Abstract
Although extensively studied, the mechanism of action of insecticidal Bacillus thuringiensis Cry toxins remains elusive and requires further elucidation. Toxin receptors in the brush border membrane demand particular attention as they presumably initiate the cascade of events leading to insect mortality after toxin activation. The 170-kDa Cry1Ac toxin-binding aminopeptidase from the tobacco budworm (Heliothis virescens) was partially purified, and its corresponding cDNA was cloned. The cDNA encodes a protein with a putative glycosyl phosphatidylinositol anchor and a polythreonine stretch clustered near the C terminus with predicted O-glycosylation. Partial purification of the 170-kDa aminopeptidase also resulted in isolation of a 130-kDa protein that was immunologically identical to the 170-kDa protein, and the two proteins had identical N termini. These proteins were glycosylated, as suggested by soybean agglutinin lectin blot results. Cry1Ac toxin affinity data for the two proteins indicated that the 130-kDa protein had a higher affinity than the 170-kDa protein. The data suggest that posttranslational modifications can have a significant effect on Cry1A toxin interactions with specific insect midgut proteins.
Insecticidal crystal proteins (Cry toxins), which are produced by the bacterium Bacillus thuringiensis during sporulation, are environmentally sound alternatives to synthetic chemical insecticides. After ingestion by susceptible larvae, protoxins are solubilized in the midgut’s alkaline environment and activated by proteases (16). The activated toxins bind with high affinity to receptors on the apical membrane, and this is followed by insertion of the toxin into the epithelial membrane. Toxin insertion is an irreversible step and is followed by toxin oligomerization and the formation of an ion pore, which results in an osmotic imbalance (16, 39). Insect mortality occurs several hours to days after ingestion of the toxin.
The noctuid Heliothis virescens, an important agricultural pest, is susceptible to B. thuringiensis toxins, such as Cry1Ac. Sensitivity to B. thuringiensis is associated with high-affinity, saturable binding of toxins to sites present on the brush border membrane of midgut epithelial cells (43). In H. virescens a three-site model has been proposed to explain the intricate relationship between toxins and the corresponding binding sites (43); however, this model may oversimplify the actual complex processes involved. One site is thought to bind Cry1Aa, Cry1Ab, and Cry1Ac toxins. The second site binds Cry1Ab and Cry1Ac, while the third site apparently binds only Cry1Ac. The model is further complicated by the fact that the site which binds all three toxins, receptor A, may consist of two binding sites for each toxin (29). Brush border membrane vesicles (BBMV) derived from a laboratory-selected Cry1Ac-resistant strain (18) exhibit Cry1Ab and Cry1Ac binding similar to that of a susceptible strain; however, there is no Cry1Aa binding. Furthermore, the Bmax values for Cry1Ab and Cry1Ac toxins in the resistant strain are slightly lower than the Bmax values in the susceptible strain, suggesting that there is a small reduction in the receptor concentration (18). Thus, the site which binds all three toxins, receptor A, is putatively the site that is altered in the resistant strain (28).
The 170-kDa aminopeptidase present in the midgut brush border was recently identified as receptor A in susceptible H. virescens; this peptidase binds the Cry1Aa, CryAb, and CryAc toxins, and the binding results in pore formation (29). Cry1Ac toxin affinity-purified 170-kDa aminopeptidase contains two Cry1A toxin-binding sites; the first site is a site with Kd values ranging from 41 to 95 nM, and the second site is a lower-affinity site with Kd values ranging from 325 to 632 nM (29).
Identification of Cry toxin-binding molecules is paramount to elucidating the mechanism of action of B. thuringiensis. Moreover, cloning the cDNAs that encode these proteins may reveal important information regarding the development of B. thuringiensis resistance in insects. To date, the Cry1A toxin-binding proteins that have been characterized are primarily leucine aminopeptidases (8, 15, 27, 45), cadherinlike proteins (32, 41), and, in one study, biotin-containing proteins (9). Aminopeptidase cDNAs have been cloned from H. virescens (15), Manduca sexta (8, 27), Plutella xylostella (8), Bombyx mori (25, 45), and Plodia interpunctella (accession no. AF034483) (46), while cadherinlike Cry1A-binding proteins have been cloned from M. sexta (41) and B. mori (32).
Complexities in Cry1A toxin-midgut receptor interactions are revealed during identification of toxin-binding molecules, as well as during affinity determinations. Kd values have been determined by utilizing toxin overlay assays, binding assays performed with BBMV, and, more recently, surface plasmon resonance. The consensus is that BBMV preparations have higher affinities for Cry1A toxins (0.2 to 0.9 nM) (6, 28, 43), while purified proteins exhibit lower affinities (30). The proposed difference in affinity between BBMV and purified proteins is due to an apparent loss of the irreversible step which involves toxin insertion into the BBMV membrane (5). Alternatively, loss of a protein complex necessary for high-affinity toxin binding resulting from protein purification may account for lower affinity values. In a model membrane environment, M. sexta aminopeptidase N (APN) exhibits an affinity for Cry1Ac toxin of 3 nM (5), a value much lower than the value obtained in a surface plasmon resonance analysis of solubilized APN (30). This suggests that at least in the case of M. sexta, toxin insertion into the membrane accounts for the higher affinity observed in BBMV preparations than in purified protein preparations (5).
In this study we determined the molecular structure of the H. virescens 170-kDa aminopeptidase previously identified as a Cry1Ac toxin-binding protein and receptor A (6, 15, 29). The BBMV protein was purified by anion-exchange chromatography and was identified as two Cry1Ac toxin-binding molecules, a 170-kDa highly glycosylated protein and a 130-kDa protein that appears to be less glycosylated. Although these two proteins have different molecular masses, they have the same N-terminal sequence. Cry1Ac toxin affinity parameters were determined for the 130- and 170-kDa proteins, and the corresponding cDNA was cloned. The 130-kDa protein has an apparent affinity for Cry1Ac of 32.1 nM, whereas the 170-kDa protein binds Cry1Ac but bound radiolabeled ligand cannot be readily displaced by an unlabeled homologous competitor. Both proteins are putatively glycosylated and contain N-linked and O-linked glycosylation sites. The difference in the molecular masses of the two toxin-binding proteins is probably accounted for by variations in posttranslational modifications. With the cloning of the 170-kDa aminopeptidase, additional molecular studies which address the mode and mechanism of toxicity of B. thuringiensis Cry toxins should now be possible.
MATERIALS AND METHODS
Toxin isolation and labeling.
Parasporal inclusions were purified from B. thuringiensis subsp. kurtstaki HD-73, which expresses only the Cry1Ac toxin (2). Recombinant Escherichia coli expressing Cry1Aa toxin was obtained from D. H. Dean (Ohio State University), and the toxin was purified as described previously (14). Iodination of the trypsin-activated and purified Cry1Ac toxin has been described previously (6). The specific activity of Cry1Ac toxin (375 Ci/mmol, based on input toxin) was determined with an LKB gamma counter.
Partial purification.
H. virescens BBMV were prepared as previously described (44) and were solubilized (6). Solubilized proteins were applied to a 0.1-ml MonoQ PC 1.6/5 anion-exchange column (Pharmacia), and bound proteins were eluted with a 0 to 500 mM KCl linear gradient in solubilization buffer containing 1.1% 3-[(3-cholamidopropyl)dimethylammonia]-1-propane sulfonate (CHAPS). Individual fractions were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and were assayed for toxin-binding ability by using the Cry1Ac toxin overlay assay (7). Fractions containing the 170-kDa protein were pooled and designated the pool I fraction. The 120-kDa aminopeptidase (15) elutes at a higher KCl concentration and is present in the pool II fraction, as previously described (6).
Affinity parameters.
One microgram of partially purified 170-kDa protein was separated by SDS-PAGE and electroblotted onto an Immobilon-P membrane (Millipore). The bands containing the 130- and 170-kDa proteins were excised, and a toxin affinity value was determined for each of the two proteins as previously described (6). Scatchard analyses were carried out with the LIGAND program (38).
Gel electrophoresis and electroblotting.
Discontinuous-buffer SDS-PAGE was performed with 8% polyacrylamide gels, unless otherwise indicated. The separated proteins were transferred to an Immobilon-P membrane (Millipore) in Tris-glycine buffer as recommended by the manufacturer. For immunoblotting, the membrane was blocked with Tris-buffered saline (pH 7.5) containing 0.1% Tween 20 (Sigma) and 5% skim milk. The primary antibody was used at a dilution of 1:10,000 in blocking buffer, and the donkey anti-rabbit horseradish peroxidase secondary antibody (Amersham) was used at a dilution of 1:5,000 in blocking buffer. Bound antibodies were detected with an enhanced chemiluminescence kit (Amersham).
Toxin overlay assays in which toxins Cry1Aa and Cry1Ac were used were performed as described by de Maagd et al. (7), with minor modifications; after incubation with toxin the membranes were washed for 20 min, and the Cry1 antibody was used at a final concentration of 1:3,000. Glycosylated proteins were detected by lectin blotting with biotin-labeled soybean agglutinin (Vector) (10 μg/ml in Tris-buffered saline supplemented with 0.1% Tween 20), followed by incubation with an avidin-biotinylated horseradish peroxidase complex (Vector) and diaminobenzidine tertrahydrochloride substrate (Sigma) as recommended by the manufacturer.
Glycosidase digestion.
Digestion with PNGase F (New England Biolabs) and O-glycosidase (Boehringer Mannheim) was performed as follows. For N-linked deglycosylation, 5 μg of pool I proteins was boiled for 10 min in denaturation buffer (5% SDS, 10% β-mercaptoethanol), 10× G7 buffer (0.5 M sodium phosphate buffer, pH 7.5), 10% NP-40, and 2.5 μl of N-glycosidase F (500 U/μl) were added, and the mixture was incubated for 3 h at 37°C. O-linked deglycosylation was performed by first denaturing 10 μg of pool I protein in a solution containing 0.1% SDS, 18 mM sodium phosphate buffer (pH 7.2), 10 mM NaN3, 0.5% NP-40, 0.5% N-octylglucoside, and 10 mM EDTA at 100°C for 2 min. Then 2 mU of neuraminidase (New England Biolabs) was added along with 2.5 mU of O-glycosidase, and the sample was incubated at 37°C for 24 h. Following deglycosylation the proteins (1 μg) were separated by SDS-PAGE, electroblotted, and visualized by soybean agglutinin lectin blotting.
N-terminal sequence analysis.
For N-terminal sequencing, the pool I proteins, which included the 170- and 130-kDa Cry1Ac toxin-binding proteins, were separated by SDS-PAGE and transferred to an Immobilon-PSQ membrane (Millipore) in 10 mM CAPS (3-[cyclohexylamino]-1-propanesulfonic acid) (pH 11)–10% methanol. The membrane was washed with several changes of deionized water and stained with 0.25% Coomassie blue G-250 in 50% methanol for 1 min. The blot was destained with 50% methanol–10% acetic acid and finally rinsed with 50% methanol. The bands of interest were excised and sequenced by automatic Edman degradation with a model 492 Applied Biosystems Procise sequencer.
cDNA library screening and sequencing.
Construction of an H. virescens midgut DNA library from size-selected mRNA (>3 kb) has been described previously (15). The primers used for PCR amplification were designed on the basis of the N-terminal sequence DPAYRLPTTTR (APN1F [GCITAYMGIYTICCNCANCANAC] and APN2R [CGTIGTIGGIARNCKRTANGCNGG]); the sequences of two conserved regions in the aminopeptidase, AFPCYDEP (APN3F [GCITTYCCITGYTAYGAYGARCC]) and AMENWGCLV (APN4R [ACIARICCCCARTTYTCCATNGC]); and a partial sequence of the 170-kDa protein (29), MGYDTRFI (APN5R [GATRAAICKIGTRTCRTANCCCAT]). Following partial sequencing, a 170-kDa protein-specific primer (APN6R [TAGTCAGGTCATTGCAGTGGAG]) was synthesized and used for PCR library screening (37). The 3.2-kb cDNA clone was sequenced by the primer walking method; automated sequencing was performed at the University of California at Berkeley sequencing facility. Both DNA strands were fully sequenced.
In vitro transcription and translation.
The 3.2-kb cDNA clone was linearized with NotI and was transcribed by using an mMessage mMachine kit (Ambion) and T7 polymerase. The resultant cRNA was translated with nuclease-treated rabbit reticulocyte lysate (Promega) in the presence of [35S]methionine (Dupont-NEN). Translation products were analyzed by SDS-PAGE and were visualized by fluorography (Entensify; Dupont-NEN).
Antibody development.
A KpnI-PstI fragment from bp 1560 to 2332 (amino acids 520 to 778) was cloned into the pQE32 E. coli expression vector (Qiagen). An IPTG (isopropyl-β-d-thiogalactopyranoside)-induced protein was produced, solubilized in urea buffer, and eluted from a nickel affinity column with buffer C supplemented with 250 mM imidazole as recommended by the manufacturer (Qiagen). The 29-kDa protein resolved on a 12% polyacrylamide SDS–PAGE gel was excised and used to immunize rabbits (Prosci Inc., Poway, Calif.) (4).
Protein concentration determination and enzyme assays.
Protein concentrations were determined with a detergent-compatible protein assay kit (Bio-Rad) and bovine serum albumin as the standard. Aminopeptidase activities were assayed as previously described (12, 15) by utilizing leucine-p-nitrophenol, lysine-p-nitrophenol, and phenylalanine-p-nitrophenol as substrates (Sigma).
RESULTS
Partial purification of H. virescens Cry1Ac toxin-binding proteins.
Cry1Ac toxin-binding proteins of H. virescens have been identified by workers in our laboratory (6, 15) and other laboratories (12, 28, 29, 34, 35) and characterized. To date, only one Heliothis protein, the 120-kDa leucine aminopeptidase, has been cloned (15). In our efforts to further identify putative Cry1A receptors, we concentrated on purification and cloning of the Cry1Ac toxin-binding 170-kDa aminopeptidase. Utilizing MonoQ anion-exchange chromatography, we fractionated CHAPS-solubilized BBMV proteins and subsequently analyzed them for toxin binding. The 170-kDa protein copurified consistently with a 130-kDa protein (Fig. 1, lane 2). Lectin blot detection with soybean agglutinin lectin revealed a strong cross-reaction with the 170-kDa protein (Fig. 1, lane 3), indicating that this protein is a glycoprotein with terminal N-acetylgalactosamine (GalNAc). The 130-kDa protein cross-reacted with the lectin weakly, perhaps due to lower protein abundance compared to the 170-kDa protein or due to less glycosylation. No reaction with the streptavidin-horseradish peroxidase complex in the absence of biotin-labeled lectin was observed with BBMV or the purified proteins (data not shown).
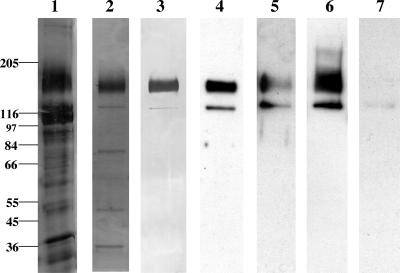
FIG. 1.
Analysis of pool I Cry1Ac and Cry1Aa toxin-binding proteins. One microgram of protein was loaded in each lane, and the proteins were separated on 8% polyacrylamide SDS-PAGE gels. For immunoblotting, toxin overlay assays, and lectin blotting, the proteins were transferred to an Immobilon-P membrane. Lane 1, silver-stained CHAPS-solubilized BBMV; lane 2, silver-stained pool I MonoQ fraction; lane 3, soybean agglutinin lectin blot detection of glycosylated proteins in pool I; lane 4, Cry1Aa toxin binding to pool I proteins; lane 5, Cry1Aa toxin binding to pool I proteins in the presence of 100 mM GalNAc; lane 6, Cry1Ac toxin binding to pool I proteins; lane 7, Cry1Ac toxin binding in the presence of 100 mM GalNAc. The positions of molecular mass markers (in kilodaltons) are indicated on the left.
Toxin overlay assays performed with activated Cry1Aa (Fig. 1, lane 4) and Cry1Ac (Fig. 1, lane 6) toxins showed that MonoQ-purified proteins bound both toxins. Competition with 100 mM GalNAc strongly inhibited binding of Cry1Ac toxin to both proteins on ligand blots (Fig. 1, lane 7). However, the 170- and 130-kDa proteins still bound Cry1Aa in the presence of excess sugar (Fig. 1, lane 5).
An analysis of the aminopeptidase activities in the partially purified fraction revealed a slight increase in leucine aminopeptidase activity in pool I and decreases in lysine and phenylalanine aminopeptidase activities (Table 1). Pool II, which contained the 120-kDa aminopeptidase, exhibited the highest leucine aminopeptidase activity; this activity was approximately 2.5 times higher than the CHAPS-solubilized BBMV activity (data not shown).
TABLE 1.
Aminopeptidase activities in isolated fractions obtained from the H. virescens midgut
| Fraction | Aminopeptidase activities (μmol/min/mg of protein)
|
||
|---|---|---|---|
| Leucine | Lysine | Phenylalanine | |
| Solubilized BBMV | 3.12 | 0.77 | 1.17 |
| Pool Ia | 3.67 | 0.345 | 0.41 |
This pool contained the 170- and 130-kDa proteins.
Cry1Ac toxin affinity determination.
Since we previously showed that a protein complex consisting of the 170- and 130-kDa proteins has a lower affinity (6) than the affinity recently demonstrated by Luo et al. (29), we determined the Cry1Ac toxin affinities to the 170- and 130-kDa proteins separately. Thus, individual 170- and 130-kDa protein bands were excised, and the affinities were monitored by using homologous competition assays. As we observed previously, the 170-kDa protein bound radiolabeled Cry1Ac; however, incubation with 100 nM cold Cry1Ac toxin resulted in an increase in toxin binding, and incubation with 1,000 nM cold Cry1Ac toxin resulted in only 25% displacement (6) (data not shown). In contrast, when Cry1Ac binding to the 130-kDa protein was tested under the same conditions, Cry1Ac was displaced, and 1,000 nM unlabeled toxin displaced more than 80% of the bound iodinated toxin (Fig. 2). Scatchard analysis yielded an apparent affinity value of 32.1 nM.
FIG. 2.
Competitive binding of 125I-labeled Cry1Ac to pool I 130-kDa protein. Pool I protein (1 μg) was separated on an 8% acrylamide SDS-PAGE gel and transferred to an Immobilon-P membrane. The 130-kDa protein band was excised from the membrane and incubated with 1 nM 125I-labeled Cry1Ac toxin in the presence of unlabeled competitor at different concentrations. The data are averages based on three replicates and include specific binding calculated by subtracting the background value from the values obtained for membrane slices coincubated with each protein slice in the same assay. The Kd value (32.1 nM) was determined by Scatchard analysis.
N-terminal sequencing of 130- and 170-kDa proteins.
To determine the structures of the 130- and 170-kDa proteins, N-terminal sequencing was performed. Both proteins were found to have the same N terminus, DPAYRLPTTTR, suggesting that the two proteins are identical and that possible posttranslational modifications account for the molecular mass difference. This sequence is nearly identical to the previously reported N-terminal amino acid sequence of a 170-kDa protein (15, 29).
Cloning and sequencing of the 170-kDa Cry1Ac toxin-binding protein.
To clone the 170-kDa Cry1Ac toxin-binding protein, a midgut size-selected plasmid cDNA library previously used to clone the 120-kDa leucine aminopeptidase (15) was utilized. Using primers for the N terminus described above, internal sequences of the 170-kDa protein (29), and conserved lepidopteran APN sequences, we identified a number of specific products amplified during PCR. In particular, primers APN1F and APN5R, a vector-specific primer and APN5R, APN3F and APN5R, and a vector primer and APN2R gave products that were 1.1, 1.3, 0.7, and 0.8 kb long, respectively. The PCR product obtained with APN1F and APN5R was cloned into a TA vector and sequenced. Sequence-specific primer APN6R was made and then used in conjunction with a vector-specific primer to screen the plasmid library as previously described (15, 37). The cDNA clone isolated was analyzed by PCR with the primer pairs mentioned above, and identical amplified products were obtained. The internal peptide sequences reported previously for the 170-kDa protein (29) corresponded to amino acids 396 to 405 and 332 to 341 in our deduced amino acid sequence. These results suggest that the N terminus described by Gill et al. (15) and the peptide sequences described by Luo et al. (29) are probably derived from the same gene.
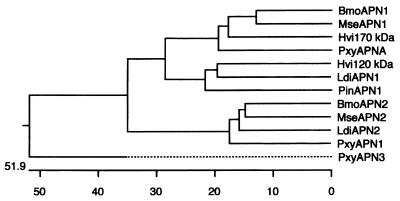
Both strands of the cDNA clone isolated were sequenced, and the deduced amino acid sequence showed that the cDNA which we isolated coded for the 170-kDa protein. This clone had 3,198-bp insert containing a 3,030-bp open reading frame that encoded a 1,010-amino-acid protein with a deduced molecular mass of 112.2 kDa (Fig. 3). A signal sequence cleavage site (33) after serine 20 was predicted. The N terminus obtained from the peptide sequencing analysis suggested that there was additional processing of the 170-kDa protein, which started at amino acid 40 in the cDNA clone. An intriguing polythreonine stretch was present at the C terminus and contained predicted sites for O glycosylation (21–23) beginning at threonine 939 and extending to threonine 984 (Fig. 4). O glycosylation was also predicted at serine residues 22 and 969. This polythreonine stretch is unique to the 170-kDa aminopeptidase, and possible O glycosylation at these sites could explain the high molecular weight of the BBMV protein compared to the deduced molecular weight of the cDNA clone. A predicted signal sequence (10), DSA, for attachment of a GPI anchor was present at amino acid 988. A hydrophobic region extended from amino acid 988 to amino acid 1011, and the GPI anchor addition sequence was preceded by the hydrophilic threonine-rich sequence. A single N-linked glycosylation was observed at amino acid 458. The 170-kDa protein also contained the canonical metal-binding motif HEXXH (26, 31) at positions 355 to 359. A dendrogram analysis of previously described insect aminopeptidases (Fig. 4) indicated that the 170-kDa protein clustered in a homology group with previously described Cry1A toxin-binding proteins.
FIG. 3.
Deduced amino acid sequence of the 170-kDa Cry1Ac-binding protein. The 3,198-bp cDNA clone encodes a 1,010-amino-acid polypeptide with a predicted cleavage site at Ser-20, which follows a signal sequence (dot underlining). The N terminus obtained from peptide sequencing (solid underlining), the canonical metal-binding site (box), the GPI anchor addition site (boldface underlining), and the cleaved C-terminal hydrophobic sequence (dash underlining) are shown. Predicted sites for N glycosylation (double underlining) and O glycosylation (dots) are also indicated. The polythreonine-rich stretch extends from amino acid 939 to amino acid 984. The GenBank accession number for this sequence is AF173552.
FIG. 4.
Sequence similarity of known insect APNs. The phylogenetic tree was constructed based on sequence similarity data by performing a Clustal analysis with the DNAstar program. GenBank accession number are as follows: B. mori APN1 (BmoAPN1), AF084257 (45); M. sexta APN1 (MseAPN1), X89081 (27); H. virescens 170-kDa protein (Hvi170 kDa), AF173552 (this study); P. xylostella APNA (PxyAPNA), AF020389 (3); H. virescens 120-kDa protein (Hvi120 kDa), U35096 (15); P. interpunctella APN1 (PinAPN1), AF034483 (46); B. mori APN2 (BmoAPN2), AB011497 (25); M. sexta APN2 (MseAPN2), X97877 (8); P. xylostella APN1 (PxyAPN1), X97878 (8); P. xylostella APN3 (PxyAPN3), AJ222699 (8a); L. dispar APN1 (LdiAPN1), AF126442 (13); and L. dispar λAPN2 (LdiAPN2), AF126443 (13).
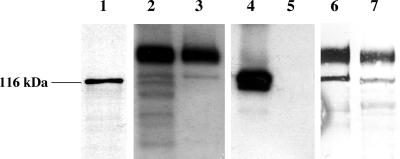
To determine the size of the translated product, the 170-kDa cDNA clone was transcribed and translated in vitro. A 116-kDa translation product (Fig. 5, lane 1) was obtained, suggesting that the 130- and 170-kDa proteins may result from additional co- and posttranslational processing which includes cleavage of the N terminus, N and O glycosylation, and addition of a GPI anchor.
FIG. 5.
In vitro transcription-translation and immunoblot analysis of the 130- and 170-kDa proteins. In vitro-produced mRNA was translated in a rabbit reticulocyte system; 35S-labeled proteins were separated on a 10% polyacrylamide SDS-PAGE gel and visualized by autoradiography; and BBMV and 1 μg of pool I protein were separated on an 8% polyacrylamide SDS-PAGE gel and transferred to an Immobilon-P membrane. Lane 1, in vitro transcripton and translation of the cDNA clone; lanes 2 through 5, immunoblot analysis of pool I proteins performed with 170-kDa protein antiserum (lanes 2 and 3) and 120-kDa protein antiserum (lanes 4 and 5) (lanes 2 and 4, BBMV; lanes 3 and 5, pool I protein); lanes 6 and 7, SBA lectin blot analysis of PNGase-treated (lane 7) and control (lane 6) pool I protein.
Antibody generation and immunoreactivity.
A 29-kDa protein fragment of the 170-kDa aminopeptidase produced in E. coli was used as an antigen to raise antibodies. The antibody recognized the 170-kDa protein in BBMV (Fig. 5, lane 2) and also reacted with the 170- and 130-kDa proteins in the pool I fraction (Fig. 5, lane 3) but not with the 120-kDa leucine aminopeptidase present in pool II (data not shown). The 120-kDa H. virescens aminopeptidase antibody recognized the 120-kDa APN in BBMV (Fig. 5, lane 4) but did not cross-react with either the 130-kDa protein or the 170-kDa protein (Fig. 5, lane 5).
Deglycosylation.
Removal of N-linked glycosylation sites with PNGase F resulted in slight decreases in the molecular masses of the 170- and 130-kDa proteins (Fig. 5, lanes 6 and 7). However, the proteins were still larger than the in vitro translated products, suggesting that possible O glycosylation occurred (Fig. 5, lane 1). Attempts to remove the O-linked sugars did not appear to reduce the molecular mass of the 170-kDa protein (data not shown). The presence of extensive localized glycosylation may impede the efficiency of the glycosidase.
DISCUSSION
Inquiries into the mechanism of action of Cry1A toxins in H. virescens resulted in the cloning of a second Cry1Ac-binding protein, the 170-kDa aminopeptidase. Our investigations uncovered additional parameters that complicate and clarify toxin binding. In this study, purification of the 170-kDa aminopeptidase resulted in isolation of an additional 130-kDa protein that binds Cry1Aa and Cry1Ac toxins on ligand blots. Scatchard analysis of the 130-kDa protein yielded an apparent Cry1Ac toxin affinity value of 32.1 nM. As previously observed, excess unlabeled Cry1Ac toxin could not displace the radiolabeled toxin bound to the 170-kDa protein. Luo et al. (29) also isolated a 170-kDa leucine aminopeptidase, which copurified with a ca. 140-kDa protein that contained a cleaved GPI anchor.
Our results indicate that the 170- and 130-kDa aminopeptidases have different affinities for Cry1Ac toxin, with the 130-kDa protein having higher affinity. When real time analysis of toxin binding with SPR was used, two binding sites on 170-kDa APN were identified, a high-affinity site and a low-affinity site (29). To determine affinity, Luo et al. (29) immobilized the 170-kDa protein; however, the protein preparation contained trace amounts of a 140-kDa protein, which was probably the 130-kDa protein found in our study. Immobilization of two proteins during SPR analysis (29) could result in identification of two binding sites for Cry1Ac toxin. The difference in the estimated molecular masses of the 130-kDa protein was probably due to variations in the SDS-PAGE analyses.
Lectin blots obtained with biotin-labeled soybean agglutinin revealed strong cross-reactions with the 170-kDa protein and, to a lesser extent, the 130-kDa protein. Cry1Ac toxin binding to the 170-kDa protein was not detected in the presence of the sugar GalNAc; however, Cry1Aa still bound, suggesting that there is another binding site which is involved in Cry1Aa toxin binding and is not readily blocked by sugars. These results are in agreement with results that show that GalNAc competes with Cry1Ac binding (15) but not with Cry1Aa and Cry1Ab binding in experiments performed with ligand blots, in binding assays, and in SPR analyses (29). We believe that Cry1Ac toxin binding to the 170-kDa aminopeptidase could be enhanced by high-level glycosylation, and this binding could not be readily eliminated in homologous competition experiments under our conditions, thus resulting in lower affinity values. The low affinity value was probably obtained because extensive glycosylation of the 170-kDa protein provided numerous Cry1Ac toxin-binding sites that were not readily saturable at nanomolar to millimolar concentrations. Therefore, the 130-kDa form, rather than the 170-kDa protein, could function as receptor A in BBMV since it has similar kinetic profiles. As observed for other partially purified proteins, the affinity of the 130-kDa protein is nevertheless lower than the affinity reported for BBMV (29, 30).
Gill et al. (15), Luo et al. (29), and we identified nearly identical N-terminal sequences in the 170-kDa protein. Although Luo et al. (29) speculated that the 140- and 170-kDa proteins are different, our finding that the N termini of the 130- and 170-kDa proteins are identical implied that these proteins are the same. Furthermore, an antibody towards the C-terminal variable region (amino acids 520 to 778) recognized both the 170-kDa protein and the 130-kDa protein, suggesting that these proteins are derived from the same gene. Furthermore, the antibody did not cross-react with a homologous 120-kDa aminopeptidase.
Like other cloned aminopeptidases, the 170-kDa protein has a GPI anchor signal sequence, a metal-binding motif, and hydrophobic N and C termini. Also, the 170-kDa protein is processed at the N terminus, yielding the mature BBMV protein. The cDNA clone encodes a 113-kDa protein with extensive predicted C-terminal O glycosylation. The single N-linked glycosylated site at amino acid 458 is utilized, as treatment with PNGase F slightly reduces the molecular masses of the 130- and 170-kDa proteins, raising the possibility that these two proteins are N glycosylated to similar extents but differ in O glycosylation. The 170-kDa protein is the highest-molecular-mass aminopeptidase described so far; however, the cDNA codes for a polypeptide whose deduced molecular mass is nearly identical to the molecular masses of the other APNs. Thus, the higher molecular mass of the mature protein can probably be attributed to extensive O glycosylation at the C terminus of the polypeptide.
O glycosylation is a posttranslational and postfolding event that has implications for protein structure and stability, recognition, and signaling molecule activity (42). The 35-amino-acid polythreonine-rich stretch with predicted O glycosylation could serve as a scaffold that brings aminopeptidase-bound toxin molecules close to the cell membrane. This process may aid in the oligomerization step necessary for toxin pore formation. Cry toxins do not form oligomers containing less than 10 subunits in solution (20); thus, it is likely that oligomerization occurs after toxin binding. In addition, it has been proposed that the sugar moiety on the GPI anchor itself plays a similar role (12). The pore-forming aerolysin toxin also binds to a GPI-anchored receptor, and the local increase in toxin concentration results in oligomerization and membrane insertion prior to cellular internalization of the toxin (1).
Dendrogram analyses of the available aminopeptidase sequences showed that insects have at least four APNs. The 170-kDa aminopeptidase exhibits 63.2% similarity to the M. sexta aminopeptidase (27), 60.2% similarity to the recently described Cry1Aa-binding B. mori aminopeptidase (45), and 57.6% similarity to P. xylostella APN-A (3). The 120-kDa aminopeptidase which we described previously (15) falls into a different homology group and exhibits 53.8% similarity to a P. interpunctella aminopeptidase sequence available from the GenBank database and 58.8% similarity to the newly identified APN1 of Lymantria dispar (13). A third homology group contains the Cry1Ab toxin-binding P. xylostella and M. sexta aminopeptidases (8) and an additional L. dispar aminopeptidase, λAPN2 (13). A fourth group contains a single P. xylostella aminopeptidase sequence available from the GenBank database (accession no. AJ222699) (8). It has been suggested that gene duplication explains the existence of multiple aminopeptidases (3, 13). Such aminopeptidase diversity could explain the heterogeneity observed with BBMV preparations used to determine toxin affinity. Clearly, more than one or two toxin-binding aminopeptidases are present in the insect midgut, and each enzyme may exhibit different affinities. For insects, the presence of multiple related aminopeptidases is important for hydrolysis of amino acids from peptides and subsequent transport of amino acids by amino acid transporters across the brush border membrane (17). It has been shown that Cry1Aa toxin specifically inhibits K+-dependent leucine transport either by acting on the transporter itself or by acting through an associated protein (36). Toxin insertion in the vicinity of the aminopeptidase that may be associated with the leucine transporter could lead to inhibition of leucine transport into the cell.
Insect resistance to B. thuringiensis toxins has been identified in field and laboratory populations (19, 40), and several resistance mechanisms appear to be involved. The mechanism of resistance in a Cry1Ac-resistant laboratory-selected H. virescens strain is apparently not related to receptor alteration (19). Proteolytic activation of Cry1Ab protoxins in this Cry1Ac-resistant H. virescens strain is slower, and processing of the active toxin is quicker, thereby reducing the amount of active toxin present in the midgut after ingestion (11). However, the same experiment was not performed with Cry1Ac toxin, although a similar result may be expected due to Cry1Ab and Cry1Ac cross-resistance in this strain.
Another Cry1Ac-resistant strain, YHD2 (18), which exhibits up to 10,000-fold resistance, lacks Cry1Aa binding (28) yet exhibits Cry1Ab and Cry1Ac binding similar to that of the susceptible strain. Cry1Ac competes with Cry1Aa toxin-binding sites; thus, it is possible that alterations in the high-affinity Cry1Aa-binding sites may be sufficient for resistance, although other lower-affinity Cry1Ac-binding sites remain intact (28). In the same study the workers detected a slight difference in binding of Cry1Aa toxin to a 120-kDa protein on ligand blots (this protein may be the same as the 130-kDa in this study). Since the ligand blot analyses were performed with solubilized BBMV rather than chromatographed proteins, as was done in this study, it is possible that slight changes in toxin binding may have been masked by proteins which migrated at the same molecular weight.
Therefore, identification of genes at the loci which affect resistance is necessary to understand the mechanism of action of B. thuringiensis (24). Our results, combined with cloning of cDNAs that encode toxin-binding proteins, should allow workers to gain more fundamental insight into the processes involved. The data that have been obtained also cannot eliminate the possibility that processing enzymes involved in glycosylation, GPI anchor addition, and lipid metabolism play a role in the development of resistance, as this study revealed that affinity differences between identical polypeptides may be due to posttranslational modifications.
ACKNOWLEDGMENTS
We thank Ruud de Maagd (CPRO-DLO, Wageningen, The Netherlands) for providing the Cry1 antiserum and Donald H. Dean (Ohio State University) for providing the recombinant Cry1Aa.
This work was supported by research grant 96-353-0-3820 from the U.S. Department of Agriculture. D. I. Oltean was supported in part by the Environmental Toxicology Graduate Program and by a T. R. Fukuto fellowship.
REFERENCES
- 1.Abrami L, Fivaz M, Glauser P E, Parton R G, van der Goot F G. A pore-forming toxin interacts with a GPI-anchored protein and causes vacuolation of the endoplasmic reticulum. J Cell Biol. 1998;140:525–540. doi: 10.1083/jcb.140.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adang M J, Staver M J, Rocheleau T A, Leighton J, Barker R F, Thompson D V. Characterized full-length and truncated plasmid clones of the crystal protein of Bacillus thuringiensis subsp. kurstaki HD-73 and their toxicity to Manduca sexta. Gene. 1985;36:289–300. doi: 10.1016/0378-1119(85)90184-2. [DOI] [PubMed] [Google Scholar]
- 3.Chang W X Z, Gahan L J, Tabashnik B E, Heckel D G. A new aminopeptidase from diamondback moth provides evidence for a gene duplication event in Lepidoptera. Insect Mol Biol. 1999;8:171–177. doi: 10.1046/j.1365-2583.1999.820171.x. [DOI] [PubMed] [Google Scholar]
- 4.Cooper H M, Patterson Y. Production of antibodies. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Straober W, editors. Current protocols in immunology. J. New York, N.Y: Wiley & Sons; 1992. pp. 2.4.1–2.4.7. [Google Scholar]
- 5.Cooper M A, Carroll J, Travis E R, Williams D H, Ellar D J. Bacillus thuringiensis Cry1Ac toxin interaction with Manduca sexta aminopeptidase N in a model membrane environment. Biochem J. 1998;333:677–683. doi: 10.1042/bj3330677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cowles E A, Yunovitz H, Charles J F, Gill S S. Comparison of toxin overlay and solid-phase binding assays to identify diverse Cry1A(c) toxin-binding proteins in Heliothis virescens midgut. Appl Environ Microbiol. 1995;61:2738–2744. doi: 10.1128/aem.61.7.2738-2744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Maagd R A, Kwa M S, van der Klei H, Yamamoto T, Schipper B, Vlak J M, Stiekema W J, Bosch D. Domain III substitution in Bacillus thuringiensis delta-endotoxin Cry1A(b) results in superior toxicity for Spodoptera exigua and altered membrane protein recognition. Appl Environ Microbiol. 1996;62:1537–1543. doi: 10.1128/aem.62.5.1537-1543.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denolf P, Hendrickx K, Van Damme J, Jansens S, Peferoen M, Degheele D, Van Rie J. Cloning and characterization of Manduca sexta and Plutella xylostella midgut aminopeptidase N enzymes related to Bacillus thuringiensis toxin-binding proteins. Eur J Biochem. 1997;248:748–761. doi: 10.1111/j.1432-1033.1997.t01-1-00748.x. [DOI] [PubMed] [Google Scholar]
- 8a.Denolf, P. Unpublished data.
- 9.Du C, Nickerson K W. The Bacillus thuringiensis insecticidal toxin binds biotin-containing proteins. Appl Environ Microbiol. 1996;62:2932–2939. doi: 10.1128/aem.62.8.2932-2939.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Englund P T. The structure and biosynthesis of glycosyl phosphatidylinositol protein anchors. Annu Rev Biochem. 1993;62:121–138. doi: 10.1146/annurev.bi.62.070193.001005. [DOI] [PubMed] [Google Scholar]
- 11.Forcada C, Alcacer E, Garcera M D, Martinez R. Differences in the midgut proteolytic activity of two Heliothis virescens strains, one susceptible and one resistant to Bacillus thuringiensis toxins. Arch Insect Biochem Physiol. 1996;31:257–272. [Google Scholar]
- 12.Garczynski S F, Adang M J. Bacillus thuringiensis Cry1A(c) δ-endotoxin binding aminopeptidase in the Manduca sexta midgut has a glycosyl-phosphatidylinositol anchor. Insect Biochem Mol Biol. 1995;25:409–415. [Google Scholar]
- 13.Garner K J, Hiremath S, Lehtoma K, Valaitis A P. Cloning and complete sequence characterization of two gypsy moth aminopeptidase-N cDNAs, including the receptor for Bacillus thuringiensis Cry1Ac toxin. Insect Biochem Mol Biol. 1999;29:527–535. doi: 10.1016/s0965-1748(99)00027-2. [DOI] [PubMed] [Google Scholar]
- 14.Ge A Z, Pfister R M, Dean D H. Hyperexpression of a Bacillus thuringiensis delta-endotoxin-encoding gene in Escherichia coli: properties of the product. Gene. 1990;93:49–54. doi: 10.1016/0378-1119(90)90134-d. [DOI] [PubMed] [Google Scholar]
- 15.Gill S S, Cowles E A, Francis V. Identification, isolation, and cloning of a Bacillus thuringiensis Cry1Ac toxin-binding protein from the midgut of the lepidopteran insect Heliothis virescens. J Biol Chem. 1995;270:27277–27282. doi: 10.1074/jbc.270.45.27277. [DOI] [PubMed] [Google Scholar]
- 16.Gill S S, Cowles E A, Pietrantonio P V. The mode of action of Bacillus thuringiensis endotoxins. Annu Rev Entomol. 1992;37:615–636. doi: 10.1146/annurev.en.37.010192.003151. [DOI] [PubMed] [Google Scholar]
- 17.Giordana B, Sacchi V F, Parenti P, Hanozet G M. Amino acid transport systems in intestinal brush-border membranes from lepidopteran larvae. Am J Physiol. 1989;257:R494–R500. doi: 10.1152/ajpregu.1989.257.3.R494. [DOI] [PubMed] [Google Scholar]
- 18.Gould F, Anderson A, Reynolds A, Bumgarner L, Moar W. Selection and genetic analysis of a Heliothis virescens (Lepidoptera: Noctuidae) strain with high levels of resistance to Bacillus thuringiensis toxins. J Econ Entomol. 1995;88:1545–1559. [Google Scholar]
- 19.Gould F, Martinez-Ramirez A, Anderson A, Ferre J, Silva F J, Moar W J. Broad-spectrum resistance of Bacillus thuringiensis toxins in Heliothis virescens. Proc Natl Acad Sci USA. 1992;89:7986–7990. doi: 10.1073/pnas.89.17.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guereca L, Bravo A. The oligomeric state of Bacillus thuringiensis Cry toxins in solution. Biochim Biophys Acta. 1999;1429:342–350. doi: 10.1016/s0167-4838(98)00241-6. [DOI] [PubMed] [Google Scholar]
- 21.Hansen J E, Lund O, Engelbrecht J, Bohr H, Nielsen J O. Prediction of O-glycosylation of mammalian proteins: specificity patterns of UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase. Biochem J. 1995;308:801–813. doi: 10.1042/bj3080801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen J E, Lund O, Rapacki K, Brunak S. O-GLYCBASE version 2.0: a revised database of O-glycosylated proteins. Nucleic Acids Res. 1997;25:278–282. doi: 10.1093/nar/25.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen J E, Lund O, Tolstrup N, Gooley A A, Williams K L, Brunak S. NetOglyc: prediction of mucin type O-glycosylation sites based on sequence context and surface accessibility. Glycoconjugate J. 1998;15:115–130. doi: 10.1023/a:1006960004440. [DOI] [PubMed] [Google Scholar]
- 24.Heckel D G, Gahan L C, Gould F, Anderson A. Identification of a linkage group with a major effect on resistance to Bacillus thuringiensis Cry1Ac endotoxin in the tobacco budworm (Lepidoptera: Noctuidae) J Econ Entomol. 1997;90:75–86. [Google Scholar]
- 25.Hua G, Tsukamoto K, Ikezawa H. Cloning and sequence analysis of the aminopeptidase N isozyme (APN2) from Bombyx mori midgut. Comp Biochem Physiol B Biochem Mol Biol. 1998;121:213–222. doi: 10.1016/s0305-0491(98)10091-3. [DOI] [PubMed] [Google Scholar]
- 26.Jongeneel C V, Bouvier J, Bairoch A. A unique signature identifies a family of zinc-dependent metallopeptidases. FEBS Lett. 1989;242:211–4. doi: 10.1016/0014-5793(89)80471-5. [DOI] [PubMed] [Google Scholar]
- 27.Knight P J, Knowles B H, Ellar D J. Molecular cloning of an insect aminopeptidase N that serves as a receptor for Bacillus thuringiensis Cry1A(c) toxin. J Biol Chem. 1995;270:17765–17770. doi: 10.1074/jbc.270.30.17765. [DOI] [PubMed] [Google Scholar]
- 28.Lee M K, Rajamohan F, Gould F, Dean D H. Resistance to Bacillus thuringiensis Cry1A delta-endotoxins in a laboratory-selected Heliothis virescens strain is related to receptor alteration. Appl Environ Microbiol. 1995;61:3836–3842. doi: 10.1128/aem.61.11.3836-3842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo K, Sangadala S, Masson L, Mazza A, Brousseau R, Adang M J. The Heliothis virescens 170 kDa aminopeptidase functions as “receptor A” by mediating specific Bacillus thuringiensis Cry1A delta-endotoxin binding and pore formation. Insect Biochem Mol Biol. 1997;27:735–743. doi: 10.1016/s0965-1748(97)00052-0. [DOI] [PubMed] [Google Scholar]
- 30.Masson L, Lu Y J, Mazza A, Brousseau R, Adang M J. The Cry1A(c) receptor purified from Manduca sexta displays multiple specificities. J Biol Chem. 1995;270:20309–20315. doi: 10.1074/jbc.270.35.20309. [DOI] [PubMed] [Google Scholar]
- 31.Murphy G J, Murphy G, Reynolds J J. The origin of matrix metalloproteinases and their familial relationships. FEBS Lett. 1991;289:4–7. doi: 10.1016/0014-5793(91)80895-a. [DOI] [PubMed] [Google Scholar]
- 32.Nagamatsu Y, Toda S, Koike T, Miyoshi Y, Shigematsu S, Kogure M. Cloning, sequencing, and expression of the Bombyx mori receptor for Bacillus thuringiensis insecticidal Cry1A(a) toxin. Biosci Biotechnol Biochem. 1998;62:727–734. doi: 10.1271/bbb.62.727. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Oddou P, Hartmann H, Geiser M. Identification and characterization of Heliothis virescens midgut membrane proteins binding Bacillus thuringiensis delta-endotoxins. Eur J Biochem. 1991;202:673–680. doi: 10.1111/j.1432-1033.1991.tb16422.x. [DOI] [PubMed] [Google Scholar]
- 35.Oddou P, Hartmann H, Radecke F, Geiser M. Immunologically unrelated Heliothis sp. and Spodoptera sp. midgut membrane-proteins bind Bacillus thuringiensis Cry1A(b) delta-endotoxin. Eur J Biochem. 1993;212:145–150. doi: 10.1111/j.1432-1033.1993.tb17644.x. [DOI] [PubMed] [Google Scholar]
- 36.Parenti P, Villa M, Hanozet G M, Tasca M, Giordana B. Interaction of the insecticidal crystal protein Cry1A from Bacillus thuringiensis with amino acid transport into brush border membranes from Bombyx mori larval midgut. J Invert Pathol. 1995;65:35–42. doi: 10.1006/jipa.1995.1005. [DOI] [PubMed] [Google Scholar]
- 37.Ross L S, Gill S S. Limited growth PCR screening of a plasmid library. BioTechniques. 1996;21:382–384. doi: 10.2144/96213bm08. [DOI] [PubMed] [Google Scholar]
- 38.Rovati G E, Rodbard D, Munson P J. DESIGN: computerized optimization of experimental design for estimating Kd and Bmax in ligand binding experiments. II. Simultaneous analysis of homologous and heterologous competition curves and analysis blocking and of “multiligand” dose-response surfaces. Anal Biochem. 1990;184:172–183. doi: 10.1016/0003-2697(90)90030-d. [DOI] [PubMed] [Google Scholar]
- 39.Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler D R, Dean D H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol Rev. 1998;62:775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tabashnik B E, Cushing N L, Finson N, Johnson M W. Field development of resistance to Bacillus thuringiensis in diamondback moth (Lepidoptera, Plutellidae) J Econ Entomol. 1990;83:1671–1676. [Google Scholar]
- 41.Vadlamudi R K, Weber E, Ji I, Ji T H, Bulla L A., Jr Cloning and expression of a receptor for an insecticidal toxin of Bacillus thuringiensis. J Biol Chem. 1995;270:5490–5494. doi: 10.1074/jbc.270.10.5490. [DOI] [PubMed] [Google Scholar]
- 42.Van den Steen P, Rudd P M, Dwek R A, Opdenakker G. Concepts and principles of O-linked glycosylation. Crit Rev Biochem Mol Biol. 1998;33:151–208. doi: 10.1080/10409239891204198. [DOI] [PubMed] [Google Scholar]
- 43.Van Rie J, Jansens S, Hofte H, Degheele D, Van Mellaert H. Specificity of Bacillus thuringiensis delta-endotoxins. Importance of specific receptors on the brush border membrane of the mid-gut of target insects. Eur J Biochem. 1989;186:239–247. doi: 10.1111/j.1432-1033.1989.tb15201.x. [DOI] [PubMed] [Google Scholar]
- 44.Wolfersberger M G, Luethy P, Maurer A, Parenti P, Sacchi F V, Giordana B, Honozet G M. Preparation and partial characterization of amino acid transporting brush border membrane vesicles from the larval midgut of the cabbage butterfly. Comp Biochem Physiol A Comp Physiol. 1987;86:301–308. [Google Scholar]
- 45.Yaoi K, Nakanishi K, Kadotani T, Imamura M, Koizumi N, Iwahana H, Sato R. cDNA cloning and expression of Bacillus thuringiensis Cry1Aa toxin binding 120 kDa aminopeptidase N from Bombyx mori. Biochim Biophys Acta. 1999;1444:131–137. doi: 10.1016/s0167-4781(98)00250-4. [DOI] [PubMed] [Google Scholar]
- 46.Zhu, Y. C., et al. Unpublished data.