Abstract
Study Objectives:
Sleep bruxism is characterized by rhythmic masticatory muscle activity (RMMA). This study aimed to determine the number and type of jaw muscles needed for a valid RMMA scoring in individuals with obstructive sleep apnea.
Methods:
Ten individuals with obstructive sleep apnea (4 males; age, 50.1 ± 8.1 years) were included in this study. RMMA was scored using 1 or more of the following jaw muscles’ electromyography (EMG) traces of polysomnography recordings: bilateral masseter and temporalis (4MT; the reference standard), unilateral masseter (1M), bilateral masseter (2M), unilateral temporalis (1T), bilateral temporalis (2T), unilateral chin EMG (1C), and bilateral chin EMG (2C).
Results:
1M, 2M, 1T, and 2T showed excellent agreement with 4MT (intraclass correlation coefficient = 0.751, 0.976, 0.815, and 0.950, respectively), while 1C and 2C presented fair agreement (intraclass correlation coefficient = 0.662 and 0.657). In addition, 2M and 2T displayed good sensitivity (87.8% and 72.0%) and positive predictive value (83.1% and 76.0%). In contrast, 1M and 1T had good sensitivity (88.4% and 87.8%) but fair positive predictive value (60.1% and 53.2%). 1C and 2C showed poor sensitivity (41.1% and 40.3%) and fair positive predictive value (62.9% and 60.6%).
Conclusions:
Polysomnography with bilateral masseter or temporalis muscle EMG traces is regarded valid in RMMA scoring in individuals with obstructive sleep apnea. In contrast, unilateral masseter or temporalis muscle EMG showed only fair accuracy, and chin EMG had poor accuracy. Consequently, these montages cannot be recommended for RMMA scoring in the presence of obstructive sleep apnea.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Name: The Effects of Oral Appliance Therapy on Masseter Muscle Activity in Obstructive Sleep Apnea; URL: https://clinicaltrials.gov/ct2/show/NCT02011425; Identifier: NCT02011425.
Citation:
Li D, Aarab G, Lobbezoo F, Arcache P, Lavigne GJ, Huynh N. Accuracy of sleep bruxism scoring based on electromyography traces of different jaw muscles in individuals with obstructive sleep apnea. J Clin Sleep Med. 2022;18(6):1609–1615.
Keywords: polysomnography, electromyography, masseter, temporalis, chin, sleep bruxism, obstructive sleep apnea, scoring accuracy
BRIEF SUMMARY
Current Knowledge/Study Rationale: Electromyography of the masseter and temporalis muscles as part of polysomnographic recordings provides key evidence of sleep bruxism. However, sleep bruxism scoring accuracy based on different jaw muscles has not yet been validated in individuals with obstructive sleep apnea.
Study Impact: Sleep physicians and polysomnographic technologists are advised to include bilateral masseter and/or temporalis muscles electromyography in their polysomnographic montage for assessment of sleep bruxism in individuals with obstructive sleep apnea.
INTRODUCTION
Sleep bruxism (SB) is a masticatory muscle activity during sleep that includes teeth grinding and clenching.1 Individuals with SB may experience conditions like severe tooth wear, orofacial pain, temporomandibular disorders, and/or fractures or failures of dental restorations or implants, while their bed partners may be disturbed by teeth grinding sounds during the night.2–5 Interestingly, recent studies suggested that SB may also play a positive, protective role in individuals with certain medical conditions,1 eg, obstructive sleep apnea (OSA; by preventing the collapse or restoring the patency of the upper airway)6–8 and gastroesophageal disorder (by increasing saliva secretion to reduce chemical tooth wear).9 A systematic review on the epidemiology of SB showed that the prevalence of frequent SB in the general population is close to 13%.10 However, in the OSA population, the SB prevalence rises to around 50%,11–13 suggesting that SB is a common comorbidity of OSA that needs the clinician’s full attention, although the exact nature of the association between SB and OSA is still inconclusive.6,11,14,15 In addition, recent studies reported that OSA therapies, such as continuous positive airway pressure and mandibular advancement appliance, can reduce the frequency of SB, while in some cases, they could induce or aggravate SB.16–19 This suggests demand for routine screening and monitoring of SB in individuals with OSA.
Currently, the gold standard of SB diagnosis is full-night polysomnography (PSG) with audio-video recordings (type I PSG), which allows the scoring of sleep, respiration, and muscle activity. SB is characterized by rhythmic masticatory muscle activity (RMMA).20 It is noteworthy that RMMA is also commonly observed in normal individuals and in individuals with OSA.11,21 According to previously published scoring criteria, RMMA is scored on the bilateral masseter and temporalis electromyography (EMG) traces when at least 3 out of 4 channels show positive EMG patterns.20,22,23 However, type I PSG is expensive and time-consuming. Given this, portable devices, such as type II PSG, type III polygraphy, or type IV EMG, have been introduced into research and utilized clinically for the detection of SB.24,25 It is noteworthy that these portable devices, which are equipped with a limited number of electrodes or a single channel (eg, type IV), do not usually allow the recording of both the bilateral masseter and temporalis muscles. A recent in-hospital type I PSG study23 reported that the RMMA index (events/h) scored on bilateral masseter muscle EMG traces was higher than that scored on 4 EMG traces (ie, bilateral masseter and temporalis muscle), suggesting that some RMMA episodes may only be visible on the masseter muscle EMG traces and not on the temporalis muscle EMG traces. To some extent, this discrepancy is supported by several other EMG studies demonstrating that during different oral tasks, the masseter and temporalis muscles presented EMG heterogeneity, including signal frequency and peak amplitudes.26–31 In addition, chin EMG is routinely collected in sleep studies to reflect motor activity and muscle tone, supplying useful information for sleep staging (ie, the identification of rapid eye movement [REM] sleep), arousal scoring, and detection of some sleep-related movement disorders, eg, REM behavior disorders.32,33 According to the American Academy of Sleep Medicine scoring manual, the characteristic changes in the masseter muscle EMG are often more prominent than changes in the chin EMG.33 All this evidence suggests that the number and type of jaw muscles used for RMMA scoring may significantly impact upon the diagnosis of SB.
No specific and systematic study has reported the possible discrepancy in RMMA scoring accuracy between different jaw muscle EMG traces (masseter, temporalis, and chin) with otherwise identical PSG montages. Also, the difference in the accuracy between unilateral and bilateral jaw muscle EMGs is unclear yet. Therefore, this study aimed to determine the number and type of jaw muscles needed for valid RMMA scoring by investigating the accuracy of different scoring montages in individuals with OSA. We hypothesized that PSG with bilateral masseter or bilateral temporalis muscle EMG traces would show good accuracy in RMMA scoring in individuals with OSA. In contrast, chin EMG, the unilateral masseter muscle EMG, or the unilateral temporalis muscle EMG would show a low accuracy in RMMA scoring in individuals with OSA.
METHODS
Participants
This is a secondary analysis of a randomized clinical trial that investigated the effects of a mandibular advancement appliance on sleep-related jaw muscle activity in participants with OSA (registered at www.clinicaltrials.gov; NCT02011425).34 The participants’ recruitment criteria have been reported in detail by Aarab et al.34 Participants aged between 35 and 65 years with moderate to severe OSA without other comorbid respiratory or sleep disorders (except SB), severe orofacial pain, severe temporomandibular disorders, untreated periodontal problems, and medication usage that could influence the respiration or sleep were included in this study.34 The PSG recordings of this study were collected at the Faculté de Médicine Dentaire, Université de Montréal, Montréal, Québec, Canada. The scientific and ethical aspects were approved by the Medical Ethics Committee of the Université de Montréal (13-105-CERES-D).
Polysomnography
PSG recordings were obtained using type II Embla Titanium hardware and analyzed by RemLogic software (Embla, Oakville Ontario, Canada). The application of PSG electrodes was performed by a trained sleep technician following the American Academy of Sleep Medicine scoring manual.35 The following channels were recorded: electroencephalogram (F3M2, F4M1, C3M2, C4M1, O1M2, O2M1), electrooculogram (right and left), electrocardiogram, EMG (bilateral chin, masseter, temporalis, and anterior tibialis muscles), airflow, abdominal and thoracic respiratory effort, oxygen saturation, and body position.
In order to avoid the possible influence of mandibular advancement appliance on muscle activity, only the baseline PSG recordings without mandibular advancement appliance in situ were used in the present study. Moreover, PSG recordings with missing data on any of the masseter, temporalis, or chin EMG traces were excluded.
Scoring criteria
Standard sleep stages (N1, N2, N3, REM, and wake) were scored manually by a single experienced and registered polysomnographic technologist from an independent company (Sleep Strategies, Ottawa, Canada), following the criteria of the American Academy of Sleep Medicine.35
In this study, RMMA was scored manually by the first author (D.L.) according to previously published criteria.20 The intrarater agreement was excellent (0.925) for RMMA scoring. Each EMG burst had a mean amplitude at least 2 times higher than the baseline EMG amplitude. A period of at least 3 seconds of baseline EMG activity had to occur between different RMMA episodes. RMMA episodes were classified as phasic (3 or more phasic EMG bursts lasting 0.25–2 seconds), tonic (1 or more tonic EMG bursts ≥ 2 seconds), and mixed (at least 1 phasic and 1 tonic burst present within a single episode). Only RMMA episodes that occurred during sleep were scored in this study.
RMMA episodes were scored in 7 rounds, using PSG scoring montages with different jaw muscle EMG trace(s): 1) unilateral masseter muscle EMG (1M), 2) bilateral masseter muscle EMG (2M), 3) unilateral temporalis muscle EMG (1T), 4) bilateral temporalis muscle EMG (2T), 5) unilateral chin EMG (1C), 6) bilateral chin EMG (2C), and 7) bilateral masseter and temporalis muscle EMG (4MT). For scoring montages with a unilateral muscle EMG trace (ie, 1M, 1T, and 1C), the left or right-side EMG trace was selected randomly for each patient. If an RMMA pattern was present on the selected side of the EMG trace, it would be scored as a positive episode. For scoring based on bilateral muscle EMG traces (ie, 2M, 2T, and 2C), the RMMA pattern should have been simultaneously and consistently visible on both muscle EMG traces. For scoring based on bilateral masseter and temporalis muscle EMG traces (ie, 4MT), the RMMA pattern should have appeared on at least 3 of the 4 EMG traces.20 During each scoring round, only the essential EMG trace(s) was (were) visible. The electroencephalogram, electrooculogram, electrocardiogram, and body position traces were always visible during RMMA scoring.
Statistical analysis
The number of RMMA episodes was transformed into indices, defined as the number of RMMA episodes per hour of sleep. Individuals with RMMA index ≥ 2 episodes/h were diagnosed with SB. 4MT was regarded as the reference standard for the analysis of the accuracy of the tested scoring montages, ie, 1M, 2M, 1T, 2T, 1C, and 2C.
The discrepancy in RMMA scoring between scoring montages was evaluated by comparing the RMMA indices obtained from different scoring montages. The normality of the RMMA index was tested by the Shapiro-Wilks test. The differences in the RMMA indices between scoring montages were analyzed by the Friedman test. Post-hoc pairwise comparisons were analyzed by the Dunn test, and the significance values were adjusted for multiple comparisons by the Bonferroni correction.
The accuracy of the tested scoring montages includes their agreement on the RMMA index with the reference standard and their validity in RMMA scoring. Bland-Altman plots and intraclass correlation coefficient (ICC) were applied to evaluate the agreement on the RMMA index between the tested scoring montages and 4MT. ICC analysis was performed using a single-measurement, 2-way random, and absolute-agreement model. ICC values larger than 0.75 indicate excellent agreement; values below 0.40 imply poor agreement; ICC values between 0.40 and 0.75 suggest fair to good agreement.36 The validity was assessed using sensitivity and positive predictive value (PPV). Since there was no true negative RMMA episode, the specificity and negative predictive value are not applicable in this study. RMMA episodes scored on the tested scoring montages were regarded as true positive RMMA episodes if they were consistent with those scored on 4MT. Sensitivity was defined as the percentage of true positive RMMA episodes on the tested scoring montage out of the total RMMA episodes scored on 4MT. PPV was defined as the percentage of true positive RMMA episodes scored on the tested scoring montage out of the total RMMA episodes scored on the tested scoring montage.
The level for statistical significance was set at .05. Data analysis was performed using SPSS (version 26; IBM SPSS Statistics, Armonk, NY).
RESULTS
Participants
Eighteen individuals with OSA were included in the original study.34 After removing PSG recordings with missing data (possibly due to loose electrodes) on any one of the masseter, temporalis, or chin EMG traces, 10 PSG recordings were eligible for this secondary analysis study. Thus, 10 participants (50.1 ± 8.1 years old), including 4 males and 6 females, were included. Their median RMMA index was 2.8 episodes/h (interquartile = 1.7). Among the 10 participants, 7 were diagnosed with SB (RMMA ≥ 2 episodes/h); the other 3 cases did show RMMA episodes but did not meet the criteria for SB diagnosis.
RMMA scoring discrepancy between scoring montages
RMMA indices obtained from all tested scoring montages (viz, 1M, 2M, 1T, 2T, 1C, and 2C) were similar to that from 4MT (all P > .05). Also, there was no significant difference in the RMMA index between 2M and 2T (P > .05), as well as between 1M and 1T (P > .05). However, 1C showed a significantly lower RMMA index than 1M (P = .023) and 1T (P = .009). In addition, the RMMA index scored on unilateral jaw muscle EMG trace did not show a significant difference with that scored on bilateral jaw muscle EMG traces (1M vs 2M, 1T vs 2T, and 1C vs 2C; all P > .05). Detailed results of the pairwise comparisons are shown in Table 1.
Table 1.
Pairwise comparisons of RMMA indices.
| Scoring Montages | RMMA Indexa | P Valuesb | ||||||
|---|---|---|---|---|---|---|---|---|
| 4MT | 1M | 2M | 1T | 2T | 1C | 2C | ||
| 4MT | 1.6|2.8|3.3 | |||||||
| 1M | 1.6|3.9|5.8 | .363 | ||||||
| 2M | 1.8|3.2|3.6 | 1.000 | 1.000 | |||||
| 1T | 1.9|3.2|4.9 | .174 | 1.000 | .624 | ||||
| 2T | 1.5|2.5|3.9 | 1.000 | 1.000 | 1.000 | 0.711 | |||
| 1C | 0.6|1.3|2.9 | 1.000 | .023 | 1.000 | .009 | 1.000 | ||
| 2C | 0.5|1.3|2.8 | 1.000 | .009 | 1.000 | .003 | 1.000 | 1.000 | |
aNonnormally distributed data are shown in quartiles (25%|median|75%). bFriedman test and Dunn test; P values have been adjusted for multiple comparisons by the Bonferroni correction; For P values larger than or equal to 1 after the correction, they are displayed as 1.000; significant differences (P < .05) are underlined. EMG = electromyography, PSG = polysomnography, RMMA = rhythmic masticatory muscle activity, 4MT = reference standard, PSG with bilateral masseter and temporalis muscle EMG traces, 1M = PSG with unilateral masseter muscle EMG trace, 2M = PSG with bilateral masseter muscle EMG traces, 1T = PSG with unilateral temporalis muscle EMG trace, 2T = PSG with bilateral temporalis muscle EMG traces, 1C = PSG with unilateral chin EMG trace, 2C = PSG with bilateral chin EMG traces.
RMMA scoring accuracy
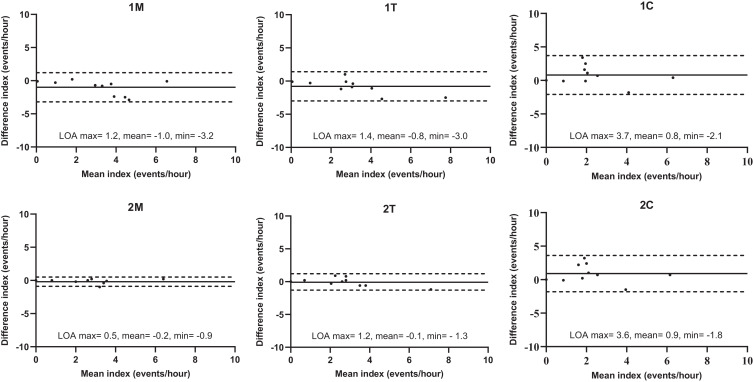
The Bland-Altman plots of the RMMA index for each scoring montage are shown in Figure 1. The bilateral masseter or temporalis muscle EMG showed better agreement with 4MT than the unilateral masseter or temporalis muscle EMG (2M vs 1M, 2T vs 1T). Besides, 2M showed a slightly better agreement with 4MT (the limits of agreement were narrower) than 2T in the RMMA index. On the other hand, the chin EMG showed a substantial disagreement with 4MT in the RMMA index, regardless of whether the scoring was based on 1C or 2C.
Figure 1. Bland-Altman plots of rhythmic masticatory muscle activity indices for tested scoring montages.
Comparisons of tested scoring montages were made against PSG with bilateral masseter and temporalis muscle EMG traces. Solid line = the mean difference, dashed line = 95% LOA (maximum: the upper 95% LOA; minimum: the lower 95% LOA). EMG = electromyography, LOA = limits of agreement, PSG = polysomnography, 1M = PSG with unilateral masseter muscle EMG trace, 2M = PSG with bilateral masseter muscle EMG traces, 1T = PSG with unilateral temporalis muscle EMG trace, 2T = PSG with bilateral temporalis muscle EMG traces, 1C = PSG with unilateral chin EMG trace, 2C = PSG with bilateral chin EMG traces.
The ICCs in the RMMA index for 1M, 2M, 1T, and 2T were 0.751, 0.976, 0.815, and 0.950, respectively (all P < 0.01), indicating excellent agreement with 4MT. In contrast, 1C and 2C showed only fair to good agreement with 4MT, with ICC of 0.662 and 0.657, respectively (both P < .01).
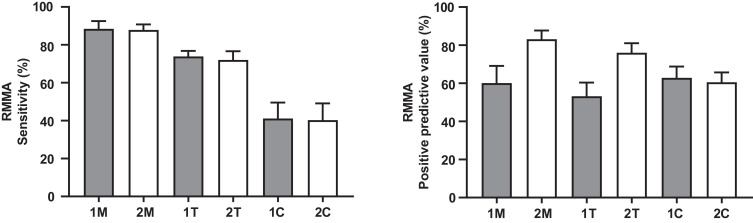
Figure 2 shows the sensitivity and PPV of each scoring montage in identifying RMMA. The masseter muscle EMG (1M and 2M) showed the best sensitivity in identifying RMMA (88.4% and 87.8%, respectively). The temporalis muscle EMG (1T and 2T) showed lower sensitivity values (73.9% and 72.0%), while the chin EMG (1C and 2C) showed the poorest sensitivity (41.1% and 40.3%). On the premise of the same muscle, unilateral jaw muscle EMG and bilateral jaw muscle EMG displayed similar sensitivity in identifying RMMA. In addition, 2M showed the best PPV in identifying RMMA (83.1%), followed by 2T (76.0%), while 1M, 1T, 1C, and 2C displayed only fair PPV (60.1%, 53.2%, 62.9%, and 60.6%, respectively).
Figure 2. Sensitivity and positive predictive values of different polysomnographic scoring montages.
The reference standard for calculating sensitivity and positive predictive value of tested scoring montages was PSG with bilateral masseter and temporalis muscle EMG traces. EMG = electromyography, PSG = polysomnography, RMMA = rhythmic masticatory muscle activity, 1M = PSG with unilateral masseter muscle EMG trace, 2M = PSG with bilateral masseter muscle EMG traces, 1T = PSG with unilateral temporalis muscle EMG trace, 2T = PSG with bilateral temporalis muscle EMG traces, 1C = PSG with unilateral chin EMG trace, 2C = PSG with bilateral chin EMG traces.
DISCUSSION
This study found that RMMA scoring based on either bilateral masseter or temporalis muscle EMG traces is valid and comparable to the reference standard of PSG with bilateral masseter and temporalis muscle EMG traces in individuals with OSA. However, the unilateral masseter or temporalis muscle EMG, in addition to unilateral or bilateral chin EMG used in RMMA scoring, showed only poor to fair accuracy.
Accuracy of different jaw muscle EMG
Both the masseter muscles and the temporalis muscles are masticatory muscles, and as reported by a previous study, they are equally activated in the majority of oral tasks.26 This is supported by our results that both the masseter and temporalis muscle EMG showed excellent agreement on the RMMA index with the reference standard, and that no significant difference in the RMMA index was found between the unilateral (or bilateral) masseter and temporalis muscle EMG in participants with OSA (Table 1).
However, the masseter muscle EMG showed higher sensitivity and PPV in RMMA scoring than the temporalis muscle EMG when scoring montages with the same number of EMG trace(s) (Figure 2) are compared, suggesting that the former has better accuracy than the latter in individuals with OSA. These discrepancies indicate that the masseter and temporalis muscle(s) sometimes showed different EMG patterns in our OSA cohort. This is in line with another study reporting that RMMA scoring based on the bilateral masseter muscles showed more RMMA episodes than scoring based on both the bilateral masseter and the bilateral temporalis muscles.23
The discrepant EMG patterns between the masseter and temporalis muscles can be explained by a heterogeneous activation theory.26–31 Anatomically, the masseter is a quadrilateral muscle with superficial and deep portions, while the temporalis muscle is a fan-shaped muscle with different fibers in different directions. The regional differences in fiber direction are the premise for various oral tasks.29 Conversely, the masseter and temporalis muscles are activated heterogeneously during different jaw movements.26–31,37 Specifically, the masseter muscle is more active than the temporalis muscle during tasks like mouth opening or closing excursions, and keeping the jaw protruded or laterotruded, while the temporalis is more active during tasks like jaw retrusion.26,30,38 As a result, the heterogeneous activation of the masseter and temporalis muscles may cause discrepancies in the amplitude as well as in the time domain (eg, start time, end time, and duration) of EMG bursts between jaw muscles. Consequently, according to the predetermined RMMA scoring criteria, the RMMA episodes scored on the masseter muscle EMG trace(s) may not be consistent with those scored on the temporalis muscle EMG trace(s), and vice versa.
As the chin EMG does not record a masticatory muscle, we hypothesized that chin EMG has poor accuracy in scoring RMMA in individuals with OSA. Based on our results, we accepted this hypothesis. As shown in Table 1, 1C showed a significantly lower RMMA index than 1M (P = 0.023) as well as 1T (P = 0.009), which suggests that scoring based only on chin EMG trace(s) may result in missing an SB diagnosis in individuals with OSA. Furthermore, chin EMG (both 1C and 2C) showed poor sensitivity (around 40%) and fair PPV (around 60%), suggesting that chin EMG has poor ability to identify true positive RMMA episodes, and that most RMMA episodes scored on chin EMG trace(s) are actually false positive ones.
The poor accuracy of chin EMG in RMMA scoring in participants with OSA can be supported by another EMG study, Farella et al,26 in which it was found that during teeth clenching, jaw elevators (ie, the masseter and temporalis muscles) showed very high activity, while the suprahyoid muscles recorded by the chin EMG showed only moderate activity.26 In contrast, the suprahyoid muscles were more active than the jaw elevators during other orofacial activities (eg, deep breathing, reading aloud, yawning, coughing, and drinking), indicating that they are mainly responsible for other mandibular movements.26 Taking all this evidence into consideration, chin EMG seems invalid for RMMA scoring; therefore, its use is not recommended as the EMG source for RMMA scoring in individuals with OSA.33
Unilateral vs bilateral jaw muscle EMG
As mentioned, chin EMG was regarded as having poor accuracy in RMMA scoring. Therefore, we omitted chin EMG from the comparison of RMMA scoring accuracy between unilateral and bilateral jaw muscle EMG. The present study found that bilateral masseter or temporalis muscle EMG (ie, 2M and 2T) displayed good sensitivity and PPV in RMMA scoring. In contrast, unilateral jaw muscle EMG (ie, 1M and 1T) displayed good sensitivity but only fair PPV (60% and 53%), indicating that around half of the RMMA episodes scored on unilateral jaw muscle EMG trace were false positive. Based on this, 2M and 2T are considered as having good accuracies in RMMA scoring, while 1M and 1T have only fair accuracy. Consequently, RMMA scoring based on unilateral EMG trace could potentially overestimate the RMMA index. To some extent, this agrees with another study in which it was reported, based on only EMG, that as fewer EMG channels were applied in the scoring, the more RMMA episodes were scored.23
The discrepancies in the accuracy between scoring montages with unilateral and bilateral muscle EMG traces suggest that RMMA episodes were present occasionally only on unilateral jaw muscle EMG trace instead of on bilateral jaw muscle EMG traces. This might be attributed mainly to the unbalanced EMG activity of the jaw muscles between 2 sides during jaw movement,39,40 resulting in only 1 side surpassing the predetermined amplitude threshold of an EMG burst (2 times higher than the baseline EMG amplitude). In addition, facial asymmetry (eg, in individuals with 1 habitual chewing side) may also contribute to the bilateral discrepancy in RMMA scoring.41–43
Limitations
First of all, this study was conducted in a small sample of participants. Therefore, we did not perform an analysis for RMMA subtypes (ie, phasic, tonic, and mixed). As RMMA subtypes could be regarded as different jaw movements, as demonstrated by their different scoring rules, the scoring accuracy of RMMA subtypes based on different jaw muscle EMG traces could be different. It is worth future studies to investigate the scoring accuracy of RMMA subtypes based on different jaw muscles. Despite this, the sample had a fair number of RMMA episodes to be analyzed, which ensures the reliability of our results. Besides, it is of importance to note that this study was performed in individuals with OSA. Although, as far as we know, no study points out any differences in the EMG pattern of SB between individuals with OSA and those without OSA, the generalization of our results to the general population needs caution. It is therefore recommended to perform similar studies to determine the accuracy of SB scoring based on different jaw muscles in individuals with SB and in the general population. Second, we did not evaluate participants’ maxillofacial morphology. Several studies44,45 reported that individuals with different maxillofacial morphology (eg, mandibular prognathism vs retrognathism, high- vs low-angle vertical facial morphology) present significant differences in the masticatory muscle function and activity. The accuracy of SB/RMMA scoring may be further improved in future studies by taking this important factor into consideration. Third, the absence of audio and video represents a critical shortcoming of this study. As reported by Carra et al,20 the absence of audio-video may lead to an overestimation of the RMMA index. However, as we mentioned before, both Carra et al20 and Miettinen et al23 concluded that PSG systems without audio-video recordings still displayed relatively good accuracy in RMMA scoring, which supports their use for both research and clinical purposes.
CONCLUSIONS
Within the limitations of this study, we concluded that polysomnography with bilateral masseter or temporalis electromyography traces yields good accuracy, and thus can be regarded as valid in the scoring of sleep bruxism in individuals with obstructive sleep apnea. In contrast, analysis using unilateral masseter or temporalis muscle electromyography results in only fair accuracy, and chin electromyography even yields poor accuracy. Consequently, these montages cannot be recommended for sleep bruxism scoring in the presence of obstructive sleep apnea.
ACKNOWLEDGMENTS
Deshui Li is supported by the China Scholarship Council (CSC), China. The grant has no role in the conception, design, and execution of this study.
ABBREVIATIONS
- EMG
electromyography
- ICC
intraclass correlation coefficient
- OSA
obstructive sleep apnea
- PPV
positive predictive value
- PSG
polysomnography
- REM
rapid eye movement
- RMMA
rhythmic masticatory muscle activity
- SB
sleep bruxism
- 1C
polysomnography with unilateral chin electromyography trace
- 1M
polysomnography with unilateral masseter muscle electromyography trace
- 1T
polysomnography with unilateral temporalis muscle electromyography trace
- 2C
polysomnography with bilateral chin electromyography traces
- 2M
polysomnography with bilateral masseter muscle electromyography traces
- 2T
polysomnography with bilateral temporalis muscle electromyography traces
- 4MT
polysomnography with bilateral masseter and temporalis muscle electromyography traces
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Faculté de Médecine Dentaire, Université de Montréal, Montreal, Canada, and Academic Centre for Dentistry Amsterdam (ACTA), Amsterdam, The Netherlands. The authors report no conflicts of interest.
REFERENCES
- 1. Lobbezoo F , Ahlberg J , Raphael KG , et al . International consensus on the assessment of bruxism: report of a work in progress . J Oral Rehabil. 2018. ; 45 ( 11 ): 837 – 844 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lobbezoo F , van der Zaag J , van Selms MKA , Hamburger HL , Naeije M . Principles for the management of bruxism . J Oral Rehabil. 2008. ; 35 ( 7 ): 509 – 523 . [DOI] [PubMed] [Google Scholar]
- 3. Lobbezoo F , Van Der Zaag J , Naeije M . Bruxism: its multiple causes and its effects on dental implants - an updated review . J Oral Rehabil. 2006. ; 33 ( 4 ): 293 – 300 . [DOI] [PubMed] [Google Scholar]
- 4. Thymi M , Shimada A , Lobbezoo F , Svensson P . Clinical jaw-muscle symptoms in a group of probable sleep bruxers . J Dent. 2019. ; 85 : 81 – 87 . [DOI] [PubMed] [Google Scholar]
- 5. Wetselaar P , Manfredini D , Ahlberg J , et al . Associations between tooth wear and dental sleep disorders: a narrative overview . J Oral Rehabil. 2019. ; 46 ( 8 ): 765 – 775 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manfredini D , Guarda-Nardini L , Marchese-Ragona R , Lobbezoo F . Theories on possible temporal relationships between sleep bruxism and obstructive sleep apnea events. an expert opinion . Sleep Breath. 2015. ; 19 ( 4 ): 1459 – 1465 . [DOI] [PubMed] [Google Scholar]
- 7. Kato T . Sleep bruxism and its relation to obstructive sleep apnea-hypopnea syndrome . Sleep Biol Rhythms. 2004. ; 2 ( 1 ): 1 – 15 . [Google Scholar]
- 8. Lavigne GJ , Kato T , Kolta A , Sessle BJ . Neurobiological mechanisms involved in sleep bruxism . Crit Rev Oral Biol Med. 2003. ; 14 ( 1 ): 30 – 46 . [DOI] [PubMed] [Google Scholar]
- 9. Ohmure H , Oikawa K , Kanematsu K , et al . Influence of experimental esophageal acidification on sleep bruxism: a randomized trial . J Dent Res. 2011. ; 90 ( 5 ): 665 – 671 . [DOI] [PubMed] [Google Scholar]
- 10. Manfredini D , Winocur E , Guarda-Nardini L , Paesani D , Lobbezoo F . Epidemiology of bruxism in adults: a systematic review of the literature . J Orofac Pain. 2013. ; 27 ( 2 ): 99 – 110 . [DOI] [PubMed] [Google Scholar]
- 11. Tan MWY , Yap AU , Chua AP , Wong JCM , Parot MVJ , Tan KBC . Prevalence of sleep bruxism and its association with obstructive sleep apnea in adult patients: a retrospective polysomnographic investigation . J Oral Facial Pain Headache. 2019. ; 33 ( 3 ): 269 – 277 . [DOI] [PubMed] [Google Scholar]
- 12. Martynowicz H , Gac P , Brzecka A , et al . The relationship between sleep bruxism and obstructive sleep apnea based on polysomnographic findings . J Clin Med. 2019. ; 8 ( 10 ): 1653 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hosoya H , Kitaura H , Hashimoto T , et al . Relationship between sleep bruxism and sleep respiratory events in patients with obstructive sleep apnea syndrome . Sleep Breath. 2014. ; 18 ( 4 ): 837 – 844 . [DOI] [PubMed] [Google Scholar]
- 14. da Costa Lopes AJ , Cunha TCA , Monteiro MCM , Serra-Negra JM , Cabral LC , Júnior PCS . Is there an association between sleep bruxism and obstructive sleep apnea syndrome? A systematic review . Sleep Breath. 2020. ; 24 ( 3 ): 913 – 921 . [DOI] [PubMed] [Google Scholar]
- 15. Mayer P , Heinzer R , Lavigne G . Sleep bruxism in respiratory medicine practice . Chest. 2016. ; 149 ( 1 ): 262 – 271 . [DOI] [PubMed] [Google Scholar]
- 16. Lobbezoo F , Li J , Koutris M , et al . Nasal CPAP therapy associated with masticatory muscle myalgia . J Clin Sleep Med. 2020. ; 16 ( 3 ): 455 – 457 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martinot JB , Borel JC , Le-Dong NN , et al . Bruxism relieved under CPAP treatment in a patient with OSA syndrome . Chest. 2020. ; 157 ( 3 ): e59 – e62 . [DOI] [PubMed] [Google Scholar]
- 18. Landry M-L , Rompré PH , Manzini C , Guitard F , de Grandmont P , Lavigne GJ . Reduction of sleep bruxism using a mandibular advancement device: an experimental controlled study . Int J Prosthodont. 2006. ; 19 ( 6 ): 549 – 556 . [PubMed] [Google Scholar]
- 19. Landry-Schönbeck A , de Grandmont P , Rompré PH , Lavigne GJ . Effect of an adjustable mandibular advancement appliance on sleep bruxism: a crossover sleep laboratory study . Int J Prosthodont. 2009. ; 22 ( 3 ): 251 – 259 . [PubMed] [Google Scholar]
- 20. Carra MC , Huynh N , Lavigne GJ . Diagnostic accuracy of sleep bruxism scoring in absence of audio-video recording: a pilot study . Sleep Breath. 2015. ; 19 ( 1 ): 183 – 190 . [DOI] [PubMed] [Google Scholar]
- 21. Lavigne GJ , Rompré PH , Poirier G , Huard H , Kato T , Montplaisir JY . Rhythmic masticatory muscle activity during sleep in humans . J Dent Res. 2001. ; 80 ( 2 ): 443 – 448 . [DOI] [PubMed] [Google Scholar]
- 22. Lavigne GJ , Rompré PH , Montplaisir JY . Sleep bruxism: validity of clinical research diagnostic criteria in a controlled polysomnographic study . J Dent Res. 1996. ; 75 ( 1 ): 546 – 552 . [DOI] [PubMed] [Google Scholar]
- 23. Miettinen T , Myllymaa K , Muraja-Murro A , et al . Polysomnographic scoring of sleep bruxism events is accurate even in the absence of video recording but unreliable with EMG-only setups . Sleep Breath. 2020. ; 24 ( 3 ): 893 – 904 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manfredini D , Ahlberg J , Castroflorio T , Poggio CE , Guarda-Nardini L , Lobbezoo F . Diagnostic accuracy of portable instrumental devices to measure sleep bruxism: a systematic literature review of polysomnographic studies . J Oral Rehabil. 2014. ; 41 ( 11 ): 836 – 842 . [DOI] [PubMed] [Google Scholar]
- 25. Casett E , Réus JC , Stuginski-Barbosa J , et al . Validity of different tools to assess sleep bruxism: a meta-analysis . J Oral Rehabil. 2017. ; 44 ( 9 ): 722 – 734 . [DOI] [PubMed] [Google Scholar]
- 26. Farella M , Palla S , Erni S , Michelotti A , Gallo LM . Masticatory muscle activity during deliberately performed oral tasks . Physiol Meas. 2008. ; 29 ( 12 ): 1397 – 1410 . [DOI] [PubMed] [Google Scholar]
- 27. Escanoela Zanato L , Maria Chiari B , Manno Vieira M , Bommarito S . Study of the electrical activity of muscles: Masseter, temporal and supra-hyoid during swallowing . Dent Oral Craniofac Res. 2016. ; 3 ( 1 ): 1 – 4 . [Google Scholar]
- 28. Blanksma NG , Van Eijden TMGJ , Weijs WA . Electromyographic heterogeneity in the human masseter muscle . J Dent Res. 1992. ; 71 ( 1 ): 47 – 52 . [DOI] [PubMed] [Google Scholar]
- 29. Blanksma NG , van Eijden TMGJ . Electromyographic heterogeneity in the human temporalis and masseter muscles during static biting, open/close excursions, and chewing . J Dent Res. 1995. ; 74 ( 6 ): 1318 – 1327 . [DOI] [PubMed] [Google Scholar]
- 30. Blanksma NG , van Eijden TMGJ , van Ruijven LJ , Weijs WA . Electromyographic heterogeneity in the human temporalis and masseter muscles during dynamic tasks guided by visual feedback . J Dent Res. 1997. ; 76 ( 1 ): 542 – 551 . [DOI] [PubMed] [Google Scholar]
- 31. Farella M , Van Eijden T , Baccini M , Michelotti A . Task-related electromyographic spectral changes in the human masseter and temporalis muscles . Eur J Oral Sci. 2002. ; 110 ( 1 ): 8 – 12 . 10.1034/j.1600-0722.2002.00128.x [DOI] [PubMed] [Google Scholar]
- 32. St Louis EK , Boeve BF . REM sleep behavior disorder: diagnosis, clinical implications, and future directions . Mayo Clin Proc. 2017. ; 92 ( 11 ): 1723 – 1736 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berry RB, Quan SF, Abreu AR, et al; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.6. Darien, IL: American Academy of Sleep Medicine; 2020.
- 34. Aarab G , Arcache P , Lavigne GJ , Lobbezoo F , Huynh N . The effects of mandibular advancement appliance therapy on jaw-closing muscle activity during sleep in patients with obstructive sleep apnea: a 3-6 months follow-up . J Clin Sleep Med. 2020. ; 16 ( 9 ): 1545 – 1553 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Berry RB , Budhiraja R , Gottlieb DJ , et al . Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events: deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine . J Clin Sleep Med. 2012. ; 8 ( 5 ): 597 – 619 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hills M , Fleiss JL . The Design and Analysis of Clinical Experiments. New York: : Wiley; ; 1987. . [Google Scholar]
- 37. Jaberzadeh S , Miles TS , Nordstrom MA . Organisation of common inputs to motoneuron pools of human masticatory muscles . Clin Neurophysiol. 2006. ; 117 ( 9 ): 1931 – 1940 . [DOI] [PubMed] [Google Scholar]
- 38. Lobbezoo F , van der Glas HW , van Kampen FM , Bosman F . The effect of an occlusal stabilization splint and the mode of visual feedback on the activity balance between jaw-elevator muscles during isometric contraction . J Dent Res. 1993. ; 72 ( 5 ): 876 – 882 . [DOI] [PubMed] [Google Scholar]
- 39. Kimoto K , Fushima K , Tamaki K , Toyoda M , Sato S , Uchimura N . Asymmetry of masticatory muscle activity during the closing phase of mastication . Cranio. 2000. ; 18 ( 4 ): 257 – 263 . [DOI] [PubMed] [Google Scholar]
- 40. Nishigawa K , Nakano M , Bando E . Study of jaw movement and masticatory muscle activity during unilateral chewing with and without balancing side molar contacts . J Oral Rehabil. 1997. ; 24 ( 9 ): 691 – 696 . [DOI] [PubMed] [Google Scholar]
- 41. Hemingway MA , Biedermann HJ , Inglis J . Electromyographic recordings of paraspinal muscles: variations related to subcutaneous tissue thickness . Biofeedback Self Regul. 1995. ; 20 ( 1 ): 39 – 49 . [DOI] [PubMed] [Google Scholar]
- 42. Kuiken TA , Lowery MM , Stoykov NS . The effect of subcutaneous fat on myoelectric signal amplitude and cross-talk . Prosthet Orthot Int. 2003. ; 27 ( 1 ): 48 – 54 . [DOI] [PubMed] [Google Scholar]
- 43. van der Glas HW , Lobbezoo F , van der Bilt A , Bosman F . Influence of the thickness of soft tissues overlying human masseter and temporalis muscles on the electromyographic maximal voluntary contraction level . Eur J Oral Sci. 1996. ; 104 : 87 – 95 . [DOI] [PubMed] [Google Scholar]
- 44. Serrao G , Sforza C , Dellavia C , Antinori M , Ferrario VF . Relation between vertical facial morphology and jaw muscle activity in healthy young men . Prog Orthod. 2003. ; 4 ( 1 ): 45 – 51 . [DOI] [PubMed] [Google Scholar]
- 45. Takeuchi-Sato T , Arima T , Mew M , Svensson P . Relationships between craniofacial morphology and masticatory muscle activity during isometric contraction at different interocclusal distances . Arch Oral Biol. 2019. ; 98 : 52 – 60 . [DOI] [PubMed] [Google Scholar]