Abstract
Objective
To retrospectively analyze and compare the relationship between the success rate of patient‐derived xenograft (PDX) modeling of osteosarcoma and prognosis (3‐year overall survival rate and disease‐free survival rate) and incidence of lung metastasis.
Methods
The sample group consisted of 57 osteosarcoma patients with definite pathological diagnoses from Shanghai General Hospital from 2015–2017. PDX models in 57 patients were analyzed by retrospective analyses. Among the patients currently inoculated, 20 were tumorigenic in the PDX model, and 37 were nontumorigenic. According to the tumorigenicity of PDXs, the corresponding osteosarcoma patients were divided into two groups. The effects of clinically related indicators on the model were retrospectively compared. The patients were followed, and the 3‐year survival, 3‐year disease‐free survival (DFS), and lung metastasis rates were collected. The relationship between the modeling success and patient prognosis was investigated.
Results
In the chemotherapy‐treated group, the PDX modeling success rate was 17.4%, and in the nonchemotherapy group, the success rate was 47.1%. The success of PDX modeling was related to whether patients received chemotherapy. The success rate of PDX modeling is significantly reduced after receiving chemotherapy. The 3‐year overall survival rate of the PDX‐grafted group was 49.23%, and that of the PDX‐nongrafted group was 65.71%. There was a significant difference between the two groups, showing a strong negative correlation between the 3‐year survival rate and the success rate of the PDX model. The 3‐year disease‐free survival rate of the PDX‐grafted group was 29.54%. The 3‐year DFS of the PDX‐nongrafted group was 50.34%. There was a significant difference between the two groups. Lower grafted rates indicate a higher DFS rate. The incidence of lung metastasis in the PDX‐grafted group was 32.4%, and that in the nongrafted group was 13.1%. There was a significant difference between the two groups. The successful establishment of the PDX model indicates that patients are more likely to have lung metastases.
Conclusions
The success of PDX modeling often indicates poor prognosis (low 3‐year overall survival rate and disease‐free survival rate) and a greater possibility of lung metastasis. Therefore, PDX modeling in osteosarcoma patients can accurately predict the prognosis of patients and the risk of lung metastasis in advance to help us develop better therapeutic strategies.
Keywords: Chemotherapy, Osteosarcoma, Patient‐derived xenograft, Prognosis
This study illustrated the relationship between success rate of osteosarcoma PDX modeling with the patient prognosis. And it focused on the success rate of PDX modeling with the DFS, OS, and lung metastasis rate.

Introduction
Osteosarcoma is the most common malignant bone tumor that occurs in children and adolescents, and the incidence is approximately three per million 1 . It is the most common malignant tumor in children's solid cancer that places a very large burden on families, society, and medical insurance. It has a high degree of malignancy and is prone to recurrence and metastasis. In the clinic, our current therapy method is surgical resection combined with chemotherapy, and with the progression of neoadjuvant chemotherapy, the 5‐year survival rate of osteosarcoma patients has increased to 60%–70% 2 . However, patients experience recurrence or metastasis in the early treatment process, and once drug resistance or metastasis occurs, the 5‐year survival rate of patients is reduced to less than 20% 3 . Therefore, we need a more accurate way to predict the malignant degree of tumors so that we can judge the state of the patient's disease more comprehensively. Some methods can predict the prognosis of patients to some extent, such as the preoperative chemotherapy necrosis rate, gene sequence, and other methods 4 , but it is still necessary to find more comprehensive and effective prognostic methods. This enables clinicians to better understand the state of the patient's disease and develop more insightful therapy strategies for dealing with osteosarcoma 5 .
Owing to the heterogeneity of osteosarcoma, it is very difficult for different patients to adopt the same methods or indicators to predict the prognosis, such as the commonly used methods of gene sequencing 6 and the preoperative chemotherapy necrosis rate. The PDX (patient‐derived xenograft) model is a kind of tumor model that is formed by inoculating human tumor tissue subcutaneously into nude mice. Tumor tissue can grow and metastasize in vivo, and tumor tissue can pass to the next generation of nude mice to perform more experiments, such as drug sensitivity tests and gene analyses 7 , 8 , 9 . To achieve this, fresh tumor tissue must be obtained from surgery, processed and chemically digested, preserved as primary cells, and planted in immunodeficient mice such as nude mice. When the tumor is large enough, it could be passed directly between mice. In the process of model establishment, tumor tissue can be transplanted heterotopically or orthotopically. The ectopic PDX model involves implanting tumors under the side skin of mice. This method can be used to more easily observe tumor growth. The advantage of this model is that it retains the heterogeneity of the tumor tissue itself and can reflect the situation of the tumor more appropriately 10 . At present, PDX models have been used in many kinds of tumors, and some scholars have used PDX models to predict the drug sensitivity of tumors 11 and to synchronize co‐preclinical trials of drugs 12 . At present, there are few reports about the use of the PDX model to predict the prognosis of osteosarcoma patients.
In this study, we collected the prognosis of 57 osteosarcoma patients in Shanghai General Hospital during 2015–2017, including the 3‐year overall survival rate, 3‐year disease‐free survival rate, and incidence of lung metastasis. Then, we retrospectively analyzed the relationship between PDX modeling and the prognosis of osteosarcoma patients.
The purpose of our research is to explore (i) the relationship between the success rate of PDX construction and the patient's 3‐year overall survival rate; (ii) the relationship between the success rate of PDX construction and the patient's disease‐free survival rate; and (iii) the relationship between the success rate of PDX model construction and the occurrence of lung metastasis. We hope that this study will provide a new prognostic model so that clinicians can be more enlightened and comprehensively deal with osteosarcoma.
Material and Methods
Patients and Tissue Specimens
All 57 osteosarcoma patients were from Shanghai General Hospital. The inclusion criteria were as follows: (i) patients who presented with osteosarcoma and had a definite pathological diagnosis; (ii) patient tumor specimens were transferred to a tissue culture room and animal facility under sterile conditions to establish the model; (iii) the success or failure of the model was observed for 4 months; (iv) the patient was treated with conventional surgical resection and chemotherapy; (v) all clinical data, including sex, age, pathology, tumor stage, tumor size, metastasis, tumor location, and chemotherapy status, were available in clinical records; and (vi) statistical analysis was conducted on the clinical data and PDX model construction to explore the correlation between them. All patient‐related studies were approved by the Shanghai General Hospital Ethics Committee, and informed consent was signed by the patients.
Establishment of the PDX Model
Tumor specimens were removed under aseptic conditions during the operation. The specimens were removed and transferred to the tissue culture room. The specimens were washed with PBS several times, cut into small pieces approximately 2 mm in diameter, and transplanted to the subcutaneous part of the flank of mice. The growth of tumors in mice was observed. The tumors were subcultured and inoculated until the diameter of the tumors reached approximately 10 mm (Figure 1A). If there was no obvious tumorigenesis in 4 months, the model was considered a failure 13 . All animal experiments were approved by the Shanghai General Hospital Ethics Committee.
Fig. 1.
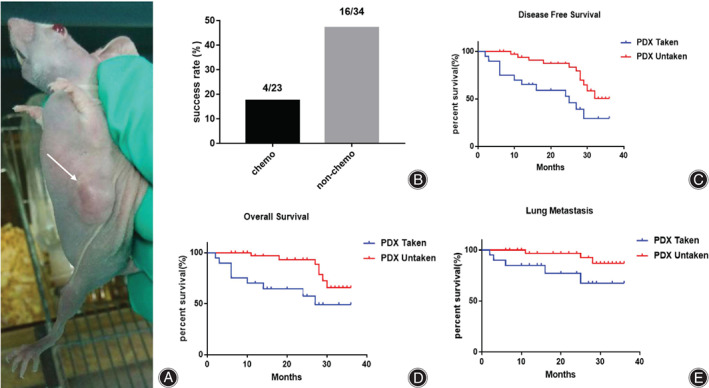
(A) PDX model of a 57‐year‐old female femur OS patient. The arrow indicates the tumor (Canon EOS 70D, scale bar = 1 cm). (B) The PDX modeling success rate correlates with chemotherapy, as the nonchemo group had an obviously higher success rate of PDX modeling than the chemo group. (C) 3‐year DFS is correlated with PDX modeling. (D) Three‐year OS is correlated with PDX modeling. The successful PDX modeling group had significantly lower disease‐free survival time and overall survival time. (E) The 3‐year lung metastasis rate is correlated with PDX modeling in osteosarcoma patients. The successful PDX modeling group had a significantly higher lung metastasis rate. *P < 0.05, significantly different compared with the unsuccessful PDX group
Parameters
Success Rate14
The success rate was used to evaluate the success rate of establishing the PDX model. The success rate was calculated by dividing the number of PDX success models by the number of PDX total inoculations. The standard for successful tumor establishment was tumor growth to approximately 10 mm in diameter.
Disease‐Free Survival (DFS)15
The disease‐free survival rate was used to indicate the time after treatment during which no sign of tumor, either recurrence or metastasis, was found. DFS was calculated by the disease‐free numbers divided by the total patient numbers.
Overall Survival (OS)15
Overall survival was defined as the time from the start of treatment, and it was expressed as the percentage of patients diagnosed with the disease who were still alive. OS was calculated by the number of living patients divided by the total number of patients. Assessment criteria: The patient should be alive when calculating the OS.
Statistics
GraphPad Prism software (version 5.01 for Windows, GraphPad Software, San Diego, California, USA) was used for statistical analysis. The χ 2 test was used to determine the relationship between all clinical indicators and the success of PDX modeling. Kaplan–Meier survival analysis and the log rank test were used for DFS, OS, and lung metastasis analysis. All tests were two‐tailed, and P values less than 0.05 indicated the statistical significance of the results.
Results
Success Rate of PDX Modeling Correlated with the Chemotherapy Situation
Since December 2015, 119 cases of sarcoma have been inoculated, of which 57 cases were osteosarcoma patients; 20 cases were successfully inoculated from osteosarcoma patients, and the success rate of xenografts was 35.1%. Among them, 32 were males and 25 were females. Twelve were successful in males, for a success rate of 37.5%, and eight were successful in females, representing a success rate of 32% (χ 2 = 0.1580; P = 0.6659). There was no significant difference in the success rate between men and women. Thirty‐four cases were inoculated under 18 years old; of these, 13 cases were successfully inoculated, and the success rate was 38.2%. Twenty‐three cases were inoculated over 18 years old; of these, seven cases were inoculated successfully, and the success rate was 30.4% (χ 2 = 0.0652; P = 0.5449). There was no significant difference in the influence of age on the success rate. According to the Ennecking stage of osteosarcoma, 20 cases were inoculated in stage I–IIA, five of which were inoculated successfully for a success rate of 25%; 37 cases were in stage IIB–III, and 15 cases were in stage IIB–III. The success rate of these cases was 40.5% (χ 2 = 0.9898; P = 0.2407).
There was no significant difference in the effect of stage on the success rate of the PDX model of osteosarcoma. Thirty‐three cases were inoculated with tumor diameters less than 8 cm, among which 13 cases were successful for a success rate of 39.4%; 24 cases were inoculated with tumor diameters greater than 8 cm, and seven of these cases were successful, representing a success rate of 29.2% (χ 2 = 0.1041; P = 0.4244). There was no significant difference in the success rate of PDX modeling based on the size of tumors. There were 47 cases with no distant metastasis at xenograft; of these, 15 cases were successful for a success rate of 31.9%. There were 10 cases with distant metastasis at xenograft, five of which were successful for a success rate of 50%. There was no significant difference in the success rate of PDX modeling between patients with and without distant metastasis. Among twenty‐seven cases of G1–G2, nine cases (33.3%) were inoculated successfully, and among 30 cases of G3–G4, 11 cases (36.7%) were inoculated successfully (χ 2 = 0.6232; P = 0.7923). There was no significant difference in the success rate of xenografts among histological grades. Seventeen cases with medial axis osteosarcoma were inoculated, and five cases were successful, for a success rate of 29.4%; 40 cases with limb extremity osteosarcoma were inoculated, and 15 cases were successful, for a success rate of 37.5% (χ 2 = 0.9995; P = 0.5583). There was no significant difference in the success rate of PDX modeling among tumor growth sites.
Twenty‐three patients received chemotherapy before xenografting, four of which were successful, and the success rate was 17.4%; 34 patients did not receive chemotherapy, 16 of which were successful, and the success rate was 47.1%. The success rate of PDX model xenografts with or without chemotherapy was significantly different (χ 2 = 0.3918; P = 0.0213), and the success rate of PDX xenografts in patients receiving chemotherapy was reduced by 29.7% (Table 1, Figure 1B). The difference in the PDX modeling success rate indicated that chemotherapy had a negative effect on the PDX modeling.
TABLE 1.
Clinical pathological features of osteosarcoma patients and their correlation with PDX modeling
| Characteristics | Cases (n) | PDX grafting | χ 2 | P value | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Gender | |||||
| Male | 32 | 12 (37.5%) | 20 (62.5%) | 0.1864 | 0.6659 |
| Female | 25 | 8 (32%) | 17 (68%) | ||
| Age (years) | |||||
| ≤18 | 34 | 13 (38.2%) | 21 (61.8%) | 0.3665 | 0.5449 |
| >18 | 23 | 7 (30.4%) | 16 (69.6%) | ||
| Enneking stage | |||||
| I–II A | 20 | 5 (25%) | 15 (75%) | 1.377 | 0.2407 |
| II B–III | 37 | 15 (40.5%) | 22 (59.5%) | ||
| Tumor size (cm) | |||||
| ≤8 | 33 | 13 (39.4%) | 20 (60.6%) | 0.6381 | 0.4244 |
| >8 | 24 | 7 (29.2%) | 17 (70.9%) | ||
| Distant metastasis | |||||
| Absence | 47 | 15 (31.9%) | 32 (68.1%) | 1.184 | 0.2765 |
| Presence | 10 | 5 (50%) | 5 (50%) | ||
| Histological grade | |||||
| G1–G2 | 27 | 9 (33.3%) | 18 (66.7%) | 0.0693 | 0.7923 |
| G3–G4 | 30 | 11 (36.7%) | 19 (63.3%) | ||
| Tumor site | |||||
| Axial skeleton | 17 | 5 (29.4%) | 12 (70.6%) | 0.3427 | 0.5583 |
| Extremities | 40 | 15 (37.5%) | 25 (62.5%) | ||
| Chemotherapy | |||||
| Yes | 23 | 4 (17.4%) | 19 (82.6%) | 5.302 | 0.0213 |
| No | 34 | 16 (47.1%) | 18 (52.9%) | ||
Success Rate of PDX Modeling Related to Patients' 3‐year DFS and OS
We found that the success of PDX modeling was related to patients' 3‐year DFS. The 3‐year DFS was 29.54% in patients with successful PDX modeling and 50.34% in patients with unsuccessful PDX modeling. There was a significant difference between the two groups (P = 0.01). The 3‐year DFS of successfully modeled patients was significantly lower than that of unsuccessful patients, and the difference was 20.8%. Successful modeling can be used as a predictor of 3‐year DFS in patients (Figure 1C). At the same time, we found that the success of PDX modeling was related to patients' 3‐year OS. The 3‐year OS was 49.23% in patients with successful PDX modeling and 65.71% in patients with unsuccessful modeling. There was a significant difference between the two groups (P = 0.01). The 3‐year OS of successfully modeled patients was significantly lower than that of unsuccessful patients. In addition, the difference was 16.48%. Successful modeling can be used as a predictor of 3‐year OS in patients (Figure 1D).
Success Rate of PDX Modeling Correlated with the Occurrence of Lung Metastasis
We found that the success of PDX modeling was correlated with lung metastasis. The 3‐year follow‐up showed that the rate of lung metastasis was 32.4% in patients who were successfully modeled. Compared with the unsuccessful group, the 3‐year incidence of lung metastasis was 13.1%. There was a significant difference between the two groups (P = 0.04). In addition, the difference was 19.3%. This finding suggests that patients with successful modeling are more prone to lung metastasis. Whether modeling is successful or not can be used as a predictor of lung metastasis (Figure 1E).
Discussion
The PDX model is a new model of transplanted tumors for cancer patients that can effectively simulate the tumors of patients. Compared with the cell line‐transplanted tumor model, PDXs retain the heterogeneity of tumor tissue better and retain the matrix components of tumors 7 . At present, many studies have applied PDX models to the establishment of cancer models 16 , drug screening, coclinical trials, cancer biology research 12 , and so on.
Chemotherapy Treatment had a Certain Effect on the Success Rate of PDX Modeling
The prognosis of osteosarcoma patients determines the survival of patients 17 . At present, many studies have found that many biomarkers can be used to predict the prognosis of osteosarcoma patients 18 . In our previous study, we found that the time of PDX tumorigenesis in osteosarcoma patients varied greatly from patient to patient. The fastest time for PDX tumorigenesis was approximately 20 days, and the slowest time was more than 3 months, or even no tumor formation. What exactly affects the success rate of PDX modeling? We studied the factors that affect the tumorigenesis of osteosarcoma patients. Factors such as sex, age, stage, size of tumors, metastasis, histological grade, location of tumors, and whether they had received chemotherapy were successfully modeled according to the PDX model. Retrospectively, we found that chemotherapy had a certain effect on the success rate of PDX modeling in osteosarcoma patients. The success rate of PDX modeling in patients after chemotherapy was significantly lower than that in patients without chemotherapy. We believe that the growth of tumors and the activity of tumor cells in patients after chemotherapy were inhibited to a certain extent; therefore, when transplanted into mice, the regeneration and tumorigenesis of tumor cells decreased, so the success rate of PDX modeling decreased.
PDX Modeling can be Used to Predict the Prognosis of Patients
We observed that the rate of tumor modeling in the PDX model was related to the degree of tumorigenesis. Tumors from a 47‐year‐old male patient with pelvic osteosarcoma developed in 20 days. When the tumors grew rapidly in the PDX model, the tumors recurred quickly, the tumors progressed rapidly, and the patients died. In some nontumorigenic PDX models, the prognosis of the corresponding patients is usually better. From the point of view of the biological behavior of tumors, the rapid growth of the PDX model also indicates that the tumors themselves have high invasiveness or strong proliferation ability, so they can grow rapidly in mice. Similarly, tumors are more prone to recurrence and metastasis in patients. Therefore, in this study, we used PDX modeling success as a variable and then analyzed the correlation between the success of PDX modeling and the prognosis of osteosarcoma patients, focusing on 3‐year DFS and OS. At the same time, we studied the lung metastasis rate of patients. Our results suggest that the 3‐year DFS and OS of patients with successful PDX modeling are significantly decreased, suggesting that the prognosis of patients with successful PDX modeling is poor. The success of PDX modeling can also be used as an indicator to predict the prognosis of patients.
PDX Modeling can be used to Predict Cancer Metastasis
Tumor metastasis is often related to the invasiveness of the tumor cells themselves. We observed that the incidence of lung metastasis in PDX successfully modeled patients was significantly higher than that in unsuccessfully modeled patients, suggesting that the success of modeling is correlated with the occurrence of lung metastasis. The invasiveness of tumor cells in modeled patients is significantly higher than that in unsuccessfully modeled patients. In addition, the prognosis was poor.
In conclusion, our study suggests that the PDX model can better simulate the growth of patients' tumors in vivo. At the same time, the success of the PDX model can be used as an important predictor of patient prognosis, which provides a new idea for the clinical application of PDXs.
Limitations
Although the success or failure of PDX modeling can be used to predict the prognosis of patients, the treatment status of patients when inoculating determines the speed of PDX model establishment, and in some cases, tumors did not even form. In addition, the establishment cost of the PDX model is high, the cost for patients is great, and thus, the economic cost limits the popularity of this method. It is necessary to study faster modeling methods, for example, organoid PDX models, and cell xenograft models, to popularize this prognosis prediction model.
Contributor Information
Chuanying Zhang, Email: zcy333333@sina.com.
Yingqi Hua, Email: hua_yingqi@163.com.
References
- 1. Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010;21 Suppl 7:vii320–5. [DOI] [PubMed] [Google Scholar]
- 2. Kansara M, Teng MW, Smyth MJ, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer. 2014;14:722–35. [DOI] [PubMed] [Google Scholar]
- 3. Jaffe N. Osteosarcoma: review of the past, impact on the future. The American experience. Cancer Treat Res. 2009;152:239–62. [DOI] [PubMed] [Google Scholar]
- 4. Cong C, Wang W, Tian J, Gao T, Zheng W, Zhou C. Identification of serum miR‐124 as a biomarker for diagnosis and prognosis in osteosarcoma. Cancer Biomark. 2018;21:449–54. [DOI] [PubMed] [Google Scholar]
- 5. Corre I, Verrecchia F, Crenn V, Redini F, Trichet V. The osteosarcoma microenvironment: a complex however, targetable ecosystem. Cells. 2020;9:976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zheng Z, Bao F, Chen X, Huang H, Zhang X. MicroRNA‐330‐3p expression indicates good prognosis and suppresses cell proliferation by targeting Bmi‐1 in osteosarcoma. Cell Physiol Biochem. 2018;46:442–50. [DOI] [PubMed] [Google Scholar]
- 7. Khandelwal G, Girotti MR, Smowton C, Taylor S, Wirth C, Dynowski M, et al. Next‐generation sequencing analysis and algorithms for PDX and CDX models. Mol Cancer Res. 2017;15:1012–6. [DOI] [PubMed] [Google Scholar]
- 8. Shi J, Li Y, Jia R, Fan X. The fidelity of cancer cells in PDX models: characteristics, mechanism and clinical significance. Int J Cancer. 2020;146:2078–88. [DOI] [PubMed] [Google Scholar]
- 9. Lee MW, Miljanic M, Triplett T, Ramirez C, Aung KL, Eckhardt SG, et al. Current methods in translational cancer research. Cancer Metastasis Rev. 2021;40:7–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blomme A, Van Simaeys G, Doumont G, Costanza B, Bellier J, Otaka Y, et al. Murine stroma adopts a human‐like metabolic phenotype in the PDX model of colorectal cancer and liver metastases. Oncogene. 2018;37:1237–50. [DOI] [PubMed] [Google Scholar]
- 11. Williams JA. Using PDX for preclinical cancer drug discovery: the evolving field. J Clin Med. 2018;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koga Y, Ochiai A. Systematic review of patient‐derived xenograft models for preclinical studies of anti‐cancer drugs in solid tumors. Cell. 2019;8:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hidalgo M, Amant F, Biankin AV, Budinská E, Byrne AT, Caldas C, et al. Patient‐derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4:998–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Conte N, Mason JC, Halmagyi C, Neuhauser S, Mosaku A, Yordanova G, et al. PDX Finder: a portal for patient‐derived tumor xenograft model discovery. Nucleic Acids Res. 2019;47:D1073–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smeele LE, Kostense PJ, van der Waal I, Snow GB. Effect of chemotherapy on survival of craniofacial osteosarcoma: a systematic review of 201 patients. J Clin Oncol. 1997;15:363–7. [DOI] [PubMed] [Google Scholar]
- 16. Braekeveldt N, Bexell D. Patient‐derived xenografts as preclinical neuroblastoma models. Cell Tissue Res. 2018;372:233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Faisham WI, Mat Saad AZ, Alsaigh LN, Nor Azman MZ, Kamarul Imran M, Biswal BM, et al. Prognostic factors and survival rate of osteosarcoma: a single‐institution study. Asia Pac J Clin Oncol. 2017;13:e104–10. [DOI] [PubMed] [Google Scholar]
- 18. Fujiwara T, Uotani K, Yoshida A, Morita T, Nezu Y, Kobayashi E, et al. Clinical significance of circulating miR‐25‐3p as a novel diagnostic and prognostic biomarker in osteosarcoma. Oncotarget. 2017;8:33375–92. [DOI] [PMC free article] [PubMed] [Google Scholar]


