Abstract
Enhanced crop growth and yield are the recurring concerns in agricultural field, considering the soaring world population and climate change. Abiotic stresses are one of the major limiting factors for constraining crop production, for several economically important horticultural crops, and contribute to almost 70% of yield gap. Salt stress is one of these unsought abiotic stresses that has become a consistent problem in agriculture over the past few years. Salinity further induces ionic, osmotic, and oxidative stress that result in various metabolic perturbations (including the generation of reactive oxygen, carbonyl, and nitrogen species), reduction in water potential (ψw), distorted membrane potential, membrane injury, altered rates of photosynthesis, leaf senescence, and reduced nitrogen assimilation, among others); thereby provoking a drastic reduction in crop growth and yield. One of the strategies to mitigate salt stress is the use of natural plant extracts (PEs) instead of chemical fertilizers, thus limiting water, soil, and environmental pollution. PEs mainly consist of seeds, roots, shoots, fruits, flowers, and leaves concentrates employed either individually or in mixtures. Since PEs are usually rich in bioactive compounds (e.g., carotenoids, flavonoids, phenolics, etc.), therefore they are effective in regulating redox metabolism, thereby promoting plant growth and yield. However, various factors like plant growth stage, doses applied, application method, soil, and environmental conditions may greatly influence their impact on plants. PEs have been reported to enhance salt tolerance in plants primarily through modulation of signaling signatures and pathways (e.g., Na+, ANNA4, GIPC, SOS3, and SCaBP8 Ca2+ sensors, etc.), and regulation of redox machinery [e.g., superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), non-specific peroxidase (POX), glutathione peroxidase (GPX), peroxiredoxin (Prx), ascorbic acid (AsA), glutathione (GSH), α-tocopherol, etc.]. The current study highlights the role of PEs in terms of their sources, methods of preparation, and mode of action with subsequent physiological changes induced in plants against salinity. However, an explicit mode of action of PEs remains nebulous, which might be explicated utilizing transcriptomics, proteomics, metabolomics, and bioinformatics approaches. Being ecological and economical, PEs might pave the way for ensuring the food security in this challenging era of climate change.
Keywords: salt stress, stress perception, signaling signatures, NaCl, bioactive compounds, climate change, antioxidants, osmoprotectants
Introduction
The most defining concern of the present and future agriculture, and of humanity, is climate change. Human dependence on wild grains began in Halocene—a geological epoch, which further laid the basis of agriculture, as it was characterized by stable and warm temperatures. However, a rapid surge in the atmospheric carbon dioxide levels from 100 ppm to over 400 ppm with an average temperature increase by 1°C, in the past 70 years, could be a steppingstone for unstable environmental temperatures. A further escalation of temperature by 3°C by the year 2100, and even by 8°C or more is also predicted. Such drastic escalations have been identified as antagonists of human civilization (Gowdy, 2020). Similarly, agricultural activities, particularly, the use of chemical fertilizers not only incites diabetes and cancer like chronic diseases in humans, but also deteriorates the environment; thereby aggravating the climate change (Ahmad et al., 2021b). In this regard, European Union (EU) has implemented a “European Green Deal” program that proposes a 20% reduction in the use of fertilizers and a 55% reduction in greenhouse gases by 2030 as compared to 1990 levels (European-Union, 2020). Apart from this, soaring human population that is expected to reach 9.7 billion by 2050, is amplifying the pressure on agriculture to meet the growing food demands globally. Substantially lower crop yield ha–1 in comparison to increasing world population is already reported by World Food Program (WFP). Likewise, by the year 2100 a decrease in the yield of maize, wheat, and rice, by 20–45, 5–50, and 5–50%, respectively, has been predicted by Food and Agriculture Organization (FAO) if the climate change remains incessant (Arora, 2019). This ever-increasing food demand has provoked intensive agriculture systems with the unprecedented use of chemical fertilizers, pesticides, herbicides, fungicides, along with the exploitation of land and water resources; thereby further aggravating the climate change. If such trends persist, they will not only compromise the global food safety and security but may also provoke a civilization collapse.
Climate change provokes a plethora of abiotic stresses (flooding, drought, salinity, etc.) in plants (Arora, 2019; Gowdy, 2020). These abiotic stresses are one of the major limiting factors for constraining crop production (Ramadoss et al., 2013), for several economically important horticultural crops, and contribute to almost 70% of yield gap (Rouphael and Colla, 2020). Salinity is one such abiotic stress that hampers plant physiological functioning in a number of ways: resulting in lower crop production. It is attributed as a measure of salt amount in soil or water content (Arif et al., 2020), and is classified as primary salinity: caused as a result of natural processes i.e., rain, weathering, wind, etc., and secondary salinity: caused as a result of anthropogenic activity, i.e., excessive irrigation, deforestation, clearing land (Munns and Tester, 2008; Arif et al., 2020). The climate change is directly responsible for primary salinity considering the excessive rains only, for example 100 mm of rainfall year–1 would deposit 10 Kg ha–1 of sodium chloride if it contains 10 mg Kg–1 of salt (Munns and Tester, 2008), whereas indirectly to secondary salinity. Subsequently, intensive agricultural systems and poor agronomic practices, like over-fertilization, desertification, excessive irrigation, etc., have increased the levels of alkalinity and salinity of cultivated soils (Arora, 2019; Li et al., 2021). In 2013, the salinity affected cultivated soils were estimated to be over 800 million ha (Ramadoss et al., 2013) globally that culminated to around 900 million ha in 2020 (Velmurugan et al., 2020). Such a rapid conversion of fertile soils into saline ones can be regarded as a major threat to food security and agricultural sustainability.
Salinity is expressed as the electric conductivity (EC) of the soil solution, and the soil is generally denominated as saline if its EC is 4 dS m–1 or more, it approximately equals to 40 mM NaCl, producing a 0.2 MPa approximate osmotic pressure (Munns and Tester, 2008). Sodium ions (Na+) are considered as major contributors to salinity, whereas Cl–, Mg2+, SO42–, or HCO3– are also responsible for soil salinity but to a lesser extent (Munns and Tester, 2008; Zörb et al., 2019). Higher concentrations of these salts trigger ionic and osmotic stress resulting in the generation of reactive oxygen species (ROS), reduced cell/leaf expansion, leaf abscission, reduction in ψw, distorted membrane potential, membrane injury, altered rates of photosynthesis, stomatal closure, protein destabilization, altered carbon portioning, cavitation, reduced nitrogen assimilation, ion cytotoxicity, and cell death among others; thereby provoking a drastic reduction in crop growth and yield (Ashraf and Harris, 2004; Munns and Tester, 2008; Acosta-Motos et al., 2017; Farooq et al., 2017; Arif et al., 2020). Therefore, it is indispensable to devise novel strategies for combating salinity.
Use of natural plant extracts (PEs) (or “botanicals”) could be one of the salinity mitigation ecofriendly strategies. PEs are potential alternatives to chemical fertilizers. PEs fall under the umbrella of plant biostimulants, and are used to enhance plant growth (Calvo et al., 2014; Drobek et al., 2019). PEs are the concentrates of plants and could be prepared using any part of the plant, i.e., seeds, roots, stems, leaves, bark, flowers, etc. (Semida and Rady, 2014; Lorenzo et al., 2019; Nessim and Kasim, 2019; Rady et al., 2019b; Zulfiqar et al., 2020b). The application of PEs could be either in liquid form as foliar spray and/or root treatment, or as soil preparations like granules, concentrates, solutions added to soil, or powders (Drobek et al., 2019). PEs can be associated with the amelioration of salinity as they are the sources of prominent phytochemicals like vitamins, carotenoids, amino acids, phytohormones, mineral nutrients, phenolics, and antioxidants (Latef et al., 2017). There are several reports where these compounds, used either individually or in mixtures, were found to be effective against salinity (Calvo et al., 2014; Iqbal et al., 2014; Bulgari et al., 2015; Drobek et al., 2019; Shukla et al., 2019; Zulfiqar et al., 2020b). However, the effect of PEs is often concentration dependent. Similarly, plant part and age of plant used as an extract also influences the PEs overall proficiency.
Currently, there are no extensive studies reported on the particular use of PEs and their subsequent mechanism of action against salinity. Previously reported studies mostly discuss the use of biostimulants that is rather a broader term and even includes microbial and non-microbial formulations, protein hydrolyzates, PEs, amino acid, seaweed extracts, etc. (Du Jardin, 2015). Furthermore, previous studies have discussed the use of biostimulants on abiotic stresses in general (Calvo et al., 2014; Drobek et al., 2019; Shukla et al., 2019; Rouphael and Colla, 2020; Zulfiqar et al., 2020b), whereas no specific study on the use of PEs against salinity is reported. Therefore, the aim of the study was to elaborate the potential of ecofriendly and natural PEs as salinity alleviators, and to underline their possible mode of action with the subsequent physiological changes thus induced. Since an explicit mode of action of PEs remains nebulous, hence this subject has been estimated considering the up or down regulation of signaling signatures, altered photosynthetic rates, and redox metabolism in general.
This review is divided into three sections. Impact of plant based biostimulants under normal conditions is discussed first followed by salinity induced physiological, biochemical, and genetic changes in plants. Subsequently, the use of PEs, including their sources and methods of preparation, as salinity mitigation strategy is discussed. Finally, all this is concluded by identifying the limitations and future perspectives of the use of PEs against salinity.
Plant Based Biostimulants
Use of plant based biostimulants is rapidly gaining popularity in agriculture. Plant based biostimulants, apart from inducing stress tolerance, are also effective in regulating a number of plants physiological processes; thereby improving plant growth and yield (Brown and Saa, 2015). They may comprise of protein hydrolyzates and amino acids, hormone-, amino acids-, or nutrients containing products, vegetable oils, etc. of plant origin (Ikrina and Kolbin, 2004; Kauffman et al., 2007; La Torre et al., 2016; Yakhin et al., 2017). Their mechanism of action might involve phosphorus (P) release from soils, activation of nitrogen (N) metabolism, stimulation of root growth, nutritional and hormonal regulation, or generic stimulation of soil microbial activity. Previous reports have documented that the application of biostimulants has enhanced various physiological processes including plant nutrient uptake and utilization, photosynthesis, water use efficiency, synthesis and concentration of growth hormones (auxins, gibberellins, and cytokinins), germination, and senescence reduction (Parađiković et al., 2011; Bulgari et al., 2015; Nasir et al., 2016; Merwad, 2017; Ur Rehman et al., 2017; Milić et al., 2018; Younis et al., 2018), which in return increase plant production, yield, post-harvest quality, and shelf life of agricultural products.
Among various plant based biostimulants, PEs are economical and easy to prepare. Several studies have reported their beneficial effects on plants growth and yield. For instances, an increase in the growth and hormonal profile was observed in eggplant and snap bean when aqueous garlic extracts were applied (Elzaawely et al., 2018; Ali et al., 2019). Similar results were reported in case of moringa leaf extracts being applied on sword lily, where they improved plant growth and vase life by regulating various physiological processes (Zulfiqar et al., 2020c). Likewise, borage extracts were reported to supplement the primary metabolism, by enhancing leaf pigments and photosynthetic activity, and reduced chlorophyl a fluorescence, by incrementing the number of active reaction centers per cross section, in lettuce plants (Bulgari et al., 2017). Likewise, vine-shoot and oak extracts were found to significantly improve wine yield and quality by triggering amino acids and volatile compounds production (Sánchez-Gómez et al., 2016). In addition, PEs are also responsible for increasing the shelf life and postharvest quality of agricultural products. For example, moringa leaf extracts were found to significantly improve avocado and citrus fruit shelf life and quality by lowering the respiration rate and water transfer from the fruits (Adetunji et al., 2012; Tesfay and Magwaza, 2017). All of these studies indicate the potential of PEs in positively regulating several physiological processes. Therefore, extrapolating the incredible potential of PEs to combat abiotic stresses, especially salinity, would be a promising approach for a sustainable agriculture.
Salinity Perception and Signaling in Plants
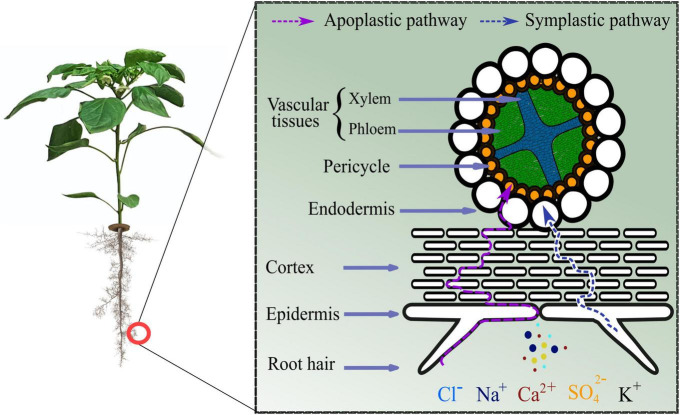
Stress perception and signaling hold an imperative role for subsequent plant behavior. Apoplastic and symplastic pathways are the recognized routes of ions entry into plant that result in salinity (Lamers et al., 2020) (Figure 1). Stress signals are perceived by plant cell surface-based receptors that stimulate the production of secondary messengers like Ca2+, ROS, and inositol phosphates, among others (Rao et al., 2016). The most common surface based receptors involved in the cation sensing (Na+ and Ca2+) are membrane bound proteins or ion channels including glycosyl inositol phosphorylceramide (GIPC) sphingolipids synthesized by MONOCATION-INDUCED [Ca2+]cyt INCREASES1 (MOCA1), Arabidopsis ANNEXIN4 (ANN4), and Arabidopsis HIGH-AFFINITY K+ TRANSPORTER1 (HKT1) (Chen et al., 2021). Similarly, ethylene receptors like Nicotiana tabacum histidine kinase 1 (NTHK1) are also reported to modulate stress signaling (Cao et al., 2008). While Ca2+ signaling is an important mechanism for salt-sensing and is modulated through SALT OVERLY SENSITIVE (SOS) pathway. SOS pathway consists of SOS1 Na+/H+ antiporter, SOS2 and SOS2-LIKE PROTEIN KINASE5 (PKB5) protein kinases, and SOS3 and SCaBP8 Ca2+ sensors (Lamers et al., 2020). The intercellular Ca2+ perturbations trigger Ca2+ sensors, e.g., SOS3 and SCaBP8, bringing about conformational changes in them in a calcium-dependent manner. These sensors further interact with their respective partners, e.g., activate SOS2, whereby a phosphorylation cascade is initiated (Quan et al., 2007; Rao et al., 2016). Subsequently, SOS1 is activated carrying out the efflux of Na+ ions (Figure 2). It is reported that within 20 s of stress (sodium) application a change in SOS1 exchanger activity can be detected (Lamers et al., 2020). Ultimately these alterations result in the genomic regulations (activation of transcription factors and stress responsive genes) by biosynthesizing metabolites and other compounds needed to combat salinity. There are two categories of stress- responsive genes, i.e., (a) early induced genes: immediate activation on receiving the stress signal with short-term persistence (including transcription factors, interfering RNAs, etc.), and (b) late induced genes: late activation with longer persistence periods (including membrane stabilizing, osmolytes, antioxidants, etc.) (Rao et al., 2016).
FIGURE 1.
Entry routes for salinity causing ions.
FIGURE 2.
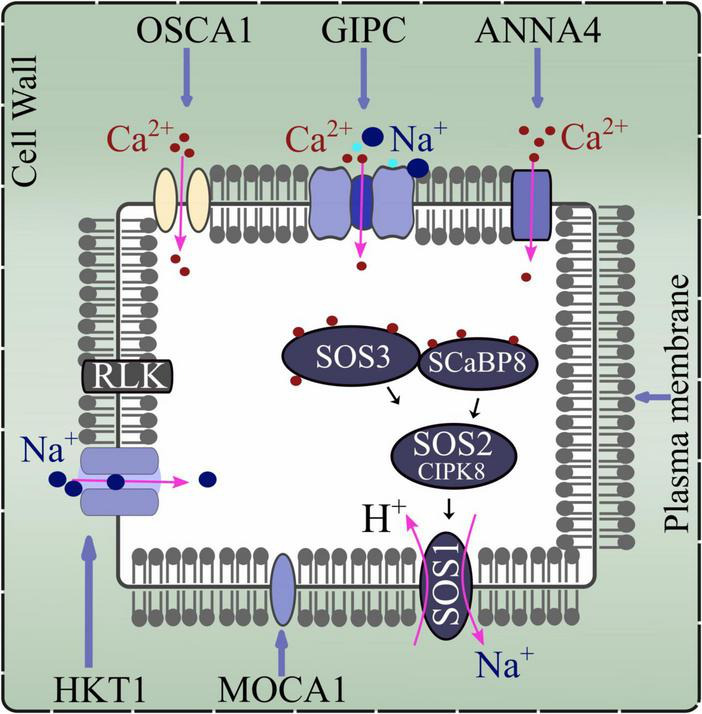
Representation of membrane-based proteins, and ion channels that regulate ions movement in plants (under saline environment).
Since plants undergo an osmotic stress under saline environment, therefore it is proposed that receptors involved in osmotic, or drought sensing can also be implicated for salinity signal transduction. It is reported that a plasma membrane located channel encoded by OSCA1 regulates the Ca2+ influx under plasma membrane tension or extracellular osmotic pressure. Similarly, the receptor-like kinases (RLKs) present on plasma membrane have also been reported to play certain role (Lamers et al., 2020) (Figure 2).
Plant Responses to Salinity
Plants generally vary in their response to salinity. Their response could be cellular- and tissue level, morphological, or physiological. Such response depends on various factors including duration and severity of stress, plant age and its developmental stage, and plant species (Peleg et al., 2011). Therefore, some plants are found to be more tolerant (less sensitive) to salinity than others. For example, barley (Hordeum vulgare) is more tolerant to salinity than rice (Oryza sativa) (Munns and Tester, 2008). Plants are generally categorized as halophytes or euhalophytes based on their genetic adaptability to salinity, whereas they are termed as glycophytes if they are less-tolerant or not adapted to salt stress (Acosta-Motos et al., 2017). The general effects of salt stress in glycophytes occur in the following two forms (Munns and Tester, 2008; Acosta-Motos et al., 2017);
-
(i)
Immediate response: onset of osmotic stress on surpassing the threshold limit of salts in the root zone resulting in the reduction of shoot biomass.
-
(ii)
Slower response: onset of ionic stress on accumulation of salt ions (Na+ and Cl–) in older leaves resulting in the impairment of photosynthetic machinery and leaf senescence.
The rapid osmotic phase response of plants begins at the root-soil periphery. The toxic concentrations of salt ions build up osmotic pressure that negatively regulates the rate of leaf expansion, emergence of new leaves, lateral buds- and shoots formation. The second phase is characterized by the higher accumulation rate (i.e., toxic concentrations) of Na+ ions in the older leaves that cannot dilute these salts due to lack of expansion, ultimately resulting in their death. Although, some plant species are sensitive to higher Cl– concentrations. This results in the reduced photosynthetic rates of plants that causes a reduction in their growth rate. Since ionic stress is time taking due to the accumulation of ions, therefore plant growth is affected much later with lesser impact as compared to osmotic stress.
Mechanisms of Salinity Tolerance in Plants
Salinity tolerance in plants is achieved through a series of complex signaling and biosynthetic responses. However, the commonly known mechanisms of salinity tolerance include morphological (roots and aerial parts), metabolic (osmotic regulation, ionic and molecular homeostasis, and hormonal homeostasis), and genetic responses. Comprehensive reports of salinity tolerance mechanisms have been reported previously (Munns and Tester, 2008; Gupta and Huang, 2014; Tang et al., 2015; Acosta-Motos et al., 2017; Farooq et al., 2017; Arif et al., 2020). Here, a description of these mechanisms is presented to elaborate the use of PEs, as they reinforce these salinity tolerance mechanisms.
Morphological Adjustments
Plants can adapt their morphological features to sustain the normal functioning and cellular homeostasis in case of any unfavorable stimulus. This is characterized as phenotypic plasticity. This also occurs in case of salinity. Although, it varies among salt tolerant and salt sensitive species. Generally, the productivity or yield of an agricultural crop can be assessed by analyzing its above-and below-ground parts. Therefore, few of the growth indices, as per Beadle (1993), taken into consideration for salt stress studies in plants are listed below:
Where W represents the total dry weight, WL is the total dry weight of leaves, A is the total leaf area, t is the time, 1 and 2 represents the start and end of a period, respectively. Considering these growth indices as standard, various studies have demonstrated relative effects of salinity on plant morphology as a decrease in RGR in five ornamental plants (Cassaniti et al., 2012), a decrease in NAR in Hibiscus cannabinus and Argyranthemum coronopifolium (Curtis and Lauchli, 1986; Morales et al., 1998), a decrease in LAR in A. coronopifolium (Morales et al., 1998), and an increase in LWR in Asteriscus maritimus (Rodrıguez et al., 2005). Similarly, other morphological changes observed in plants (salt sensitive) under salinity include decrease in leaf thickness, succulence values, surface to volume ratio of cells and tissue density, spongy parenchyma, and number of mitochondrial cristae, whereas an increase in the lower area/volume ratio of mesophyll cells, mesophyll thickness, leaf water balance, leaf size, palisade cell size, succulence values, intercellular space, and palisade parenchyma has been reported (Longstreth and Nobel, 1979; Romero-Aranda et al., 1998; Franco-Navarro et al., 2016; Acosta-Motos et al., 2017). Most of these changes suggest that plants under salt stress intend to increase the CO2 diffusion so that the energy production should not be disrupted along with an increased water use efficiency through higher photosynthetic performance. Similarly, leaf senescence and leaf color change are also salinity mitigation mechanisms, in which chlorophyll is gradually degraded resulting in the accumulation of carotenoids and anthocyanins that provide protection against oxidative stress (Hörtensteiner, 2006; Garriga et al., 2014).
Plant roots experience some morphological changes in their size, diameter, and number so as to maximize the nutrients and water uptake. Increased root to shoot ratio helps plants in compartmentalization and ions retention. Likewise, root proliferation helps plant to curb toxic ions accumulations. Also, root density and electrical conductivity increases under saline environment (Acosta-Motos et al., 2017; Arif et al., 2020). The other anatomical and ultra-structural change under salinity is the development of casparian strip and suberin lamella serving as apoplastic barrier. Similarly, plants’ chloroplast number and stable size structure increases under salinity (Arif et al., 2020) to maintain the effective photosynthetic rates.
Metabolic Adjustments
Salinity creates an ionic imbalance in plants that may result in denaturation or damage to subcellular organelles like chloroplasts and mitochondria. Therefore, plants compartmentalize the excessive ions into their vacuoles, and usually put into play their inclusion and exclusion mechanisms. This is generally regulated by (a) sequestration of excessive salt into vacuole with the help of various pumps (e.g., Na+/H+ antiporters); (b) ionic equilibrium: modulation of Na+, Cl–, K+, and Ca2+ in the plant cell via SOS and non-selective cationic channels (NSCCs) (Gupta and Huang, 2014; Arif et al., 2020). Osmotic potential (ψs) of plant cell is critical for its growth, development, and yield. That is why its proper regulation is important. Plants generally produce osmoprotectants to keep in check their ψs. These compounds are also described as compatible solutes. Unlike ions they neither paralyze the metabolic functions of enzymes, nor they destabilize the cellular membranes. In comparison to inorganic compounds, higher concentrations of these compounds are non-toxic to cellular metabolism (Nahar et al., 2016). Additionally, osmoprotectants play a diverse role in plant physiology under harsh conditions. Some of their prominent roles regarding metabolic adjustments in plants under stress include stabilization of proteins structures, regulation of protein folding, detoxification of ROS, stabilization of thylakoid membranes, protection of antioxidant enzymes, regulation of redox balance, and activation of stress responsive genes that result in redox homeostasis, stress signaling, upregulation of photosynthesis, and scavenging of toxic radicals (Zulfiqar et al., 2020a). These compatible solutes include betaines [proline betaine, hydroxyproline betaine, glycine betaine (GB), and pipecolate betaine], proline, sugars (fructose, glucose, sucrose, and fructans), and sugar alcohols (mannitol, sorbitol, and inositol). Of these compatible solutes, GB, a quaternary ammonium compound, usually ameliorates the toxic effects by accumulating in the cell and by distinguishing Na+ to K+ ratio. It is also reported to safeguard PSII under salt stress. Also, proline acts as an osmoprotectant as well as a molecular chaperone sustaining the structural integrity of macromolecules. Similarly, higher amounts of reduced sugars, i.e., fructose, glucose, sucrose, and fructans, stabilize the membrane integrity and prevent them from denaturation. Likewise, mannitol, sorbitol, and inositol facilitate in maintaining turgor, Na+ sequestration into the vacuole, and quenching ROS (Tang et al., 2015; Acosta-Motos et al., 2017; Arif et al., 2020).
One of the most common abiotic stress indicators in plants is the induction of oxidative stress. Salt stress also results in oxidative stress that comprises of ROS, reactive carbonyl species (RCS), and reactive nitrogen species (RNS) (Mano, 2012; Corpas, 2016; Fancy et al., 2017). However, these indicators of stress are also found in plant cells under normal conditions and a proper regulation of their intrinsic cellular concentration exists because they are also involved in plant growth and development, and signaling at subcellular and intercellular level (Corpas, 2016; Fancy et al., 2017; Zulfiqar and Ashraf, 2021a). Under any unfavorable condition, homeostatic balance of these reactive species disrupts resulting in altered cellular redox potential that results in the denaturation of various vital compounds including nucleic acids, proteins, lipids, etc. and disruption of cellular structures (Mano, 2012; Hasanuzzaman et al., 2020; Zulfiqar et al., 2020a). Nitric oxide (NO) and derived molecules altogether constitute RNS, whereas methylglyoxal (MG) and other α,β-unsaturated carbonyl compounds constitute RCS that are more stable than ROS (Mano et al., 2019; Nareshkumar et al., 2020). Mitigation of these radicals protect plant organelles in a number of ways. For example, the photoproduction and removal of ROS not only protects the chloroplast from the damaging effects of ROS but also acts as an escape valve for excess photons (Hasanuzzaman et al., 2020). Similarly, MG detoxification may result in improved cell proliferation, miotic index, seed germination, photosynthesis, stress-related gene expression, etc. (Mostofa et al., 2018). Preferred sites of ROS generation have been identified as chloroplast, mitochondria, and peroxisomes, whereas for NO as peroxisomes—although this remains a subject of further research (Hernandez et al., 1995; Hernández et al., 2001; Corpas, 2016). Similarly, RCS are generated as a by-product in various metabolic pathways, i.e., sugar metabolism, oxidative degradation of glucose and glycated proteins, glycolysis, lipid peroxidation, photosynthesis, etc., (Kaur et al., 2016; Mostofa et al., 2018), and therefore can be associated to be present in chloroplast, mitochondria, peroxisomes, cell membranes, nucleus, endoplasmic reticulum, and cytosol.
Ascorbate–glutathione (AsA–GSH) is a key metabolic pathway that keeps the oxidative stress of plants in check through enzymatic [catalase (CAT) EC 1.11.1.6, ascorbate peroxidase (APX) EC 1.11.1.11, dehydroascorbate reductase (DHAR) EC 1.8.5.1, monodehydroascorbate reductase (MDHAR) EC 1.6.5.4, glutathione-S-transferase (GST) EC 2.5.1.18, glutathione reductase (GR) EC 1.6.4.2, guaiacol peroxidase (GPX) EC 1.11.1.7, glutathione peroxidase (GPX) EC 1.11.1.9) and non-enzymatic (ascorbic acid (AsA), glutathione (GSH)] antioxidant players (Acosta-Motos et al., 2017; Hasanuzzaman et al., 2020). Similarly, RCS scavenging system also comprises of enzymatic and non-enzymatic compounds (Mano et al., 2019). A rise in MG levels is usually observed under salt stress that triggers the synthesis of glyoxalase: enzyme responsible for the detoxification of MG (Kaur et al., 2016). Under salt stress, mitochondria and chloroplast are specifically found to be affected. Consequently, electron transport chain is disrupted due to stomatal closure. Accordingly, the final electron acceptor in PSI (NADP+) of the electron chain suffers a halt in its regeneration that triggers Mehler Reaction (transfer of electron from ferredoxin to oxygen to form O2–) (Gururani et al., 2015; Acosta-Motos et al., 2017). This O2– is further converted to hydrogen peroxide (H2O2) and superoxide (O2–) by superoxide dismutase (SOD). Similarly, O2– generation in peroxisomes is modulated by APX and CAT activities. Protein denaturation and other structural damages have been reported previously in salt stressed cells, affecting particularly chloroplast and mitochondria due to the accumulation of H2O2 and O2– radicals. Therefore, in order to protect the photosynthetic machinery from ROS, plants regulate ‘xanthophyll cycle’ in which violaxanthin de-epoxidase converts carotenoid violaxanthin to zeaxanthin. This cycle helps in excessive energy dissipation in the form of heat, constituting the main mechanism of excessive energy dissipation, from PSII through non-photochemical quenching (NPQ). Zeaxanthin serves as an antioxidant for photoinhibition and photo-oxidation by scavenging ROS in thylakoid membranes. Similarly, salinity also results in the decrease in chlorophyl content that causes an increase in the anthocyanin and carotenoid accumulation, which help in toxic radicals scavenging and chloroplasts protection from photoinhibition and photooxidation. In the same way, another adapted mechanism for salinity tolerance is photorespiration that constantly recycles carbon dioxide from the decarboxylation of glycine in the mitochondria, so that the Calvin cycle is kept operational. Consequently, it diminishes ROS generation in electron transport chain. In addition, plants also use the water-water cycle to scavenge the ROS and dissipate excessive energy. In this cycle, water generated electrons in PSII are used to; (a) photo-reduce the dioxygen to superoxide in PSI, and (b) recycle ascorbate; thereby sustaining a linear electron flow for ATP generation. Furthermore, NO is involved in the glutathione metabolism by regulating GSH-dependent enzymes, i.e., GST, GR, and GSH. Also, it is reported that NO is a multifunctional molecule that regulates salt stress through genetic and molecular level regulations. Besides the aforementioned mechanisms, plants can also mitigate the oxidative stress through selective up-regulation of antioxidant enzymes, as found in Lycopersicon pennellii (Hernandez et al., 1995; Asada, 1999; Hernández et al., 2001; Mittova et al., 2003; Hörtensteiner, 2006; Garriga et al., 2014; Corpas, 2016; Acosta-Motos et al., 2017; Mano et al., 2019).
Phytohormonal Adjustments
Phytohormones modulate salinity by participating in signaling pathways and gene regulation. Abscisic acid (ABA) is known to regulate genes responsible for stomatal closure and osmoprotectant biosynthesis. It also helps in plant acclimation and inhibition of lateral root growth (Arif et al., 2020). Indole-3-acetic acid (IAA) promotes ion homeostasis by upregulating the expression of various genes including auxin/indoleacetic acid (Aux/IAA), small auxin-up RNA (SAUR) and GH3 (Sun et al., 2018), apart from regulating plant growth and development. Likewise, brassinosteroids (BRs) help plant to cope up with salinity by playing their role in the pollen tube growth, reproduction, proton pump activation, vascular differentiation, photosynthesis, and by improving antioxidant and osmoprotectant contents (Nolan et al., 2020). Also, cytokinins (CKs) are involved in salinity mitigation by increasing shoot to root ratio and antioxidants gene expression (Arif et al., 2020). Furthermore, ethylene is involved in salinity signaling perception and upregulating the expression of osmoprotectant genes, e.g., GB (Fahad et al., 2015). As well, gibberellins (GAs) are increased under salinity and modulate it by improving redox metabolism, sugar signaling, and osmolyte production (Fahad et al., 2015; Rao et al., 2016). Additionally, jasmonic acid (JA) reinforces the expression of arginine decarboxylase, invertase, and Rubisco genes to mitigate salinity. It is also involved in the metabolism of fatty acid along with methyl jasmonate (MeJA). The upregulation of arginine decarboxylase genes results in the modulation of polyamines biosynthesis that serve as osmolytes. Furthermore, it facilitates protein synthesis and CO2 fixation under saline conditions (Arif et al., 2020). Additionally, higher amounts of polyamines (PAs), nitrogen-containing aliphatic compounds, are accumulated in salt stressed cells to modulate signaling, cell proliferation, genetic expression, cell turgidity, and senescence (Ismail and Horie, 2017). In addition, salicylic acid (SA) modulates plant salinity to a great deal by participating in signaling pathways and regulating various genes expression. It also modulates ion homeostasis, i.e., limits Na+ influx in roots, Na+ regulates sequestration and exclusion, facilitates root H+-ATPase activity, and augments K+ concentration in aerial parts. As well, it upregulates genes of various ion channels to avoid K+ leakage (Arif et al., 2020).
Genomic Adjustments
Plants affected by salinity undertake various genomic adjustments in which various genes are up- and down-regulated. Currently, several advanced genomic techniques have made it possible to assess the molecular changes going-on in a plant under salt stress. Although, this is a set of complex mechanisms that range from transcription to post-translational modifications. Such genetic variation of expression results in the higher production of RNAs and proteins necessary to mitigate salinity. For instance, an upregulation of genes responsible for osmoprotectants biosynthesis is certainly a desired behavior for combating salinity (Amirbakhtiar et al., 2019). Nevertheless, an upregulation of genes is not always the case, rather the genomic behavior of plants may result in down-regulation, moderate expression, or even no expression. Similarly, gene expression can also be altered by the involvement of transcription factors or interfering RNAs. It has been discovered that the endogenous small interfering RNAs (siRNAs) and microRNAs (miRNAs) e.g., miR530a, miR1445, miR1446a-e, miR1447, miR396, miR394, miR393, miR319, miR171 miR169, miR168, etc., also play important roles in stress mitigation (Mangrauthia et al., 2013; Rao et al., 2016).
Following four functional categories of stress-responsive genes of plants have been established by Rao et al. (2016):
-
(a)
Molecular chaperones (e.g., HSP genes).
-
(b)
Ion transport or homeostasis (e.g., SOS genes, AtNHX1, and H+-ATPase).
-
(c)
Dehydration-related transcription factors (e.g., DREB).
-
(d)
Senescence-associated genes (e.g., SAG).
Some representative and differentially expressed genes (DEGs) are presented in Table 1, where genes or their families are grouped based on their function and involvement in signaling transduction, stress (ionic, osmotic, and oxidative), and metabolites biosynthesis. Generally, all these sets of genes are upregulated under saline environment with few exceptions (Arif et al., 2020). In a recent study, around 5128 DEGs for Triticum aestivum, treated with 150 mM NaCl, have been reported (Amirbakhtiar et al., 2019). This huge number of transcripts indicate the level of complexity involved in the regulation of salt stress, although it varies from species to species. Moreover, this study also underlined the upregulation of a set of genes involved particularly in signaling pathways, ion transporters, and oxidative stress.
TABLE 1.
Representative salt stress regulating genes and their respective functions in plants.
| Gene/gene family | Function | References |
| Signaling transduction pathways | ||
| SOS1, SOS2, SOS3, AtNHX1 | Vacuolar Na+/K+ antiporter, plasma membrane Na+/K+ antiporter, protein kinase, Calcium-binding protein. | Sottosanto et al., 2007; Chakraborty et al., 2012; Ismail and Horie, 2017 |
| ANN4, ACA7, NCL2, and GLR | Ca2+ transporters: adjust Ca2+ cytosolic concentrations | Amirbakhtiar et al., 2019 |
| CaM, CIPK, and CPK | Ca2+ signaling pathway | Amirbakhtiar et al., 2019 |
| HAK25, ABAC15, SOS3/CBL4 | Ion homeostasis, and coding for calcium sensing molecules | Fahad et al., 2015; Amirbakhtiar et al., 2019 |
| GmSALT3 | Encodes various membrane transporters | Yousefirad et al., 2020 |
| CaM1, CML37, CML27, CML29, and CDPK1 | Responsible for the activation of kinase and Ca2+ pathway | Amirbakhtiar et al., 2019; Arif et al., 2020 |
| Ionic, osmotic, and oxidative stress | ||
| MAPKKKA, MAPKKK2, and MAPKKK3 | Involved in ion homeostasis | Amirbakhtiar et al., 2019; Arif et al., 2020 |
| MYB, NAC, bHLH, WRKY, bZIP,s and AP2/ERF | Regulate the expression of the genes engaged in dealing with osmotic, ionic, and oxidative stresses arising from salinity | Deinlein et al., 2014; Amirbakhtiar et al., 2019 |
| TP4-1-like and NIP1-1-like; Wrab18, LEA1, LEA3, LEA-D34-Like, and LEA14-A; DHN3, DHN4, DHN7, and DHN9; P5CS, and P5CS | Genes coding for aquaporins, LEA proteins, dehydrins, and proline synthesis. Involved in plant metabolic pathway | Amirbakhtiar et al., 2019 |
| CAT, GRXC1, GST, CCOMT, SAM, GAPDH, and LAX AP2/ERF | Mediating in oxidative stress | Amirbakhtiar et al., 2019 |
| GmLAX3 and GmST1 | Improve salt tolerance by promoting antioxidant machinery and scavenging ROS | Khan et al., 2019 |
| MPK3 and MEKK2 | Ion homeostasis | Amirbakhtiar et al., 2019; Arif et al., 2020 |
| SUS1, TPS, and TPP | Involved in plant metabolic pathway | Ghaffari et al., 2016 |
| OsHsp17.0, OsHSP23.7, OsHSP71.1, and OsHSP80.2 | Heat-shock proteins, molecular chaperones, proteins transportation | Zou et al., 2009 |
| GAPDH | Participates in the glycolytic cycle | Albaladejo et al., 2018 |
| OSCA1 | Acts as a putative osmosensor | Zörb et al., 2019 |
| Plant growth and development | ||
| CRR-RLK, LRR-RLK, CRR-RLK, PERK, and SRK | Modulates plant growth, development, yield, and stabilizes the cell membrane under salinity | Amirbakhtiar et al., 2019; Guo et al., 2019; Zörb et al., 2019 |
| AtSTO1 | Biomass, photosynthesis, and pith size | Michler, 2014 |
| SWEET15 | Modulates vacuolar storage and transport of sugar | Jithesh et al., 2019 |
| LAX | Increases vascular development, xylem differentiation, and plant growth | Khan et al., 2019 |
| RCA1 and AOX1A | Promote photosynthetic efficiency | Albaladejo et al., 2018 |
| CYP94 (cytochrome P450) | Enhanced expression of CYP94C2b | Mochida and Shinozaki, 2011 |
| CCOMT and SAM | Involved in suberin and lignin biosynthesis | Arif et al., 2020 |
| OsRab7 | Seedling growth and increased proline content | Dhanaraj et al., 2015 |
Furthermore, it has been reported that genes coding for calcium sensing molecules (SOS3/CBL4) are up regulated in saline conditions. Their activation leads to the formation of a protein complex resulting in the transcription of Na+ antiporter gene (SOS1). Where Ca2+ causes Na+/H+ EXCHANGER 1 (NHX1) antiporter assisted Na+ sequestration into the vacuole, SOS1 gene induces Na+ efflux from cytosol (Arif et al., 2020). Likewise, genes encoding for proteins of photosynthetic machinery, ROS scavenging activity, SOD, cytochrome production, and isoflavone reductase production have also been found to be upregulated (Fahad et al., 2015; Rao et al., 2016; Amirbakhtiar et al., 2019; Khan et al., 2019). Briefly, all of the enzymes, proteins, osmoprotectants, co-factors, transporters, metabolites, etc. involved in ionic, oxidative, and osmotic stress (as described above: in morphological adjustments and metabolic adjustments) get their respective genes upregulated under the salt stress. For example, calcium pathways and SOS signaling genes have been reported to play their role in cell homeostasis and salt acclimation (Fahad et al., 2015). Although, several transcription factors play their intermediate role in these processes and post-translational modifications, but a comprehensive elucidation of their role remains ambiguous.
Salinity Mitigation Through Conventional Methods
Salts accumulation around the plants root cause salinity in plants. In order to remove these toxic concentrations of salts so as to gain maximum plant yield, commonly implied strategies include flushing, scraping, and leaching. Of these leaching is most widely used strategy, in which irrigation is sustained over the evapotranspiration rates. The excessive amount of water retains the concentrations of salts below their critical limits. For instance, in a study 20–30% extra irrigated water was found to leach 70% of salts from the maize roots (Plaut et al., 2013; Rao et al., 2016). Various irrigation models (e.g., SALTMED, CWPF, Enviro-Gro, WATSUIT, TETRANS, UNSATCHEM, MOPECO-Salt, etc.) based on the leaching principle have also been suggested as salinity mitigation approach and to improve crop yield (Plaut et al., 2013). But these models either considered the salt concentration as constant for any given time or were limited in their performance under various environmental factors; thereby constraining crop yield.
Soil mulching is another conventional approach for salinity mitigation. For this, soil surface is covered with mulch or plastic sheet to enhance water availability by limiting water evaporation. Previous studies conducted on cotton plants have demonstrated positive impact of mulching against salinity in terms of reduced activity of MDA, decreased accumulation of Na+ in leaves and roots, inhibition of lipid oxidation, improved photosynthesis, and higher biomass (Dong et al., 2008, 2009). However, this approach has short term effects and is only efficient to affect the upper soil layer. Irrigation water treatment through aeration and/or magnetic processing is another salinity mitigation technique (Plaut et al., 2013). Nevertheless, this is not widely adopted technique due to associated costs and intricacy of process. Another conventional approach is the cultivation of halophytes in saline soils for eliminating or reducing the accumulated salts to the threshold levels for glycophytes. Some halophytes are reported to have salt glands for this purpose that possess the ability to exclude salts, whereas others are reported to have salt hairs that serve to accumulate salts. Additionally, better agronomic and farm management practices can also improve salinity. For instance, with the drip irrigation a controlled amount of water can be applied to the soil, whereby limiting the soil salination. Also, surface and sprinkler irrigations might prove effective to leach down the excessive salts from root zone. Similarly, crop rotation with perennial crops can be practiced particularly in rain-fed areas. Deep roots of perennial crops might help restore the salt-water equilibrium in the soil (Plaut et al., 2013; Rao et al., 2016).
Selection, conventional breeding, and/or genetic engineering for salinity tolerant crops are also reported as salinity mitigation techniques (Athar and Ashraf, 2009; Plaut et al., 2013). In this regard, halophytes or salinity tolerant genotypes could be bred with desired salinity susceptible crop plants to get salt tolerant progeny. Similarly, salinity susceptible plants can be genetically transformed with salt tolerant genes or can be engineered for having salt glands/hairs. Equally, elicitation of plant bioregulators, osmolytes, antioxidants, or other metabolites biosynthesis has also been regarded as a valuable approach (Ashraf and Akram, 2009; Zulfiqar and Ashraf, 2021a). However, despite of the remarkable potential, these strategies are rather limited due to huge amounts of time required and the associated costs. Equally, salinity tolerance is a complex process that is regulated by a large number of genes that obscures the crop breeding and genetic transformation processes. Therefore, attaining a salt resistant transgenic line with its subsequent adoption in field conditions still remains a challenge.
In the same way, use of microbial inoculants, chemical and organic soil amendments, and electro remediation are other promising salinity mitigation strategies that are gaining increased scientific attention lately (Sahab et al., 2020). As chemical soil amendments pose threats to soil microbiota and indirectly to human life, therefore this cannot be regarded as a sustainable approach. An alternative method of salinity mitigation is the exogenous application of nutrients and metabolites that relieves plants from Na+ and Cl– injury (Munns, 2002; Plaut et al., 2013). Use of natural PEs, in this regard, can be associated with this strategy of salinity mitigation, although PEs do not only have phytonutrients but also other stress relieving metabolites, e.g., GB, proline, melatonin, etc.
Use of Plant Extracts Against Salinity
Use of PEs to mitigate salinity can be regarded as an environment friendly and sustainable way of fighting abiotic stress, as it contains no synthetic chemicals. Depending upon the parts of plants used to prepare PE, it may contain various amounts of bioactive compounds (flavonols, phenolics, betaines, amino-polysaccharides, sterols, glucosinolates, terpenoids, furostanol glycosides, etc.), phytohormones, mineral elements, photosynthetic pigments, amino acids, nucleotides or nucleosides, lipids, etc. Due to the associated detoxifying and ROS quenching capabilities of these compounds, PEs are frequently used in pharmacological industry for providing protection against neurodegeneration, diabetes, muscular dystrophy, and cancer like chronic diseases (Pehlivan, 2018). Since these natural compounds are also effective in preventing macromolecules like lipids, proteins, and DNA from damage in animal cells (Pehlivan, 2018), therefore it can be deduced that they might also be effective in plants against salinity as it disrupts redox balance in plants. This has been demonstrated in previous studies that PEs contribute to a better growth, development, yield, disease-, and stress-resistance in plants given the presence of aforementioned compounds (Howladar, 2014; Drobek et al., 2019; Desoky et al., 2020; Zulfiqar et al., 2020b). Nevertheless, further scientific evidences are yet to be excavated to ensure that such a wide variety of molecules in PEs is functional or not. Similarly, viability and quality of PEs is also an aspiring research area.
Sources of PEs, methods of application, and their implications against salinity are discussed below.
Sources, Preparation, and Application Methods
Plant extracts or botanicals are prepared from natural resources like higher plants. They can be prepared either from a whole plant or from any specific part of the plant, i.e., fruits, flowers, bark, roots, leaves, stems, seeds, pollens, grains, etc. (Semida and Rady, 2014; Lorenzo et al., 2019; Nessim and Kasim, 2019; Rady et al., 2019a; Zulfiqar et al., 2020b; Suryaman et al., 2021). Whereas plant derived products like protein hydrolyzates, polyamines, polyols, amides, etc. fall under the category of plant derived biostimulants (PDBs), as PEs are multicomponent mixtures. However, the extraction of a particular compound or a mixture of compounds can be reinforced by selecting an appropriate method of extract preparation.
Conventionally PEs are prepared by maceration. The extraction is done in some solvent either hydrous or organic. For aqueous extraction, desired plant part is macerated or processed mechanically in deionized H2O, followed by its purification and centrifugation. The resultant analyte is diluted as per requirement and applied to plant (Rady and Mohamed, 2015; Abd El-Mageed et al., 2017; Yakhin et al., 2017; Ali et al., 2018; Zulfiqar et al., 2020b). In organic solvent extraction, the desired plant part is homogenized in an organic solvent, e.g., ethanol, followed by fractionated extraction with hexane, ethyl acetate, and/or butanol like solvents. Further, the resultant extractants are purified by removing organic solvents through evaporation (Salama et al., 2013; Lim et al., 2014; Brockman and Brennan, 2017; Yakhin et al., 2017; Pehlivan, 2018). Aqueous extraction is considered relatively easier, faster, and economical as compared to the organic solvent-based extraction. Furthermore, several other methods for homogenates preparation can be implied such as bead impact methods, rotor–stator homogenizer, high pressure batch/flow, low-pressure droplet method, ultrasonic processors, etc. (Goldberg, 2008). Besides, these basic methods can be further modified based on the desired extractant, i.e., lipophilic, or hydrophilic. However, an appropriate method of PE preparation is important as it affects the stability characteristics of the formulation (Lötze and Hoffman, 2016). In addition, the extractions carried out using organic solvent, like ethanol, may vary in triggering the physiological response as compared to the extracts prepared through aqueous extraction. The possible reason of such difference could be the variation in physiochemical properties, i.e., pH, temperature, electric charge, surface tension, solubility, etc., of the aqueous and organic extracts. For instance, a subsequent increase in the extract viscosity was observed when pH and temperature were increased (Briceño-Domínguez et al., 2014). Similarly, penetration and assimilation of applied extract may vary depending upon its hydrophilic nature, mode of application, environmental conditions (light, temperature, relative humidity, etc.), ontogenesis, and permeability of plant surface (Fernández and Brown, 2013; Fernández et al., 2017). A turgid cell might not absorb more water resulting in no absorption of aqueous extract. Likewise, a PE prepared through organic solvent-based extraction might also not get absorbed due to the hydrophilic nature of plant cuticle. All these variable factors greatly influence plant physiological response to PEs. A stepwise illustration of the preparation of PEs is presented in Figure 3.
FIGURE 3.
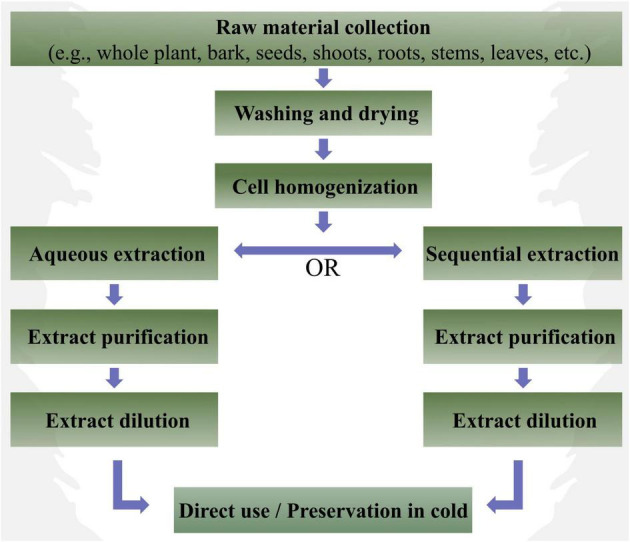
A stepwise illustration of the preparation of plant extracts (PEs).
Usually, PEs are applied to plants through following three methods;
-
(a)
Foliar spray (Habib et al., 2012; Lorenzo et al., 2019).
-
(b)
Soil based application (Pehlivan, 2018; Hassanein et al., 2019; Brazales-Cevallos et al., 2022).
-
(c)
Biopriming (seed priming) (Panuccio et al., 2018; ElSayed et al., 2022).
For a good penetration and assimilation of ingredients, PEs should be water soluble (or in any other suitable solvent). To overcome the lipophilicity and molecular size like uptake problems might be solved by mixing PEs with surfactants or other additives (Yakhin et al., 2017). Similarly, the absorbability of PEs also depends upon the molecular structure of cuticle of the plant under study, environmental conditions, and other extrinsic factors (Bulgari et al., 2015).
Implications of Plant Extracts Against Salinity
The basic aim of the use of PEs is reinforcing the plant responses to salinity so as to sustain the cellular homeostasis. Exogenous application of PEs is found to take part in the signaling, primary and secondary metabolic pathways, and other physiological processes of plant (Table 2). Similarly, morphological, and anatomical adjustments are important mechanisms for stress regulation in plants. Improvement in growth traits including plant height, root and shoot length, fresh and dry weight of shoots, fresh and dry weight of roots, root/shoot ratio, number of leaves, leaf area, leaf thickness leaf relative water content (RWC), number of pods, pods weight, number of seeds, seed weight, grain yield, biological yield, and harvest index results on the use of PEs on salt stressed plants (Habib et al., 2012; Rady et al., 2015; Latif and Mohamed, 2016; Merwad, 2020; Suryaman et al., 2021). These altered characteristics might help glycophytes in better acclimation and tolerance, presumably, by enhanced robustness, a higher accumulation of reserves, photosynthetic pigments, gaseous exchange, and ionic compartmentalization. Nevertheless, nutrients are the fundamental players for such alterations providing energy and substrates. Nutrient uptake is greatly challenged under salinity conditions (Munns and Tester, 2008; Zörb et al., 2019) that can be assuaged by the exogenous application of PEs. Several studies have reported an improvement in nutrient (particularly NPK, Fe, Zn, and Mn) uptake and assimilation in salt stressed plant upon the application of PEs (Bulgari et al., 2015; Rady et al., 2019a; Merwad, 2020).
TABLE 2.
Use of different plant extracts (PEs) against salinity in various plant species.
| Plant extract | Extract type |
Application method |
Species under study |
Salt concentration/ salinity |
Results | References |
| Seed extracts | ||||||
| Foeniculum vulgare and Ammi visnaga | Seed extracts (2,000 ppm) | Foliar spray | Vigna unguiculata | Seawater (EC: 3.5 and 7 dS m–1) | Improved growth and yield traits, osmoprotectants content, antioxidant system, RWC, MSI, photosynthetic efficiency, nutrient contents, K+/Na+ ratio, and anatomical features. Reduced Na+ content, EL, and oxidative stress biomarkers. |
Desoky et al., 2020 |
| Garcinia mangostana | Pericarp extract (1%) | Seed priming | Vigna radiata R. Wilczek | 0.5 and 1% of NaCl | Increase plant height, leaf area, and yield components. | Suryaman et al., 2021 |
| Leaf extracts | ||||||
| Moringa oleifera | Leaf extract (1:30) | Foliar spray | Phaseolus vulgaris | 200 mM NaCl | Mitigation of oxidative stress and improved morphological and physiological parameters. | Latif and Mohamed, 2016 |
| Moringa oleifera | Leaf extract (1:25) | Foliar spray | Trigonella foenum-graecum | 0, 50, 100 and 200 mM NaCl | Improved ion homeostasis, growth traits, photosynthetic pigments, organic solutes, and total phenols. Increased activities of POD, CAT, APX, and SOD. Identification of new 12 polypeptides. | Latef et al., 2017 |
| Moringa oleifera | Leaf extract (3%) | Foliar spray | Sorghum vulgare var. sudanense | Non-saline (EC: 3.01 dS m–1), medium saline (EC: 6.12 dS m–1), highly saline (EC: 12.33 dS m–1) |
Increased cumulative yield and nutrient uptake. | Merwad, 2017 |
| Moringa oleifera | Leaf extract (1:30) | Foliar spray and seed priming | Helianthus annuus | Sandy loam (EC: 6.42–6.48 dS m–1) | Improved growth traits, RWC, MSI, concentrations of total chlorophylls, total carotenoids, total soluble sugars, free proline and ascorbic acid, ion homeostasis, antioxidant enzymes, seed yield, and seed oil and protein contents. | Taha, 2016 |
| Moringa oleifera | Leaf extract (1:30) | Foliar spray and seed priming | Phaseolus vulgaris | Saline soil (EC = 6.23–6.28 dS m–1) | Improved growth traits, RWC, MSI, concentrations of total chlorophylls, total carotenoids, total soluble sugars, free proline and ascorbic acid, ion homeostasis, antioxidant enzymes, green pods and dry seed yield. | Rady and Mohamed, 2015 |
| Moringa oleifera | Leaf extract (1:30) | Foliar spray | Phaseolus vulgaris | 90 mM NaCl, 1 mM Cd2+ (CdCl2) |
Enhanced growth traits, level of photosynthetic pigments, green pod yield and pod protein, antioxidant enzymes and proline content. No effect on EL and lipid peroxidation |
Howladar, 2014 |
| Ocimum basilicum | Leaf extract (20%) | Foliar spray | Vicia faba | 0.0, 50, 100, or 150 mM NaCl | Increased activity of antioxidant enzymes, organic solutes, lipid peroxidation, and ions content | Aboualhamed and Loutfy, 2020 |
| Moringa oleifera and Moringa peregrina | Leaf extract (2.5, 5, 10, and 20%) | Soil based | Ocimum basilicum cv. Cispum | 100 mM NaCl | Increased content of proline, MDA, anthocyanin, total carbohydrates, and SOD. Significant increase in growth traits. | Hassanein et al., 2019 |
| Cupressus macrocarpa | Leaf Extract (0.5%) | Seed priming | Cucurbita pepo cv. Kavili | 100 mM NaCl | Enhanced growth, photosynthetic capacity, antioxidant enzyme and rubisco activities, increased contents of AsA, GSH, ratio of K+/Na+, and proline. Genes upregulation (CuZnSOD2, CAT1, APX, GR, DHAR, and PrxQ) |
ElSayed et al., 2022 |
| Moringa oleifera | Leaf extract (3%) | Seed priming and foliar spray | Triticum aestivum Cv. Sakha 93 | Saline soil (EC: 9.10 dS m–1) | Enhanced osmotic stress tolerance by stabilizing membrane integrity and decreasing EL. Improved endogenous GSH, AsA, photosynthetic efficiency, photosynthetic pigments, growth traits, ionic- and hormonal-homeostasis. | ur Rehman et al., 2021 |
| Moringa oleifera | Leaf extract (1:30) | Foliar spray | Rosa damascena var. trigintipetala Dieck | 200 mM NaCl | Enhanced growth attributes, chlorophyll content, RWC, proline content, and MSI. Increased radical scavenging activity, total phenols, ratio of K+/Na+, and antioxidant enzyme activity. |
Hassan et al., 2020 |
| Moringa oleifera | Leaf extract (–) | Seed priming | Phaseolus vulgaris cv. Bronco | 100 mM NaCl | Improved growth, yield, content of osmoprotectants, activity of enzymatic and non-enzymatic antioxidants and ratio of K+/Na+ | Rady et al., 2013 |
| Moringa oleifera | Leaf extract (6%) | Seed priming | Triticum aestivum cultivar Giza 168 | 120 mM NaCl | Significant amelioration on biomass, yield, osmoprotectants and antioxidant systems | Rady et al., 2019b |
| Typha angustifolia | Leaf extract (4%) | Seed priming | Pisum sativum var. Lincoln | 120, 240, and 320 mM NaCl | Membrane integrity, increased values of osmotica (proline, total soluble sugars, K+, and P), chlorophyll and carotenoid content, and lower EL. | Ghezal et al., 2016 |
| Rosmarinus officinalis and Artemisia herba-alba | Leaf extracts (1:5) | Seed priming | Zea mays | 100 mM NaCl | Increased germination percentage and germination indexes, ion compartmentalization of cations and anions, root/shoot ration photosynthetic pigments, and antioxidant system. | Panuccio et al., 2018 |
| Moringa oleifera | Leaf extract (1, 3, and 10%) | Seed priming and soil based (irrigation) | Arabidopsis thaliana | 100 mM NaCl | Activation of ABA-, SA-, AUX-, and ET-related signaling pathways. | Brazales-Cevallos et al., 2022 |
| Root extracts | ||||||
| Glycyrrhiza glabra | Root extract (0.5%) | Seed priming | Pisum sativum cv. Master-B | 150 mM NaCl | Enhanced seedling growth, photosynthetic attributes, AsA, GSH, proline, soluble sugars, α-tocopherols, ratio of K+/Na+, and antioxidant enzyme activities. Upregulation of CAT-, SOD-, APX-, GR-, DHAR-, and PrxQ-encoding genes. |
Desoky et al., 2019 |
| Beta vulgaris | Root extract (50 mmol Kg–1 of GB) | Foliar spray | Abelmoschus esculentus cv. Arka-anamika and Sabaz-pari | 100 mM NaCl | Improved biomass production, plant yield, various gas exchange characteristics, and leaf ion homeostasis (K+, Ca2+, Cl–, Na+, K+/Na+ ratio in shoot and root). | Habib et al., 2012 |
| Glycyrrhiza glabra and Moringa oleifera | Licorice: root extract (0.5%); Moringa: leaf extracts (3%) | Foliar spray | Triticum aestivum cv. Sakha 93 | Saline soil (EC: 9.12 dS m–1) | Increased yield, protein content, photosynthetic pigments, and nutrient uptake (NPK, Fe, Zn, and Mn). | Merwad, 2020 |
| Beta vulgaris | Root extract (50 mmol Kg–1 of GB) | Foliar spray | Solanum melongena cv. Dilnasheen and Bemisal | 100 mM NaCl | Improved growth, yield, photosynthetic rate, transpiration, stomatal conductance, GB accumulation, and leaf K+, Ca+, Cl–, and Na+ content. | Abbas et al., 2010 |
| Daucus carota | Root extract (20%) | Seed priming | Lupinus termis cv. Gemmeza R2 | 150 mM NaCl | Enhanced growth traits, leaf water content and photosynthetic pigments, total soluble sugars, proteins, alkaloids, MDA, CAT, peroxidase activities and ascorbate content. Preserved cell wall, integrity of chloroplast membranes, normal grana organization and nuclear structure with well-defined nucleoli. |
Nessim and Kasim, 2019 |
| Daucus carota | Root extract (2%) | Seed priming | Zea mays | Seawater induced (Na+: 10 mg L–1; Cl–: 784 mg L–1) | Improved growth traits, protection of the photosynthetic pigments, chlorophylls, carotenoids, ion homeostasis, osmolytes, and ROS mitigation. | Latef et al., 2019 |
| Glycyrrhiza glabra | Root extract (0.5%) | Seed priming | Phaseolus vulgaris cv. Bronco | Saline soil (EC = 7.2 dS m–1) | Increased plant growth, yield, photosynthetic pigments, free proline, total soluble carbohydrates, total soluble sugars TSS, nutrients, and selenium, ion homeostasis, RWC, MSI, activities of all enzymatic antioxidants, and anatomical features. Decreased EL, MDA, and ROS content. |
Rady et al., 2019a |
| Fruit and grains extracts | ||||||
| Zea mays | Grains extract (6%) | Seed priming and foliar spray | Phaseolus vulgaris cv. Paulista | Saline soils (EC = 7.43–7.51 dS m–1) | Improved growth and yield components, RWC, MSI, photosynthetic pigments, soluble sugars, proline, N, P, K+, Ca2+, IAA, GA, and CKs concentrations; K+/Na+ and Ca2+/Na+ ratios; SOD, and CAT activities; GSH and AsA contents. | Rady et al., 2019b |
| Vaccinium arctostaphylos | Fruit extract (6%) | Soil based (irrigation) | Zea mays Samada 07 | 200 mM NaCl | Reduced pigment loss, biomass loss, damage to roots and shoots, lipid oxidation, proline synthesis and endogenous H2O2 concentrations. Improved growth, and levels of antioxidant enzymes. |
Pehlivan, 2018 |
| Bark extracts | ||||||
| Acacia dealbata | Bark extract (0, 450, or 900 ppm) | Foliar spray | Allium cepa | 60 and 120 mM NaCl | Attenuation of salinity by increased height, leaf-, root-, total biomass, sugar, and protein content. | Lorenzo et al., 2019 |
| Salix babylonica | Bark extract (2, 4%) and leaf extracts (2, 4%) | Seed priming | Zea mays | 100 mM NaCl | Increased growth traits (shoot fresh weight, root area, etc.), leaf protein concentration. Reduced lipid peroxidation and specific activities of antioxidative enzymes. |
Mutlu-Durak and Yildiz Kutman, 2021 |
| Whole plant extracts | ||||||
| Sorghum bicolor | Whole plant extract (5%) | Seed priming | Camelina sativa | Saline soil (EC: 10 dS m–1) | Improved growth traits (emergence percentage, root length, shoot length etc.), α-amylase activity, chlorophyll content, antioxidant enzymes activity and shoot K+ ion. Reduced concentrations of H2O2, MDA, and shoot Na+ ion. |
Huang et al., 2021 |
| Rosmarinus officinalis | Whole plant extract (10 and 20%) |
– | Malus domestica (seedlings) | 50 and 100 mM NaCl | Increased concentrations of ascorbic acid, phenols, trehalose and flavonoids. | Mahmoudand and Dahab, 2018 |
| Sorghum bicolor | Whole plant extract (5%) | Seed priming | Triticum aestivum | Saline soil (EC: 4 and 10 dS m–1) | Increased total phenolics, total soluble sugars, proteins, α-amylase activity, chlorophyll contents, and K+ ions. Decreased Na+ content. |
Bajwa et al., 2018 |
ABA, abscisic acid; AsA, ascorbic acid; APX, ascorbate peroxidase; AUX, auxin; CAT, catalase; CKs, cytokinins; DHAR, dehydroascorbate reductase; EL, electrolyte leakage; ET, ethylene; GA, gibberellic acid; GSH, Glutathione; GR, glutathione reductase; H2O2, hydrogen peroxide; IAA, indole-3-acetic acid; MDA, malondialdehyde; MSI, membrane stability index; POD, guaiacol peroxidase; PrxQ, peroxiredoxins; ROS, reactive oxygen species; Rubisco, ribulose-1,5-bisphosphate carboxylase/oxygenase; RWC, relative water content; SOD, superoxide dismutase; SA, salicylic acid.
The PEs rich in antioxidants can be associated to facilitate the stress mitigation processes through enzymatic and non-enzymatic processes. For instance, salinity caused osmotic stress results in lower ψs, photosynthetic performance (Fv/Fm, intracellular CO2 concentration, CO2 assimilation rate, net photosynthetic rate, transpiration rate, and stomatal conductance), altered ions concentrations (K+/Na+, Ca2+/Na+, K+ + Ca2+/Na+), and disrupted hormonal content. These perturbations could be mitigated by the enhanced content of GSH and AsA that improve the tolerance by decreasing electrolyte leakage and stabilizing membrane integrity. Enhanced content of osmoprotectants/osmolytes (GB, proline, pipecolate betaine mannitol, sorbitol, etc.) could be attributed to enhanced ionic, hormonal, and osmotic adjustments that result in improved acclimation, photosynthetic efficiency, growth, and yield (Bulgari et al., 2015; ur Rehman et al., 2021). Overall, lower concentrations of PEs have been found to induce these positive results as higher concentrations might produce harmful results.
Similarly, PEs are efficient sources of ROS and RNS scavengers that are needed to mitigate toxic radicals and stabilize cell homeostasis. They can restore the K+/Na+ ratios of both root and leaf (Abbas et al., 2010). Desoky et al. (2020) reported a decreased in the contents of these reactive species on the application of seed extracts on Vigna unguiculata irrigated with seawater (EC: 3.5 and 7 dS m–1). Among others, they attributed the improved K+/Na+ ratio and membrane integrity to antioxidants and polyphenols rich seed extracts. Analogous results have been reported in case of Phaseolus vulgaris, Vicia faba, Triticum aestivum, Lupinus termis, and Zea mays plants subjected to varying degree of salt concentrations (Rady and Mohamed, 2015; Latif and Mohamed, 2016; Latef et al., 2019; Nessim and Kasim, 2019; Aboualhamed and Loutfy, 2020; ur Rehman et al., 2021). In another study, presence of phenols, flavonoids, and AsA in Rosmarinus officinalis L. extracts was associated to salinity alleviation in apple seedlings (Mahmoudand and Dahab, 2018). Each plant responds differently as per PEs used and therefore various oxidative stress mitigation strategies can be observed. Likewise, the negligible or no activity of some antioxidants might be attributed to various fluctuations in the activation of corresponding transcription factor/genes. A demonstration of negative effects of salinity on plant and its mitigation by PEs is illustrated in Figure 4.
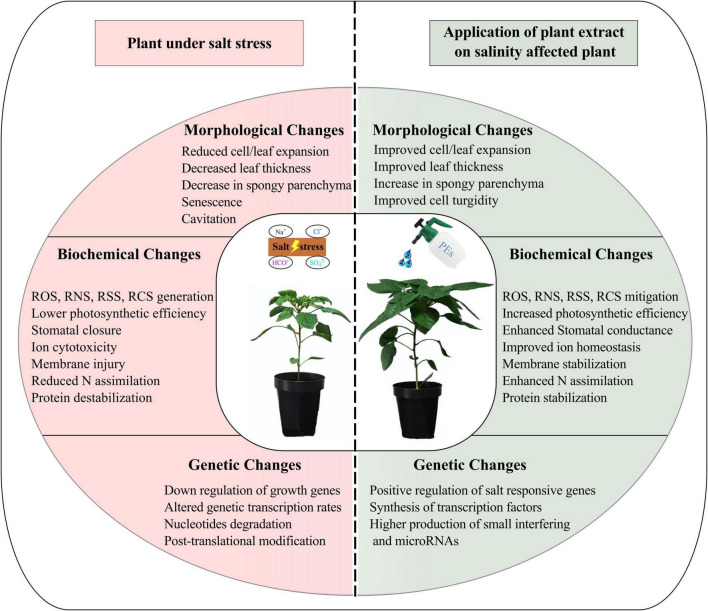
FIGURE 4.
Illustration of the impact of salinity, in terms of morphological, biochemical, and genetic changes, on plants (Left) in comparison with the plants supplied with PEs (Right).
In the same way, PEs carried phytohormones, particularly ABA, JA, ethylene, and SA, stimulate the signaling pathway; whereby triggering various transcription factors and stress related genes. Equally, phytohormones might also be associated to bolster the photosynthetic machinery and overall ionic balance in the cell. In a recent study, Cupressus macrocarpa foliar extract primed seed were found to have upregulated various stress related genes (CuZnSOD2, CAT1, DHAR, APX, PrxQ, and GR) in Cucurbita pepo (ElSayed et al., 2022). Upregulation of PrxQ-, APX-, SOD-, DHAR-, GR-, and CAT-encoding genes have also reported in Pisum sativum when its seeds were primed with Glycyrrhiza glabra root extracts and subjected to 150 mM NaCl stress (Desoky et al., 2019). Another evidence of the possible role of PEs in signaling was backed in a study on Arabidopsis, where moringa leaf extracts facilitated salinity mitigation by transcriptionally activating ABA-, SA-, AUX-, and ET-related signaling pathways (Brazales-Cevallos et al., 2022). ABA is also reported to regulate the transcription factor ABI5 (ABSCISIC ACID INSENSIVE 1) that is required by plants to activate ABI5 expression and salt acclimation (Zörb et al., 2019). However, comprehensive studies to establish the molecular basis of PEs phytohormones in signaling are either in dearth or non-existent.
Limitations and Future Perspective
Plant extracts being amalgams of various biological compounds make it difficult to map out their exact mode of action. Studies undertaken to compare the individual effects of various osmolytes (e.g., GB) or phytohormones (e.g., SA) along with PEs, concluded the supremacy of PEs as being more efficient (Abbas et al., 2010; Rady et al., 2015). Similarly, the role of PEs in signal transduction remains ambiguous as exogenous application of relevant compounds (i.e., ethylene, AsA, etc.) can also elicit the plant response. Either PEs work as elicitors of natural compounds or PEs carried molecule assimilation results in the desired results, needs further investigation. Similarly, the involvement of protein kinases has already been documented in saline environments (Zörb et al., 2019; Arif et al., 2020), but no such studies have been undertaken upon the use of PEs. Likewise, elucidation of the potential role of reactive sulfur species (RSS) (Corpas and Barroso, 2015) and RCS with respect to salinity and its subsequent mitigation through PEs might open new avenues of research. In addition, application of nanoparticles (NPs) coated PEs might improve their efficiency exponentially as various NPs have been documented effective against salinity (Zulfiqar and Ashraf, 2021b). Nevertheless, intensive research is needed for the application of PEs coated with NPs because of the reported toxicities of NPs based on their physiochemical properties and plant species (Ahmad et al., 2021a).
The role of PEs in morphological and anatomical traits like shape and size of palisade and mesophyll cells, integrity of grana and thylakoids, integrity of cristae, the number and size of plastoglobuli, number and diameter of xylem and phloem tissues, width of cortex, suberin and casparian strips development, root-, shoot-apex, endodermis, and exodermis etc. needs more scientific attention, as these factors play crucial role in gaseous exchanges, ions permeability, ψw, ψs, and energy generation processes (Acosta-Motos et al., 2017). Analogously, comprehensive studies using omics approaches (genomics, transcriptomics, proteomics, metabolomics, and bioinformatics) can further shed lights on positive or negative regulators of morphological-, metabolic-, and genomic-adjustments, target molecules, and the potential receptors activated by the use of PEs. The already identified signaling signatures, genes, and other key metabolites can be used to investigate such processes on the use of PEs. The influence of PEs on interference RNA mechanism to combat salinity also remains an enticing research area.
Use of PEs to combat salinity is a green, ecofriendly, and sustainable approach. This also opens further doors of investigation on the use of invasive plant species to be used as salinity moderator. Another important aspect is that plant response owing to PEs varies greatly from species to species and even within the same species. This might be answered by undertaking further comparative studies. Furthermore, use of salinity to trigger the production of various osmolytes and antioxidants can be utilized as an elicitation approach. Plants subjected to salinity can be used to prepare PEs that might prove more promising against salinity than conventional ones.
Conclusion
Salt stress has become a consistent problem in agriculture over the past few years, and was reported to culminate around 900 million ha in 2020. Plants perceive salinity by sensors, e.g., cell surface-based receptors, protein kinases, etc., resulting in a cascade of phosphorylation that regulates subsequent genetic expression. Salinity results in ionic, osmotic, and oxidative stress, which further disrupts various physiological and metabolic processes in plants. Use of PEs to combat salinity is an efficient, economical, and sustainable approach. Whole plants or parts of plants, i.e., roots, leaves, flowers, bark, seeds, pollens, etc. can be used to prepare PEs through aqueous or organic-solvent extraction techniques. PEs are multicomponent organic mixtures, containing vitamins, carotenoids, amino acids, phytohormones, mineral nutrients, phenolics, and antioxidants, etc., which facilitate stress signaling, genes regulation, redox metabolism, and synthesis of various proteins and metabolites. The degree of impact of PEs depends on various factors like plant species, age of plant, application method, etc. Molecular characterization of the PEs produced effects can pave the way for elucidating their comprehensive mechanism of action.
Author Contributions
AA, BB, and VM: conceptualization. AA: study design, data collection and analysis, draft writing and editing, and Illustrations. VM and BB: critical analysis, revision, and supervision. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
This work was supported by the projects: “VIRTUOUS” funded from the European Union’s Horizon 2020 Project H2020-MSCA-RISE-2019. Ref. 872181, “SUSTAINABLE” funded from the European Union’s Horizon 2020 Project H2020-MSCA-RISE-2020. Ref. 101007702, and the “Project of Excellence” from FEDER (Fondo Europeo de Desarrollo Regional)- Junta de Andalucia 2018. Ref. P18-H0-4700. The funding organizations had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Abbas W., Ashraf M., Akram N. A. (2010). Alleviation of salt-induced adverse effects in eggplant (Solanum melongena L.) by glycinebetaine and sugarbeet extracts. Sci. Hortic. 125 188–195. 10.1016/j.scienta.2010.04.008 [DOI] [Google Scholar]
- Abd El-Mageed T. A., Semida W. M., Rady M. M. (2017). Moringa leaf extract as biostimulant improves water use efficiency, physio-biochemical attributes of squash plants under deficit irrigation. Agric. Water Manag. 193 46–54. 10.1016/j.agwat.2017.08.004 [DOI] [Google Scholar]
- Aboualhamed M. F., Loutfy N. (2020). Ocimum basilicum Leaf Extract Induces Salinity Stress Tolerance in Faba Bean Plants. Egypt. J. Bot. 60 681–690. [Google Scholar]
- Acosta-Motos J. R., Ortuño M. F., Bernal-Vicente A., Diaz-Vivancos P., Sanchez-Blanco M. J., Hernandez J. A. (2017). Plant responses to salt stress: adaptive mechanisms. Agronomy 7:18. 10.3390/agronomy7010018 [DOI] [Google Scholar]
- Adetunji C., Fawole O., Arowora K., Nwaubani S., Ajayi E., Oloke J., et al. (2012). Quality and safety of Citrus Sinensis coated with Hydroxypropylmethylcellulose edible coatings containing Moringa oleifera extract stored at ambient temperature. Global J. Sci. Front. Res. Biol. Technol. Genet. 12 29–33. [Google Scholar]
- Ahmad A., Ordoñez J., Cartujo P., Martos V. (2021b). Remotely Piloted Aircraft (RPA) in Agriculture: a Pursuit of Sustainability. Agronomy 11:7. 10.3390/agronomy11010007 [DOI] [Google Scholar]
- Ahmad A., Hashmi S. S., Palma J. M., Corpas F. J. (2021a). Influence of metallic, metallic oxide, and organic nanoparticles on plant physiology. Chemosphere 290:133329. 10.1016/j.chemosphere.2021.133329 [DOI] [PubMed] [Google Scholar]
- Albaladejo I., Egea I., Morales B., Flores F. B., Capel C., Lozano R., et al. (2018). Identification of key genes involved in the phenotypic alterations of res (restored cell structure by salinity) tomato mutant and its recovery induced by salt stress through transcriptomic analysis. BMC Plant Biol. 18:213. 10.1186/s12870-018-1436-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali E., Hassan F., Elgimabi M. (2018). Improving the growth, yield and volatile oil content of Pelargonium graveolens L. Herit by foliar application with moringa leaf extract through motivating physiological and biochemical parameters. S. Afr. J. Bot. 119 383–389. 10.1016/j.sajb.2018.10.003 [DOI] [Google Scholar]
- Ali M., Cheng Z.-H., Hayat S., Ahmad H., Ghani M. I., Tao L. (2019). Foliar spraying of aqueous garlic bulb extract stimulates growth and antioxidant enzyme activity in eggplant (Solanum melongena L.). J. Integr. Agric. 18 1001–1013. 10.1016/s2095-3119(18)62129-x [DOI] [Google Scholar]
- Amirbakhtiar N., Ismaili A., Ghaffari M. R., Nazarian Firouzabadi F., Shobbar Z.-S. (2019). Transcriptome response of roots to salt stress in a salinity-tolerant bread wheat cultivar. PLoS One 14:e0213305. 10.1371/journal.pone.0213305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif Y., Singh P., Siddiqui H., Bajguz A., Hayat S. (2020). Salinity induced physiological and biochemical changes in plants: an omic approach towards salt stress tolerance. Plant Physiol. Biochem. 156 64–77. 10.1016/j.plaphy.2020.08.042 [DOI] [PubMed] [Google Scholar]
- Arora N. K. (2019). Impact of climate change on agriculture production and its sustainable solutions. Environ. Sustain. 2 95–96. 10.1007/s42398-019-00078-w [DOI] [Google Scholar]
- Asada K. (1999). The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Ann. Rev. Plant Biol. 50 601–639. 10.1146/annurev.arplant.50.1.601 [DOI] [PubMed] [Google Scholar]
- Ashraf M., Akram N. A. (2009). Improving salinity tolerance of plants through conventional breeding and genetic engineering: an analytical comparison. Biotechnol. Adv. 27 744–752. 10.1016/j.biotechadv.2009.05.026 [DOI] [PubMed] [Google Scholar]
- Ashraf M., Harris P. (2004). Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 166 3–16. 10.1016/j.plantsci.2003.10.024 [DOI] [Google Scholar]
- Athar H. R., Ashraf M. (2009). “Strategies for crop improvement against salinity and drought stress: An overview,” in Salinity and water stress, eds Ashraf M., Ozturk M., Athar H. (Dordrecht: Springer; ), 1–16. 10.3390/ijms22116119 [DOI] [Google Scholar]
- Bajwa A. A., Farooq M., Nawaz A. (2018). Seed priming with sorghum extracts and benzyl aminopurine improves the tolerance against salt stress in wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 24 239–249. 10.1007/s12298-018-0512-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadle C. (1993). “Growth analysis,” in Photosynthesis and Production in a Changing Environment, ed. Hall D. O. (Berlin: Springer; ), 36–46. [Google Scholar]
- Brazales-Cevallos D. K., Romero-Contreras Y. J., Vences-Guzmán M. Á, Torres M., Aviles-Baltazar N. Y., Sohlenkamp C., et al. (2022). Transcriptional characterization of the biostimulant effect of Moringa oleifera leaf extracts using Arabidopsis thaliana as a model. S. Afr. J. Bot. 144 250–256. 10.1016/j.sajb.2021.09.011 [DOI] [Google Scholar]
- Briceño-Domínguez D., Hernández-Carmona G., Moyo M., Stirk W., van Staden J. (2014). Plant growth promoting activity of seaweed liquid extracts produced from Macrocystis pyrifera under different pH and temperature conditions. J. Appl. Phycol. 26 2203–2210. 10.1007/s10811-014-0237-2 [DOI] [Google Scholar]
- Brockman H. G., Brennan R. F. (2017). The effect of foliar application of Moringa leaf extract on biomass, grain yield of wheat and applied nutrient efficiency. J. Plant Nutr. 40 2728–2736. 10.1080/01904167.2017.1381723 [DOI] [Google Scholar]
- Brown P., Saa S. (2015). Biostimulants in agriculture. Front. Plant Sci. 6:671. 10.3389/fpls.2015.00671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgari R., Cocetta G., Trivellini A., Vernieri P., Ferrante A. (2015). Biostimulants and crop responses: a review. Biol. Agric. Hortic. 31 1–17. 10.1080/01448765.2014.964649 [DOI] [Google Scholar]
- Bulgari R., Morgutti S., Cocetta G., Negrini N., Farris S., Calcante A., et al. (2017). Evaluation of borage extracts as potential biostimulant using a phenomic, agronomic, physiological, and biochemical approach. Front. Plant Sci. 8:935. 10.3389/fpls.2017.00935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo P., Nelson L., Kloepper J. W. (2014). Agricultural uses of plant biostimulants. Plant Soil 383 3–41. 10.1007/s11104-014-2131-8 [DOI] [Google Scholar]
- Cao Y.-R., Chen S.-Y., Zhang J.-S. (2008). Ethylene signaling regulates salt stress response: an overview. Plant Signal. Behav. 3 761–763. 10.4161/psb.3.10.5934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassaniti C., Romano D., Timothy J. F. (2012). “The Response of Ornamental Plants to Saline Irrigation Water,” in Irrigation-Water Management, Pollution and Alternative Strategies, eds García-Garizábal I., Abrahao R. (London, UK: IntechOpen Limited; ), 130–158. [Google Scholar]
- Chakraborty K., Sairam R. K., Bhattacharya R. (2012). Differential expression of salt overly sensitive pathway genes determines salinity stress tolerance in Brassica genotypes. Plant Physiol. Biochem. 51 90–101. 10.1016/j.plaphy.2011.10.001 [DOI] [PubMed] [Google Scholar]
- Chen X., Ding Y., Yang Y., Song C., Wang B., Yang S., et al. (2021). Protein kinases in plant responses to drought, salt, and cold stress. J. Integr. Plant Biol. 63 53–78. 10.1111/jipb.13061 [DOI] [PubMed] [Google Scholar]
- Corpas F. J. (2016). Reactive nitrogen species (RNS) in plants under physiological and adverse environmental conditions: current view. Prog. Bot. 78 97–119. 10.1007/124_2016_3 33311142 [DOI] [Google Scholar]
- Corpas F. J., Barroso J. B. (2015). Reactive sulfur species (RSS): possible new players in the oxidative metabolism of plant peroxisomes. Front. Plant Sci. 6:116. 10.3389/fpls.2015.00116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis P. S., Lauchli A. (1986). The role of leaf area development and photosynthetic capacity in determining growth of kenaf under moderate salt stress. Funct. Plant Biol. 13 553–565. 10.1071/pp9860553 [DOI] [Google Scholar]
- Deinlein U., Stephan A. B., Horie T., Luo W., Xu G., Schroeder J. I. (2014). Plant salt-tolerance mechanisms. Trends Plant Sci. 19 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desoky E.-S. M., El-maghraby L. M., Awad A. E., Abdo A. I., Rady M. M., Semida W. M. (2020). Fennel and ammi seed extracts modulate antioxidant defence system and alleviate salinity stress in cowpea (Vigna unguiculata). Sci. Hortic. 272:109576. 10.1016/j.scienta.2020.109576 [DOI] [Google Scholar]
- Desoky E.-S. M., ElSayed A. I., Merwad A.-R. M., Rady M. M. (2019). Stimulating antioxidant defenses, antioxidant gene expression, and salt tolerance in Pisum sativum seedling by pretreatment using licorice root extract (LRE) as an organic biostimulant. Plant Physiol. Biochem. 142 292–302. 10.1016/j.plaphy.2019.07.020 [DOI] [PubMed] [Google Scholar]
- Dhanaraj B., Papanna M. K., Adinarayanan S., Vedachalam C., Sundaram V., Shanmugam S., et al. (2015). Prevalence and risk factors for adult pulmonary tuberculosis in a metropolitan city of South India. PLoS One 10:e0124260. 10.1371/journal.pone.0124260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Li W., Tang W., Zhang D. (2008). Furrow seeding with plastic mulching increases stand establishment and lint yield of cotton in a saline field. Agron. J. 100 1640–1646. 10.2134/agronj2008.0074 [DOI] [Google Scholar]
- Dong H., Li W., Tang W., Zhang D. (2009). Early plastic mulching increases stand establishment and lint yield of cotton in saline fields. Field Crops Res. 111 269–275. 10.1016/j.fcr.2009.01.001 [DOI] [Google Scholar]
- Drobek M., Frąc M., Cybulska J. (2019). Plant biostimulants: importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy 9:335. 10.3390/agronomy9060335 [DOI] [Google Scholar]
- Du Jardin P. (2015). Plant biostimulants: definition, concept, main categories and regulation. Sci. Hortic. 196 3–14. 10.1016/j.scienta.2015.09.021 [DOI] [Google Scholar]
- ElSayed A. I., Rafudeen M. S., Ganie S. A., Hossain M. S., Gomaa A. M. (2022). Seed priming with cypress leaf extract enhances photosynthesis and antioxidative defense in zucchini seedlings under salt stress. Sci. Hortic. 293:110707. 10.1016/j.scienta.2021.110707 [DOI] [Google Scholar]
- Elzaawely A. A., Ahmed M. E., Maswada H. F., Al-Araby A. A., Xuan T. D. (2018). Growth traits, physiological parameters and hormonal status of snap bean (Phaseolus vulgaris L.) sprayed with garlic cloves extract. Arch. Agron. Soil Sci. 64 1068–1082. 10.1080/03650340.2017.1410543 [DOI] [Google Scholar]
- European-Union (2020). European Green Deal [Online]. Available: https://ec.europa.eu/clima/eu-action/european-green-deal_en (accessed Oct 1, 2022). [Google Scholar]
- Fahad S., Hussain S., Matloob A., Khan F. A., Khaliq A., Saud S., et al. (2015). Phytohormones and plant responses to salinity stress: a review. Plant Growth Reg. 75 391–404. 10.1007/s10725-014-0013-y [DOI] [Google Scholar]
- Fancy N. N., Bahlmann A. K., Loake G. J. (2017). Nitric oxide function in plant abiotic stress. Plant Cell Environ. 40 462–472. 10.1111/pce.12707 [DOI] [PubMed] [Google Scholar]
- Farooq M., Gogoi N., Hussain M., Barthakur S., Paul S., Bharadwaj N., et al. (2017). Effects, tolerance mechanisms and management of salt stress in grain legumes. Plant Physiol. Biochem. 118 199–217. 10.1016/j.plaphy.2017.06.020 [DOI] [PubMed] [Google Scholar]
- Fernández V., Brown P. H. (2013). From plant surface to plant metabolism: the uncertain fate of foliar-applied nutrients. Front. Plant Sci. 4:289. 10.3389/fpls.2013.00289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández V., Bahamonde H. A., Javier Peguero-Pina J., Gil-Pelegrín E., Sancho-Knapik D., Gil L., et al. (2017). Physico-chemical properties of plant cuticles and their functional and ecological significance. J. Exp. Bot. 68 5293–5306. 10.1093/jxb/erx302 [DOI] [PubMed] [Google Scholar]
- Franco-Navarro J. D., Brumós J., Rosales M. A., Cubero-Font P., Talón M., Colmenero-Flores J. M. (2016). Chloride regulates leaf cell size and water relations in tobacco plants. J. Exp. Bot. 67 873–891. 10.1093/jxb/erv502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriga M., Retamales J. B., Romero-Bravo S., Caligari P. D., Lobos G. A. (2014). Chlorophyll, anthocyanin, and gas exchange changes assessed by spectroradiometry in Fragaria chiloensis under salt stress. J. Integr. Plant Biol. 56 505–515. 10.1111/jipb.12193 [DOI] [PubMed] [Google Scholar]
- Ghaffari M. R., Ghabooli M., Khatabi B., Hajirezaei M. R., Schweizer P., Salekdeh G. H. (2016). Metabolic and transcriptional response of central metabolism affected by root endophytic fungus Piriformospora indica under salinity in barley. Plant Mol. Biol. 90 699–717. 10.1007/s11103-016-0461-z [DOI] [PubMed] [Google Scholar]
- Ghezal N., Rinez I., Sbai H., Saad I., Farooq M., Rinez A., et al. (2016). Improvement of Pisum sativum salt stress tolerance by bio-priming their seeds using Typha angustifolia leaves aqueous extract. S. Afr. J. Bot. 105 240–250. 10.1016/j.sajb.2016.04.006 [DOI] [Google Scholar]
- Goldberg S. (2008). Mechanical/physical methods of cell disruption and tissue homogenization. Methods Mol. Biol. 424 3–22. 10.1007/978-1-60327-064-9_1 [DOI] [PubMed] [Google Scholar]
- Gowdy J. (2020). Our hunter-gatherer future: climate change, agriculture and uncivilization. Futures 115:102488. 10.1016/j.futures.2019.102488 [DOI] [Google Scholar]
- Guo S.-M., Tan Y., Chu H.-J., Sun M.-X., Xing J.-C. (2019). Transcriptome sequencing revealed molecular mechanisms underlying tolerance of Suaeda salsa to saline stress. PLoS One 14:e0219979. 10.1371/journal.pone.0219979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta B., Huang B. (2014). Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int. J. Genomics 2014:701596. 10.1155/2014/701596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururani M. A., Venkatesh J., Tran L. S. P. (2015). Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 8 1304–1320. 10.1016/j.molp.2015.05.005 [DOI] [PubMed] [Google Scholar]
- Habib N., Ashraf M., Ali Q., Perveen R. (2012). Response of salt stressed okra (Abelmoschus esculentus Moench) plants to foliar-applied glycine betaine and glycine betaine containing sugarbeet extract. S. Afr. J. Bot. 83 151–158. 10.1016/j.sajb.2012.08.005 [DOI] [Google Scholar]
- Hasanuzzaman M., Bhuyan M., Zulfiqar F., Raza A., Mohsin S. M., Mahmud J. A., et al. (2020). Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9:681. 10.3390/antiox9080681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan F., Al-Yasi H., Ali E., Alamer K., Hessini K., Attia H., et al. (2020). Mitigation of salt-stress effects by moringa leaf extract or salicylic acid through motivating antioxidant machinery in damask rose. Can. J. Plant Sci. 101 157–165. 10.1016/j.plaphy.2021.05.042 [DOI] [PubMed] [Google Scholar]
- Hassanein R. A., Abdelkader A. F., Faramawy H. M. (2019). Moringa leaf extracts as biostimulants-inducing salinity tolerance in the sweet basil plant. Egypt. J. Bot. 59 303–318. [Google Scholar]
- Hernández J. A., Ferrer M. A., Jiménez A., Barceló A. R., Sevilla F. (2001). Antioxidant systems and O2.-/H2O2 production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol. 127 817–831. 10.1104/pp.010188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez J., Olmos E., Corpas F., Sevilla F., Del Rio L. (1995). Salt-induced oxidative stress in chloroplasts of pea plants. Plant Sci. 105 151–167. 10.1016/0168-9452(94)04047-8 [DOI] [Google Scholar]
- Hörtensteiner S. (2006). Chlorophyll degradation during senescence. Annu. Rev. Plant Biol. 57 55–77. 10.1146/annurev.arplant.57.032905.105212 [DOI] [PubMed] [Google Scholar]
- Howladar S. M. (2014). A novel Moringa oleifera leaf extract can mitigate the stress effects of salinity and cadmium in bean (Phaseolus vulgaris L.) plants. Ecotoxicol. Environ. Saf. 100 69–75. 10.1016/j.ecoenv.2013.11.022 [DOI] [PubMed] [Google Scholar]
- Huang P., He L., Abbas A., Hussain S., Hussain S., Du D., et al. (2021). Seed priming with sorghum water extract improves the performance of camelina (camelina sativa (L.) crantz.) under salt stress. Plants 10:749. 10.3390/plants10040749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikrina M. A., Kolbin A. M. (2004). Peptides as universal biological regulators. Her. Russ. Acad. Sci. 80 419–429. 10.1134/S1019331610050011 [DOI] [Google Scholar]
- Iqbal N., Umar S., Khan N. A., Khan M. I. R. (2014). A new perspective of phytohormones in salinity tolerance: regulation of proline metabolism. Environ. Exp. Bot. 100 34–42. 10.1016/j.envexpbot.2013.12.006 [DOI] [Google Scholar]
- Ismail A. M., Horie T. (2017). Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Ann. Rev. Plant Biol. 68 405–434. 10.1146/annurev-arplant-042916-040936 [DOI] [PubMed] [Google Scholar]
- Jithesh M., Shukla P. S., Kant P., Joshi J., Critchley A. T., Prithiviraj B. (2019). Physiological and transcriptomics analyses reveal that Ascophyllum nodosum extracts induce salinity tolerance in Arabidopsis by regulating the expression of stress responsive genes. J. Plant Growth Reg. 38 463–478. 10.1007/s00344-018-9861-4 [DOI] [Google Scholar]
- Kauffman G. L., Kneivel D. P., Watschke T. L. (2007). Effects of a biostimulant on the heat tolerance associated with photosynthetic capacity, membrane thermostability, and polyphenol production of perennial ryegrass. Crop Sci. 47 261–267. 10.2135/cropsci2006.03.0171 [DOI] [Google Scholar]
- Kaur C., Sharma S., Singla-Pareek S. L., Sopory S. K. (2016). Methylglyoxal detoxification in plants: role of glyoxalase pathway. Ind. J. Plant Physiol. 21 377–390. 10.1007/s40502-016-0260-1 [DOI] [Google Scholar]
- Khan T. A., Yusuf M., Ahmad A., Bashir Z., Saeed T., Fariduddin Q., et al. (2019). Proteomic and physiological assessment of stress sensitive and tolerant variety of tomato treated with brassinosteroids and hydrogen peroxide under low-temperature stress. Food Chem. 289 500–511. 10.1016/j.foodchem.2019.03.029 [DOI] [PubMed] [Google Scholar]
- La Torre A., Battaglia V., Caradonia F. (2016). An overview of the current plant biostimulant legislations in different European Member States. J. Sci. Food Agric. 96 727–734. 10.1002/jsfa.7358 [DOI] [PubMed] [Google Scholar]
- Lamers J., Van Der Meer T., Testerink C. (2020). How plants sense and respond to stressful environments. Plant Physiol. 182 1624–1635. 10.1104/pp.19.01464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latef A. A. H. A., Mostofa M. G., Rahman M. M., Abdel-Farid I. B., Tran L.-S. P. (2019). Extracts from yeast and carrot roots enhance maize performance under seawater-induced salt stress by altering physio-biochemical characteristics of stressed plants. J. Plant Growth Reg. 38 966–979. 10.1007/s00344-018-9906-8 [DOI] [Google Scholar]
- Latef A. A., Alhmad M. F. A., Hammad S. A. (2017). Foliar application of fresh moringa leaf extract overcomes salt stress in fenugreek (Trigonellafoenum-graecum) plants. Egypt. J. Bot. 57 157–179. [Google Scholar]
- Latif H., Mohamed H. (2016). Exogenous applications of moringa leaf extract effect on retrotransposon, ultrastructural and biochemical contents of common bean plants under environmental stresses. S. Afr. J. Bot. 106 221–231. 10.1016/j.sajb.2016.07.010 [DOI] [Google Scholar]
- Li H., Yue H., Li L., Liu Y., Zhang H., Wang J., et al. (2021). Seed biostimulant Bacillus sp. MGW9 improves the salt tolerance of maize during seed germination. AMB Express 11 1–15. 10.1186/s13568-021-01237-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S. M., Goh Y. M., Kuan W. B., Loh S. P. (2014). Effect of germinated brown rice extracts on pancreatic lipase, adipogenesis and lipolysis in 3T3-L1 adipocytes. Lipids Health Dis. 13:169. 10.1186/1476-511X-13-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstreth D. J., Nobel P. S. (1979). Salinity effects on leaf anatomy: consequences for photosynthesis. Plant Physiol. 63 700–703. 10.1104/pp.63.4.700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo P., Souza-Alonso P., Guisande-Collazo A., Freitas H. (2019). Influence of Acacia dealbata Link bark extracts on the growth of Allium cepa L. plants under high salinity conditions. J. Sci. Food Agric. 99 4072–4081. 10.1002/jsfa.9637 [DOI] [PubMed] [Google Scholar]
- Lötze E., Hoffman E. W. (2016). Nutrient composition and content of various biological active compounds of three South African-based commercial seaweed biostimulants. J. Appl. Phycol. 28 1379–1386. 10.1007/s10811-015-0644-z [DOI] [Google Scholar]
- Mahmoudand R. A., Dahab A. A. (2018). Response of Apple Seedlings Grown Under Saline Conditions to Natural Plant Extracts. Biosci. Res. 15 589–601. [Google Scholar]
- Mangrauthia S. K., Agarwal S., Sailaja B., Madhav M. S., Voleti S. (2013). “MicroRNAs and their role in salt stress response in plants,” in in Salt stress in plants, eds Ahmad P., Azooz M. M., Prasad M. N. V. (New York, NY: Springer; ), 15–46. 10.1007/978-1-4614-6108-1_2 [DOI] [Google Scholar]
- Mano J. I, Biswas M., Sugimoto K. (2019). Reactive carbonyl species: a missing link in ROS signaling. Plants 8:391. 10.3390/plants8100391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano J. I. (2012). Reactive carbonyl species: their production from lipid peroxides, action in environmental stress, and the detoxification mechanism. Plant Physiol. Biochem. 59 90–97. 10.1016/j.plaphy.2012.03.010 [DOI] [PubMed] [Google Scholar]
- Merwad A.-R. M. (2017). Effect of humic and fulvic substances and Moringa leaf extract on Sudan grass plants grown under saline conditions. Can. J. Soil Sci. 97 703–716. [Google Scholar]
- Merwad A.-R. M. (2020). Mitigation of salinity stress effects on growth, yield and nutrient uptake of wheat by application of organic extracts. Commun. Soil Sci. Plant Anal. 51 1150–1160. 10.1080/00103624.2020.1751188 [DOI] [Google Scholar]
- Michler C. (2014). Overexpression of AtSTO1 leads to improved salt tolerance in Populus tremula× P. alba. Transgenic Res. 23 817–826. 10.1007/s11248-014-9808-x [DOI] [PubMed] [Google Scholar]
- Milić B., Tarlanović J., Keserović Z., Magazin N., Miodragović M., Popara G. (2018). Bioregulators can improve fruit size, yield and plant growth of northern highbush blueberry (Vaccinium corymbosum L.). Sci. Hortic. 235 214–220. 10.1016/j.scienta.2018.03.004 [DOI] [Google Scholar]
- Mittova V., Tal M., Volokita M., Guy M. (2003). Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Environ. 26 845–856. 10.1046/j.1365-3040.2003.01016.x [DOI] [PubMed] [Google Scholar]
- Mochida K., Shinozaki K. (2011). Advances in omics and bioinformatics tools for systems analyses of plant functions. Plant Cell Physiol. 52 2017–2038. 10.1093/pcp/pcr153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M., Sánchez-Blanco M., Olmos E., Torrecillas A., Alarcon J. (1998). Changes in the growth, leaf water relations and cell ultrastructure in Argyranthemum coronopifolium plants under saline conditions. J. Plant Physiol. 153 174–180. 10.1016/s0176-1617(98)80062-x [DOI] [Google Scholar]
- Mostofa M. G., Ghosh A., Li Z.-G., Siddiqui M. N., Fujita M., Tran L.-S. P. (2018). Methylglyoxal–a signaling molecule in plant abiotic stress responses. Free Radic. Biol. Med. 122 96–109. 10.1016/j.freeradbiomed.2018.03.009 [DOI] [PubMed] [Google Scholar]
- Munns R. (2002). Comparative physiology of salt and water stress. Plant Cell Environ. 25 239–250. 10.1046/j.0016-8025.2001.00808.x [DOI] [PubMed] [Google Scholar]
- Munns R., Tester M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59 651–681. [DOI] [PubMed] [Google Scholar]
- Mutlu-Durak H., Yildiz Kutman B. (2021). Seed Treatment with Biostimulants Extracted from Weeping Willow (Salix babylonica) Enhances Early Maize Growth. Plants 10:1449. 10.3390/plants10071449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahar K., Hasanuzzaman M., Fujita M. (2016). “Roles of osmolytes in plant adaptation to drought and salinity, Iqbal,” in Osmolytes and plants acclimation to changing environment: Emerging omics technologies, eds Nazar N., Khan R. A. (Berlin: Springer; ), 37–68. 10.1007/978-81-322-2616-1_4 [DOI] [Google Scholar]
- Nareshkumar A., Subbarao S., Vennapusa A. R., Ashwin V., Banarjee R., Kulkarni M. J., et al. (2020). Enzymatic and non-enzymatic detoxification of reactive carbonyl compounds improves the oxidative stress tolerance in cucumber, tobacco and rice seedlings. J. Plant Growth Reg. 39 1359–1372. 10.1007/s00344-020-10072-w [DOI] [Google Scholar]
- Nasir M., Khan A. S., Basra S. A., Malik A. U. (2016). Foliar application of moringa leaf extract, potassium and zinc influence yield and fruit quality of ‘Kinnow’mandarin. Sci. Hortic. 210 227–235. 10.1016/j.scienta.2016.07.032 [DOI] [Google Scholar]
- Nessim A., Kasim W. (2019). Physiological impact of seed priming with CaCl2 or Carrot root extract on Lupinus termis plants fully grown under salinity stress. Egypt. J. Bot. 59 763–777. [Google Scholar]
- Nolan T. M., Vukašinović N., Liu D., Russinova E., Yin Y. (2020). Brassinosteroids: multidimensional regulators of plant growth, development, and stress responses. Plant Cell 32 295–318. 10.1105/tpc.19.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panuccio M., Chaabani S., Roula R., Muscolo A. (2018). Bio-priming mitigates detrimental effects of salinity on maize improving antioxidant defense and preserving photosynthetic efficiency. Plant Physiol. Biochem. 132 465–474. 10.1016/j.plaphy.2018.09.033 [DOI] [PubMed] [Google Scholar]
- Parađiković N., Vinković T., Vrček I. V., Žuntar I., Bojić M., Medić-Šarić M. (2011). Effect of natural biostimulants on yield and nutritional quality: an example of sweet yellow pepper (Capsicum annuum L.) plants. J. Sci. Food Agric. 91 2146–2152. 10.1002/jsfa.4431 [DOI] [PubMed] [Google Scholar]
- Pehlivan N. (2018). Salt stress relief potency of whortleberry extract biopriming in maize. 3 Biotech 8 1–10. 10.1007/s13205-018-1113-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg Z., Apse M. P., Blumwald E. (2011). Engineering salinity and water-stress tolerance in crop plants: getting closer to the field. Adv. Bot. Res. 57 405–443. 10.1016/b978-0-12-387692-8.00012-6 [DOI] [Google Scholar]
- Plaut Z., Edelstein M., Ben-Hur M. (2013). Overcoming salinity barriers to crop production using traditional methods. Crit. Rev. Plant Sci. 32 250–291. 10.1080/07352689.2012.752236 [DOI] [Google Scholar]
- Quan R., Lin H., Mendoza I., Zhang Y., Cao W., Yang Y., et al. (2007). SCABP8/CBL10, a putative calcium sensor, interacts with the protein kinase SOS2 to protect Arabidopsis shoots from salt stress. Plant Cell 19 1415–1431. 10.1105/tpc.106.042291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rady M. M., Mohamed G. F. (2015). Modulation of salt stress effects on the growth, physio-chemical attributes and yields of Phaseolus vulgaris L. plants by the combined application of salicylic acid and Moringa oleifera leaf extract. Sci. Hortic. 193 105–113. 10.1016/j.scienta.2015.07.003 [DOI] [Google Scholar]
- Rady M. M., Mohamed G. F., Abdalla A., Ahmed Y. H. (2015). Integrated application of salicylic acid and Moringa oleifera leaf extract alleviates the salt-induced adverse effects in common bean plants. Int. J. Agric. Technol. 11 1595–1614. [Google Scholar]
- Rady M. M., Talaat N. B., Abdelhamid M. T., Shawky B. T., Desoky E.-S. M. (2019b). Maize (Zea mays L.) grains extract mitigates the deleterious effects of salt stress on common bean (Phaseolus vulgaris L.) growth and physiology. J. Hortic. Sci. Biotechnol. 94 777–789. 10.1080/14620316.2019.1626773 [DOI] [Google Scholar]
- Rady M. M, Desoky E.-S., Elrys A., Boghdady M. (2019a). Can licorice root extract be used as an effective natural biostimulant for salt-stressed common bean plants? S. Afr. J. Bot. 121 294–305. 10.1016/j.sajb.2018.11.019 [DOI] [Google Scholar]
- Rady M. M., Varma B., Howladar S. M. (2013). Common bean (Phaseolus vulgaris L.) seedlings overcome NaCl stress as a result of presoaking in Moringa oleifera leaf extract. Sci. Hortic. 162 63–70. 10.1016/j.scienta.2013.07.046 [DOI] [Google Scholar]
- Ramadoss D., Lakkineni V. K., Bose P., Ali S., Annapurna K. (2013). Mitigation of salt stress in wheat seedlings by halotolerant bacteria isolated from saline habitats. SpringerPlus 2 1–7. 10.1186/2193-1801-2-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A. Q., Ud S., Akhtar S., Sarwar M. B., Ahmed M., Rashid B., et al. (2016). Genomics of salinity tolerance in plants. Plant Genomics 2016 273–299. 10.1007/978-3-7643-8554-5_25 [DOI] [Google Scholar]
- Rodrıguez P., Torrecillas A., Morales M., Ortuno M., Sánchez-Blanco M. (2005). Effects of NaCl salinity and water stress on growth and leaf water relations of Asteriscus maritimus plants. Environ. Exp. Bot. 53 113–123. 10.1016/j.envexpbot.2004.03.005 [DOI] [Google Scholar]
- Romero-Aranda R., Moya J., Tadeo F., Legaz F., Primo-Millo E. (1998). 623 Talon M (1998) Physiological and anatomical disturbances 624 induced by chloride salts in sensitive and tolerant citrus: 625 beneficial and detrimental effects of cations. Plant Cell Environ. 626 1243–1253. 10.1046/j.1365-3040.1998.00349.x [DOI] [Google Scholar]
- Rouphael Y., Colla G. (2020). Biostimulants in agriculture. Front. Plant Sci. 11:40. 10.3389/fpls.2020.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahab S., Suhani I., Srivastava V., Chauhan P. S., Singh R. P., Prasad V. (2020). Potential risk assessment of soil salinity to agroecosystem sustainability: current status and management strategies. Sci. Total Environ. 764:144164. 10.1016/j.scitotenv.2020.144164 [DOI] [PubMed] [Google Scholar]
- Salama Z. A., El Fouly M. M., Gaafar A. A. (2013). Mitigation of the adverse effect of salinity through stimulation some secondary metabolites and antioxidant enzymes of methanolic extract of maize cultivars by exogenous ascorbic acid. J. Food Agric. Environ. 11 1328–1335. [Google Scholar]
- Sánchez-Gómez R., Garde-Cerdán T., Zalacain A., Garcia R., Cabrita M., Salinas M. (2016). Vine-shoot waste aqueous extract applied as foliar fertilizer to grapevines: effect on amino acids and fermentative volatile content. Food Chem. 197 132–140. 10.1016/j.foodchem.2015.10.034 [DOI] [PubMed] [Google Scholar]
- Semida W. M., Rady M. M. (2014). Presoaking application of propolis and maize grain extracts alleviates salinity stress in common bean (Phaseolus vulgaris L.). Sci. Hortic. 168 210–217. 10.1016/j.scienta.2014.01.042 [DOI] [Google Scholar]
- Shukla P. S., Mantin E. G., Adil M., Bajpai S., Critchley A. T., Prithiviraj B. (2019). Ascophyllum nodosum-based biostimulants: sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 10:655. 10.3389/fpls.2019.00655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottosanto J. B., Saranga Y., Blumwald E. (2007). Impact of AtNHX1, a vacuolar Na+/H+ antiporter, upon gene expression during short-and long-term salt stress in Arabidopsis thaliana. BMC Plant Biol. 7:18. 10.1186/1471-2229-7-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L.-J., Zhou J.-J., Pan J.-L., Liang Y.-Y., Fang Z.-J., Xie Y., et al. (2018). Electrochemical mapping of indole-3-acetic acid and salicylic acid in whole pea seedlings under normal conditions and salinity. Sens. Actuators B Chem. 276 545–551. 10.1016/j.snb.2018.08.152 [DOI] [Google Scholar]
- Suryaman M., Sunarya Y., Istarimila I., Fudholi A. (2021). Effect of salinity stress on the growth and yield of mungbean (Vigna radiata (L.) R. Wilczek) treated with mangosteen pericarp extract. Biocatal. Agric. Biotechnol. 36:102132. 10.1016/j.bcab.2021.102132 [DOI] [Google Scholar]
- Taha R. (2016). Improving salt tolerance of Helianthus annuus (L.) plants by Moringa oleifera leaf extract. Egypt. J. Agron. 38 117–140. 10.21608/agro.2016.301 [DOI] [Google Scholar]
- Tang X., Mu X., Shao H., Wang H., Brestic M. (2015). Global plant-responding mechanisms to salt stress: physiological and molecular levels and implications in biotechnology. Crit. Rev. Biotechnol. 35 425–437. 10.3109/07388551.2014.889080 [DOI] [PubMed] [Google Scholar]
- Tesfay S. Z., Magwaza L. S. (2017). Evaluating the efficacy of moringa leaf extract, chitosan and carboxymethyl cellulose as edible coatings for enhancing quality and extending postharvest life of avocado (Persea americana Mill.) fruit. Food Packag. Shelf Life 11 40–48. 10.1016/j.fpsl.2016.12.001 [DOI] [Google Scholar]
- Ur Rehman H. U., Basra S., Rady M. M., Ghoneim A. M., Wang Q. (2017). Moringa leaf extract improves wheat growth and productivity by affecting senescence and source-sink relationship. Int. J. Agric. Biol. 19 479–484. 10.17957/ijab/15.0316 29653435 [DOI] [Google Scholar]
- ur Rehman H., Alharby H. F., Bamagoos A. A., Abdelhamid M. T., Rady M. M. (2021). Sequenced application of glutathione as an antioxidant with an organic biostimulant improves physiological and metabolic adaptation to salinity in wheat. Plant Physiol. Biochem. 158 43–52. 10.1016/j.plaphy.2020.11.041 [DOI] [PubMed] [Google Scholar]
- Velmurugan A., Swarnam P., Subramani T., Meena B., Kaledhonkar M. (2020). “Water demand and salinity,” in Desalination - Challenges and Opportunities. eds Farahani M. H. D. A., Vatanpour V., Taheri A. H. (London: IntechOpen; ). 10.5772/intechopen.88095 [DOI] [Google Scholar]
- Yakhin O. I., Lubyanov A. A., Yakhin I. A., Brown P. H. (2017). Biostimulants in plant science: a global perspective. Front. Plant Sci. 7:2049. 10.3389/fpls.2016.02049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younis A., Akhtar M. S., Riaz A., Zulfiqar F., Qasim M., Farooq A., et al. (2018). Improved cut flower and corm production by exogenous moringa leaf extract application on gladiolus cultivars. Acta Sci. Pol. Hortorum Cultus 17 25–38. 10.24326/asphc.2018.4.3 [DOI] [Google Scholar]
- Yousefirad S., Soltanloo H., Ramezanpour S. S., Zaynali Nezhad K., Shariati V. (2020). The RNA-seq transcriptomic analysis reveals genes mediating salt tolerance through rapid triggering of ion transporters in a mutant barley. PLoS One 15:e0229513. 10.1371/journal.pone.0229513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zörb C., Geilfus C. M., Dietz K. J. (2019). Salinity and crop yield. Plant Biol. 21 31–38. 10.1111/plb.12884 [DOI] [PubMed] [Google Scholar]
- Zou J., Liu A., Chen X., Zhou X., Gao G., Wang W., et al. (2009). Expression analysis of nine rice heat shock protein genes under abiotic stresses and ABA treatment. J. Plant Physiol. 166 851–861. 10.1016/j.jplph.2008.11.007 [DOI] [PubMed] [Google Scholar]
- Zulfiqar F., Casadesús A., Brockman H., Munné-Bosch S. (2020b). An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant Sci. 295:110194. 10.1016/j.plantsci.2019.110194 [DOI] [PubMed] [Google Scholar]
- Zulfiqar F., Younis A., Finnegan P. M., Ferrante A. (2020c). Comparison of soaking corms with moringa leaf extract alone or in combination with synthetic plant growth regulators on the growth, physiology and vase life of sword lily. Plants 9:1590. 10.3390/plants9111590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulfiqar F., Akram N. A., Ashraf M. (2020a). Osmoprotection in plants under abiotic stresses: new insights into a classical phenomenon. Planta 251:3. 10.1007/s00425-019-03293-1 [DOI] [PubMed] [Google Scholar]
- Zulfiqar F., Ashraf M. (2021a). Bioregulators: unlocking their potential role in regulation of the plant oxidative defense system. Plant Mol. Biol. 105 11–41. 10.1007/s11103-020-01077-w [DOI] [PubMed] [Google Scholar]
- Zulfiqar F., Ashraf M. (2021b). Nanoparticles potentially mediate salt stress tolerance in plants. Plant Physiol. Biochem. 160 257–268. 10.1016/j.plaphy.2021.01.028 [DOI] [PubMed] [Google Scholar]