Abstract
Preclinical studies reveal maternal exercise as a promising intervention to reduce the transmission of multigenerational metabolic dysfunction caused by maternal obesity. The benefits of maternal exercise on offspring health may arise from multiple factors and have recently been shown to involve DNA demethylation of critical hepatic genes leading to enhanced glucose metabolism in offspring. Histone modification is another epigenetic regulator, yet the effects of maternal obesity and exercise on histone methylation in offspring are not known. Here, we find that maternal high-fat diet (HFD; 60% kcal from fat) induced dysregulation of offspring liver glucose metabolism in C57BL/6 mice through a mechanism involving increased reactive oxygen species, WD repeat-containing 82 (WDR82) carbonylation, and inactivation of histone H3 lysine 4 (H3K4) methyltransferase leading to decreased H3K4me3 at the promoters of glucose metabolic genes. Remarkably, the entire signal was restored if the HFD-fed dams had exercised during pregnancy. WDR82 overexpression in hepatoblasts mimicked the effects of maternal exercise on H3K4me3 levels. Placental superoxide dismutase 3 (SOD3), but not antioxidant treatment with N-acetylcysteine was necessary for the regulation of H3K4me3, gene expression, and glucose metabolism. Maternal exercise regulates a multicomponent epigenetic system in the fetal liver resulting in the transmission of the benefits of exercise to offspring.
Introduction
Maternal overnutrition during pregnancy increases the risks of obesity and type 2 diabetes (T2D) in offspring, an effect that persists throughout the lifetime (1–3). Rodent studies have shown that offspring exposed to a maternal high-fat diet (HFD) present with increased weight gain, hyperlipidemia, hepatic triglycerides accumulation, impaired hepatic mitochondrial respiration, and glucose intolerance (4–9). Since the rates of obesity and T2D are increasing among women of childbearing age (10), drastically impacting the health of the next generation, establishing effective means to prevent this intergenerational transmission of metabolic dysfunction is urgently needed.
Previous studies have demonstrated maternal exercise improves metabolic parameters, including glucose tolerance and insulin sensitivity, in adult offspring (1,3). Remarkably, the detrimental effects of a maternal HFD on offspring metabolism are completely negated by maternal exercise (1,3,7,8,11,12). We established the liver mainly governs the metabolic improvement in offspring of exercise-trained mothers (8,13,14). Moreover, we recently discovered that superoxide dismutase 3/extracellular superoxide dismutase (SOD3/EC-SOD), a redox secretory protein increased in the placenta of offspring of trained mothers, improves offspring glucose homeostasis (14). This effect involves SOD3-induced DNA demethylation at hepatic gene promoters, leading to enhanced gene expression and glucose metabolism in offspring liver. These results suggest that maternal exercise-induced placental SOD3 may be a primary means by which the development of obesity and T2D can be mitigated in adult offspring
Perturbation of DNA hypermethylation (12,14) and impaired demethylation of promoters (14,15) are key epigenetic events contributing to the harmful effects of maternal HFD on offspring. Additionally, two earlier studies reported offspring livers of HFD-fed dams have altered histone H3 lysine 9 trimethylation (H3K9me3) at the promoters of Wnt1, Pparg, Ppara, Rxra, and Rora (16,17). Ten-eleven translocation (TET) methylcytosine demethylase, an enzyme mediating DNA demethylation, is recruited to specific promoters that have enriched histone H3 lysine 4 (H3K4)me3 (18,19). Therefore, we hypothesize that maternal exercise results in specific histone modification leading to TET recruitment and DNA methylation at the promoters of glucose metabolic genes.
We found that maternal exercise recovered the inhibitory effects of maternal HFD on H3K4me3 levels at the promoters of the hepatic glucose metabolism genes in offspring. Altered H3K4me3 was associated with H3K4 methyltransferase (HKMT) activity and reactive oxygen species (ROS) production in the offspring livers of HFD-fed dams, and maternal exercise prevented these harmful effects on protein function through the protection of WD repeat-containing protein 82 (WDR82) carbonylation. Exercise-induced placental SOD3, but not treatment with N-acetylcysteine (NAC), rescued the negative impact of maternal HFD on offspring liver metabolism.
Research Design and Methods
Placenta-Specific Sod3-Knockout Mice
The Tpbpa/Ada Cre/loxP was used to generate trophoblast-specific Sod3-knockout (Sod3−/−) and flox control (Sod3f/f) mice (14).
Training Program
Virgin female C57Bl/6 mice (8 weeks old) and Sod3f/f mice (7–12 weeks old) were fed chow (21% kcal from fat, LabDiet) or an HFD (60% kcal from fat, Research Diets) for 2 weeks preconception and during gestation. Mice were divided into two subgroups: trained (housed with running wheels) and sedentary (housed in static cages). Male breeders were 10-week-old C57BL/6 mice or 10- to 12-week-old Sod3f/f Tpbpa/Ada Cre+/− (Sod3−/−) sedentary mice maintained on the chow diet. Average and cumulative running distance during both preconception and gestation were not different between chow- and HFD-fed C57BL/6 and Sod3f/f dams (Supplementary Fig. 1A–D). To control for sire differences, breeding was conducted using harems. Each group contained 6 dams. Litters were culled to six, and offspring were fed chow and housed in static cages from birth onward.
Primary Embryonic Hepatoblast and Hepatocyte Culture
Hepatoblasts and hepatocytes were collected in the random fed state as previously described (14). The hepatoblasts were a mixture of male and female fetuses. Transfection of siRNA duplexes specific to Wdr82 (SR409957A; rCrCrUrArCrUrGrCrArArGrArArUrCrGrArArArGrArArCAG, SR423353B; rCrCrCrUrArGrArArArGrUrCrArCrArArUrCrUrUrGrAAA, SR423353C; rCrCrUrGrCrArArUrArArGrArArGrUrArArArGrArCrCrAAG, Origene) and control (SR30004, Origene) was performed using Lipofectamine RNAiMAX (13778-030, Invitrogen) for 24 h. Transfection of pcDNA3-Myc-Wdr82 was performed using jetPEI-Hepatocyte (102-05N, Polyplus-transfection).
In Vitro Glucose Production Assay
Hepatic glucose production was determined as previously described (14). Glucose in culture medium was normalized to total protein levels.
Chromatin Immunoprecipitation
Hepatoblasts (1 × 108) isolated from embryonic day (E) 13.5 livers of sedentary or trained dams were analyzed as previously described (14). Antibodies against H3K4me3 (9751, CST) or rabbit IgG (2729, CST) were captured on Dynabeads M-280 anti-rabbit IgG (11203D, Thermo Fisher). Primer sequences are listed in Supplementary Table 2.
Methylation-Specific PCR
DNA methylation levels were analyzed as previously described (14).
Real-Time Quantitative PCR
Gene expression was analyzed as previously described (14). Each mRNA expression was calculated relative to that of Rpl13a. Primer sequences are listed in Supplementary Table 3 or were previously presented (14).
Biochemical Assays
HKMT activity was analyzed by EpiSeeker Histone Methyltransferase H3 (K4) Activity Quantification Assay Kit (ab113452, Abcam). Cellular ROS was determined by OxiSelect In Vitro ROS/RNS Assay (STA-347, Cell Biolabs). Antioxidant capacity was determined using the Total Antioxidant Capacity Assay (ab65329, Abcam). Total protein carbonylation was analyzed by OxiSelect Protein Carbonyl ELISA (STA-310, Cell Biolabs).
Immunoprecipitation
Hepatoblasts were incubated in PBS with 20 mmol/L dimethyl pimelimidate for 1 h at 37°C. WDR82 antibody (99715, CST) or IgG (2729, CST) were captured with Dynabeads Protein G (10003D, Thermo Fisher) in PBS at room temperature for 40 min. Following immunoprecipitation methods were performed as previously described (14).
Western Blotting
Lysates were analyzed as previously described (14). Primary antibodies against H3K4me3 (9751), H3K9me3 (13969), H3K27me3 (9733), H3K27ac (8173), histone-H3 (4499), WDR82 (99715), AMPKα (5831), and phospho-AMPKα (2535) were obtained from CST. Carbonylated proteins were detected using OxiSelect Protein Carbonyl Immunoblot (STA-308, Cell Biolabs).
Protein Sequence Analysis by Liquid Chromatography–Tandem Mass Spectrometry
Gel pieces of 25 and 35 kDa upon Western blotting with the DNP antibody were analyzed by proteomics, as previously described (14). We assessed both values to exclude any potential error that may occur when only using a sum intensity measurement. Since we used samples only from the offspring livers from HFD-fed dams, there was no statistical analysis. We screened the candidates based on total peptides and sum intensity.
Intraperitoneal Glucose Tolerance Test
Glucose metabolism was analyzed as previously described (14).
Purification of SOD3 Protein
Human SOD3 (hSOD3) cDNA was obtained from Origene (RC204156) and was cloned into p3xFLAG-CMV8 vector (E9908, Sigma-Aldrich). Recombinant hSOD3 was produced by transient transfection of p3xFLAG-CMV8-hSOD3 in ExpiCHO Expression System (A29133, Gibco). FLAG-tagged hSOD3 proteins were captured with anti–FLAG-M2 Magnetic Beads affinity isolated antibody (M8823, Sigma-Aldrich) and eluted with 5 mg/mL 3×-FLAG-Peptide (F4799, Sigma-Aldrich) in TBS (pH 7.4). The amount of hSOD3 was measured by ELISA (ELH-SOD3-1, RayBiotech), and hSOD3 activity was evaluated with the SOD assay kit-WST (S311, Dojindo).
Exo Utero Developmental System
Manipulation was performed as previously described (14,20). C57BL/6 female mice (8 weeks old) were fed the HFD for 2 weeks preconception and during gestation. For each dam, three embryos on one side of the uterine horn were designated the experimental group (hSOD3 or NAC), and three embryos on the other side of the uterine horn were designated controls (saline). NAC was dissolved in 20 mmol/L in water. Eluted hSOD3 was dissolved in TBS (20 μg/mL, pH 7.4); then, 1 μL of solution was injected near the liver of each embryo through the fetal membrane. To analyze embryonic livers, fetuses were collected at E15.5. To analyze adult livers, embryos at E18.5 were removed from the abdomen, and the resuscitated newborns were parented by C57BL/6 foster mothers. Litters were culled to three mice per treatment, and offspring were fed chow and housed in static cages from birth onward.
Statistical Analysis
All data are reported as means ± SEM. Statistical significance was defined as P < 0.025 or 0.01 and determined by one- or two-way ANOVA, with Tukey and Bonferroni post hoc analysis. For experiments conducted at various ages, statistical analyses were determined based on the control group at each time point, and comparisons among ages were not performed.
Data and Resource Availability
Comprehensive data that show the list of all carbonylated proteins in this study are available in Supplementary Table 1.
Results
Maternal Exercise Increases H3K4me3 Levels at the Promoters of Glucose Metabolism Genes in Livers of Offspring
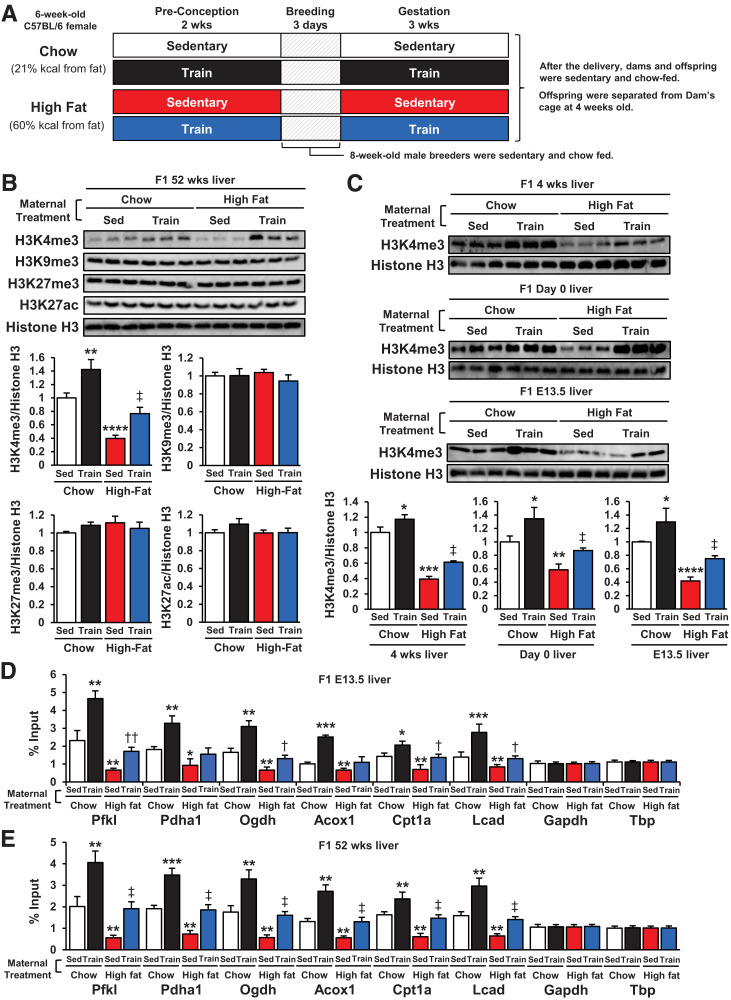
We measured total H3K4me3, H3K9me3, H3K27me3, and H3K27ac, which represent active or inactive promoters (21), in the livers of 52-week-old offspring of dams that were sedentary chow fed, trained chow fed, sedentary HFD fed, or trained HFD fed (Fig. 1A and B). Maternal exercise increased hepatic H3K4me3 in the offspring from chow-fed dams. Conversely, offspring livers of sedentary HFD-fed dams had significantly lower H3K4me3 levels. The suppressive effects of maternal HFD on offspring liver H3K4me3 were partially reversed toward the levels of chow-fed dams when the HFD-fed dams were trained. H3K9me3, H3K27me3, and H3K27ac levels were unaffected by maternal diet and exercise. The influence of maternal exercise on H3K4me3 status was present in offspring livers at 4 weeks, day 0, and E13.5 (Fig. 1C). Next, we performed H3K4me3 chromatin immunoprecipitation-quantitative PCR of key glucose metabolism genes in the livers of E13.5 offspring. These genes are regulated by maternal exercise (14) and diet (Supplementary Fig. 2A and B) and have an apparent H3K4me3 peak in the −1,000 upstream region as determined from the University of California Santa Cruz genomic database. The H3K4me3 levels at the promoters of specific genes involved in pyruvate metabolism (Pfkl), Krebs cycle activity (Pdha1, Ogdh), and fatty acid metabolism (Acox1, Cpt1a, Lcad) were significantly increased in E13.5 and 52-week-old offspring livers of trained dams compared with sedentary dams, whereas offspring from HFD-fed dams had markedly decreased H3K4 trimethylation at these sites (Fig. 1D and E). Maternal exercise in HFD-fed dams partially restored H3K4 trimethylation to baseline levels in offspring livers. Neither maternal HFD nor exercise affected the H3K4me3 levels at the promoters of the housekeeping genes Gapdh and Tbp. Furthermore, DNA methylation levels and H3K4me3 levels at Ogdh and Cpt1a promoters were significantly correlated in E13.5 offspring livers (Supplementary Fig. 2C). These data indicate that maternal exercise upregulates H3K4me3 levels at the promoters of glucose metabolism genes in the offspring livers of chow- or HFD-fed dams.
Figure 1.
Maternal exercise increases H3K4me3 at the promoters of offspring hepatic genes. A: Schematic illustration of the maternal exercise and diet program. B: H3K4me3, H3K9me3, H3K27me3, and H3K27ac levels in livers of 52-week-old offspring of chow- or HFD-fed and sedentary (Sed) or trained dams. C: H3K4me3 levels in livers of 4-week-old, day 0, and E13.5 offspring of chow- or HDS-fed and sedentary or trained dams. H3K4me3 levels at promoters of glucose metabolism genes in livers of E13.5 (D) and 52-week-old (E) offspring of chow- or HFD-fed and sedentary or trained dams (n = 6). All data are reported as means ± SEM. *P < 0.025 vs. Chow-Sed; **P < 0.01 vs. Chow-Sed; ***P < 0.001 vs. Chow-Sed; ****P < 0.0001 vs. Chow-Sed; †P < 0.025 High Fat-Sed vs. High Fat-Train; ‡P < 0.01 High Fat-Sed vs. High Fat-Train; ††P < 0.01 High Fat-Sed vs. High Fat-Train. Statistical significance was determined by one- or two-way ANOVA, with Tukey and Bonferroni post hoc analysis.
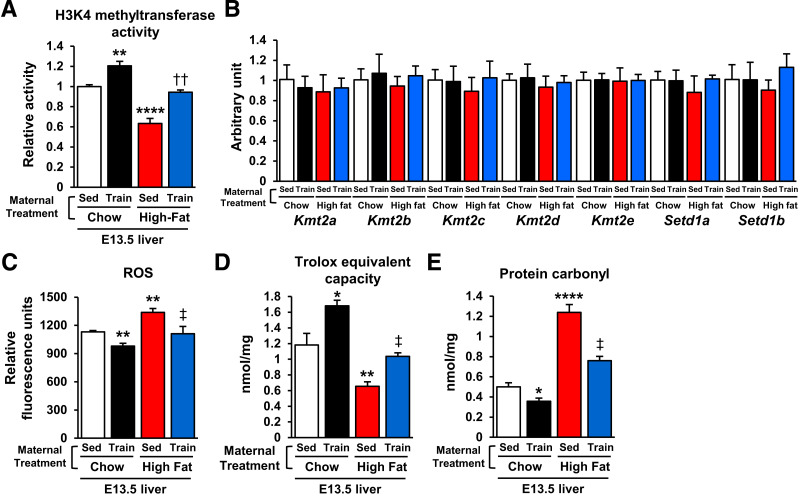
Maternal Exercise Increases HKMT Activity and Decreases ROS-Induced Protein Carbonylation in Offspring Livers
Total HKMT activity was significantly decreased in E13.5 offspring livers of HFD-fed dams and significantly increased in livers from exercise-trained chow- and HFD-fed dams (Fig. 2A), effects not due to changes in gene expression of HKMTs (Kmt2a, Kmt2b, Kmt2c, Kmt2d, Kmt2e, Setd1a, and Setd1b) (22) (Fig. 2B). Protein carbonylation is a posttranslational modification resulting in protein dysfunction (23). With HFD, carbonylation is generally caused by the ROS generation (24,25). Hence, we hypothesized the HFD-activated ROS/carbonylation axis disrupts HKMT activity. We found that E13.5 offspring livers of HFD-fed dams had significantly higher ROS levels (Fig. 2C). Conversely, maternal exercise reduced hepatic ROS levels in the offspring of chow- and HFD-fed dams. Total hepatic antioxidant capacity was increased in offspring of trained chow-fed dams, and the suppressive effects of maternal HFD on the antioxidant capacity were reversed by maternal exercise (Fig. 2D). Total hepatic protein carbonylation was significantly higher in the offspring of HFD-fed dams than in those of chow-fed dams, while hepatic protein carbonylation was suppressed by maternal exercise in the offspring of both chow- and HFD-fed dams (Fig. 2E). These data suggest that maternal exercise protects the offspring liver of HFD-fed dams from ROS-induced carbonylation.
Figure 2.
Maternal exercise promotes H3K4 methyltransferase activity and protects against ROS-induced protein carbonylation in offspring livers. Enzymatic activity of total H3K4 methyltransferase (A), mRNA expression of histone methylation-related gene expression (B), ROS level (C), Trolox equivalent capacity (D), and carbonylated protein content (E) in livers of E13.5 offspring of chow- or HFD-fed and sedentary (Sed) or trained dams (n = 6). All data are reported as means ± SEM. *P < 0.025 vs. Chow-Sed; **P < 0.01 vs. Chow-Sed; ****P < 0.0001 vs. Chow-Sed; ‡P < 0.01 High Fat-Sed vs. High Fat-Train; ††P < 0.01 High Fat-Sed vs. High Fat-Train. Statistical significance was determined by one- or two-way ANOVA, with Tukey and Bonferroni post hoc analysis.
Maternal Exercise Reverses the Detrimental Effects of Maternal HFD Feeding on HKMT Activity via Protection From WDR82 Carbonylation
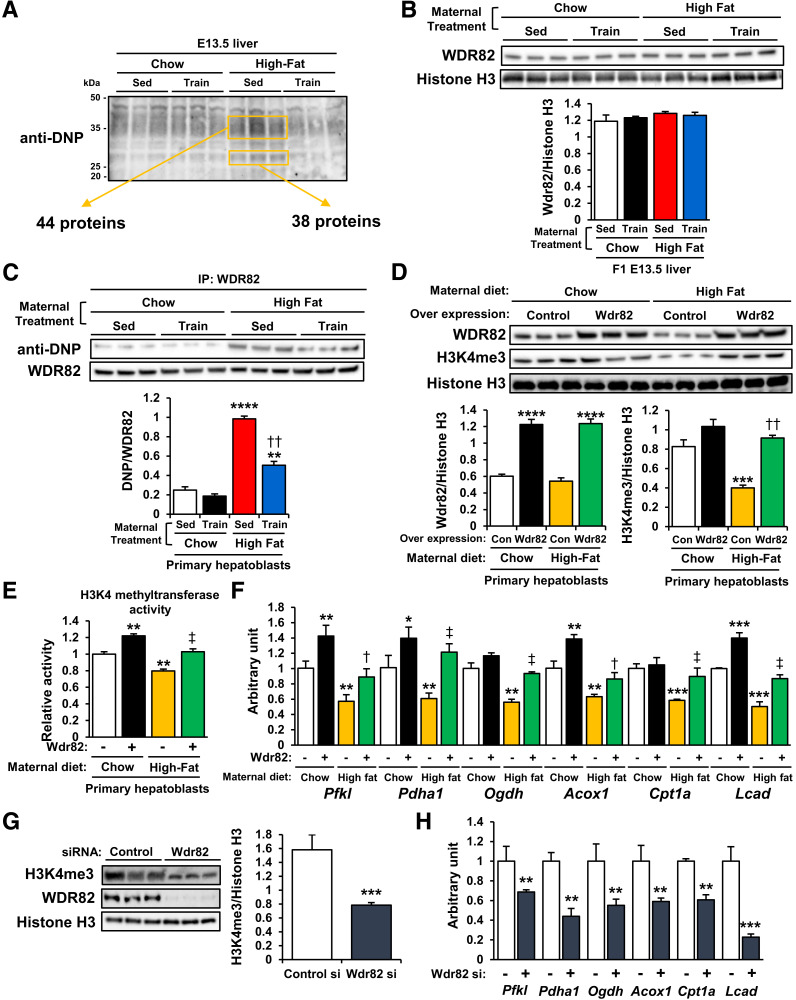
To identify the specific carbonylated proteins in the offspring liver regulated by maternal diet and exercise, we labeled lysates of E13.5 offspring livers with dinitrophenylhydrazine (DNPH), which forms hydrazone with carbonylated proteins (26). Immunoblotting with DNP antibody detected DNPH-derivatized proteins and showed that maternal HFD strongly induced carbonylation of proteins of ∼35 and 25 kDa in the E13.5 offspring liver (Fig. 3A and Supplementary Table 1). An effect was not present in offspring livers from HFD-fed dams that were exercise trained.
Figure 3.
Maternal exercise-induced H3K4me3 stabilization is mediated through protection against WDR82 carbonylation. A: DNP-labeled carbonylated proteins in livers of offspring of chow- or HFD-fed and sedentary (Sed) or trained dams. B: WDR82 protein expression in livers of offspring of chow- or HFD-fed and sedentary or trained dams. C: DNP immunoblotting of WDR82-immunoprecipitated proteins in livers of offspring of chow- or HDS-fed and sedentary or trained dams (n = 3). **P < 0.001 vs. Chow-Sed; ****P < 0.0001 vs. Chow-Sed; ††P < 0.001 High Fat-Sed vs. High Fat-Train. Effects of Wdr82 overexpression on H3K4me3 levels (D), H3K4 methyltransferase activity (E), and mRNA expression of glucose metabolism genes (F) in primary hepatoblasts of chow- or HFD-fed dams (n = 3). *P < 0.025 vs. Chow-control (Con) (Wdr82−); **P < 0.01 vs. Chow-Con (Wdr82−); ***P < 0.001 vs. Chow-Con (Wdr82−); ****P < 0.0001 vs. Chow-Con (Wdr82−); †P < 0.025 High Fat-Wdr82− vs. High Fat-Wdr82 overexpression (Wdr81+); ‡P < 0.01 High Fat-Con (Wdr82−) vs. High Fat-Wdr82+; ††P < 0.001 High Fat-Wdr82− vs. High Fat-Wdr82+). Effects of Wdr82 knockdown on H3K4me3 levels (G) and mRNA expression of glucose metabolism genes (H) in primary hepatoblasts (n = 3). All data are reported as means ± SEM. **P < 0.001; ***P < 0.001. Statistical significance was determined by one- or two-way ANOVA, with Tukey and Bonferroni post hoc analysis.
We then isolated hepatic carbonylated proteins from the offspring of HFD-fed dams by DNPH labeling and DNP-immunoprecipitation and analyzed these proteins by liquid chromatography–tandem mass spectrometry. There were 44 proteins identified in the 35-kDa samples and 38 proteins in the 25-kDa samples (Fig. 3A and Supplementary Table 1). Of these, only WDR82 is recognized as an important regulator of histone-trimethylation (27,28). Neither maternal diet nor maternal exercise affected WDR82 protein expression in offspring livers (Fig. 3B). To evaluate WDR82 carbonylation, we immunoprecipitated lysates of the E13.5 and 24-week-old offspring livers with WDR82 antibodies and blotted the immunoprecipitants with DNP antibody. The E13.5 offspring livers of sedentary HFD-fed dams had increased levels of carbonylated WDR82, and the effects of maternal HFD on offspring liver WDR82 carbonylation were reversed by maternal exercise (Fig. 3C). However, WDR82 carbonylation was not changed by maternal diet or exercise at 24 weeks old (Supplementary Fig. 4). Next, we isolated primary hepatoblasts from the E13.5 embryos of chow- or HFD-fed dams and transfected them with Wdr82 overexpression vectors (Fig. 3D). The primary hepatoblasts from the offspring of HFD-fed dams showed low H3K4me3 levels. Wdr82 overexpression upregulated H3K4me3 levels in the offspring hepatoblasts of HFD-fed dams. Moreover, Wdr82 overexpression promoted HKMT activity (Fig. 3E) and mRNA expression of Pfkl, Pdha1, Ogdh, Acox1, Cpt1a, and Lcad (Fig. 3F) in the offspring hepatoblasts of chow- or HFD-fed dams. Transient transfection of hepatoblasts with Wdr82-specific siRNA suppressed H3K4me3 levels (Fig. 3G) and hepatic gene expression (Fig. 3H). Taken together, these results indicate that maternal exercise recovers HKMT activity by protecting against WDR82 carbonylation in the offspring liver of HFD-fed dams.
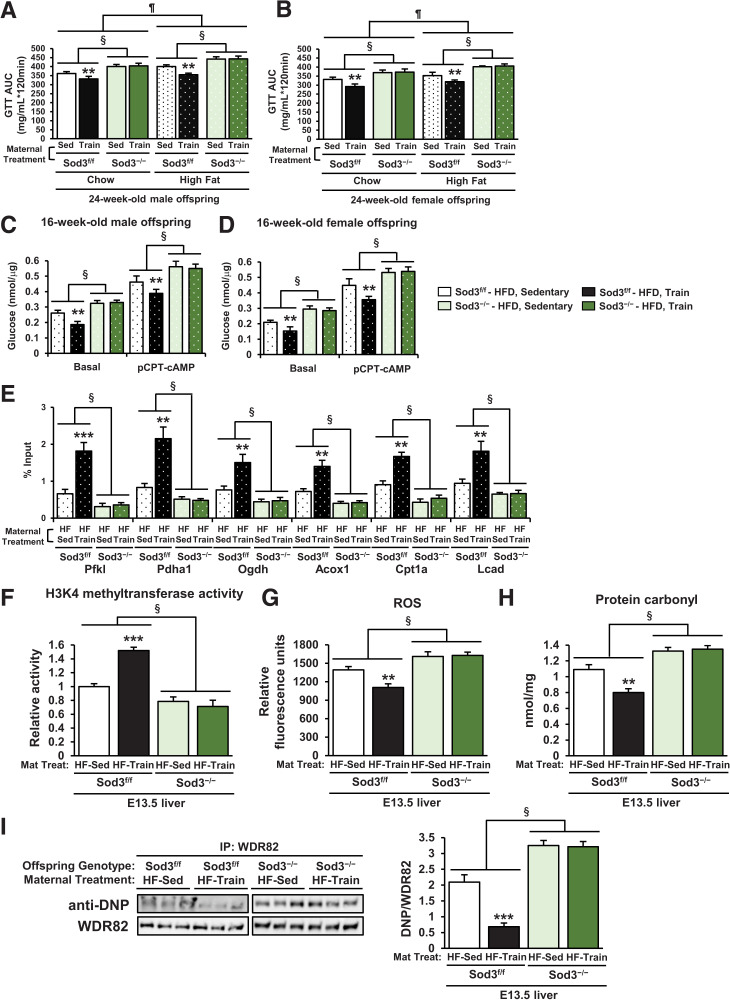
Exercise-Induced, Placenta-Derived SOD3 Mediates the Protective Effects of Maternal Exercise on Glucose Metabolism in the Offspring Livers From HFD-fed Dams
We set out to determine whether placental SOD3 (14) was necessary for the effects of maternal exercise to reverse the detrimental effects of HFD on offspring metabolism. The detrimental effects of maternal HFD on glucose tolerance were worse in 24-week-old male and female Sod3−/− offspring compared with Sod3f/f (Supplementary Fig 5A and B and Fig. 4A and B). Maternal exercise improved glucose tolerance in male and female Sod3f/f offspring of HFD-fed dams. However, there was no effect of maternal exercise to improve glucose tolerance in Sod3−/− offspring. Basal and (4-chlorophenylthio)adenosine 3′,5′-cyclic monophosphate (pCPT-cAMP)-mediated glucose production was increased in hepatocytes from 16-week-old male and female Sod3f/f offspring of HFD-fed dams (Fig. 4C and D). Sod3−/− hepatocytes had impaired function compared with Sod3f/f, and the beneficial effects of maternal exercise to decrease glucose production were not present in Sod3−/−. Hepatocytes from 16-week-old male and female Sod3−/− offspring of HFD-fed dams showed decreased gene expression (Supplementary Fig. 5C and D) and reduced H3K4me3 levels at the promoter of Pfkl, Pdha1, Ogdh, Acox1, Cpt1a, and Lcad (Fig. 4E). The positive effects of maternal exercise on gene expression and H3K4me3 levels at the promoters of hepatic genes were also diminished in Sod3−/− offspring. HKMT activity was attenuated in Sod3−/− offspring livers from both chow- and HFD-fed dams (Fig. 4F). The levels of ROS (Fig. 4G), total carbonylated proteins (Fig. 4H), and WDR82 carbonylation (Fig. 4I) were significantly higher in Sod3−/− offspring livers than Sod3f/f of HFD-fed dams. These data demonstrate that maternal exercise did not improve these harmful effects of maternal HFD on offspring livers in Sod3−/−. Placental SOD3 is indispensable for the protection of offspring from the metabolic dysfunction caused by maternal HFD-induced HKMT dysfunction and WDR82 carbonylation.
Figure 4.
Beneficial effects of maternal exercise on glucose metabolism and WDR82 carbonylation in offspring of HFD-fed dams were blocked by placenta-specific Sod3 knockout. A and B: Glucose tolerance measured at 24 weeks in Sod3f/f or Sod3−/− offspring of dams that were sedentary or trained and fed chow or the HFD. Glucose area under the curve (AUC) of male (A) and female (B) offspring is shown. GTT, glucose tolerance test. Data are means ± SEM (n = 5–7/group). **P < 0.01 vs. Chow-Sod3f/f-Sed; §P < 0.01 effect of genotype; ¶P < 0.01 effect of diet. Glucose production in hepatocytes of 16-week-old male (C) and female (D) Sod3f/f or Sod3−/− offspring of dams that were sedentary (Sed) or trained and fed the HFD. Data are means ± SEM (n = 3). **P < 0.01 vs. Sod3f/f-HFD-Sedentary, §P < 0.01 effect of genotype. Effects of placenta-specific Sod3−/− on mRNA expression of glucose metabolism genes (E), H3K4 methyltransferase activity (F), ROS levels (G), carbonylated protein content (H), and carbonylated WDR82 levels (I) in livers of E13.5 offspring of sedentary or trained HFD-fed dams (n = 3). HF, high-fat diet; IP, immunoprecipitation; Mat Treat. Maternal treatment. All data are reported as means ± SEM.**P < 0.01 vs. Sod3f/f-HF-Sed; ***P < 0.001 vs. Sod3f/f-HF-Sed; §P < 0.01 effect of genotype. Statistical significance was determined by one- or two-way ANOVA, with Tukey and Bonferroni post hoc analysis.
Benefits of Placental SOD3 for Offspring Hepatic Function Are Not Provided by an Antioxidant
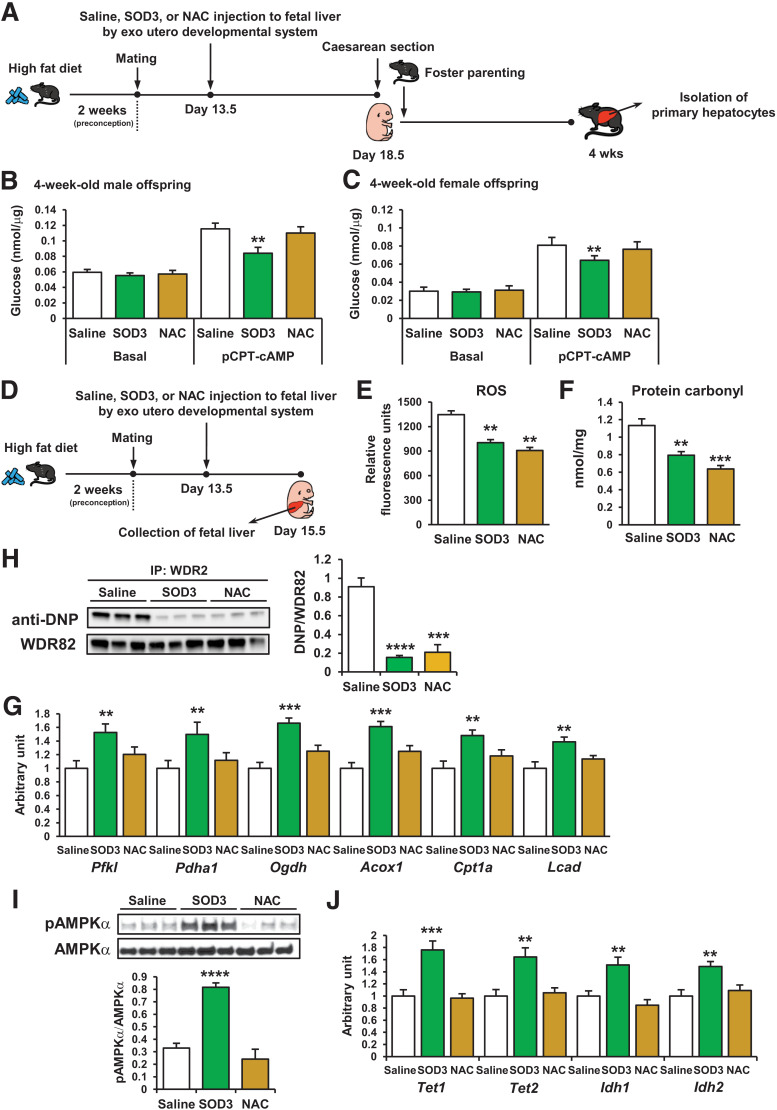
SOD3 has been recognized as a redox enzyme that reduces the potential toxicity of ROS-induced cellular damage (29). To determine whether antioxidant reagents can substitute for SOD3 to protect offspring metabolism from maternal HFD-induced ROS reaction in offspring livers, we compared the effects of recombinant SOD3 protein and the antioxidant NAC (30) on offspring hepatic function of HFD-fed dams. E13.5 offspring livers were injected with SOD3, NAC, or saline using an exo utero developmental system (14,20). Dams were fed the HFD for 5 days after these injections. Offspring were delivered by caesarean section on E18.5 and were parented by chow-fed foster mothers (Fig. 5A). The recombinant SOD3 treatment decreased glucose production in the hepatocytes from 4-week-old male and female offspring; however, the NAC treatment had no effect (Fig. 5B and C). To investigate why NAC did not alter glucose production, dams were fed the HFD preconception and during gestation. SOD3, NAC, or saline were injected into the offspring livers at day E13.5, and the fetal livers were collected at E15.5 (Fig. 5D). The SOD3 and NAC treatments significantly decreased ROS levels (Fig. 5E), total carbonylated protein (Fig. 5F), and carbonylated WDR82 (Fig. 5G) in offspring livers, and the reductions were greater in response to NAC compared with SOD3. The mRNA expression levels of Pfkl, Pdha1, Ogdh, Acox1, Cpt1a, and Lcad were improved by SOD3 treatment in offspring livers; however, NAC treatment did not affect these gene expressions (Fig. 5H). Since our previous study showed that placental SOD3 activates the AMPK/isocitrate dehydrogenase (IDH)/TET axis regulating the improvement of glucose metabolism in offspring (14), we examined this signal in SOD3- and NAC-treated offspring livers. SOD3 treatment significantly increased AMPKα phosphorylation (Fig. 5I) and mRNA expression of Tet1, Tet2, Idh1, and Idh2 (Fig. 5J) in E15.5 offspring livers; however, NAC had no effect. Taken together, these results suggest that both decreased ROS signaling and SOD3-mediated AMPK/TET signaling are necessary for the beneficial effects of maternal exercise on offspring liver.
Figure 5.
Effects of SOD3 on offspring glucose metabolism are distinct from NAC. A and D: Developmental system used to treat offspring livers with recombinant SOD3 or NAC exo utero. Offspring livers were collected at 4 weeks (A) or at E13.5 (D). Glucose production in primary hepatocytes of 4-week-old male (B) and female (C) offspring of HFD-fed, saline-, SOD3-, or diethyldithiocarbamate (DETCA)-treated dams (n = 3). **P < 0.01 vs. pCPT-saline. Effects of SOD3 or NAC treatment in utero on ROS levels (E), carbonylated protein content (F), WDR82 carbonylation levels (G), mRNA expression of glucose metabolism genes (H), AMPKα phosphorylation (pAMPKα) levels (I), and mRNA expression of Tet and Idh (J) in livers of E13.5 offspring of HFD-fed dams (n = 3). IP, immunoprecipitation. All data are reported as means ± SEM. **P < 0.01 vs. pCPT-saline; ***P < 0.01 vs. pCPT-saline; ****P < 0.01 vs. pCPT-saline. Statistical significance was determined by one- or two-way ANOVA, with Tukey and Bonferroni post hoc analysis.
Discussion
The detrimental effects of maternal HFD-feeding behavior on offspring health are difficult to reverse in later life. Epigenetic mechanisms are implicated in maternal diet-induced metabolic changes in adult offspring (12,14–16,31). Maternal exercise protects the offspring against maternal HFD-induced metabolic disorders by reversing maternal HFD-induced disturbances in the epigenetic profiles of offspring. Here, we establish that the mechanism of this protective effect involves stabilization of H3K4me3 levels leading to the reversal of altered glucose metabolic gene expressions in offspring of HFD-fed dams. Maternal exercise prevents WDR82 from being carbonylated by maternal HFD-induced ROS in offspring liver. Placenta-specific Sod3−/− mice showed higher protein carbonylation and reduced metabolic function compared with Sod3f/f, indicating that basal exposure of the fetal liver to placental SOD3 fundamentally maintains glucose homeostasis in offspring of HFD-fed dams.
Since mothers and pups did not exercise once the pups were born in our maternal exercise program, we eliminated the direct effect of postnatal exercise on offspring. Through recombinant SOD3 injection using the exo utero developmental system and fostering by sedentary mothers, we also showed that the protective effects of SOD3 on HFD-induced ROS production during the embryonic period are beneficial for offspring metabolism. Previous studies have indicated that exercise during the gestation period alone can confer some but not all of the beneficial effects to offspring (1,3), and we have reported that maternal exercise can alter the components of breast milk (13).
Most of the H3K4me3 peaks have been shown to be highly enriched at the active promoters of most genes (32). We found that maternal HFD decreases H3K4me3 levels at the promoters of glucose metabolism-related genes in the offspring liver and that this suppressive effect was eliminated by maternal exercise. These H3K4me3 abundant sequences overlapped with the maternal exercise-induced DNA demethylation sites (14). Hypomethylated CpG regions have been shown to have high levels of H3K4me3, whereas sites with hypermethylated CpG lacked H3K4me3 (33). Other work has demonstrated that TETs are specifically recruited to H3K4me3 elevated sites (18). We found that neither maternal diet nor exercise affect H3K4me3 levels at the promoters of housekeeping genes, suggesting that TET may regulate the selective effects of maternal exercise and diet on hepatic gene expression in offspring through changes in H3K4me3 and corresponding DNA demethylation. The causes of the specific changes to H3K4me3 levels remain unknown. However, the differences in H3K4me3 domains between housekeeping and cell-type specific genes may regulate chromatin accessibility (34,35), and/or enriched H3K4me3 may control transcriptional activation by interacting with superenhancers (36). Taken together, dynamic maternal exercise- and diet-induced changes in gene expression may be related to combined epigenetic regulation via DNA demethylation and high H3K4me3 sites.
The detrimental effects of the maternal HFD on offspring metabolism were caused by ROS accumulation in fetal offspring livers and were reversed by maternal exercise. Another study investigating the effects of maternal exercise in mothers with streptozotocin-induced pregestational diabetes found that maternal diabetes increased ROS levels in fetal offspring hearts and that maternal exercise mitigated ROS-induced heart failure (37). Maternal metabolic dysfunction-induced increases in ROS accumulation in fetal tissues may negatively impact offspring organs, and maternal exercise offers a promising means to prevent these harmful phenotypes. ROS exposure in Caenorhabditis elegans was shown to decrease global levels of H3K4me3 in early life (38). In accordance with our finding, ROS inhibited HKMT activity rather than the expression of the HKMT complex in C elegans. These results suggest that H3K4me3 is a maternal HFD-induced, ROS-sensitive epigenetic modification affecting gene expression in offspring organs. Furthermore, maternal exercise prevents ROS-sensitive epigenetic changes during offspring development.
We found that the decrease in H3K4me3 levels in offspring livers of HFD-fed dams was caused by ROS-induced WDR82 carbonylation. WDR82 is required to target methylation sites near transcription start sites (27,28). A previous study reported that WDR-deficient embryos have high apoptotic rates at the blastocyst stage (39), which is interesting in light of our previous finding that HFD-fed dams presented with decreased litter size whereas exercise-trained dams had increased litters (7). This observation may be related to maternal diet and exercise-regulated WDR82 function during development, suggesting the effects of maternal HFD on WDR82 in offspring manifests as both embryonic lethality and offspring metabolic phenotypes. Other dysfunctional proteins may also contribute to metabolic disorders in adult offspring; however, irreversible ROS-induced protein carbonylation does not always cause protein dysfunction (40,41). Our study is the first to establish a putative relationship between functional abnormalities of WDR82 carbonylation in the fetus and the risk of glucose intolerance in adult offspring. Other types of ROS-induced posttranslational modification, including advanced protein oxidation and tyrosine-nitration, may be involved in the harmful effects of ROS accumulation (42). Further studies are needed to elucidate the deleterious effects of ROS in offspring livers from HFD-fed dams.
Another important finding of this study is that NAC antioxidant treatment cannot adequately substitute for the beneficial effects of exercise-induced placental SOD3 on offspring metabolism. SOD3 protein treatment mimicked the improvements in hepatic function in offspring from trained dams, whereas no effect of NAC on hepatocyte glucose production was observed despite decreased ROS levels. Relatively, human studies have shown that the application of NAC in sepsis patients reduced oxidative stress but did not improve disease outcome (43). Previous studies have shown that NAC treatment does not affect AMPK phosphorylation in mouse (44) and human (45) skeletal muscle, and we found that NAC did not activate the AMPK-TET axis, which regulates the SOD3-induced signals leading to DNA demethylation (14). Therefore, it is likely that both prevention of high levels of ROS-stimulated protein carbonylation and AMPK activation are needed for the benefits of maternal exercise on offspring. It should be noted that while an overactive ROS-protein carbonylation cycle has been linked to metabolic dysfunction in adults (46,47), ROS-mediated signaling is essential for maintaining the function of trophoblasts (48) and placenta (49), suggesting that a baseline level of ROS is necessary for normal development. The importance of a physiological balance between oxidant/antioxidant is also cited as a limitation in the treatment of lung disease pathogenesis (50). The precise mechanism by which SOD3 regulates ROS level in the fetal liver is not known and will be an important area of future investigation.
In conclusion, we have determined the epigenetic mechanisms underlying the effects of maternal exercise to reverse the deleterious effects of maternal HFD on glucose metabolism in offspring. This reversal occurs through protection of WDR82 from ROS-induced protein carbonylation, allowing for increased H3K4 methyltransferase activity and increased H3K4me3 levels at the promoter of glucose metabolism genes. Placental SOD3 is necessary for these effects of maternal exercise on the ROS-mediated carbonylation/H3K4me3 axis and thus has a vital role in regulating offspring epigenetics and transmitting the benefits of exercise during pregnancy to the next generation. These finding may have enormous clinical relevance, as maternal exercise is both a powerful and inexpensive tool for addressing the transmission of metabolic disease.
Article Information
Acknowledgments. The authors thank Ross Tomaino, from Taplin Mass Spectrometry Facility at Harvard Medical School, and Laura Hernandez-Lagunas, from University of Colorado Anschutz Medical Center. The authors also thank Drs. Roeland J.W. Middelbeek, Pasquale Nigro, Maria Vamvini, Susana Rovira Llopis, Sarah Lessard, and Tara MacDonald, all from the Joslin Diabetes Center at Harvard Medical School, for helpful scientific discussions.
Funding. This work was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK101043 (to L.J.G.) and P30DK036836 (Diabetes Research Center to Joslin Diabetes Center) and National Heart, Lung, and Blood Institute grant 1R35HL139726 (to E.S.N.), by the American Diabetes Association (training grant number 1-17-PMF-009 to A.B.A.-W.), by the Tohoku Initiative for Fostering Global Researchers for Interdisciplinary Sciences (TI-FRIS) (to J.K.), and by JSPS KAKENHI grant number 21H03315 (to J.K.). J.K. was supported by individual research fellowships from Sunstar Foundation, JSPS Overseas Research Fellowships, Kanae Foundation for the Promotion of Medical Science, and Meiji Yasuda Life Foundation of Health and Welfare.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.K. designed the study, conducted most of the experiments, analyzed data, and wrote the paper. N.S.M., B.G.A., A.B.A.-W., R.H.C., and N.B.P. assisted with animal experiments. Y. Xiu and Y. Xia provided the Ada-Cre mice. C.R.R.A. assisted with the analysis of carbonylated proteins. K.R. helped with histone analysis. C.K., T.H., and R.N. assisted with exo utero developmental system and analysis of embryos. M.F.H. managed the generation Sod3-knockout mice and supervised all experiments. E.S.N. supervised the experiments for SOD3 analysis. L.J.G. directed the research project, designed experiments, and wrote the paper. All authors reviewed the manuscript and approved the final manuscript. J.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.19337927.
References
- 1. Kusuyama J, Alves-Wagner AB, Makarewicz NS, Goodyear LJ. Effects of maternal and paternal exercise on offspring metabolism. Nat Metab 2020;2:858–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Perng W, Oken E, Dabelea D. Developmental overnutrition and obesity and type 2 diabetes in offspring. Diabetologia 2019;62:1779–1788 [DOI] [PubMed] [Google Scholar]
- 3. Harris JE, Baer LA, Stanford KI. Maternal exercise improves the metabolic health of adult offspring. Trends Endocrinol Metab 2018;29:164–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Franco JG, Fernandes TP, Rocha CP, et al. Maternal high-fat diet induces obesity and adrenal and thyroid dysfunction in male rat offspring at weaning. J Physiol 2012;590:5503–5518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Volpato AM, Schultz A, Magalhães-da-Costa E, Correia ML, Águila MB, Mandarim-de-Lacerda CA. Maternal high-fat diet programs for metabolic disturbances in offspring despite leptin sensitivity. Neuroendocrinology 2012;96:272–284 [DOI] [PubMed] [Google Scholar]
- 6. Pereira TJ, Fonseca MA, Campbell KE, et al. Maternal obesity characterized by gestational diabetes increases the susceptibility of rat offspring to hepatic steatosis via a disrupted liver metabolome. J Physiol 2015;593:3181–3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stanford KI, Lee MY, Getchell KM, So K, Hirshman MF, Goodyear LJ. Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring. Diabetes 2015;64:427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stanford KI, Takahashi H, So K, et al. Maternal exercise improves glucose tolerance in female offspring. Diabetes 2017;66:2124–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stevanović-Silva J, Beleza J, Coxito P, et al. Maternal high-fat high-sucrose diet and gestational exercise modulate hepatic fat accumulation and liver mitochondrial respiratory capacity in mothers and male offspring. Metabolism 2021;116:154704. [DOI] [PubMed] [Google Scholar]
- 10. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 2018;14:88–98 [DOI] [PubMed] [Google Scholar]
- 11. Carter LG, Lewis KN, Wilkerson DC, et al. Perinatal exercise improves glucose homeostasis in adult offspring. Am J Physiol Endocrinol Metab 2012;303:E1061–E1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laker RC, Lillard TS, Okutsu M, et al. Exercise prevents maternal high-fat diet-induced hypermethylation of the Pgc-1α gene and age-dependent metabolic dysfunction in the offspring. Diabetes 2014;63:1605–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harris JE, Pinckard KM, Wright KR, et al. Exercise-induced 3′-sialyllactose in breast milk is a critical mediator to improve metabolic health and cardiac function in mouse offspring. Nat Metab 2020;2:678–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kusuyama J, Alves-Wagner AB, Conlin RH, et al. Placental superoxide dismutase 3 mediates benefits of maternal exercise on offspring health. Cell Metab 2021;33:939–956.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pang H, Ling D, Cheng Y, et al. Gestational high-fat diet impaired demethylation of Pparα and induced obesity of offspring. J Cell Mol Med 2021;25:5404–5416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang KF, Cai W, Xu JL, Shi W. Maternal high-fat diet programs Wnt genes through histone modification in the liver of neonatal rats. J Mol Endocrinol 2012;49:107–114 [DOI] [PubMed] [Google Scholar]
- 17. Suter MA, Ma J, Vuguin PM, et al. In utero exposure to a maternal high-fat diet alters the epigenetic histone code in a murine model. Am J Obstet Gynecol 2014;210:463.e1–463.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu H, D’Alessio AC, Ito S, et al. Dual functions of Tet1 in transcriptional regulation in mouse embryonic stem cells. Nature 2011;473:389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu Y, Wu F, Tan L, et al. Genome-wide regulation of 5hmC, 5mC, and gene expression by Tet1 hydroxylase in mouse embryonic stem cells. Mol Cell 2011;42:451–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yamada M, Hatta T, Otani H. Mouse exo utero development system: protocol and troubleshooting. Congenit Anom (Kyoto) 2008;48:183–187 [DOI] [PubMed] [Google Scholar]
- 21. Jambhekar A, Dhall A, Shi Y. Roles and regulation of histone methylation in animal development. Nat Rev Mol Cell Biol 2019;20:625–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hughes AL, Kelley JR, Klose RJ. Understanding the interplay between CpG island-associated gene promoters and H3K4 methylation. Biochim Biophys Acta Gene Regul Mech 2020;1863:194567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hecker M, Wagner AH. Role of protein carbonylation in diabetes. J Inherit Metab Dis 2018;41:29–38 [DOI] [PubMed] [Google Scholar]
- 24. Roberts CK, Vaziri ND, Wang XQ, Barnard RJ. Enhanced NO inactivation and hypertension induced by a high-fat, refined-carbohydrate diet. Hypertension 2000;36:423–429 [DOI] [PubMed] [Google Scholar]
- 25. Matsuzawa-Nagata N, Takamura T, Ando H, et al. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism 2008;57:1071–1077 [DOI] [PubMed] [Google Scholar]
- 26. Suzuki YJ, Carini M, Butterfield DA. Protein carbonylation. Antioxid Redox Signal 2010;12:323–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu M, Wang PF, Lee JS, et al. Molecular regulation of H3K4 trimethylation by Wdr82, a component of human Set1/COMPASS. Mol Cell Biol 2008;28:7337–7344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee JH, Skalnik DG. Wdr82 is a C-terminal domain-binding protein that recruits the Setd1A Histone H3-Lys4 methyltransferase complex to transcription start sites of transcribed human genes. Mol Cell Biol 2008;28:609–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Y, Branicky R, Noë A, Hekimi S. Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J Cell Biol 2018;217:1915–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rushworth GF, Megson IL. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol Ther 2014;141:150–159 [DOI] [PubMed] [Google Scholar]
- 31. Liang X, Yang Q, Fu X, et al. Maternal obesity epigenetically alters visceral fat progenitor cell properties in male offspring mice. J Physiol 2016;594:4453–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Park S, Kim GW, Kwon SH, Lee JS. Broad domains of histone H3 lysine 4 trimethylation in transcriptional regulation and disease. FEBS J 2020;287:2891–2902 [DOI] [PubMed] [Google Scholar]
- 33. Dahl JA, Jung I, Aanes H, et al. Broad histone H3K4me3 domains in mouse oocytes modulate maternal-to-zygotic transition. Nature 2016;537:548–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Benayoun BA, Pollina EA, Ucar D, et al. H3K4me3 breadth is linked to cell identity and transcriptional consistency. Cell 2014;158:673–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen K, Chen Z, Wu D, et al. Broad H3K4me3 is associated with increased transcription elongation and enhancer activity at tumor-suppressor genes. Nat Genet 2015;47:1149–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dhar SS, Zhao D, Lin T, et al. MLL4 is required to maintain broad H3K4me3 peaks and super-enhancers at tumor suppressor genes. Mol Cell 2018;70:825–841.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Saiyin T, Engineer A, Greco ER, et al. Maternal voluntary exercise mitigates oxidative stress and incidence of congenital heart defects in pre-gestational diabetes. J Cell Mol Med 2019;23:5553–5565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bazopoulou D, Knoefler D, Zheng Y, et al. Developmental ROS individualizes organismal stress resistance and lifespan. Nature 2019;576:301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bi Y, Lv Z, Wang Y, et al. WDR82, a key epigenetics-related factor, plays a crucial role in normal early embryonic development in mice. Biol Reprod 2011;84:756–764 [DOI] [PubMed] [Google Scholar]
- 40. Bachi A, Dalle-Donne I, Scaloni A. Redox proteomics: chemical principles, methodological approaches and biological/biomedical promises. Chem Rev 2013;113:596–698 [DOI] [PubMed] [Google Scholar]
- 41. Souza JM, Peluffo G, Radi R. Protein tyrosine nitration--functional alteration or just a biomarker? Free Radic Biol Med 2008;45:357–366 [DOI] [PubMed] [Google Scholar]
- 42. Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol 2020;21:363–383 [DOI] [PubMed] [Google Scholar]
- 43. Szakmany T, Hauser B, Radermacher P. N-acetylcysteine for sepsis and systemic inflammatory response in adults. Cochrane Database Syst Rev 2012;2012:CD006616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Merry TL, Steinberg GR, Lynch GS, McConell GK. Skeletal muscle glucose uptake during contraction is regulated by nitric oxide and ROS independently of AMPK. Am J Physiol Endocrinol Metab 2010;298:E577–E585 [DOI] [PubMed] [Google Scholar]
- 45. Merry TL, Wadley GD, Stathis CG, et al. N-Acetylcysteine infusion does not affect glucose disposal during prolonged moderate-intensity exercise in humans. J Physiol 2010;588:1623–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Newsholme P, Cruzat VF, Keane KN, Carlessi R, de Bittencourt PI Jr. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem J 2016;473:4527–4550 [DOI] [PubMed] [Google Scholar]
- 47. Hauck AK, Huang Y, Hertzel AV, Bernlohr DA. Adipose oxidative stress and protein carbonylation. J Biol Chem 2019;294:1083–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Banerjee P, Malik A, Malhotra SS, Gupta SK. Role of STAT signaling and autocrine action of chemokines during H2O2 induced HTR-8/SVneo trophoblastic cells invasion. J Cell Physiol 2019;234:1380–1397 [DOI] [PubMed] [Google Scholar]
- 49. Nezu M, Souma T, Yu L, et al. Nrf2 inactivation enhances placental angiogenesis in a preeclampsia mouse model and improves maternal and fetal outcomes. Sci Signal 2017;10:eaam5711. [DOI] [PubMed] [Google Scholar]
- 50. Villegas L, Stidham T, Nozik-Grayck E. Oxidative stress and therapeutic development in lung diseases. J Pulm Respir Med 2014;4:194. [DOI] [PMC free article] [PubMed] [Google Scholar]