Abstract
Parkinsonism is a clinical syndrome presenting with bradykinesia, tremor, rigidity, and postural instability. Nonmotor symptoms have recently been included in the parkinsonian syndrome, which was traditionally associated with motor symptoms only. Various pathologically distinct and unrelated diseases have the same clinical manifestations as parkinsonism or parkinsonian syndrome. The etiologies of parkinsonism are classified as neurodegenerative diseases related to the accumulation of toxic protein molecules or diseases that are not neurodegenerative. The former class includes Parkinson’s disease (PD), multiple-system atrophy, progressive supranuclear palsy, and corticobasal degeneration. Over the past decade, clinical diagnostic criteria have been validated and updated to improve the accuracy of diagnosing these diseases. The latter class of disorders unrelated to neurodegenerative diseases are classified as secondary parkinsonism, and include drug-induced parkinsonism (DIP), vascular parkinsonism, and idiopathic normal-pressure hydrocephalus (iNPH). DIP and iNPH are regarded as reversible and treatable forms of parkinsonism. However, studies have suggested that the absence of protein accumulation in the nervous system as well as managing the underlying causes do not guarantee recovery. Here we review the differential diagnosis of PD and parkinsonism, mainly focusing on the clinical aspects. In addition, we describe recent updates to the clinical criteria of various disorders sharing clinical symptoms with parkinsonism.
Keywords: Parkinsonian disorders; Parkinson’s disease; Parkinson-plus syndromes; Parkinson disease, secondary; differential diagnosis
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative disease in which Lewy bodies accumulate in the substantia nigra within the midbrain,1 leading to motor dysfunction characterized by bradykinesia, rigidity, tremor, and postural instability.2,3 These characteristic symptoms are caused by the degeneration of dopaminergic neurons due to the deposition of Lewy bodies, which leads to dopamine dysregulation in the basal ganglia motor circuit.4 These complex motor symptoms, called parkinsonian symptoms or simply parkinsonism, were initially considered to be specific to PD until similar symptoms were found in various other neurodegenerative diseases and conditions, including progressive supranuclear palsy (PSP), dementia with Lewy bodies, multiple-system atrophy (MSA), and corticobasal degeneration (CBD).5,6 These neurodegenerative diseases show neuropathological findings distinct from those of PD and may be termed “Parkinson-plus syndromes.”7,8 Differentiating these diseases is difficult, particularly in their early stages, because they commonly present as parkinsonism. Neuroimaging studies, including brain magnetic resonance imaging (MRI), dopamine transporter (DAT) imaging, and fluorodeoxyglucose (FDG) positron-emission tomography (PET) have improved the diagnostic accuracy in differentiating diseases presenting with parkinsonism.9,10,11,12 However, the relatively low sensitivity and specificity of brain imaging mean that clinical evaluations and neurological examinations are important for ensuring accurate diagnoses of parkinsonism patients. Various neurodegenerative diseases manifest parkinsonian symptoms, but each has distinct neuropathological findings that distinguish it from PD, as well as different clinical courses and outcomes.6,13
In addition to neurodegenerative diseases, various conditions classified as secondary parkinsonism and other heredodegenerative diseases are listed in the differential diagnosis of parkinsonism (Table 1). Here we review the clinical aspects of PD and Parkinson-plus syndromes, including PSP, MSA, and CBD. In addition, we review the clinical characteristics and diagnosis of secondary parkinsonism.
Table 1. Classification of parkinsonism and the etiologies in each category.
| Neurodegenerative diseases | Secondary parkinsonism | Heredodegenerative diseases |
|---|---|---|
| Parkinson's disease | Drug-induced parkinsonism | Spinocerebellar ataxia |
| Progressive supranuclear palsy | Vascular parkinsonism | Huntington's disease |
| Multiple-system atrophy | Normal-pressure hydrocephalus | Neurodegeneration with brain-iron degeneration |
| Corticobasal degeneration | Traumatic encephalopathy | Dopa responsive dystonia |
| Dementia with Lewy bodies | Toxic encephalopathy | Wilson's disease |
PARKINSON’S DISEASE
The clinical syndrome of PD was first described in “An Essay on the Shaking Palsy” by James Parkinson, and is clinically characterized by bradykinesia, resting tremor, rigidity, and postural instability, with these symptoms collectively termed parkinsonism.14 The motor symptoms in PD are caused by dysfunction of the basal ganglia cortical motor circuit due to the degeneration of dopaminergic neurons in the substantia pars compacta, which is a pathological hallmark of PD.15,16,17 Bradykinesia is a key motor symptom of PD, which presents as difficulties in planning, initiating, and executing movement, and performing sequential tasks. Other manifestations of bradykinesia include loss of spontaneous movements and drooling due to swallowing difficulty, monotonic and hypophonic speech, masked face (loss of facial expression), decreased eye blinking, small-amplitude movements of the limbs (e.g., reduced arm swing while walking), micrographia, and stooped posture. Rigidity manifests as increased resistance to passive limb movements over all ranges induced by an examiner. When rigidity is associated with an underlying tremor, the “cogwheel” phenomenon can be present. Rigidity can be associated with pain, and it is sometimes misdiagnosed as arthritis or bursitis. Resting tremor is an easily recognized symptom of PD. It is initially unilateral, at a frequency of 4–6 Hz, and prominent in the distal part of the extremities. The tremor increases during walking, and disappears when the involved arm is moving and during sleep. The “pill-rolling” tremor involves alternating contractions of the supinator and pronator muscles in one hand. Resting tremors can be present in the lips and chin. Postural instability refers to the loss of postural reflexes, which manifests in the advanced stage of PD. The degree of postural instability (retropulsion) can be assessed by rapidly pulling the patient backward by the shoulder (“pull test”). Postural instability and gait disturbance (PIGD) usually occur in patients with advanced PD, but also develop in the early stage of disease in about 20% of patients.18
Patients with PD can be classified based on the main motor manifestations into tremor-dominant and PIGD subtypes19,20 The clinical course is worse in the PIGD subtype than in the tremor-dominant subtype.19,21 Freezing of gait (FOG), which has been defined as “brief, episodic absence or marked reduction of forward progression of the feet despite the intention to walk,” usually occurs in patients with an advanced stage of PD.22 PIGD and FOG contribute to the risk of falls and bone fractures. The Hoehn and Yahr23 stage is commonly used to evaluate the functional severity of PD, and provides a gross assessment of disease progression, which ranges from no sign (stage 0) to bedridden (stage 5). The Unified Parkinson’s Disease Rating Scale (UPDRS) is another well-established scale for assessing disability and impairment.24 The revised MDS-UPDRS (sponsored by the Movement Disorder Society) is more sensitive to detecting small changes and integrating nonmotor symptoms of PD.25,26 Various nonmotor manifestations present with the progression of motor symptoms or even before motor manifestations develop.27,28 Nonmotor manifestations of PD include sensory dysfunction, autonomic dysfunction, psychiatric symptoms, cognitive dysfunction, and autonomic dysfunctions such as constipation, urinary incontinence, and postural hypotension. Anosmia, depression, and sleep disorders such as rapid-eye-movement sleep behavior disorder (RBD) are nonmotor manifestations preceding the development of motor symptoms that indicate the prodromal stage of PD.28
Pathologically, PD results from dopaminergic neuronal degeneration in the substantia nigra and is associated with Lewy bodies, which are intracellular eosinophilic inclusion bodies containing aggregates of α-synuclein.29 Neuron loss is found in many other brain areas beyond the substantia nigra, including the locus coeruleus, raphe nucleus, and hypothalamus.30 Braak et al.31 hypothesized that Lewy body pathology progresses from the olfactory bulb to the cerebral cortex (from stage 1 to stage 6). According to that hypothesis, PD patients experiencing the onset of parkinsonian motor symptoms would already be in pathological stage 3. Braak’s staging has provided insight into the concept of prodromal PD as well as improved the awareness of the importance of nonmotor manifestations as symptoms in the premotor stage of PD.32
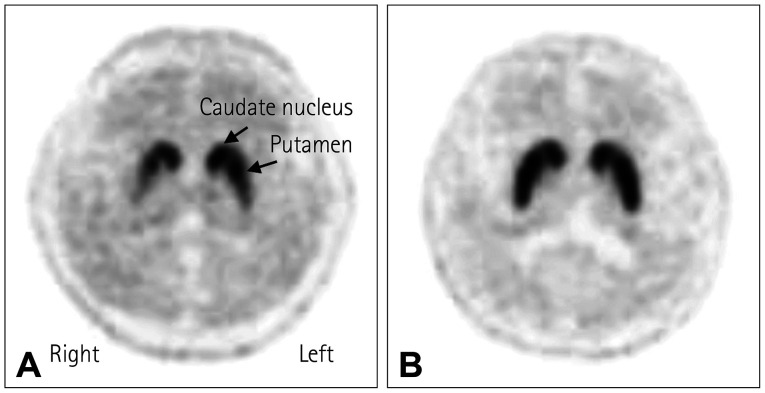
PD is primarily diagnosed by neurological examinations, and is also based on the cardinal symptom of an excellent response to levodopa administration. Structural neuroimaging is mostly used to exclude structural lesions that might be responsible for parkinsonism, such as hydrocephalus, brain tumor, or vascular lesions. A clinicopathological study found that about a quarter of patients clinically diagnosed with PD were subsequently confirmed as having other diseases in postmortem pathology.33 With the recent development of neuroimaging tools, DAT imaging can now detect presynaptic dopaminergic degeneration with high sensitivity and specificity, which aids in the diagnosis of PD (Fig. 1). This test can be useful for the differential diagnosis of PD from parkinsonism caused by drug-induced parkinsonism (DIP), vascular parkinsonism (VaP), and normal-pressure hydrocephalus (NPH), and there is increasing evidence of its effectiveness in discriminating neurodegenerative diseases defined as Parkinson-plus syndrome.34,35 Standard clinical diagnostic criteria and validation processes have improved the accuracy of premortem diagnoses of PD. The clinical diagnostic criteria for PD were first described by the UK Brain Bank in 1988,33,36 and they were widely used until the MDS proposed new clinical criteria in 2015.37 These criteria employ a two-step process to diagnose PD. The first step involves the definition of parkinsonism as bradykinesia plus rigidity or tremor at rest. Postural instability is omitted from the definition of parkinsonism in the MDS criteria. In particular, the definition of bradykinesia has become stricter when the response decreases during repetitive movement (“sequential effect”). This first step helps to avoid the false-positive inclusion of patients with walking disorders and posture abnormalities due to musculoskeletal problems. In the second step, a diagnosis of clinically probable or clinically possible PD is made by combining supportive features, exclusion criteria, and red flags. PD could be excluded if any exclusion criterion are present, which strongly suggests a parkinsonian syndrome other than PD. This includes overt cerebellar dysfunction, supranuclear gaze limitation, dementia consistent with the diagnostic criteria of behavioral variant frontotemporal dementia (FTD) or primary progressive aphasia within 5 years, evidence of DIP, unequivocal neurological signs supporting corticobasal syndrome (CBS), normal imaging of the presynaptic dopaminergic system, and documentation of an alternative condition that is more likely than PD. Four supporting PD criteria and ten red flags are counterbalanced when diagnosing clinically probable PD, with more than two red flags not being allowed. Clinically established PD patients need to have two or more supportive criteria and no red flags. A validation study showed that the MDS clinical diagnostic criteria improved the diagnostic accuracy for PD compared with the UK Brain Bank criteria.38
Fig. 1. Dopamine transporter image in Parkinson’s disease. A: [18F]-N-3-fluoropropyl-2-betacarboxymethoxy-3-beta-(4-iodophenyl) nortropane positron emission tomography computed tomography in a patient with Parkinson’s disease shows decreased uptake of the dopamine transporter (DAT) in the bilateral putamen, severely on the right side, with an anterior-posterior gradient. B: Normal DAT uptake in the striatum in a healthy subject.
The mean survival duration from diagnosis to death in patients with PD was 14 years in a clinicopathological study at Queen Square Brain Bank. A lower age at onset, midline symptoms, and absence of depression were related to longer survival.39 Research on the prognosis of PD related to clinical subtypes is ongoing, and nonmotor symptoms at the time of diagnosis are known to be related to the prognosis.40,41,42 Nonmotor manifestations associated with a poor prognosis include postural hypotension, cognitive impairment, and RBD.40 Along with providing consensus diagnostic criteria, the MDS proposed research criteria for prodromal PD.43 The likelihood ratio was calculated based on eight risk markers (male sex, regular pesticide exposure, occupational solvent exposure, nonuse of caffeine, nonsmoking, family history of PD, known gene mutations, and substantia nigra hyperechogenicity) and nine prodromal markers (RBD, abnormal motor movements, olfactory loss, constipation, excessive daytime somnolence, symptomatic hypotension, severe erectile dysfunction, urinary dysfunction, and depression). Applying the prodromal PD criteria in prospective cohorts produced high specificity and negative predictive values (>98%), but low sensitivity and positive predictive values.44 It has recently been proposed that the research criteria for prodromal PD need to be updated.45
Diagnosing PD is currently based on cardinal motor symptoms including bradykinesia, rigidity, and tremors. Differentiating PD from Parkinson-plus syndromes (e.g., PSP, MSA, and CBD) and different types of secondary parkinsonism (e.g., DIP, VaP, and idiopathic normal-pressure hydrocephalus [iNPH]) is the main step in diagnosing PD. It is likely that a consensus on the application of diagnostic criteria for the prodromal stage of PD will appear in the near future, along with disease-modifying therapeutic strategies.
PROGRESSIVE SUPRANUCLEAR PALSY
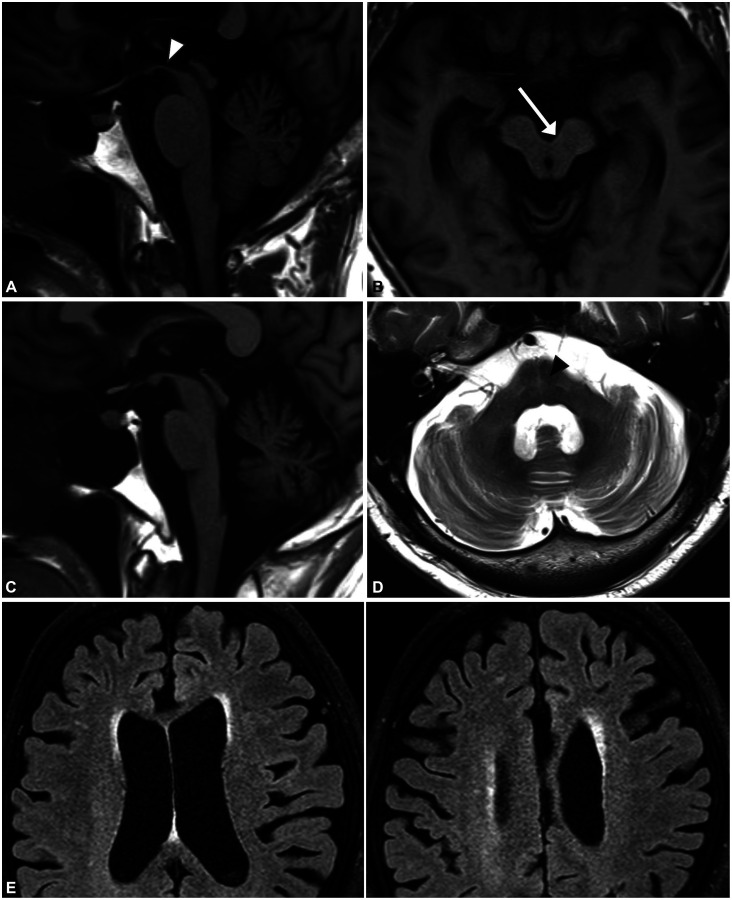
PSP is a rare neurodegenerative disorder characterized by supranuclear gaze palsy, gait disturbance, frequent falling, and various neuropsychiatric manifestations, with imaging revealing neurofibrillary tangles and neuropil threads in the pons, substantia nigra, subthalamic nucleus, and pallidum.46 PSP is one of the most common primary tauopathies, and it shares pathology with CBD.47 The mean age at onset is typically 65 years in patients with classic PSP-RS (Richardson syndrome). In classic PSP-RS, supranuclear vertical gaze limitation (patients typically complain of difficulty looking down, but this vertical gaze paresis will correct when the examiner passively rolls the patient’s head in the vertical line) presents with limitations of pursuits, slow saccade movements, and the around-the-house sign. These eye-movement abnormalities are associated with midbrain atrophy and characteristic MRI findings in PSP (Fig. 2A and B). Prominent axial rigidity with upright posture and frequent falling within 3 years due to early balance difficulties are typical clinical manifestations of PSP-RS. Initial medical attention is needed when patients show postural instability and a tendency to fall. Cognitive abnormalities are also observed, especially in processing speed during frontal executive function. Memory impairment and visuospatial dysfunction are unusual because frontal lobe involvement is dominant. Until the new diagnostic criteria were proposed in 2017 by the MDS,48 the clinical criteria proposed by the National Institute of Neurological Disorders and Stroke and Society for PSP (NINDS-SPSP) in 1996 were widely used. Because vertical supranuclear gaze palsy or slow vertical saccades and postural instability with early falls are mandatory for diagnosing PSP, the diagnostic specificity for pathologically proven PSP was excellent in the NINDS-SPSP diagnostic criteria.49 However, the diagnostic sensitivity for the early stage of PSP-RS was low. In addition, various clinical phenotypes have been reported, with 74% of patients with PSP reportedly showing variant presentations other than PSP-RS.50,51
Fig. 2. Brain MRI in Parkinson-plus syndromes. A and B: Brain MRI in a patient with progressive supranuclear palsy shows midbrain atrophy. The arrowhead indicates the hummingbird sign in a sagittal T1-weighted image (A) and the arrow indicates the morning-glory sign in an axial T1-weighted image (B). C and D: Brain MRI in a patient with multiple-system atrophy. C: Cerebellar-pontine atrophy is shown in a T1-weighted sagittal image. D: Atrophy in the middle cerebellar peduncle and the hot-cross-bun sign (arrowhead) are shown in a T2-weighed axial image. E: Brain MRI FLAIR images in a patient with corticobasal syndrome showing severe asymmetric parietofrontal atrophy on the left side.
The MDS clinical diagnostic criteria of 2017 based on the basic features of mandatory inclusion, exclusion criteria, and context-dependent exclusion criteria subdivide the core features into ocular dysfunction, postural instability, akinesia, and cognitive dysfunction domains for diagnosing the variant form of PSP.48 By integrating clinical features and clues, the diagnostic criteria defined definite, probable, possible, and “suggestive of” PSP according to the certainty of a PSP diagnosis, adding the predominant type according to the combination of core features. PSP variants other than PSP-RS include PSP-ocular motor, PSP-postural instability, PSP-parkinsonism (PSP-P), PSP-frontal (PSP-F), PSP-progressive gait freezing (PSP-PGF), PSP-CBS, PSP-speech/language (PSP-SL) disorder, and PSP-primary lateral sclerosis. PSP-P and PSP-PGF are classified as “brainstem” variants.52 These subtypes have a benign clinical course, with a survival time exceeding 10 years. Patients with PSP-P are frequently misdiagnosed clinically as PD because they present with asymmetric parkinsonism without supranuclear palsy in the early stage. In addition, they exhibit moderate levodopa responses and sometimes show a sustained response with long-term levodopa-induced dyskinesia. “Cortical” variants of PSP include PSP-CBS, PSP-F, and PSP-SL. These clinical variants are difficult to diagnose because they overlap with the clinical manifestations of CBD and frontotemporal lobar degeneration belonging to the same tauopathies. Autonomic dysfunction rarely develops in PSP, in contrast to in PD. Cerebellar ataxia, which is common in MSA, is also unusual, and is distinct from gait unsteadiness. Although the cerebellar variant of PSP has been reported in Japanese patents, it is extremely rare.53 Levodopa responsiveness to parkinsonism and postural instability is temporary and limited, although long-term benefits are frequently observed in patients with PSP-P.54 Botulinum toxin might be helpful in improving severe retrocollis and eyelid-opening apraxia. Deep brain stimulation targeting the pedunculopontine nucleus was expected to improve symptoms of postural instability in patients with PSP, but its effectiveness has not been demonstrated. Frequent falls are a substantial cause of morbidity in patients with PSP due to head trauma and fractures. The terminal stage of the disease is characterized by severe difficulty in speech articulation, axial rigidity, immobility, and swallowing difficulty, which can lead to frequent aspiration pneumonia. The mean disease duration from onset to death across all variants is approximately 8.7 years, ranging widely from 2 to 28 years. The PSP-RS phenotype shows the worse clinical course, whereas the PSP-P phenotype is relatively benign.46
PSP is a tauopathy presenting with a Parkinsonism phenotype. Tauopathy diseases—PSP, CBD, frontotemporal lobar degeneration, and Alzheimer’s disease—share certain clinical syndromes and pathological disease entities. PSP-P and PSP-PGF may be difficult to treat in the early stages of PD. Careful neurological examinations for detecting the slowing of vertical saccadic eye movements and convergence impairment in the accommodation reflex might be important. Hypometabolism of the frontal cortices, basal ganglia, thalamus, and midbrain on FDG-PET would be helpful in diagnosing PSP, despite imaging findings not being included in the consensus criteria for PSP.55
MULTIPLE SYSTEM ATROPHY
MSA is a fatal and progressive neurodegenerative disorder characterized by autonomic dysfunction, parkinsonism, and cerebellar ataxia due to glial and neuronal cytoplasmic α-synuclein-positive inclusions throughout the nervous system.56 Parkinsonism and cerebellar dysfunction appear as symptoms associated with autonomic dysfunction. Parkinsonism-dominant MSA and cerebellar-dysfunction-dominant MSA are classified as MSA-P and MSA-C subtypes, respectively.57,58 This classification is straightforward at the beginning of the disease, but it is common for all symptoms to appear as the disease progresses.59 Marked autonomic dysfunction early in the disease course is a key feature of MSA and is mandatory for a clinical diagnosis.57,58 Urogenital dysfunction and orthostatic hypotension are characteristic of dysautonomia in MSA.60 The MSA-P subtype is about four times more prevalent than the MSA-C subtype in Western patients, whereas the MSA-C subtype is more frequent in Japanese patients. It was reported recently that the MSA-P subtype is approximately twice as common as the MSA-C subtype in China,61 but the subtype prevalence rates in other Asian countries remain unclear.
Parkinsonism in patients with MSA is difficult to differentiate from that in patients with PD in the early stage. Moreover, akinetic-rigid syndrome in parkinsonism is almost identical to that in PD patients. Parkinsonism in the MSA-P subtype typically responds poorly to levodopa, whereas more than 30% of patients with MSA-P show transient levodopa-induced improvement early in the disease course.62 The dystonic type of levodopa-induced dyskinesia affecting the cranium and neck muscles occurs in about half of the patients, and this feature is considered to be distinct from levodopa-induced dyskinesia occurring in patients with PD.63 Patients with the MSA-C subtype show gait ataxia, scanning dysarthria, and uncoordinated limb movements due to cerebellar dysfunction. Neurological examinations frequently reveal oculomotor disturbances, including gaze-evoked nystagmus, saccadic pursuit, and hyper- or hypometric saccades. It is very difficult to differentiate the early stage of MSA-C when only mild cerebellar ataxia is present, including mild tandem gait instability, from other forms of cerebellar ataxia such as spinocerebellar ataxia and fragile-X-associated tremor ataxia syndrome.59,64,65
Tremor is common in both MSA subtypes, with a characteristic pattern of postural tremor including a jerky myoclonic component that distinguishes it from PD.66 RBD, sleep apnea, frontal executive dysfunction, and rare visual hallucinations may occur in patients with MSA.59 RBD, which is known to be marker of synucleinopathy,67 often appears several years before the occurrence of key features of MSA, as in the case of PD. Diagnosing MSA relies on clinical diagnostic criteria consisting of probable and possible MSA-C or MSA-P subtypes.58,68 The second consensus criteria included brain imaging findings as additional features for diagnosing possible MSA.58 These include atrophy of the putamen, cerebellum, middle cerebellar peduncle, or pons in brain MRI (Fig. 2C and D); hypometabolism in the putamen, brainstem, and cerebellum in FDG-PET; and presynaptic nigrostriatal dopaminergic degeneration in DAT imaging. The sensitivities of the second consensus criteria were 41% and 18% for possible and probable MSA, respectively.65 Additional features supporting (red flags) or opposing MSA have been proposed to increase the initial diagnostic accuracy. Six red-flag categories were identified: early instability, rapid progression, abnormal posture, bulbar dysfunction, respiratory dysfunction, and emotional incontinence.69 Applying red flags increased the sensitivity and specificity of diagnostic criteria to 92% and 63%, respectively, and additionally led to possible MSA-P and probable MSA-P being diagnosed a mean of 15.9 months earlier compared with only applying the consensus criteria of MSA.69 Since more than 10 years have passed since the secondary clinical consensus criteria were established, it has been suggested that “clinically established MSA” needs to be added to support the existence of a mixed type of MSA that cannot be clearly divided into the MSA-C or MSA-P subtype, and to consider new variants of MSA that include MSA with pure autonomic failure and MSA with a corticobasal presentation. The low diagnostic accuracy of the latest consensus diagnostic criteria, the clinical heterogeneity of MSA, and the need to diagnose in the prodromal stage encourage the development of new criteria for MSA.64 At present, only symptomatic management is practically available, although the promising results of stem cell treatments for patients with MSA are raising expectations for future disease-modifying treatments.70,71 The importance of diagnosing prodromal MSA would be emphasized for the application of disease-modifying treatment.72
CORTICOBASAL DEGENERATION
CBD is a distinct pathological disease entity characterized by the deposition of hyperphosphorylated four-repeat tau in cortical and striatal neurons and glia.73,74 Until various clinical subtypes had been reported in patients with pathologically proven CBD and the clinical phenotypes associated with the pathology of CBD had been established,75,76,77 the terminology of CBD was used interchangeably when referring to both the clinical syndrome and the pathology. The typical clinical manifestations of CBD, which are now referred to as CBS, are characterized by asymmetric ideomotor or limb kinetic apraxia, dystonia and myoclonus, and “the useless arm” with marked asymmetric rigidity and bradykinesia. Postmortem pathological studies of CBD studies have confirmed that a wide spectrum of clinical symptoms and signs other than CBS can be present in patients with pathologically confirmed CBD,75,78 including progressive supranuclear palsy syndrome (PSPS), frontal behavioral-spatial syndrome (FBS), and nonfluent/agrammatic variants of primary progressive aphasia.77 Conversely, CBS is a clinical subtype with various pathological disease entities in addition to CBD, including PSP, FTD, and Alzheimer’s disease,78 which makes antemortem clinical diagnoses of CBD difficult. CBD-CBS is the classic presentation of CBD, and parkinsonism is included in the CBS phenotype of CBD. Rigidity and bradykinesia appear asymmetrically as in PD, and more-severe limb rigidity and dystonia with dystonic posturing can be used to differentiate between CBD and PD.
Levodopa responsiveness has been reported in 25% of patients with CBD, but parkinsonism in patients with CBD usually shows poor and transient levodopa responsiveness or levodopa unresponsiveness.79,80 Myoclonus is frequently stimulus-sensitive. Dystonia and myoclonus are less frequent in patients with PD than in those with CBD-CBS. Some patients present with alien-hand phenomena characterized by involuntary grasping and purposeless movements. CBS can manifest as oculomotor apraxia (saccadic movement dysfunction but normal optokinetic nystagmus) and subsequently develop into vertical supranuclear palsy. Brain MRI typically demonstrates asymmetric cortical atrophy in the posterior frontal and the parietal cortices (Fig. 2E).81 CBD-PSP can present with PSP symptoms, symmetric parkinsonism, postural instability, and oculomotor disturbances, which are difficult to differentiate from PSP-RS. However, frontal executive dysfunction is usually more severe in CBD-PSP than in pathologically proven PSP.
The symptoms of CBD may shift from one phenotype to another as the disease progresses, such as starting with CBS and progressing to PSPS and FBS. The survival time is usually 7–8 years from symptom onset.82 As described in PSP, CBD is one of the tauopathies and has many clinical variants. The CBD-CBS clinical variant presenting as an asymmetric akinetic-rigid syndrome may impede the early differential diagnosis of PD. A careful neurological examination for detecting cortical sensory impairment, arm posture (mainly observed during walking), and ideomotor or limb-kinetic apraxia may be important for the early differential diagnosis.
DRUG-INDUCED PARKINSONISM
DIP is the second most common cause of parkinsonism after PD.83 Typical and atypical antipsychotics, gastrointestinal motility drugs, calcium-channel blockers, and antiepileptic drugs are known to be offending drugs that cause parkinsonism.84 DIP is difficult to distinguish clinically from idiopathic PD. DIP typically has a symmetric presentation, but clinical observational studies have found that more than 30% of patients have asymmetric tremor at rest, similar to the prevalence in PD. Parkinsonism in DIP is difficult to distinguish from PD based on clinical characteristics alone. A report of 7% of patients diagnosed with PD and who were taking medication being subsequently diagnosed with DIP reflects the difficulty in diagnosing DIP.85 It is expected that parkinsonism develops after administering dopamine antagonists due to their antipsychotic effects.86 Being older and female are known to be risk factors for DIP, and several genetic risk factors have recently been reported.84 The therapeutic effect of antipsychotics involves blocking dopamine D3 and D4 receptors in the limbic system, with parkinsonism resulting from the dopamine D2 receptors in the striatum also being blocked. The onset of parkinsonism occurs from several days to years after taking offending drugs. DIP usually improves within weeks to months after discontinuing the causative agent, but the symptoms persist or temporarily improve before subsequently progressing again in 10%–50% of patients. In these patients it is thought that dopamine receptor blockers taken during the preclinical stage of PD trigger parkinsonian symptoms. The introduction of DAT imaging improved the accuracy of DIP diagnoses.12,34 Because dopamine receptor antagonists do not affect the presynaptic dopamine receptor or DAT in DIP, the uptake of DAT appears normal in single-photon-emission computed tomography and PET, whereas it is decreased in PD or other conditions related to presynaptic dopaminergic neuronal degeneration. In addition, DAT imaging has provided insight into the pathophysiological mechanisms of DIP. The patterns of PET scans in approximately 20% of patients diagnosed with DIP are the same as those in typical PD.87 These patients may have actually had PD rather than DIP, but the effect of the dopamine-receptor-blocking agent was not completely excluded. Discontinuing the offending drugs was found to improve parkinsonism in most patients.88
VASCULAR PARKINSONISM
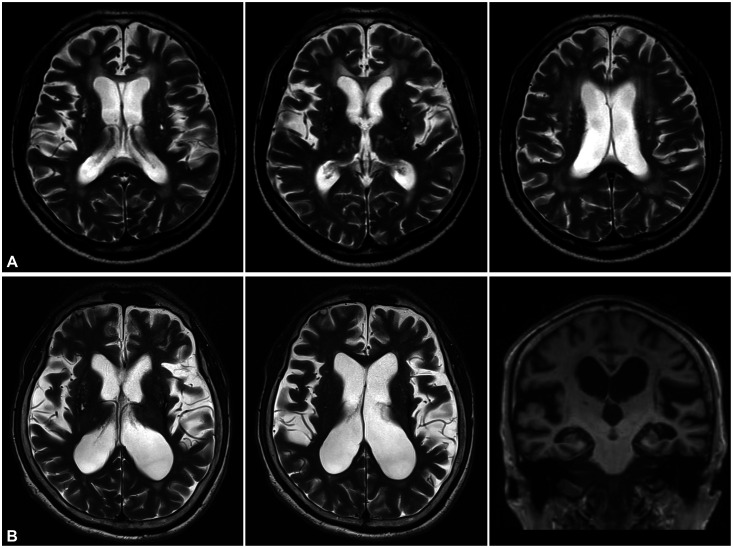
VaP is defined as parkinsonism associated with ischemic (rarely hemorrhagic) cerebrovascular disease.89 Since patients with VaP present with marked gait difficulty combined with minimal or no deficit in the upper limbs, “lower body parkinsonism” has been used as a typical designating clinical feature of VaP.90 Brain MRI of patients typically shows extensive subcortical white-matter lesions (Fig. 3A). Gait impairment in patients with VaP sometimes presents as narrow-base gait similar to that in patients with PD, but patients with VaP show a wide range of gait patterns including wide-base gait with ataxia, spastic gait, FOG, and gait initiation failure.91 These various patterns of gait disorders in patients with VaP have led to proposals to reconsider the “lower body parkinsonism” term.92,93 Gait disorders and neurological deficits that do not conform to the definition of parkinsonism could be referred to as “vascular pseudoparkinsonism” or “cerebrovascular gait disorder.”92,93 A recent expert working group proposed the following three subtype definitions of VaP according to the clinical, anatomical, and imaging findings of cerebrovascular disease:94 1) acute/subacute poststroke VaP, which is defined as clinically identical to PD due to stroke in the unilateral substantia nigra or nigrostriatal pathway region; 2) insidious VaP, which is a common form of VaP presenting as symmetric neurological involvement with predominant lower body parkinsonism, and cognitive or urinary symptoms due to ischemic white-matter ischemic lesions; and 3) mixed neurodegenerative parkinsonism and cerebrovascular disease, which can be defined as syndromes of VaP overlapping with PD or other types of neurodegenerative parkinsonism confirmed by molecular imaging biomarkers in DAT imaging. The appropriateness of classifying subtypes of VaP might be controversial, but the attempts to classify heterogeneous clinical and imaging phenotypes in the VaP syndrome have been valuable.95
Fig. 3. Brain MRI in vascular parkinsonism and idiopathic normal pressure hydrocephalus. A: Brain MRI showing multiple ischemic lesions in the bilateral periventricular white matter and basal ganglia in a patient with lower body parkinsonism. B: Brain MRI showing ventriculomegaly with disproportionately enlarged subarachnoid space in coronal views suggesting idiopathic normal hydrocephalus.
IDIOPATHIC NORMAL-PRESSURE HYDROCEPHALUS
NPH is characterized by gait disturbance, cognitive impairment, and urinary dysfunction as a clinical triad and ventriculomegaly disproportionate to cortical atrophy in brain imaging.96 iNPH is the most common form of NPH and is distinguished from secondary NPH that occurs after meningitis, subarachnoid hemorrhage, or head trauma.97 iNPH is an important disorder in the differential diagnosis of various diseases that cause parkinsonism. The clinical manifestations of iNPH are attributed to dysfunction of the basal ganglia-thalamo-cortical pathway due to ventriculomegaly secondary to increased cerebrospinal fluid (CSF) volume.98 Bradykinesia, rigidity, and postural instability, which are cardinal motor symptoms in patients with PD, are also common in patients with iNPH and are correlated with the severity of ventriculomegaly.99 Whereas patients with PD show short steps with decreased and asymmetric arm swing, iNPH is characterized by a wide-base, small-steps gait with initiation difficulty, which is known as a higher level frontal lobe gait disorder.100 Gait difficulty and mostly preserved upper limb movements (i.e., lower body parkinsonism) are typical motor symptoms and signs in patients with iNPH.101 Despite these differences in patterns of gait dysfunction, the similarity of clinical symptoms, the presence of asymptomatic hydrocephalus in old age, and the overlap with iNPH and neurodegenerative diseases related to parkinsonism make diagnosing iNPH difficult.102 Unfortunately there is no specific pathological marker or biomarker for diagnosing iNPH. iNPH can be suspected in patients with cardinal symptoms and confirmed by enlarged ventricles disproportionate to the cortical atrophy in brain MRI or computed tomography (Fig. 3B). It is also helpful to check whether clinical symptoms are improved by removing the CSF through lumbar puncture.103 The differential diagnosis of iNPH is particularly important because this disorder has been considered treatable. Since the ultimate treatment for iNPH is a ventriculoperitoneal or ventriculolumbar shunt operation, it is important to accurately diagnose iNPH for selecting good surgical candidates.104 A recent review suggested that clinical improvement after CSF removal, —which has been regarded as a factor predicting the prognosis of surgery—is not related with the long-term outcome of a shunt operation in patients with iNPH.102 In addition, there is increasing evidence for the importance of comorbidities in patients with iNPH such as Alzheimer’s disease and Parkinson-plus syndrome.105,106,107 It is therefore important to find biomarkers for diagnosing iNPH as well as for selecting good candidates for surgery.
CONCLUSION
Many diseases are considered etiologies of parkinsonism. PD is the most common disease causing parkinsonism, and its differential diagnosis from other neurodegenerative diseases referred to as Parkinson-plus syndrome is challenging. Since a definite diagnosis is not possible in an alive subject, the diagnostic accuracy must be improved through clinical diagnostic criteria. It is necessary to be familiar with the clinical diagnostic criteria of each disease, particularly the red flags that distinguish PD from other diseases. DIP is the second most common cause of parkinsonism after PD, and its diagnosis is particularly important because symptoms can be improved by discontinuing the offending drugs in many patients. VaP and iNPH are generally diagnosed based on brain imaging findings along with those related to parkinsonism (mainly gait disorder). However, there are controversies regarding the pathophysiology of the two diseases, the possibility of concomitant neurodegenerative disease, and the causal relationship between the abnormal findings in brain imaging and parkinsonism or other gait disorders. Molecular brain imaging (especially DAT imaging) plays a role in the differential diagnosis of parkinsonism by reflecting the degeneration of presynaptic dopaminergic neurons. The value of DAT imaging in the differential diagnosis of PD and Parkinson-plus syndrome remains to be established, and we expect this to become possible based on the results of current research.
Footnotes
- Conceptualization: Hae-Won Shin, Young Chul Youn.
- Data curation: Hae-Won Shin, Sang-Wook Hong.
- Methodology: Hae-Won Shin, Sang-Wook Hong.
- Project administration: Young Chul Youn.
- Resources: Hae-Won Shin, Young Chul Youn.
- Supervision: Young Chul Youn.
- Visualization: Hae-Won Shin, Sang-Wook Hong.
- Writing—original draft: Hae-Won Shin.
- Writing—review & editing: Young Chul Youn, Sang-Wook Hong.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding Statement: None
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
References
- 1.Kalia LV, Kalia SK. α-Synuclein and Lewy pathology in Parkinson’s disease. Curr Opin Neurol. 2015;28:375–381. doi: 10.1097/WCO.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 2.Sveinbjornsdottir S. The clinical symptoms of Parkinson’s disease. J Neurochem. 2016;139 Suppl 1:318–324. doi: 10.1111/jnc.13691. [DOI] [PubMed] [Google Scholar]
- 3.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 4.Gao LL, Zhang JR, Chan P, Wu T. Levodopa effect on basal ganglia motor circuit in Parkinson’s disease. CNS Neurosci Ther. 2017;23:76–86. doi: 10.1111/cns.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menšíková K, Tučková L, Kolařiková K, Bartoníková T, Vodička R, Ehrmann J, et al. Atypical parkinsonism of progressive supranuclear palsy-parkinsonism (PSP-P) phenotype with rare variants in FBXO7 and VPS35 genes associated with Lewy body pathology. Acta Neuropathol. 2019;137:171–173. doi: 10.1007/s00401-018-1923-y. [DOI] [PubMed] [Google Scholar]
- 6.Keener AM, Bordelon YM. Parkinsonism. Semin Neurol. 2016;36:330–334. doi: 10.1055/s-0036-1585097. [DOI] [PubMed] [Google Scholar]
- 7.Schnaberth G. “Parkinson plus.” Multisystem involvement in Parkinson disease. Fortschr Med. 1986;104:205–212. [PubMed] [Google Scholar]
- 8.Olfati N, Shoeibi A, Litvan I. Progress in the treatment of Parkinson-Plus syndromes. Parkinsonism Relat Disord. 2019;59:101–110. doi: 10.1016/j.parkreldis.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Seppi K. MRI for the differential diagnosis of neurodegenerative parkinsonism in clinical practice. Parkinsonism Relat Disord. 2007;13 Suppl 3:S400–S405. doi: 10.1016/S1353-8020(08)70038-5. [DOI] [PubMed] [Google Scholar]
- 10.Chougar L, Pyatigorskaya N, Degos B, Grabli D, Lehéricy S. The role of magnetic resonance imaging for the diagnosis of atypical parkinsonism. Front Neurol. 2020;11:665. doi: 10.3389/fneur.2020.00665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brajkovic L, Kostic V, Sobic-Saranovic D, Stefanova E, Jecmenica-Lukic M, Jesic A, et al. The utility of FDG-PET in the differential diagnosis of Parkinsonism. Neurol Res. 2017;39:675–684. doi: 10.1080/01616412.2017.1312211. [DOI] [PubMed] [Google Scholar]
- 12.Weber WE, Vlaar AM. Role of DAT-SPECT in diagnostic work-up of Parkinsonism. Mov Disord. 2008;23:774. doi: 10.1002/mds.21952. [DOI] [PubMed] [Google Scholar]
- 13.Williams DR, Litvan I. Parkinsonian syndromes. Continuum (Minneap Minn) 2013;19(5 Movement Disorders):1189–1212. doi: 10.1212/01.CON.0000436152.24038.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herzberg L. An essay on the shaking palsy: reviews and notes on the journals in which they appeared. Mov Disord. 1990;5:162–166. doi: 10.1002/mds.870050213. [DOI] [PubMed] [Google Scholar]
- 15.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 16.DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 17.Rinne JO. Nigral degeneration in Parkinson’s disease. Mov Disord. 1993;8 Suppl 1:S31–S35. doi: 10.1002/mds.870080507. [DOI] [PubMed] [Google Scholar]
- 18.Simuni T, Caspell-Garcia C, Coffey C, Lasch S, Tanner C, Marek K PPMI Investigators. How stable are Parkinson’s disease subtypes in de novo patients: analysis of the PPMI cohort? Parkinsonism Relat Disord. 2016;28:62–67. doi: 10.1016/j.parkreldis.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 19.Foltynie T, Brayne C, Barker RA. The heterogeneity of idiopathic Parkinson’s disease. J Neurol. 2002;249:138–145. doi: 10.1007/pl00007856. [DOI] [PubMed] [Google Scholar]
- 20.Schapira AH, Schrag A. Parkinson disease: Parkinson disease clinical subtypes and their implications. Nat Rev Neurol. 2011;7:247–248. doi: 10.1038/nrneurol.2011.40. [DOI] [PubMed] [Google Scholar]
- 21.van der Heeden JF, Marinus J, Martinez-Martin P, Rodriguez-Blazquez C, Geraedts VJ, van Hilten JJ. Postural instability and gait are associated with severity and prognosis of Parkinson disease. Neurology. 2016;86:2243–2250. doi: 10.1212/WNL.0000000000002768. [DOI] [PubMed] [Google Scholar]
- 22.Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10:734–744. doi: 10.1016/S1474-4422(11)70143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 24.Martínez-Martín P, Gil-Nagel A, Gracia LM, Gómez JB, Martínez-Sarriés J, Bermejo F. Unified Parkinson’s Disease Rating Scale characteristics and structure. Mov Disord. 1994;9:76–83. doi: 10.1002/mds.870090112. [DOI] [PubMed] [Google Scholar]
- 25.Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003;18:738–750. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 26.Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord. 2007;22:41–47. doi: 10.1002/mds.21198. [DOI] [PubMed] [Google Scholar]
- 27.Lee HM, Koh SB. Many faces of Parkinson’s disease: non-motor symptoms of Parkinson’s disease. J Mov Disord. 2015;8:92–97. doi: 10.14802/jmd.15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18:435–450. doi: 10.1038/nrn.2017.62. [DOI] [PubMed] [Google Scholar]
- 29.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 30.Dickson DW. Parkinson’s disease and parkinsonism: neuropathology. Cold Spring Harb Perspect Med. 2012;2:a009258. doi: 10.1101/cshperspect.a009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 32.Mahlknecht P, Seppi K, Poewe W. The concept of prodromal Parkinson’s disease. J Parkinsons Dis. 2015;5:681–697. doi: 10.3233/JPD-150685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poewe W, Scherfler C. Role of dopamine transporter imaging in investigation of parkinsonian syndromes in routine clinical practice. Mov Disord. 2003;18 Suppl 7:S16–S21. doi: 10.1002/mds.10573. [DOI] [PubMed] [Google Scholar]
- 35.Jin S, Oh M, Oh SJ, Oh JS, Lee SJ, Chung SJ, et al. Differential diagnosis of parkinsonism using dual-phase F-18 FP-CIT PET imaging. Nucl Med Mol Imaging. 2013;47:44–51. doi: 10.1007/s13139-012-0182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Dis ord. 2015;30:1591–1601. doi: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 38.Postuma RB, Poewe W, Litvan I, Lewis S, Lang AE, Halliday G, et al. Validation of the MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2018;33:1601–1608. doi: 10.1002/mds.27362. [DOI] [PubMed] [Google Scholar]
- 39.Selikhova M, Williams DR, Kempster PA, Holton JL, Revesz T, Lees AJ. A clinico-pathological study of subtypes in Parkinson’s disease. Brain. 2009;132(Pt 11):2947–2957. doi: 10.1093/brain/awp234. [DOI] [PubMed] [Google Scholar]
- 40.Fereshtehnejad SM, Romenets SR, Anang JB, Latreille V, Gagnon JF, Postuma RB. New clinical subtypes of Parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol. 2015;72:863–873. doi: 10.1001/jamaneurol.2015.0703. [DOI] [PubMed] [Google Scholar]
- 41.Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB. Clinical criteria for subtyping Parkinson’s disease: biomarkers and longitudinal progression. Brain. 2017;140:1959–1976. doi: 10.1093/brain/awx118. [DOI] [PubMed] [Google Scholar]
- 42.Rajput AH, Rajput ML, Ferguson LW, Rajput A. Baseline motor findings and Parkinson disease prognostic subtypes. Neurology. 2017;89:138–143. doi: 10.1212/WNL.0000000000004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berg D, Postuma RB, Adler CH, Bloem BR, Chan P, Dubois B, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2015;30:1600–1611. doi: 10.1002/mds.26431. [DOI] [PubMed] [Google Scholar]
- 44.Pilotto A, Heinzel S, Suenkel U, Lerche S, Brockmann K, Roeben B, et al. Application of the movement disorder society prodromal Parkinson’s disease research criteria in 2 independent prospective cohorts. Mov Disord. 2017;32:1025–1034. doi: 10.1002/mds.27035. [DOI] [PubMed] [Google Scholar]
- 45.Heinzel S, Berg D, Gasser T, Chen H, Yao C, Postuma RB MDS Task Force on the Definition of Parkinson's Disease. Update of the MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2019;34:1464–1470. doi: 10.1002/mds.27802. [DOI] [PubMed] [Google Scholar]
- 46.Armstrong MJ. Progressive supranuclear palsy: an update. Curr Neurol Neurosci Rep. 2018;18:12. doi: 10.1007/s11910-018-0819-5. [DOI] [PubMed] [Google Scholar]
- 47.Coughlin DG, Litvan I. Progressive supranuclear palsy: advances in diagnosis and management. Parkinsonism Relat Disord. 2020;73:105–116. doi: 10.1016/j.parkreldis.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, et al. Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord. 2017;32:853–864. doi: 10.1002/mds.26987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 50.Williams DR, de Silva R, Paviour DC, Pittman A, Watt HC, Kilford L, et al. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson’s syndrome and PSP-parkinsonism. Brain. 2005;128(Pt 6):1247–1258. doi: 10.1093/brain/awh488. [DOI] [PubMed] [Google Scholar]
- 51.Respondek G, Stamelou M, Kurz C, Ferguson LW, Rajput A, Chiu WZ, et al. The phenotypic spectrum of progressive supranuclear palsy: a retrospective multicenter study of 100 definite cases. Mov Disord. 2014;29:1758–1766. doi: 10.1002/mds.26054. [DOI] [PubMed] [Google Scholar]
- 52.Ling H. Clinical approach to progressive supranuclear palsy. J Mov Disord. 2016;9:3–13. doi: 10.14802/jmd.15060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ando S, Kanazawa M, Onodera O. Progressive supranuclear palsy with predominant cerebellar ataxia. J Mov Disord. 2020;13:20–26. doi: 10.14802/jmd.19061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boxer AL, Yu JT, Golbe LI, Litvan I, Lang AE, Höglinger GU. Advances in progressive supranuclear palsy: new diagnostic criteria, biomarkers, and therapeutic approaches. Lancet Neurol. 2017;16:552–563. doi: 10.1016/S1474-4422(17)30157-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eckert T, Barnes A, Dhawan V, Frucht S, Gordon MF, Feigin AS, et al. FDG PET in the differential diagnosis of parkinsonian disorders. Neuroimage. 2005;26:912–921. doi: 10.1016/j.neuroimage.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 56.Papp MI, Kahn JE, Lantos PL. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome) J Neurol Sci. 1989;94:79–100. doi: 10.1016/0022-510x(89)90219-0. [DOI] [PubMed] [Google Scholar]
- 57.Vidailhet M, Bonnet AM, Agid Y. Clinical diagnostic criteria for multiple system atrophy. Rev Neurol (Paris) 1998;154:17–21. [PubMed] [Google Scholar]
- 58.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watanabe H, Riku Y, Hara K, Kawabata K, Nakamura T, Ito M, et al. Clinical and imaging features of multiple system atrophy: challenges for an early and clinically definitive diagnosis. J Mov Disord. 2018;11:107–120. doi: 10.14802/jmd.18020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sakakibara R, Hattori T, Uchiyama T, Kita K, Asahina M, Suzuki A, et al. Urinary dysfunction and orthostatic hypotension in multiple system atrophy: which is the more common and earlier manifestation? J Neurol Neurosurg Psychiatry. 2000;68:65–69. doi: 10.1136/jnnp.68.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Du JJ, Wang T, Huang P, Cui S, Gao C, Lin Y, et al. Clinical characteristics and quality of life in Chinese patients with multiple system atrophy. Brain Behav. 2018;8:e01135. doi: 10.1002/brb3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seppi K, Yekhlef F, Diem A, Luginger Wolf E, Mueller J, Tison F, et al. Progression of parkinsonism in multiple system atrophy. J Neurol. 2005;252:91–96. doi: 10.1007/s00415-005-0617-2. [DOI] [PubMed] [Google Scholar]
- 63.Boesch SM, Wenning GK, Ransmayr G, Poewe W. Dystonia in multiple system atrophy. J Neurol Neurosurg Psychiatry. 2002;72:300–303. doi: 10.1136/jnnp.72.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin DJ, Hermann KL, Schmahmann JD. Multiple system atrophy of the cerebellar type: clinical state of the art. Mov Disord. 2014;29:294–304. doi: 10.1002/mds.25847. [DOI] [PubMed] [Google Scholar]
- 65.Stankovic I, Quinn N, Vignatelli L, Antonini A, Berg D, Coon E, et al. A critique of the second consensus criteria for multiple system atrophy. Mov Disord. 2019;34:975–984. doi: 10.1002/mds.27701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaindlstorfer C, Granata R, Wenning GK. Tremor in multiple system atrophy - a review. Tremor Other Hyperkinet Mov (N Y) 2013;3:tre-03-165-4252-1. doi: 10.7916/D8NV9GZ9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iranzo A, Tolosa E, Gelpi E, Molinuevo JL, Valldeoriola F, Serradell M, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013;12:443–453. doi: 10.1016/S1474-4422(13)70056-5. [DOI] [PubMed] [Google Scholar]
- 68.Gilman S, Low PA, Quinn N, Albanese A, Ben-Shlomo Y, Fowler CJ, et al. Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci. 1999;163:94–98. doi: 10.1016/s0022-510x(98)00304-9. [DOI] [PubMed] [Google Scholar]
- 69.Köllensperger M, Geser F, Seppi K, Stampfer-Kountchev M, Sawires M, Scherfler C, et al. Red flags for multiple system atrophy. Mov Disord. 2008;23:1093–1099. doi: 10.1002/mds.21992. [DOI] [PubMed] [Google Scholar]
- 70.Lee PH, Lee JE, Kim HS, Song SK, Lee HS, Nam HS, et al. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol. 2012;72:32–40. doi: 10.1002/ana.23612. [DOI] [PubMed] [Google Scholar]
- 71.Kim HN, Kim DY, Oh SH, Kim HS, Kim KS, Lee PH. Feasibility and efficacy of intra-arterial administration of mesenchymal stem cells in an animal model of double toxin-induced multiple system atrophy. Stem Cells Transl Med. 2017;6:1424–1433. doi: 10.1002/sctm.16-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Osaki Y, Morita Y, Miyamoto Y, Ohtsuru S, Shogase T, Furushima T, et al. Identification of a pre-possible multiple system atrophy phase. Acta Neurol Scand. 2021;143:313–317. doi: 10.1111/ane.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, et al. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61:935–946. doi: 10.1093/jnen/61.11.935. [DOI] [PubMed] [Google Scholar]
- 74.Kouri N, Whitwell JL, Josephs KA, Rademakers R, Dickson DW. Corticobasal degeneration: a pathologically distinct 4R tauopathy. Nat Rev Neurol. 2011;7:263–272. doi: 10.1038/nrneurol.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wadia PM, Lang AE. The many faces of corticobasal degeneration. Parkinsonism Relat Disord. 2007;13 Suppl 3:S336–S340. doi: 10.1016/S1353-8020(08)70027-0. [DOI] [PubMed] [Google Scholar]
- 76.Boeve BF. The multiple phenotypes of corticobasal syndrome and corticobasal degeneration: implications for further study. J Mol Neurosci. 2011;45:350–353. doi: 10.1007/s12031-011-9624-1. [DOI] [PubMed] [Google Scholar]
- 77.Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, et al. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ling H, O’Sullivan SS, Holton JL, Revesz T, Massey LA, Williams DR, et al. Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain. 2010;133(Pt 7):2045–2057. doi: 10.1093/brain/awq123. [DOI] [PubMed] [Google Scholar]
- 79.Kompoliti K, Goetz CG, Boeve BF, Maraganore DM, Ahlskog JE, Marsden CD, et al. Clinical presentation and pharmacological therapy in corticobasal degeneration. Arch Neurol. 1998;55:957–961. doi: 10.1001/archneur.55.7.957. [DOI] [PubMed] [Google Scholar]
- 80.Constantinescu R, Richard I, Kurlan R. Levodopa responsiveness in disorders with parkinsonism: a review of the literature. Mov Disord. 2007;22:2141–2148. doi: 10.1002/mds.21578. [DOI] [PubMed] [Google Scholar]
- 81.Grisoli M, Fetoni V, Savoiardo M, Girotti F, Bruzzone MG. MRI in corticobasal degeneration. Eur J Neurol. 1995;2:547–552. doi: 10.1111/j.1468-1331.1995.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 82.Wenning GK, Litvan I, Jankovic J, Granata R, Mangone CA, McKee A, et al. Natural history and survival of 14 patients with corticobasal degeneration confirmed at postmortem examination. J Neurol Neurosurg Psychiatry. 1998;64:184–189. doi: 10.1136/jnnp.64.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wenning GK, Kiechl S, Seppi K, Müller J, Högl B, Saletu M, et al. Prevalence of movement disorders in men and women aged 50-89 years (Bruneck Study cohort): a population-based study. Lancet Neurol. 2005;4:815–820. doi: 10.1016/S1474-4422(05)70226-X. [DOI] [PubMed] [Google Scholar]
- 84.Shin HW, Chung SJ. Drug-induced parkinsonism. J Clin Neurol. 2012;8:15–21. doi: 10.3988/jcn.2012.8.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Esper CD, Factor SA. Failure of recognition of drug-induced parkinsonism in the elderly. Mov Disord. 2008;23:401–404. doi: 10.1002/mds.21854. [DOI] [PubMed] [Google Scholar]
- 86.Jones HM, Pilowsky LS. Dopamine and antipsychotic drug action revisited. Br J Psychiatry. 2002;181:271–275. doi: 10.1192/bjp.181.4.271. [DOI] [PubMed] [Google Scholar]
- 87.Shin HW, Kim JS, Oh M, You S, Kim YJ, Kim J, et al. Clinical features of drug-induced parkinsonism based on [18F] FP-CIT positron emission tomography. Neurol Sci. 2015;36:269–274. doi: 10.1007/s10072-014-1945-8. [DOI] [PubMed] [Google Scholar]
- 88.Hong JY, Sunwoo MK, Oh JS, Kim JS, Sohn YH, Lee PH. Persistent drug-induced parkinsonism in patients with normal dopamine transporter imaging. PLoS One. 2016;11:e0157410. doi: 10.1371/journal.pone.0157410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mostile G, Nicoletti A, Zappia M. Vascular parkinsonism: still looking for a diagnosis. Front Neurol. 2018;9:411. doi: 10.3389/fneur.2018.00411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.FitzGerald PM, Jankovic J. Lower body parkinsonism: evidence for vascular etiology. Mov Disord. 1989;4:249–260. doi: 10.1002/mds.870040306. [DOI] [PubMed] [Google Scholar]
- 91.Korczyn AD. Vascular parkinsonism--characteristics, pathogenesis and treatment. Nat Rev Neurol. 2015;11:319–326. doi: 10.1038/nrneurol.2015.61. [DOI] [PubMed] [Google Scholar]
- 92.Rektor I, Rektorová I, Kubová D. Vascular parkinsonism--an update. J Neurol Sci. 2006;248:185–191. doi: 10.1016/j.jns.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 93.Vizcarra JA, Lang AE, Sethi KD, Espay AJ. Vascular Parkinsonism: deconstructing a syndrome. Mov Disord. 2015;30:886–894. doi: 10.1002/mds.26263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rektor I, Bohnen NI, Korczyn AD, Gryb V, Kumar H, Kramberger MG, et al. An updated diagnostic approach to subtype definition of vascular parkinsonism - Recommendations from an expert working group. Parkinsonism Relat Disord. 2018;49:9–16. doi: 10.1016/j.parkreldis.2017.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Levin OS. Correspondence relating to “an updated diagnostic approach to subtype definition of vascular parkinsonism - Recommendations from an expert working group”. Parkinsonism Relat Disord. 2018;52:109–110. doi: 10.1016/j.parkreldis.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 96.Frank E, Tew JM., Jr Normal-pressure hydrocephalus: clinical symptoms, diagnosis, pathophysiology, and treatment. Heart Lung. 1982;11:321–326. [PubMed] [Google Scholar]
- 97.Nassar BR, Lippa CF. Idiopathic normal pressure hydrocephalus: a review for general practitioners. Gerontol Geriatr Med. 2016;2:2333721416643702. doi: 10.1177/2333721416643702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Curran T, Lang AE. Parkinsonian syndromes associated with hydrocephalus: case reports, a review of the literature, and pathophysiological hypotheses. Mov Disord. 1994;9:508–520. doi: 10.1002/mds.870090503. [DOI] [PubMed] [Google Scholar]
- 99.Molde K, Söderström L, Laurell K. Parkinsonian symptoms in normal pressure hydrocephalus: a population-based study. J Neurol. 2017;264:2141–2148. doi: 10.1007/s00415-017-8598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bugalho P, Alves L, Miguel R. Gait dysfunction in Parkinson’s disease and normal pressure hydrocephalus: a comparative study. J Neural Transm (Vienna) 2013;120:1201–1207. doi: 10.1007/s00702-013-0975-3. [DOI] [PubMed] [Google Scholar]
- 101.Stolze H, Kuhtz-Buschbeck JP, Drücke H, Jöhnk K, Illert M, Deuschl G. Comparative analysis of the gait disorder of normal pressure hydrocephalus and Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2001;70:289–297. doi: 10.1136/jnnp.70.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Espay AJ, Da Prat GA, Dwivedi AK, Rodriguez-Porcel F, Vaughan JE, Rosso M, et al. Deconstructing normal pressure hydrocephalus: ventriculomegaly as early sign of neurodegeneration. Ann Neurol. 2017;82:503–513. doi: 10.1002/ana.25046. [DOI] [PubMed] [Google Scholar]
- 103.Halperin JJ, Kurlan R, Schwalb JM, Cusimano MD, Gronseth G, Gloss D. Practice guideline: idiopathic normal pressure hydrocephalus: response to shunting and predictors of response: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2015;85:2063–2071. doi: 10.1212/WNL.0000000000002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pujari S, Kharkar S, Metellus P, Shuck J, Williams MA, Rigamonti D. Normal pressure hydrocephalus: long-term outcome after shunt surgery. J Neurol Neurosurg Psychiatry. 2008;79:1282–1286. doi: 10.1136/jnnp.2007.123620. [DOI] [PubMed] [Google Scholar]
- 105.Ohara M, Hattori T, Yokota T. Progressive supranuclear palsy often develops idiopathic normal pressure hydrocephalus-like magnetic resonance imaging features. Eur J Neurol. 2020;27:1930–1936. doi: 10.1111/ene.14322. [DOI] [PubMed] [Google Scholar]
- 106.Constantinides VC, Paraskevas GP, Velonakis G, Toulas P, Stefanis L, Kapaki E. Midbrain morphology in idiopathic normal pressure hydrocephalus: a progressive supranuclear palsy mimic. Acta Neurol Scand. 2020;141:328–334. doi: 10.1111/ane.13205. [DOI] [PubMed] [Google Scholar]
- 107.Cabral D, Beach TG, Vedders L, Sue LI, Jacobson S, Myers K, et al. Frequency of Alzheimer’s disease pathology at autopsy in patients with clinical normal pressure hydrocephalus. Alzheimers Dement. 2011;7:509–513. doi: 10.1016/j.jalz.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.