Abstract
Academic industry partnership (AIP) represents an important alliance between academic researchers and industry that helps translate technology and complete the innovation cycle within academic health systems. Despite diverging missions and skillsets the culture for academia and industry is changing in response to the current digital era which is spawning greater collaboration between physicians and businesses in this marketplace. In the field of pathology, this is further driven by the fact that traditional funding sources cannot keep pace with the innovation needed in digital pathology and artificial intelligence. This concept article from the Digital Pathology Association (DPA) describes the rules of engagement for pathology innovators in academia and for their corporate partners to help establish best practices in this critical area. Stakeholders include pathologists, basic and translational researchers, university technology transfer and sponsored research offices, as well as industry relations officers. The article discusses the benefits and pitfalls of an AIP, reviews different partnership models, examines the role of pathologists in the innovation cycle, explains various agreements that may need to be signed, covers conflict of interest and intellectual property issues, and offers recommendations for ensuring successful partnerships.
Keywords: Academic-industry partnership, Artificial intelligence, Conflict of interest, Digital pathology, Industry, Innovation, Intellectual property, Patent, Sponsored research, University
1. Introduction
Academic Industry Partnership (AIP) represents an important alliance between academic researchers and industry that helps identify and translate technology into clinical practice.1,2 AIP stimulates innovation, lowers barriers to develop marketable technology, and offers a mechanism to fund translational research.3 Reliance on industry resources has become increasingly more important as federal research funds diminish. A prior study illustrated that in 2012 in the United States approximately 36% ($38 billion) of all research and development (R&D) funding comes from traditional federal funding sources (e.g. National Institutes of Health, National Science Foundation, Department of Defense) and approximately 64% ($67.9 billion) from industry.4 This gap continues to widen, favoring industry over federal funding. At academic medical centers where sustaining clinical revenues are under constant pressure, some departments may search for additional revenue streams through industry collaboration. AIP is being further driven by the fact that traditional grant funding sources are unlikely to keep pace with the innovation needed in digital imaging and artificial intelligence (AI).
A strategic alliance allows academic researchers who discover novel technology solutions to team up with experts in industry to help commercialize their fruitful discoveries for widespread adoption.5,6 AIP thereby allows many patients to benefit from academic research. Apart from financial support, the benefits of publications, and gaining early or low-cost and sometimes even free access to new and sophisticated products, academia can further benefit from an AIP because faculty and trainees get to broaden their experience and employment opportunities.7,8 Engaging with industry allows faculty to also keep ahead in their field, which helps them prioritize research and the relevance of their work for the private sector.8 Today, a proportion of academic faculty is expected to be entrepreneurial.9 Although this may vary by department, of that faculty whose efforts result in a commercial product or service, only a few will eventually form a company or venture partnership. For industry allies, an academic medical center is an excellent source of novel technology and knowledge, access to scientific and medical experts, ethical access to clinical patients and data, fuels publications that help with marketing, and presents an opportunity to receive market feedback that results in making functional products users want.10
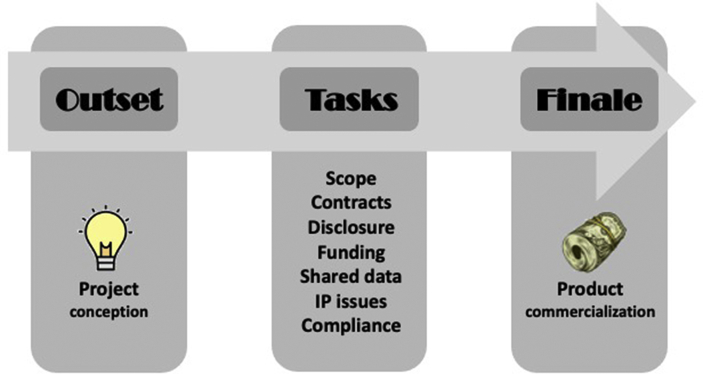
A successful AIP includes a clear definition of the goals and scope of the project, data sharing plan, timeline, execution of contracts, clarification of each party's responsibilities, development of a budget, managing disclosures, compliance with regulations, as well as establishing intellectual property (IP) ownership (Fig. 1).11 Examples of successful AIPs in healthcare include collaborations between academia and pharmaceutical companies (e.g. drug development, clinical trials),12 as well as with biotechnology companies (e.g. medical devices, cancer research, etc.).13 In the current digital era of healthcare including pathology where AI applications are rapidly emerging, we are witnessing a plethora of AIPs to try to meet the demand for innovative products and services for use in clinical practice.14,15 Indeed, such AIPs have the potential to catalyze the use of AI in healthcare, including their role in the field of pathology. These AIPs have taken on many forms including partaking in clinical trials for digital pathology system regulatory approval, alpha/beta prototype testing, co-development of AI algorithms, and sponsored research agreements to validate commercial tools in a clinical laboratory setting. By leveraging digital pathology, pathologists have been enabled to contribute digital slides, including large public datasets, to the AIP process, as well as assist with supervised learning of AI-based image algorithms.
Fig. 1.
Schematic showing the critical path for a successful AIP. The priority assigned to the various tasks will be dictated by partnering stakeholders. Failure to successfully navigate these steps in a timely fashion can delay AIP efforts. (IP = Intellectual property).
Much has been learned from AIPs arising from the digital transformation in radiology,16,17 and from highly successful collaborations in the realm of clinical pathology.18 In the realm of device development, both in radiology and surgery, several successful models have been employed, including the “embedded scientist” model, the graduate/undergraduate student model, externally supported commercial development, industry supported researchers, and the incubator and accelerator models.19 Others have likewise identified pitfalls to be avoided relative to funding, purchasing, integrity of results/publications, and disclosures.20 In all of these, the way academia and business ventures can successfully play to their relative strengths in a win–win manner rests on understanding the motives, priorities and operational realities of both stakeholders. It is thus imperative that pathologists and other academics looking to commercialize their innovation or otherwise collaborate are aware of the pertinent industry ecosystem.
Investigator-initiated trials are conducted when clinical investigators drive researching new uses for a product, compare multiple products, or seek to expand the safety profile and overall knowledge of existing products. It is typically the responsibility of university investigators to manage the ethical and regulatory elements of investigator-initiated trials.21 Funded AIPs were vitally important to enable pathologists from several academic and private pathology practices across the United States to participate with companies seeking Food and Drug Administration (FDA) approval to market their digital pathology systems to aid pathologists in examining digital surgical pathology slides for primary diagnosis.22,23 For data to be considered valid by the FDA, companies initiating such pre-market studies must ensure that their partner sites comply with FDA guidelines including good clinical practice (GCP) and appropriate human subjects protection. Companies may also initiate and fund studies after products receive FDA approval to achieve expanded FDA claims or demonstrate additional use cases.
There are, unfortunately, risks associated with an AIP that may include conflict of interest (COI) and undermining of academic standards (e.g. publication bias).7,24, 25, 26 Misappropriation and malevolence arising from various partnerships have sometimes eroded public trust in research.27 There has also been notable gender disparity in AIPs, where female physicians in some fields have received fewer and lower industry payments.28 Regrettably, some partnerships have drawn unwanted bad press or resulted in litigation. In the current AI era, there are also unique challenges that have emerged due to data ownership and control over assets (e.g. transformation of personal digital data into a private asset).29
Managing a successful AIP can thus be challenging. Currently, there are no widely published “rules of engagement” in pathology that help guide all parties in an AIP. Some effort has been made by federal regulators, professional associations, and continuing medical education programs by offering policies that address disclosures and COI. Hence, the Digital Pathology Association (DPA) embarked on producing this concept article to safely promote AIPs in the era of digital pathology and AI. The aim of this article is to accordingly educate the pathology community, and vendors working in the digital pathology and AI market, for them to better manage partnerships and proactively mitigate potential risks. The article discusses different types of AIP that may exist, various phases of the innovation cycle and how entrepreneurial pathologists and their spin-off companies fit into this sequence, numerous agreements that may need to be signed, potential COI that may arise, and matters relevant to IP ownership. The goal is to encourage AIP and provide guidance for a successful partnership that results in a win for academia, industry, and patients.
2. Contracts to support different partnerships
Common AIP contracts include an industry Sponsored Research Agreement (SRA), Technology Transfer Agreement (TTA), and industry consulting agreement (Table 1). Understanding how these different AIPs can catalyze technology validation and adoption are important to entrepreneurs focused on translating their innovative ideas into reality.
Table 1.
Different types of contractual arrangements to develop an AIP.
| Partnership type | Key elements | Strengths | Weaknesses |
|---|---|---|---|
| Sponsored Research Agreement (SRA) | Specified research | Variable funding sources possible | Ownership of resultant IP may become an issue |
| Specified timeframe | Contractual customization for deliverables | ||
| Widely understood | Incentives may not align completely | ||
| Technology Transfer Agreement (TTA) | Facilitates transfer of existing (and future) IP to potential commercial venture | Specifies what IP is involved, protecting rights of developers | Does not cover funding of commercial venture |
| Categorizes type of transfer (license, sale, etc.) | Reward on IP may be limited by commercial success or failure due to other factors | ||
| Consulting Agreement | Specific individual services retained | Contract specifies nature of services, term and incentives | University may only be a bystander to these agreements |
IP = intellectual property.
2.1. Sponsored research agreement (SRA)
The most common, and potentially impactful, AIP is the SRA. A SRA is a formal contract established between a university and sponsor intended to fund and conduct specific research during a defined timeframe at the university. If a company funds such research without receiving rights in return (e.g. license IP), it is considered a gift. SRAs can be supported by funding received from a non-profit (e.g. federal government, foundation) or for-profit (e.g. private industry) sponsor. A SRA contains terms that govern several key aspects of the collaboration such as scope of work to be performed, deliverables (e.g. publication of research results), budget for the research, payment obligations, options to license IP arising from this research, compliance with regulations, and rights regarding termination of the project.
2.2. Technology Transfer Agreement (TTA)
These agreements can be used to form a relationship between an academic partner who develops an innovation and registers it as IP, and subsequently seeks to transfer such IP to an industry partner. This can happen in many ways, but the most common mechanisms include: 1) licensing of an academic partner's IP to an industry partner (licensor) through a technology transfer office (TTO), 2) the academic inventor (licensee) develops a start-up company, licenses their own IP from their university and subsequently seeks Small Business Innovation Research (SBIR) funding from the federal government, and 3) a company approaches an academic partner via a Small Business Technology Transfer (STTR) agreement to develop technology in partnership. Both SBIR and STTR awards–based programs encourage small domestic businesses to engage in federal R&D which thereby stimulates high-tech innovation. The STTR program, in particular, fosters AIP. STTR grants are suitable for early-stage spin-off companies, whereas an SBIR is more appropriate for self-sufficient companies. Details about SBIR/STTR seed funding eligibility and award amounts are available online (https://www.sbir.gov/about).
2.3. Consulting agreement
For such consulting agreements there is a personal contract established between an academic faculty member (i.e. consultant) and an industry client who wants to retain specific services from the consultant. Such an agreement defines the scope and financial terms of the consulting activity. The consultant's university is typically not a party in these personal agreements. Compensation is usually, but not always, paid directly to the faculty depending on their university and/or healthcare system employment contract.
3. The innovation cycle
Innovation in the context of this concept article refers to the invention of new products and/or services that may result in commercialization. Without a successful business model, there is no innovation, just invention. According to Peter Drucker, the father of modern-day management, innovation can serve as an instrument of entrepreneurship.30 Innovation is a continuous cycle of product discovery, development, and commercialization. AIPs can fuel both the efficiency and effectiveness of this process (Fig. 2). In fact, AIPs commonly arise during the innovation cycle that helps reduce the cost of resources as well as advance development and evaluation of new products. Healthcare commercialization programs (HCPs) exist, such as the National Science Foundation's Innovation Corps (I-Corps™) program that encourages AIPs to enable the transformation of invention to impact.31 However, this program is aimed mainly at individual faculty rather than larger institutional programs. There is a long history of enlightened universities creating centers and institutes with industry partnerships as a primary goal. The intent is to remove barriers for initiating and sustaining industry collaborations. These modern academic incubators typically manage many sponsored projects, provide stewardship, administer research funds, and thereby allow their students and staff to connect with entrepreneurs and interested financiers. Some industry partners pay a subscription to belong to these institutions. The MIT Media Lab is a famous example that is unconstrained by traditional disciplines and promotes interdisciplinary research. Alumni of this lab have become successful inventors, entrepreneurs, owners of spinoff companies, and consultants. The University of Utah has created a similar Scientific Computing and Imaging (SCI) Institute that fosters considerable cross-disciplinary research, much of which is in the form of contract research for industry.
Fig. 2.
The innovation cycle of academic industry partnerships. Principal Investigators (PI) of federally funded grants focused on software and algorithm innovation (e.g. Computational Pathology innovations) develop Intellectual Property (IP) as part of their Research and Development (R&D) plans. Federal funding in academic health care systems comes from National Institute of Health (NIH) or National Science Foundation (NSF) grants. In addition, academic entrepreneurs who seek to validate their technology innovations seek Collaborative Research and Development Agreements (CRADA), industry Sponsored Research Agreements (SRA) as well as Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) grants to develop their innovations into commercially hardened solutions. The Early University innovation ecosystem then helps to de-risk technology, perform market analysis and early commercial adoption/management plans. At this stage, Early Investment “angel” or regional investment entities help to further validate innovations. Sometimes health system ecosystems and local business development apply together for additional rounds of NIH or NSF funding to do clinical trials leading to formal capital venture investment. Finally, commercial entities license or option technology (IP including patents) and create a new company. The funds generated from this financial “success” can lead to reinvestment in R&D and software engineering for the academic entrepreneurs who were the original inventors of the IP.
The life cycle of innovation can be broken down into four phases.32 The first phase (ideation) is when a researcher (principal investigator or PI) performs R&D and invents a novel device, software or an AI-based algorithm that was funded by a grant or other mechanism (e.g. SRA, SBIR, STTR). The second phase (project selection) is when a decision gets made to invest in commercializing this invention. During this critical phase, tactics such as advocacy and market analysis are employed to gauge the likelihood an invention has towards commercial success. In this phase, AIPs can be very useful as industry partners have far better expertise and infrastructure for this process (e.g. product pipelines, customers). Funding during this phase may involve an angel or seed investment from a private high-net-worth individual who offers financial backing to the entrepreneur or small start-up in exchange for ownership equity in their new company.33
The third phase (product development) involves building the product. This requires developing a prototype or pilot within a health system, often with local business developers which translate the derisked technology into an advanced product. This allows for validation of the technology in a clinical setting, including possibly dealing with reimbursement and regulatory issues. This phase is also often deeply reliant on AIPs, where the inventor can rely on existing products that industry partners already have in place (e.g. laboratory information system, whole slide imaging system). Investment at this stage is generally secured from the capital venture community, as well as industry partners critical to the commercialization and integration of the product. The fourth phase (commercialization) requires bringing the product to market and adapting it to meet customer demands. This entails licensing of the product and/or new company (newco) creation that is dependent on a sales pipeline and building a customer base. A new company is likely to establish success only when significant customer sales are created during this fourth phase of the life cycle. The funds generated from this financial “success” during the fourth phase can lead to reinvestment in R&D for academic entrepreneurs and inventors of the product, to test their new ideas. This process can continue as a cycle for additional innovations. Of note, a “Lean Startup” approach is a method sometimes used to launch a new product or start a new company based on an existing company or based upon products that consumers have already demonstrated are desirable.
Companies interested in an AIP should ideally seek academic partnership early in the development cycle to align objectives and ensure adequate resources are invested to guarantee product success. Once an innovation reaches its final phase, AIPs can still be helpful by scaling up the innovation into a commercial success. At the final stage and beyond, AIPs help generate financial revenue through licensing revenues shared by the inventor and their academic institution. After the final product is launched, reinvestment from this financial success will make engaged faculty provide continuous feedback to improve the end product, help drive regulatory approval, promote adoption by acting as an individual consultant or serving on the company's advisory board, and develop related or new products. The digital pathology industry is filled with companies that spun out of academia (e.g. InterScope, Inspirata, Omnyx, Paige.AI, PathPresenter, SpIntellx).34 This is not surprising, because when commercializing a university invention spin off companies are often the best route to ensure the original inventors remain involved.35
4. Academia versus industry priorities
Academia and industry are critically dependent on specialized and innovative talent for success. In this manner they share traditional core missions such as research and education. However, both face different constraints to achieve these goals with divergent agendas and varying prioritization (Table 2). Typically, education and clinical care for universities coupled with a medical center are of highest institutional priority, followed by research and then innovation. In contrast, industry and particularly small start-up companies try to achieve a competitive advantage by prioritizing innovation over research and education.36 Unlike corporate culture which is more guarded because they want to monetize their innovations, academic culture usually encourages researchers to share and publish their novel findings. Despite these different agendas and competing priorities, there are still propelling forces for partnership between academia and industry.37,38 Of interest, one group of researchers in Switzerland found that scientific institutes more oriented toward applied research and/or lower teaching obligations were more strongly inclined to get involved in technology transfer activities.39
Table 2.
Academic and industry priorities. The top three priorities for a university versus an industry or start-up company are tabulated in the order of relevance to the organizational goal. Note that education and innovation are prioritized in reverse order depending on the designation of the organization.
| Prioritization | University partner | Industry partner |
|---|---|---|
| High priority | Education | Innovation |
| Medium priority | Research | Research |
| Low priority | Innovation | Education |
Establishing a successful partnership between academia and industry clearly requires understanding of the factors that influence the success of such a partnership. A recent systematic review of the literature identified numerous critical factors that influence a successful collaboration between industry and universities.3 Flexibility in understanding and accepting cultural differences emerged as the most significant institutional factor for success in such partnerships. Transparency and clarity in communication between institutions were also significant. In addition, awareness of how economic, legal, political and social developments influenced such collaborations was important to assess and monitor. An AIP can be further strengthened by putting into place the correct governance structure (e.g. allowing academics to be decision makers),37 as well as careful management of resources offered by each partner. Some of the critical resources include finances, time, staff, laboratory space, equipment, and access to published literature (e.g. library). Whilst an industry partner can be heavily motivated to deliver resources fast and on command, the academic institute–based resources may be limited in availability and dependent on the timing of the institutional curriculum. Hence, it is evident that these partnerships will benefit from appropriate project management to improve coordination and communication between partners with diverse priorities and approaches to decision making.40, 41, 42
5. Role of the pathologist
Pathologists may be involved at any stage of an AIP (Table 3). This could be at the beginning of an invention, during the commercialization phase, while validating alpha or beta products, as the principal investigator on a clinical trial, serving as an industry consultant, or as a member on a scientific advisory board. In the field of digital pathology and AI, pathologists are frequently summoned by industry associates to serve as subject-matter experts (SME) who can provide scientific and practical guidance on product development regarding their feasibility, design, workflow impact, and clinical utility. Participating in preclinical and clinical trials requires that pathologists and their institutions, address relevant ethical (e.g. animal use committee and institution review board approval), regulatory, financial and logistical issues (e.g. hiring study coordinators).43
Table 3.
The role of pathologists during different phases of an AIP project.
| Project phase | Pathologist role |
|---|---|
| Feasibility |
|
| Verification |
|
| Validation |
|
For issues related to technology transfer (e.g. filing patents) and commercialization of research (e.g. legal counsel), academic pathologists will likely need to work with their university technology transfer office (TTO) or technology licensing office (TLO). These offices act as a conduit between academia and industry, and are intended to protect an institution's IP and license novel products developed by their faculty to industry partners. After the Bayh–Dole Act (or Patent and Trademark Law Amendments Act) was passed in the United States (1980), many university policies created IP policies that obligated faculty to assign their inventions to the university. Finally, while universities may allow faculty to participate as individual consultants and advisory board members in outside activities, they are often still regulated. For example, the pathologist is responsible to ensure that the industry consulting agreement they sign is consistent with their university rules (e.g. employment obligations, requirement to disclose, and assign inventions to the university).
6. Agreements
When industry and universities engage in studies, partnerships, or joint ventures there is a natural order of agreements that are essential to ensure legal protection and ethical conduct for both sides (Table 4). Prior to sharing detailed study information or other IP, both sides should execute a non-disclosure agreement (NDA) or confidential disclosure agreement (CDA). A mutual NDA or CDA is warranted when both parties plan to jointly design a study or exchange proprietary information for product development.44 One-way agreements may occur in some industry sponsored clinical trial cases where only the university is receiving confidential information needed to execute a study. Typically, the university TTO or sponsored program office (SPO) has templates to start NDA or CDA negotiations. Of note, most universities do not allow investigators to sign such agreements on behalf of the university.
Table 4.
Overview of transactional agreements typically used in Academic-Industry Partnerships and collaborations. The table summarizes agreements between companies and universities only, excluding other third parties.
| Name of agreement | Main purpose | Agreement coverage | University office | Process and time investment | Agreement considerations |
|---|---|---|---|---|---|
| Confidential Disclosure Agreement or Non-Disclosure Agreement (CDA or NDA) | Exchange of confidential non-public information between two or more parties to facilitate a common objective | Scope and nature of information for disclosure, definition of confidential information, permitted use of information, and consequences for violations. | TTO | Mostly simple and straight forward, complete in days or weeks. |
|
| Material Transfer Agreement (MTA) | To access or provide unique proprietary materials and resources | Definition of material or resource, permitted use, limitations on use, rights on materials and derivatives, publication of results and data, and consequences for violations. | TTO | Mostly simple and straight forward, complete in days or weeks |
|
| Data Use/Transfer Agreement (DUA) | To govern the transfer and use of data between two or more parties where the data is non-public or is otherwise subject to some restrictions on its use | Scope and nature of data, subject matter of data, ownership, allowed uses for data, publication or patenting of data, clauses about compliance with appropriate regulations, policies and guidelines, term and expiration, data disposal and liability | TTO | Depends on nature of data (proprietary, personal information about human subjects etc.) |
|
| Sponsored Research Agreement (SRA) | To enable collaboration between university researcher/lab and company scientists to advance a common scientific objective | Research plan, parties' contribution and responsibilities, management of IP and data rights, publication, financial terms, ability to change course, dispute resolution, and termination | TTO or SPO | Complex research could make this very involved, complete in weeks or months |
|
| Clinical Trial Agreement (CTA) | Establishes drug or device supply for a clinical trial | Terms related to drug or device supply (e.g. quantity, labelling, shipping), reporting, conditions for termination, data and IP rights, liabilities, indemnification, dispute resolution, monitoring, publication, regulatory application and related terms | SPO | Can be very involved with multiple stakeholders from each party, can take significant amount of time as this can involve high risk |
|
HIPAA = Health Insurance Portability and Accountability Act, IP = intellectual property, SPO = sponsored programs office, TTO = technology transfer office.
The next step required to advance an academic-industry relationship involves a material transfer agreement (MTA) or data use agreement (DUA). MTAs typically pertain to the acquisition of various biological and research materials, including data. While options for open MTAs exist, many universities may insist on using agreements specific to their own institution. MTAs may disallow the redistribution of materials, which many argue impedes multisite research.45 Some universities use research collaboration agreements, which combine elements of the NDA/CDA and MTA/DUA to expedite these important partnership agreements. Research collaboration agreements further include language regarding publication rights and IP ownership related to the scope of work. Investigators should utilize their respective university TTO or SPO to negotiate and provide final sign-off on any MTA, DUA, or research collaboration agreement. Because views may differ, negotiations can sometimes be time consuming. Issues of contention that may arise often relate to ownership of technology generated by the research, compliance with policies, and the use and dissemination of research results (e.g. restricting publication of unfavorable results).
Studies arising from collaborations involving human subjects should only proceed with Institutional Review Board (IRB) approval.46 It is the responsibility of both the university investigators and industry entities to ensure that new protocols are reviewed by an IRB. Many industry sponsored trials utilize a centralized IRB to achieve general approval for a new protocol, which can expedite approval on multi-centered trials. Most universities require a local IRB review (that may be expedited if agreements are in place with a centralized IRB) and centralized IRB approval in order for sponsored research to proceed. Industry sponsored trials also require a clinical trials agreement (CTA) between the company and university. While university research teams or individual investigators can work directly with sponsors to negotiate budgets and study details, investigators should ultimately utilize their SPO for negotiation and final execution of a CTA.
If collaborations result in a funded grant, a grant subaward agreement may need to be negotiated to ensure proper allocation of responsibilities and funds (subaward) among the party that submitted the grant (pass-through entity) and their partner (subrecipient). Grant subaward agreements can be initiated by either party and should always be led by the party that applied for the grant. Such agreements are usually tailored to the specifications of the grant. Like grant recipients, the collaborating subrecipient will need to comply with a grant's requirements. Improper distribution of funds to outside entities could lead to scrutiny and potential loss of an award.
7. Disclosure and conflict of interest
The key to successfully navigating all disclosure requirements and COI reporting for AIPs are appropriate communication with federal funding agencies, university and hospital compliance offices, and during public announcements (e.g. meeting presentations, publications, public messages, broadcasts, etc.). Perceptions of COI must be avoided at every step of the innovation process. In essence, the safest policy is to disclose everything, every time, especially when there are significant financial conflicts involved (e.g. ownership of stock, licensing revenues or other IP entanglements with an industry partner). It is important to also disclose any COI when having a conversation with key decision makers about making a financial recommendation related to a company partner or their industry. Disclosures often need to be updated annually, or any time a new industry collaboration is initiated. Disclosures usually also extend to family members.
At many universities researchers are permitted to spend a proportion (e.g. up to 20%) of their time consulting with industry and or engage with other entities including “for profit” concerns. These activities should be disclosed and made transparent not only to the university, but also to federal funding agencies and other associations (e.g. professional societies, accredited continuing medical education providers), as well as in teaching settings. It is equally important that a pathologist who owns a company should not sponsor research in their own laboratory or have graduate students from their own institution work in their company. Moreover, as an equity owner in an established company that is generating revenue at some universities a pathologist cannot take on a high-ranking executive title (e.g. CEO, CMO). In such cases, most academic entrepreneurs instead evolve into clinical or scientific advisors and should not maintain direct decision making/fiscal authority. However, these rules may vary among academic institutions, with some relaxation at enlightened institutions. Academic leaders should also be cautious of drawing negative attention if they are offered equity, board seats, or other perks in exchange for facilitating arrangements rather than on the basis of their individual R&D contributions.
In the United States, the Physician Payments Sunshine Act (PPSA) necessitates that medical product manufacturers disclose to the Centers for Medicare and Medicaid Services (CMS) any payments or transfers of value (e.g. covered travel expenses) they have given to physicians or teaching hospitals.47 The PPSA additionally requires certain manufacturers and group purchasing organizations to disclose any physician ownership or investment interests held in those companies.48 Since 2014, this information has been published annually in a publicly searchable database (https://www.cms.gov/OpenPayments). It remains unclear how this reporting affects the behavior of different stakeholders (e.g. providers, patients, and industry) and what impact this has on AIPs.
7.1. Intellectual property
IP refers to an invention to which one has legal rights and may accordingly apply for patent, copyright, trademark or trade secret protection. This enables individuals to earn recognition and financial benefit from their invention. Patents can incentivize innovation.49 It is critical to choose an appropriate type of protection for any valuable IP. For example, inventions in digital pathology can be protected using either patents or trade secrets, but each of these provide different advantages and disadvantages. A fundamental difference between these two is that a patent relies on mandatory public disclosure whereas a trade secret relies on guarded secrecy from the public. From the perspective of public policy, a patent is granted with exclusive rights for a limited time when an inventor provides sufficient disclosure of their invention which can benefit the public. Patent protection lasts 20 years from the filing and trade secret protection can be perpetual, if one is able to keep its secrecy. However, trade secret protection is lost if someone reverse engineers the invention whereas a patented invention is protected against reverse engineering. Thus, it is important to carefully consider which type of IP protection is optimal for an invention.
Driven by major advances in imaging technology, we have witnessed a significant increase in digital pathology patent applications and patents granted.50 This trend is likely to continue in the future, especially with the rapidly emerging application of AI in pathology. Hence, it is important for inventors in this field to understand the patent system that enables them to protect their IP (Supplemental Appendix; Table 5). The most requested patents in digital pathology are utility (Table 6) and design patents. For digital pathology most patents requested or granted can be broadly classified into the following technological categories: digital scanners, telepathology, pathology consultation/diagnostic networks, digital image analysis, and computer-aided diagnosis (CAD) tools. While not all partnerships generate IP, they may still yield business opportunity for departments of pathology and laboratory medicine.
Table 5.
Stages in a typical utility patenting process.
| Stage | Task |
|---|---|
| 1 | Identify invention |
| 2 | Patentability evaluation and preparation (including invention disclosure) of the patent application (via patent attorney or self) |
| 3 | Filing of the application with a patent office |
| 4 | Prosecuting the application (negotiating with the Patent Examiner regarding what can be granted) to reach an outcome (issuance or abandonment or restart the negotiation) |
| 5 | Patent issuance |
| 6 | Post-issuance procedures (optional) |
| 7 | Payment of maintenance fee to keep the patent alive |
| 8 | Enforcement of patent against infringers (if necessary) |
Table 6.
Utility patent protection details in the USA for digital pathology inventions.
| Type of IP | Statutory basis for Protection in USA | What is protected | Legal requirement | Rights granted in USA | Term of protection | Process, time and cost involved | International rights | Digital Pathology example |
|---|---|---|---|---|---|---|---|---|
| Utility patent | U.S. Constitution and 1952 Patent Act, as amended, see 35 U.S.C. §§ 1–390 | New and useful process, machine, manufacture, or composition of matter or improvements | Novelty; non-obviousness; utility and sufficiency of disclosure | To make, use, offer to sell, sell, and import the patented invention | 20 years from filing | Very involved, can take years and be very expensive | Rights are country specific, hence need to obtain a patent in each country where one is desired | Auto-focus methods and systems for multi-spectral imaging US Patent: 10,768,403 |
8. Challenges associated with AIP in digital pathology
Much of the discipline of pathology is an image intensive specialty that generates enormous datasets that includes both structured and unstructured clinical data. With the advent and progressive global adoption of whole slide imaging in pathology, large digital datasets are being established that have ushered in the field of computational pathology. Not surprisingly, there is an increasing desire for industry vendors in the field of digital pathology and AI to partner with pathology laboratories, particularly those at academic institutions, to gain exclusive access to these huge, annotated datasets to generate novel AI-based applications. However, data ownership is currently one of the key challenges for AIP in pathology. It is often unclear who owns the data: the patients, institutions hosting these data, pathologists who generated and curated these datasets that reside in institutional databases, or their industry partners.51 Unfortunately, practical guidelines that deal with this issue in AIP are lacking. Another big challenge is difficulty related to licensing software, which is key for the use of AI-based systems. Although the FDA identifies Software as a Medical Device (SaMD), regulations are still emerging to cover the vast array of unique software solutions and their applications in digital pathology.
9. Recommendations for successful partnerships
A successful AIP (Table 7) for responsible innovation relies heavily on setting clear goals and expectations in terms of action and impact, continued active communication throughout the partnership involving all stakeholders, an agile approach to adjust to unforeseen parameters as well as shifting landscapes in a fast-evolving space, and resilience to redefine the collaboration if necessary. Most importantly, an AIP should be built on a strong foundation of trust, sound ethical principles, and solid strategic decisions.52,53 Ethical actions in physician relationships with industry should also be guided by professional standards, medical society guidelines, and local institutional policies.54 These principles apply to all governance modes of AIPs; viz. an institutional mode where interactions are mediated via the university's administrative structures (e.g. TTO, SPO) and the personal mode where interactions are dictated by binding contractual agreements between a company and individual academics executed without direct involvement by the university. Prior experience with collaborative research has been found to lower barriers to a successful AIP. There are myriad reasons why an AIP may fail including conflict between stakeholders, overpromising, over/under budgeting, unclear or changing aims, poor communication, inadequate governance, lack of a troubleshooting mechanism, milestones not met, failed deliverables, loss of marketability and/or commercialization, and an undesirable scandal.55, 56, 57, 58
Table 7.
Practical recommendations and checklist for sustainable and successful Academic-Industry Partnerships.
| Win–Win relationship: The partnership should benefit all engaged parties. |
| Building trust: There must be trust, integrity and compatibility of values. |
| Legal review: Consult legal counsel including a TTO early on. |
| Upfront review: Sign all necessary partnership agreements upfront. |
| Define vision and scope: Clearly define vision, expectations, as well as short and long term plans. |
| Create boundaries: Determine institutional restrictions on both sides. |
| Identify champions: Identify a champion from each party to drive the relationship. |
| Get the C-suite engaged: Garner support from senior leadership on both ends. |
| Financial review: Establish a credible financial budget. |
| Data sharing: Generate an ongoing data sharing plan. |
| Pilot program: Initiate a pilot program to determine fit and scope. |
| Workload balance: There should be fair distribution of roles. |
| Review resources: Manage resources judiciously. |
| Governance review: Ensure good governance and project management. |
| Be transparent: Maintain transparency throughout the partnership. |
| Compliance review: Maintain compliance with all involved institutions and regulatory bodies. |
| Full disclosures: Adhere to full disclosure and acknowledgement. |
| IP review: Address monetizing IP with all stakeholders early on. |
| Local versus International: For international partnerships explore geographical considerations, especially as they apply to data sharing and compliance with specific regulatory bodies. |
| Engage academicians: Mitigate fear from academicians getting involved in entrepreneurship. |
IP = Intellectual property; TTO = Technology transfer office.
10. Conclusion
In summary, successful and sustainable academic and industry partnerships must be designed to benefit all parties and create win–win engagements from the outset (Table 7). This will enable all stakeholders to stay engaged and ensure that the relationship is productive and collaborative. As in any relationship, there must be trust, integrity, and compatibility of values. Organizational work cultures also need to be compatible. Early key steps include engaging legal counsel to set the boundaries for how the AIP will operate in the near and long term. It is highly beneficial to have a leader or champion from each side drive this relationship forward and facilitate well balanced involvement of stakeholders from within their organizations. A vital aspect of a successful AIP is addressing intellectual property issues, licensing, and revenue opportunities upfront. In addition, there needs to be an ongoing data sharing plan, especially if there is intent to build adaptive deep learning algorithms that continuously utilize new data and feedback for improvement. Involving several intra-organizational teams is essential with representation from scientific, information technology, legal, regulatory compliance, business development and domain experts. Attending to all the aforementioned important details discussed in this review will help facilitate a fruitful AIP.
Disclosures
Michael J. Becich is a founder and equity owner in SpIntellx and has industry sponsored research agreements with Owkin, Inc. and Pfizer. Dr. Becich is a paid consultant to the following academic medical centers and cancer centers: Indiana University, Medical College WI, Northwestern University, Rockefeller University, Rutgers, UC Davis, UCSD, University of Chicago, University of Cincinnati, University of IL Chicago, University of New Mexico, USC, UTSA, University WI/Marshfield Clinic, Wake Forest Baptist Cancer Center. He receives federal funding from CDC NIOSH, NCATS and PCORI. Dr. Becich is an NIH employee as a member of the NCI Board of Scientific Advisors and the Frederic National Labs NCI/DOE Advisory Committee.
Marilyn M. Bui is an employee of the H. Lee, Moffitt Cancer Center and Research Institute. She received a Cancer Center Support Grant from NIH as the Scientific Director of the Analytic Microscopy Core of Moffitt Cancer Center. She received US patents (No. 14/373,277 and No. 15/034,683). She received an honorarium serving on the medical/scientific advisory board of ContextVision, Visiopharm, and AstraZeneca. She donated her honorarium serving as the scientific advisory board of Aiforia to a pathology foundation.
Ehab A. ElGabry is the Sr. Medical Director for Roche Tissue Diagnostics (RTD) Companion Diagnostics and the Pharma Services Medical Director.
Liron Pantanowitz is on the medical advisory board for Ibex, a consultant for Hamamatsu and Nano Tech Projects, and an equity owner in LeanAP Innovators.
Anil Parwani is on the medical advisory board for Visiopharm, Context Vision and PathPresenter. He is also the sponsor for a research agreement with Hamamatsu.
David Tulman is a founder, equity owner, and board member of Instapath. Dr. Tulman receives federal funding from the NSF and state funding from the Cancer Prevention Research Institute of Texas.
Mohamed Salama is on the board of directors and has stock options at Techcyte Inc.
Manu Sebastian is on the medical advisory board for OptraScan and PathPresenter.
The following individuals have no pertinent disclosures: Lewis Hassell, Chhavi Chauhan, Zaibo Li, and Sury Vepa.
Acknowledgments
The authors thank Abbey Norris for facilitating this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.acpath.2022.100026.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Cyert R.M., Goodman P.S. Creating effective University-industry alliances: an organizational learning perspective. Organ Dynam. 1997;25:45–57. [Google Scholar]
- 2.Ankrah S., AL-Tabbaa O. Universities–industry collaboration: a systematic review. Scand J Manag. 2015;31(3):387–408. [Google Scholar]
- 3.Rybniceck R., Konigsgruber R. What makes industry–university collaboration succeed? A systematic review of the literature. J Bus Econ. 2019;89:221–250. [Google Scholar]
- 4.Tierney W.M., Meslin E.M., Kroenke K. Industry support of medical research: important opportunity or treacherous pitfall? J Gen Intern Med. 2016;31(2):228–233. doi: 10.1007/s11606-015-3495-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kneller R. Technology transfer: a review for biomedical researchers. Clin Cancer Res. 2001;7(4):761–774. [PubMed] [Google Scholar]
- 6.Van Norman G.A., Eisenkot R. Technology transfer: from the research bench to commercialization: Part 2: the commercialization process. JACC Basic Transl Sci. 2017;2(2):197–208. doi: 10.1016/j.jacbts.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prigge G.W. University-industry partnerships: what do they mean to Universities?: a review of the literature. Ind High Educ. 2005;19(3):221–229. [Google Scholar]
- 8.Gregory E.H. University-industry strategic partnerships: benefits and impediments. Ind High Educ. 1997;11(4):253–254. [Google Scholar]
- 9.Rao B., Mulloth B. The role of universities in encouraging growth of technology-based new ventures. Int J Innovat Technol Manag. 2017;14(4):1–22. [Google Scholar]
- 10.Schofield T. Critical success factors for knowledge transfer collaborations between university and industry. J Res Adm. 2013;44:38–56. [Google Scholar]
- 11.Rose D.M., Marshall R., Surber M.W. Pharmaceutical industry, academia and patient advocacy organizations: what is the recipe for synergic (win-win-win) collaborations? Respirology. 2015;20(2):185–191. doi: 10.1111/resp.12458. [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen K., Bero L., Redberg R., Gotzsche P.C., Lundh A. Collaboration between academics and industry in clinical trials: cross sectional study of publications and survey of lead academic authors. BMJ. 2018;363:k3654. doi: 10.1136/bmj.k3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George G., Zahra S.A., Wood D.R. The effects of business-university alliances on innovative output and financial performance: a study of publicly traded biotechnology companies. J Bus Ventur. 2002;17:577–609. [Google Scholar]
- 14.Jain S.H., Rosenblatt M., Duke J. Is big data the new frontier for academic-industry collaboration? JAMA. 2014;311(21):2171–2172. doi: 10.1001/jama.2014.1845. [DOI] [PubMed] [Google Scholar]
- 15.Colling R., Pitman H., Oien K., Rajpoot N., Macklin P., CM-Path AI in Histopathology Working Group. Snead D., Sackville T., Verrill C. Artificial intelligence in digital pathology: a roadmap to routine use in clinical practice. J Pathol. 2019;249(2):143–150. doi: 10.1002/path.5310. [DOI] [PubMed] [Google Scholar]
- 16.Eusemann C.D., Sammons B.E., Holmes D.R., Brady T.J., Erenburg I., Toneguzzo F. Academic technology transfer and radiology. A strong partnership for the future. Acad Radiol. 2007;14(11):1289–1295. doi: 10.1016/j.acra.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Huang H.K. Medical imaging, PACS, and imaging informatics: retrospective. Radiol Phys Technol. 2014;7(1):5–24. doi: 10.1007/s12194-013-0245-y. [DOI] [PubMed] [Google Scholar]
- 18.Baker M. In Biomarkers we trust? Nat Biotechnol. 2005;23:297–304. doi: 10.1038/nbt0305-297. [DOI] [PubMed] [Google Scholar]
- 19.Srimathveeravalli G., Balesh E., Cheng C.P., Chen D. If you build it, they will come: how to establish an academic innovation enterprise. Tech Vasc Intervent Radiol. 2017;20(2):121–126. doi: 10.1053/j.tvir.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Lewin J.S. Industrial-academic research relationships: departmental collaborations. Radiology. 2009;250:23–27. doi: 10.1148/radiol.2493081306. [DOI] [PubMed] [Google Scholar]
- 21.Li R.H., Wacholtz M.C., Barnes M., et al. Incorporating ethical principles into clinical research protocols: a tool for protocol writers and ethics committees. J Med Ethics. 2016;42(4):229–234. doi: 10.1136/medethics-2014-102540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukhopadhyay S., Feldman M.D., Abels E., et al. Whole slide imaging versus microscopy for primary diagnosis in surgical pathology: a multicenter blinded randomized noninferiority study of 1992 cases (pivotal study). Am. J Surg Pathol. 2018;42(1):39–52. doi: 10.1097/PAS.0000000000000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borowsky A.D., Glassy E.F., Wallace W.D., et al. Digital whole slide imaging compared with light microscopy for primary diagnosis in surgical pathology. Arch Pathol Lab Med. 2020;144(10):1245–1253. doi: 10.5858/arpa.2019-0569-OA. [DOI] [PubMed] [Google Scholar]
- 24.Meslin E.M., Rager J.B., Schwartz P.H., et al. Benchmarks for ethically credible partnerships between industry and academic health centers: beyond disclosure of financial conflicts of interest. Clin Transl Med. 2015;4:36. doi: 10.1186/s40169-015-0077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hillebrand R., Werker C. Values in university–industry collaborations: the case of academics working at universities of technology. Sci Eng Ethics. 2019;25:1633–1656. doi: 10.1007/s11948-019-00144-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonnell J.M., Dalton D.M., Ahern D.P., Welch-Phillips A., Butler J.S. Methods to mitigate industry influence in industry sponsored research. Clin Spine Surg. 2021;34(4):143–145. doi: 10.1097/BSD.0000000000001098. [DOI] [PubMed] [Google Scholar]
- 27.Singh A., Singh S. The connection between academia and industry. Mens Sana Monogr. 2005;3(1):5–35. doi: 10.4103/0973-1229.27876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deipolyi A.R., Becker A.S., Covey A.M., et al. Gender disparity in industry relationships with academic interventional radiology physicians. Am J Roentgenol. 2020;215(2):494–501. doi: 10.2214/AJR.19.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birch K., Chiappetta M., Artyushina A. The problem of innovation in technoscientific capitalism: data rentiership and the policy implications of turning personal digital data into a private asset. Pol Stud. 2020;41(5):468–487. [Google Scholar]
- 30.Drucker P.F. The discipline of innovation. Harv Bus Rev. August 2002 https://hbr.org/2002/08/the-discipline-of-innovation [PubMed] [Google Scholar]
- 31.Collins J.M., Reizes O., Dempsey M.K. Healthcare commercialization programs: improving the efficiency of translating healthcare innovations from academia into practice. IEEE J Transl Eng Health Med. 2016;4:3500107. doi: 10.1109/JTEHM.2016.2609915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Kluyver C.A. Innovation and industrial product life cycles. Calif Manag Rev. 1977;20(1):21–33. [Google Scholar]
- 33.Mas J.P., Hsueh B. An investor perspective on forming and funding your medical device start-up. Tech Vasc Intervent Radiol. 2017;20(2):101–108. doi: 10.1053/j.tvir.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Pantanowitz L., Carter A., Kurc T., Sharma A., Sussman A., Saltz J. Twenty Years of Digital Pathology: an overview of the road travelled, what is on the horizon, and emergence of vendor neutral archives. J Pathol Inf. 2018;9:40. doi: 10.4103/jpi.jpi_69_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DePasse J.W., Chen C.E., Sawyer A., Jethwani K., Sim I. Academic Medical Centers as digital health catalysts. Healthcare. 2014;2(3):173–176. doi: 10.1016/j.hjdsi.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Tsai W., Erickson S. Early-stage biotech companies: strategies for survival and growth. Biotechnol Healthc. 2006;3(3):49–53. [PMC free article] [PubMed] [Google Scholar]
- 37.Canhoto A.I., Quinton S., Jackson P., Dibb S. The co-production of value in digital, university–industry R&D collaborative projects. Ind Market Manag. 2016;56:86–96. [Google Scholar]
- 38.Galan-Muros V., Plewa C. What drives and inhibits university-business cooperation in Europe? A comprehensive assessment. R D Manag. 2016;46:369–382. [Google Scholar]
- 39.Arvanitis S., Kubli U., Woerter M. University-industry knowledge and technology transfer in Switzerland: what university scientists think about co-operation with private enterprises. Res Pol. 2008;37(10):1865–1883. [Google Scholar]
- 40.Claus T., Kesting T. How businesses should govern knowledge-intensive collaborations with universities: an empirical investigation of university professors. Ind Market Manag. 2017;62:185–198. [Google Scholar]
- 41.Starbuck E. Optimizing university research collaborations. Res Technol Manag. 2001;44:40–44. [Google Scholar]
- 42.Boardman C., Bozeman B. Academic faculty as intellectual property in university-industry research alliances. Econ Innovat N Technol. 2015;24(5):403–420. [Google Scholar]
- 43.Rajalo S., Vadi M. University-industry innovation collaboration: Reconceptualization. Technovation. 2017;62–63:42–54. [Google Scholar]
- 44.Lader E.W., Cannon C.P., Ohman E.M., et al. The clinician as investigator: participating in clinical trials in the practice setting. Circulation. 2004;109(21):2672–2679. doi: 10.1161/01.CIR.0000128702.16441.75. [DOI] [PubMed] [Google Scholar]
- 45.Sunderland J.J. The academic NDA: justification, process, and lessons learned. J Nucl Med. 2020;61(4):480–487. doi: 10.2967/jnumed.119.238287. [DOI] [PubMed] [Google Scholar]
- 46.Scheibner J., Ienca M., Kechagia S., et al. Data protection and ethics requirements for multisite research with health data: a comparative examination of legislative governance frameworks and the role of data protection technologies. J Law Biosci. 2020;7(1):1–30. doi: 10.1093/jlb/lsaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grady C. Institutional review boards: purpose and challenges. Chest. 2015;148(5):1148–1155. doi: 10.1378/chest.15-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richardson E. The physician payments sunshine act. Health Affairs Health Policy Brief. October 2, 2014 doi: 10.1377/hpb20141002.272302. [DOI] [Google Scholar]
- 49.Trerise J. The influence of patents on science. Polit Philos Econ. 2016;15(4):424–450. [Google Scholar]
- 50.Cucoranu I., Parwani A.V., Vepa S., Weinstein R., Pantanowitz L. Digital Pathology: a systemic evaluation of the patent landscape. J Pathol Inf. 2014;5(1):16. doi: 10.4103/2153-3539.133112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chauhan C., Gullapalli R.R. Ethics of AI in Pathology. Current paradigms and emerging issues. Am J Pathol. 2021;10:1673–1683. doi: 10.1016/j.ajpath.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berbegal-Mirabent J., Sánchez García J.L., Ribeiro-Soriano D.E. University–industry partnerships for the provision of R&D services. J Bus Res. 2015;68(7):1407–1413. [Google Scholar]
- 53.Van Norman G.A., Eisenkot R. Technology transfer: from the research bench to commercialization: Part 1: intellectual property rights-basics of patents and copyrights. JACC Basic Transl Sci. 2017;2(1):85–97. doi: 10.1016/j.jacbts.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pandza K., Ellwood P. Strategic and ethical foundations for responsible innovation. Res Pol. 2013;42(5):1112–1125. [Google Scholar]
- 55.Plewa C., Korff N., Baaken T., Macpherson G. University–industry linkage evolution: an empirical investigation of relational success factors. R D Manag. 2013;43(4):365–380. [Google Scholar]
- 56.Breault J.L., Knafl E. Pitfalls and safeguards in industry-funded research. Ochsner J. 2020;20(1):104–110. doi: 10.31486/toj.19.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bodas Freitas I.M., Aldo G., Rossi F. Finding the right partners: institutional and personal modes of governance of university-industry interactions. Res Pol. 2013;42(1):50–62. [Google Scholar]
- 58.Bruneel J., D'Este P., Salter A. Investigating the factors that diminish the barriers to university-industry collaboration. Res Pol. 2010;39(7):858–868. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.