Abstract
Study Objectives:
Data on adherence and outcome of upper airway stimulation (UAS) for patients with obstructive sleep apnea (OSA) are collected in an international registry (ADHERE). Previous publications report significant improvement in self-reported and objective OSA outcomes, durable effectiveness, and high adherence. Debate remains whether the effectiveness of UAS is influenced by preoperative OSA severity; therefore, we aimed to evaluate this using data from the ADHERE Registry.
Methods:
ADHERE is a postmarket, ongoing, international multicenter registry. Adult patients were included if they had undergone UAS implantation and had at least 1 follow-up visit recorded in the database on June 8, 2021. We divided the patients into 5 subgroups, based on OSA severity at baseline (AHI in events/h): subgroup 1 (0–15), 2 (15–30), 3 (≥ 30–50), 4 (> 50–65), and 5 (> 65). We compared results regarding objective and self-reported treatment outcomes.
Results:
A total of 1,963 patients were included. Twelve months after implantation, there was a significant (P < .0001) improvement in objective sleep parameters in all subgroups with an AHI above 15 events/h. Patients in subgroup 1 had the lowest AHI at the final visit and the AHI reduction in patients in subgroup 5 was the largest (P < .0001). No significant difference was found between the subgroups in overall treatment success (66.6%) and improvement in self-reported outcomes.
Conclusions:
Our results suggest that UAS is an effective treatment for patients with an AHI ≥ 15 events/h, independent of preoperative OSA severity. Self-reported outcomes and treatment success did not differ significantly between the 5 subgroups. These results clearly support that the indication of UAS could be broadened for patients with an AHI above 65 events/h, which, to date, is not common practice.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Title: Adherence and Outcome of Upper Airway Stimulation (UAS) for OSA International Registry (ADHERE Registry); Identifier: NCT02907398; URL: https://www.clinicaltrials.gov/ct2/show/NCT02907398
Citation:
Bosschieter PFN, de Vries N, Mehra R, et al. Similar effect of hypoglossal nerve stimulation for obstructive sleep apnea in 5 disease severity categories. J Clin Sleep Med. 2022;18(6):1657–1665.
Keywords: sleep apnea, obstructive, therapy
BRIEF SUMMARY
Current Knowledge/Study Rationale: Previous publications on upper airway stimulation (UAS) for patients with obstructive sleep apnea (OSA) report significant improvement in self-reported and objective OSA outcomes, durable effectiveness, and high adherence. Whether preoperative severity of OSA influences the effectiveness of UAS remains debatable; hence, we investigated this question using analyses within the ADHERE Registry.
Study Impact: Our results suggest that UAS is an effective treatment for patients with an apnea-hypopnea index ≥15 events/h, independent of preoperative OSA severity. Self-reported outcomes and treatment success did not differ significantly between the 5 subgroups. These results clearly support that the indication of UAS could be broadened for patients with an apnea-hypopnea index above 65 events/h, which, to date, is not common practice.
INTRODUCTION
The ADHERE Registry (Adherence and Outcome of Upper Airway Stimulation for OSA International Registry) is a postmarket, multicenter, observational registry that was designed to collect data related to the use and effectiveness of upper airway stimulation (UAS) therapy (Inspire Medical Systems, Inc, Minneapolis, MN). The latter is a treatment developed for patients with obstructive sleep apnea (OSA) who are not effectively treated with positive airway pressure (PAP), which has been elaborately described in the literature previously.1
All patients who are eligible for an Inspire implant may be invited to participate in the ADHERE Registry. There are no limitations for the registry for age, sex, body mass index (BMI), or OSA severity, so therefore some patients who are enrolled have been implanted outside of the current indication criteria. Participants who are enrolled in the ADHERE Registry are followed for at least 1 year post-implantation. Information related to demographics, severity of OSA (as measured by polysomnography [PSG] or home sleep test [HST]) and symptoms are collected at baseline. Implant details are collected and recorded at the time of implantation. Follow-up information related to severity of OSA (as measured by PSG or HST), symptoms, surgical outcomes, complications, quality of life, therapy usage, patient improvement, and satisfaction with UAS is collected at approximately 6 months (post-titration) and 12 months (final follow-up) post-implantation.
Previous publications on the ADHERE Registry report significant improvement in self-reported and objective OSA outcomes, and of greater novelty, that the UAS therapy effect is durable and adherence remains high.2–5 These studies suggest that female sex,2 a lower baseline BMI2,3 and increasing age3,5 are significant positive predictors of therapeutic outcome. Heiser et al3 found that OSA severity was not a predictor for UAS treatment success. Thaler et al,2 however, found that a higher apnea-hypopnea index (AHI) was a negative predictor for success and suggested that this specific predictor bears further investigation.
Bearing this in mind, we specifically aimed to evaluate the effectiveness of UAS in 5 subgroups stratified by preoperative severity of OSA, using data from the ADHERE Registry. A second reason to evaluate whether preoperative OSA severity is a predictor for success is that, in some countries, OSA reimbursement is restricted to certain cutoff values concerning the AHI, due to lack of evidence concerning patients treated with UAS therapy beyond the scope of this range.
We hypothesized that objective and self-reported therapy efficacy, adherence, and patient-reported outcomes after UAS implantation are independent of preoperative OSA severity.
METHODS
We adhered to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for reporting of prospective studies.6
Study design, setting, and participants
The ADHERE Registry, which began enrolment in October 2016 is an ongoing international, multicenter observational study. The registry collects patient and physician-reported outcomes before and after UAS implantation at over 50 clinical centers in the United States, Belgium, Germany, Switzerland, and the Netherlands and was approved by the ethics committee or institutional review board at every implantation center. Data are collected once the patient has given informed consent. The UAS system, indication for implantation, and implantation procedure have been described previously.2
The ADHERE Registry collects and reports on real-world data, which may include data on patients who are outside of the current indications for use (at the discretion of the physician). Collection and reporting of these data allow us to determine prevalence and outcomes related to off-label usage. Patients were included in this analysis if they had undergone implantation of the Inspire system and had at least 1 follow-up visit (post-titration or final visit) recorded in the database on June 8, 2021. For this dataset, a post-titration visit is marked as missed if the patient is more than 9 months past their implant date as of May 2021. A final visit is marked as missed if the patient is more than 2 years past their implant date.
Variables and data collection
The primary outcome measure of this study was the efficacy of UAS. Patients were stratified into 5 subgroups, according to baseline AHI:
• Subgroup 1. Zero to 15 events/h: non- or mild OSA
• Subgroup 2. Greater than 15 events/h and less than 30 events/h: moderate OSA
• Subgroup 3. Greater than or equal to 30 events/h and less than or equal to 50 events/h: severe OSA
• Subgroup 4. Greater than 50 events/h and less than or equal to 65 events/h: severe OSA, not investigated during the original Stimulation Therapy for Apnea Reduction (STAR) trial, Conformitè Europëenne (CE) and Food and Drug Administration approved
• Subgroup 5. Greater than 65 events/h: severe OSA, no CE and no Food and Drug Administration approval
Secondary outcomes measures were self-reported therapy efficacy, adherence, and patient-reported outcomes stratified by preoperative severity of OSA. All data are recorded in an online cloud-based platform (iMEDnet, Mednet, Minnetonka, MN).
OSA severity information, including AHI and oxygen desaturation index, is captured using either in-laboratory PSG or an HST at baseline and follow-up time points. AHI was based on a full night; we did not use the treatment AHI obtained from the titration.
The Epworth Sleepiness Scale (ESS) was used to acquire information about patient symptoms.7 The ESS is a validated, self-report instrument that rates an individual’s tendency to fall asleep in 8 common daily situations. Scores range from 0 to 24, with a lower score indicating less daytime sleepiness. An ESS score of 10 or less is equivalent to the normalized population.
Therapy usage (hours of active Inspire UAS use per week) was captured by the device when the device is turned on and is reported during follow-up visits when the device data are downloaded onto the physician programmer. Patient improvement was recorded using the Clinical Global Impression–Improvement (CGI-I) Scale.8 The CGI-I is a 7-point scale that requires the clinician to assess how much the patient’s illness has improved or worsened at follow-up, relative to baseline. Patient satisfaction was collected by questionnaires regarding patients’ experience with UAS at follow-up visits.
The mean disease alleviation (MDA) is a measure of therapeutic effectiveness and is the product of therapeutic efficacy and adjusted compliance. Therapeutic efficacy is defined as baseline AHI minus the AHI at 12 months, expressed as the percentage of the baseline AHI. Since we do not know the total sleep time of the patients, we assumed that patients sleep 7 hours per night. The adjusted compliance was defined as hours of use corrected for total sleep time.
Statistical analysis
Statistical analysis was performed using R (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria). Quantitative data were reported as means and standard deviations or as medians and quartiles 1 and 3 when not normally distributed. Statistical comparisons were made between the subgroups in terms of demographic and outcome variables. Statistical significance testing was done using 1 of 4 tests: Wilcoxon rank-sum test, paired t test, Kruskal-Wallis, Fisher’s exact test, chi-square test, or ANOVA (analysis of variance). A P value of less than .05 was considered to indicate statistical significance. To investigate the influence of the use of 2 types of sleep test (HST and PSG) on our outcomes we performed additional analyses. A chi-square test was performed with exclusion of patients whereby the type of sleep test was unknown or did not have a final AHI. In addition, we performed 2 multiple linear regressions (final AHI and change in AHI) and 1 multiple logistic regression for Sher’s response.9
RESULTS
A total of 2,824 patients were enrolled in the ADHERE database in May 2021. The baseline AHI was unknown in 91 patients. A total of 1,921 participants had completed a post-titration (∼6 months post-implant) visit and 1,170 participants had completed a final (∼12 months post-implant) visit. In this study 1,963 participants were analyzed. The average age was 60.2 ± 10.7 years, BMI was 29.2 ± 3.8 kg/m2, and 72.8% were male.
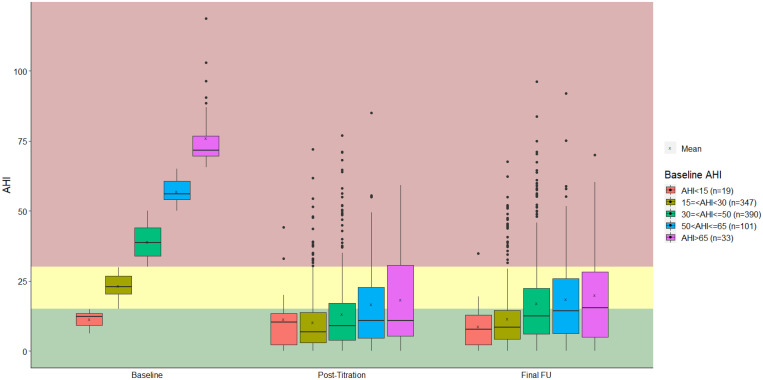
In comparing the demographic variables between the 5 subgroups, there was a significant difference in BMI (P < .0001); subgroup 1 had the lowest BMI (28.4 ± 3.5 kg/m2), while subgroup 5 had the highest BMI (30.6 ± 3.6 kg/m2). The sex distribution differed significantly (P = .01) between the 5 subgroups; however, in all subgroups, the majority of patients were male. Baseline demographic characteristics are shown in Table 1. A comparison of outcome variables is provided in Table 2 and Table 3. Forty-two patients (2.1%) had an AHI of 0–15 events/h (subgroup 1), 765 patients (39.0%) an AHI of 15–30 events/h (subgroup 2), 821 patients (41.8%) an AHI of ≥ 30–50 events/h (subgroup 3), 258 patients (13.1%) an AHI of ≥ 50–65 events/h (subgroup 4), and 77 patients (3.9%) an AHI > 65 events/h (subgroup 5). There was a statistically significant reduction in AHI between baseline and the final visit in subgroups 2–5. The AHI at the final visit and change in AHI significantly differed (both P < .000) between the subgroups, with the lowest AHI at the final visit in subgroup 1 and the greatest reduction in AHI in subgroup 5 (Figure 1).
Table 1.
Demographic information.
| Demographic Variables | All Patients (n = 1,963) | Subgroup 1 | Subgroup 2 | Subgroup 3 | Subgroup 4 | Subgroup 5 | P∼ |
|---|---|---|---|---|---|---|---|
| AHI < 15 events/h (n = 42) | 15 ≥ AHI < 30 events/h (n = 765) | 30 ≥ AHI ≤ 50 events/h (n = 821) | 50 > AHI ≤ 65 events/h (n = 258) | AHI > 65 events/h (n = 77) | |||
| Age (years) | n = 1,957; 60.2 ± 10.7 | n = 42; 59.6 ± 10.8 | n = 763; 60.3 ± 10.7 | n = 817; 60.2 ± 10.7 | n = 258; 60.1 ± 11.2 | n = 77; 59.0 ± 10.8 | .89* |
| Body mass index (kg/m2) | n = 1,918; 29.2 ± 3.8 | n = 41; 28.4 ± 3.5 | n = 748; 28.9 ± 3.9 | n = 796; 29.1 ± 3.6 | n = 257; 29.8 ± 4.2 | n = 76; 30.6 ± 3.6 | < .0001* |
| Sex, male | 1,423 (72.8%) | 27 (64.3%) | 527 (69.2%) | 613 (75.0%) | 194 (75.5%) | 62 (81.6%) | .01** |
| Race∞ | |||||||
| White | 1,860 (94.0%) | 39 (90.7%) | 722 (93.4%) | 785 (95.0%) | 244 (94.6%) | 70 (88.6%) | .47** |
| Black | 36 (1.8%) | 1 (2.3%) | 15 (1.9%) | 11 (1.3%) | 6 (2.3%) | 3 (3.8%) | |
| Asian | 15 (0.76%) | 1 (2.3%) | 6 (0.8%) | 6 (0.7%) | 2 (0.8%) | 0 (0.0%) | |
| American Indian or Alaska Native | 5 (0.25%) | 0 (0.0%) | 3 (0.39%) | 2 (0.24%) | 0 (0%) | 0 (0%) | |
| Other | 40 (2.0%) | 1 (2.3%) | 17 (2.2%) | 14 (1.7%) | 4 (1.6%) | 4 (5.1%) | |
| Unknown | 23 (1.2%) | 1 (2.3%) | 10 (1.3%) | 8 (0.97%) | 2 (0.78%) | 2 (2.5%) |
Data are presented as mean ± SD, median (minimum, maximum) or count (%). *ANOVA (analysis of variance); **chi-square test. ∼P values compare 5 subgroups: ∞Chi-square compared White vs non-White patients; some patients selected multiple races, which is why the cumulative number is higher than the total amount of patients. AHI = apnea-hypopnea index, SD = standard deviation.
Table 2.
Outcome variables.
| Outcome Variables | All Patients (n = 1,963) | |||
|---|---|---|---|---|
| Baseline | 6 Months (Post-titration) | 12 Months (Final) | P† | |
| AHI (events/h) | n = 1,963; 33.0 (0.60, 118.7) | n = 1,852; 7.8 (0.0, 103.0) | n = 890; 10.2 (0.0, 96.2) | <.0001* |
| Change in AHI (events/h) | — | n = 1,852; 23.0 ± 18.3 | n = 890; 20.7 ± 18.4 | See above |
| ODI (events/h) | n = 461; 22.9 (0.0, 242.0) | n = 930; 8.4 (0,126.0) | n = 557; 9.4 (0.0, 198.0) | < .0001* |
| ESS | n = 1,712; 11.0 (0.0, 24.0) | n = 1,528; 7 (0.0, 24.0) | n = 994; 6.0 (0.0, 23.0) | < .0001* |
| Therapy usage (h/night) | — | n = 1,573; 6.4 ± 2.1 | n = 913; 5.7 ± 2.2 | < .0001** |
Data are presented as mean ± SD or median (minimum, maximum) †P values compare baseline and final visit using a paired t test, *p < 0.05, **Compared with post-titration. AHI = apnea-hypopnea index, ESS = Epworth Sleepiness Scale, ODI = oxygen desaturation index, SD = standard deviation.
Table 3.
Outcome variables.
| Outcome Variables | Subgroup 1: AHI < 15 events/h (n = 42) | Subgroup 2: 15 ≥ AHI < 30 events/h (n = 765) | Subgroup 3: 30 ≥ AHI ≤ 50 events/h (n = 821) | Subgroup 4: 50 < AHI ≤ 65 events/h (n = 258) | Subgroup 5: AHI > 65 events/h (n = 77) | P‡ | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 Months (Post-titration) | 12 Months (Final) | P† | Baseline | 6 Months (Post-titration) | 12 Months (Final) | P† | Baseline | 6 Months (Post-titration) | 12 Months (Final) | P† | Baseline | 6 Months (Post-titration) | 12 Months (Final) | P† | Baseline | 6 Months (Post-titration) | 12 Months (Final) | P† | ||
| AHI | n = 42; 11.2 (0.6, 14.9) | n = 39; 6.1 (0.0, 44.2) | n = 19; 7.8 (0.0, 34.8) | .17* | n = 765; 22.3 (15.0, 29.9) | n = 723; 6.0 (0.0, 72.0) | n = 347; 8.5 (0.0, 67.6) | < .0001* | n = 821; 38.2 (30, 50.0) | n = 773; 8.7 (0.0, 80.0) | n = 390; 12.6 (0.0, 96.2) | < .0001* | n = 258; 56.3 (50.1, 65.0) | n = 242; 10.4 (0.0, 85.0) | n = 101; 14.4 (0.0, 92.0) | < .0001* | n = 77; 72.4 (65.5, 118.7) | n = 75; 17.9 (0.0, 103) | n = 33; 15.5 (0.0, 70.0) | < .0001* | < .0001** |
| Change in AHI | — | n = 39; 0.28 ± 10.19 | n = 19; 2.57 ± 7.9 | See above | — | n = 723; 13.0 ± 11.0 | n = 347; 11.6 ± 11.7 | See above | — | n = 773; 25.5 ± 15.0 | n = 390; 22.1 ± 16.0 | See above | — | n = 242; 40.4 ± 18.4 | n = 101; 38.4 ± 17.4 | See above | — | n = 75; 50.3 ± 24.8 | n = 33; 56.0 ± 21.1 | See above | < .0001*** |
| ODI | n = 12; 4.75 (0.0, 29.2) | n = 21; 6.15 (0.0, 126.0) | n = 11; 8.45 (0.5, 74.5) | .63* | n = 192; 16.8 (0.2,92) | n = 370; 6.4 (0,66.7) | n = 217; 7.8 (0.0, 198) | .01* | n = 205; 28.2 (1.0, 94.0) | n = 410; 9.0 (0.0, 96.0) | n = 251; 10.7 (0.0, 61.5) | < .0001* | n = 35; 45.9 (15.2, 242.0) | n = 98; 14.6 (0.2, 87.0) | n = 63; 13.2 (0.0, 89.0) | NA | n = 17; 53.9 (4.0, 90.4) | n = 31; 18.7 (0.0, 62.4) | n = 15; 21.5 (0.0, 70.0) | NA | < .0001** |
| ESS | n = 37; 11.0 (0.0, 20.0) | n = 26; 5.5 (0.0, 16.0) | n = 24; 4.0 (0.0, 14.0) | < .0001* | n = 664; 11.0 (0.0, 24.0) | n = 598; 7.0 (0.0, 24.0) | n = 371; 6.0 (0.0, 21.0) | < .0001* | n = 726; 11.0 (0.0, 24.0) | n = 647; 7.0 (0.0, 23.0) | n = 442; 6.0 (0.0, 23.0) | < .0001* | n = 215; 12.0 (0, 24.0) | n = 200; 8.0 (0.0, 24.0) | n = 116; 7.0 (0.0, 22.0) | < .0001* | n = 70; 11.0 (0.0, 22.0) | n = 57; 9.0 (0.0, 21.0) | n = 41; 6.0 (0.0, 20.0) | < .0001* | .21** |
| Therapy usage (h/night) | — | n = 30; 6.55 ± 1.61 | n = 20; 5.67 ± 2.21 | .45§ | — | n = 609; 6.5 ± 2.0 | n = 343; 6.0 ± 2.1 | < .0001§ | — | n = 670; 6.5 ± 2.0 | n = 398; 5.7 ± 2.2 | < .0001§ | — | n = 206; 6.1 ± 2.3 | n = 115; 5.4 ± 2.4 | < .0001§ | — | n = 58; 5.7 ± 2.1 | n = 37; 4.7 ± 2.6 | .054§ | .005*** |
Data are presented as mean ± SD or median (minimum, maximum). *Paired t test, **Kruskal-Wallis, ***ANOVA (analysis of variance). †P values compare baseline and final visit; ‡P values compare groups at final visit; §P values compare post-titration to final visit. AHI = apnea-hypopnea index, ESS = Epworth Sleepiness Scale, NA = not enough data to run statistical test, ODI = oxygen desaturation index, SD = standard deviation.
Figure 1. Therapy outcomes (AHI).
AHI = apnea-hypopnea index.
Excessive daytime sleepiness (ESS) significantly improved in all subgroups from baseline to the final visit. There was no significant difference (P = .21) in ESS score between the 5 subgroups at final follow-up.
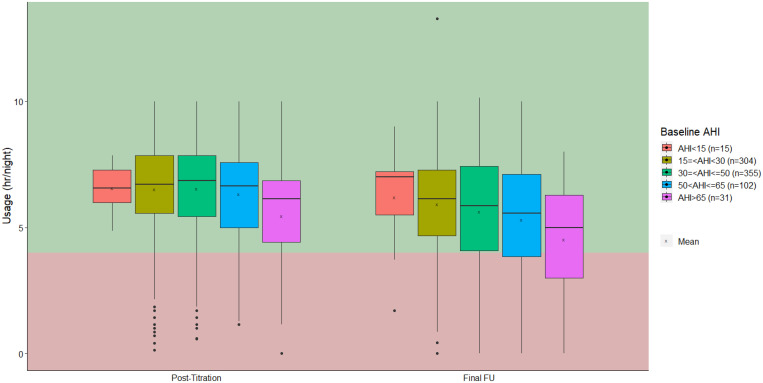
Overall, therapy usage decreased between 6 and 12 months post-implant; this was not significant in subgroups 1 and 5. Subgroup 5 had the lowest therapy usage of 4.7 hours/night at the final visit, which exceeds the commonly used criteria for continuous positive airway pressure (CPAP) tolerance of 4 hours per night.10 Adherence differed significantly (P = .005) between all subgroups, with the highest adherence in subgroup 2 (Figure 2). The MDA was 24.6% for subgroup 1, 53.0% for subgroup 2, 54.5% for subgroup 3, 57.4% for subgroup 4, and 52.7% for subgroup 5.
Figure 2. Therapy usage.
AHI = apnea-hypopnea index, FU = follow-up.
Treatment success was calculated according to Sher’s criteria: at least a 50% decrease in AHI and treatment AHI ≤ 20 events/h.9 Overall treatment success for all patients was 66.6%. Treatment success as determined by Sher’s criteria ranged from 42.1% (subgroup 1) to 68.3% (subgroup 2), but did not differ significantly between the subgroups (Table 4).
Table 4.
Therapy response.
| Therapy Response | All Patients | Subgroup 1 | Subgroup 2 | Subgroup 3 | Subgroup 4 | Subgroup 5 | P |
|---|---|---|---|---|---|---|---|
| AHI < 15 events/h | 15 ≥ AHI < 30 events/h | 30 ≥ AHI ≤ 50 events/h | 50 > AHI ≤ 65 events/h | AHI > 65 events/h | |||
| Sher et al9 response | n = 593 (66.6%) | n = 8 (42.1%) | n = 237 (68.3%) | n = 260 (66.7%) | n = 66 (65.4%) | n = 22 (66.7%) | .23* |
| AHI < 21 events/h | n = 678 (76.2%) | n = 18 (94.7%) | n = 295 (85.0%) | n = 277 (71.0%) | n = 66 (65.4%) | n = 22 (66.7%) | < .0001** |
| 50% reduction AHI | n = 618 (69.4%) | n = 8 (42.1%) | n = 237 (68.3%) | n = 267 (68.5%) | n = 80 (79.2%) | n = 26 (78.8%) | .01* |
P value (chi-square test) compares the response rates between the 5 subgroups: *chi-square test, **Fisher’s exact test. AHI = apnea-hypopnea index
For subgroup 5, patient improvement (CGI-I) was rated slightly less positive, although this outcome was not significantly different compared with the other subgroups (P = .58) (Table 5). Patient satisfaction was impressive in all subgroups, without significant differences between the subgroups. At least 90% of all participants rated UAS better than CPAP (Table 6).
Table 5.
CGI-I scale.
| CGI-I | All Patients (n = 917) | Subgroup 1 | Subgroup 2 | Subgroup 3 | Subgroup 4 | Subgroup 5 | P* |
|---|---|---|---|---|---|---|---|
| AHI < 15 events/h (n = 21) | 15 ≥ AHI < 30 events/h (n = 349) | 30 ≥ AHI ≤ 50 events/h (n = 399) | 50 < AHI ≤ 65 events/h (n = 109) | AHI > 65 events/h (n = 39) | |||
| Very much improved | 41.4% | 33.3% | 45.0% | 40.1% | 43.1% | 23.1% | .58 |
| Much improved | 36.1% | 47.6% | 35.0% | 37.3% | 33.0% | 35.9% | |
| Minimally improved | 13.1% | 9.5% | 12.0% | 12.8% | 13.8% | 25.6% | |
| No change | 6.2% | 4.8% | 5.7% | 5.5% | 8.3% | 12.8% | |
| Minimally worse | 2.0% | 4.8% | 1.4% | 2.8% | 0.9% | 0% | |
| Much worse | 1.1% | 0.0% | 0.86% | 1.3% | 0.9% | 2.6% | |
| Very much worse | 0.11% | 0.0% | 0.0% | 0.25% | 0.0% | 0.0% |
*P value (chi-square test) compares subgroups at final visit; improved vs not improved (including no change). AHI = apnea-hypopnea index, CBI-I = Clinical Global Impression–Improvement.
Table 6.
Patient satisfaction with therapy.
| Patient Satisfaction | All Patients | Subgroup 1 | Subgroup 2 | Subgroup 3 | Subgroup 4 | Subgroup 5 | P* |
|---|---|---|---|---|---|---|---|
| AHI < 15 events/h | 15 ≥ AHI < 30 events/h | 30 ≥ AHI ≤ 50 events/h | 50 > AHI ≤ 65 events/h | AHI > 65 events/h | |||
| Subject rated Inspire as better than CPAP | 90.5% | 89.5% | 92.5% | 90.2% | 84.3% | 92.3% | .16 |
| Subject would choose Inspire again | 91.5% | 89.5% | 92.7% | 90.9% | 90.9% | 88.5% | .41 |
| Subject would recommend Inspire to friends or family | 92.8% | 89.5% | 94.9% | 91.7% | 92.1% | 88.5% | .47 |
| Subject is satisfied with Inspire | 89.8% | 89.5% | 91.2% | 89.3% | 90.0% | 80.8% | .44 |
*P value (Fisher’s exact) compares subgroups at final visit. AHI = apnea-hypopnea index.
DISCUSSION
Not only is this the largest cohort study of patients with UAS therapy to date it is also the first to focus on the efficacy of treatment on both objective and self-reported values, as well as adherence of therapy stratified by baseline AHI severity. After 12 months, there was a significant improvement in objective sleep parameters in all subgroups with a baseline AHI of 15 events/h and above. The AHI at the final visit was the lowest in the subgroup with the lowest preoperative AHI. The subgroup with the highest preoperative AHI had the greatest reduction in AHI at final visit. There was significant improvement regarding symptoms of excessive daytime sleepiness (ESS) and patient improvement as measured by the CGI-I at 12 months after implantation, without differences between the 5 subgroups. Overall treatment success was 66.6%, without significant differences between the subgroups.
Despite the increasing baseline AHI, objective sleep parameters were significantly improved in subgroups 2–5 at the final visit. This is particularly interesting when considering that it is generally easier to reach surgical success in patients with a low AHI.10 Whether preoperative OSA severity is a predictor for success is not reserved for UAS therapy alone, it remains a question of debate in all types of upper airway surgery for OSA. For example, in a meta-analysis performed to assess the effects of isolated uvulopalatopharyngoplasty and tonsillectomy in patients with OSA, the authors concluded that it may be hypothesized that clinical anatomy is of higher relevance than disease severity.11 In another meta-analysis, the surgical success rate of maxillomandibular advancement was greater with a lower mean preoperative AHI.12 For most surgical treatment modalities it remains unclear. A possible explanation for the found equality between the severity subgroups is that, due to the strict inclusion criteria, only perfectly suitable patients are included. Patients with a higher baseline AHI often have a higher BMI (> 35 kg/m2) and a complete concentric collapse of the soft palate. Both are negative predictors for surgical success and in case of UAS exclusion criteria. In addition, recent studies found that UAS not only resolves obstruction at the tongue-base level but also at the epiglottic level as well as isolated palatal obstruction (palatal coupling and tethering). Due to its multilevel effect, UAS is, in particular, suitable for patients with multilevel collapse, which is more often associated with a higher baseline AHI.13 Compared with other surgical treatment options, UAS is dynamic. In general, surgical treatment success depends on wound healing and fibrotic tissue. In the case of UAS, treatment is adjustable (titration) to the patient’s need, synchronized with the respiration, and surgery does not cause fibrotic tissue in the upper airway. A recent study suggests that patients with a BMI up to 35 kg/m2 have a positive treatment response with UAS therapy14; these recent findings, together with the results of the present analysis, suggest that the current indications for Inspire could be broadened.
One remarkable finding was that treatment adherence was significantly lower in patients with an AHI > 65 events/h. In general, adults with severe disease tend to be more adherent to treatment due to a combination of relatively more self-reported improvement and the understanding of long-term consequences of untreated severe disease.15 In CPAP use, severe disease is a predictor for adherence.16,17 We hypothesize that this is related to therapy response and airway collapsibility. We experienced that patients with a higher baseline AHI require higher stimulation levels, potentially leading to treatment discomfort. The functional higher amplitudes are likely due to greater collapsibility of the airway. A recent study by Op de Beeck et al18 illustrated that higher arousal threshold leads to an increased tolerability for stimulation and vice versa. Taking this into account, patients with an AHI higher than 65 events/h in combination with high-enough arousal threshold should certainly be good candidates for UAS in the future. Another possible explanation is a slightly higher residual AHI adversely affecting compliance with therapy. Nevertheless, even in the highest disease-severity subgroup, treatment use was high (4.7 hours/night) and exceeds the commonly used arbitrary criteria for CPAP adherence of 4 hours per night. The effectiveness of conservative treatment regarding the reduction in AHI depends both on the impact on the airway obstruction and compliance.19 Bearing this in mind, we calculated the MDA, representing therapeutic effectiveness, which showed no differences between the subgroups 2–5; the calculated MDA was similar for subgroup 2 and 5 (53.0% and 52.7%, respectively). Subgroup 1 had a lower MDA (24%) due to the small change in AHI.
We did not have actual data on usage of the device as a percentage of total sleep time and assumed patients sleep 7 hours per night.
The positive effect of UAS in patients with an AHI above 50 events/h has important clinical consequences. For example, the findings from this study suggest that patients with an AHI greater than 50 events/h are suitable candidates for UAS therapy and the chance of improvement in objective and self-reported parameters remains parallel to patients with less severe OSA. The findings are in line with the indications for UAS according to CE mark and FDA approval. This is indeed an avenue that should be pursued for the patients with AHI > 50 events/h. This is of particular importance since these patients need alternative treatment when CPAP is not tolerated, due to the health and functional risks associated with untreated severe OSA.
In subgroup 1 the AHI did not decrease significantly but self-reported outcomes significantly improved and did not differ significantly from the other subgroups. However, it remains debatable if patients with an AHI below 15 events/h, and in general less disease burden, should be treated in this manner since many other less-invasive treatment options are available.
There are limitations to be noted. The ADHERE Registry does not record patients who refused participation. The distribution among the severity subgroups was not equal. Additionally, the majority of participants were White males, which does not represent the general population, influencing external validation. However, it must be noted that, due to the strict privacy laws in Europe, documentation of race is not performed as standard of care, it may only be documented when specifically agreed on by the patient. Since the ADHERE Registry only collects standard-of-care date, information on race in Europe remains missing, thereby further influencing report of race on the registry. The chosen stratification is based on OSA severity, reimbursement, and Food and Drug Administration approval. While the original STAR trial was a randomized controlled trial with a treatment withdrawal arm after 12 months, the present study is a cohort study. As described previously, both home and in‐laboratory studies were used in the analysis, with potential lack of uniformity of AHI recording. Home sleep studies may underestimate AHI—this may have affected both the pre‐ and post‐implantation studies. Therefore, we performed additional analysis; the type of sleep test (HST vs PSG) done at the final visit was significantly different between the 5 subgroups (P = .01). However, when controlling for the type of sleep test, change in AHI remains highest in subgroup 5 and final AHI remains lowest in subgroup 1 and there is still no significant difference in response rates between the subgroups. Therefore, while the type of sleep test at final visit indeed differs between the 5 AHI groups, it is not the reason why the subgroups’ results differ. When interpreting the self-reported results on satisfaction with therapy regarding CPAP vs UAS, one must consider that patients are candidates for UAS if they had CPAP intolerance or failure. Therefore, the chance that they prefer UAS more than CPAP is likely to be higher. There are multiple reasons why a patient may not tolerate CPAP and, if not addressed, may affect the adherence to UAS treatment. The greatest strength of this study is that the ADHERE Registry has a large sample size and is an ongoing international effort.
CONCLUSIONS
UAS is a safe and effective treatment for moderate to severe OSA, independent of the degree of severity (AHI). This analysis demonstrates that there are no significant differences between the subgroups based on preoperative disease severity regarding treatment success, excessive daytime sleepiness symptoms (ESS), or self-reported improvement (CGI-I). Mean therapy usage in each subgroup is at least 4.7 hours per night. Patient satisfaction remains high in all subgroups. These results support the broader indication for UAS therapy in patients with an AHI above 50 events/h and even above 65 events per hour of sleep. These are the patients with the highest burden of disease, in whom no other effective treatment options are available in case of CPAP failure.
ACKNOWLEDGEMENTS
The data that support the finding of this study are available upon reasonable request.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CGI-I
Clinical Global Impression–Improvement
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- HST
home sleep test
- MDA
mean disease alleviation
- OSA
obstructive sleep apnea
- PSG
polysomnography
- UAS
upper airway stimulation
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. The ADHERE Registry is sponsored by Inspire Medical Systems, Inc (Minneapolis, MN). The ADHERE Registry is a real-world registry that collects data on all individuals who were implanted with the Inspire UAS System and have consented to having their data collected; this includes patients who were implanted outside of labeling (including patients with an apnea-hypopnea index > 65 events/h, etc). Prof. Dr. O. Vanderveken reports research grants from Philips and Somnomed at Antwerp University Hospital, research support from Inspire Medical Systems and Nyxoah at Antwerp University Hospital, and a Senior Clinical Fellowship Grant (Fundamenteel Klinisch Mandaat) from Research Foundation–Flanders–Vlaanderen (FWO). Prof. Dr. S. Manchanda is consultant on the Physician Advisory Council for Inspire Medical Systems. Prof. Dr. N. de Vries is a member of the Medical Advisory Board of NightBalance and a consultant for Philips Healthcare, Inspire Medical Systems, and Nyxoah. The other authors report no conflicts of interest.
REFERENCES
- 1. Strollo PJ Jr , Soose RJ , Maurer JT , et al. ; STAR Trial Group . Upper-airway stimulation for obstructive sleep apnea . N Engl J Med. 2014. ; 370 ( 2 ): 139 – 149 . [DOI] [PubMed] [Google Scholar]
- 2. Thaler E , Schwab R , Maurer J , et al . Results of the ADHERE upper airway stimulation registry and predictors of therapy efficacy . Laryngoscope. 2020. ; 130 ( 5 ): 1333 – 1338 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Heiser C , Steffen A , Boon M , et al. ; ADHERE Registry Investigators . Post-approval upper airway stimulation predictors of treatment effectiveness in the ADHERE registry . Eur Respir J. 2019. ; 53 ( 1 ): 1801405 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boon M , Huntley C , Steffen A , et al. ; ADHERE Registry Investigators . Upper airway stimulation for obstructive sleep apnea: results from the ADHERE registry . Otolaryngol Head Neck Surg. 2018. ; 159 ( 2 ): 379 – 385 . [DOI] [PubMed] [Google Scholar]
- 5. Withrow K , Evans S , Harwick J , Kezirian E , Strollo P . Upper airway stimulation response in older adults with moderate to severe obstructive sleep apnea . Otolaryngol Head Neck Surg. 2019. ; 161 ( 4 ): 714 – 719 . [DOI] [PubMed] [Google Scholar]
- 6. von Elm E , Altman DG , Egger M , Pocock SJ , Gøtzsche PC , Vandenbroucke JP ; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies . Ann Intern Med. 2007. ; 147 ( 8 ): 573 – 577 . [DOI] [PubMed] [Google Scholar]
- 7. Johns MW . A new method for measuring daytime sleepiness: the Epworth sleepiness scale . Sleep. 1991. ; 14 ( 6 ): 540 – 545 . [DOI] [PubMed] [Google Scholar]
- 8. Busner J , Targum SD . The clinical global impressions scale: applying a research tool in clinical practice . Psychiatry (Edgmont). 2007. ; 4 ( 7 ): 28 – 37 . [PMC free article] [PubMed] [Google Scholar]
- 9. Sher AE , Schechtman KB , Piccirillo JF . The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome . Sleep. 1996. ; 19 ( 2 ): 156 – 177 . [DOI] [PubMed] [Google Scholar]
- 10. Ravesloot MJL , de Vries N . Reliable calculation of the efficacy of non-surgical and surgical treatment of obstructive sleep apnea revisited . Sleep. 2011. ; 34 ( 1 ): 105 – 110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stuck BA , Ravesloot MJL , Eschenhagen T , de Vet HCW , Sommer JU . Uvulopalatopharyngoplasty with or without tonsillectomy in the treatment of adult obstructive sleep apnea—a systematic review . Sleep Med. 2018. ; 50 : 152 – 165 . [DOI] [PubMed] [Google Scholar]
- 12. Holty J-EC , Guilleminault C . Maxillomandibular advancement for the treatment of obstructive sleep apnea: a systematic review and meta-analysis . Sleep Med Rev. 2010. ; 14 ( 5 ): 287 – 297 . [DOI] [PubMed] [Google Scholar]
- 13. Safiruddin F , Vanderveken OM , de Vries N , et al . Effect of upper-airway stimulation for obstructive sleep apnoea on airway dimensions . Eur Respir J. 2015. ; 45 ( 1 ): 129 – 138 . [DOI] [PubMed] [Google Scholar]
- 14. Suurna MV , Steffen A , Boon M , et al . Impact of body mass index and discomfort on upper airway stimulation: ADHERE Registry 2020 update . Laryngoscope. 2021. ; 131 ( 11 ): 2616 – 2624 . [DOI] [PubMed] [Google Scholar]
- 15. Blinder H , Momoli F , Bokhaut J , et al . Predictors of adherence to positive airway pressure therapy in children: a systematic review and meta-analysis . Sleep Med. 2020. ; 69 : 19 – 33 . [DOI] [PubMed] [Google Scholar]
- 16. Patil SP , Ayappa IA , Caples SM , Kimoff RJ , Patel SR , Harrod CG . Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment . J Clin Sleep Med. 2019. ; 15 ( 2 ): 301 – 334 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mehrtash M , Bakker JP , Ayas N . Predictors of continuous positive airway pressure adherence in patients with obstructive sleep apnea . Lung. 2019. ; 197 ( 2 ): 115 – 121 . [DOI] [PubMed] [Google Scholar]
- 18. Op de Beeck S , Wellman A , Dieltjens M , et al. ; STAR Trial Investigators . Endotypic mechanisms of successful hypoglossal nerve stimulation for obstructive sleep apnea . Am J Respir Crit Care Med. 2021. ; 203 ( 6 ): 746 – 755 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ravesloot MJ , de Vries N , Stuck BA . Treatment adherence should be taken into account when reporting treatment outcomes in obstructive sleep apnea . Laryngoscope. 2014. ; 124 ( 1 ): 344 – 345 . [DOI] [PubMed] [Google Scholar]