Abstract
An Mn2+ and Cd2+ uptake gene, mntA, was cloned from Lactobacillus plantarum ATCC 14917 into Escherichia coli. Its expression conferred on E. coli cells increased Cd2+ sensitivity as well as energy-dependent Cd2+ uptake activity. Both transcription and translation of mntA were induced by Mn2+ starvation in L. plantarum, as indicated by reverse transcriptase PCR and immunoblotting. Two Cd2+ uptake systems have been identified in L. plantarum: one is a high-affinity Mn2+ and Cd2+ uptake system that is expressed in Mn2+-starved cells, and the other is a nonsaturable Cd2+ uptake system that is expressed in Cd2+-sufficient cells (Z. Hao, H. R. Reiske, and D. B. Wilson, Appl. Environ. Microbiol. 65:592–99, 1999). MntA was not detected in an Mn2+-dependent mutant of L. plantarum which had lost high-affinity Mn2+ and Cd2+ uptake activity. The results suggest that mntA is the gene encoding the high-affinity Mn2+ and Cd2+ transporter. On the basis of its predicted amino acid sequence, MntA belongs to the family of P-type cation-translocating ATPases. The topology and potential Mn2+- and Cd2+-binding sites of MntA are discussed. A second clone containing a low-affinity Cd2+ transport system was also isolated.
Active uptake systems for metal ions, such as Mn2+ and Zn2+, exist in a variety of bacteria. Some of them have been shown to take up other metal ions, such as Cd2+. In Escherichia coli, Cd2+ enters the cells via a Zn2+ transport system (11). In gram-positive bacteria, such as Bacillus subtilis and Staphylococcus aureus, Cd2+ competes for transport with Mn2+ (6, 10, 28). Two Cd2+ uptake systems have been identified in Lactobacillus plantarum (7a). One is a high-affinity, high-velocity Mn2+ and Cd2+ uptake system which is induced by Mn2+ starvation. Cd2+ and Mn2+ are competitive inhibitors of each other for this system, and its affinity for Cd2+ is higher than that for Mn2+. The other is expressed in Mn2+-sufficient cells, and Cd2+ uptake by this system is nonsaturable. Of all the known bacterial Cd2+ uptake systems, the one in Mn2+-starved L. plantarum has the highest Cd2+ affinity, and it also has a high velocity. Two Mn2+-dependent mutants have been isolated from L. plantarum ATCC 8014. Their growth requirements for Mn2+ are more than 5,000 times higher than those of the parental strain. Mn2+ starvation-induced Cd2+ uptake in both mutants is less than 5% the wild-type rate (7a).
The best-studied Cd2+ transport system comprises a Cd2+ efflux ATPase present on a Cd2+ resistance plasmid of S. aureus in which two separate loci confer different levels of Cd2+ resistance. cadA confers high-level resistance to Cd2+, and cadB mediates low-level resistance (26). The mechanism of cadB function is not clear; it may confer resistance by enhancing the binding of Cd2+ to cells (16). The cadA Cd2+ resistance determinant was cloned and expressed in B. subtilis (19, 32). The DNA sequence contains two open reading frames (ORFs): cadC and cadA, cadA encodes a protein of 727 amino acids (19). A comparison of the predicted amino acid sequence of CadA with those in the protein databases showed that it belongs to the class of P-type cation-translocating ATPases (22, 23). This system expels Cd2+ and Zn2+ but not Mn2+ from cells and is induced by Cd2+, Zn2+, Co2+, Pb2+, and Bi2+ (31). It uses only ATP as an energy source (27).
So far, there has been no molecular characterization of genetic systems that mediate Mn2+ and Cd2+ uptake from any source. In this study, we report the cloning and characterization of a high-affinity Mn2+ and Cd2+ uptake gene from L. plantarum. The cloned gene, encoding an Mn2+ transporter (MntA), conferred on E. coli increased Cd2+ uptake activity, and the regulation of its expression in L. plantarum was the same as that for the high-affinity Mn2+ and Cd2+ uptake system of L. plantarum. On the basis of its predicted amino acid sequence, MntA belongs to the family of P-type cation-translocating ATPases.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The plasmids constructed in this study are described below. E. coli GI 724 and GI 698 (Invitrogen) were grown in RM medium (Invitrogen). Other E. coli strains—DH5α-IQ (Gibco BRL), JM109 (30), RB791 (4), and XL1-blue (5)—were grown in Luria-Bertani (LB) medium. Low-Mn2+ APT medium (1) was used for the growth of E. coli for measuring Cd2+ uptake or accumulation. L. plantarum ATCC 14917 or ATCC 8014 (American Type Culture Collection) was grown in APT complex medium (1) or MRS medium (Difco Laboratories). The Mn2+-dependent mutants of L. plantarum—mnd11-06 and mnd15-26—were grown in modified APT medium containing 100 mM Mn2+ (7a).
Cd2+ uptake and accumulation assays.
The Cd2+ uptake assay for L. plantarum was carried out as described elsewhere (7a).
To prepare E. coli cells for Cd2+ uptake, overnight cultures were inoculated by 100-fold dilution into fresh low-Mn2+ APT medium and grown to an optical density at 600 nm (OD600) of 0.5. To induce the cloned gene, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 1 mM, and the cells were harvested after 2 to 3 h of induction, when the OD600 of the culture reached 0.5 to 0.6. Control cells containing only the vector were induced and harvested at about the same cell density. Cells were harvested by centrifugation at 4°C, washed, resuspended to a final OD600 of 0.5 in fresh low-Mn2+ APT medium containing 25 μg of chloramphenicol per ml, and kept at 4°C for 30 min.
To measure Cd2+ uptake, cells were incubated in a shaking water bath (37°C) for 10 min, and a mixture of 109CdCl2 and nonradioactive CdCl2 was added. Duplicate 0.2-ml samples were removed, filtered through 0.45-μm-pore-diameter nitrocellulose filters (Millipore), rinsed twice with 4 ml of ice-cold low-Mn2+ APT medium, and placed in a vial for radioactivity counting with a Beckman LS-7500 scintillation counter. To compare uptake activities in different media, LB medium, MOPS medium (20 mM morpholinepropanesulfonic acid [MOPS] [pH 6.7], 56 mM KCl, 86 mM NaCl, 13.5 mM MgCl2, 55 mM glucose), or phosphate buffer (20 mM K2HPO4 or KH2PO4 [pH 6.7], 56 mM KCl, 86 mM NaCl, 13.5 mM MgCl2, 55 mM glucose) with or without citrate was used.
To measure relatively long-term Mn2+ or Cd2+ accumulation, cells were prepared as described above. After recovery at 37°C for 10 min, CdCl2 was added, and the cells were incubated in a shaking water bath (37°C) for 1 h. Cells were harvested at 4°C and washed three times with ice-cold low-Mn2+ APT medium by centrifugation. The cell pellets were lyophilized, and the dried cells were digested overnight in 70% nitric acid at 45°C. The digestion mixture was diluted sixfold with water, and the total Mn2+ or Cd2+ content of the cells was measured with a Perkin-Elmer model 2380 atomic absorption spectrophotometer.
Construction of a genomic DNA library.
To extract genomic DNA from L. plantarum ATCC 14917, 250 ml of cells was harvested by centrifugation at 7,000 × g for 20 min. The cell pellet was suspended in 20 ml of TEN buffer (10 mM Tris-HCl [pH 7.5], 1 mM EDTA, 10 mM NaCl), centrifuged at the same speed, resuspended in 1 ml of SET buffer (50 mM Tris-HCl [pH 7.5], 20% sucrose, 50 mM EDTA, 15 mg of lysozyme), and incubated at 37°C for 10 min. Then, 10 ml of TEN buffer and 1 ml of 25% sodium dodecyl sulfate (SDS) were added, and the solution was mixed thoroughly by inversion. After lysis, 2 ml of 5 M NaCl was added, followed by 20 ml of buffer-saturated phenol; the tube was gently inverted. The aqueous phase was separated by centrifugation at 3,500 × g for 20 min and transferred to a fresh tube. Residual protein was extracted with an equal volume of chloroform-isoamyl alcohol (24:1), and the separated aqueous phase was transferred to a fresh beaker. Genomic DNA was precipitated by the addition of 2 volumes of ethanol, spooled out of solution on a glass rod, dried in air, and resuspended in TE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA).
After treatment with RNase A, the DNA was partially digested with Sau3AI. The digested DNA was size fractionated on a sucrose density gradient (2). DNA fragments of 3 to 7 kb were ligated into the BamHI site of low-copy-number plasmid pCL1921 (12). The ligation mixture was used to electrotransform E. coli XL1-blue, with selection for spectinomycin resistance. The library was screened for clones containing Cd2+ uptake genes.
Screening of colonies containing Cd2+ uptake genes.
The primary screen, for Cd2+-hypersensitive colonies, was done by transferring transformants by use of toothpicks onto low-Mn2+ APT medium plates containing 250 μM Cd2+. Colonies which did not grow were retested for growth inhibition by Cd2+ in liquid medium containing different levels of Cd2+ to confirm their hypersensitivity to Cd2+. The hypersensitive clones were then tested for their ability to accumulate Cd2+ in low-Mn2+ APT medium containing 20 or 100 μM Cd2+. Clones which had both increased Cd2+ sensitivity and accumulation were then tested for Cd2+ uptake activity with 109CdCl2.
To confirm that the increased sensitivity, accumulation, and uptake were due to the effects of recombinant plasmids, plasmid DNA was extracted from the recombinant strains, and the presence of an insertion was confirmed by restriction enzyme digestion. These plasmids were used to retransform XL1-blue, and the transformants were tested again for the above properties.
Identification of functional ORFs.
Plasmid DNA from each clone which showed increased Cd2+ sensitivity, accumulation, and uptake was isolated, and the DNA sequence of each inserted fragment was determined. For each possible ORF, a partial or total deletion was made and the activities of the remaining ORFs were tested to identify functional ORFs.
mRNA detection by RT-PCR.
Total RNA was isolated from 1 ml of Mn2+-starved or Mn2+-sufficient L. plantarum ATCC 14917 cells at an OD600 of 1 by use of a Qiagen RNeasy Mini Kit. Lysozyme (20 mg/ml) and mutanolysin (1 mg/ml) were used to digest the cell wall of Mn2+-sufficient and Mn2+-starved cells, respectively, at 37°C for 10 min. The total RNA obtained was treated with RNase-free DNase I as described by Dilworth and McCarey (7). To amplify a 1.1-kb fragment of mntA, reverse transcription (RT) was carried out with a specific antisense primer (2.5 μM) and Moloney murine leukemia virus reverse transcriptase (5 U) in a 100-μl reaction mixture, and PCR was performed with the RT mixture as a template and primers 5′ TTGATGCGAAGGCTTTAGTTGTGG and 5′ GCGAGTGCGTTTTAAAGGTCTGGT as described by Innis et al. (9).
Antibody production and immunoblotting.
The Invitrogen ThioFusion Expression System was used to overexpress MntA fragments as thioredoxin C-terminal fusion proteins. The DNA fragment encoding either the N-terminal 103 or the C-terminal 137 amino acids of MntA was amplified by PCR and cloned into plasmid pTrxFus between the BamHI and PstI sites, producing pTrx3-5N or pTrx3-5C, respectively, to generate an in-frame fusion with thioredoxin. The expression of the fusion protein was induced by tryptophan. The fusion protein was separated from total cell proteins by SDS-polyacrylamide gel electrophoresis (PAGE). Washed and homogenized gel slices containing the fusion protein were used to obtain polyclonal rabbit antibodies as described previously (8). The presence of antibodies to the MntA protein was verified by use of broken cells of L. plantarum. Proteins were separated by SDS-PAGE and electrophoretically transferred to nitrocellulose membranes. Goat anti-rabbit immunoglobulin G-alkaline phosphatase conjugate (Bio-Rad) was used as the second antibody and detected as described by the supplier.
Cell membrane preparation.
E. coli cells were grown and induced as described above with LB medium. Cell membrane extracts were prepared as described by Wulff-Strobel and Wilson (29). Mn2+-starved or Mn2+-sufficient cells of L. plantarum strains were prepared as described elsewhere (7a) and harvested when the OD600 of the culture (100 ml) was about 0.5. The cells were washed twice with 10 mM MOPS-KOH (pH 7.0) and resuspended in sucrose buffer (50 mM MOPS-KOH [pH 7.0], 250 mM sucrose, 200 mM KCl, 10 mM MgSO4) to 1/50 the original culture volume. Lysozyme (20 mg/ml) or mutanolysin (2 mg/ml) was added to digest the cell wall of Mn2+-sufficient or Mn2+-starved cells, respectively. After incubation at 37°C for 15 min, the cells were diluted to 1/10 the original culture volume in sucrose buffer and lysed by passage through a French pressure cell at 18,000 lb/in2; cell debris and unbroken cells were removed by centrifugation at 12,000 × g. Membranes were pelleted by centrifugation of the low-speed supernatant at 160,000 × g for 90 min. The pellets were suspended in 0.2 ml of 100 mM MOPS-KOH (pH 7.0). The protein concentrations of cell membrane extracts were determined by the assay of Lowry et al. (13). Proteins were separated by SDS-PAGE, and MntA protein was identified by Western blotting as described above.
Computer analysis.
Analysis of DNA and protein sequences was performed on a computer with Lasergene Sequence Analysis Software (DNASTAR Inc.). The National Center for Biotechnology Information sequence similarity search tool BLAST was used for sequence similarity searches. The DAS server and TMpred server were used for the prediction of transmembrane segments. The ScanProsite program was used to scan protein sequences for the occurrence of patterns stored in the PROSITE database.
Nucleotide sequence accession number.
The nucleotide sequence of the mntA gene cloned from L. plantarum ATCC 14917 has been assigned GenBank accession no. AF136521.
RESULTS
Cloning and screening of Cd2+ and Mn2+ uptake genes.
We have isolated two Cd2+ and Mn2+ uptake mutants of L. plantarum (7a). However, transformation of the mutant strains has not been successful, despite many attempts. Therefore, E. coli was used for cloning of the Cd2+ and Mn2+ uptake genes from L. plantarum. Cd2+ uptake in E. coli XL1-blue was characterized to establish conditions which minimized interference from the background uptake of the host strain during screening for cloned genes.
Another study of ours (7a) indicated that citrate only weakly inhibits Cd2+ uptake in L. plantarum. To study whether citrate can reduce background E. coli Cd2+ uptake, the effect of citrate on E. coli XL1-blue Cd2+ uptake was tested. Citrate at 5 mM inhibited 80% of the uptake of 0.1 μM Cd2+ in MOPS buffer or LB broth, and the Cd2+ uptake rate was lowest in low-Mn2+ APT complex medium, which contains 17 mM citrate. Low-Mn2+ APT medium has been used for cultivation and for Cd2+ uptake assays with L. plantarum (1, 7a). In this study, it was used for screening of clones expressing Cd2+ uptake genes because of its strong inhibition of background E. coli Cd2+ uptake.
Table 1 shows a comparison of Cd2+ uptake in APT medium for E. coli and L. plantarum. In this medium, the rates in E. coli were only 0.2 to 2% those obtained with the high-affinity Cd2+ uptake system in L. plantarum when the Cd2+ concentration was in the range of 0.02 to 200 μM. Even the rates obtained with the low-affinity Cd2+ uptake system in L. plantarum were about 10 times those in E. coli when the Cd2+ concentration was above 2 μM. These data indicated that in APT medium, Cd2+ uptake in E. coli was inhibited to a low enough level to prevent interference with the screening of cloned Cd2+ uptake genes.
TABLE 1.
Initial rates of cadmium uptake by E. coli and L. plantarum in APT mediuma
| Cd2+ (μM) | Cd2+ uptake (nmol g of dry cell−1 min−1) by:
|
||
|---|---|---|---|
|
L. plantarum ATCC 14917
|
E. coli XL1-blue | ||
| Mn2+ starved | Mn2+ sufficient | ||
| 0.02 | 73 ± 6 | 0.5 ± 0.1 | 0.3 ± 0.1 |
| 0.2 | 802 ± 42 | 6.1 ± 0.3 | 1.8 ± 0.2 |
| 2 | 2,821 ± 112 | 52 ± 3 | 5.8 ± 0.52 |
| 20 | 3,563 ± 308 | 258 ± 16 | 23 ± 1 |
| 100 | 3,214 ± 382 | ND | 41 ± 10 |
| 200 | 3,082 ± 203 | 520 ± 42 | 45 ± 7 |
Cells were prepared and preincubated for 10 min at 37°C in low-Mn2+ APT medium containing different amounts of Cd2+ as described in the text. Initial uptake values were determined with 0.2-ml samples filtered 15 s after radioactive Cd2+ was added. Values are means ± ranges from replicate experiments. ND, not determined.
After construction of an L. plantarum ATCC 14917 genomic DNA library in E. coli XL1-blue, about 10,000 colonies from the library were screened for Cd2+ hypersensitivity on APT medium plates containing 250 μM Cd2+. Those which did not grow were further tested in liquid APT medium to confirm their hypersensitivity. Sixty-four clones were found to be at least twice as sensitive as the control. When tested for Cd2+ accumulation, 18 of them appeared to accumulate at least 25% more Cd2+ than the control at both 20 and 100 μM Cd2+. These clones were further assayed for Cd2+ uptake with 109CdCl2. Only two clones, 3-5 and 2-29, which contained recombinant plasmids pCL3-5 and pCL2-29, respectively, showed increased Cd2+ uptake activity over the E. coli host strain background activity.
Identification of the functional ORF, subcloning, and sequencing.
The inserted DNA fragment in pCL3-5 was sequenced, and it contains 2,122 bp (data not shown). There is one ORF starting at bp 259 and running to the end of the cloned fragment without a stop codon. To clone the complete gene, an SmaI fragment from the cloned fragment was labeled and used as a probe to hybridize to 7- to 8-kb HindIII fragments from a total digest of L. plantarum genomic DNA. DNA fragments of about this size were cloned into plasmid pCL1921, and clones that were positive after colony hybridization were picked. A recombinant plasmid containing a 7.8-kb inserted fragment with the ATPase gene in the same direction as the lac promoter (pCL3-5L) was subcloned by KpnI digestion, followed by MscI/EcoRI and ClaI/KpnI double digestion. The resulting plasmid has an insertion of 2,996 bp containing the entire coding region. The deduced polypeptide contains 758 amino acids starting from the first ATG. This plasmid was designated pZH3-5 and was used for further study. The functional ORF was tentatively named mntA, assuming that it encodes an Mn2+ transporter (MntA). The amino acid sequence predicted from the coding region was checked against protein databases for related proteins. MntA showed significant matches (20 to 30% identity) with a family of P-type cation-translocating ATPases from both bacterial and eukaryotic sources (data not shown).
The upstream DNA sequence encodes a protein significantly homologous to bacterial as well as eukaryotic enolases. There is 68 bp between the two ORFs, and no inverted or direct repeats of 10 bp or longer or any apparent transcription termination or initiation signals are present in this region; these data suggest that the MntA ORF and the enolase ORF may be in the same operon. Removal of an enolase-encoding DNA fragment by PCR did not affect Cd2+ uptake activity, while removal of the fragment encoding the C-terminal 358 amino acids of MntA caused a complete loss of Cd2+ uptake activity (data not shown); these data indicate that mntA is the functional gene.
The same methods were used to screen for the ORF responsible for Cd2+ uptake activity in pCL2-29 and to clone its complete gene, tentatively designated cdtB. CdtB shares no overall homology with any sequence in the databases (data not shown). Characterization of the cdtB gene will be detailed in another paper.
Cd2+ uptake by MntA.
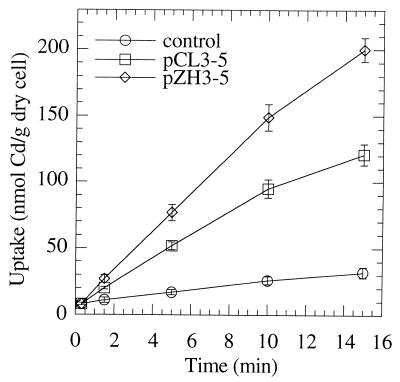
Figure 1 shows a comparison of Cd2+ uptake by both the complete and the partial MntA proteins. Complete MntA (758 amino acids) appeared to have a higher Cd2+ uptake activity than truncated MntA (621 amino acids), indicating that the C-terminal 137 amino acids were needed for the full activity of this ATPase in E. coli. Cd2+ uptake by MntA at various Cd2+ concentrations is summarized in Table 2 and compared with uptake by a control. At the lowest concentration used in this study (0.02 μM), the rate of mntA strain Cd2+ uptake was more than eightfold above the background rate. The difference between the control and the mntA strain decreased with increasing Cd2+ concentration, indicating a high-affinity uptake system. In contrast, the difference in Cd2+ uptake between a strain expressing cdtB and a control increased with increasing Cd2+ concentration (data not shown). These results are consistent with those of the Cd2+ sensitivity assays, as clone 3-5 showed a higher Cd2+ sensitivity than clone 2-29.
FIG. 1.
Cd2+ uptake by E. coli cells expressing MntA. Cells containing pCL1921 (control), pCL3-5, or pZH3-5 were prepared and preincubated for 10 min at 37°C in ATP medium containing 0.2 μM Cd2+. Samples (0.2 ml) were harvested at intervals after 0.1 μM radioactive Cd2+ was added. Each point represents the mean ± ranges (n = 2). pCL1921 is a cloning vector; pCL3-5 is pCL1921 containing part of the mntA gene, which encodes 621 amino acids; pZH3-5 is pCL1921 containing the entire mntA gene, which encodes 759 amino acids.
TABLE 2.
Initial rates of Cd2+ uptake by mntA and control E. coli strainsa
| Cd2+ (μM) | Cd2+ uptake (nmol g of dry cell−1 min−1) by the following strain:
|
|
|---|---|---|
| Control (pCL1921) | mntA (pZH3-5) | |
| 0.02 | 0.4 ± 0.1 | 3.4 ± 0.2 |
| 0.2 | 2.0 ± 0.1 | 14 ± 1 |
| 2 | 5.5 ± 0.4 | 26 ± 3 |
| 20 | 26 ± 1 | 61 ± 2 |
The assay was performed with ATP medium containing the indicated concentrations of Cd2+ as described in the legend to Fig. 1. Values are means ± ranges from replicate experiments.
Energy dependency and cation specificity of Cd2+ uptake by MntA.
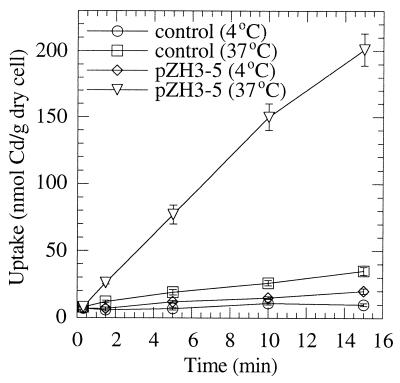
It has been shown that Cd2+ uptake by L. plantarum requires energy (7a). The effect of low temperature or the presence of CCCP on Cd2+ uptake by MntA was investigated in this study. As shown in Fig. 2, Cd2+ uptake activity by cells expressing MntA was inhibited over 90% when the temperature was decreased from 37 to 4°C. The same effect was observed when cells were preincubated with 100 μM carbonyl cyanide p-trifluoromethoxyphenylhydrazone at 37°C for 10 min before the addition of 109Cd2+ (data not shown). These results suggest that Cd2+ uptake by MntA in E. coli is an energy-dependent process.
FIG. 2.
Temperature dependence of Cd2+ uptake by MntA in E. coli. The assay was performed as described in the legend to Fig. 1 with ATP medium containing 0.2 μM Cd2+, except that the cells were incubated at both 37 and 4°C during the assay to study the effect of temperature on uptake activity. Each point represents the mean ± ranges (n = 2).
The cation specificity of Cd2+ uptake by MntA was investigated by measuring the effect of Zn2+ or Mn2+ on Cd2+ uptake by induced E. coli cells containing pZH3-5 or pCL1921. In the presence of a 200-fold excess of Zn2+ at 0.1 μM Cd2+, no significant inhibition of Cd2+ uptake by cells expressing MntA was observed, while Zn2+ inhibited Cd2+ uptake by control cells by about 65 to 75% (data not shown). Mn2+ did not affect Cd2+ uptake by control cells (11); however, it inhibited Cd2+ uptake by cells expressing MntA by about 30%. These results confirmed that the increased Cd2+ uptake activity resulted from the expression of the cloned Cd2+ uptake system, MntA, rather than the intrinsic Zn2+ and Cd2+ uptake system of E. coli.
Effect of Mn2+ starvation on mntA mRNA synthesis in L. plantarum.
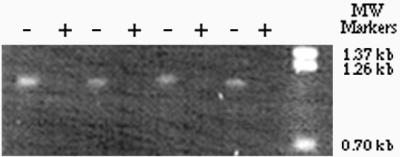
To study whether the transcription of mntA in L. plantarum is induced by Mn2+ starvation, total RNA was extracted from Mn2+-starved and Mn2+-sufficient cells of L. plantarum ATCC 14917. After treatment with RNase-free DNase, the RNA was reverse transcribed with a specific antisense primer designed from the DNA sequence of mntA. The product of RT was then used as a template for PCR amplification of a 1.1-kb mntA fragment. As shown in Fig. 3, only RNA from starved cells produced PCR products. In a control experiment, no PCR products could be seen on the gel when reverse transcriptase was omitted and Taq DNA polymerase (which has very low intrinsic reverse transcriptase activity) was used for PCR (data not shown). These results show that the PCR products were truly derived from mntA mRNA and that Mn2+ starvation induced the synthesis of mntA mRNA in L. plantarum, consistent with the induction by Mn2+ starvation of a high-affinity Cd2+ and Mn2+ uptake system.
FIG. 3.
Induction of the synthesis of mntA mRNA by Mn2+ starvation in L. plantarum ATCC 14917. Total RNA was isolated from L. plantarum ATCC 14917 Mn2+-starved (−) or Mn2+-sufficient (+) cells, treated with DNase I, and reverse transcribed with a specific antisense primer. The RT reaction mixture was used as a template to perform PCR to amplify a 1.1-kb fragment of mntA as described in the text. Each lane represents a separate experiment with four independent RNA preparations. MW, molecular weight.
Identification of MntA in wild-type L. plantarum and mutants.
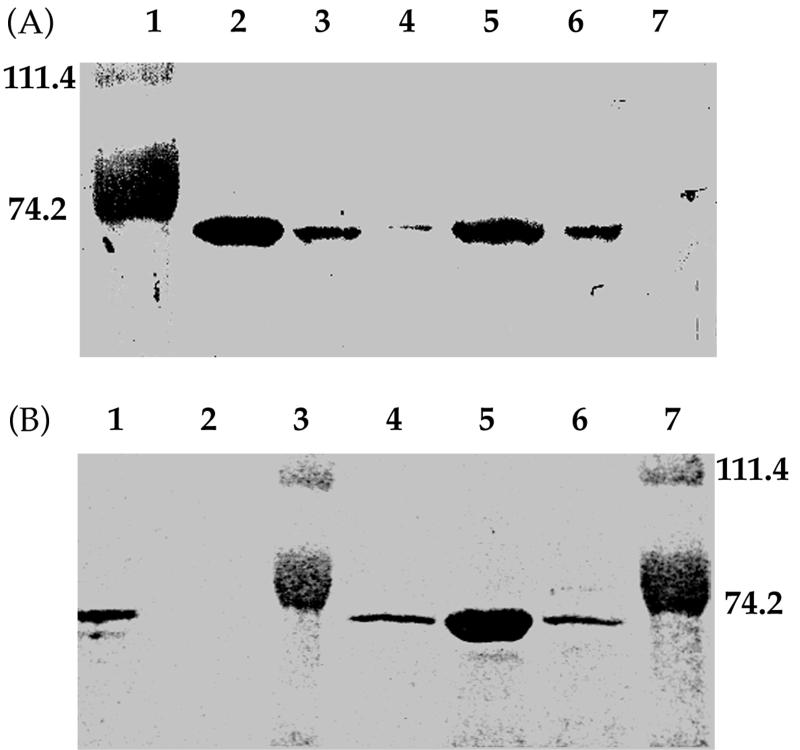
To identify MntA expression, crude cell extracts or total membrane proteins of two wild-type L. plantarum strains (ATCC 14917 and ATCC 8014) as well as two Mn2+-dependent mutants (mnd15-26 and mnd11-06) were prepared, separated by SDS-PAGE, and immunoblotted with an MntA antibody. As shown in Fig. 4A, immunoblotting of either total or membrane protein from Mn2+-starved wild-type L. plantarum strains resulted in a unique band with an apparent molecular mass of approximately 70 kDa, close to the predicted molecular mass of MntA (81 kDa). When the same amount of total protein was loaded, this band was not observed with Mn2+-sufficient cells of L. plantarum ATCC 14917, and only a low level was detected with Mn2+-sufficient ATCC 8014 cells. These results further demonstrate that Mn2+ starvation induced the expression of MntA in L. plantarum. The protein was present in the membrane fraction of both wild-type L. plantarum strains, as expected for a transmembrane protein (Fig. 4A). Immunoblot analysis also confirmed the expression of MntA in IPTG-induced E. coli cells containing the cloned mntA gene (data not shown). Again, the MntA band was detected only in the membrane fraction.
FIG. 4.
Identification of MntA in wild-type L. plantarum strains (A) and Mn2+-dependent mutants (B). Western blotting was done with an MntA antibody and protein extracts from L. plantarum strains and Cd2+ uptake mutants. (A) Lane 1, molecular mass standards (in kilodaltons); lanes 2 and 5, membrane protein from Mn2+-starved cells of ATCC 8014 and ATCC 14917, respectively; lanes 3 and 6, total protein from Mn2+-starved cells of ATCC 8014 and ATCC 14917, respectively; lanes 4 and 7, total protein from Mn2+-sufficient cells of ATCC 8014 and ATCC 14917, respectively. (B) Lanes 1 and 6, total protein from Mn2+-starved cells of ATCC 8014 and ATCC 14917, respectively; lanes 2 and 4, total protein from Mn2+-starved cells of mnd15-26 and mnd11-06, respectively; lanes 3 and 7, molecular weight standards; lane 5, membrane protein from Mn2+-starved cells of ATCC 8014.
The antibody was also used to look for the presence of MntA in the two Mn2+-dependent mutants of strain ATCC 8014, mnd11-06 and mnd15-26 (7a). These two mutants had lost high-affinity Mn2+ and Cd2+ uptake activity and required a high level of Mn2+ to grow (7a). As shown in Fig. 4B, while the unique MntA band was still observed with Mn2+-starved cells of mnd11-06, no band was detected with Mn2+-starved cells of mnd15-26. These results suggest that mnd11-06 produced nonfunctional MntA which still reacted with the antibody, while in mnd15-26, either mutant MntA was readily degraded or no MntA was produced.
In another experiment, membrane proteins from L. plantarum Mn2+-starved or Mn2+-sufficient cells were prepared and separated by SDS-PAGE, and the two intense Mn2+ starvation-induced bands were excised from the gel and subjected to Edman N-terminal sequence analysis. When compared with sequences in databases, the first 20 amino acids of these two proteins showed 85 to 95% identity with the N-terminal sequences of two glycolytic enzymes, enolase and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Given the facts that the DNA sequence upstream of mntA encodes an enolase and that the expression of both the enolase gene and mntA was induced by Mn2+ starvation of cells, it is possible that mntA and the enolase gene belong to the same Mn2+-regulated operon.
DISCUSSION
Bacterial cells accumulate inorganic cations and anions by specific membrane transport systems, each of which consists of one or a few proteins. Frequently, there are several transport systems for an ion: a constitutively synthesized system for times of nutrient abundance and an inducible, highly specific system for times of nutrient starvation. Regulation of ion transport occurs at the level of physiological function as well as at the level of synthesis of the proteins. Two Cd2+ uptake systems have been identified in L. plantarum. One is a high-affinity, high-velocity Mn2+ and Cd2+ transport system. Its expression is induced by Mn2+ starvation. The other is a low-affinity Cd2+ uptake system which is constitutively expressed in L. plantarum and whose function is not clear. We cloned two Cd2+ uptake genes from L. plantarum, mntA and cdtB. MntA appears to function as a high-affinity Mn2+ and Cd2+ uptake system, and its expression is induced by Mn2+ starvation of L. plantarum cells, while CdtB appears to function as a low-affinity Cd2+ uptake system.
Transport systems operating under conditions of starvation or stress are frequently coupled to ATP (25). There are two families, P-type (or E1-E2) ATPases and ATP-binding-cassette transporter complexes. The P-type ATPases are always cation-translocating membrane enzymes and include transporters for H+, Ca2+, Na+, K+, Mg2+, and Cd2+ (25) and for Cu2+ (20). Sequence analysis indicated that MntA falls into the family of P-type ATPases. mntA is the first Mn2+ and Cd2+ uptake gene that has been cloned and sequenced.
P-type ATPases are primarily cytoplasmic globules formed by at least four interactive domains, each of which is connected by a narrow stalk to a hydrophobic transmembrane segment (15). The transmembrane hairpins are postulated to form a channel through which cations are transported (3, 15). Figure 5 shows the model of MntA predicted by the TMpred program (European Molecular Biology network).
FIG. 5.
Model for the secondary structure of MntA. Each cylinder represents a transmembrane helix. The numbers above each cylinder refer to the location of the helix in the primary structure of MntA.
MntA shares significant homology with other P-type ATPases in the “core structure” regions, which are thought to be directly involved in the translocation of cations coupled to ATP hydrolysis (14). Figure 6 shows a comparison of the relatively conserved sections of MntA with the corresponding regions of other P-type ATPases. The first conserved region is the transduction domain, which is involved in the translocation of cations. The transduction domain in MntA consists of about 120 residues and is highly hydrophilic. It is separated by the first transmembrane segment from the hydrophilic N-terminal region. The homology in this region covers a stretch of about 50 residues, with several conserved aspartate, glutamate, and glycine residues (Fig. 6). Following the transduction domain is a transmembrane hairpin, with the second half of the hairpin containing a proline residue (Pro-249). In all P-type ATPases, this proline is located 43 residues before the aspartate (Asp-292 in MntA) that is phosphorylated in P-type ATPases. Asp-292 in MntA is the first of a string of 7 amino acids (Asp-Lys-Thr-Gly-Thr-Leu or Ile-Thr) that are conserved in all P-type ATPases and are flanked by conservative replacements (Fig. 6). From a comparison with other P-type ATPases, Asp-292 in MntA is the residue which undergoes phosphorylation (14). The next and most extended region of homology between MntA and other P-type ATPases starts at about residue 480 and continues for about 60 residues. This region is believed to include the end of the nucleotide-binding domain (3). In all of the P-type ATPases, the phosphorylation and ATP-binding regions constitute a single intracellular domain of 250 to 400 amino acids. After three other putative transmembrane hairpins, MntA ends at His-758.
FIG. 6.
Conserved sequences in MntA and other bacterial and eukaryotic P-type ATPases. Three putative functional domains of P-type ATPases are aligned. Identical residues in all sequences are boxed. Sequence accession numbers: Rat Ca-ATPase (P18596), S. aureus Cd-ATPase (J04551), Enterococcus faecalis Cu-ATPase (P32113), Heterosigma akashiwo H-ATPase (S53302), Methanococcus jannaschii H-ATPase (A64453), E. coli KdpB (K02670), Salmonella typhimurium Mg-ATPase (P36640), human Na,K-ATPase (P050123).
Unlike CadA and MerP, MntA does not contain the paired cysteine motif (CXXC) that is hypothesized to play a role in the initial binding of Cd2+ or Hg2+. Possible metal-binding sites in MntA include (i) the N-terminal domain, which is rich in negatively charged residues (E3, D4, and E7; D17, D21, and E24; E39 and E46; and E52 and E57); (ii) HADMIQM (residues 230 to 236), which contains histidine, aspartate, and the putative heavy-metal-binding motif (MXXM); and (iii) HRPEQWDM (residues 627 to 634), which is located in a hydrophilic region of MntA. A transmembrane helix at residues 702 to 718 may be involved in cation translocation and is in the same location as the cation translocation element identified in CadA, a Cd2+ efflux ATPase (24). Their alignment is as follows: MntA 708 GLNCLLTIGLASS 720 : : : : : CadA 370 GCPCALVISTPIS 382
Upstream of mntA is an ORF encoding a polypeptide significantly homologous to bacterial and eukaryotic enolases, a multifunctional, manganese-containing enzyme. The expression of MntA, enolase, and another glycolytic enzyme, GAPDH, appears to be induced by Mn2+ starvation. In both eukaryotes and prokaryotes, enolase and GAPDH are found to be membrane associated and to be involved in a variety of functions in addition to their catalytic function, such as activities related to plasmin and transferrin (17, 18, 21). Enolase and GAPDH may be involved in Mn2+ transport and/or homeostasis in L. plantarum. Further work is needed to identify and characterize the Mn2+ starvation-induced operon and the proteins encoded by this operon.
ACKNOWLEDGMENTS
We thank Peter C. Hinkle and John D. Helmann for critical reading of the manuscript.
This work was supported by a grant from the Cornell Superfund Basic Research and Education Program of the National Institute of Environmental Health Sciences.
REFERENCES
- 1.Archibald F S, Duong M. Manganese acquisition by Lactobacillus plantarum. J Bacteriol. 1984;158:1–8. doi: 10.1128/jb.158.1.1-8.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates and Wiley Interscience; 1987. [Google Scholar]
- 3.Brandl C J, Green N M, Korczak B, MacLennan D H. Two Ca2+ ATPase genes: homologies and mechanistic implications of deduced amino acid sequences. Cell. 1986;44:597–608. doi: 10.1016/0092-8674(86)90269-2. [DOI] [PubMed] [Google Scholar]
- 4.Brent R, Ptashne M. The luxA gene product represses its own promoter. Proc Natl Acad Sci USA. 1980;77:1932–1936. doi: 10.1073/pnas.77.4.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullock W O, Fernandez J M, Short J M. XL1-blue: a high efficiency plasmid transforming recA Escherichia coli strain with beta-galactosidase selection. BioTechniques. 1987;5:376. [Google Scholar]
- 6.Burke B E, Pfister R M. Cadmium transport by a Cd2+-sensitive and a Cd2+-resistant strain of Bacillus subtilis. Can J Microbiol. 1986;32:539–542. doi: 10.1139/m86-100. [DOI] [PubMed] [Google Scholar]
- 7.Dilworth D D, McCarey J R. Single-step elimination of contaminating DNA prior to reverse transcriptase PCR. PCR Methods Applications. 1992;1:279–281. doi: 10.1101/gr.1.4.279. [DOI] [PubMed] [Google Scholar]
- 7a.Hao Z, Reiske H R, Wilson D B. Characterization of cadmium uptake in Lactobacillus plantarum and isolation of cadmium and manganese uptake mutants. Appl Environ Microbiol. 1999;65:592–99. doi: 10.1128/aem.65.11.4741-4745.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 9.Innis M A, Gelfand D H, Sninsky J J, White T J. PCR protocols: a guide to methods and applications. San Diego, Calif: Academic Press, Inc.; 1990. [Google Scholar]
- 10.Laddaga R A, Bessen R, Silver S. Cadmium-resistant mutant of Bacillus subtilis 168 with reduced cadmium transport. J Bacteriol. 1985;162:1106–1110. doi: 10.1128/jb.162.3.1106-1110.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laddaga R A, Silver S. Cadmium uptake in Escherichia coli K-12. J Bacteriol. 1985;162:1100–1105. doi: 10.1128/jb.162.3.1100-1105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lerner C G, Inouye M. Low copy number plasmids for regulated low-level expression of cloned genes in Escherichia coli with blue/white insert screening capacity. Nucleic Acids Res. 1990;18:4631. doi: 10.1093/nar/18.15.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 14.Lustsenko S, Kaplan J. Organization of P-type ATPases: significance of structural diversity. Biochemistry. 1995;34:15607–15613. doi: 10.1021/bi00048a001. [DOI] [PubMed] [Google Scholar]
- 15.MacLennan D H, Brandl C J, Korczak B, Green N M. Amino-acid sequence of a Ca2++Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985;316:696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- 16.Mobley H L T, Summers A O. Plasmid-encoded ion transport systems. In: Rosen B P, Silver S, editors. Ion transport in prokaryotes. San Diego, Calif: Academic Press, Inc.; 1987. pp. 305–326. [Google Scholar]
- 17.Modun B, Williams P. The staphylococcal transferrin-binding protein is a cell wall glyceraldehyde-3-phosphate dehydrogenase. Infect Immun. 1999;67:1086–1092. doi: 10.1128/iai.67.3.1086-1092.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakajima K, Hamanoue M, Takemoto N, Kato K, Kohsaka S. Plasminogen binds specifically to alpha-enolase on rat neuronal plasma membrane. J Neurochem. 1994;63:2048–2057. doi: 10.1046/j.1471-4159.1994.63062048.x. [DOI] [PubMed] [Google Scholar]
- 19.Nucifora G, Chu L, Misra T K, Silver S. Cadmium resistance from Staphylococcus aureus plasmid pI258 cadA gene results from a cadmium efflux ATPase. Proc Natl Acad Sci USA. 1989;86:3544–3548. doi: 10.1073/pnas.86.10.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odermatt A, Suter H, Krapf R, Solioz M. Primary structure of two P-type ATPases involved in copper homeostasis in Enterococcus hirae. J Biol Chem. 1993;268:12775–12779. [PubMed] [Google Scholar]
- 21.Pancholi V, Fischetti V A. A major surface protein on group A streptococci is a glycoaldehyde-3-phosphate-dehydrogenase with multiple binding activity. J Exp Med. 1992;176:415–426. doi: 10.1084/jem.176.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silver S, Laddaga R A. Molecular genetics of heavy metal resistance systems of Staphylococcus plasmids. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH Publishers; 1990. pp. 531–549. [Google Scholar]
- 23.Silver S, Nucifora G, Chu L, Misra T K. Bacterial-resistance ATPases: primary pumps for exporting toxic cations and anions. Trends Biochem Sci. 1989;14:76–80. doi: 10.1016/0968-0004(89)90048-0. [DOI] [PubMed] [Google Scholar]
- 24.Silver S, Phung L T. Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol. 1996;50:753–789. doi: 10.1146/annurev.micro.50.1.753. [DOI] [PubMed] [Google Scholar]
- 25.Silver S, Walderhaug M. Ion transport. Encyclopedia Microbiol. 1992;2:549–560. doi: 10.1128/mr.56.1.195-228.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith H W, Novick R P. Genetic studies on plasmid-linked cadmium resistance in Staphylococcus aureus. J Bacteriol. 1972;112:761–762. doi: 10.1128/jb.112.2.761-772.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai K, Yoon K P, Lynn A R. ATP-dependent cadmium transport by the cadA cadmium resistance determinant in everted membrane vesicles of Bacillus subtilis. J Bacteriol. 1992;174:116–121. doi: 10.1128/jb.174.1.116-121.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tynecka Z, Gos Z, Zajac J. Reduced cadmium transport determined by a resistance plasmid in Staphylococcus aureus. J Bacteriol. 1981;147:305–312. doi: 10.1128/jb.147.2.305-312.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wulff-Strobel C R, Wilson D B. Cloning, sequencing, and characterization of a membrane-associated Prevotella ruminicola B14 β-glucosidase with cellodextrinase and cyanoglycosidase activities. J Bacteriol. 1995;177:5884–5890. doi: 10.1128/jb.177.20.5884-5890.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors and new Escherichia coli host strain construction. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 31.Yoon K P, Misra T K, Silver S. Regulation of the cadA cadmium resistance determinant of Staphylococcus aureus plasmid pI258. J Bacteriol. 1991;173:7643–7649. doi: 10.1128/jb.173.23.7643-7649.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoon K P, Silver S. A second gene in the Staphylococcus aureus cadmium resistance determinant of plasmid pI258. J Bacteriol. 1991;173:7636–7642. doi: 10.1128/jb.173.23.7636-7642.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]