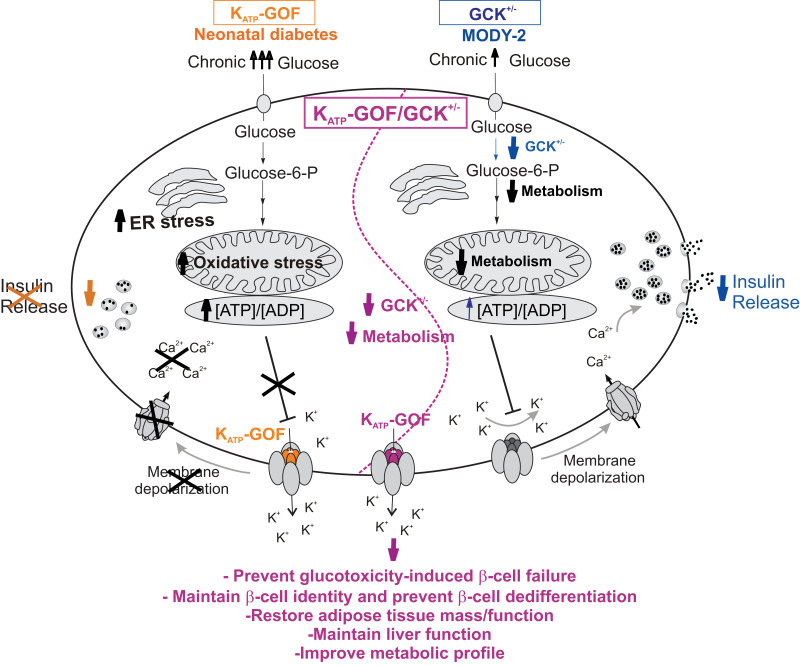
We demonstrate that reduction of β-cell glucose metabolism protects against reduction of insulin content and loss of β-cell identity and dedifferentiation in a KATP-GOF model of neonatal diabetes (Fig. 6). By extrapolation to findings in other forms of diabetes that result from failure of glucose-dependent insulin secretion, reduced glucose metabolism paradoxically emerges as a mechanism to prevent glucotoxicity-induced loss of functional β-cell mass in diabetes and to maintain adipose tissue and liver function, as well as metabolic parameters.
Figure 6.
Schematic representation of the glucose metabolism and insulin secretory pathway in a pancreatic β-cell: 1) mice with β-cell–specific KATP-GOF mutation (orange, left side of the cell); 2) mice with β-cell–specific GCK+/− haploinsufficiency (blue, right side of the cell), and 3) a combination of both β-cell–specific KATP-GOF and GCK+/− (purple, middle of the cell). MODY-2, maturity-onset diabetes of the young 2.
Article Information
Funding. This work was supported by National Institutes of Health grants R01DK098584 and R01DK123163 (to M.S.R.). The authors also acknowledge the Diabetes Models Phenotyping Core and the Metabolic Tissue Function Core, Diabetes Research Center, Washington University in St Louis, MO, National Institutes of Health grant P30 DK020579.
The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Z.Y., M.Fo., Z.A.S., A.L.C., M.Fu., and M.S.R. performed the experiments and data analysis. Z.Y. and M.S.R. wrote the manuscript. C.G.N. and M.S.R. conceptualized the study and edited the manuscript. All authors read and approved the final version of the manuscript. M.S.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in oral form at the 78th Scientific Sessions of the American Diabetes Association, 22–26 June 2018.
References
- 1. Eizirik DL, Pasquali L, Cnop M. Pancreatic β-cells in type 1 and type 2 diabetes mellitus: different pathways to failure. Nat Rev Endocrinol 2020;16:349–362 [DOI] [PubMed] [Google Scholar]
- 2. Gloyn AL, Pearson ER, Antcliff JF, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med 2004;350:1838–1849 [DOI] [PubMed] [Google Scholar]
- 3. Flanagan SE, Clauin S, Bellanné-Chantelot C, et al. Update of mutations in the genes encoding the pancreatic beta-cell K(ATP) channel subunits Kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat 2009;30:170–180 [DOI] [PubMed] [Google Scholar]
- 4. Nielsen EM, Hansen L, Carstensen B, et al. The E23K variant of Kir6.2 associates with impaired post-OGTT serum insulin response and increased risk of type 2 diabetes. Diabetes 2003;52:573–577 [DOI] [PubMed] [Google Scholar]
- 5. Koster JC, Marshall BA, Ensor N, Corbett JA, Nichols CG. Targeted overactivity of beta cell K(ATP) channels induces profound neonatal diabetes. Cell 2000;100:645–654 [DOI] [PubMed] [Google Scholar]
- 6. Remedi MS, Kurata HT, Scott A, et al. Secondary consequences of beta cell inexcitability: identification and prevention in a murine model of K(ATP)-induced neonatal diabetes mellitus. Cell Metab 2009;9:140–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Remedi MS, Agapova SE, Vyas AK, Hruz PW, Nichols CG. Acute sulfonylurea therapy at disease onset can cause permanent remission of KATP-induced diabetes. Diabetes 2011;60:2515–2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang Z, York NW, Nichols CG, Remedi MS. Pancreatic β cell dedifferentiation in diabetes and redifferentiation following insulin therapy. Cell Metab 2014;19:872–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bensellam M, Jonas JC, Laybutt DR. Mechanisms of β-cell dedifferentiation in diabetes: recent findings and future research directions. J Endocrinol 2018;236:R109–R143 [DOI] [PubMed] [Google Scholar]
- 10. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102–110 [DOI] [PubMed] [Google Scholar]
- 11. Cinti F, Bouchi R, Kim-Muller JY, et al. Evidence of β-cell dedifferentiation in human type 2 diabetes. J Clin Endocrinol Metab 2016;101:1044–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo S, Dai C, Guo M, et al. Inactivation of specific β cell transcription factors in type 2 diabetes. J Clin Invest 2013;123:3305–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moin ASM, Butler AE. Alterations in beta cell identity in type 1 and type 2 diabetes. Curr Diab Rep 2019;19:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matschinsky F, Liang Y, Kesavan P, et al. Glucokinase as pancreatic beta cell glucose sensor and diabetes gene. J Clin Invest 1993;92:2092–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Magnuson MA, She P, Shiota M. Gene-altered mice and metabolic flux control. J Biol Chem 2003;278:32485–32488 [DOI] [PubMed] [Google Scholar]
- 16. Chen C, Hosokawa H, Bumbalo LM, Leahy JL. Mechanism of compensatory hyperinsulinemia in normoglycemic insulin-resistant spontaneously hypertensive rats. Augmented enzymatic activity of glucokinase in beta-cells. J Clin Invest 1994;94:399–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liang Y, Najafi H, Matschinsky FM. Glucose regulates glucokinase activity in cultured islets from rat pancreas. J Biol Chem 1990;265:16863–16866 [PubMed] [Google Scholar]
- 18. Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 2012;150:1223–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robertson RP, Harmon J, Tran PO, Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 2004;53(Suppl. 1):S119–S124 [DOI] [PubMed] [Google Scholar]
- 20. Wajchenberg BL. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 2007;28:187–218 [DOI] [PubMed] [Google Scholar]
- 21. Benninger RK, Remedi MS, Head WS, Ustione A, Piston DW, Nichols CG. Defects in beta cell Ca²+ signalling, glucose metabolism and insulin secretion in a murine model of K(ATP) channel-induced neonatal diabetes mellitus. Diabetologia 2011;54:1087–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Terauchi Y, Sakura H, Yasuda K, et al. Pancreatic beta-cell-specific targeted disruption of glucokinase gene. Diabetes mellitus due to defective insulin secretion to glucose. J Biol Chem 1995;270:30253–30256 [DOI] [PubMed] [Google Scholar]
- 23. Remedi MS, Koster JC, Patton BL, Nichols CG. ATP-sensitive K+ channel signaling in glucokinase-deficient diabetes. Diabetes 2005;54:2925–2931 [DOI] [PubMed] [Google Scholar]
- 24. Emfinger CH, Yan Z, Welscher A, et al. Contribution of systemic inflammation to permanence of KATP-induced neonatal diabetes in mice. Am J Physiol Endocrinol Metab 2018;315:E1121–E1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yan Z, Shyr ZA, Fortunato M, et al. High-fat-diet-induced remission of diabetes in a subset of KATP -GOF insulin-secretory-deficient mice. Diabetes Obes Metab 2018;20:2574–2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yasuhara D, Naruo T, Nagai N, Tanaka M, Muranaga T, Nozoe S. Insulinogenic index at 15 min as a marker of nutritional rehabilitation in anorexia nervosa. Am J Clin Nutr 2003;77:292–299 [DOI] [PubMed] [Google Scholar]
- 27. Shyr ZA, Wang Z, York NW, Nichols CG, Remedi MS. The role of membrane excitability in pancreatic β-cell glucotoxicity. Sci Rep 2019;9:6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lodhi IJ, Dean JM, He A, et al. PexRAP Inhibits PRDM16-Mediated Thermogenic Gene Expression. Cell Rep 2017;20:2766–2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reid BN, Ables GP, Otlivanchik OA, et al. Hepatic overexpression of hormone-sensitive lipase and adipose triglyceride lipase promotes fatty acid oxidation, stimulates direct release of free fatty acids, and ameliorates steatosis. J Biol Chem 2008;283:13087–13099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim-Muller JY, Zhao S, Srivastava S, et al. Metabolic inflexibility impairs insulin secretion and results in MODY-like diabetes in triple FoxO-deficient mice. Cell Metab 2014;20:593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jonas JC, Bensellam M, Duprez J, Elouil H, Guiot Y, Pascal SM. Glucose regulation of islet stress responses and beta-cell failure in type 2 diabetes. Diabetes Obes Metab 2009;11(Suppl. 4):65–81 [DOI] [PubMed] [Google Scholar]
- 32. Brereton MF, Iberl M, Shimomura K, et al. Reversible changes in pancreatic islet structure and function produced by elevated blood glucose. Nat Commun 2014;5:4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 2008;371:1753–1760 [DOI] [PubMed] [Google Scholar]
- 34. Torella R, Salvatore T, Cozzolino D, Giunta R, Quatraro A, Giugliano D. Restoration of sensitivity to sulfonylurea after strict glycaemic control with insulin in non-obese type 2 diabetic subjects. Diabete Metab 1991;17:443–447 [PubMed] [Google Scholar]
- 35. Alvarsson M, Sundkvist G, Lager I, et al. Effects of insulin vs. glibenclamide in recently diagnosed patients with type 2 diabetes: a 4-year follow-up. Diabetes Obes Metab 2008;10:421–429 [DOI] [PubMed] [Google Scholar]
- 36. Greenwood RH, Mahler RF, Hales CN. Improvement in insulin secretion in diabetes after diazoxide. Lancet 1976;1:444–447 [DOI] [PubMed] [Google Scholar]
- 37. Qvigstad E, Kollind M, Grill V. Nine weeks of bedtime diazoxide is well tolerated and improves beta-cell function in subjects with type 2 diabetes. Diabet Med 2004;21:73–76 [DOI] [PubMed] [Google Scholar]
- 38. Porat S, Weinberg-Corem N, Tornovsky-Babaey S, et al. Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab 2011;13:440–449 [DOI] [PubMed] [Google Scholar]
- 39. Nichols CG, Remedi MS. The diabetic β-cell: hyperstimulated vs. hyperexcited. Diabetes Obes Metab 2012;14(Suppl. 3):129–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jahan I, Corbin KL, Bogart AM, et al. Reducing glucokinase activity restores endogenous pulsatility and enhances insulin secretion in islets from db/db mice. Endocrinology 2018;159:3747–3760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kassem S, Bhandari S, Rodríguez-Bada P, et al. Large islets, beta-cell proliferation, and a glucokinase mutation. N Engl J Med 2010;362:1348–1350 [DOI] [PubMed] [Google Scholar]
- 42. Cuesta-Muñoz AL, Huopio H, Otonkoski T, et al. Severe persistent hyperinsulinemic hypoglycemia due to a de novo glucokinase mutation. Diabetes 2004;53:2164–2168 [DOI] [PubMed] [Google Scholar]
- 43. Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B. Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes 2000;49:1325–1333 [DOI] [PubMed] [Google Scholar]
- 44. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 45. Alvarsson M, Sundkvist G, Lager I, et al. Beneficial effects of insulin versus sulphonylurea on insulin secretion and metabolic control in recently diagnosed type 2 diabetic patients. Diabetes Care 2003;26:2231–2237 [DOI] [PubMed] [Google Scholar]
- 46. Ilkova H, Glaser B, Tunçkale A, Bagriaçik N, Cerasi E. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients by transient intensive insulin treatment. Diabetes Care 1997;20:1353–1356 [DOI] [PubMed] [Google Scholar]
- 47. Ha J, Satin LS, Sherman AS. A mathematical model of the pathogenesis, prevention, and reversal of type 2 diabetes. Endocrinology 2016;157:624–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Omori K, Nakamura A, Miyoshi H, et al. Glucokinase inactivation paradoxically ameliorates glucose intolerance by increasing β-cell mass in db/db mice. Diabetes 2021;70:917–931 [DOI] [PubMed] [Google Scholar]
- 49. Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes 1997;46:887–894 [DOI] [PubMed] [Google Scholar]
- 50. Shirakawa J, Togashi Y, Sakamoto E, et al. Glucokinase activation ameliorates ER stress-induced apoptosis in pancreatic β-cells. Diabetes 2013;62:3448–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Riahi Y, Israeli T, Yeroslaviz R, et al. Inhibition of mTORC1 by ER stress impairs neonatal β-cell expansion and predisposes to diabetes in the Akita mouse. eLife 2018;7:e38472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Denroche HC, Huynh FK, Kieffer TJ. The role of leptin in glucose homeostasis. J Diabetes Investig 2012;3:115–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tudurí E, Marroquí L, Soriano S, et al. Inhibitory effects of leptin on pancreatic alpha-cell function. Diabetes 2009;58:1616–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Badman MK, Kennedy AR, Adams AC, Pissios P, Maratos-Flier E. A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independently of weight loss. Am J Physiol Endocrinol Metab 2009;297:E1197–E1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lu B, Kurmi K, Munoz-Gomez M, et al. Impaired β-cell glucokinase as an underlying mechanism in diet-induced diabetes. Dis Model Mech 2018;11:dmm033316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chan SM, Sun RQ, Zeng XY, et al. Activation of PPARα ameliorates hepatic insulin resistance and steatosis in high fructose-fed mice despite increased endoplasmic reticulum stress. Diabetes 2013;62:2095–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ip E, Farrell GC, Robertson G, Hall P, Kirsch R, Leclercq I. Central role of PPARalpha-dependent hepatic lipid turnover in dietary steatohepatitis in mice. Hepatology 2003;38:123–132 [DOI] [PubMed] [Google Scholar]
- 58. Saito M, Matsushita M, Yoneshiro T, Okamatsu-Ogura Y. Brown adipose tissue, diet-induced thermogenesis, and thermogenic food ingredients: from mice to men. Front Endocrinol (Lausanne) 2020;11:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sapiro JM, Mashek MT, Greenberg AS, Mashek DG. Hepatic triacylglycerol hydrolysis regulates peroxisome proliferator-activated receptor alpha activity. J Lipid Res 2009;50:1621–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Montagner A, Polizzi A, Fouché E, et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 2016;65:1202–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chakravarthy MV, Pan Z, Zhu Y, et al. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab 2005;1:309–322 [DOI] [PubMed] [Google Scholar]