Abstract
Study Objectives:
Chronic pain is associated with insomnia. The objective of this clinical study was to compare the efficacy and safety of different prescribed doses of zopiclone and clonidine for the management of insomnia in patients with chronic pain.
Methods:
This prospective observational crossover study included 160 consenting adult patients receiving pain management treatment. For insomnia treatment, each patient ingested different prescribed doses of zopiclone or clonidine on alternate nights. Each patient used a special validated sleep diary to collect data including pain score, sleep scores, sleep duration, sleep medication dose, and adverse effects. Each patient completed the diary for 3 continuous weeks. Pain was measured using a numeric pain rating scale. Sleep score was measured using the Likert Sleep Scale. A change in the pain or sleep scores by 2 points was considered significant. Of the 160 study participants, 150 (93.8%) completed the study successfully, and their data were analyzed with IBM SPSS Statistics 25 (IBM Corporation, Armonk, NY) using Student’s t test, analysis of variance, Pearson chi-square test, and regression analysis. A P value < .05 was considered significant.
Results:
Pain score was lower with clonidine than zopiclone (P = .025). Time to fall asleep was shorter with clonidine than zopiclone (P = .001). Feeling rested on waking in the morning was better with clonidine than zopiclone (P = .015). Overall sleep quality was better with clonidine than zopiclone (P = .015). Total Likert sleep score was better with clonidine than zopiclone (P = .005). Total sleep duration was better with clonidine than zopiclone (P = .013). Adverse effects were commoner with zopiclone, including collapse, fall, confusion, amnesia, mood disorder, hallucination, nightmare, nocturnal restlessness, locomotor dysfunction, nausea and headache. A minor adverse effect of dry mouth was commoner with clonidine.
Conclusions:
Clonidine is significantly better than zopiclone with respect to sleep quality, analgesia, tolerability profile, and patient safety. Further studies comparing clonidine with other insomnia medications will be beneficial.
Citation:
Bamgbade OA, Tai-Osagbemi J, Bamgbade DO, et al. Clonidine is better than zopiclone for insomnia treatment in chronic pain patients. J Clin Sleep Med. 2022;18(6):1565–1571.
Keywords: insomnia, sleep, clonidine, zopiclone, chronic pain, sedative hypnotic, sleep score
BRIEF SUMMARY
Current Knowledge/Study Rationale: Good sleep management will improve quality of life outcomes for patients with insomnia and/or pain. Sleep medications may cause adverse effects, but clonidine may offer better qualities for sleep management. The current study is the first research to report that clonidine provides better sleep and analgesia compared to zopiclone.
Study Impact: Clonidine is efficacious and safe for insomnia treatment and will have significant positive impacts in the clinical management of patients with insomnia and/or pain.
INTRODUCTION
Chronic pain has negative impacts on sleep and is a major cause of insomnia.1,2 Insomnia is part of the vicious cycle of pain propagation and central pain sensitization syndrome.2,3 Good sleep hygiene is essential for chronic pain management and improvement.4–6 Insomnia should be treated with nonpharmacologic modalities such as diet, psychotherapy, hypnotherapy, and cognitive behavioral therapy.4,5,7 However, a significant proportion of chronic pain patients may require pharmacologic therapy for insomnia.5,6,8 Nonprescription sleep medicines include melatonin, antihistamines, chamomile, valerian, theanine, rhodiola, scutellaria, and passionflower.1,5,8 Prescription sleep medicines include zolpidem, zopiclone, lemborexant, lorazepam, nitrazepam, trazodone, temazepam, triazolam, flurazepam, and quetiapine.8–11
Zopiclone is a popular nonbenzodiazepine drug and is categorized as a cyclopyrrolone.9–11 Although zopiclone is molecularly different from benzodiazepines, it has a similar mode of action to benzodiazepines by increasing the transmission of the inhibitory neurotransmitter gamma-aminobutyric acid in the central nervous system. Zopiclone use is controversial and associated with unpredictable effects. Adverse effects include nausea, residual drowsiness, prolonged tiredness, dry mouth, headache, amnesia, confusion, depression, hallucination, sleepwalking, nightmares, incoordination, collapse, paradoxical excitation, drug dependence, withdrawal, and polysubstance use.9–14
Clonidine is an α2-adrenoreceptor agonist drug that is used to treat hypertension, bruxism, attention-deficit/hyperactivity disorder, drug or substance withdrawal, menopausal hot flush, spasticity, and anxiety.15–18 Clonidine provides effective therapy for restless legs, nightmare, and other sleep disorders.19–21 It produces predictable adverse effects of dry mouth, dizziness, and postural hypotension. Clonidine provides reliable anxiolysis, analgesia, and sleep but is not commonly employed for these useful qualities.15,16,20,21 Compared to zopiclone, clonidine may afford better sleep, better analgesia, and fewer adverse effects and these outcomes may be particularly beneficial in chronic pain patients.
There is no previous clinical study that compares the sleep or other beneficial effects of clonidine and zopiclone in chronic pain patients. The objective of this prospective observational clinical study was to compare zopiclone and clonidine for insomnia treatment in chronic pain patients. The study aimed to compare the 2 drugs in terms of sleep quality, onset, duration, efficacy, and reliability. The study also aimed to compare both drugs with respect to tolerability, safety, adverse effects, and impact on chronic pain management outcomes.
METHODS
We conducted a prospective observational crossover study of consecutive adult patients who underwent pain management at a Canadian pain clinic during May 2020 to April 2021. This was a quality assurance study of routine clinical practice and patient preference. The study was approved by the local research committee and the pain clinic. Informed consent was obtained from every patient. All the patients were initially taking prescribed zopiclone 7.5 mg tablet at every bedtime or night as insomnia therapy. All the patients were on multimodal analgesia therapy comprising interventional injections, acetaminophen, nonsteroidal anti-inflammatory drug (NSAID), magnesium, topical nonopioid agents, and low-dose codeine or tramadol.
The study inclusion criteria were adult patients with chronic pain, good treatment compliance, severe chronic insomnia, failure of nonpharmacologic sleep therapy, regular zopiclone therapy for 3 months or more, regular sleep diary, regular pain diary, informed consent for diary review, and consent for clinical record quality assurance review. Chronic pain was defined as frequent and/or significant pain for more than 3 months.1–6 Chronic insomnia was defined as significantly inadequate sleep on 3 or more nights per week for more than 3 months.1–6
The exclusion criteria were obstructive sleep apnea, body mass index ≥ 40 kg/m2, organ insufficiency, cognitive disorder, inability to provide consent, major neuropsychiatric disorder, unreliable diary, cannabis use, regular alcohol intake, stimulant use, substance abuse, poor treatment compliance, high dose opioid, gabapentinoid use, sedative use, and nonsevere insomnia. Medication-related exclusion criteria were irregular zopiclone intake, regular zopiclone therapy for less than 3 months, and previous adverse/allergic reactions to clonidine or zopiclone.
The estimated sample size was 150 patients. This was based on our average annual caseload of 300 patients with severe chronic insomnia. It was also based on our previous clinical audit of 250 patients with severe chronic insomnia that suggested a 5% incidence of adverse effects of zopiclone. We calculated that, with 150 patients, the 95% confidence intervals on a 5% proportion would be 2.5%–8.5%, ie, better than 50% relative error on observed incidence.
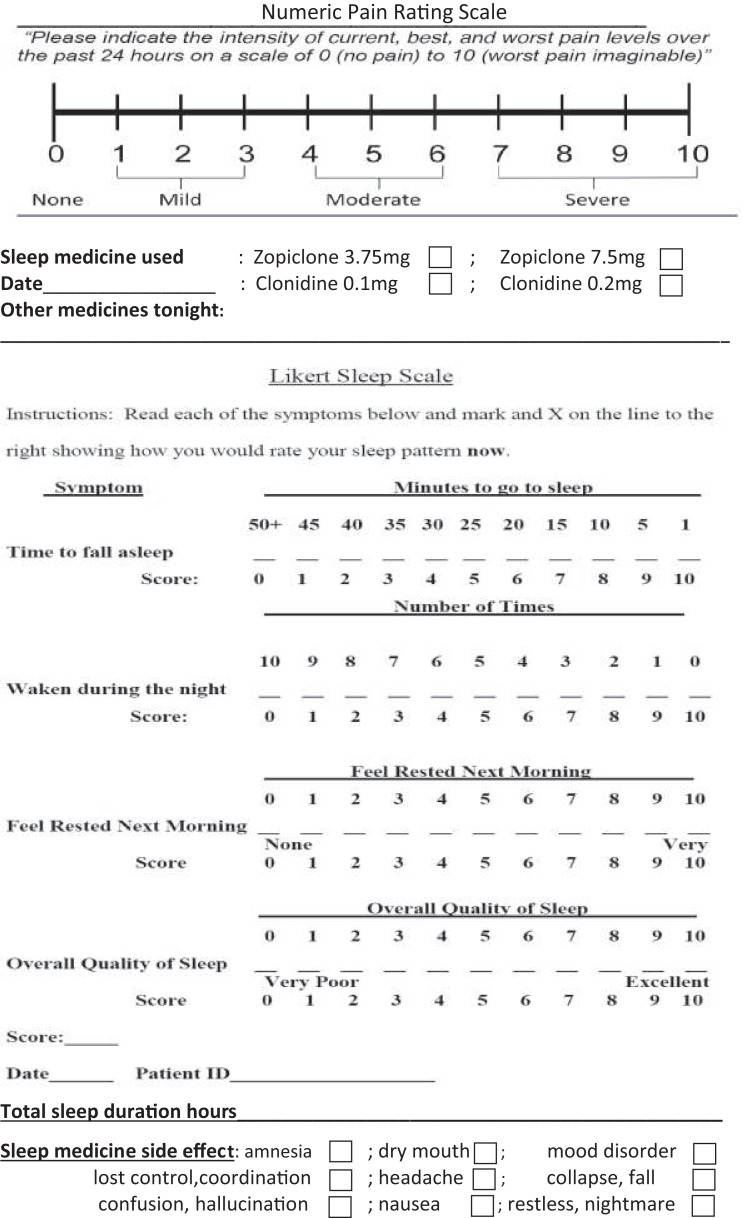
The patients were subsequently prescribed and provided zopiclone 3.75-mg tablet (1–2 tablets per dose) and clonidine 0.1 mg tablet (1–2 tablets per dose). They were advised to take either clonidine or zopiclone on alternate nights. For each medication, they alternated the doses of 1 tablet or 2 tablets at subsequent nightly administrations. The 4 possible medication doses were zopiclone 3.75 mg, zopiclone 7.5 mg, clonidine 0.1 mg, and clonidine 0.2 mg, as shown on the sleep diary in Figure 1. The recommended orders of treatment were zopiclone 7.5 mg, clonidine 0.1 mg, zopiclone 3.75 mg, clonidine 0.2 mg, or clonidine 0.2 mg, zopiclone 3.75 mg, clonidine 0.1 mg, zopiclone 7.5 mg. Each patient participated in the study treatment and completed the sleep diary for 3 continuous weeks. Therefore, each of the 4 medication doses was used 5 times by each patient. This allowed collection of adequate data for averaging and analysis.
Figure 1. Sleep diary.
The special validated sleep diary was used to collect data including pain score, sleep score, sleep duration, sleep medication and dose, sleep medication side-effect, and other nighttime medication. Pain score was measured using a 10-point numeric pain rating scale that categorized pain severity as mild (score 1–3), moderate (score 4–6), or severe (score 7–10). A change in the pain score by 2 points was considered significant. Sleep score was measured with the Likert Sleep Scale, which uses 10-point numeric scales to measure 4 variables including time to fall asleep, sleep disruption during the night, feeling rested on waking up, and overall sleep quality. A change in the sleep scores by 2 points was considered significant. Frequency-of-waking during night sleep was used to measure the reliability of the sleep medication. Feeling rested on waking in the morning was used to measure sleep efficacy and the efficacy of the sleep medication. The validated pain and sleep scales were incorporated into the sleep diary and explained to the patients.
Other data collected included age, sex, body mass index, sleep alone or with spouse, opioid analgesia type and dose, and pain diagnosis. Data was also collected regarding sleep medication complication types and incidence and this was used to measure medication tolerability and safety. All the quantitative and qualitative data are presented as tables. The data include ranges, numbers, categories, and descriptions. The data were compared, analyzed, and interpreted appropriately. Data were analyzed with IBM SPSS Statistics 25 (IBM Corporation, Armonk, NY) using Student’s t test, analysis of variance, Pearson chi-square test, and regression analysis. A P value < .05 was considered significant.
A total of 160 patients consented and participated in the study, and 150 patients (93.8%) completed the study and sleep diary for the recommended period of 3 continuous weeks. About 10 patients dropped out of the study and were not included in the data analysis. The 150 patients who completed the study were all compliant and they were included in the “per protocol” analysis, but “intention-to-treat” analysis was not required because the patients completed the study successfully.
This clinical study was approved by the health authority and health care facility, which confirmed that it was a quality assurance study and did not require formal research ethics board review.
RESULTS
Table 1 shows the general characteristics of the 150 patients who successfully completed the study. The majority of patients (61%) were nonelderly adults. Most patients (66%) were female. A majority of patients (59%) were obese (body mass index > 30 kg/m2). Most patients (61%) slept regularly with a spouse or partner. A majority of patients (68%) were being treated for severe spinal or paraspinal pain syndrome. About 69% of patients regularly used codeine analgesic, and 31% regularly used tramadol analgesic.
Table 1.
Patient characteristics, total number = 150.
| Category | Population Number | % |
|---|---|---|
| Age | ||
| 33–64 years | 91 | 60.7 |
| 65–88 years | 59 | 39.3 |
| Sex | ||
| Male | 51 | 34 |
| Female | 99 | 66 |
| BMI | ||
| 21–29.9 kg/m2 | 62 | 41.3 |
| 30–39.9 kg/m2 | 88 | 58.7 |
| Regular sleep partner | ||
| Yes | 92 | 61.3 |
| No | 58 | 38.7 |
| Chronic pain diagnosis | ||
| Spinal or paraspinal | 102 | 68 |
| Non–spinal limb pain | 48 | 32 |
| Weak opioid type | ||
| Codeine 30 mg TID | 104 | 69.3 |
| Tramadol 37.5 mg TID | 46 | 30.7 |
BMI = body mass index, TID = 3 times daily.
Table 2 shows the mean and standard deviation values for the patients’ pain scores, sleep measurements, and sleep medication efficacy. Table 3 shows the 95% confidence interval values for the patients’ pain scores, sleep measurements, and sleep medication efficacy. Pain score was lower with clonidine than zopiclone use (P = .025). Pain score was similar with either clonidine doses. Pain score was similar with either zopiclone doses. Time to fall asleep was shorter with clonidine than zopiclone intake (P = .001). Time to fall asleep was shorter with clonidine 0.2 mg than with clonidine 0.1 mg or zopiclone use (P = .031). Waking during night sleep was similar with clonidine 0.2 mg, clonidine 0.1 mg, or zopiclone 7.5 mg, but better than with zopiclone 3.75 mg intake (P = .023). Feeling rested on waking in the morning was better with clonidine than zopiclone use (P = .015). Feeling rested on waking in the morning was better with clonidine 0.2 mg than with clonidine 0.1 mg or zopiclone intake (P = .035). Generally, feeling rested on waking in the morning had patterns or measurements similar to overall sleep quality. Overall sleep quality was better with clonidine than zopiclone use (P = .015). Overall sleep quality was better with clonidine 0.2 mg than with clonidine 0.1 mg or zopiclone intake (P = .035). Total Likert sleep score was better with clonidine than zopiclone use (P = .005). Total sleep duration was better with clonidine than zopiclone intake (P = .013).
Table 2.
Sleep measurements, pain scores, and sleep medication efficacy.
| Parameter | Zopiclone 3.75 mg | Zopiclone 7.5 mg | Clonidine 0.1 mg | Clonidine 0.2 mg |
|---|---|---|---|---|
| Pain score | 5 ± 3 (2–8) | 5 ± 3 (2–8) | 3 ± 1 (2–4) | 2 ± 1 (1–3) |
| Time to fall asleep | ||||
| Minutes | 45 ± 9 (36–54) | 40 ± 5 (40–55) | 35 ± 5 (30–40) | 25 ± 5 (20–30) |
| Score out of 10 | 1 ± 1 (0–2) | 1 ± 1 (0–2) | 3 ± 1 (2–4) | 4 ± 1 (3–5) |
| Waking during night sleep | ||||
| Number of times | 2 ± 1 (1–3) | 1 ± 1 (0–2) | 1 ± 0 (0–1) | 1 ± 0 (0–1) |
| Score out of 10 | 7 ± 1 (6–8) | 9 ± 1 (8–10) | 9 ± 1 (8–10) | 9 ± 1 (8–10) |
| Feeling rested in morning, score out of 10 | 4 ± 1 (3–5) | 5 ± 1 (4–6) | 6 ± 1 (5–7) | 8 ± 1 (7–9) |
| Overall sleep quality, score out of 10 | 4 ± 1 (3–5) | 5 ± 1 (4–6) | 6 ± 1 (5–7) | 8 ± 1 (7–9) |
| Total Likert sleep score | 16 ± 4 (12–20) | 20 ± 3 (17–23) | 24 ± 1 (23–25) | 29 ± 1 (28–30) |
| Total sleep duration, hours | 4 ± 1 (3–5) | 5 ± 2 (3–7) | 6 ± 1 (5–7) | 8 ± 1 (7–9) |
Values are given as mean ± standard deviation (range). For total sleep scores and duration, higher scores are better.
Table 3.
Sleep measurements, pain scores, sleep medication efficacy.
| Parameter | Zopiclone 3.75 mg | Zopiclone 7.5 mg | Clonidine 0.1 mg | Clonidine 0.2 mg |
|---|---|---|---|---|
| Pain score | 4.5–5.5 | 4.5–5.5 | 2.8–3.2 | 1.8–2.2 |
| Time to fall asleep | ||||
| Minutes | 43.5–46.5 | 39.2–40.8 | 34.2–35.8 | 24.2–25.8 |
| Score out of 10 | 0.8–1.2 | 0.8–1.2 | 2.8–3.2 | 3.8–4.2 |
| Waking during night sleep | ||||
| Number of times | 1.8–2.2 | 0.8–1.2 | 1 | 1 |
| Score out of 10 | 6.8–7.2 | 8.8–9.2 | 8.8–9.2 | 8.8–9.2 |
| Feeling rested in morning, score out of 10 | 3.8–4.2 | 4.8–5.2 | 5.8–6.2 | 7.8–8.2 |
| Overall sleep quality, score out of 10 | 3.8–4.2 | 4.8–5.2 | 5.8–6.2 | 7.8–8.2 |
| Total Likert sleep score | 15.3–16.7 | 19.5–20.5 | 23.8–24.2 | 28.8–29.2 |
| Total sleep duration, hours | 3.8–4.2 | 4.8–5.2 | 5.8–6.2 | 7.8–8.2 |
Values are given as 95% confidence intervals. For total sleep scores and duration, higher scores are better.
Table 4 shows the pattern of sleep medication complications. Amnesia was commoner with zopiclone than clonidine use (P = .001). Dry mouth was commoner with clonidine 0.2 mg than with clonidine 0.1 mg or zopiclone intake (P = .041). Mood disorder occurred exclusively with zopiclone intake but rarely with clonidine use (P = .001). Motor coordination impairment occurred almost exclusively with zopiclone use and rarely with clonidine intake (P = .001). Headache occurred only with zopiclone use but it did not occur with clonidine use (P = .013). Collapse or fall occurred only after zopiclone intake but not after clonidine intake (P = .011). Confusion or hallucination occurred only with zopiclone use but these did not occur with clonidine use (P = .004). Nausea was commoner with zopiclone than with clonidine intake (P = .025). Nightmare or nocturnal restlessness occurred only with zopiclone intake but these did not occur with clonidine intake (P = .021).
Table 4.
Sleep medication complications.
| Complication Type | Zopiclone 3.75 mg | Zopiclone 7.5 mg | Clonidine 0.1 mg | Clonidine 0.2 mg |
|---|---|---|---|---|
| Amnesia | 60% | 80% | 1% | 5% |
| Dry mouth | 5% | 10% | 10% | 20% |
| Mood disorder | 40% | 40% | 1% | 1% |
| Motor coordination impaired | 40% | 50% | 0 | 1% |
| Headache | 10% | 10% | 0 | 0 |
| Collapse or fall | 5% | 10% | 0 | 0 |
| Confusion or hallucination | 5% | 30% | 0 | 0 |
| Nausea | 10% | 10% | 1% | 1% |
| Restlessness or nightmare | 10% | 20% | 0 | 0 |
Values are given as average rate %.
DISCUSSION
Chronic pain and insomnia are interrelated major comorbidities.1,5 Insomnia constitutes a major physiologic and socioeconomic burden and it increases health care costs and utilization.8–10 Old age is a known major risk factor for chronic pain and insomnia.6,7 However, in the current study the majority of the patients were nonelderly adults. It is important to note that all the patients in this study had chronic pain, insomnia, and regular zopiclone use. This is the first study to highlight a higher prevalence of chronic pain and insomnia in nonelderly adults compared to elderly patients; and this is in contrast to previous studies.6,7 The current study also included a preponderance of obese and female patients. These are unique and interesting findings that will encourage clinicians to proactively provide better care for chronic pain patients with insomnia.
Regular opioid analgesic use is a known major risk factor for significant insomnia.2 This was corroborated by the current study, in which showed all patients used opioid analgesic regularly. Therefore, chronic pain management should involve a more effective multimodal approach that combines psychotherapy, physical therapy, nonopioid pharmacotherapy and opioid analgesic. This multimodal therapy will reduce patients’ use of opioid analgesic and may improve their sleep quality. The current study also revealed that the majority of patients had a regular sleep partner or spouse. Therefore, the achievement of better sleep quality for such patients will also benefit their partner or spouse. Better sleep quality will have exponential positive impacts on the wider population, society, and economy. A higher quality of nocturnal sleep will reduce stress and enhance the activities of daily living.22
The current study revealed that analgesia was significantly better with clonidine intake than with zopiclone intake. This confirms that clonidine reliably provides and augments analgesia.16,18,21 This is a welcome additional benefit for patients with chronic insomnia who also experience chronic pain. This reliable study is the first publication to highlight these interesting and beneficial analgesia qualities of clonidine compared to zopiclone.
This study highlighted that clonidine is significantly better than zopiclone with respect to overall sleep quality, total Likert sleep score, time to fall asleep, feeling rested on waking in the morning, and total sleep duration. Feeling rested on waking in the morning was used to measure sleep efficacy and the efficacy of the sleep medication. The study outcomes show that feeling rested on waking has similar patterns to overall sleep quality. These unique and exciting outcomes will have major positive clinical impacts on insomnia therapy. The study emphasized that clonidine 0.2 mg is significantly better than clonidine 0.1 mg or zopiclone with respect to overall sleep quality, time to fall asleep, and feeling rested on waking in the morning. This higher dose of clonidine is very effective and safe. This dose-dependent effect will be beneficial for most patients with insomnia. The frequency of waking during night sleep was used to measure the reliability of the sleep medication. The study revealed that waking during night sleep was worse or more frequent with zopiclone 3.75 mg compared to clonidine 0.2 mg, clonidine 0.1 mg, or zopiclone 7.5 mg. This indicates that clonidine is superior to zopiclone in reducing nocturnal sleep disruption, especially since zopiclone also causes more adverse effects at higher doses.
The study explored sleep medication adverse effects, and this was used to measure medication tolerability and safety. It showed that neuropsychiatric complications of memory and mood disorders were significantly more common with zopiclone than clonidine. The study recorded central nervous system abnormalities of headache, confusion, hallucination, nightmare and nocturnal restlessness with zopiclone use but none with clonidine use. These findings confirm that zopiclone causes major neurologic, memory, cognitive, subconscious and mood anomalies, which have potentially serious consequences.9,10,12–14 The study showed that the adverse effects of collapse, fall, and impaired motor coordination are significantly common with zopiclone use but not with clonidine use. These findings confirm that zopiclone causes major neuromotor complications, with potentially catastrophic or lethal outcomes.10,12,14
The current study highlights that clonidine is better than zopiclone in terms of clinical tolerability, effects, profile, and outcomes, especially for patients with chronic insomnia and pain. This study is the first to emphasize these interesting and better qualities of clonidine in comparison to zopiclone. The study also reported that nausea occurred exclusively with zopiclone use but dry mouth was more common with clonidine use. However, these orogastric or enteric side effects are minor and easily managed.
The power calculation and crossover methodology of the study are reliable. Each crossover patient served as their own control, which reduced the influence of confounding variables, required fewer patients, and increased statistical efficiency. The study had some limitations, such as the consecutive patient recruitment method and the consequentially longer study recruitment period. The order in which medication treatments were administered may potentially have affected some study outcome measurements and adverse effect estimates. The interval between treatment administration may potentially have confounded the estimates of the treatment effects. However, these potential confounding effects were insignificant in this study because of the short elimination half-life of oral clonidine or zopiclone, which is 5–7 hours.9–18 The elimination half-life is significantly shorter than the regular 24-hour interval between the treatments. These reliable time factors ensure adequate elimination time for each treatment, before a subsequent treatment and outcome measurement.
CONCLUSIONS
Chronic insomnia and pain are common interrelated comorbidities. There is a higher prevalence of chronic pain and insomnia in nonelderly adults compared to elderly patients. Regular opioid analgesic use is a risk factor for insomnia, but multimodal nonopioid analgesia may minimize insomnia. Clonidine reliably provides and augments analgesia. Clonidine is significantly better than zopiclone with respect to analgesia, tolerability profile, and patient safety. Clonidine is significantly better than zopiclone in terms of overall sleep quality, total Likert sleep score, time to fall asleep, feeling rested on waking in the morning, and total sleep duration. Further and larger clinical studies comparing clonidine with other insomnia medications would be beneficial.
ACKNOWLEDGMENTS
Author contributions: All the authors were involved in the conception, design, data collection, data analysis, writing, proof-reading, critical review, and approval of the final article draft. All authors are responsible for the content and similarity index of the article.
DISCLOSURE STATEMENT
All authors have read and approved the final manuscript. Research support was provided by Salem Anaesthesia pain clinic, Surrey, Canada. Institutional support is acknowledged, but this research did not receive any grant from funding agencies in the public, commercial or nonprofit sectors. The authors report no conflicts of interest.
REFERENCES
- 1. Cheatle MD , Foster S , Pinkett A , Lesneski M , Qu D , Dhingra L . Assessing and managing sleep disturbance in patients with chronic pain . Sleep Med Clin. 2016. ; 11 ( 4 ): 531 – 541 . [DOI] [PubMed] [Google Scholar]
- 2. Miller MB , Chan WS , Curtis AF , et al . Pain intensity as a moderator of the association between opioid use and insomnia symptoms among adults with chronic pain . Sleep Med. 2018. ; 52 : 98 – 102 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Afolalu EF , Moore C , Ramlee F , Goodchild CE , Tang NK . Development of the Pain-Related Beliefs and Attitudes about Sleep (PBAS) Scale for the assessment and treatment of insomnia comorbid with chronic pain . J Clin Sleep Med. 2016. ; 12 ( 9 ): 1269 – 1277 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilson KG , Kowal J , Ferguson EJ . Clinically important change in insomnia severity after chronic pain rehabilitation . Clin J Pain. 2016. ; 32 ( 9 ): 784 – 791 . [DOI] [PubMed] [Google Scholar]
- 5. Husak AJ , Bair MJ . Chronic pain and sleep disturbances: a pragmatic review of their relationships, comorbidities, and treatments . Pain Med. 2020. ; 21 ( 6 ): 1142 – 1152 . [DOI] [PubMed] [Google Scholar]
- 6. Curtis AF , Williams JM , McCoy KJM , McCrae CS . Chronic pain, sleep, and cognition in older adults with insomnia: a daily multilevel analysis . J Clin Sleep Med. 2018. ; 14 ( 10 ): 1765 – 1772 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vitiello MV , Rybarczyk B , Von Korff M , Stepanski EJ . Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis . J Clin Sleep Med. 2009. ; 5 ( 4 ): 355 – 362 . [PMC free article] [PubMed] [Google Scholar]
- 8. Thelus Jean R , Hou Y , Masterson J , Kress A , Mysliwiec V . Prescription patterns of sedative hypnotic medications in the military health system . J Clin Sleep Med. 2019. ; 15 ( 6 ): 873 – 879 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tubbs AS , Fernandez FX , Ghani SB , et al . Prescription medications for insomnia are associated with suicidal thoughts and behaviors in two nationally representative samples . J Clin Sleep Med. 2021. ; 17 ( 5 ): 1025 – 1030 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi JW , Lee J , Jung SJ , Shin A , Lee YJ . Use of sedative-hypnotics and mortality: a population-based retrospective cohort study . J Clin Sleep Med. 2018. ; 14 ( 10 ): 1669 – 1677 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yen CF , Yen CN , Ko CH , et al . Correlates of dependence and beliefs about the use of hypnotics among zolpidem and zopiclone users . Subst Use Misuse. 2015. ; 50 ( 3 ): 350 – 357 . [DOI] [PubMed] [Google Scholar]
- 12. Ferentinos P , Paparrigopoulos T . Zopiclone and sleepwalking . Int J Neuropsychopharmacol. 2009. ; 12 ( 1 ): 141 – 142 . [DOI] [PubMed] [Google Scholar]
- 13. Wong CP , Chiu PK , Chu LW . Zopiclone withdrawal: an unusual cause of delirium in the elderly . Age Ageing. 2005. ; 34 ( 5 ): 526 – 527 . [DOI] [PubMed] [Google Scholar]
- 14. Berry SD , Lee Y , Cai S , Dore DD . Nonbenzodiazepine sleep medication use and hip fractures in nursing home residents . JAMA Intern Med. 2013. ; 173 ( 9 ): 754 – 761 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huynh N , Lavigne GJ , Lanfranchi PA , Montplaisir JY , de Champlain J . The effect of 2 sympatholytic medications--propranolol and clonidine--on sleep bruxism: experimental randomized controlled studies . Sleep. 2006. ; 29 ( 3 ): 307 – 316 . [DOI] [PubMed] [Google Scholar]
- 16. Bamgbade OA . Dexmedetomidine for peri-operative sedation and analgesia in alcohol addiction . Anaesthesia. 2006. ; 61 ( 3 ): 299 – 300 . [DOI] [PubMed] [Google Scholar]
- 17. Uhde TW , Stein MB , Vittone BJ , et al . Behavioral and physiologic effects of short-term and long-term administration of clonidine in panic disorder . Arch Gen Psychiatry. 1989. ; 46 ( 2 ): 170 – 177 . [DOI] [PubMed] [Google Scholar]
- 18. Camí J , de Torres S , San L , Solé A , Guerra D , Ugena B . Efficacy of clonidine and of methadone in the rapid detoxification of patients dependent on heroin . Clin Pharmacol Ther. 1985. ; 38 ( 3 ): 336 – 341 . [DOI] [PubMed] [Google Scholar]
- 19. Aurora RN , Kristo DA , Bista SR , et al. American Academy of Sleep Medicine . Update to the AASM Clinical Practice Guideline: “The Treatment of Restless Legs Syndrome and Periodic Limb Movement Disorder in Adults—An Update for 2012: Practice Parameters with an Evidence-Based Systematic Review and Meta-Analyses”. Sleep. 2012. ; 35 ( 8 ): 1039 – 1062 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morgenthaler TI , Auerbach S , Casey KR , et al . Position Paper for the Treatment of Nightmare Disorder in Adults: an American Academy of Sleep Medicine position paper . J Clin Sleep Med. 2018. ; 14 ( 6 ): 1041 – 1055 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bamgbade OA , Alfa JA . Dexmedetomidine anaesthesia for patients with obstructive sleep apnoea undergoing bariatric surgery . Eur J Anaesthesiol. 2009. ; 26 ( 2 ): 176 – 177 . [DOI] [PubMed] [Google Scholar]
- 22. Talbot LS , Neylan TC , Metzler TJ , Cohen BE . The mediating effect of sleep quality on the relationship between PTSD and physical activity . J Clin Sleep Med. 2014. ; 10 ( 7 ): 795 – 801 . [DOI] [PMC free article] [PubMed] [Google Scholar]