Abstract
Study Objectives:
Auditory stimulation devices (white and pink noise) are used to mask sounds and facilitate relaxation and sleep; however, the effectiveness of this intervention is not well established. This systematic review examined the scientific literature for the effect of specific types of auditory stimulation on sleep outcomes in adults.
Methods:
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement guided this review. Searches were conducted in 9 databases for intervention studies that could easily be employed in clinical practice. We excluded other types of auditory stimulation (music alone, binaural tones, and synchronization). Two reviewers screened abstracts and full-text articles for eligibility, with conflicts resolved by a third reviewer, and extracted data. Risk of bias was assessed with the Effective Public Health Practice Project Quality Assessment Tool for Quantitative Studies.
Results:
Thirty-four studies reported results of 1,103 persons participating in 3 categories of interventions: white noise (18), pink noise (11), and 6 multiaudio (some combination of white, pink, music, or silence). Nineteen studies had positive findings in terms of improving sleep outcomes: 6 white noise (33%), 9 pink noise (81.9%), and 4 multiaudio (66.7%). Multiaudio had the lowest (better) risk of bias (mean/standard deviation: 1.67/0.82) compared to white (2.38/0.69) and pink noise (2.36/0.81).
Conclusions:
Although there was no strong evidence to support use of auditory stimulation, none of the studies reported any adverse effects with short-term application of auditory stimulation during sleep. Future research needs to include confounding factors that can affect outcomes, including one’s noise sensitivity, personality, and other conditions or medications that may affect sleep.
Citation:
Capezuti E, Pain K, Alamag E, Chen XQ, Philibert V, Krieger AC. Systematic review: auditory stimulation and sleep. J Clin Sleep Med. 2022;18(6):1697–1709.
Keywords: systematic review, sleep, sleep quality, white noise, pink noise, music, insomnia
INTRODUCTION
Although consciousness is reduced during sleep, the auditory system continues to evaluate sounds in the environment by changing brain wave patterns, boosting delta patterns, and augmenting sleep spindles during non–rapid eye movement sleep.1,2 Ambient sound or external noise may adversely affect sleep both in a person’s home or an institutional (eg, hotel, hospital, nursing home, etc) environment.3,4 Sleep disturbance depends on the stage of sleep, background sound level, and individual factors such as hearing ability.2 Despite the frequent use of barriers to absorb (eg, acoustic wall panels) or reduce (eg, soundproof windows) sound, none of them effectively overcome all noises. As a result, measures to mask sounds through various forms of auditory stimulation have been used to reduce the undesirable effects of noise and facilitate sleep. It is theorized that insomnia is due to altered neuronal activity in structures of the thalamus, prefrontal cortex, parietal lobe, brain stem, cerebellum, and caudate nucleus,5 and that auditory stimulation works by improving the functional connectivity among these brain areas.6
Auditory stimulation approaches include the use of white and pink noise, as well as music, binaural tones, and sound synchronization. Despite the fact that noise is defined as an unwanted sound, white and pink “noise” are terms commonly used in the clinical literature and commercial products.7 White noise results from combining sounds of all different frequencies so that the intensity is the same at every frequency. It is analogous to white light, which contains all visible frequencies. It is most often used to mask unwanted sounds such as background noise on a hospital unit or in an office because, as the most intense sensory stimulus, it will decrease the audibility of background sounds.8 It is also used to treat tinnitus (ringing in the ears)9 and hyperacusis (an increased sensitivity to normal environmental sounds).10 Examples include television static, steam hissing from a radiator or humidifier, or the whir of a fan. Commercial white noise generators mimic these sounds with brief, prerecorded audio-tracks that repeat at the end of the track.
Pink noise also contains all frequencies that the human ear can hear, but the intensity of the sound decreases as frequency increases. It increases the lower frequency range so that bass frequencies are louder and higher frequencies are turned down. This is considered equivalent to the spectrum of natural sound.11 Examples include nature sounds, such as waves lapping on the beach, leaves rustling in the trees, or a steady rainfall.
Multiaudio interventions use either white or pink noise in combination with music or silence. Music is meant to promote muscle relaxation and provide a distraction from other sounds or inhibit intrusive, distressing thoughts about life situations. Soothing, relaxing music used to promote sleep is usually lyric-free with a slow beat (a rhythm of about 60–80 beats per minute) played from a speaker, headphones, or earbuds.12 It has been shown to result in decreased heart rate, respiratory rate, and blood pressure, thus indicating relaxation and reduced stress.13 Other auditory interventions are binaural tones and synchronization. Binaural tones or beats plays 2 different sounds in each ear, with frequencies lower than 1500 Hz and fewer than 40 Hz between the two. Sound synchronization plays audio that matches brainwaves indicated by neurofeedback.14,15
Phone apps, smart speakers (eg, Amazon Echo, Seattle WA), and sound machines or conditioners employing white or pink noise are in increasing use, but the effectiveness of these devices in initiating or maintaining sleep is not well established. Nonetheless, these devices may provide an easily employed intervention to improve sleep quality. The purpose of this systematic review was to examine the scientific literature for the effect of select auditory stimulation interventions (white noise, pink noise, or a combination) on sleep outcomes in adults.
METHODS
Literature search
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was used to guide this review.16 A library specialist (K.P.) performed systematic searches of MEDLINE, Embase, CINAHL, PsycINFO, Scopus, Engineering Village, IEEE Xplore, and the Cochrane Library for all available years through February 1, 2021. Major search terms for all databases included both controlled vocabulary and keywords (Table 1) on the topic of auditory stimulation and sleep. Complete database search strategies are available in the supplemental material.
Table 1.
Search terms.
| Concepta | Keywordsb | Controlled Vocabularyc |
|---|---|---|
| 1. Auditory stimulation | ((white or pink) adj2 noise) or (sound* adj2 (ambient or enrichment or generator* or nature or modulat* or therap* or masking or condition* or broadband or oscillat*)) or (stimulat* adj2 (acoustic or auditory)) or (soundscape* or binaural beat* or audio* relax*) or (noise* adj (mask* or block*)) or ((sleep adj (machine* or device* or app or apps)) and (sound* or noise*)) | Medline and Cochrane: Acoustic Stimulation Embase: auditory stimulation CINAHL: Acoustic Stimulation PsycINFO: Auditory Stimulation or White Noise or Auditory Masking |
| 2. Sleep | (sleep* or insomnia* or circadian or “biological clock*” or “night* wak*” or “night* awak*”) | Medline and Cochrane: exp Sleep or exp Sleep Wake Disorders Embase: exp sleep or exp sleep disorder CINAHL: Sleep+ or Sleep Disorders+ PsycINFO: Sleep or Napping or NREM Sleep or REM Sleep or Sleep Disorders or Hypersomnia or Insomnia or Kleine Levin Syndrome or Narcolepsy or Parasomnias or Sleepwalking |
| 3. Tinnitus | (tinnitus or tinnitis) | Medline and Cochrane: Tinnitus Embase: tinnitus CINAHL: Tinnitus PsycINFO: Tinnitus |
Search term modifiers: * = truncation, adj = adjacency, () = nested terms, “” = locked terms, exp = narrower terms included. aSearch term concepts were combined using Boolean operators: (1 AND 2) NOT 3. Full search strategies for each database are available in the supplemental material. bKeywords were searched in all available text fields across 8 databases: Medline, Embase, CINAHL, PsycINFO, Cochrane, Engineering Village, Scopus, and IEEE Xplore. cControlled vocabulary terms were used only per specified database.
Study selection and classification
Table 2 describes the inclusion/exclusion criteria. Searches, where appropriate, were restricted to adult, English-language studies but were not otherwise limited by study design, setting, or date of publication. Since we were interested in interventions that could easily be employed in clinical practice, we excluded music by itself, binaural tones, and those that conveyed auditory interventions based on neurofeedback (synchronization). Since there were few studies, we also included abstracts from scientific meetings.
Table 2.
Inclusion and exclusion criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
*White noise includes sound generators that mimic television static, steam hissing from a radiator or humidifier, or the whir of a fan. **Pink noise includes nature sounds such as waves lapping on the beach, leaves rustling in the trees, or a steady rainfall.
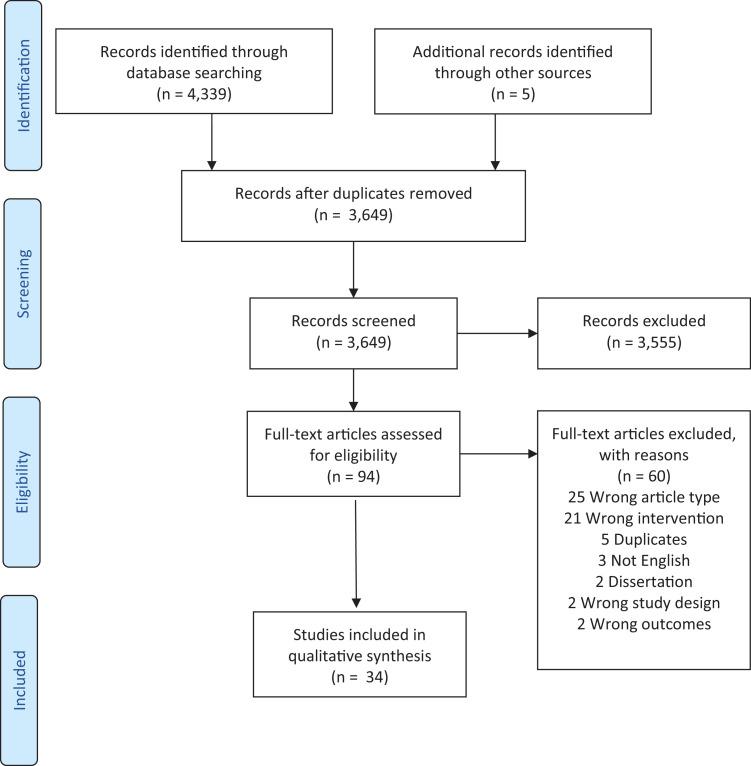
The search results were uploaded to a web-based software platform, Covidence.17 As indicated in the PRISMA diagram (Figure 1), after 695 duplicates were removed, 2 reviewers (E.A., E.C., or X.Q.C.) independently screened 3,649 titles/abstracts with 1 coauthor (A.K.) resolving any conflicts. This resulted in the exclusion of 3,555 studies that did not meet the inclusion criteria. The remaining 94 full-text studies were accessed by 2 reviewers (E.A., E.C., X.Q.C., or V.P.) for eligibility and another (A.K.) made the final determination. Sixty studies were excluded since they were the wrong study type (25; lay article, study protocol, letter, literature summary), the wrong intervention (21), were duplicates (5), dissertations (2), in another language (3), used the wrong design (2), or did not measure a sleep outcome (2). This resulted in 34 articles retained for this review.
Figure 1. PRISMA flow diagram.
PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred doi:10.1371/journal.pmed1000097. For more information, visit www.prisma-statement.org; accessed January 8, 2022.
Data extraction
Two research assistants (RAs: E.A., X.Q.C.) extracted the study characteristics of each study onto an Excel worksheet. One author (E.C.) checked 10 article extractions and reviewed errors with the RAs. Then each RA checked the other while 1 author (E.C.) randomly reviewed 25% of articles until there was 100% agreement. The authors worked independently in the research, data collection, and risk of bias assessment. Audio interventions were categorized into 3 categories: white noise, pink noise, and multiaudio.
Methodological quality assessment
The 2 RAs also assessed study quality with the Effective Public Health Practice Project (EPHPP) Quality Assessment Tool (QAT) for Quantitative Studies18 and another coauthor discussed any discrepancies until consensus was reached. The QAT includes 7 domains: selection bias, study design, confounders, blinding, data collection methods, withdrawals and dropouts, and intervention integrity. The cumulative score provided a global rating of 1 (strong or no weak ratings), 2 (moderate or 1 weak rating), or 3 (weak or 2 or more weak ratings). The QAT has content and construct validity and acceptable test-retest reliability (kappa = 0.74 and interrater reliability (kappa = 0.61).19 Additionally, we compared studies by how sleep was measured, specifically if objective measures were used; we further differentiated if measured by the gold standard of polysomnography or by actigraphy, which is known to overestimate total sleep time and efficiency and underestimate sleep onset.
RESULTS
Overview of the included studies
The 34 studies that met criteria included 27 articles8,11,20–44 and 7 abstracts45–51 published between 1988 and 2021. The characteristics of included studies are presented in Table 3. A third of all studies were randomized controlled trials (RCTs) (12) or controlled clinical trials (2), while the other study designs included cohort (18), cohort analytic (1), and a 2 × 3 design (1). Most (n = 28) studies excluded those with any sleep problems or did not screen for sleep problems, while 7 studies included those with insomnia.22,25,28,31,32,34,44 Nine studies specifically excluded those with hearing problems, while 1 included those with tinnitus,28 and the remainder did not indicate it as an exclusion criterion. One study included participants with dementia.43
Table 3.
Characteristics of included studies (n = 34).
| Study | Intervention Description | Design, Setting, and Location | Sample Size; Participant Inclusion Criteria [Mean Age (SD, Range), % Women] | Sleep Measure(s) Risk of Bias (RoB)* | Sleep Result (Positive, Mixed, None, Negative) and Specific Outcomes for Intervention Group |
|---|---|---|---|---|---|
| White Noise (n = 18) | |||||
| Alkahtani 20198 | White noise (simulated air conditioner sound at 43 dB) to mask ambient sounds | Cohort; Community–home; Saudi Arabia–Riyadh | n = 48. Healthy young adults not disturbed by night noises, had sleep disorder or hearing problem. Age 21.3 (1.9, 18–25); 58.9% women | Actigraphy, Sleep diary RoB = 2 | None. No improvement in sleep latency, duration, efficiency, WASO, or sleep quality |
| Daum 198821 | White noise– monotonous stimulation of 186 tones (each for 2 seconds) for 40 minutes, 500 Hz at 38 dB vs silence | 2 × 3 factorial design; Lab; Europe–Germany | n = 55. Healthy male university students; Age 18–22; 0% woman** | PSG RoB = 3 | None. No significant differences in sleep latency |
| Ebben 202122 | White noise to mask sounds | Cohort; Community–home; USA-NY | n = 8. Complaints of noise disturbing sleep; Age 39–74: 62.5% women | Actigraphy, Sleep log RoB = 2 | Positive. Reduced WASO and awakenings from sleep |
| Farokhnezhad 201623 | White noise to mask hospital sounds for 120 minutes at 40–50 dB | Cohort analytic; Hospital–CCU; Iran-Tehran | n = 60. > 30 years older on CCU at least 3 nights; Age 58.9 (10.9); 43.3% women** | PSQI, Sleep log RoB = 2 | Mixed. Improved perceived sleep quality but no change in total sleep time |
| Forquer 200725 | White noise–generator for 480 minutes at 60–75 dB | Cohort; Community– college dorm rooms; USA-MI | n = 4. With sleep latency > 30 minutes and/or wakes up > 1/night. Age 19, 75% women | Sleep diary, Sleep survey RoB = 3 | Positive. Reduced sleep latency and night awakenings |
| Gao 202026 | White noise vs REM brain-wave music vs SWS brain-wave music × 20 min for 6 days | RCT (3 groups); Sleep center; China | n = 33. History of staying up late, right-handed with subhealthy sleep quality; Age 21.4 (5.6), 48.5% women | PSG RoB = 3 | None. Nonsignificant sleep efficacy decreased in white noise and REM and increased in SWS group |
| Handscomb 200628 | White noise sound generator–heartbeat, nature sounds for 480 minutes | Cohort; Hospital; Europe-UK | n = 35. With complaints tinnitus-related sleep problems; Age 52.9 (28–78); 71.8% women | PSQI RoB = 2 | Positive. Improved perceived sleep quality |
| Messineo 201731 | White noise–broadband sound at 46 dB | RCT; Lab; USA-MA | n = 18. Exclude if history of insomnia or drug use that impairs sleep; Age 28.5 (13.5, 20–65); 50% women** | PSG, Visual analog scale for sleep quality, Stanford Sleepiness Scale RoB = 1 | Mixed. Reduced N2 sleep latency but not latency to stage N3 |
| Montgomery-Downs 201032*** | White noise–low frequency fan for 480 minutes < 50 Hz with subtle heartbeat < 100 Hz at 63 dB vs Pink Noise (nature sounds) > 2000 Hz at < 50 dB | Cohort; Community-home; USA-VA | n = 25. History of insomnia but not any other sleep disorder; Age 40 (14); 64% women** | Actigraphy, PSQI RoB = 1 | None. None for white noise and mixed for Pink. Positive for improvements in sleep latency for pink noise but reduced duration. |
| Owen 201945 Abstract | Active noise cancelling headphones with white noise masking for 1 night and 1 night no intervention | Randomized crossover; Hospital–ICU; England | n = 14; Critically ill, nondelirious adults not receiving ventilation | Richards-Campbell Sleep Questionnaire RoB = 3 | None. No significant differences in sleep (5.7 score improvement in intervention group, P = .55) |
| Shaikh 201749 Abstract | Masking sound for 360 minutes that reduced environmental sound from > 75 dB to 60.3 (0.5) dB | Cohort; Hospital–ICU; USA-IL | n = 5. Adults weaning from prolonged mechanical ventilation in ICU without hearing problems, delirium, sedation or agitation | PSG, Survey RoB = 3 | Positive. Improved sleep efficiency– fewer episodes of awakening to a sound event (9% vs 21%); 3/5 participants reported improved sleep quality and 2 with no difference |
| Stanchina 200537 | White noise mixed in with ICU noise for 480 minutes, 22.05 KHz, 62 dB | Cohort; Hospital; USA-RI | n = 5. No history of sleep problems; Age 27 (1.7, 18–65); 40% women | PSG RoB = 3 | Positive. Substantially reduced arousals, especially during REM sleep (not NREM) while slow wave sleep with white noise remained the same as baseline (no noise), compared to reduction with ICU noise. |
| Taranto-Monemurro 201751 Abstract | White noise, filtered by 4 speakers at 45.3 (1.2) dB | Cohort; Lab; USA-MA | n = 8. | PSG RoB = 3 | Mixed. Positive for reduced sleep latency but no difference in sleep maintenance, total time or slow wave activity |
| Topf 199239 | White noise available for personal control over CCU noise for 480 minutes at 84 dB | RCT; Lab–simulated hospital; USA-CA | n = 105. No hearing or sleeping problems. Age 35.6, 100% women with mixed racial/ethnic groups | PSG, Sleep summary sheets RoB = 2 | Negative. Personal control with white noise resulted in poorer sleep efficiency and more difficulty falling and staying asleep |
| Waye 200440 | White noise–low frequency noise for 360 minutes, 50 Hz at 40 dB | Cohort; Lab-Europe | n = 26. Age 26 (4.3); 0% women | Cortisol levels (saliva) and Self-reported Sleep Disturbance Questionnaire RoB = 2 | Mixed. No differences with sleep latency and maintenance or changes in cortisol secretion but participants felt more tired in the morning |
| Webb 197941 | White noise–recording of a fan motor for 30 minutes at 70 dB vs intermittent tones for 30 minutes, 80 Hz at 70 dB vs silence | RCT, 3 groups; Lab; USA-FL | n = 48. Age 18–22. | PSG RoB = 2 | None. No improvement for white noise while intermittent tones resulted in reduced latency and better sleep maintenance |
| Young 198843 | Modified white noise (water) generator at the bedside from 9 pm to 7 am × 4 nights (4 nights acclimate to lab + 4 night no intervention) | Randomized crossover; Lab; USA-MD | n = 8; Adults > 60 years with Alzheimer’s disease and history of nocturnal wandering. Age 70 (60–82); 50% women | Sleep behavioral observation every 30 minutes by nurse RoB = 3 | None. No differences in sleep though 2 participants displayed reduced agitation |
| Zabrecky 202044 | White noise–vibroacoustic stimulator included a 24-minute vibroacoustic (8–10 Hz) program twice/week in lab and audio × 60 minutes nightly in bed. Both for 1 month | RCT; Lab twice/week, audio in community -home; USA-PA | n = 30. Adults with insomnia. Age 43.3 (19.6, 27–35); 46.7% women | Actigraphy (5 nights prior and following intervention), Insomnia Severity Index RoB = 3 | Positive. Significant improvement in minutes slept and sleep quality. |
| Multiaudio (n = 6) | |||||
| Clark 201720 | Multiaudio. Audio sleep menu of white noise machine, music, ear plugs, close door/blinds, tea | Controlled clinical trial; Inpatient for > 1 day; Hospital-Medical unit; USA-VA | n = 62. Age 42–90; 56% women | Sleep Satisfaction Survey RoB = 3 | Mixed. Intervention group participants were more satisfied with sleep though men preferred white noise compared to women |
| Farrehi 201624 | Multiaudio. Choice of white noise, sleep mask, or ear plugs | RCT; Hospital-non-ICU for > 4 days; USA-MI | n = 86. Age 56.22 (11.41); 44% women | Patient-Reported Outcomes Measurement Information System survey RoB = 1 | Positive. Reported decreased sleep, wake disturbance, and fatigue. White noise used most (50–53%) |
| Goel 200527 | Multiaudio. Bird song with classical music background × 120 minutes at 60 dB | Cohort; Lab; USA-CT | n = 10. Age 44.7 (21.4, 18–72); 50% women | Melatonin (saliva), Profile of Moods States, Fatigue subscale RoB = 2 | Positive. Improved fatigue level |
| Hu 201529 | Multiaudio. Classical music and nature sounds in evening (birds) and morning (frogs/waves) × 30 minutes at 69.8 ± 2 dB + eye masks and ear plugs | RCT; Postcardiac surgery without sleep disorder; Hospital-SICU; Asia-China | n = 45. Age 56.6 (11)**; 55.5% women | Nocturnal urine, melatonin, and cortisol levels, Richards-Campbell Sleep Questionnaire RoB = 2 | Positive. Increased perceived sleep quality (latency, maintenance, total sleep time and depth); no differences in melatonin or cortisol levels |
| Rybarczk 200234 | Multiaudio. Audio relaxation (music, breathing, nature sounds) at home × 30 minutes for 1 week vs classroom CBT vs control | RCT, 3 groups; Community-Home; USA-IL; Mixed racial/ethnicity | n = 38. Adults > 54 years with insomnia and chronic illness; Age 67.8; 58% women | Actigraphy, PSQI, Sleep logs, Dysfunctional Beliefs and Attitudes About Sleep Scale RoB = 1 | Mixed. CBT demonstrated the most improvement in self-reported measures while Multiaudio improved total sleep time, latency, efficiency, and perceived sleep quality. No changes in any group with actigraphy. |
| Ryu 201235 | Multiaudio. Music–nature sounds, delta wave control music and Goldberg variations with earphones × 53 minutes + eye bandage vs ear plugs | RCT; Hospital-CCU; South Korea | n = 58. With CAD after PTCA; Age 61.2; 35% women | Verran and Snyder-Halpern Sleep Scale, Quantity of sleeping questionnaire RoB = 1 | Positive. Both total sleep time and perceived quality of sleep improved. |
| Pink Noise (n = 11; 1 Compared to White Noise) | |||||
| Kawada 199330 | Pink Noise—SF-05 noise generator through loudspeaker × 480 minutes at 60 dB | Cohort–within participants design; Lab; Asia-Japan | n = 5. Age 19–28; 25% women | PSG RoB = 3 | Positive. Improved sleep latency |
| Montgomery-Downs 201032*** | Pink Noise (nature sounds) > 2000 Hz at < 50 dB vs (see above) White noise–low frequency fan for 480 minutes < 50 Hz with subtle heartbeat < 100 Hz at 63dB vs | Cohort; Community-home; USA-VA | n = 25. History of insomnia but not any other sleep disorder; Age 40 (14); 64% women** | Actigraphy, PSQI RoB = 1 | Mixed. Positive for improvements in sleep latency but reduced duration. |
| Nasari 201833 | Nature sounds (rain, birds, forest) with MP player for 30 minutes at 60–70 dB silence (muted headphones) vs control. | RCT (3 groups, single blind); Hospital– CCU; Iran-Tehran | n = 93. No history of sleep disorders, on CCU unit for at least 2 days; Age 56.25 (14); 38.7% women** | Richards-Campbell Sleep Questionnaire RoB = 1 | Positive. Both nature sounds and silence improved sleep latency, maintenance, efficiency, and self-reported sleep quality |
| Papalambros 201546 Abstract | Pink Noise, alternating pulses on vs off of 0.8–2.1 Hz | Cohort; Lab; USA-IL | n = 7. Healthy adults older ≥ 50 years; Age 66.85 (10); 85.7% women | PSG (EEG–power spectral analysis) RoB = 3 | Mixed. Positive for enhancing slow wave activity during pink noise pulses but no difference between stimulation vs sham night |
| Santostasi 201747 Abstract | Pink Noise | Cohort; Lab; USA-IL | n = 7. Age 66.85 (10); 86.7% women | PSG RoB = 3 | Positive. Enhances slow wave activity and spindle amplitude |
| Santostasi 201548 Abstract | 50-minute bursts in blocks of 5 of pink noise, then 5 seconds refractory time | Cohort; Lab; USA-IL | n = 4. Healthy young adults; Age 20.3 (1.2) | EEG RoB = 3 | Positive. Enhanced slow wave sleep |
| Simor 201650 Abstract | Alternate 1 Hz pulses of pink noise with silence via 1 earplug during NREM sleep | Cohort; Lab: Europe-Hungary | n = 16. Right-handed healthy adults | PSG RoB = 3 | Positive. Enhanced slow wave sleep and sleep spindling activity during deep sleep. |
| Simor 201836 | After 5 minutes in daytime NREM sleep, unilaterally given 12 bursts of pink noise at 1 Hz and < 60 dB for 12 seconds, alternated with 15 seconds of silence, × 120 minutes | Cohort; Lab: Europe-Hungary | n = 35. Right-handed adults with no psychiatric, neurological, or sleep complaints; Age 27.25 (5.3); 57.1% women | PSG RoB = 2 | Positive. Enhanced slow wave activity during NREM sleep |
| Suzuki 199138 | 420 minutes of pink noise at 40–60 dB | Cohort; Lab; Asia-Japan | n = 6. Age 21–28; 25% women | PSG RoB = 3 | Positive. Deepened mean sleep depth at 60 dB vs 35 dB and decrease in sleep latency |
| Williamson 199242 | Ocean sounds for 750 minutes | RCT; Hospital; USA-AL | n = 56. No sleep disorders or repeat CABG. Age 58.6 (7.7, 35–69); 30% women** | Richards-Campbell Sleep Questionnaire RoB = 2 | Positive. Greater perceived sleep latency, maintenance, deeper and total sleep |
| Zhou 201211 | Pink Noise–brain activity synchronization with 10 min quiet + 10 min pink noise × 50 minutes (group 1); 480 minutes (group 2); afternoon 60 minutes | Cohort, 3 groups; Lab; Asia-China | n = 40. Age 21–30; 50% women | PSG, Sleep quality questionnaire RoB = 2 | Positive. Increased stable sleep time (maintenance) and self-reported sleep quality |
*The Risk of Bias is the summary score of the Effective Public Health Practice Project–Quality Assessment Tool for Quantitative Studies. **If total sample demographics not given then only experimental group demographics are listed. ***The study by Montgomery-Downs et al 2010 compared white to pink noise so it appears twice in the table. CABG = coronary artery bypass grafting, CAD = coronary artery disease, CBT = cognitive behavioral therapy, CCU = coronary care unit, EEG = electroencephalogram, ICU = intensive care unit, NREM = non-REM, PSG = polysomnography, PSQI = The Pittsburgh Sleep Quality Index, PTCA = percutaneous transluminal coronary angioplasty, RCT = randomized controlled trial, REM = rapid eye movement, SICU = surgical intensive care unit, SWS = slow wave sleep, WASO = wake time after sleep onset.
Most interventions were implemented in a lab (15) or hospital (12, with 4 on critical care units) in North America (19). Others took place in Asia (6), Europe (6) and the Middle East (3). There was a total of 1,103 participants with a mean number of 32.44 (standard deviation [SD] = 21.21) per study and a range of 4–105 participants. Of the 24 studies reporting age, the mean was 56.7 years (SD = 18.88) with a range of 17–70 years. Among the 29 studies that included sex, the mean proportion of women was 52.12%. Twenty-four studies described the race/ethnicity of participants as White (11), Asian (5), and 2 mixed (White, African American, Hispanic, and American Indian).
From the 34 articles that met criteria, a total of 35 (Montgomery-Downs et al32 compared white to pink noise) interventions were identified: white noise (18), pink noise (11), and 6 multiaudio (some combination of white, pink, music and/or silence). The duration of the auditory intervention, reported in 26 studies, was an average of 267.04 minutes (SD = 225.94; range: 30–750) with 13 providing sound all night (360–750 minutes), while the others provided sound for 30–120 minutes prior to sleep initiation (13). Most studies (19) provided sound intensity with an average of 56.38 dB (SD = 13.68; range = 30–93), while only 8 studies indicated sound frequency with an average of 82.43 (SD = 170.85; median = 31; range = 1–500) Hz. Five studies provided both sound intensity and frequency, 14 indicated only intensity, 3 reported only frequency, and 12 studies did not indicate either sound intensity or frequency.
In half (17) of the studies, a single measure for sleep outcome was used, including objective measurements such as polysomnography (PSG; 16), actigraphy (5), cortisol/melatonin urine levels (2), or melatonin (1) or cortisol (1) saliva levels. Others used validated self-reports: The Pittsburgh Sleep Quality Index (4),52 Richards-Campbell Sleep Questionnaire (4),53 Verran and Snyder-Halpern Sleep Scale (1),54 The Profile of Mood States (1),55 Patient-Reported Outcomes Measurement Information System survey (1),56 the Insomnia Severity Index (1),57 and the Stanford Sleepiness Scale (1).58 Four studies used a sleep diary or log and another 10 used investigator-developed tools. Among sleep outcomes, onset latency (15), sleep maintenance (16), slow wave activity (14), and/or total sleep time (10) were reported.
Nineteen (56%) studies showed an improvement in sleep: 6 (38%) white noise, 4 (66.7%) multiaudio, and 9 (81.9%) pink noise. Improvements were observed in slow wave activity (8), wake time after sleep onset (8), sleep latency (7), duration (4), efficiency (3), as well as 9 studies reporting positive perceptions of sleep quality. The proportion of positive findings was similar in a simulated lab environment (50%) vs those conducted in either a community or institutional setting (59%).
Methodological quality assessment
Table 4 summarizes the QAT global rating score, study design, objective sleep measure, and whether the intervention improved sleep quality. The QAT risk of bias varied across studies with 6 studies scoring “1,” 14 scoring “2,” and 15 scoring “3” (lower scores indicate less bias). The average score (range 1–3) was 2.29 (0.76) with most studies not reporting that either the assessor or the participant was blinded. Also, since 8 studies were presented in a conference abstract, we were not able to determine risks of confounding or the rate of withdrawals/dropouts in those studies. When the QAT global rating score was compared by audio type, multiaudio (mean [M]/SD = 1.67/0.82) intervention studies had the lowest (better) quality ratings vs the white noise (M/SD = 2.38/0.69) and pink noise (M/SD = 2.36/0.81) studies. Thus, multiaudio had the best risk of bias scores. Although most studies examined white noise (20), white noise had the lowest proportion of positive findings and highest risk of bias.
Table 4.
Methodological quality of studies.
| Global Rating | Design | PSG/ACT | |
|---|---|---|---|
| White noise (n = 18) | |||
| Alkahtani 20198 | 2 | Cohort | ACT |
| Daum 198821 | 3 | 2 × 3 | PSG |
| Ebben 202122 | 2 | Cohort | ACT |
| Farokhnezhad 201623 | 2 | Cohort analytic | |
| Forquer 200725 | 3 | Cohort | |
| Gao 202026 | 3 | RCT | PSG |
| Handscomb 200628 | 2 | Cohort | |
| Messineo 201731 | 1 | RCT | PSG |
| Montgomery-Downs 201032* | 1 | Cohort | ACT |
| Owen 201945 | 3 | RCT | |
| Shaikh 2017?49 | 3 | Cohort | PSG |
| Stanchina 200537 | 3 | Cohort | PSG |
| Taranto-Monemurro 201751 | 3 | Cohort | PSG |
| Topf 199239 | 2 | RCT | PSG |
| Waye 200440 | 2 | Cohort | |
| Webb 197941 | 2 | RCT | PSG |
| Young 198843 | 3 | RCT | |
| Zabrecky 202044 | 3 | RCT | ACT |
| Multiaudio (n = 6) | |||
| Clark 201720 | 3 | RCT | |
| Farrehi 201624 | 1 | RCT | |
| Goel 200527 | 2 | Cohort | |
| Hu 201529 | 2 | RCT | |
| Rybarczyk 200234 | 1 | RCT | ACT |
| Ryu 201235 | 1 | RCT | |
| Pink noise (n = 11) | |||
| Kawada 199330 | 3 | Cohort | PSG |
| Montgomery-Downs 201032* | 1 | Cohort | ACT |
| Nasari 201833 | 1 | RCT | |
| Papalambros 201546 | 3 | Cohort | PSG |
| Santostasi 201747 | 3 | Cohort | PSG |
| Santostasi 201548 | 3 | Cohort | PSG |
| Simor 201650 | 3 | Cohort | PSG |
| Simor 201836 | 2 | Cohort | PSG |
| Suzuki 199138 | 3 | Cohort | PSG |
| Williamson 199242 | 2 | RCT | |
| Zhou 201211 | 2 | Cohort | PSG |
| Summary | No. Positive/Total* | Percentage Positive | Mean/SD Global Rating |
| Multiaudio | 4/6 | 66.7 | 1.67 (0.82) |
| White noise | 7/18 | 38.0 | 2.45 (0.69) |
| Pink noise | 9/11 | 81.8 | 2.36 (0.80) |
Italic rows indicate positive results. Global rating is the summary of the 7 domains of the Effective Public Health Practice Project (EPHPP) Quality Assessment Tool (QAT) for Quantitative Studies. Scores: 1 = strong or no weak ratings, 2 = moderate or one weak rating, or 3 = weak or 2 or more weak ratings. *The study by Montgomery-Downs et al 2010 compared white to pink noise so it appears twice in the table and the total is 35 instead of 34. CCT = controlled clinical trial, PSG/ACT = measured sleep outcomes with polysomnography and/or actigraphy, RCT = randomized controlled trial, SD = standard deviation.
Comparison of studies by category
From the 18 white noise studies, we identified 2 studies with a low risk of bias (11.1%), 7 RCTs (38.9%), with 8 employing PSG. The 2 low-risk studies reported no32 or mixed results.31 Of the 7 studies of moderate risk of bias, 2 had a positive, 2 a mixed, 2 none, and 1 a negative result. Of the 9 studies conducted in a lab, only 1 had positive results,44 while 5 of 9 studies in a hospital or home had positive results. White noise was produced with either a sound generator, a low-frequency fan, or a recording of a fan motor. Twelve (66.7%) studies reported intensity of the sound with a range from 38 to 84 dB and a mean of 55.55 dB (SD = 14.65). Frequency ranged from 22 to 500 Hz (M/SD = 140/202) among the 5 studies reporting Hz. Of the14 studies reporting duration, most exposed participants to the sound all night (9); however only 4 had positive results.25,28,37,49
Most (9) of the 11 pink noise studies reported positive sleep outcomes. However, only 2 studies had a low risk of bias32,33; 1 of which had mixed findings32 and the other had a positive finding.33 Seven of the 9 studies with positive findings were conducted in a lab environment. In the 1 study that compared white and pink noise, there was no improvement in sleep among those in the white noise group, while the results from the pink noise group were mixed.32 In 5 studies, the participants were exposed to pink noise for most of the night (420–750 minutes),11,30,32,38,42 while others were exposed only during sleep initiation (3; 30–120 minutes)33,48,50; the proportion of those with positive findings were similar (4/5 vs 5/6, respectfully). Fewer than half reported sound intensity (5; M/SD = 57/6.7) or frequency (4; M/SD = 501/999; range = 1–2000).
The multiaudio interventions had the highest proportion of positive findings and the best risk of bias scores. Of the 6 multiaudio interventions, half had a low risk of bias and used an RCT design,24,34,35 but none measured sleep with PSG. The combination of audio interventions included either nature sounds plus different types of music (4)27,29,34,35 or provided a choice to participants of white noise, ear plugs, or music (2).20,24 Of the latter, most chose white noise. Four (66.7%) reported positive sleep findings,24,27,29,35 but there was no consistent pattern of multiaudio type or risk of bias. The sounds were played an average of 58 minutes (SD = 43; range 30–120; n = 4) for an intensity of approximately 65 dB (2), and none reported sound frequency. Only 1 study was conducted in a lab. Of the 4 with positive results, 1 was in a lab. Compared to the white noise group, a higher proportion of those with positive findings had been conducted in a lab (1 out of 4 vs 1 out of 9). The pink noise group had a relatively higher proportion of those with positive findings when studied in the lab (7 out of 9).
DISCUSSION
Overview of the qualitative analyses
Although a quiet environment is suggested for inducing and maintaining sleep, it is not always possible, especially in urban settings. Paradoxically, many have resorted to using music or the audio of a television as a sleep aid,59,60 without describing a clear negative impact on sleep duration or quality.61 Audio stimulation to induce sleep with sound “conditioners” or “generators” first appeared in the United States in the 1960s and now accounts for over a billion dollars in sales each year.62 Nonetheless, scientific research has been lacking in this field. This is the first known systematic review to examine the evidence of auditory stimulation effects on sleep outcomes in adults. Our review identified only 34 studies, and the findings are conflicting. Moreover, it was not possible to conduct a meta-analysis due to the variation among study design, measurement, and intervention characteristics.
Delivery of audio stimulation varied by duration of exposure, volume setting, and device (speakers vs earbuds/headphones), making it difficult to compare within and across sound categories. Although this is a scientific review of audio interventions, only 5 studies provided both sound intensity and frequency, thus making it difficult to replicate their study findings.
Most of the studies evaluated white noise, but fewer than a third had positive findings and each of these studies demonstrated moderate or weak risk of bias. The white noise was mostly played all night since the primary function was to mask sounds. However, as others have pointed out, “listening to a sound without meaning can actually be an unpleasant rather than a helpful experience.”28 Comparatively, pink noise may potentially be viewed as more effective than white noise, but the rigor of these studies was poor. This indicates the need for more research, especially since only 1 study compared white to pink noise.32
Multiaudio sound had a higher proportion of positive findings with the lowest (better) risk of bias and most used an RCT design. None, however, measured sleep with PSG and there were only 4 positive studies, with a total of 199 participants. Most (5 of 6) studies used a combination of white or pink noise and music. Music is meant to induce sleep by relaxation. It is not possible to test every type of music but to use music perceived as pleasant to the study participant. Several systematic reviews and meta-analyses have reported music as effective for improving self-reported sleep quality among those with both acute and chronic insomnia.63–65 An online survey, not included in this review, was conducted in the United Kingdom and reported that among 403 respondents who used music at bedtime, younger people who listened to music regularly were significantly more likely to use music to aid sleep. Respondents viewed music as stimulating sleep, part of a normal sleep routine, facilitating a physical or mental state for sleep, and/or blocking internal or external stimuli.66 A secondary analysis of 15 studies evaluating audio stimulus as a sleep aid found similar results.67
Several important methodological issues were identified in this review. Only 5 studies specifically included participants with insomnia complaints22,25,28,32,34 with only 1 study indicating it was specifically due to environmental noise22 Also, only 1 study included those with complaints of tinnitus.32 Further, the exclusion criteria did not account for potentially confounding factors of the participant’s usual sleep environment or the testing sites other environmental characteristics (eg, light and humidity levels). Thus, it calls into question the usability of the findings in clinical practice. These conclusions are consistent with another systematic review of studies that examined the association of continuous white noise with sleep.68
Implications for clinical practice
Although there is no strong evidence to support use of auditory stimulation, none of the studies reported any adverse effects with short-term application of auditory stimulation during sleep. While 1 study indicated that white noise “induces maladaptive changes in the brain that degrade neurological health and compromise cognition,”69 there is not enough evidence to support this concern and discourage a patient from using white noise if the person believes it helps with sleep. Among studies conducted in the hospital (not simulated) environment, those using multiaudio20,24,29,35 had the highest proportion of positive findings (50% within their intervention category) and the lowest risk of bias (1.33/0.6). The 3 positive studies either combined nature sounds with music29,35 or gave patients the choice of white noise or earplugs.24
Hospitals and other institutional patient settings have been working toward reducing overall noise levels,70 and combining strategies for noise reduction with audio interventions may be effective.71 The delivery of auditory sounds could be included as part of the hospital television or radio channel choices or the patient’s own electronic device; however, this would best work within a single-patient room. In shared rooms, the delivery of sounds by headphones or earplugs could be considered, depending on the patient’s level of comfort with these devices. It seems reasonable to ask patients their preference in terms of delivery mode of audio interventions to improve sleep during their hospital stay.
Limitations
We did not include studies of audio interventions that cannot be easily applied in a person’s home or a clinical setting, such as binaural tones, and sound synchronization. Furthermore, studies evaluating the effect of music on sleep were also not included since it is difficult to standardize and implement it in an institutional setting due to individual preferences. Our study also did not include newer technologies such as noise “canceling” headphones or earbuds. Given that conference abstracts were included in this review, the risk of bias in these studies may have been underestimated, given that many essential elements of the study methodology were not included in these brief synopses.
Recommendations for future research
This review revealed favorable results with the multiaudio interventions in terms of improving sleep quality, and more studies are needed to compare the various audio interventions individually and in combination. Future research also needs to take into consideration other methodological factors that can affect outcomes, including typical sleeping environment (including those that use television audio, radio, podcasts, or music to sleep), an individual’s noise sensitivity and preferences, environmental noise levels, sleep hygiene practices, and any health conditions or medications that may affect sleep. Auditory stimulation also needs to be examined in those with personality traits that are associated with sleep disturbances, such as those with neuroticism who are highly responsive to environmental stressors as well as those with anxiety and who are perfectionists.72 Since participants of the studies in this review were mostly young and middle-aged white adults, future studies should address this lack of age and racial/ethnic diversity which may have biased the results.
CONCLUSIONS
Although the use of auditory stimulation interventions to improve sleep is a common practice, there was no clear evidence from this review that it effectively improves sleep. A more rigorous, randomized trial of the various audio interventions among those with insomnia, while controlling for the participant’s usual bedtime schedule and environmental factors, would be useful to help determine the potential effect of auditory stimulation on sleep both at home and in institutional settings. For the latter, knowing an individual’s preference in terms of audio intervention would also be desirable.
ABBREVIATIONS
- PSG
polysomnography
- QAT
Quality Assessment Tool for Quantitative Studies
- RA
research assistant
- RCT
randomized controlled trial
- RoB
risk of bias
- SD
standard deviation
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Hunter College and Weill Cornell Medical College. The authors report no conflicts of interest.
REFERENCES
- 1. Lustenberger C , Patel YA , Alagapan S , et al . High-density EEG characterization of brain responses to auditory rhythmic stimuli during wakefulness and NREM sleep . Neuroimage. 2018. ; 169 : 57 – 68 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lustenberger C, Boyle MR, Alagapan S, Mellin JM, Vaughn BV, Fröhlich F. Feedback-controlled transcranial alternating current stimulation reveals a functional role of sleep spindles in motor memory consolidation. Curr Bio. 2016;26(16):2127–2136. [DOI] [PMC free article] [PubMed]
- 3. Medrzycka-Dabrowska W , Lewandowska K , Kwiecień-Jaguś K , Czyż-Szypenbajl K . Sleep deprivation in intensive care unit–systematic review . Open Med (Wars). 2018. ; 13 ( 1 ): 384 – 393 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Basner M , McGuire S . WHO environmental noise guidelines for the European region: a systematic review on environmental noise and effects on sleep . Int J Environ Res Public Health. 2018. ; 15 ( 3 ): 519 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Riemann D , Spiegelhalder K , Feige B , et al . The hyperarousal model of insomnia: a review of the concept and its evidence . Sleep Med Rev. 2010. ; 14 ( 1 ): 19 – 31 . [DOI] [PubMed] [Google Scholar]
- 6. Li C, Dong M, Yin Y, Hua K, Fu S, Jiang G. Aberrant effective connectivity of the right anterior insula in primary insomnia. Front Neurol. 2018;9:317. [DOI] [PMC free article] [PubMed]
- 7. Muzet A . Environmental noise, sleep and health . Sleep Med Rev. 2007. ; 11 ( 2 ): 135 – 142 . [DOI] [PubMed] [Google Scholar]
- 8. Alkahtani MN , Alshathri NA , Aldraiweesh NA , et al . The effect of air conditioner sound on sleep latency, duration, and efficiency in young adults . Ann Thorac Med. 2019. ; 14 ( 1 ): 69 – 74 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jastreboff PJ , Jastreboff MM . Tinnitus Retraining Therapy (TRT) as a method for treatment of tinnitus and hyperacusis patients . J Am Acad Audiol. 2000. ; 11 ( 3 ): 162 – 177 . [PubMed] [Google Scholar]
- 10. Fackrell K , Potgieter I , Shekhawat GS , Baguley DM , Sereda M , Hoare DJ . Clinical interventions for hyperacusis in adults: a scoping review to assess the current position and determine priorities for research . BioMed Res Int. 2017. ; 2017 : 2723715 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou J , Liu D , Li X , Ma J , Zhang J , Fang J . Pink noise: effect on complexity synchronization of brain activity and sleep consolidation . J Theor Biol. 2012. ; 306 : 68 – 72 . [DOI] [PubMed] [Google Scholar]
- 12. Yeo KS , Kim MS , Bae MJ . Acoustic characteristics of the forest sounds inducing sleep . International Information Institute (Tokyo) Information. 2015. ; 18 ( 10 ): 4407 – 4412 . [Google Scholar]
- 13. Vaajoki A , Kankkunen P , Pietilä AM , Vehviläinen-Julkunen K . Music as a nursing intervention: effects of music listening on blood pressure, heart rate, and respiratory rate in abdominal surgery patients . Nurs Health Sci. 2011. ; 13 ( 4 ): 412 – 418 . [DOI] [PubMed] [Google Scholar]
- 14. Gao X , Cao H , Ming D , et al . Analysis of EEG activity in response to binaural beats with different frequencies . Int J Psychophysiol. 2014. ; 94 ( 3 ): 399 – 406 . [DOI] [PubMed] [Google Scholar]
- 15. Choi J , Han S , Won K , Jun SC . The neurophysiological effect of acoustic stimulation with real-time sleep spindle detection . Annu Int Conf IEEE Eng Med Biol Soc. 2018. ; 2018 : 470 – 473 . [DOI] [PubMed] [Google Scholar]
- 16. Liberati A , Altman DG , Tetzlaff J , et al . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration . J Clin Epidemiol. 2009. ; 62 ( 10 ): e1 – e34 . [DOI] [PubMed] [Google Scholar]
- 17. Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia . Available at www.covidence.org ; accessed June 1, 2021. .
- 18. Jackson N , Waters E ; Guidelines for Systematic Reviews in Health Promotion and Public Health Taskforce. Criteria for the systematic review of health promotion and public health interventions . Health Promot Int. 2005. ; 20 ( 4 ): 367 – 374 . [DOI] [PubMed] [Google Scholar]
- 19. Thomas BH , Ciliska D , Dobbins M , Micucci S . A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions . Worldviews Evid Based Nurs. 2004. ; 1 ( 3 ): 176 – 184 . [DOI] [PubMed] [Google Scholar]
- 20. Clark A , Mills M . Can a sleep menu enhance the quality of sleep for the hospitalized patient? J Adv Med Surg Nurs. 2017. ; 26 ( 4 ); https://www.thefreelibrary.com/Can+a+sleep+menu+enhance+the+quality+of+sleep+for+the+hospitalized...-a0503466845 ; accessed Jan. 8, 2022. [Google Scholar]
- 21. Daum I , Leonard JP , Hehl FJ . Development of sleep during monotonous stimulation as related to individual differences . Pavlov J Biol Sci. 1988. ; 23 ( 3 ): 118 – 124 . [DOI] [PubMed] [Google Scholar]
- 22. Ebben MR , Yan P , Krieger AC . The effects of white noise on sleep and duration in individuals living in a high noise environment in New York City . Sleep Med. 2021. ; 83 : 256 – 259 . [DOI] [PubMed] [Google Scholar]
- 23. Farokhnezhad Afshar P , Bahramnezhad F , Asgari P , Shiri M . Effect of white noise on sleep in patients admitted to a coronary care . J Caring Sci. 2016. ; 5 ( 2 ): 103 – 109 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farrehi PM , Clore KR , Scott JR , Vanini G , Clauw DJ . Efficacy of sleep tool education during hospitalization: A randomized controlled trial . Am J Med. 2016. ; 129 ( 12 ): 1329.e9 – 1329.e17 . [DOI] [PubMed] [Google Scholar]
- 25. Forquer LM , Johnson CM . Continuous white noise to reduce sleep latency and night walkings in college students . Sleep Hypn. 2007. ; 9 ( 2 ); http://www.sleepandhypnosis.org/ing/Pdf/a72d260d52c84ec99e7a751a4c6a1d83.pdf . Accessed January 8, 2022. [Google Scholar]
- 26. Gao D , Long S , Yang H , et al . SWS brain-wave music may improve the quality of sleep: an EEG study . Front Neurosci. 2020. ; 14 : 67 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goel N . Late-night presentation of an auditory stimulus phase delays human circadian rhythms . Am J Physiol Regul Integr Comp Physiol. 2005. ; 289 ( 1 ): R209 – R216 . [DOI] [PubMed] [Google Scholar]
- 28. Handscomb L . Use of bedside sound generators by patients with tinnitus-related sleeping difficulty: which sounds are preferred and why? Acta Otolaryngol Suppl. 2006. ; Dec ( 556 ): 59 – 63 . [DOI] [PubMed] [Google Scholar]
- 29. Hu RF , Jiang XY , Hegadoren KM , Zhang YH . Effects of earplugs and eye masks combined with relaxing music on sleep, melatonin and cortisol levels in ICU patients: a randomized controlled trial . Crit Care. 2015. ; 19 ( 1 ): 115 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kawada T , Suzuki S . Sleep induction effects of steady 60 dB (A) pink noise . Ind Health. 1993. ; 31 ( 1 ): 35 – 38 . [DOI] [PubMed] [Google Scholar]
- 31. Messineo L , Taranto-Montemurro L , Sands SA , Oliveira Marques MD , Azabarzin A , Wellman DA . Broadband sound administration improves sleep onset latency in healthy subjects in a model of transient insomnia . Front Neurol. 2017. ; 8 : 718 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Montgomery-Downs HE , Insana SP , Miller EA . Effects of two types of ambient sound during sleep . Behav Sleep Med. 2010. ; 8 ( 1 ): 40 – 47 . [DOI] [PubMed] [Google Scholar]
- 33. Nasari M , Ghezeljeh TN , Haghani H . Effects of nature sounds on sleep quality among patients hospitalized in coronary care units: a randomized controlled clinical trial . Nurs Midwifery Stud. 2018. ; 7 ( 1 ): 18 – 23 . [Google Scholar]
- 34. Rybarczyk B , Lopez M , Benson R , Alsten C , Stepanski E . Efficacy of two behavioral treatment programs for comorbid geriatric insomnia . Psychol Aging. 2002. ; 17 ( 2 ): 288 – 298 . [PubMed] [Google Scholar]
- 35. Ryu MJ , Park JS , Park H . Effect of sleep-inducing music on sleep in persons with percutaneous transluminal coronary angiography in the cardiac care unit . J Clin Nurs. 2012. ; 21 ( 5-6 ): 728 – 735 . [DOI] [PubMed] [Google Scholar]
- 36. Simor P , Steinbach E , Nagy T , et al . Lateralized rhythmic acoustic stimulation during daytime NREM sleep enhances slow waves . Sleep. 2018. ; 41 ( 12 ): zsy176 . [DOI] [PubMed] [Google Scholar]
- 37. Stanchina ML , Abu-Hijleh M , Chaudhry BK , Carlisle CC , Millman RP . The influence of white noise on sleep in subjects exposed to ICU noise . Sleep Med. 2005. ; 6 ( 5 ): 423 – 428 . [DOI] [PubMed] [Google Scholar]
- 38. Suzuki S , Kawada T , Ogawa M , Aoki S . Sleep deepening effect of steady pink noise . J Sound Vibrat. 1991. ; 151 ( 3 ): 407 – 414 . [Google Scholar]
- 39. Topf M . Effects of personal control over hospital noise on sleep . Res Nurs Health. 1992. ; 15 ( 1 ): 19 – 28 . [DOI] [PubMed] [Google Scholar]
- 40. Waye KP , Agge A , Clow A , Hucklebridge F . Cortisol response and subjective sleep disturbance after low-frequency noise exposure . J Sound Vibrat. 2004. ; 277 ( 3 ): 453 – 457 . [Google Scholar]
- 41. Webb WB , Agnew HW Jr . Sleep onset facilitation by tones . Sleep. 1978. ; 1 ( 3 ): 281 – 286 . [DOI] [PubMed] [Google Scholar]
- 42. Williamson JW . The effects of ocean sounds on sleep after coronary artery bypass graft surgery . Am J Crit Care. 1992. ; 1 ( 1 ): 91 – 97 . [PubMed] [Google Scholar]
- 43. Young SH , Muir-Nash J , Ninos M . Managing nocturnal wandering behavior . J Gerontol Nurs. 1988. ; 14 ( 5 ). [DOI] [PubMed] [Google Scholar]
- 44. Zabrecky G , Shahrampour S , Whitely C , et al . An fMRI study of the effects of vibroacoustic stimulation on functional connectivity in patients with insomnia . Sleep Disord. 2020. ; 2020 : 7846914 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Owen JD , Dunn MJ . Active noise cancelling headphones with white noise to aid sleep in non-ventilated, non-delirious critical care patients: a randomised crossover trial . Br J Anaesth. 2019. ; 123 ( 4 ): e503 . [Google Scholar]
- 46. Papalambros PA , Santostasi G , Malkani RG , Weintraub S , Poller KA , Zee PC . Acoustic stimulation increases slow wave activity in older adults . Sleep. 2015;38(Abstract Suppl): A402. [Google Scholar]
- 47. Santostasi G . Acoustic stimulation increases slope, amplitude and time in between slow waves in older adullts . Sleep. 2017. ; 40 ( suppl_1 ): A81 . [Google Scholar]
- 48. Santostasi G, Malkani, R, Zee PC, Palier KA. A phase-locked loop for acoustic stimulation during slow-wave sleep . Sleep. 2015. ;38(Abstract Suppl):A98. [Google Scholar]
- 49. Shaikh H , Ghods F , Jubran A , Tobin M , Laghi F . The effect of sound masking on sleep consolidation in patients weaning from prolonged mechanical ventilation . Am J Respir Crit Care Med. 2017. ; 195 : A5770 . [Google Scholar]
- 50. Simor P, Farthouat J, Gilson M, Gombos F, Bodizs R, Peigneux P. Lateralized rhythmic acoustic stimulation during NREM sleep . J Sleep Res. 2016. ; 25 ( 1 ): 104 . [DOI] [PubMed] [Google Scholar]
- 51. Taranto-Montemurro L , Messineo L , Sands S , Azarbarin A , Marques M , Wellman A . Effect of background noise on sleep quality . Sleep. 2017. ; 40 ( suppl_1 ): A146 – A147 . [Google Scholar]
- 52. Buysse DJ , Reynolds CF III , Monk TH , Berman SR , Kupfer DJ . The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research . Psychiatry Res. 1989. ; 28 ( 2 ): 193 – 213 . [DOI] [PubMed] [Google Scholar]
- 53. Richards KC , O’Sullivan PS , Phillips RL . Measurement of sleep in critically ill patients . J Nurs Meas. 2000. ; 8 ( 2 ): 131 – 144 . [PubMed] [Google Scholar]
- 54. Snyder-Halpern R , Verran JA . Instrumentation to describe subjective sleep characteristics in healthy subjects . Res Nurs Health. 1987. ; 10 ( 3 ): 155 – 163 . [DOI] [PubMed] [Google Scholar]
- 55. Curran SL , Andrykowski MA , Studts JL . Short form of the Profile of Mood States (POMS-SF): psychometric information . Psychol Assess. 1995. ; 7 ( 1 ): 80 – 83 . [Google Scholar]
- 56. Buysse DJ , Yu L , Moul DE , et al . Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments . Sleep. 2010. ; 33 ( 6 ): 781 – 792 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Morin CM , Belleville G , Bélanger L , Ivers H . The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response . Sleep. 2011. ; 34 ( 5 ): 601 – 608 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hoddes E , Zarcone V , Smythe H , Phillips R , Dement WC . Quantification of sleepiness: a new approach . Psychophysiology. 1973. ; 10 ( 4 ): 431 – 436 . [DOI] [PubMed] [Google Scholar]
- 59. Gooneratne NS , Tavaria A , Patel N , et al . Perceived effectiveness of diverse sleep treatments in older adults . J Am Geriatr Soc. 2011. ; 59 ( 2 ): 297 – 303 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Exelmans L , Van den Bulck J . The use of media as a sleep aid in adults . Behav Sleep Med. 2016. ; 14 ( 2 ): 121 – 133 . [DOI] [PubMed] [Google Scholar]
- 61. Custers K , Van den Bulck J . Television viewing, internet use, and self-reported bedtime and rise time in adults: implications for sleep hygiene recommendations from an exploratory cross-sectional study . Behav Sleep Med. 2012. ; 10 ( 2 ): 96 – 105 . [DOI] [PubMed] [Google Scholar]
- 62. Hagood M . How a bad night’s sleep birthed the sound conditioner . Atlantic. June 23, 2019. . [Google Scholar]
- 63. Feng F , Zhang Y , Hou J , et al . Can music improve sleep quality in adults with primary insomnia? A systematic review and network meta-analysis . Int J Nurs Stud. 2018. ; 77 : 189 – 196 . [DOI] [PubMed] [Google Scholar]
- 64. Jespersen KV , Koenig J , Jennum P , Vuust P . Music for insomnia in adults . Cochrane Database Syst Rev. 2015. ( 8 ):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang CF , Sun YL , Zang HX . Music therapy improves sleep quality in acute and chronic sleep disorders: a meta-analysis of 10 randomized studies . Int J Nurs Stud. 2014. ; 51 ( 1 ): 51 – 62 . [DOI] [PubMed] [Google Scholar]
- 66. Trahan T , Durrant SJ , Müllensiefen D , Williamson VJ . The music that helps people sleep and the reasons they believe it works: a mixed methods analysis of online survey reports . PLoS One. 2018. ; 13 ( 11 ): e0206531 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dickson GT , Schubert E . How does music aid sleep? literature review . Sleep Med. 2019. ; 63 : 142 – 150 . [DOI] [PubMed] [Google Scholar]
- 68. Riedy SM , Smith MG , Rocha S , Basner M . Noise as a sleep aid: a systematic review . Sleep Med Rev. 2021. ; 55 : 101385 . [DOI] [PubMed] [Google Scholar]
- 69. Attarha M , Bigelow J , Merzenich MM . Unintended consequences of white noise therapy for tinnitus—otolaryngology’s cobra effect: a review . JAMA Otolaryngol Head Neck Surg. 2018. ; 144 ( 10 ): 938 – 943 . [DOI] [PubMed] [Google Scholar]
- 70. Garside J , Stephenson J , Curtis H , Morrell M , Dearnley C , Astin F . Are noise reduction interventions effective in adult ward settings? A systematic review and meta analysis . Appl Nurs Res. 2018. ; 44 : 6 – 17 . [DOI] [PubMed] [Google Scholar]
- 71. Schade MM , Mathew GM , Roberts DM , Gartenberg D , Buxton OM . Enhancing slow oscillations and increasing N3 sleep proportion with supervised, non-phase-locked pink noise and other non-standard auditory stimulation during NREM sleep . Nat Sci Sleep. 2020. ; 12 : 411 – 429 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Emert SE , Tutek J , Lichstein KL . Associations between sleep disturbances, personality, and trait emotional intelligence . Pers Individ Dif. 2017. ; 107 : 195 – 200 . [Google Scholar]