This nonrandomized clinical trial evaluates if the HPV vaccination is associated with a lower number of recurrences requiring surgical intervention in patients with new and recurrent respiratory papillomatosis.
Key Points
Question
Is human papillomavirus (HPV) vaccine containing HPV-6–specific and HPV-11–specific antigens associated with lower frequency of recurrences in patients with recurrent respiratory papillomatosis (RRP)?
Findings
This nonrandomized clinical trial of 50 patients with RRP found a significant decline in postvaccination frequency of recurrences requiring surgical interventions compared with prevaccination period; no difference in postvaccination recurrences was found between patients with newly diagnosed and recurrent disease. Both groups appeared to show equally beneficial RRP outcomes following HPV vaccination.
Meaning
The findings suggest that the earlier that patients with RRP receive adjuvant HPV vaccine, the sooner they may experience beneficial outcomes and reduced burden of disease.
Abstract
Importance
Recurrent respiratory papillomatosis (RRP) is a rare benign chronic disease of the larynx etiologically linked with the infection of low-risk human papillomavirus (HPV). Combination of surgical and immunomodulatory therapy has limited success. Possible use of prophylactic HPV vaccine that includes HPV-6 and HPV-11 antigens has been studied.
Objective
To evaluate if the HPV vaccination is associated with a lower number of recurrences requiring surgical intervention in patients with new and recurrent RRP.
Design, Setting, and Participants
This was a non–placebo-controlled intervention study. Enrollment data were collected from October 2011 to August 2013. The patients were followed up at 1 month, 12 months, and 5 years after the third dose of the vaccine and clinically monitored until December 31, 2018. Data were analyzed from 2019 to 2021. Altogether, 50 adults with active RRP were enrolled and followed up in referral centers. For the final outcome, follow-up data for 42 patients were available. Eight patients who did not fulfill the protocol were excluded.
Interventions
All patients received HPV vaccine as an adjuvant treatment and were clinically followed up. When RRP progression or a significant recurrent lesion was detected, surgical removal via direct laryngoscopy was indicated. No adjuvant therapy with antiviral or biological agents was used.
Main Outcomes and Measures
This study compared the prevaccination and postvaccination positivity for HPV-specific antibodies. The main outcome was the difference in the frequency of RRP recurrences in the prevaccination and postvaccination period.
Results
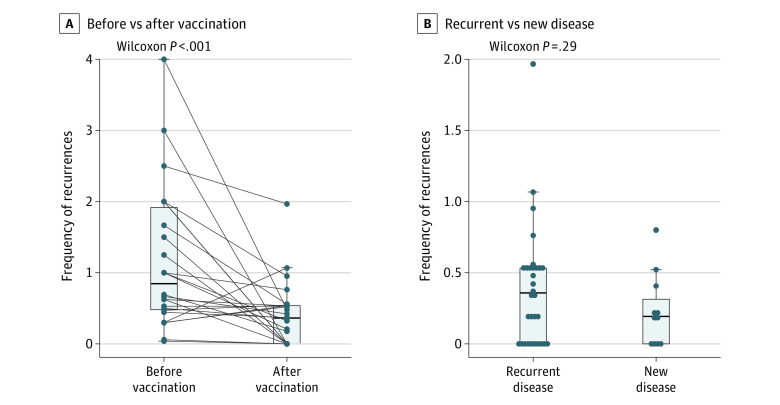
A total of 50 patients with RRP were enrolled (median [SD] age, 41.5 [12.3] years [range, 21-73 years]; 39 [78%] men and 11 [22%] women). After HPV vaccination, patients with previously no HPV-specific antibodies showed seroconversion, and all patients developed 100-fold higher levels of HPV vaccine type-specific antibodies compared with the prevaccination period. In patients with recurrent RRP, decreased frequency of recurrences requiring surgical treatment was present after vaccination (from 0.85 to 0.36 recurrences/y). No difference in postvaccination recurrences was found between patients with newly diagnosed and recurrent RRP.
Conclusions and Relevance
In this nonrandomized clinical trial, the frequency of RRP recurrences was significantly lower after HPV vaccination, and patients with RRP thus had a reduced burden of disease. Because no difference was detected in the frequency of recurrent postvaccination lesions in patients with new and recurrent disease, it appears that both groups showed equal benefit following HPV vaccination. These findings suggest that the earlier that patients with RRP receive HPV vaccine, the sooner they may show reduced burden of disease.
Trial Registration
EudraCT Identifier: 2011-002667-14; ClinicalTrials.gov Identifier: NCT01375868
Introduction
Recurrent respiratory papillomatosis (RRP) is a rare benign disease with high morbidity characterized by the growth of papillomas in the airways, which negatively affects the voice and breathing and substantially reduces the quality of life. According to the patients’ age at disease onset, RRP is classified as either juvenile or adult, with estimated prevalences of 4.3 and 1.8 cases per 100 000 persons, respectively.1 The incidence may vary by country, studied population, and diagnostic method. Men are slightly more affected than women. Etiologically, RRP is associated with infection of the squamocolumnar junction of mucosal areas in the upper respiratory tract with human papillomavirus (HPV) types 6 and 11. The detection of other HPV types, including high-risk types, in RRP is rare, but it cannot be ruled out. Type HPV-11 was linked with a more aggressive course of the disease, although recent studies did not confirm previous observations.2,3 The papillomas have the potential to spread to the lower respiratory tract and even convert to malignant neoplasm.
The clinical examination of patients with RRP consists of in-office laryngoscopy. The most precise of the endoscopic imaging methods is flexible videoendoscopy with narrow-band imaging, which is capable of detecting even the smallest lesion.4,5 Treatment of RRP is mostly surgical removal of papillomas. Surgical procedures have evolved from the use of cold (nonpowered) instruments to lasers and microdebriders. Carbon dioxide lasers have most frequently been used during the several past decades.6 Of other therapeutic options, intralesional application of cidofovir has been used.7 Several other candidate drugs are currently being tested as adjuvant therapies for RRP, such as monoclonal antibodies against vascular endothelial growth factor (bevacizumab)8,9 and the checkpoint inhibitor molecule programmed cell death 1 ligand 1 (avelumab).10
Since 2006, HPV vaccines have been available for primary prevention of HPV-associated diseases that include low-risk types, HPV-6/HPV-11, in addition to high-risk types. The HPV vaccines are safe and highly effective in the naive population. In comparison with natural infections, they induce much higher titers of neutralizing HPV-specific antibodies in sera, cervicovaginal secretions,11 and oral mucosal fluids.12 Increased titers of HPV-specific antibodies and higher positivity rates have also been detected in patients with RRP.13,14 The efficacy of HPV vaccines in reducing anogenital HPV-associated lesions and tumors has been documented.15,16,17,18,19 Recently, the protective effect of these vaccines against HPV type–specific oral infections20 and HPV-6/HPV-11–associated diseases, including RRP in the young population, has also been shown.21,22,23 Additionally, HPV vaccine has proved to be efficient in reducing the frequency of recurrences of HPV-associated diseases, for example, cutaneous/genital warts and cervical, vulvar, vaginal, and anal intraepithelial lesions.24,25,26,27 Also, several case and small series reports have shown positive outcome with increasing the intervals between surgeries in patients with RRP. Recent meta-analyses of 12 studies confirmed the outcomes of the case report studies.28 However, most of the studies had the following limitations: low numbers of patients, diversity of methodologies used for outcome evaluation, and limited follow-up periods.
The main aim of the clinical trial was to prospectively monitor the outcome of the tetravalent HPV vaccine on the recurrence of papillomatous lesions in comparison with the prevaccination outcomes in patients with RRP to assess if the vaccine is associated with lower burden of disease in adults with RRP.
Methods
Study Population
Altogether 50 patients diagnosed with or treated for RRP were enrolled between October 2011 and August 2013. On enrollment, the patients signed an informed consent form. The study included patients 18 years or older who were not pregnant. Besides consequent patients treated in the enrollment center, patients from other otorhinolaryngology offices who agreed with participation in the study were also included. Data were obtained through medical records, and blood samples, biopsy specimens, and/or smear specimens were collected. From patients who underwent surgical removal of a laryngeal lesion, a biopsy specimen was available for analysis. When surgical intervention was not indicated, a smear from an RRP lesion or the site of a previous lesion under laryngoscopy control was taken. The smear and/or biopsy specimens were used for HPV DNA detection and typing. The first dose of the vaccine was typically given within 1 month after enrollment, the second dose followed within 2 months, and the third one within 6 months after the first dose. The patients were then invited for blood sampling and examination at 1 month, 12 months, and 5 years after the last dose. A single-dose recipient was excluded from the study, and 49 patients presented for follow-up 1 month after the third dose. Two patients who did not present at the 1-year follow-up were excluded. One patient died owing to inoperable oral spinocellular cancer with metastasis. Five-year follow-up data were missing for 4 patients. All requirements specified in the protocol of the study were met by 42 patients.
All patients were clinically followed up by means of in-office flexible videolaryngoscopy with narrow-band imaging. The follow-up interval was 6 months in case of a negative finding or in the presence of a small recurrent nonprogressive papillomatous lesion. When progression or a significant recurrent lesion aggravating voice disorder was detected, surgical removal via direct laryngoscopy was indicated. The procedures were performed with cold instruments or carbon dioxide laser with a micromanipulator and a scanner coupled to an operating microscope. In case of anterior commissure involvement, surgery was carried out as a 2-stage procedure to eliminate the risk of anterior commissure webbing. No adjuvant therapy with antiviral or biological agents was used.
For the final clinical evaluation based on the presence or absence of recurrences, patients were divided into 3 groups: (1) no recurrence, (2) stationary papilloma with no need for treatment, and (3) progressive recurrent disease requiring repeated treatment. The frequency of recurrences was calculated as the ratio of the number of interventions to the number of years with the disease.
The last follow-up visit was scheduled at 5 years plus or minus 6 months after the third vaccine dose. The study was finished on December 31, 2018. The clinical trial registered under EudraCT No. 2011-002667-14 and ClinicalTrials.gov identifier NCT01375868 was monitored. Adverse effects were reported to the qualified person responsible for pharmacovigilance in Merck Sharp & Dohme. The adverse effects reported by patients were minor and consistent with the summary of product characteristics (pain in the injection site, fatigue, headache, malaise, vomiting). The patients were enrolled in 1 center, Medical Healthcom Ltd, Voice and Hearing Centre, and the study was approved by the Ethical Committee of this center (reference No. 3/2011).
HPV DNA Detection and Serological Assays
Patients’ DNA was extracted from biopsy specimens and/or smears by the QIAamp DNA Mini Kit (Qiagen) according to the manufacturer’s protocol. The detection and genotyping of HPV DNA were performed as described elsewhere.29 The presence of antibodies to HPV was tested using an in-house L1 virus-like particles (VLPs)–based enzyme-linked immunosorbent assay as described previously30 (eMethods in Supplement 1).
Statistical Analyses
For contingency tables, the Fisher exact test was used. To compare the antibody levels in groups of patients with newly diagnosed and recurrent disease, the Mann-Whitney test was used. All tests were 2 sided, and the significance level α was .05. The prevaccination and postvaccination frequency of RRP recurrences were compared by the Wilcoxon signed-rank test. A 1-sided alternative of lower frequency after vaccination was considered. The postvaccination frequency of recurrences (the total of recurrences) in patients with newly diagnosed and recurrent RRP were compared by means of the Poisson regression. The statistical analyses were performed using GraphPad Prism, version 8.4.2 (GraphPad Software) and R, version 4.0.2 (R Foundation for Statistical Computing).
Results
Study Population
The median (SD) age of the patients with RRP (n = 50) was 41.5 (12.3) years (range, 21-73 years); 39 (78%) were men, and 11 (22%) were women (Table 1). Of the 50 enrolled patients, 13 were newly diagnosed with RRP and underwent no or a single treatment in the 3 preceding months. The other 37 patients had recurrent disease. Mean (SD) time of disease duration before enrollment was 10.2 (10.8) years (range, 1-30 years; median, 4 years). According to the frequency of pre-enrollment surgical interventions, 37 of 50 (74%) patients needed 1 or fewer interventions per year (median, 0.44 surgical sessions per year), while 13 of 50 (26%) patients required more frequent interventions (median, 2.0 surgical sessions per year). When comparing the enrollment characteristics of the excluded patients (n = 8) and those who completed the protocol of the study (n = 42), we did not find any major differences (data not shown).
Table 1. Patient Characteristics at Enrollment.
| Characteristic | No./total No. (%) |
|---|---|
| Age, y | |
| Total | |
| No./total No. (%) | 50/50 (100) |
| Median (SD) [range] | 41.5 (12.3) [21-73] |
| Men | |
| No./total No. (%) | 39/50 (78) |
| Median (SD) [range] | 44 (13.5) [21-73] |
| Women | |
| No./total No. (%) | 11/50 (22) |
| Median (SD) [range] | 39 (4.4) [37-48] |
| HPV DNA | |
| Biopsy and/or smear | 39/50 (78) |
| HPV-6 biopsy and/or smear | 30/39 (77) |
| HPV-11 biopsy and/or smear | 7/39 (18) |
| HPV-16, HPV-18, HPV-31 biopsy and/or smear | 2/39 (5) |
| Anti–HPV-6 and/or HPV-11 antibody | 42/50 (84) |
| Anti–HPV-6 antibody | 40/50 (80) |
| GMT | 35.8 |
| Anti–HPV-11 antibody | 28/50 (56) |
| GMT | 9.9 |
| Low frequency, ≤1/y | 37/50 (74) |
| Median No. of surgeries/y | 0.44 |
| High frequency, >1/y | 13/50 (26) |
| Median No. of surgeries/y | 2.0 |
Abbreviations: GMT, geometric mean titer; HPV, human papillomavirus.
HPV DNA Detection
Biopsy specimens were available from 30 of 50 patients. All of them (100%, 30 of 30) were HPV positive, 80% (24 of 30) for HPV-6 and 13% (4 of 30) for HPV-11. One biopsy specimen was positive for HPV-31 (3%), and HPV-31/HPV-16 coinfection was detected in the smear from the same patient. One smear contained HPV-18, and the respective patient was histologically diagnosed with papillary carcinoma.
Smears were available from 48 of 50 patients. Smears were taken from a clinically visible lesion, and if not seen, from the site of a previous lesion. Smears positive for HPV were found in 54% (26 of 48) of patients. In concordance with HPV types present in biopsies, HPV-6 was detected more often, in 73% (19 of 26) of HPV-positive smears, followed by HPV-11 in 15% (4 of 26). One smear contained HPV-16, and HPV-6 was present in the biopsy specimen from the same patient, with no tumor found on histologic evaluation.
Presence of HPV in the biopsy specimen and/or smear was detected in 78% of patients (39 of 50). Of these, 77% (30 of 39) were positive for HPV-6 and 18% (7 of 39) for HPV-11 (Table 1).
Prevalence of HPV-Specific Antibodies
In all but 8 patients, ie, in 42 of 50 (84%), HPV-6–specific and/or HPV-11–specific antibodies were detected prior to vaccination. There was a difference of 11 percentage points in the positivity rate for HPV-6/HPV-11–specific antibodies between patients newly diagnosed with RRP, 12 of 13 (92%), and those with recurrent disease, 30 of 37 (81%) (odds ratio [OR], 0.44; 95% CI, 0.04-3.14). Women were more often positive for HPV-6 and HPV-11 antibodies than men (100% and 64% for HPV-6; 64% and 51% for HPV-11 positivity among women and men, respectively; OR, 4.40; 95% CI, 0.66-51.02 for HPV-6; OR, 1.66; 95% CI, 0.40-5.67 for HPV-11). The wide CI prevents making a definitive conclusion regarding the true effect size. Geometric mean titers of both HPV-6–specific and HPV-11–specific antibodies increased with disease duration (Table 2).
Table 2. Geometric Mean Titer (GMT) of Human Papillomavirus (HPV)–Specific Antibodies at Enrollment Depending on Disease Duration.
| Time from RRP diagnosis, y | No. | GMT of specific antibodies | |
|---|---|---|---|
| HPV-6 antibodies | HPV-11 antibodies | ||
| ≤1 | 21 | 16.7 | 4.3 |
| 2-4 | 11 | 46.2 | 14.2 |
| >4 | 18 | 74.1 | 13.7 |
Abbreviation: RRP, recurrent respiratory papillomatosis.
All patients for whom the samples were available (n = 49) showed seroconversion after the third dose of HPV vaccine. The levels of antibodies specific to all vaccine types increased 100-fold within 1 month after vaccination. The immune response to the cross-reactive types HPV-31 and HPV-33 was detected. One month after the third dose, all samples were seropositive for HPV-31 antibodies, and 47 of 49 (96%) for HPV-33 antibodies.
For the comparison of HPV-specific vaccine antibody levels, serum samples of 42 patients with 5-year follow-up samplings were available. After 5 years of follow-up, antibody levels against the vaccine types decreased but still remained 10 times above the prevaccination levels (Figure 1). No statistically significant difference was found in the prevaccination and postvaccination levels of HPV-specific antibodies between patients newly diagnosed with RRP (n = 11) and those who underwent 1 to 4 (n = 12), 5 to 9 (n = 14), and more than 9 lifetime papilloma surgeries (n = 5) before enrollment (Figure 1).
Figure 1. Geometric Mean Titer (GMT) of Human Papillomavirus (HPV)–Specific Antibodies Before Vaccination, 1 Month, 1 Year, and 5 Years After the Third Vaccine Dose.
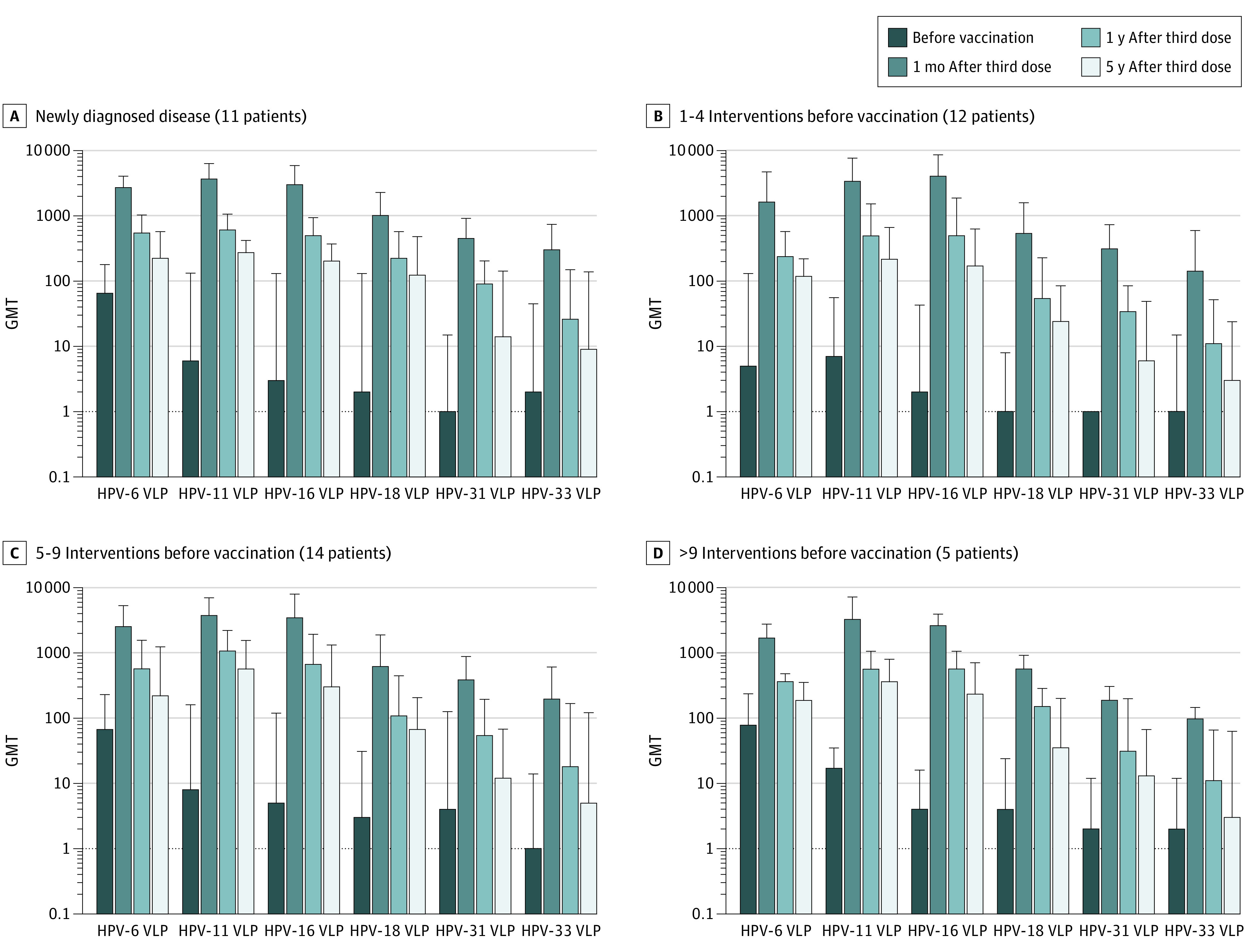
On the y-axis, the logarithmic scale was used. Four groups of participants are shown, divided according to lifetime number of interventions prior to vaccination: (A) newly diagnosed disease (≤1 surgical intervention at enrollment), (B) 1 to 4 interventions, (C) 5 to 9 interventions, and (D) more than 9 interventions. VLP indicates virus-like particle. The error bars represent 1 SD.
Clinical Evaluation
The final clinical evaluation revealed 3 patient groups: (1) no recurrence (n = 12; 28.6%), (2) stationary papilloma with no need for treatment (n = 4; 9.5%), and (3) progressive recurrent disease requiring repeated treatment (n = 26; 61.9%). The frequency of recurrences was calculated for 26 patients with progressive recurrent disease (Table 3).
Table 3. Frequency of Prevaccination and Postvaccination Recurrences in Patients With Recurrent Disease (n = 26).
| Time | Median (range) | ||
|---|---|---|---|
| No. of interventions | No. of years | Frequency (No. of interventions/No. of years) | |
| Before enrollment | 4.5 (1-10) | 4.0 (1.0-30.0) | 0.85 (0.04-4.00) |
| After vaccination | 2.0 (0-10) | 5.1 (3.6-6.2) | 0.36 (0.00-1.97) |
Figure 2A shows the decline in the frequency of recurrences in patients with recurrent disease (n = 26). The median (IQR) follow-up was 5.1 (0.9) years. The use of surgical interventions decreased from 0.85 per year before vaccination to 0.36 after vaccination, with median decrease equal to 0.52 (95% CI, 0.08 to 1.11). The difference between the postvaccination recurrences in patients with recurrent disease and patients with newly diagnosed RRP was 0.11 (95% CI, −0.04 to 0.34) on 5-year follow-up (Figure 2B).
Figure 2. Frequency of Recurrences .
Frequency of recurrences (A) before and after vaccination in patients with recurrent disease (n = 26) and (B) after vaccination in patients newly diagnosed with recurrent respiratory papillomatosis (n = 11) and those with recurrent disease (n = 26). Horizontal lines in the boxes represent the median and quartiles, and whiskers are given by the maximal (minimal) value that is not considered as an outlier, ie, not more than 1.5 times IQR distant from the box.
Discussion
This phase 3b clinical trial enrolled 50 patients with RRP and followed them up over a period of 5 years to evaluate the frequency of recurrences before and after receipt of the tetravalent vaccine directed against HPV types causally linked with 100% of RRPs. Findings confirmed the rare case reports and results of small series of patients showing decreased frequency of recurrences and/or prolongation of time between recurrences requiring surgical treatment and thus reducing the burden of disease in vaccinated patients.
Results showed that HPV-6 or HPV-11 DNA positivity in biopsy specimens and/or smears was confirmed in 78% of the 50 study patients with active RRP, and 84% of the patients had HPV-6–specific and/or HPV-11–specific antibodies at enrollment. The level of HPV type–specific seroreactivity in patients with RRP is usually lower despite long-term HPV-6/HPV-11 infection and disease duration.13,14 In the study by Buchinsky et al,13 almost half (47%) of adult and adolescent participants with a median (range) duration of symptoms of 11 (1-69) years possessed no antibodies against HPV-6 and HPV-11. The proportion of seropositive people with RRP according to some studies may be positively correlated with the duration of symptomatic disease and the number of surgery-necessitating interventions.13,31,32 The duration of the disease before enrollment was slightly shorter in our study in comparison with that of Buchinsky et al,13 so the higher percentage of seropositive patients may be more likely attributable to the use of diverse methods for antibody level testing. Serological assays for the detection of antibodies to HPV are, except for neutralization assays, used mainly for the assessment of antibody response after HPV vaccination. In principle, VLPs composed of L1 protein-binding or competitive immunoassay are used. In enzyme-linked immunosorbent assays, VLPs are bound to a solid phase, and antibodies are bound directly. The test measures all binding antibodies of immunoglobulin class given by the secondary antibody. In the competitive assay, labeled type-specific monoclonal antibody competes with antibodies in the sample to bind to VLPs. This assay detects antibodies of all immunoglobulin classes but only to 1 epitope present on VLPs, omitting antibodies to all other epitopes. Therefore, it can explain the lower number of seropositive patients in the study by Buchinsky et al,13 especially when natural HPV infection induces lower levels of antibodies. In such situation, it might be extremely difficult to compare results of HPV antibody testing among various studies that use different types of tests.33 Additionally, we cannot exclude the effect of age because in the study by Buchinsky et al,13 also younger participants were enrolled, and as has been shown by us and others, the prevalence of HPV-specific antibodies increases with age.34,35,36
Some researchers postulated that next to individual genetic predisposition, defects in the immune response can pose a risk for RRP. It has been shown that HPV infection is relatively common in the upper respiratory tract of children and adults, including HPV-6 and HPV-11, but the incidence of RRP is very low.37,38,39 An insufficient local immune response with the predominant component of TH2 and Treg immune responses can lead to tolerance to HPV-infected cells and inability of the immune system to clear HPV infection.40,41 Prophylactic HPV vaccines, in addition to high serum neutralization antibody levels, are able to induce antibody titers in mucosal secretions and oral fluids.11,12 Their reducing effect on recurrences of HPV-associated diseases has been demonstrated.25,28,42 Therefore, it is reasonable to assume the same effect of HPV vaccine on RRP lesions.
All study participants developed substantial levels of HPV-specific antibodies after the third dose of vaccine compared with baseline at enrollment and were followed up for 5 years after the vaccination. During the follow-up, the levels of HPV-specific antibodies slightly decreased but were still several times higher than after natural infection. The mechanism behind the therapeutic effect of HPV vaccines is not yet fully understood. The induction of high serum levels of neutralizing antibodies is thought to reduce possible mucosal reinfections at the site of papilloma and could prevent the spread of RRP lesions. In this study, we observed a significant decline in postvaccination recurrences requiring surgical interventions in patients with RRP and confirmed the benefit of adjuvant HPV vaccine. In a meta-analysis published recently,28 higher reduction in surgical procedures per month was calculated (0.35 to 0.06 per month before and after vaccination, respectively). However, the authors stated that only a few studies were eligible for quantitative analyses of the therapeutic benefit of HPV vaccine in patients with RRP.
Somewhat surprising was the absence of a significant difference in postvaccination recurrences between the patients with new vs recurrent disease. It needs to be pointed out that the number of patients with newly diagnosed RRP in our study was nearly 3 times lower than that of patients with recurrent disease. Additionally, our results imply that HPV vaccine given as part of adjuvant treatment early after RRP diagnosis could have some positive association with the course of the disease and reduce the burden over the years. However, it is evident that the maximum preventive effect of the vaccine is seen when it is given prior to infection.21,22,23
Limitations
The absence of a placebo group in our study might be considered as a limitation. However, the course of the disease is greatly variable among patients, and we determined that comparison of the disease outcome before and after the vaccination for the same participant would be more informative. Possible bias could have been introduced by the changes in the management of patients in the pre-enrollment vs postenrollment period. However, the possibility to detect the lesions at an earlier stage led to the less aggressive approach in the treatment of the lesion. In the pre-enrollment period, when the videoendoscopy with narrow-band imaging was not available, the recurrent papillomas were more likely to be detected at an advanced stage and thus were often referred for immediate surgical removal. Therefore, we assumed that the frequency of surgical procedures was not influenced by the changes in the management of patients; however, we do not have sufficient data to prove this assumption.
Conclusions
In this nonrandomized clinical trial, patients with RRP showed benefit after receiving vaccines containing HPV-6–specific and HPV-11–specific antigens. Patients with no HPV-specific antibodies showed seroconversion, and all patients developed 100-fold higher levels of HPV vaccine type-specific antibodies, and after 5 years, the level was still 10 times as high compared with the prevaccination period. Because no difference in postvaccination recurrences was found between patients with new and recurrent disease, both groups appear to benefit equally from HPV vaccination. Therefore, the earlier that patients with RRP receive HPV vaccine, the earlier they could benefit from the effect we observed—less frequent recurrences. In conclusion, we have shown that vaccination against HPV is associated with a lower number of recurrences and thus a reduced the burden of disease in patients with RRP. As routine vaccination of children before first sexual intercourse has been associated with lower incidence of diseases linked to HPV infection, we will most likely see elimination of RRP in the future.21,22,23 But the effect will be visible sooner and be more evident in populations with gender-neutral vaccination of children.
eMethods.
Data Sharing Statement
References
- 1.Derkay CS, Bluher AE. Recurrent respiratory papillomatosis: update 2018. Curr Opin Otolaryngol Head Neck Surg. 2018;26(6):421-425. doi: 10.1097/MOO.0000000000000490 [DOI] [PubMed] [Google Scholar]
- 2.Gallagher TQ, Derkay CS. Recurrent respiratory papillomatosis: update 2008. Curr Opin Otolaryngol Head Neck Surg. 2008;16(6):536-542. doi: 10.1097/MOO.0b013e328316930e [DOI] [PubMed] [Google Scholar]
- 3.Buchinsky FJ, Valentino WL, Ruszkay N, et al. Age at diagnosis, but not HPV type, is strongly associated with clinical course in recurrent respiratory papillomatosis. PLoS One. 2019;14(6):e0216697. doi: 10.1371/journal.pone.0216697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lukes P, Zabrodsky M, Lukesova E, et al. The role of NBI HDTV magnifying endoscopy in the prehistologic diagnosis of laryngeal papillomatosis and spinocellular cancer. Biomed Res Int. 2014;2014:285486. doi: 10.1155/2014/285486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tjon Pian Gi REA, Halmos GB, van Hemel BM, et al. Narrow band imaging is a new technique in visualization of recurrent respiratory papillomatosis. Laryngoscope. 2012;122(8):1826-1830. doi: 10.1002/lary.23344 [DOI] [PubMed] [Google Scholar]
- 6.Ivancic R, Iqbal H, deSilva B, Pan Q, Matrka L. Current and future management of recurrent respiratory papillomatosis. Laryngoscope Investig Otolaryngol. 2018;3(1):22-34. doi: 10.1002/lio2.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazia F, Galletti B, Freni F, et al. Use of intralesional cidofovir in the recurrent respiratory papillomatosis: a review of the literature. Eur Rev Med Pharmacol Sci. 2020;24(2):956-962. doi: 10.26355/eurrev_202001_20081 [DOI] [PubMed] [Google Scholar]
- 8.Sidell DR, Balakrishnan K, Best SR, et al. Systemic bevacizumab for treatment of respiratory papillomatosis: international consensus statement. Laryngoscope. 2021;131(6):E1941-E1949. doi: 10.1002/lary.29343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tkaczuk A, Trivedi S, Mody MD, et al. Parenteral bevacizumab for the treatment of severe respiratory papillomatosis in an adult population. Laryngoscope. 2021;131(3):E921-E928. doi: 10.1002/lary.29133 [DOI] [PubMed] [Google Scholar]
- 10.Allen CT, Lee S, Norberg SM, et al. Safety and clinical activity of PD-L1 blockade in patients with aggressive recurrent respiratory papillomatosis. J Immunother Cancer. 2019;7(1):119. doi: 10.1186/s40425-019-0603-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pattyn J, Van Keer S, Tjalma W, Matheeussen V, Van Damme P, Vorsters A. Infection and vaccine-induced HPV-specific antibodies in cervicovaginal secretions: a review of the literature. Papillomavirus Res. 2019;8:100185. doi: 10.1016/j.pvr.2019.100185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Handisurya A, Schellenbacher C, Haitel A, Senger T, Kirnbauer R. Human papillomavirus vaccination induces neutralising antibodies in oral mucosal fluids. Br J Cancer. 2016;114(4):409-416. doi: 10.1038/bjc.2015.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchinsky FJ, Ruszkay N, Valentino W, et al. In RRP, serologic response to HPV is frequently absent and slow to develop. PLoS One. 2020;15(3):e0230106. doi: 10.1371/journal.pone.0230106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tjon Pian Gi REA, San Giorgi MRM, Pawlita M, et al. Immunological response to quadrivalent HPV vaccine in treatment of recurrent respiratory papillomatosis. Eur Arch Otorhinolaryngol. 2016;273(10):3231-3236. doi: 10.1007/s00405-016-4085-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drolet M, Bénard É, Pérez N, Brisson M; HPV Vaccination Impact Study Group . Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. 2019;394(10197):497-509. doi: 10.1016/S0140-6736(19)30298-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falcaro M, Castañon A, Ndlela B, et al. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: a register-based observational study. Lancet. 2021;398(10316):2084-2092. doi: 10.1016/S0140-6736(21)02178-4 [DOI] [PubMed] [Google Scholar]
- 17.Kjaer SK, Nygård M, Sundström K, et al. Long-term effectiveness of the nine-valent human papillomavirus vaccine in Scandinavian women: interim analysis after 8 years of follow-up. Hum Vaccin Immunother. 2021;17(4):943-949. doi: 10.1080/21645515.2020.1839292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med. 2020;383(14):1340-1348. doi: 10.1056/NEJMoa1917338 [DOI] [PubMed] [Google Scholar]
- 19.Luostarinen T, Apter D, Dillner J, et al. Vaccination protects against invasive HPV-associated cancers. Int J Cancer. 2018;142(10):2186-2187. doi: 10.1002/ijc.31231 [DOI] [PubMed] [Google Scholar]
- 20.Chaturvedi AK, Graubard BI, Broutian T, et al. Effect of prophylactic human papillomavirus (HPV) vaccination on oral HPV infections among young adults in the United States. J Clin Oncol. 2018;36(3):262-267. doi: 10.1200/JCO.2017.75.0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meites E, Stone L, Amiling R, et al. Significant declines in juvenile-onset recurrent respiratory papillomatosis following human papillomavirus (HPV) vaccine introduction in the United States. Clin Infect Dis. 2021;73(5):885-890. doi: 10.1093/cid/ciab171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novakovic D, Cheng ATL, Zurynski Y, et al. A prospective study of the incidence of juvenile-onset recurrent respiratory papillomatosis after implementation of a national HPV vaccination program. J Infect Dis. 2018;217(2):208-212. doi: 10.1093/infdis/jix498 [DOI] [PubMed] [Google Scholar]
- 23.Teutsch SM, Nunez CA, Morris A, et al. Australian Paediatric Surveillance Unit (APSU) annual surveillance report 2019. Commun Dis Intell (2018). 2020;44:44. doi: 10.33321/cdi.2020.44.60 [DOI] [PubMed] [Google Scholar]
- 24.Bossart S, Imstepf V, Hunger RE, Seyed Jafari SM. Nonavalent human papillomavirus vaccination as a treatment for skin warts in immunosuppressed adults: a case series. Acta Derm Venereol. 2020;100(6):adv00078. doi: 10.2340/00015555-3437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dion GR, Teng S, Boyd LR, et al. Adjuvant human papillomavirus vaccination for secondary prevention: a systematic review. JAMA Otolaryngol Head Neck Surg. 2017;143(6):614-622. doi: 10.1001/jamaoto.2016.4736 [DOI] [PubMed] [Google Scholar]
- 26.Jentschke M, Kampers J, Becker J, Sibbertsen P, Hillemanns P. Prophylactic HPV vaccination after conization: a systematic review and meta-analysis. Vaccine. 2020;38(41):6402-6409. doi: 10.1016/j.vaccine.2020.07.055 [DOI] [PubMed] [Google Scholar]
- 27.Swedish KA, Factor SH, Goldstone SE. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: a nonconcurrent cohort study. Clin Infect Dis. 2012;54(7):891-898. doi: 10.1093/cid/cir1036 [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg T, Philipsen BB, Mehlum CS, et al. Therapeutic use of the human papillomavirus vaccine on recurrent respiratory papillomatosis: a systematic review and meta-analysis. J Infect Dis. 2019;219(7):1016-1025. doi: 10.1093/infdis/jiy616 [DOI] [PubMed] [Google Scholar]
- 29.Tachezy R, Smahelova J, Kaspirkova J, Salakova M. Human papillomavirus type-specific prevalence in the cervical cancer screening population of Czech women. PLoS One. 2013;8(11):e79156. doi: 10.1371/journal.pone.0079156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamsikova E, Smahelova J, Ludvikova V, et al. The prevalence of HPV infections in HPV-vaccinated women from the general population. APMIS. 2017;125(6):585-595. doi: 10.1111/apm.12677 [DOI] [PubMed] [Google Scholar]
- 31.Tachezy R, Hamsikova E, Valvoda J, et al. Antibody response to a synthetic peptide derived from the human papillomavirus type 6/11 L2 protein in recurrent respiratory papillomatosis: correlation between Southern blot hybridization, polymerase chain reaction, and serology. J Med Virol. 1994;42(1):52-59. doi: 10.1002/jmv.1890420111 [DOI] [PubMed] [Google Scholar]
- 32.Durzyńska J, Błazejewska P, Szydłowski J, Goździcka-Józefiak A. Detection of anti-HPV11-L1 antibodies in immune sera from patients suffering from recurrent respiratory papillomatosis using ELISA. Viral Immunol. 2010;23(4):415-423. doi: 10.1089/vim.2010.0014 [DOI] [PubMed] [Google Scholar]
- 33.Pinto LA, Dillner J, Beddows S, Unger ER. Immunogenicity of HPV prophylactic vaccines: serology assays and their use in HPV vaccine evaluation and development. Vaccine. 2018;36(32 pt A):4792-4799. doi: 10.1016/j.vaccine.2017.11.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamsikova E, Ludvikova V, Stasikova J, Tachezy R. Cross-sectional study on the prevalence of HPV antibodies in the general population of the Czech Republic. Sex Transm Infect. 2013;89(2):133-137. doi: 10.1136/sextrans-2012-050486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markowitz LE, Sternberg M, Dunne EF, McQuillan G, Unger ER. Seroprevalence of human papillomavirus types 6, 11, 16, and 18 in the United States: National Health and Nutrition Examination Survey 2003-2004. J Infect Dis. 2009;200(7):1059-1067. doi: 10.1086/604729 [DOI] [PubMed] [Google Scholar]
- 36.Newall AT, Brotherton JML, Quinn HE, et al. Population seroprevalence of human papillomavirus types 6, 11, 16, and 18 in men, women, and children in Australia. Clin Infect Dis. 2008;46(11):1647-1655. doi: 10.1086/587895 [DOI] [PubMed] [Google Scholar]
- 37.Abramson AL, Steinberg BM, Winkler B. Laryngeal papillomatosis: clinical, histopathologic and molecular studies. Laryngoscope. 1987;97(6):678-685. doi: 10.1288/00005537-198706000-00005 [DOI] [PubMed] [Google Scholar]
- 38.Rautava J, Willberg J, Louvanto K, et al. Prevalence, genotype distribution and persistence of human papillomavirus in oral mucosa of women: a six-year follow-up study. PLoS One. 2012;7(8):e42171. doi: 10.1371/journal.pone.0042171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szydłowski J, Jonczyk-Potoczna K, Pucher B, et al. Prevalence of human papillomavirus (HPV) in upper respiratory tract mucosa in a group of pre-school children. Ann Agric Environ Med. 2014;21(4):822-824. doi: 10.5604/12321966.1129940 [DOI] [PubMed] [Google Scholar]
- 40.Bonagura VR, Hatam LJ, Rosenthal DW, et al. Recurrent respiratory papillomatosis: a complex defect in immune responsiveness to human papillomavirus-6 and -11. APMIS. 2010;118(6-7):455-470. doi: 10.1111/j.1600-0463.2010.02617.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivancic R, Iqbal H, deSilva B, Pan Q, Matrka L. Immunological tolerance of low-risk HPV in recurrent respiratory papillomatosis. Clin Exp Immunol. 2020;199(2):131-142. doi: 10.1111/cei.13387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yiu Y, Fayson S, Smith H, Matrka L. Implementation of routine HPV vaccination in the management of recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol. 2019;128(4):309-315. doi: 10.1177/0003489418821695 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
Data Sharing Statement