Abstract
Study Objectives:
It has been suggested that treatment for obstructive sleep apnea (OSA) reduces cardiovascular risk. So far, knowledge is limited about the difference in the reduction of this risk between mandibular advancement device (MAD) and continuous positive airway pressure (CPAP) therapy. The aim of this study was to compare the cardiovascular effects of MAD vs CPAP therapy in patients with moderate OSA.
Methods:
Patients with an apnea-hypopnea index of 15–30 events/h were randomized to either MAD or CPAP therapy. At baseline and after 12-month follow-up, 24-hour ambulant blood pressure measurements and laboratory measurements were performed. Ambulant blood pressure measurements consisted of 24-hour, daytime and night-time systolic and diastolic blood pressure and heart rate measurements. Laboratory measurements consisted of serum lipid values, creatinine, high-sensitivity C-reactive protein, plasma glucose, hemoglobin A1c glycated hemoglobin, proinflammatory cytokines, soluble receptor for advanced glycation end-products, chemokines, and adhesion molecules.
Results:
Of the 85 randomized patients with moderate OSA, data were available for 54 patients (n = 24 MAD, n = 30 CPAP) at 12-month follow-up and showed that apnea-hypopnea index significantly decreased with either therapy. In the MAD group, soluble receptor for advanced glycation end-products and glycated hemoglobin were significantly higher after 12 months’ follow-up compared to baseline. No significant changes were found between MAD and CPAP treatments for all outcomes.
Conclusions:
Treatment of patients with moderate OSA with either MAD or CPAP therapy had no profound effects on major cardiovascular risk factors after 12 months.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Title: MRA Therapy vs CPAP Therapy in Moderate OSAS; Identifier: NCT01588275; URL: https://clinicaltrials.gov/ct2/show/NCT01588275
Citation:
Uniken Venema JAM, Knol-de Vries GE, van Goor H, Westra J, Hoekema A, Wijkstra PJ. Cardiovascular and metabolic effects of a mandibular advancement device and continuous positive airway pressure in moderate obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2022;18(6)1547–1555.
Keywords: obstructive sleep apnea, treatment, mandibular advancement device, continuous positive airway therapy, cardiovascular effects, metabolic effects
BRIEF SUMMARY
Current Knowledge/Study Rationale: It has been suggested that obstructive sleep apnea increases the risk of developing sustained periods of hypertension, arrhythmias, and cardiovascular disease. This study aimed to compare the cardiovascular effects of mandibular advancement device and continuous positive airway pressure therapy in moderate obstructive sleep apnea.
Study Impact: At 12-month follow-up, no profound effects on cardiovascular risk factors were seen. In addition, both therapies did not differ significantly. These results do not support an earlier finding that continuous positive airway pressure and mandibular advancement device therapies reduce systolic blood pressure and diastolic blood pressure in patients with moderate obstructive sleep apnea.
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by repetitive upper airway collapse during sleep, resulting in a complete (apnea) or partial (hypopnea) obstruction in airflow and reduced oxygen saturation levels (hypoxia).1 The increased respiratory effort to restore oxygen levels (reoxygenation) results in activation of the sympathetic nervous system and brief awakenings from sleep (arousals).2,3 The sympathetic nervous system activation contributes to peripheral vasoconstriction and increases heart rate and arterial blood pressure. OSA is independently associated with an increased risk of developing sustained periods of hypertension, arrhythmias, and cardiovascular disease.4–7
The underlying mechanisms explaining the association between OSA and cardiovascular disease are not completely understood.4,5,8,9 It has been suggested that intermittent hypoxia leads to enhanced oxidative stress due to increased formation of reactive oxygen species, decreased nitric oxide levels and lipid peroxidation, and subsequent release of proinflammatory cytokines, chemokines, and adhesion molecules.10 Besides, the activation of the sympathetic nervous system can also result in hypertension.6,7 These events eventually promote endothelial dysfunction and atherosclerosis.11,12
In addition, OSA is strongly associated with metabolic diseases, including obesity, insulin resistance, and type 2 diabetes mellitus. The incidence of these metabolic diseases increases each year.13 A large proportion of patients with OSA fulfill the criteria of metabolic syndrome (MetS). This condition is a diagnosis of a combination of metabolic and cardiovascular abnormalities and includes measurements of fasting glucose, blood pressure, triglycerides, high density lipoprotein cholesterol, and waist circumference.14,15
It has been suggested that OSA treatment lowers cardiovascular risks by reducing blood pressure and inflammation and improving a patient’s lipid profile.4,16 However, to date not many clinical studies have been performed evaluating these specific treatment outcomes. It has been shown that continuous positive airway pressure (CPAP) reduces systolic (SBP) and diastolic (DBP) blood pressure, and has a positive effect on high-sensitivity C-reactive protein, inflammation, improvement in endothelial function, and a reduction in arterial stiffness.17–20 Therapy with a mandibular advancement device (MAD) shows effects on SBP and DBP comparable to CPAP.8 However, to date, there remains a paucity of studies assessing the effect of MAD therapy on other cardiovascular and metabolic outcomes. In particular, clinical studies directly comparing MAD and CPAP therapies in moderate OSA on cardiovascular and metabolic biomarkers are scarce.
Therefore, the aim of this study was to compare the cardiovascular and metabolic effects of MAD vs CPAP therapy in patients with moderate OSA. We hypothesized that there would be a comparable effect between MAD and CPAP therapies on these outcome measures.
METHODS
The measurements of the cardiovascular and metabolic effects were part of a randomized controlled trial. The primary outcome measure was apnea-hypopnea index (AHI) reduction by MAD vs CPAP therapy in patients with moderate OSA.21 Cardiovascular and metabolic effects were secondary outcomes in this trial.8
Participants
Adult participants, who had been diagnosed with moderate OSA (AHI 15–30 events/h), based on a single-night polysomnography, were included. Candidates were excluded when diagnosed with reported or documented unstable endocrine dysfunction, or clinically concurrent severe cardiovascular and/or pulmonary comorbidity. Participants were scheduled for a baseline visit at which a physical examination (measurements of weight, waist-and neck circumference, fat percentage, and skin autofluorescence with an advanced glycation end-products reader), 24-hour blood pressure assessment, and fasting blood and urine sampling were performed. After the baseline visit patients were randomized to either MAD or CPAP therapy. Randomization was performed using a computer program, thereby considering baseline hypercholesterolemia, diabetes, and hypertension status to minimize any imbalance between the numbers of patients in each treatment group. Consequently, the effect of a priori cardiovascular differences between patients receiving MAD therapy and CPAP therapy was minimized. After 12 months a full examination was performed, including all the measurements determined at the baseline visit.
The study was approved by the Ethical Committee of University Medical Center Groningen (number NL34138.042.10). Furthermore, the study was registered at ClinicalTrials.gov (number NCT01588275). All patients provided written informed consent before inclusion in the study.
Intervention therapy
MAD therapy
Patients randomized to MAD therapy were treated with a titratable bibloc MAD (SomnoDent MAS; SomnoMed Australia/Europe AG), customized by dental specialists experienced in the field of dental sleep medicine. At baseline, the mandible was set at 60%–70% of the patient’s maximum advancement. During follow-up, the mandible was further advanced until symptoms abated or until further protrusion of the mandible resulted in discomfort.
CPAP therapy
Patients randomized to CPAP therapy were treated with fixed-pressure CPAP (autoCPAP, Philips Respironics REMstar Auto A-Flex, provided by VitalAire BV The Netherlands). The proper pressure for each patient was set using a predefined protocol.22 Patients were fitted with a comfortable CPAP mask before the titration procedure started. Patients were allowed to change masks and to use chinstraps or a humidifier, if necessary.
Cardiovascular risk outcomes
Ambulatory blood pressure monitoring
Systolic and diastolic blood pressure and heart rate were measured every 30 minutes during a 24-hour period (Spacelab 90207; SpaceLabs Inc, Redmond, WA). Hypertension was defined as use of antihypertensive medication or SBP > 140 mm Hg and/or DBP > 90 mm Hg. Dipping was calculated by subtracting mean blood pressure at night from mean blood pressure during the day and dividing this outcome by the mean blood pressure during the day.23
Blood and urine sample assessment
Venous blood samples and urine samples were collected in the morning after polysomnography testing. During venous blood sampling patients remained in a sitting position. Samples were directly frozen after collection. When all samples were collected, they were analyzed at the same time in the same laboratory. Fasting concentrations of creatinine, triglycerides, total cholesterol, low-density lipoprotein cholesterol and high density lipoprotein cholesterol, high-sensitivity C-reactive protein, and plasma glucose were analyzed following the Roche cobas c module (Roche Diagnostics, Basel, Switzerland). Fasting concentrations of glycated hemoglobin (HbA1c) were analyzed following the Tosoh G8 module (Tosoh, Tessenderlo, Belgium). Fasting concentrations of N-terminal pro–hormone B-type natriuretic peptide (NTproBNP) were analyzed following the Roche cobas e module. Finally, fasting concentrations of interleukin (IL)-6, IL-18, tumor necrosis factor α, and soluble receptor for advanced glycation end-products (sRAGE) were analyzed following the R&D Systems enzyme-linked immunosorbent assay (ELISA) module (Minneapolis, MN).
Assessment of advanced glycation end-products
Advanced glycation end-products (AGEs) are considered to be markers of oxidative and glycemic stress and to be involved in the atherosclerotic process. Tissue accumulation of AGEs in the skin tissue was measured noninvasively using the AGE-Reader (DiagnOptics BV, Groningen, The Netherlands). This device assesses skin autofluorescence, expressed in arbitrary units (AU) of AGEs on the volar side of the forearm, using the fluorescent properties of the AGEs. More detailed information about this protocol has previously been described by Koetsier et al24
Other assessments
For each patient, presence of MetS was assessed, based on fasting glucose, SBP, triglycerides, high-density lipoprotein, and waist circumference at baseline and after 12 months’ follow-up according to the ATP III criteria.25,26
Furthermore, an estimation of 10-year risk of fatal cardiovascular disease was assessed with the Systematic Coronary Risk Evaluation (SCORE) scale for each patient using age, sex, smoking status, systolic blood pressure, and total cholesterol. The risk score was obtained using the SCORE chart for populations at low cardiovascular disease risk. According to the European Society of Cardiology guidelines, a SCORE risk score of 5% or higher was considered high risk.27
Smoking status was divided into current smokers, ex-smokers, and never smokers.
Statistical analysis
Descriptive statistics for continuous variables are presented as means and standard deviations (normal distribution) or medians and interquartile ranges (skewed distribution). Categorical variables are presented in terms of proportions.
Differences between baseline and follow-up variables within the groups were compared using the paired Student’s t test for normal distributions or Wilcoxon’s signed rank test for variables with skewed distributions. Differences between groups were compared using the independent Student’s t test for normal distributions or Mann-Whitney U test for variables with skewed distributions.
“Intention-to-treat” and “per protocol” analyses were performed. Since both analyses yielded comparable results, only the per protocol analysis was reported in case of similar outcomes. Correlations between inflammatory markers and AHI (at baseline, at follow-up, and the difference between baseline and follow-up) were performed with Pearson’s correlation coefficient. Differences were considered to be statistically significant when P < .05. Statistical analyses are performed using IBM SPSS Statistics 23 (Armonk, New York).
RESULTS
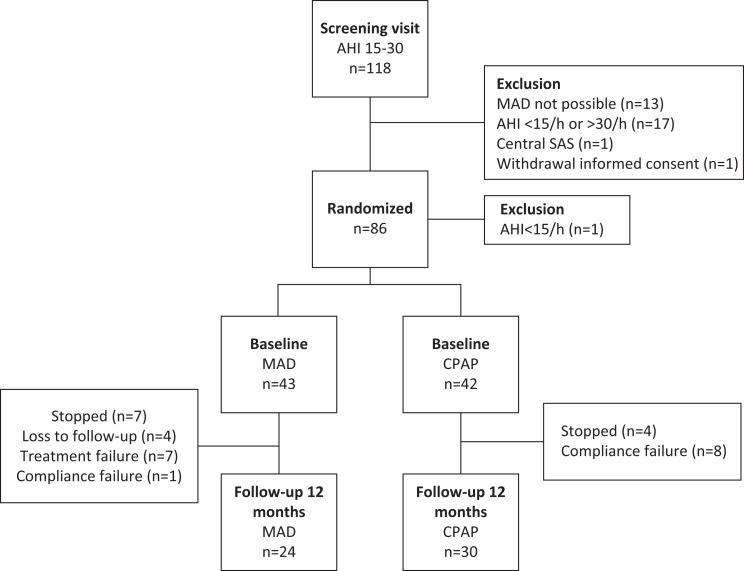
Between June 2012 and September 2016, 118 patients were screened. A total of 32 patients were excluded after the screening visit (Figure 1). Eighty-six patients were randomized (n = 44 MAD; n = 42 CPAP), of which 1 patient (randomized to MAD) was excluded after unjustified randomization due to having mild OSA at baseline, yielding 85 study participants (50.7 ± 9.7 years, body-mass index [BMI] 30.2 ± 4.9 kg/m2, men/women 70/15) with AHI 15–30 events/h (mean AHI 20.9 ± 4.5 events/h). In total, 54 patients (n = 24 MAD; n = 30 CPAP) completed the study period having received the therapy to which they were initially randomized (per protocol group) (Figure 1).
Figure 1. Flowchart showing allocation, and dropout of patients.
AHI = apnea-hypopnea index, CPAP = continuous positive airway pressure, MAD = mandibular advancement device, OSA = obstructive sleep apnea, SAS = sleep apnea syndrome.
There were no significant baseline differences in patient characteristics between participants randomized to MAD therapy or CPAP therapy (Table 1).
Table 1.
Baseline characteristics of patients randomized to MAD and CPAP.
| MAD (n = 43) | CPAP (n = 42) | |
|---|---|---|
| Age (years) | 50 ± 9.7 | 51 ± 9.8 |
| Male sex, n (%) | 37 (86) | 33 (79) |
| AHI (events/h) | 20.9 ± 4.4 | 21.0 ± 4.7 |
| Minimal oxygen saturation (%) | 83.6 ± 4.4 | 82.3 ± 4.2 |
| DM, n (%) | 4 (9) | 4 (10) |
| Type 1/type 2 | 1/3 | 0/4 |
| Smoking status, n (%) | ||
| Never-smoker | 18 (42) | 17 (40) |
| Ex-smoker | 10 (23) | 8 (19) |
| Current smoker | 15 (35) | 16 (38) |
| Medication use, n (%) | ||
| Antihypertensive medication | 10 (23) | 16 (38) |
| Cholesterol-lowering medication | 7 (16) | 5 (12) |
| Anticoagulation | 4 (9) | 4 (10) |
| Arrhythmias | 1 (2) | 1 (2) |
| Hypertension (140/90 and/or medication), n (%) | 14 (33) | 20 (48) |
Data are presented as mean ± SD or number, percentage. Differences between the MAD and CPAP group were not statistically significant. AHI = apnea-hypopnea index, CPAP = continuous positive airway pressure, DM = diabetes mellitus, MAD = mandibular appliance design, SD = standard deviation.
Of the 85 randomized patients, data on cardiovascular outcomes were available for 65 patients (n = 29 MAD; n = 36 CPAP) after 12 months. However, a total of n = 24 MAD and n = 30 CPAP randomized patients fulfilled the study according to protocol. In addition, 2 patients of the per-protocol analyses had no fasting lipid profile and glucose at baseline and were excluded from analysis with respect to these outcome parameters.
AHI was significantly reduced with both MAD and CPAP therapies after 12-month follow-up (Table 2). The reduction in AHI was significantly greater (P < .01) with CPAP (mean reduction AHI 19.1 ± 4.3 events/h) compared to MAD therapy (mean reduction AHI 10.3 ± 12.0 events/h after 12 months) (Table 2).
Table 2.
Cardiovascular and metabolic effects in patients fulfilling the study (per protocol).
| Baseline | 12 Months | |||
|---|---|---|---|---|
| MAD (n = 24) | CPAP (n = 30) | MAD (n = 24) | CPAP (n = 30) | |
| AHI (events/h) | 21.1 ± 4.6 | 20.4 ± 4.3 | 10.8 ± 11.9*# ^ | 1.5 ± 1.6*# ^ |
| BMI (kg/m2) | 28.9 ± 4.5 | 30.7 ± 5.3 | 29.2 ± 4.2 | 31.7 ± 5.1^ |
| Waist circumference (cm) | 104.1 ± 7.8 | 106.5 ± 13.1 | 105.7 ± 8.2 | 108.4 ± 12.58^ |
| Neck circumference (cm) | 40.7 ± 3.4 | 41.3 ± 3.6 | 40.5 ± 3.1 | 41.3 ± 3.4 |
| Minimal oxygen saturation (%) | 83.6 ± 4.4 | 82.3 ± 4.2 | 86.0 (84.3–89.8)* | 92.0 (90.2–93.0)* |
| hsCRP mg/l | 2.1 (0.9–5.0) | 1.5 (0.9–4.3) | 1.5 (0.9–6.3) | 2.4 (1.0–3.7) |
| Triglycerides (mmol/L) | 1.45 (1.09–2.06) | 1.22 (1.09–1.68) | 1.32 (0.98–2.36) | 1.33 (1.03–1.77) |
| Creatinine (mmol/L) | 83.3 ± 13.1 | 80.6 ± 15.8 | 84.0 ± 15.2 | 82.1 ± 17.2 |
| eGFR (mmol/L) | 86.6 ± 15.1 | 86.2 ± 12.5 | 85.0 ± 15.6 | 84.3 ± 16.0 |
| Total cholesterol (mmol/L | 5.8 ± 1.0* | 5.0 ± 1.0* | 5.6 ± 1.1 | 5.1 ± 1.1 |
| HDL-cholesterol (mmol/L) | 1.4 ± 0.4 | 1.3 ± 0.4 | 1.4 ± 0.3 | 1.2 ± 0.4 |
| LDL-cholesterol (mmol/L) | 3.9 ± 0.9* | 3.3 ± 0.9* | 3.7 ± 1.0* | 3.3 ± 1.0* |
| Glucose (mmol/L) | 5.7 ± 0.7 | 5.8 ± 1.0 | 5.9 ± 0.6 | 5.7 ± 0.8 |
| HbA1c (mmol/mol) | 37.4 ± 4.3 | 37.5 ± 6.5 | 38.3 ± 4.3^ | 37.6 ± 6.5 |
| Skin AF (AU) | 2.06 ± 0.54 | 2.25 ± 0.55 | 2.19 ± 0.50 | 2.21 ± 0.58 |
| NTproBNP (pg/mL) | 24.2 (12.3–57.8) | 39.3 (17.3–61.3) | 26.0 (11.3–52.0) | 31.4 (12.2–56.8) |
| IL-6 (pg/mL) | 1.3 (0.8–2.8) | 1.4 (1.0–2.5) | 1.4 (0.7–2.3) | 1.5 (1.2–2.7) |
| IL-18 (pg/mL) | 7.2 (6.3–8.5) | 7.4 (5.8–9.2) | 7.1 (6.2–7.9) | 7.9 (5.7–9.6) |
| TNFα (pg/mL) | 1.1 (1.0–1.3) | 1.3 (1.1–1.5) | 1.2 (1.2–1.4) | 1.3 (1.1–1.5) |
| sRAGE (pg/mL) | 235.1 ± 92.7 | 223.2 ± 92.7 | 248.8 ± 102.3^ | 224.0 ± 90.0 |
| 24-h SBP (mm Hg) | 125.6 ± 14.2 | 129.2 ± 10.9 | 125.5 ± 13.2 | 129.9 ± 12.6 |
| 24-h DBP (mm Hg) | 77.5 ± 9.8 | 79.9 ± 7.7 | 77.0 ± 8.9 | 79.3 ± 8.8 |
| 24-h HR (bpm) | 71.1 ± 10.6 | 70.0 ± 9.1 | 71.1 ± 8.3 | 70.7 ± 11.2 |
| Daytime SBP (mm Hg) | 131.7 ± 13.8 | 134.6 ± 11.2 | 131.4 ± 13.3 | 135.7 ± 13.7 |
| Daytime DBP (mm Hg) | 81.7 ± 9.9 | 84.1 ± 8.1 | 81.8 ± 9.2 | 83.4 ± 9.3 |
| Daytime HR (bpm) | 76.1 ± 11.3 | 73.6 ± 10.4 | 74.9 ± 9.2 | 73.4 ± 10.0 |
| Nighttime SBP (mm Hg) | 113.3 ± 14.7 | 118.1 ± 10.2 | 113.7 ± 13.5 | 118.3 ± 12.8 |
| Nighttime DBP (mm Hg) | 68.4 ± 10.3 | 70.8 ± 7.8 | 67.1 ± 8.7 | 71.1 ± 8.9 |
| Nighttime HR (bpm) | 62.2 ± 9.7 | 62.9 ± 8.7 | 63.4 ± 8.0 | 61.3 ± 8.4 |
| Dipping % SBP (%) | 14.6 ± 5.0 | 12.1 ± 4.8 | 13.5 ± 5.2 | 12.7 ± 6.3 |
| Dipping % DBP (%) | 17.1 ± 7.2 | 15.8 ± 5.7 | 17.9 ± 5.8 | 14.5 ± 6.7 |
| Dipping % HR (%) | 17.5 ± 7.6 | 13.8 ± 10.4 | 15.1 ± 7.0 | 16.1 ± 6.1 |
| MetS, n (%) | 11 (45.8) | 11 (36.7) | 9 (37.5) | 11 (36.7) |
| SCORE (n) | 1 (0–2) | 1 (0–1) | 1 (0–1) | 1 (0–1) |
| SCORE ≥ 5%, n (%) | 0 (0) | 1 (2.4) | 0 (0) | 0 (0) |
Data presented as mean ± SD or median (interquartile range). *Significant difference between MAD and CPAP at baseline or follow-up (independent samples t-test or Mann-Whitney U test). #Significant difference in delta change from baseline to 12-months between MAD and CPAP (independent samples t test or Mann-Whitney U test). ^Significant difference between baseline and follow-up (paired Student’s t test or Wilcoxon’s signed rank test). AHI = apnea-hypopnea index, AU = arbitrary units, BMI = body mass index, bpm = beats per minute, CPAP = continuous positive airway pressure, DBP = diastolic blood pressure, eGFR = estimated glomerular filtration rate, HbA1c = glycated hemoglobin, HDL = high-density lipoproteins, HR = heart rate, hsCRP = high-sensitivity C-reactive protein, IL = interleukin, LDL = low-density lipoprotein, MAD = mandibular appliance design, MetS = metabolic syndrome, NTproBNP = N-terminal pro–hormone B-type natriuretic peptide, SBP = systolic blood pressure, SCORE = Systematic Coronary Risk Evaluation, skin AF = skin autofluorescence, sRAGE = soluble receptor for advanced glycation end-products, TNFα = tumor necrosis factor α.
Scores on the Epworth Sleepiness Scale (ESS) were significantly reduced in both MAD and CPAP from baseline to follow-up. Besides, both therapies improved health-related quality of life. Further analyses of ESS and quality of life scores in this cohort were published by de Vries et al21,28
Blood pressure measurement
Twenty-four-hour, daytime and night-time SBP, DBP, and heart rate (HR) showed no changes after 12 months with either MAD or CPAP therapy (Table 2). Besides, comparing percentages of dipping during the night, 24-hour HR, daytime HR, and night-time HR, no significant differences were found with the intention-to-treat nor with the per protocol analysis.
Blood and urine sample assessment
HbA1c increased significantly in the MAD group from baseline (37.4 ± 4.3) to follow-up (38.3 ± 4.3) (P = .02). In the intention-to-treat analysis this significant difference was not observed. In addition, sRAGE significantly increased in the MAD group from baseline (235.1 ± 92.7) to follow-up (248.8 ± 102.3) (P = .049). Otherwise, there were no significant effects of both MAD and CPAP therapies on laboratory outcomes (Table 2). Baseline values for total cholesterol and low-density lipoprotein cholesterol were significantly higher in the MAD group (5.8 ± 1.0 and 3.9 ± 0.9) compared with CPAP (5.0 ± 1.0 and 3.3 ± 0.9) (P = .007 and P = .014, respectively). This resulted in a significant difference in total cholesterol and low-density lipoprotein cholesterol reduction between MAD and CPAP after 12 months (P = .03).
When performing a Pearson correlation with delta AHI, a significant negative correlation was found with sRAGE in the MAD group. Delta AHI correlated negatively with sRAGE at baseline r(21) = –.519 (P = .016) and at follow-up r(20) = –.499 (P = .025) in MAD group.
Assessment of skin autofluorescence as a measure of AGEs
No significant differences were found between baseline and follow-up measurements of skin autofluorescence. However, in the per protocol analysis a significant difference between the delta AU of MAD and CPAP was observed (respectively, 0.144 ± 0.21 and –0.058 ± 0.25, P = .038) respectively, 0.14 ± 0.21 and −0.05 ± 0.25, P = .038, favoring MAD therapy.
Other assessments
Medication use did change in 7 patients, 4 in the CPAP group and 3 in the MAD group. In the CPAP group, n = 1 had a higher dosage of arrhythmias (verapamil), n = 1 started anticoagulation medication after 6 months (acetylsalicylic acid), n = 1 had a higher dosage of blood pressure lowering medication (perindopril), n = 1 started with cholesterol-lowering medication (simvastatin). In the MAD group, n = 1 had a lower dosage of anticoagulation medication (acetylsalicylic acid), n = 1 had a lower dosage of cholesterol-lowering medication (simvastatin), and n = 1 started blood pressure lowering medication (enalapril).
Of all patients 41% (n = 22) were diagnosed with metabolic syndrome, with 3 out of 5 criteria, at baseline. Thirty-eight percent (n = 20) of patients were diagnosed with MetS at follow-up; MetS data for 2 patients were missing. In the MAD group 11 out of 24 patients fulfilled the criteria for MetS at baseline, and at follow-up this was the case for 9 patients. In CPAP group 11 out of 30 patients fulfilled the criteria for MetS at baseline and at follow-up. Three MAD patients switched from MetS at baseline to no MetS at follow-up, and 1 patient switched from no MetS at baseline to MetS at follow-up. Two CPAP patients switched from no MetS at baseline to MetS at follow-up, and 2 patients switched from MetS at baseline to no MetS at follow-up.
In patients with MetS at baseline, a significant difference between MAD and CPAP therapies was calculated for AHI at follow-up (P = .013) and for minimal oxygen saturation (P = .009). These differences were in favor of CPAP therapy. SCORE risk score at baseline was 5% for only 1 patient in CPAP group (73-year-old man). Finally, in the MAD group DBP at night was significantly reduced in the MetS group (P = .028).
DISCUSSION
This study showed that treatment of patients with moderate OSA with either MAD or CPAP therapy had no profound effect on major cardiovascular risk factors after 12 months of therapy. While cardiovascular treatment outcome was similar between both therapies, AHI and minimal oxygen saturation differed significantly between groups at follow-up and favored CPAP therapy.
The mean age in this cohort was 50 ± 9.7 years in the MAD group and 51 ± 9.8 years in the CPAP group. This is relatively young compared to other studies of OSA treatment, which mostly reported a mean age around 60 years.29–31 This could explain the relatively normal baseline cardiovascular and metabolic values of the study participants, and the fact that no major improvements in the outcomes and cardiovascular risk, such as HR, SBP, and DBP, were seen.
Comparing this trial with previous studies, generally less profound effects on cardiovascular outcomes are observed. Khan et al32 suggested in a meta-analysis that in participants with moderate-to-severe OSA, sufficient CPAP compliance (≥ 4 hours per night) reduces the risk of major adverse cardiovascular events, primarily driven by a reduction in the risk of stroke. It has been shown that CPAP and MAD reduce SBP and DBP, and that CPAP has a positive effect on high-sensitivity C-reactive protein.8,17–20 In our study, however, similar results were not observed. This could be explained by the relatively short-term follow-up of 12 months in our study. In the meta-analysis of Khan et al the mean follow-up was 39 months.
Abuzaid and colleagues33 suggested, in a meta-analysis with 3780 patients, that in participants with OSA, sufficient CPAP compliance (≥ 4 hours per night) is also associated with improved cardiac outcome. Dipping of HR is important in reducing cardiovascular risk. In patients whose heart rate did not exhibit the nocturnal decline, the risk of future cardiovascular disease was 2.4 times greater, independent of nondipping of SBP or diabetes status or BP level.34 In a general population a 10%–20% nighttime dip of BP is normal.35 In our study population this dip was 12.1%–17.9%, which is in line with a normal BP dip. The HR dip in our study population was 13.8%–17.5%, also in line with a normal HR dip which is 10%–20%.36 The absence of significant differences in most cardiovascular outcomes could be explained by the relatively healthier and younger study population of the present study. Therefore, an improved cardiac outcome was difficult to reach.
Polysomnographic outcomes differed significantly between both therapies. AHI and minimal oxygen saturation dropped significantly more in the CPAP group compared to the MAD group. From literature it is known that patients with moderate to severe OSA generally perform better with CPAP therapy compared to MAD therapy in terms of AHI reduction.37,38 Since, in this study, all patients were diagnosed with moderate OSA and were randomly allocated to MAD or CPAP therapy, this could explain the significant difference in AHI outcome in favor of the CPAP group. Striking is that the difference in AHI outcome did not result in differences in cardiovascular outcomes.
In this trial we focused on cardiac biomarkers in blood and plasma such as NTproBNP, IL-6, IL-18, tumor necrosis factor α, and sRAGE. Only sRAGE and HbA1c were significantly increased following therapy in the MAD group; no other significant differences were found. NTproBNP values provide information about the heart filling and cardiac dysfunction; the main stimulus for synthesis and secretion is myocardial wall stress.39 NTproBNP may be elevated due to sympathetic activity, a rise in blood pressure, and apnea-induced wall stress. Tasci et al40 investigated the level of NTproBNP in 60 OSA patients and concluded that CPAP lowers the concentration of NTproBNP. However, Hubner et al41 found neither a relation between concentration of NTproBNP and AHI nor an elevated level of NTproBNP in OSA patients compared to patients with suspected sleep apnea. In our trial, no relation was found between NTproBNP and AHI in either the MAD or CPAP groups, independent of AHI reductions. This could again be explained by the relatively healthy and younger study population. As reported by Tasci et al40, the amount of NTproBNP was 60.8 ± 9.9 pg/ml in hypertensive participants, 43.2 ± 6.8 pg/ml in normotensive OSA patients, and 36.5 ± 8.5 pg/ml in participants without OSA. In our study population the amount of NTproBNP was even lower. Furthermore, IL-6 and tumor necrosis factor α have a role in promoting vascular inflammation. IL-6 especially is elevated as an early biomarker in patients with moderate to severe OSA.42 Comparing the results of our study with the moderate to severe OSA patients in the study by Motamedi et al,42 we observed lower levels of IL-6 at baseline. However, in the CPAP group, IL-6 follow-up correlated negatively (P = .036) with AHI at baseline. In patients with a high AHI, a larger difference in IL-6 was seen. This could possibly be explained by the fact that patients with a higher baseline AHI may have been affected by OSA for a longer period of time and therefore had more pronounced IL-6 levels. IL-18 is a risk factor for coronary events and was significantly reduced by CPAP therapy in the study of Jin et al.43 However, in our population a similar reduction following CPAP or MAD therapy was not observed. HbA1c is a predictor for diabetes mellitus but also for heart failure.44 In a study by Monneret et al,45 HbA1c was correlated with SaO2. In the study by Labarca et al,46 CPAP therapy did not change HbA1c levels after 12- or 24-weeks follow-up. It is expected that after a long period of time the level of HbA1c would decrease due to treatment and reduction of AHI. However, in our study HbA1c, as well as glucose levels, did increase after MAD therapy. This could be explained by the short follow-up period and the smaller reduction in AHI in the MAD group. In the CPAP group the AHI reduction was more pronounced; however, no correlation with HbA1c was observed. The concentration of sRAGE is low in patients with hypertension, as it is in many patients with OSA. sRAGE predicts oxidative stress and correlates negatively with AHI and BMI.47 In our study sRAGE levels significantly increased in the MAD but not CPAP group at 12-months follow-up. This could partly be explained by the significant difference in BMI in our groups between MAD and CPAP therapies at follow-up. In our study baseline and follow-up sRAGE correlated positively with follow-up AHI in MAD group and in the total group, while delta AHI correlated negatively with baseline and follow-up sRAGE in the MAD group and in the total group. Cai et al48 also showed a strong negative correlation between sRAGE and AHI. As sRAGE levels are associated with a decline in AHI, it might be used as a biomarker in this group of patients.
A well-known issue in prospective randomized controlled trials is the dropout rate. In this study 24 participants out of 43 MAD and 30 out of 42 CPAP completed follow-up. This paper was written to compare the cardiovascular and metabolic effects of MAD and CPAP therapies in patients with moderate OSA; however, the study’s statistical power derives from differences in AHI reduction between MAD and CPAP therapies. If the study’s statistical power was based on differences in cardiovascular outcomes, a larger treatment group would have been needed, and possibly more significant differences would have been seen. At baseline, the subgroup of hypertensive patients especially was small and lacked power.
A weakness of the study is the follow-up period. One-year follow-up under treatment might be too short to observe any effects on cardiovascular biomarkers. Furthermore, effects on long-term outcomes like cardiovascular events and death were not assessed. In a future study, other biomarkers such as coronary artery calcium and carotid ultrasound of the intima-media thickness could be added. Also aortic pulse-wave velocity, ankle-brachial index, and echocardiography could be used to measure preclinical vascular damage.49
In general, comorbidities such as obesity, gastroesophageal reflux disease, and type 2 diabetes mellitus play an important role in OSA.50 This is why interpretations of studies on cardiovascular outcomes are difficult to make. Confounders such as weight, BMI, neck circumference, and cholesterol interplay in development of both OSA and cardiovascular diseases. The relationship between specific biomarkers and OSA is often influenced by comorbidities but also by age, sex, and ethnicity. This is why a single biomarker suitable for the assessment of cardiovascular risk in OSA has not yet been identified.51 However, there is increasing evidence that CPAP therapy could lead to a significant reduction in cardiovascular diseases.52 Still more research is needed to address these elements of the cardiovascular effects of both MAD and CPAP therapies on the long term.
CONCLUSIONS
Treatment of patients with moderate OSA with either MAD or CPAP therapy had no major effect on major cardiovascular risk factors after 12 months. Further research is suggested in patients with more severe OSA and cardiac stress outcomes with a longer-term follow-up.
ABBREVIATIONS
- AGE
advanced glycation end-product
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- DBP
diastolic blood pressure
- HbA1c
glycated hemoglobin
- HR
heart rate
- IL
interleukin
- MAD
mandibular advancement device
- MetS
metabolic syndrome
- NTproBNP
N-terminal pro–hormone B-type natriuretic peptide
- OSA(S)
obstructive sleep apnea (syndrome)
- SBP
systolic blood pressure
- SCORE
Systematic Coronary Risk Evaluation
- sRAGE
soluble receptor for advanced glycation end-products
DISCLOSURE STATEMENT
All authors have read and approved the final manuscript. Work for this study was performed at the University Medical Center, Groningen, the Netherlands. A.H. is a medical advisor for Airway Management Inc., SomnoMed, and Zephyr Sleep Technologies. P.J.W. reports grants and personal fees from Philips, grants and personal fees from RESMED, grants from VIVISOL, personal fees from Synapse, and from Bresotec, outside the submitted work. All other authors report no conflicts of interest.
REFERENCES
- 1. Peppard PE , Young T , Barnet JH , Palta M , Hagen EW , Hla KM . Increased prevalence of sleep-disordered breathing in adults . Am J Epidemiol. 2013. ; 177 ( 9 ): 1006 – 1014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Banno K , Kryger MH . Sleep apnea: clinical investigations in humans . Sleep Med. 2007. ; 8 ( 4 ): 400 – 426 . [DOI] [PubMed] [Google Scholar]
- 3. Malhotra A , White DP . Obstructive sleep apnoea . Lancet. 2002. ; 360 ( 9328 ): 237 – 245 . [DOI] [PubMed] [Google Scholar]
- 4. Marin JM , Carrizo SJ , Vicente E , Agusti JGN . Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study . Lancet 2005. ; 365 : 1046 – 1053 . [DOI] [PubMed] [Google Scholar]
- 5. Al-Falahi Z , Williamson J , Dimitri H . Atrial fibrillation and sleep apnoea: guilt by association? Hear Lung Circ 2017. ; 26 ( 9 ): 902 – 910 . [DOI] [PubMed] [Google Scholar]
- 6. Marin JM , Agusti A , Villar I , et al . Association between treated and untreated obstructive sleep apnea and risk of hypertension . JAMA. 2012. ; 307 ( 20 ): 2169 – 2176 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Floras JS . Hypertension and sleep apnea . Can J Cardiol. 2015. ; 31 ( 7 ): 889 – 897 . [DOI] [PubMed] [Google Scholar]
- 8. de Vries GE , Wijkstra PJ , Houwerzijl EJ , Kerstjens HAM , Hoekema A . Cardiovascular effects of oral appliance therapy in obstructive sleep apnea: a systematic review and meta-analysis . Sleep Med Rev. 2018. ; 40 : 55 – 68 . [DOI] [PubMed] [Google Scholar]
- 9. Chowdhuri S , Quan SF , Almeida F , et al. ATS Ad Hoc Committee on Mild Obstructive Sleep Apnea . An official American Thoracic Society research statement: Impact of mild obstructive sleep apnea in adults . Am J Respir Crit Care Med. 2016. ; 193 ( 9 ): e37 – e54 . [DOI] [PubMed] [Google Scholar]
- 10. McNicholas WT . Obstructive sleep apnea and inflammation . Prog Cardiovasc Dis. 2009. ; 51 ( 5 ): 392 – 399 . [DOI] [PubMed] [Google Scholar]
- 11. Drager LF , Polotsky VY , Lorenzi-Filho G . Obstructive sleep apnea: an emerging risk factor for atherosclerosis . Chest. 2011. ; 140 ( 2 ): 534 – 542 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoyos CM , Melehan KL , Liu PY , Grunstein RR , Phillips CL . Does obstructive sleep apnea cause endothelial dysfunction? A critical review of the literature . Sleep Med Rev. 2015. ; 20 : 15 – 26 . [DOI] [PubMed] [Google Scholar]
- 13. Li M , Li X , Lu Y . Obstructive sleep apnea syndrome and metabolic diseases . Endocrinology. 2018. ; 159 ( 7 ): 2670 – 2675 . [DOI] [PubMed] [Google Scholar]
- 14. Castaneda A , Jauregui-Maldonado E , Ratnani I , Varon J , Surani S . Correlation between metabolic syndrome and sleep apnea . World J Diabetes. 2018. ; 9 ( 4 ): 66 – 71 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parish JM , Adam T , Facchiano L . Relationship of metabolic syndrome and obstructive sleep apnea . J Clin Sleep Med. 2007. ; 3 ( 5 ): 467 – 472 . [PMC free article] [PubMed] [Google Scholar]
- 16. Giles T , Lasserson T , Smith B , et al . Continuous positive airways pressure for obstructive sleep apnoea in adults . Cochrane Database Syst Rev. 2006. ; 25 . [DOI] [PubMed] [Google Scholar]
- 17. Xu H , Wang Y , Guan J , Yi H , Yin S . Effect of CPAP on endothelial function in subjects with obstructive sleep apnea: a meta-analysis . Respir Care. 2015. ; 60 ( 5 ): 749 – 755 . [DOI] [PubMed] [Google Scholar]
- 18. Ning Y , Zhang T-S , Wen W-W , et al . Effects of continuous positive airway pressure on cardiovascular biomarkers in patients with obstructive sleep apnea: a meta-analysis of randomized controlled trials . Sleep Breath. 2019. ; 23 ( 1 ): 77 – 86 . [DOI] [PubMed] [Google Scholar]
- 19. Baessler A , Nadeem R , Harvey M , et al . Treatment for sleep apnea by continuous positive airway pressure improves levels of inflammatory markers–a meta-analysis . J Inflamm (Lond). 2013. ; 10 : 13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bazzano LA , Khan Z , Reynolds K , He J . Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea . Hypertension. 2007. ; 50 ( 2 ): 417 – 423 . [DOI] [PubMed] [Google Scholar]
- 21. de Vries GE , Hoekema A , Claessen JQPJ , et al . Long-term objective adherence to mandibular advancement device therapy versus continuous positive airway pressure in patients with moderate obstructive sleep apnea . J Clin Sleep Med. 2019. ; 15 ( 11 ): 1655 – 1663 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miljeteig H , Hoffstein V . Determinants of continuous positive airway pressure level for treatment of obstructive sleep apnea . Am Rev Respir Dis. 1993. ; 147 ( 6 Pt 1 ): 1526 – 1530 . [DOI] [PubMed] [Google Scholar]
- 23. Taylor KS , Heneghan CJ , Stevens RJ , Adams EC , Nunan D , Ward A . Heterogeneity of prognostic studies of 24-hour blood pressure variability: systematic review and meta-analysis . PLoS One. 2015. ; 10 ( 5 ): e0126375 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koetsier M , Lutgers HL , de Jonge C , Links TP , Smit AJ , Graaff R . Reference values of skin autofluorescence . Diabetes Technol Ther. 2010. ; 12 ( 5 ): 399 – 403 . [DOI] [PubMed] [Google Scholar]
- 25. Carmelli D , Swan GE , Bliwise DL . Relationship of 30-year changes in obesity to sleep-disordered breathing in the Western Collaborative Group Study . Obes Res. 2012. ; 8 ( 9 ): 632 – 637 . [DOI] [PubMed] [Google Scholar]
- 26. Moy FM , Bulgiba A . The modified NCEP ATP III criteria maybe better than the IDF criteria in diagnosing Metabolic Syndrome among Malays in Kuala Lumpur . BMC Public Health. 2010. ; 10 ( 1 ): 678 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McMurray JJV , Adamopoulos S , Anker SD , et al. ESC Committee for Practice Guidelines . ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC . Eur Heart J. 2012. ; 33 ( 14 ): 1787 – 1847 . [DOI] [PubMed] [Google Scholar]
- 28. De Vries , GE , Hoekema A , Vermeulen KM , et al . Clinical- and cost-effectiveness of a mandibular advancement device vs continuous positive airway pressure in moderate obstructive sleep apnea . J Clin Sleep Med. 2019. ; 15 ( 10 ): 1477 – 1485 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McEvoy RD , Antic NA , Heeley E , et al. SAVE Investigators and Coordinators . CPAP for prevention of cardiovascular events in obstructive sleep apnea . N Engl J Med. 2016. ; 375 ( 10 ): 919 – 931 . [DOI] [PubMed] [Google Scholar]
- 30. Fietze I , Laharnar N , Obst A , et al . Prevalence and association analysis of obstructive sleep apnea with gender and age differences - Results of SHIP-Trend . J Sleep Res. 2019. ; 28 ( 5 ): e12770 . [DOI] [PubMed] [Google Scholar]
- 31. Hua-Huy T , Rouhani S , Nguyen X-Y , Luchon L , Meurice JC , Dinh-Xuan AT . Cardiovascular comorbidities in obstructive sleep apnoea according to age: a sleep clinic population study . Aging Clin Exp Res. 2015. ; 27 ( 5 ): 611 – 619 . [DOI] [PubMed] [Google Scholar]
- 32. Khan SU , Duran CA , Rahman H , Lekkala M , Saleem MA , Kaluski E . A meta-analysis of continuous positive airway pressure therapy in prevention of cardiovascular events in patients with obstructive sleep apnoea . Eur Heart J. 2018. ; 39 ( 24 ): 2291 – 2297 . [DOI] [PubMed] [Google Scholar]
- 33. Abuzaid AS , Al Ashry HS , Elbadawi A , et al . Meta-analysis of cardiovascular outcomes with continuous positive airway pressure therapy in patients with obstructive sleep apnea . Am J Cardiol. 2017. ; 120 ( 4 ): 693 – 699 . [DOI] [PubMed] [Google Scholar]
- 34. Kazuo E , Satoshi H , Ishikawa J , et al . Nocturnal non-dipping of heart rate predicts cardiovascular events in hypertensive patients . Hypertension. 2009. ; 27 ( 11 ): 2265 – 2270 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bloomfield D , Park A . Night time blood pressure dip . World J Cardiol. 2015. ; 7 ( 7 ): 373 – 376 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Loredo JS , Nelesen R , Ancoli-Israel S , Dimsdale JE . Sleep quality and blood pressure dipping in normal adults . Sleep. 2004. ; 27 ( 6 ): 1097 – 1103 . [DOI] [PubMed] [Google Scholar]
- 37. Ramar K , Dort LC , Katz SG , et al . Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: An update for 2015 . J Clin Sleep Med. 2015. ; 11 ( 7 ): 773 – 827 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sharples LD , Clutterbuck-James AL , et al . Meta-analysis of randomised controlled trials of oral mandibular advancement devices and continuous positive airway pressure for obstructive sleep apnoea-hypopnoea . Sleep Med Rev. 2016. ; 27 : 108 – 124 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weber M , Hamm C . Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine . Heart. 2006. ; 92 ( 6 ): 843 – 849 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tasci S , Manka R , Scholtyssek S , et al . NT-pro-BNP in obstructive sleep apnea syndrome is decreased by nasal continuous positive airway pressure . Clin Res Cardiol. 2006. ; 95 ( 1 ): 23 – 30 . [DOI] [PubMed] [Google Scholar]
- 41. Hübner RH , El Mokhtari NE , Freitag S , et al . NT-proBNP is not elevated in patients with obstructive sleep apnoea . Respir Med. 2008. ; 102 ( 1 ): 134 – 142 . [DOI] [PubMed] [Google Scholar]
- 42. Motamedi V , Kanefsky R , Matsangas P , et al . Elevated tau and interleukin-6 concentrations in adults with obstructive sleep apnea . Sleep Med. 2018. ; 43 : 71 – 76 . [DOI] [PubMed] [Google Scholar]
- 43. Jin F , Liu J , Zhang X , et al . Effect of continuous positive airway pressure therapy on inflammatory cytokines and atherosclerosis in patients with obstructive sleep apnea syndrome . Mol Med Rep. 2017. ; 16 ( 5 ): 6334 – 6339 . [DOI] [PubMed] [Google Scholar]
- 44. Kishimoto I , Makino H , Ohata Y , et al . Hemoglobin A1c predicts heart failure hospitalization independent of baseline cardiac function or B-type natriuretic peptide level . Diabetes Res Clin Pract. 2014. ; 104 ( 2 ): 257 – 265 . [DOI] [PubMed] [Google Scholar]
- 45. Monneret D , Tamisier R , Ducros V , et al . Glucose tolerance and cardiovascular risk biomarkers in non-diabetic non-obese obstructive sleep apnea patients: effects of long-term continuous positive airway pressure . Respir Med. 2016. ; 112 : 119 – 125 . [DOI] [PubMed] [Google Scholar]
- 46. Labarca G , Reyes T , Jorquera J , Dreyse J , Drake L . CPAP in patients with obstructive sleep apnea and type 2 diabetes mellitus: Systematic review and meta-analysis . Clin Respir J. 2018. ; 12 ( 8 ): 2361 – 2368 . [DOI] [PubMed] [Google Scholar]
- 47. Volná J , Kemlink D , Kalousová M , et al . Biochemical oxidative stress-related markers in patients with obstructive sleep apnea . Med Sci Monit. 2011. ; 17 ( 9 ): CR491 – CR497 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cai W , Sun JF , Liu Y , et al . Relationship between serum levels of endogenous secretory RAGE and blood pressure in male nondiabetic patients with obstructive sleep apnea . J Hum Hypertens. 2015. ; 29 ( 12 ): 713 – 718 . [DOI] [PubMed] [Google Scholar]
- 49. Piepoli MF , Hoes AW , Agewall S , et al. ESC Scientific Document Group . 2016 European Guidelines on cardiovascular disease prevention in clinical practice . Eur Heart J. 2016. ; 37 ( 29 ): 2315 – 2381 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sweed RA , Hassan S , ElWahab NHA , Aref SR , Mahmoud MI . Comorbidities associated with obstructive sleep apnea: a retrospective Egyptian study on 244 patients . Sleep Breath. 2019. ; 23 ( 4 ): 1079 – 1085 . [DOI] [PubMed] [Google Scholar]
- 51. de Araújo Freitas IG , de Bruin PF , Bittencourt L , de Bruin VM , Tufik S . What can blood biomarkers tell us about cardiovascular risk in obstructive sleep apnea? Sleep Breath. 2015. ; 19 ( 3 ): 755 – 768 . [DOI] [PubMed] [Google Scholar]
- 52. Vrints H , Shivalkar B , Hilde H , et al . Cardiovascular mechanisms and consequences of obstructive sleep apnoea . Acta Clin Belg. 2013. ; 68 ( 3 ): 169 – 178 . [DOI] [PubMed] [Google Scholar]