Summary
Background
To date, only dexamethasone and tocilizumab have been shown to reduce mortality in patients with COVID-19. Baricitinib is a Janus kinase 1/2 inhibitor with known anti-inflammatory and anti-viral properties. We performed a meta-analysis of RCTs assessing the role of baricitinib in hospitalised patients with COVID-19.
Methods
Electronic databases such as MEDLINE, EMBASE, and Cochrane Central were searched up until March 31, 2022, for RCTs evaluating the efficacy of baricitinib in hospitalised patients with COVID-19. The outcomes assessed were 28-day mortality, progression to invasive mechanical ventilation (IMV) or ECMO, progression to respiratory failure needing positive pressure ventilation, IMV or death, duration of hospitalisation and time to discharge. The meta-analysis was registered in the PROSPERO database (CRD42022314579).
Findings
Four studies (with 10,815 patients) were included in the analysis. Pooled analysis using random-effects model showed a statistically significant reduction in 28-day mortality (OR 0.69, 95% CI 0.50-0.94; p=0.04, I2=65%) and composite outcome of progression to severe disease needing positive pressure ventilation, IMV or death (OR 0.89, 95% CI 0.80-0.99, p= 0.03, I2=0%). There was a favorable trend towards reduced progression to IMV or ECMO (OR 0.76, 95% CI 0.58-1.01; p=0.06, I2=49%) in the baricitinib arm compared to standard therapy, even though it was not statistically significant. Statistical significance was achieved for all outcomes with fixed-effects model analysis.
Interpretation
In hospitalised patients with COVID-19, baricitinib was associated with reduced 28-day mortality although there was not a statistically significant reduction in progression to IMV or ECMO. Baricitinib used in conjunction with standard of care treatments is associated with improved mortality in hospitalised patients with COVID-19 disease.
Funding
None.
Keywords: Baricitinib, JAK, COVID19, COVID, Janus kinase inhibitors
Research in context.
Evidence before this study
Multiple clinical trials and observational studies have presented heterogenous results about the use of baricitinib in hospitalised patients with COVID-19 disease. We conducted a systematic search in MEDLINE, EMBASE, and Cochrane Central to identify all relevant articles with restrictions to the English language using the following search terms: (“SARS-CoV2” OR “COVID-19”) AND (“baricitinib” OR “Janus kinase inhibitor” OR “JAK” OR “JAK inhibitor”) till March 31, 2022, to identify all relevant randomised controlled trials evaluating the efficacy of baricitinib in hospitalised COVID-19 patients, including preprint and non-peer reviewed studies. Baricitinib has been added to the treatment guidelines, but data from various randomised controlled trials remain conflicting.
Added value of this study
Our updated meta-analysis provides a comprehensive scrutiny of the available randomized trials on the efficacy of baricitinib therapy in hospitalised patients with COVID-19 disease. The results from our meta-analysis showed a mortality benefit with baricitinib treatment in hospitalised patients with moderate to severe COVID-19 disease. The use of baricitinib therapy was also associated with shorter duration of hospitalisation and early discharge compared to standard therapy in hospitalised patients with COVID-19 disease.
Implications of all the available evidence
Our findings suggest that the use of baricitinib in conjunction with dexamethasone and/or anti-IL6 inhibitors is associated with reduced mortality, and early discharge from the hospital.
Alt-text: Unlabelled box
Introduction
Despite treatment advances, reducing mortality among hospitalised patients with COVID-19 remains a crucial unmet need. Current adult models of COVID-19 disease typically involve three clinical phases. First, there is an initial viral response phase where patients mostly have mild constitutional symptoms, followed by a pulmonary phase where there is an overlap of host inflammatory response and viral replication effects. Lastly, there is a hyperinflammatory phase where the pathophysiology is driven primarily by the host immune response.1 Baricitinib, an oral inhibitor of Janus Kinase (JAK) 1/2, was originally approved for treatment of rheumatoid arthritis.2 JAKs are involved in the inflammatory pathways that modulate the signaling pathway, preventing activation of signal transducers and activators of transcription for cytokines like interleukin-2, interleukin-6, interleukin-10, and interferon- γ. JAK inhibitors can prevent the dysregulated production of proinflammatory cytokines primarily involved in cellular survival, proliferation, and differentiation, proving to be clinically useful in immune, and inflammatory diseases.3,4
Multiple studies have demonstrated anti-viral activity of baricitinib through different mechanisms: (1) inhibition of numb associated kinases (NAKs) (2) prevention of type 1 interferon mediated increase in expression for the SARS-CoV2 receptor (angiotensin converting enzyme-2), thereby reducing viral endocytosis (3) impairment of the adaptor protein (AP)2-associated protein kinase 1.5, 6, 7 The Adaptive COVID-19 Treatment Trial-2 (ACTT-2) first suggested improved outcomes in hospitalised patients with COVID when baricitinib was added to remdesivir therapy and several additional observational cohort studies have been done that showed evidence of clinical improvement with baricitinib treatment.8, 9, 10, 11 The JAK inhibitors tofacitinib and ruxolitinib have also been associated with reduced mortality in small, multicenter, randomised controlled trials (RCTs).12, 13, 14, 15 Although the ACTT-2 trial did not detect a significant mortality difference between treatment groups, recent trials such as COV-BARRIER and RECOVERY showed mortality benefit in hospitalised patients that were treated with baricitinib compared to standard of care.16, 17, 18 The US Food and Drug Administration (FDA) issued an emergency use authorization (EUA) for baricitinib use in combination with remdesivir, for the treatment of hospitalised, hypoxic patients with COVID-19 requiring invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO).19
Tocilizumab was added to the standard of care along with dexamethasone, especially in severe or critically ill patients, as it was shown to reduce mortality in hospitalised patients with COVID-19 with severe disease (requiring invasive mechanical ventilation).20,21 Given shortages in tocilizumab supply and increasing mortality in hospitalised patients with COVID-19 despite improvements in the standard of care, other treatments such as baricitinib are still urgently needed to reduce the high frequency of deaths. To address this critical gap in knowledge, we conducted a meta-analysis of the RCTs assessing the efficacy of baricitinib in hospitalised patients with COVID-19.
Methods
Search strategy and selection criteria
We conducted a systematic search in MEDLINE, EMBASE, and Cochrane Central to identify all relevant articles using the following search terms: (“SARS-CoV2” OR “COVID-19”) AND (“baricitinib” OR “Janus kinase inhibitor” OR “JAK” OR “JAK inhibitor”). Databases were searched till March 31, 2022, to identify all relevant RCTs evaluating the efficacy of baricitinib in hospitalised patients with COVID-19, including preprint and non-peer reviewed studies. Review articles, observational studies, case reports, letters, abstracts, opinion articles, brief reports were excluded. All results were imported into EndNote version 20 and identical results were identified and removed. Results were limited to humans and the English language. This study was conducted according to the Cochrane Collaboration and the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement.22 Ethics approval of the study was waived.
Two reviewers with similar experience and expertise independently (VS and AL) screened the retrieved papers based on the title and abstract. The whole paper was retrieved if the data was not clear from the title and abstract. Any disagreements between the two reviewers were discussed and resolved by consensus. A study was considered eligible for inclusion in the analysis if it was 1) randomised controlled trial 2) reported outcomes of interest in hospitalised patients with COVID-19 with baricitinib therapy compared to standard treatment or placebo. The outcomes assessed were all-cause mortality, progression to severe disease needing non-invasive positive pressure ventilation (NIPPV), invasive mechanical ventilation (IMV), need for ECMO or death, time to hospital discharge, and duration of hospitalisation.
Data extraction
Two reviewers (VS and AL) independently extracted data from the included studies. Extracted data included 1) study characteristics - design, sites of study, dates of study, and type of randomisation 2) details of the study population and the interventions utilised, including demographics of participants in both intervention and control arms, presence of comorbidities and treatment received 3) primary outcome and follow up. The meta-analysis was registered in the PROSPERO database (CRD42022314579).23 The risk of bias was assessed for the domains suggested by the Cochrane collaboration, emphasizing sequence generation, allocation concealment, blinding, outcomes assessment, and selective reporting.24 Any divergence was resolved by consensus.
Data synthesis and statistical analysis
Statistical analysis was performed as per recommendations from the Cochrane collaboration and the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) guidelines.25,26 The Mantel-Haenszel method was used to calculate aggregated odds ratios (ORs) with corresponding 95% confidence intervals (CIs). A p value of 0.05 or less was considered to be statistically significant. Heterogeneity was assessed using Higgins and Thompson's I2 statistic, which assesses unexplained statistical heterogeneity among studies. I2 is the proportion of total variation observed between the trials attributable to differences between trials rather than sampling error, with I2 values of 75%, corresponding to low, moderate, and high levels of heterogeneity.27 The meta-analysis was performed with a random-effects model. Statistical analysis was performed using Review Manager, version 5. Different RCTs reported medians and means for hospitalisation duration and recovery time. To standardise this, they were converted to the mathematically implied means based on an exponential distribution, as is often assumed for time to event data.
Role of the funding source
There was no funding source for this study. All authors had full access to all the data in the study and accept responsibility to submit for publication.
Results
Search results and characteristics of included trials
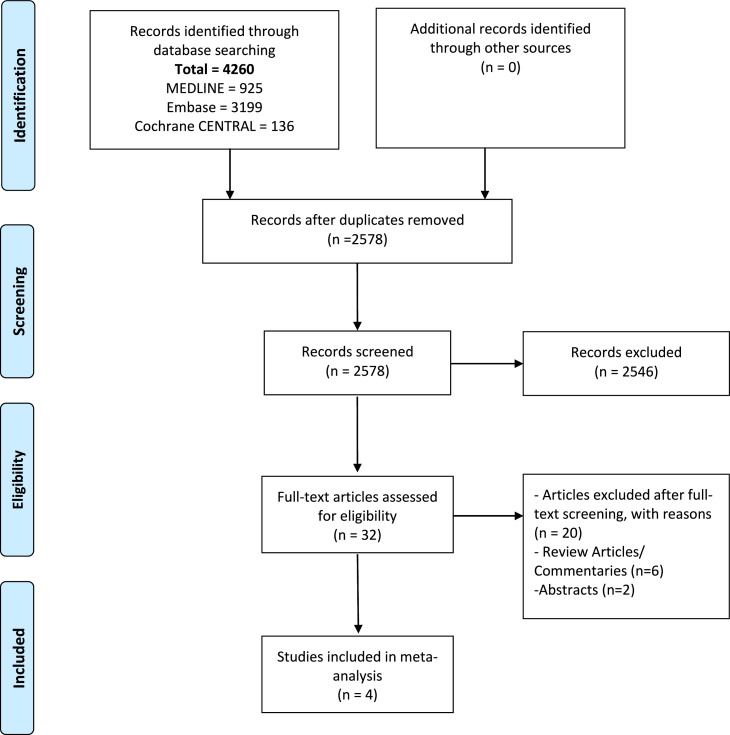
Figure 1 shows the PRISMA flow chart summarising the search strategy. The literature search identified 32 full-text articles, of which four RCTs were eligible for inclusion in this study after full read.9,16, 17, 18 A total of 10,815 patients were included, of which 5,478 patients received baricitinib, and 5,377 received standard care. Baseline characteristics were similar across the intervention and standard care groups. Detailed characteristics of the studies are described in Table 1.
Figure 1.
PRISMA flow chart outlining literature search.
Table 1.
Study Characteristics.
| Reference | Groups | Sample size | Median Age (years) | Race/ethnic group– white % | Race/ ethnic group– black or African American% | Race/ ethnic group– Asian % | Males (%) | Females (%) | DM2 (%) | HTN (%) | Obesity (%) | Corticosteroids (%) | Remdesivir (%) | Mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACTT-2 | Baricitinib + Remdesivir | 515 | 55.0±15.4 | 48.7 | 15 | 9.5 | 62 | 38 | 39 | 51 | 58 | N/A | 100 | 4.67% |
| Placebo + Remdesivir | 518 | 55.8±16.0 | 47.3 | 15.3 | 10 | 64 | 36 | 35 | 52 | 53 | N/A | 100 | 7.14% | |
| COV-BARRIER | Baricitinib | 764 | N/A | 64 | 5 | 11 | 64 | 36 | 29 | 48 | 33 | 80 | 18 | 8% |
| Placebo | 761 | N/A | 59 | 5 | 13 | 62 | 38 | 31 | 48 | 33 | 78 | 19 | 13% | |
| COV-BARRIER (severe) | Baricitinib + Usual care | 51 | 58.4 | 64 | 2 | 0 | 49 | 51 | 39 | 61 | 55 | 84 | 0 | 39.2% |
| Placebo + Usual care | 50 | 58.8 | 61 | 2 | 2 | 60 | 40 | 32 | 48 | 58 | 88 | 2 | 58% | |
| RECOVERY | Baricitinib | 4148 | 58.5 | 77 | 11 | 66 | 34 | 23 | N/A | N/A | 96 | 21 | 12% | |
| Usual care | 4008 | 57.7 | 77 | 11 | 66 | 34 | 23 | N/A | N/A | 95 | 20 | 14% | ||
All trials included in the analysis studied hospitalised patients with COVID-19 and were multicenter in design. Three international trials were conducted across multiple countries in Europe, Mexico, South Korea, Japan, and Brazil while one was conducted in the UK. Three trials were double-blinded, and placebo controlled while one had open label, platform design. Detailed study designs and study criteria are described in Table 2. Exclusion criteria are described in Supplementary Table 1.
Table 2.
Study Design and Criteria.
| Reference | Site | Design | Dates | Follow up | Inclusion criteria | Primary outcome |
|---|---|---|---|---|---|---|
| ACTT-2 | Global | Double-blinded, placebo-controlled, multicenter trial | May 8, 2020, to July 1, 2020 | 28 days | Participants aged ≥18 years of age with positive laboratory confirmed RT-PCR assay result of SARS-CoV2 infection + one of the following: radiographic infiltrates by imaging study, peripheral oxygen saturation (SpO2) ≤94% on room air, or requiring supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO). | The time to recovery, with the day of recovery defined as the first day, during the 28 days after enrollment, on which a patient attained category 1, 2, or 3 on the eight-category ordinal scale |
| COV-BARRIER | Global | Multicenter, randomized, double-blind, placebo controlled, parallel-group, phase 3 trial | June 11, 2020, to Jan 15, 2021 | 60 days | Patients aged ≥18 years of age, were hospitalized with laboratory confirmed SARS-CoV-2 infection, had evidence of pneumonia or active and symptomatic COVID-19, and had at least one elevated inflammatory marker (C-reactive protein, D-dimer, lactate dehydrogenase, or ferritin) | Proportion who progressed to high-flow oxygen, non-invasive ventilation, invasive mechanical ventilation, or death by day 28 |
| COV-BARRIER (Severe) | Global | Multicenter, randomized, double-blind, placebo controlled, parallel-group, phase 3 trial | December 23, 2020, to April 10, 2021 | 60 days | Eligible participants were ≥18 years of age, hospitalized with laboratory-confirmed SARS-CoV-2 infection, use of IMV or ECMO at study entry and randomization, had evidence of pneumonia or clinical symptoms of COVID-19, and had at least one elevated inflammatory marker above the upper limit of normal range based on the local laboratory result (C-reactive protein, D-dimer, lactate dehydrogenase, or ferritin) | All-cause mortality through days 28 and 60, and number of ventilator-free days, duration of hospitalization, and time to recovery through day 28 |
| RECOVERY | UK | Randomized, controlled, open-label, platform trial. | February 2, to December 29, 2021 | 180 days | Clinically suspected or laboratory confirmed SARS-CoV-2 infection in patients > 2 years | 28-day mortality |
Risk of Bias Assessment
The risk of bias assessment for the included trials is presented in Supplementary Figure 1. All trials reported using random sequence generation and mentioned using allocation concealment. One trial had open label study design. The risk of performance and selection bias was high in this trial as participants and personnel were not blinded to the assigned treatment.17 Risk of attrition bias was deemed low in all trials.
Assessment of Outcomes
28-day Mortality
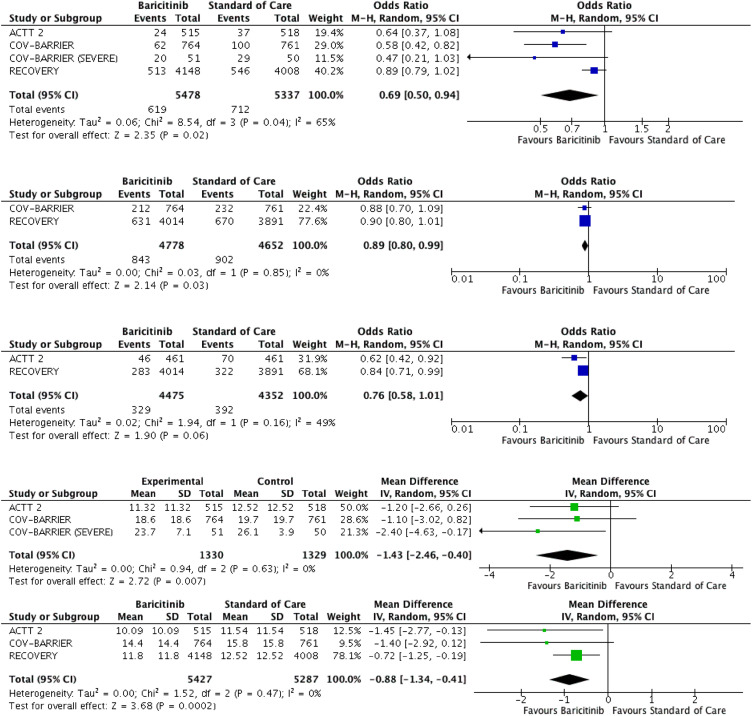
All four RCTs reported the outcome of 28-day mortality.9,16, 17, 18 A total of 619 deaths out of 5478 participants (11.30%) were reported in the baricitinib arm compared to 712 deaths out of 5337 participants (13.34%) in the standard care or placebo arm. Pooled analysis showed a reduction in 28-day mortality with baricitinib therapy than standard care or placebo (OR 0.69, 95% CI 0.50-0.94; p=0.04). Moderate heterogeneity was observed in the analysis (I2=65%) (Figure 2A).
Figure 2.
Forest plots for primary and secondary outcomes. A: 28-day mortality outcome B: Progression to respiratory failure needing positive pressure ventilation, IMV or death C: Progression to IMV or ECMO D: Duration of hospitalisation E: Time to recovery.
Progression to respiratory failure needing positive pressure ventilation (PPV), IMV or death
Two RCTs reported the composite outcome of progression to respiratory failure needing PPV or death.17,18 843 out of 4778 participants (17.64%) progressed to PPV or died in the baricitinib group compared to 902 out of 4652 participants (19.39%) in the other group. Statistical significance was observed with OR of 0.89 and 95% CI 0.80-0.99 with a p value = 0.03. Low heterogeneity was observed in the analysis (I2=0%) (Figure 2B).
Progression to invasive mechanical ventilation (IMV) or ECMO
Two RCTs reported the outcome of progression to IMV or ECMO.9,17 329 out of 4475 participants (7.35%) progressed to IMV or ECMO in the baricitinib group compared to 392 out of 4352 participants (9.01%) in the standard care or placebo group. (OR 0.76, 95% CI 0.58-1.01; p=0.06) Moderate heterogeneity was observed in the analysis (I2=49%) (Figure 2C).
Duration of Hospitalisation
Three RCTs reported outcome of duration of hospitalisation.9,16,18 Pooled analysis showed significantly improved duration of hospitalisation with baricitinib therapy than standard therapy or placebo (Mean difference -1.43, 95% CI -2.46, -0.40, p=0.007). Low heterogeneity was observed in the analysis (I2=0%). (Figure 2D).
Time to Recovery
Three RCTs reported outcome of time to recovery or discharge.9,17,18 Pooled analysis showed significantly improved outcome of time to recovery with baricitinib therapy than standard therapy or placebo (Mean difference -0.88, 95% CI -1.34, -0.41, p=0.0002). Low heterogeneity was observed in the analysis (I2=0%). (Figure 2E).
Sensitivity analysis and Publication Bias assessment
Sensitivity analysis of 28-day mortality after excluding COV-BARRIER (SEVERE) study demonstrated similar results. A total of 599 patients out of 5427 patients (11.04%) died in the baricitinib arm compared to 683 patients out of 5287 patients (12.92%) in the standard care arm. Pooled analysis showed a reduction in 28-day mortality with baricitinib therapy compared to standard care (OR 0.72, 95% C.I. 0.52-1.00, p=0.05). (Supplementary Figure 2). Sensitivity analysis using a fixed-effect model yielded disparate results. Statistical significance was achieved with outcomes of 28-day mortality (OR 0.82, 95% CI 0.73-0.92; p=0.001), progression to respiratory failure needing PPV, IMV or death (OR 0.89, 95% CI 0.80-0.99; p=0.03) and progression to IMV or ECMO (OR 0.80, 95% CI 0.69-0.94; p=0.005) (Supplementary Table 2).
Pooled analysis of standardised mean differences for outcome of duration of hospitalisation was not statistically significant for patients treated with baricitinib compared to usual care (MD -0.10, 95% C.I. -02, 0.01, p=0.06). However, standardised mean differences for outcome of time to recovery was statistically significant for patients treated with baricitinib compared to usual care (MD -0.07, 95% C.I. -0.11, -0.03, p=0.0002). Quantitative analysis of publication bias and subgroup analysis were not performed due to the limited number of studies included in the meta-analysis.
Discussion
This meta-analysis provides a comprehensive aggregate analysis of the available randomised trials to date on the efficacy of baricitinib therapy in hospitalised patients with COVID-19 infection. The results of this study showed favorable trend towards mortality benefit with baricitinib treatment in hypoxic patients with COVID-19. Baricitinib therapy was also associated with reduced progression to PPV, IMV or death. The results remained consistent supporting a reduced duration of hospitalisation and early hospital discharge than standard therapy in hospitalised patients with COVID-19.
Baricitinib, an IL-6 receptor antibody, is an inhibitor of JAK1/JAK2 enzymes and exhibits anti-viral activity in tolerable therapeutic dose ranges and clinically relevant serum concentrations.28 It does this by inhibiting upregulated INF-1 caused by the ACE-2 receptor, thereby blocking the cell entry of viruses. Therefore, it can block inhibit cell entry through clathrin-mediated endocytosis inhibition.29 In observational studies, patients with COVID-19 treated with baricitinib showed marked reduction in serum levels of cytokines and increased level of antibody against the SARS-CoV2 spike protein.30 A meta-analysis done by Lin et al. showed improved intensive care unit admission, requirement for invasive mechanical ventilation, and reduced mortality with baricitinib compared to usual care.31 Another systematic review done by Sampath et al. revealed reduced mortality risk and significant clinical improvements in hospitalised participants with moderate to severe COVID-19, particularly those requiring high flow oxygen or mechanical ventilation.32 Our results in this updated meta-analysis of randomised controlled trials are similar to the results published in the prior meta-analyses.
The hyperinflammatory phase in COVID-19 involves several cytokines. Baricitinib interrupts cytokines signaling pathway, including IL-2, IL-6, IL-10, INF-Y, and GM-CSF, resulting in reduction of downstream immune cell function.33 In the ACTT-2 trial, combination treatment with baricitinib and remdesivir did not reveal mortality benefit when compared to remdesivir treatment alone. With concomitant dexamethasone administration, there may be a synergistic effect on other inflammatory pathways additionally.9 In the COV-BARRIER trial, baricitinib plus standard of care showed a 38·2% relative reduction in 28-day mortality compared with placebo plus standard of care.18 In the RECOVERY trial, 12% of the patients allocated to baricitinib group died compared to 14% of allocated to usual care within 28 days. 23% of the patients received tocilizumab concurrently, and only ∼20% of the patients received remdesivir.17 Greater than 80% patients in the COV-BARRIER trials and > 90% of the patients in RECOVERY trial received corticosteroids/dexamethasone.17,18 Our meta-analysis is congruent with these results and showed mortality benefit with baricitinib when used in conjunction with dexamethasone and/or IL-6 inhibitors.
The primary outcome of the COV-BARRIER trial - progression to high-flow oxygen, non-invasive ventilation, invasive mechanical ventilation, or death by day 28, was not met.18 In the ACTT-2 trial, there was a higher likelihood of improved clinical status at day 15 in the combination group patients compared to those that received only remdesivir.9 In the RECOVERY trial, 7% of the patients in the baricitinib group progressed to IMV or ECMO compared to 8.3% of the patients in the usual care group.17 Our meta-analysis showed that patients treated with baricitinib had reduced progression to IMV or ECMO compared to standard care. Improvement in clinical status was most noticeable among patients with moderate to severe disease, that is in hospitalised patients on high flow oxygen or noninvasive ventilation (WHO group 6 and above).34 Baricitinib has a short half-life, acts on targeted pathways to reduce inflammation and biologic redundancy with less immunosuppression. The peak concentrations of baricitinib are rapidly achieved within half to one hour following administration. However, plasma concentrations decline rapidly following attainment of peak concentrations, with a mean terminal half-life of 5 to 7 hours.35 This may explain why approximately 22% of the COV-BARRIER participants progressed on the first day.18 However, steady state plasma concentrations of baricitinib are usually achieved after the second day of once daily dosing, with minimal accumulation in plasma.
The Infectious Diseases Society of America (IDSA) issued a moderate recommendation for the use of baricitinib along with remdesivir and corticosteroids in patients with severe or critical COVID-19 infection. They recommended against usage of baricitinib in addition to IL-6 receptor inhibitors such as tocilizumab due to lack of any clinical studies evaluating combination therapy.36 In January 2022, the World Health Organization (WHO) included a strong recommendation for using baricitinib as an alternative to an IL-6 receptor blocker, in combination with corticosteroids, in patients with severe or critical conditions COVID-19.37 In February 2022, the National Institutes of Health (NIH) updated its guidelines recommending the use of baricitinib for patients on dexamethasone who have rapidly increasing oxygen needs and systemic inflammation. The Panel recommended against the use of baricitinib in combination with IL-6 receptor inhibitors to treat COVID-19.38 In total, the results of our meta-analysis strengthen the evidence that baricitinib when used along with dexamethasone and/or IL-6 inhibitors, can reduce mortality, time to discharge, and progression to mechanical ventilation.
Our meta-analysis has certain limitations. Firstly, the number of patients in the RECOVERY trial was much higher than other RCTs.17 Secondly, there were differences in enrollment criteria, heterogeneity of clinical practice across different geographical regions, and differences in measuring clinical progression. There was also substantial heterogeneity in the statistical analysis. Thirdly, one of the included trials had an open-label design, implying a high risk of performance and selection bias due to the lack of blinding of participants and personnel to intervention.17 Lastly, as the pandemic has evolved, so has the use of concomitant therapy across trials. In the ACTT-2 trial, there was limited use of corticosteroids at baseline.9 In the RECOVERY trial, there was limited use of remdesivir at baseline.17 Other factors that may differ between the trials include the predominant circulating SARS-CoV-2 variant(s) and the prevalence of SARS-CoV-2 vaccination.
In conclusion, our meta-analysis suggests baricitinib, when used along with dexamethasone and/or anti-IL6 inhibitor, could be an effective therapeutic option with favorable evidence on reduced mortality, shortened duration of hospitalization, and early discharge from hospital. Future studies could assess the effect of baricitinib at higher doses or with a loading dose to prevent progression events. Head-to-head trials and platform trials comparing the efficacy of baricitinib to tocilizumab (in addition to use of baricitinib with and without use of other immunomodulators) could also be considered.
Contributors
VS, AF, and AL conceptualised the original study. VS and AL developed the methodology. VS and AL have verified the data. MSK performed the analysis. VS and AF wrote the original manuscript. KDA, AL and GC critically reviewed the manuscript. GC supervised the research. VS, AL, AF, MSK, KDA, and GC had full access to all the data in the study and accept responsibility to submit for publication.
Declaration of interests
We declare no competing interests.
Data sharing statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
Funding
None.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101489.
Appendix. Supplementary materials
References
- 1.Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodriguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Markham A. Baricitinib: First Global Approval. Drugs. 2017;77(6):697–704. doi: 10.1007/s40265-017-0723-3. [DOI] [PubMed] [Google Scholar]
- 3.Damsky W, Peterson D, Ramseier J, et al. The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J Allergy Clin Immunol. 2021;147(3):814–826. doi: 10.1016/j.jaci.2020.10.022. [DOI] [PubMed] [Google Scholar]
- 4.Gao Q, Liang X, Shaikh AS, Zang J, Xu W, JAK/STAT Zhang Y. Signal Transduction: Promising Attractive Targets for Immune, Inflammatory and Hematopoietic Diseases. Curr Drug Targets. 2018;19(5):487–500. doi: 10.2174/1389450117666161207163054. [DOI] [PubMed] [Google Scholar]
- 5.Stebbing J, Phelan A, Griffin I, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20(4):400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Zhang Y, Qiao W, Zhang J, Qi Z. Baricitinib, a drug with potential effect to prevent SARS-COV-2 from entering target cells and control cytokine storm induced by COVID-19. Int Immunopharmacol. 2020;86 doi: 10.1016/j.intimp.2020.106749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stebbing J, Sanchez Nievas G, Falcone M, et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021;7(1) doi: 10.1126/sciadv.abe4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantini F, Niccoli L, Matarrese D, Nicastri E, Stobbione P, Goletti D. Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J Infect. 2020;81(2):318–356. doi: 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021;384(9):795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantini F, Niccoli L, Nannini C, et al. Beneficial impact of Baricitinib in COVID-19 moderate pneumonia; multicentre study. J Infect. 2020;81(4):647–679. doi: 10.1016/j.jinf.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Titanji BK, Farley MM, Mehta A, et al. Use of Baricitinib in Patients With Moderate to Severe Coronavirus Disease 2019. Clin Infect Dis. 2021;72(7):1247–1250. doi: 10.1093/cid/ciaa879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Y, Wei J, Zou L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146(1):137–146. doi: 10.1016/j.jaci.2020.05.019. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guimaraes PO, Quirk D, Furtado RH, et al. Tofacitinib in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med. 2021;385(5):406–415. doi: 10.1056/NEJMoa2101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murugesan H, Cs G, Nasreen HS, et al. An Evaluation of Efficacy and Safety of Tofacitinib, A JAK Inhibitor in the Management of Hospitalized Patients with Mild to Moderate COVID-19 - An Open-Label Randomized Controlled Study. J Assoc Physicians India. 2022;69(12):11–12. [PubMed] [Google Scholar]
- 15.Stanevich OV, Fomina DS, Bakulin IG, et al. Ruxolitinib versus dexamethasone in hospitalized adults with COVID-19: multicenter matched cohort study. BMC Infect Dis. 2021;21(1):1277. doi: 10.1186/s12879-021-06982-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ely EW, Ramanan AV, Kartman CE, et al. Baricitinib plus Standard of Care for Hospitalised Adults with COVID-19 on Invasive Mechanical Ventilation or Extracorporeal Membrane Oxygenation: Results of a Randomised. Placebo-Controlled Trial. 2021 doi: 10.1016/S2213-2600(22)00006-6. medRxiv 202110.11.21263897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Group RC, Horby PW, Emberson JR, et al. Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analysis. medRxiv2022: 2022.03.02.22271623. [DOI] [PMC free article] [PubMed]
- 18.Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9(12):1407–1418. doi: 10.1016/S2213-2600(21)00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.US Food & Drug Administration Letter of authorization: EUA for baricitinib (Olumiant) for treatment of coronavirus disease 2019 (COVID-19). https://www.fda.gov/media/143822/download. Accessed 25 March 2022.
- 20.WHO recommends life-saving interleukin-6 receptor blockers for COVID-19 and urges producers to join efforts to rapidly increase access. https://www.who.int/news/item/06-07-2021-who-recommends-life-saving-interleukin-6-receptor-blockers-for-covid-19-and-urges-producers-to-join-efforts-to-rapidly-increase-access. Accessed 25 March 2022.
- 21.Selvaraj V, Khan MS, Bavishi C, et al. Tocilizumab in Hospitalized Patients with COVID-19: A Meta Analysis of Randomized Controlled Trials. Lung. 2021;199(3):239–248. doi: 10.1007/s00408-021-00451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiavo JH. PROSPERO: An International Register of Systematic Review Protocols. Med Ref Serv Q. 2019;38(2):171–180. doi: 10.1080/02763869.2019.1588072. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JPTG. John Wiley & Sons Ltd; Chichester, England: 2011. S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. [updated March] [Google Scholar]
- 27.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 28.Frediansyah A, Tiwari R, Sharun K, Dhama K, Harapan H. Antivirals for COVID-19: A critical review. Clin Epidemiol Glob Health. 2021;9:90–98. doi: 10.1016/j.cegh.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 2020;181(5):1016–1035. doi: 10.1016/j.cell.2020.04.035. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bronte V, Ugel S, Tinazzi E, et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Invest. 2020;130(12):6409–6416. doi: 10.1172/JCI141772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Z, Niu J, Xu Y, Qin L, Ding J, Zhou L. Clinical efficacy and adverse events of baricitinib treatment for coronavirus disease-2019 (COVID-19): A systematic review and meta-analysis. J Med Virol. 2022;94(4):1523–1534. doi: 10.1002/jmv.27482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sampath A, Banerjee A, Atal S, Jhaj R. Use of Baricitinib in Treatment of COVID-19: A Systematic Review. medRxiv2021: 2021.12.26.21268434. [DOI] [PubMed]
- 33.Richardson P, Griffin I, Tucker C, et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30. doi: 10.1016/S0140-6736(20)30304-4. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. The Lancet Infectious Diseases. 2020;20(8):e192–e1e7. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao X, Sheng XY, Payne CD, Zhang X, Wang F, Cui YM. Pharmacokinetics, Safety, and Tolerability of Single- and Multiple-Dose Once-Daily Baricitinib in Healthy Chinese Subjects: A Randomized Placebo-Controlled Study. Clin Pharmacol Drug Dev. 2020;9(8):952–960. doi: 10.1002/cpdd.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Infectious Diseases Society of America (IDSA) guidelines on the Treatment and Management of Patients with COVID-19. https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/. Accessed 25 March 2022.
- 37.WHO . 2022. Therapeutics and COVID-19: living guideline. V9.2. 14 Jan. https://app.magicapp.org/#/guideline/nBkO1E/rec/E5AOaN. Accessed 25 March 2022. [PubMed] [Google Scholar]
- 38.NIH . 2022. Therapeutic Management of Hospitalized Adults With COVID-19. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults–therapeutic-management/. Accessed 25 March. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.