Abstract
Objective
To review the clinical outcomes of revision total knee arthroplasty (RTKA) with massive proximal tibial bone defects using patient‐customized three‐dimensional (3D) printed highly porous metaphyseal cones.
Methods
A retrospective study of all patients at our institution who underwent RTKA with the Anderson Orthopaedic Research Institute type III tibial defects using patient‐customized 3D‐printed highly porous metaphyseal cones was performed from 2016 to 2018. Seven patients were enrolled in this study. General results (age, sex, and body mass index); intraoperative results (interface compatibility and stability, and operating time); and perioperative complications (total blood loss, blood transfusion rate, and deep venous thrombosis) were recorded and analyzed. Clinical improvement and functional evaluation (survivorship of implant, improvement of Hospital for Special Surgery Score and McMaster Universities Osteoarthritis Index, and improvement of range of motion [ROM]), and radiographic improvement and implant evaluation (progressive radiolucent lines or radiographic loosening, and mechanical alignment) were evaluated at 2 weeks, 6 weeks, 3 months, 6 months, 1 year, 2 years, and then annually, postoperatively.
Results
The mean age at diagnosis was 68 (61–77) years. The mean follow‐up was 25.3 (19–36) months. At the latest follow‐up, no aseptic loosening, prosthetic joint infection, or other complications were noted. The mean Hospital for Special Surgery Score increased from 49 (39–63) to 78 (70–83) (P < 0.01), whereas the mean Western Ontario and McMaster Universities Osteoarthritis Index increased from 59 (46–73) to 26 (12–38) (P < 0.01). All patients achieved improved postoperative ROM with the mean flexion angle increasing from 66° (30°–80°) to 93° (80°–100°), and improved mechanical alignment with all hip–knee–ankle (HKA) angles within ±3°.
Conclusions
The patient‐customized 3D‐printed metaphyseal cone is useful technique for reconstructing massive proximal tibial bone defects, with encouraging clinical and radiological outcomes in RTKA.
Keywords: 3D printing, Bone defects, Metaphyseal cone, Patient‐customized, Revision total knee arthroplasty
We reviewed the efficacy of using patient‐customized 3D‐printed cones in massive proximal tibial defects (AORI type III) RTKAs in our institution. Our results suggest encouraging clinical and radiological outcomes in an average of 25‐month follow‐up.
Introduction
The volume of revision total knee arthroplasty (RTKA) is increasing with the aging population and the consequent increasing demand for primary TKA. A total of 268,000 RTKAs are expected in the United States by 2030, six times the number in 2005. 1 RTKA can be challenging owing to preexisting bone defects caused by periprosthetic infection, aseptic loosening, polyethylene wear, or periprosthetic fractures, which can occur on either the proximal tibia or distal femur. 2 A full preoperative assessment of the features of bone defects, including size, severity, and location, is usually needed to determine the most suitable surgical plan. The Anderson Orthopaedic Research Institute (AORI) classification 3 is the most effective and frequently used system for this purpose. 4
Recently, cones and sleeves have become mainstream in the reconstruction of massive proximal tibial defects (AORI types IIB and III). Cones perform better in filling defects, 5 whereas sleeves focus more on stability. 6 Zanirato et al. 7 systemically reviewed 37 published studies on RTKA with bone defect restoration and found that both the cone and sleeve groups showed promising clinical and functional outcomes. The aseptic survivorship of the implants was 97.3% and 97.8% in the cone and sleeve groups, respectively.
However, precise anatomic reconstruction and biomechanical restoration are challenging to accomplish in that uncustomized cones and sleeves cannot perfectly fit into various bone defects. 5 During cone or sleeve insertion, further sculpturing and reaming of the host bone with a broach or high‐speed burr are required. However, it is still difficult to fit an uncustomized cone or sleeve to the defective site. In addition, additional bone sculpturing increases the complexity of the procedure, prolongs the operating time, and introduces iatrogenic bone loss. Intraoperative fracture caused by implant insertion is another unneglected risk, which increases the difficulty of the procedure even more. 8 Previous studies have shown a relatively low survival rate for prostheses and patient satisfaction in RTKA with massive tibial defects. Surgical complications such as postoperative pain, are common. 9 , 10 Innovative technology is urgently required to address this dissatisfaction.
The maturity of 3D‐printing technology makes the customized cone, modeled following the patient's anatomical construction, a novel solution for bone‐defect restoration. A cone composed of trabecular titanium imitating natural trabecular bone morphology meets biomechanical demands, facilitates osseointegration, and induces bone ingrowth. 11 , 12 High friction from the porous surface also provides immediate fixation. A simplified procedure with a shortened operating time is another advantage, particularly when massive bone defects are involved. 13 However, few studies have focused on the utilization of patient‐customized 3D‐printed cones are less reported in RTKA with massive bone defects.
In this study, we present a retrospective review of massive proximal tibial defects RTKAs treated with this novel patient‐customized 3D‐printed highly porous metaphyseal cones in our institution. This study aimed to: (i) describe the preoperative preparation, surgical procedure, and perioperative management of this technology; (ii) evaluate the implant survivorship, complications, and clinical and radiographic outcomes; and (iii) assess the feasibility, advantages, and application prospects of this technology.
Methods
Study Design and Participants
A single‐center case series study was conducted. This study reviewed nine patients with massive proximal tibial defects (AORI type III) who underwent RTKA and received patient‐customized 3D‐printed highly porous metaphyseal cones in our institution from 2016 to 2018. Two patients with co‐existing massive distal femoral defects or incomplete medical records were excluded, leaving seven patients for further evaluation.
This study was approved by the Ethics Committee of Peking University Third Hospital(IRB00006761‐M2019029). All patients provided signed informed consent.
Preoperative Preparation
Computed Tomography (CT) Scanning and Modeling of the Tibia
Before establishing surgical procedures, patients underwent non‐contrast CT scans (slice thickness: 0.625) of their affected knees from the mid‐femur to mid‐tibia. The CT imaging set (DICOM format) was imported into MIMICS 17 software (Materialise, Leuven, Belgium) to obtain a 3D model of the proximal tibia. The 3D model (STL format) was processed using Unigraphics NX 10.0, software (Siemens PLM Software, Munich, Germany).
Design of Patient‐Customized 3D‐Printed Highly Porous Metaphyseal Cone
The appropriate size and model of the tibial prosthesis and diaphyseal stem (ACCK revision knee system, AK Medical, Beijing, China) were determined by performing virtual bone cutting and prosthesis installation in Unigraphics according to the patient's axial alignment of the lower extremity. A suitable cone was designed on the basis of the contour of the bone defects and the preselected prosthesis. The upper margin of the cone was designed to sit at the level of the head of the fibula after being appropriately inserted into the host bone, which makes joint line restoration effortlessly achievable.
Three‐Dimensional Printing of Patient‐Customized Highly Porous Metaphyseal Cone
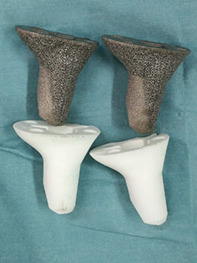
The 3D model of the cone (STL format) was exported into an electron beam melting rapid prototyping machine (Arcam, Sweden), in which fine titanium powders (Ti6Al4V) were fused into a highly porous cone layer‐by‐layer. The diameters of the pores and wires were 600–1000 μm and 350–750 μm, respectively, with an average porosity of 50%–80%. These features are beneficial for bone ingrowth, both in vitro and in vivo. 14 , 15 Another identical polyamide cone was printed as a trial and sterilized with radiation for surgical use (Fig. 1). In addition, two cones with diameters 2mm larger or smaller than the standard one were also printed, given the uncertainty of intraoperative bone modification.
Fig. 1.
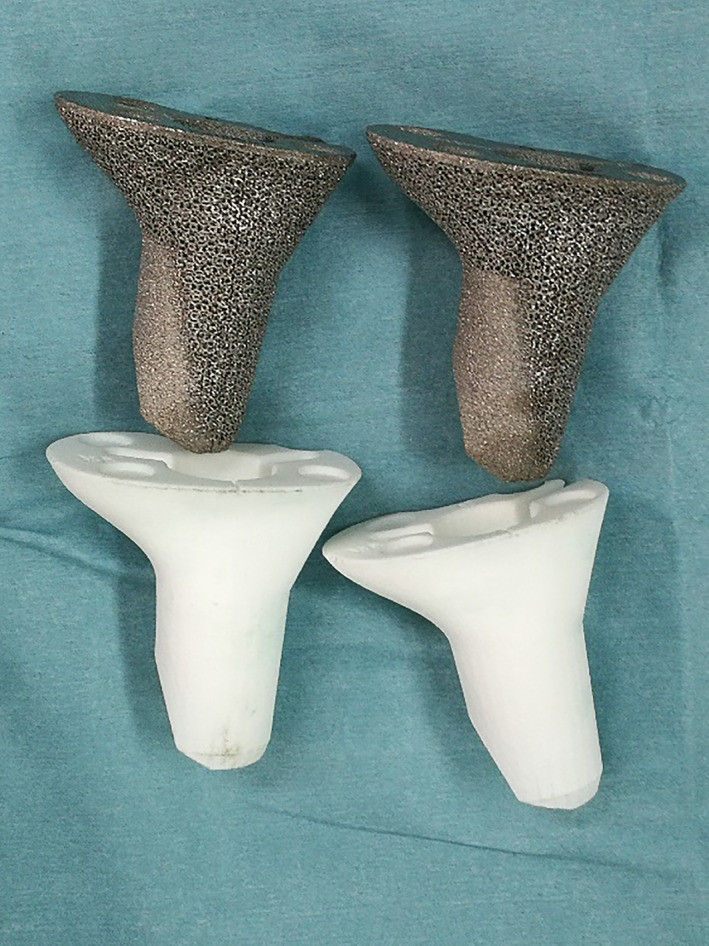
Patient‐customized three‐dimensional (3D)‐printed highly porous metaphyseal cones and their trials (Photograph courtesy: Dr. Yang Li, during the operation; we have the copyright of this image).
Surgical Procedure and Perioperative Management
Surgical Procedure
All patient‐customized 3D‐printed highly porous cones were used along with an ACCK revision knee system. Sequentially, the loosening prosthesis or antibiotic‐loaded bone cement spacer and all necrotizing soft tissues were removed completely. The articular cavity was then irrigated thoroughly. The bone defect characteristics were double‐checked. The cone trial was inserted. The compatibility of the interface was observed and the stability of the insertion was tested. The host bone was modified using a high‐speed burr or spatula if the initial compatibility was unsatisfactory. The customized cone was inserted and impacted into the metaphyseal bone defect appropriately. The implant was placed, and tibial plates and stems were cemented.
Perioperative Management
A tourniquet was used during the operation, and tranexamic acid was administered intravenously at 1 g before skin incision and wound closure.
All patients underwent routine blood examinations within 3 days after surgery. When the hemoglobin (Hb) level was above 100 g/L, blood transfusion was not required; however, it was needed when the level was below 70 g/L. When the Hb was 70–100 g/L, blood transfusion was determined according to whether the patient had symptoms of anemia, such as dizziness and fatigue. All patients received low‐molecular‐weight heparin (LMWH) (100 AXaIu/kg, qd) to prevent lower extremity deep vein thrombosis. The dose of LMWH was adjusted to 100 AXaIu/kg (Bid) if the patient was diagnosed with deep vein thrombosis of the lower extremity usingq2q22ultrasound performed 2 days after surgery.
Assessment of Outcome
Patient age, sex, body mass index (BMI), diagnosis, preoperative mechanical axis angle, range of motion (ROM), Hospital for Special Surgery Score (HSS), and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score were recorded. Additionally, the tibial bone loss AORI classification was determined using preoperative radiographs and intraoperative assessment. Operational characteristics were also recorded, including operative time, total blood loss (TBL), postoperative transfusion, and postoperative complications. TBL was calculated using the Gross equation. 16
Patients were followed up for 2 weeks, 6 weeks, 3 months, 6 months, 1 year, 2 years, and annually after surgery. Follow‐up data, including the postoperative mechanical axis angle, HSS, WOMAC, and ROM, were recorded from the latest follow‐up. The preoperative and postoperative mechanical axis angles were determined using the hip–knee–ankle (HKA) angle.
Hospital for Special Surgery Score (HSS)
HSS was used to evaluate the postoperative recovery of knee function in an adult population. The HSS score system primarily includes seven aspects: pain, function, ROM, myodynamia, absence of deformity, stability, and subtraction items. The scoring standard had a maximum of 100 points (best possible outcome). A total score <60 was considered poor, 60–69 fair, 70–84 good, and 85–100 excellent.
McMaster Universities Osteoarthritis Index (WOMAC)
WOMAC was used to evaluate the symptoms of patients with knee osteoarthritis or after surgery in an adult population. The WOMAC score system has 24 questions, with five levels each (0 = none, 4 = severe), including pain, stiffness, and function scores. Patients with total WOMAC osteoarthritis index scores of <21, 21–48, and >48 were considered to have mild, moderate, and severe clinical symptoms, respectively.
Statistical Analysis
Statistical significance for preoperative and postoperative HSS and WOMAC was determined using two‐tailed t‐tests using SPSS 25.0 (SPSS, Inc., Chicago, IL, USA), and P‐values less than 0.05 were considered statistically significant.
Results
Perioperative Characteristics
General Results
The mean age of the enrolled patients was 68 (61–77) years, with a mean BMI of 28.1 (24.9–32.0). The primary diagnoses leading to RTKA were aseptic loosening (5/7) and prosthetic joint infection (2/7; stage II revision). Table 1 shows the demographic and preoperative characteristics of each patient.
TABLE 1.
Demographics and preoperative characteristics of patients
| Patient† | Age at Diagnosis (years) | Sex | BMI (kg/m2) | Diagnosis | Preoperative ROM (°) | Preoperative HSS | Preoperative WOMAC | Preoperative HKA angle (°) |
|---|---|---|---|---|---|---|---|---|
| 1 | 65 | F | 30.84 | Prosthetic joint infection | 10–80 | 39 | 73 | +7.2 |
| 2 | 61 | F | 32.04 | Prosthetic joint infection | 0–30 | 44 | 66 | +8.1 |
| 3 | 67 | F | 27.24 | Aseptic loosening | 10–70 | 51 | 51 | +19.7 |
| 4 | 69 | F | 26.18 | Aseptic loosening | 15–80 | 54 | 62 | +20.6 |
| 5 | 77 | F | 28.40 | Aseptic loosening | 0–80 | 63 | 46 | +10.8 |
| 6 | 73 | F | 24.97 | Aseptic loosening | 5–60 | 42 | 59 | +18.5 |
| 7 | 64 | F | 27.06 | Aseptic loosening | 10–65 | 48 | 55 | +7.6 |
Abbreviations: †, number; F, female; +, varus, BMI, body mass index; ROM, range of motion; HSS, Hospital for Special Surgery Score; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; HKA, hip–knee–ankle.
Intraoperative Results
The loosening prosthesis or antibiotic‐loaded bone cement spacer was removed effortlessly. The interface compatibility and stability of the cone trials were perfect when cone trials were performed. Therefore, modification of the host bone with a high‐speed burr or spatula was rarely required. Moreover, all the customized cones were inserted with perfect interface compatibility and stability. The mean operative time was 108 (95–129) min.
Perioperative Complications
The mean TBL was 1202 (890–1540) mL. Three patients experienced anemia postoperatively and required blood transfusion during hospitalization. Patient 5 had muscular calf vein thrombosis in both legs, which was corrected after a 2‐week anticoagulation therapy with low‐molecular‐weight heparin (LMWH) (Table 2).
TABLE 2.
Surgical characteristics and follow‐up data
| Patient† | Operating time (min) | Estimated blood loss (mL) | Postoperative transfusion | Postoperative complications | Follow‐up (months) | Postoperative ROM (°) | Postoperative SS | Postoperative WOMAC | Postoperative HKA angle (°) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 129 | 890.6 | No | No | 36 | 0–100 | 83 | 12 | −1.1 |
| 2 | 102 | 1445.9 | No | No | 27 | 0–90 | 70 | 38 | +1.8 |
| 3 | 95 | 1540.2 | Yes, 400 mL | No | 19 | 0–95 | 80 | 35 | +2.9 |
| 4 | 112 | 1064.2 | Yes, 200 mL | No | 24 | 0–95 | 82 | 27 | +1.3 |
| 5 | 103 | 1140.3 | No | Calfthrombosis | 21 | 5–95 | 78 | 16 | −2.3 |
| 6 | 115 | 1383.2 | Yes, 400 mL | No | 24 | 0–80 | 74 | 26 | 0.3 |
| 7 | 99 | 949.8 | No | No | 26 | 10–95 | 77 | 29 | −1.9 |
Abbreviations: †, number; +, varus; −, valgus; ROM, range of motion; HSS, Hospital for Special Surgery Score; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; HKA, hip–knee–ankle.
Clinical and Radiologic Outcomes of Follow‐Up
Clinical Improvement and Functional Evaluation
The mean follow‐up was 25.3 (19–36) months. At the latest follow‐up, no aseptic loosening or prosthetic joint infections were observed. The mean HSS increased from 49 (39–63) to 78 (70–83) (p < 0.01), and the mean WOMAC increased from 59 (46–73) to 26 (12–38) (p < 0.01). The mean flexion angle increased from 66° (30–80) to 93° (80–100).
Radiographic Improvement and Implant Evaluation
There were no traces of progressive radiolucent lines or radiographic loosening. Moreover, the mechanical axial was well restored, with all HKA angles within ±3°. Table 2 lists the patients' operational characteristics and follow‐up data. Figures 2A‐F and 3A‐F display the perioperative radiographs of patients 1 and 3.
Fig. 2.
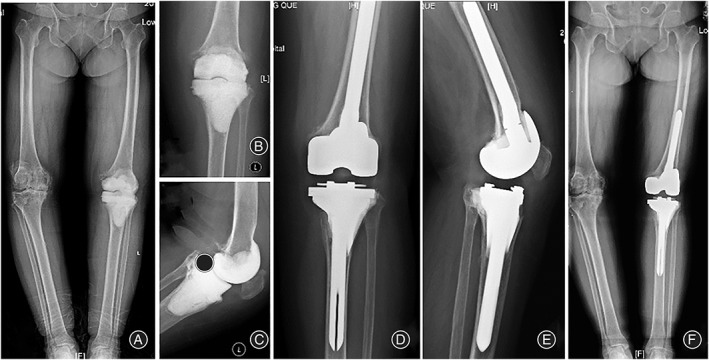
Representative images for patient 1. (A, B, C) Preoperative anterioposterior (AP)/lateral radiographs, which demonstrate antibiotic‐loaded cement spacers. The patient is debrided due to the periprosthetic infection. (D, E, F) AP/lateral radiographs at the latest follow‐up (36 months).
Fig. 3.
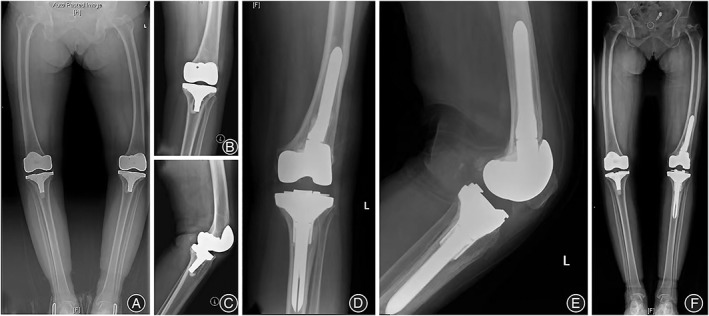
Representative images for patient 3. (A, B, C) Preoperative anterioposterior (AP)/lateral radiographs, which demonstrate aseptic loosening. (D, E, F) AP/lateral radiographs at the latest follow‐up (19 months).
Discussion
Feasibility of this Technology
In this study, we retrospectively reviewed seven RTKAs with AORI type III tibial defects using patient‐customized 3D‐printed highly porous metaphyseal cones. The surgical procedure was simplified, and the incidence of perioperative complications was quite low. The mean follow‐up durations were 25.3 months. At the latest follow‐up, no aseptic loosening or prosthetic joint infections were observed. All patients showed great postoperative improvement in the HSS score, WOMAC index, ROM, and mechanical alignment. These results demonstrated the feasibility of this technology.
Advantages of this Technology
Excellent Clinical and Radiographic Outcomes
All cones utilized in our study for RTKA with AORI type III tibial defects were modeled based on the patients' preoperative CT scans and printed layer‐by‐layer with titanium alloy. From our experience, immediate rigid metaphyseal fixation was much more accessible to achieve with customized cones compared with fixed‐size ones. Both the operating time and procedural complexity decreased. At the 25‐month follow‐up, the clinical and radiographic outcomes were excellent, without complications such as aseptic loosening, periprosthetic infection, and fracture.
High Stability
Our study revealed several advantages of the application of 3D‐printed patient‐customized cones in RTKA with massive proximal tibial defects. High stability stands first. By combining the excellent filling performance of conventional cones with the prominent stability of sleeves, customized cones can achieve reliable initial metaphyseal fixation. 17 The pore size and porosity of the cones can be appropriately configured in modeling to increase friction and promote osseointegration, which maximizes the initial stability and long‐term stability. 18 Owing to their incredible immediate stability, the demand for diaphyseal stem fixation can be minimized or even eliminated to avoid stem‐related postoperative leg pain. 19 Further studies, including finite element analysis, are required to verify this benefit, which will be our next step.
Excellent Fitness
An excellent fitness, both functionally and anatomically, is another important advantage. The 3D‐printed patient‐customized cone modeled according to the preoperative images fits the bone defects much more precisely than conventional fixed‐size cones, particularly when dealing with massive bone defects in complicated revisions. The 3D‐printed patient‐customized cone was also different from the 3D‐printed titanium metaphyseal cones reported in recent articles, which were only manufactured using 3D printing technology; however, their shapes were not patient‐customized according to the patient's CT and other conditions. 20
Simplified Surgical Procedure
In addition, as extra bone cutting and reaming are minimized by the utilization of customized cones, iatrogenic bone loss is significantly avoided, and the procedure is greatly simplified by converting a complicated revision TKA into a straightforward “primary” TKA. Metal 3D printing technology also greatly reduces the cost of a customized cone and shortens its production cycle because traditional manufacturing techniques, such as casting, forging, and cutting, are no longer involved, making it more practical and affordable solution. 21 Moreover, the 3D‐printed porous cone can accurately mimic the elastic modulus gradient from cancellous bone to cortical bone to diminish stress shielding and osteolysis. 22
Technical Challenges and Surgical Experience
The design of a patient‐customized 3D‐printed highly porous metaphyseal cone is undoubtedly the hardest part, in which excellent fitness and high stability must be realized. The solution to this problem was that doctors and engineers cooperated to recognize the patient‐customized bone defect and left host bone, and then designed the patient‐customized 3D‐printed highly porous metaphyseal cone. From our experience, printing another identical polyamide cone as a trial was essential, which was inserted before the cone implant to observe the compatibility of the interface and test the stability of the insertion. The host bone was modified using a high‐speed bur or spatula if the initial compatibility was unsatisfactory. In addition, two cones with diameters 2mm larger or smaller than the standard one were also printed, given the uncertainty of intraoperative bone modification.
Our study has some limitations. We enrolled seven patients without a control group; nevertheless, it is by far the largest case series regarding the utilization of the patient‐customized 3D‐printed metaphyseal cone in RTKA with massive bone defects. The follow‐up period was short. Thus, a comparison with the long‐term clinical and radiological outcomes of traditional cones in RTKA is unavailable. Coincidentally, all seven cases were women, which may have induced selection bias to some extent. To further verify the advantages of this technique, expansion of the sample size and follow‐up time are needed for subsequent studies.
In conclusion, the patient‐customized 3D‐printed highly porous metaphyseal cone could be a promising technique for addressing severe tibial defects (AORI type III) in RTKA, as it effectively simplifies the procedure, shortens the operating time, and achieves articular biomechanical stability. Our study showed encouraging short‐term clinical and radiological outcomes with no aseptic loosening, periprosthetic infection, or fracture. Nevertheless, further follow‐up and expansion of the sample size are needed to fully demonstrate the advantages of this innovative technique.
Conflicts of Interest
All authors have read and understood the declaration of interests and declare that they have no competing interests.
Acknowledgments
No funding was received for this study. The authors would like to thank Beijing 3D Printing Orthopedic Application Engineering Technology Research Center for helpful discussions on the design and production of 3D‐printed patient‐customized cone.
References
- 1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–5. [DOI] [PubMed] [Google Scholar]
- 2. Ponzio DY, Austin MS. Metaphyseal bone loss in revision knee arthroplasty. Curr Rev Musculoskelet Med. 2015;8:361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Engh GA, Parks NL. The management of bone defects in revision total knee arthroplasty. Instr Course Lect. 1997;46:227–36. [PubMed] [Google Scholar]
- 4. Shafaghi R, Rodriguez O, Schemitsch EH, et al. A review of materials for managing bone loss in revision total knee arthroplasty. Mater Sci Eng C Mater Biol Appl. 2019;104:109941. [DOI] [PubMed] [Google Scholar]
- 5. Burastero G, Cavagnaro L, Chiarlone F, Alessio‐Mazzola M, Carrega G, Felli L. The use of tantalum metaphyseal cones for the management of severe bone defects in septic knee revision. J Arthroplasty. 2018;33:3739–45. [DOI] [PubMed] [Google Scholar]
- 6. Klim SM, Amerstorfer F, Bernhardt GA, et al. Excellent mid‐term osseointegration and implant survival using metaphyseal sleeves in revision total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2020;28:3843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zanirato A, Formica M, Cavagnaro L, Divano S, Burastero G, Felli L. Metaphyseal cones and sleeves in revision total knee arthroplasty: two sides of the same coin? Complications, clinical and radiological results‐a systematic review of the literature. Musculoskelet Surg. 2020;104:25–35. [DOI] [PubMed] [Google Scholar]
- 8. Barnett SL, Mayer RR, Gondusky JS, Choi L, Patel JJ, Gorab RS. Use of stepped porous titanium metaphyseal sleeves for tibial defects in revision total knee arthroplasty: short term results. J Arthroplasty. 2014;29:1219–24. [DOI] [PubMed] [Google Scholar]
- 9. Abdelaziz H, Jaramillo R, Gehrke T, Ohlmeier M, Citak M. Clinical survivorship of aseptic revision total knee arthroplasty using hinged knees and tantalum cones at minimum 10‐year follow‐up. J Arthroplasty. 2019;34:3018–22. [DOI] [PubMed] [Google Scholar]
- 10. Petersen KK, Simonsen O, Laursen MB, Nielsen TA, Rasmussen S, Arendt‐Nielsen L. Chronic postoperative pain after primary and revision total knee arthroplasty. Clin J Pain. 2015;31:1–6. [DOI] [PubMed] [Google Scholar]
- 11. Faizan A, Bhowmik‐Stoker M, Alipit V, et al. Development and verification of novel porous titanium metaphyseal cones for revision total knee arthroplasty. J Arthroplasty. 2017;32:1946–53. [DOI] [PubMed] [Google Scholar]
- 12. Cheng A, Humayun A, Cohen DJ, Boyan BD, Schwartz Z. Additively manufactured 3D porous Ti‐6Al‐4V constructs mimic trabecular bone structure and regulate osteoblast proliferation, differentiation and local factor production in a porosity and surface roughness dependent manner. Biofabrication. 2014;6:045007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brune JC, Hesselbarth U, Seifert P, et al. CT lesion model‐based structural allografts: custom fabrication and clinical experience. Transfus Med Hemother. 2012;39:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lv J, Jia Z, Li J, et al. Electron beam melting fabrication of porous Ti6Al4V scaffolds: cytocompatibility and osteogenesis. Adv Eng Mater. 2015;17(9):1391–8. [Google Scholar]
- 15. Geng X, Li Y, Li F, et al. A new 3D printing porous trabecular titanium metal acetabular cup for primary total hip arthroplasty: a minimum 2‐year follow‐up of 92 consecutive patients. J Orthop Surg Res. 2020;15:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gross JB. Estimating allowable blood loss: corrected for dilution. Anesthesiology. 1983;58:277–80. [DOI] [PubMed] [Google Scholar]
- 17. Morgan‐Jones R, Oussedik SI, Graichen H, Haddad FS. Zonal fixation in revision total knee arthroplasty. Bone Joint J. 2015;97‐B:147–9. [DOI] [PubMed] [Google Scholar]
- 18. Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–91. [DOI] [PubMed] [Google Scholar]
- 19. Albino RB, Santos LS, Gobbi RG, et al. Pain at the tip of the stem after revision total knee arthroplasty. Rev Bras Ortop. 2012;47:73–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anderson LA, Christie M, Blackburn BE, et al. 3D‐printed titanium metaphyseal cones in revision total knee arthroplasty with cemented and cementless stems. Bone Joint J. 2021;103‐B:150–7. [DOI] [PubMed] [Google Scholar]
- 21. Malik HH, Darwood AR, Shaunak S, et al. Three‐dimensional printing in surgery: a review of current surgical applications. J Surg Res. 2015;199:512–22. [DOI] [PubMed] [Google Scholar]
- 22. Parthasarathy J, Starly B, Raman S, Christensen A. Mechanical evaluation of porous titanium (Ti6Al4V) structures with electron beam melting (EBM). J Mech Behav Biomed Mater. 2010;3:249–59. [DOI] [PubMed] [Google Scholar]


