Abstract
Young adults with type 1 diabetes (T1D) often have difficulty co-managing weight and glycemia. The prevalence of overweight and obesity among individuals with T1D now parallels that of the general population and contributes to dyslipidemia, insulin resistance, and risk for cardiovascular disease. There is a compelling need to develop a program of research designed to optimize two key outcomes–weight management and glycemia–and to address the underlying metabolic processes and behavioral challenges unique to people with T1D. For an intervention addressing these dual outcomes to be effective, it must be appropriate to the unique metabolic phenotype of T1D, and to biological and behavioral responses to glycemia (including hypoglycemia) that relate to weight management. The intervention must also be safe, feasible, and accepted by young adults with T1D. In 2015, we established a consortium called ACT1ON: Advancing Care for Type 1 Diabetes and Obesity Network, a transdisciplinary team of scientists at multiple institutions. The ACT1ON consortium designed a multi-phase study which, in parallel, evaluated the mechanistic aspects of the unique metabolism and energy requirements of individuals with T1D, alongside a rigorous adaptive behavioral intervention to simultaneously facilitate weight management while optimizing glycemia. This manuscript describes the design of our integrative study—comprised of an inpatient mechanistic phase and an outpatient behavioral phase—to generate metabolic, behavioral, feasibility, and acceptability data to support a future, fully powered sequential, multiple assignment, randomized trial to evaluate the best approaches to prevent and treat obesity while co-managing glycemia in people with T1D.
Clinicaltrials.gov identifiers: NCT03651622 and NCT03379792. The present study references can be found here: https://clinicaltrials.gov/ct2/show/NCT03651622 https://clinicaltrials.gov/ct2/show/NCT03379792?term=NCT03379792&draw=2&rank=1
Submission Category: “Study Design, Statistical Design, Study Protocols”.
Keywords: Glycemia, Obesity, Type 1 Diabetes, Calorimetry, Sequential Multiple Assignment Randomized Trial
1. Introduction
1.1. Obesity and Metabolic Disease Risk in Type 1 Diabetes
There has been an alarming increase in the prevalence of obesity in individuals with type 1 diabetes (T1D) in recent years. Thirty-one percent of young adults enrolled in the T1D Exchange are overweight and 15% have obesity (1).The prevalence of overweight and obesity now exceeds 50% in adults with T1D, approaching that of the general population (1-3). Obesity contributes to insulin resistance, dyslipidemia, and cardiometabolic complications in T1D (4-6). The Diabetes Control and Complications Trial demonstrated that the cardioprotective benefits of intensive insulin therapy were attenuated among study participants in the upper quartile of weight gain compared to those in the lowest quartile of weight gain (7-10).
1.2. Guidelines are Needed for Co-management of Glycemia and Adiposity in Type 1 Diabetes
Young adults with T1D face challenges with managing the competing priorities of weight and glycemia (11). Hemoglobin A1c (HbA1c) peaks at 9.3% (78 mmol/mol) between ages 15 and 18 and remains elevated above 8% (64 mmol/mol) until age 28 (1, 12). Fear of hypoglycemia, diabetes distress, and excess energy intake in response to hypoglycemia all pose T1D-specific challenges to co-management of glycemia and weight (13).
The American Diabetes Association (ADA) Standards of Medical Care make specific recommendations for weight management for adults with type 2 diabetes (T2D) and overweight or obesity, but not for people with T1D and overweight and obesity (14). Evidence is lacking on accurate determination of energy requirements in people with T1D across the spectrum of body mass index (BMI) and glycemia (15-18). In addition, there are virtually no prospective studies of obesity treatment in people with T1D on which to base recommendations (19).
Given the prevalence and impact of obesity in people with T1D, there is a compelling need to develop a research program designed to optimize weight management and glycemia while addressing underlying metabolic processes and behavioral challenges unique to people with T1D. To facilitate this, we established a transdisciplinary and integrated approach consisting of three phases, in which we: 1) addressed metabolic differences across the spectrum of weight status and 2) tested the effectiveness, safety, feasibility, and acceptability of three dietary approaches for weight management utilizing a sequential multiple assignment randomization trial (SMART) pilot design. The data generated will guide Phase 3: the design of a fully powered SMART to determine the efficacy of nutritional interventions for achieving optimal weight and glycemic targets for people with T1D.
2. Study Design and Methods
2.1. Overview
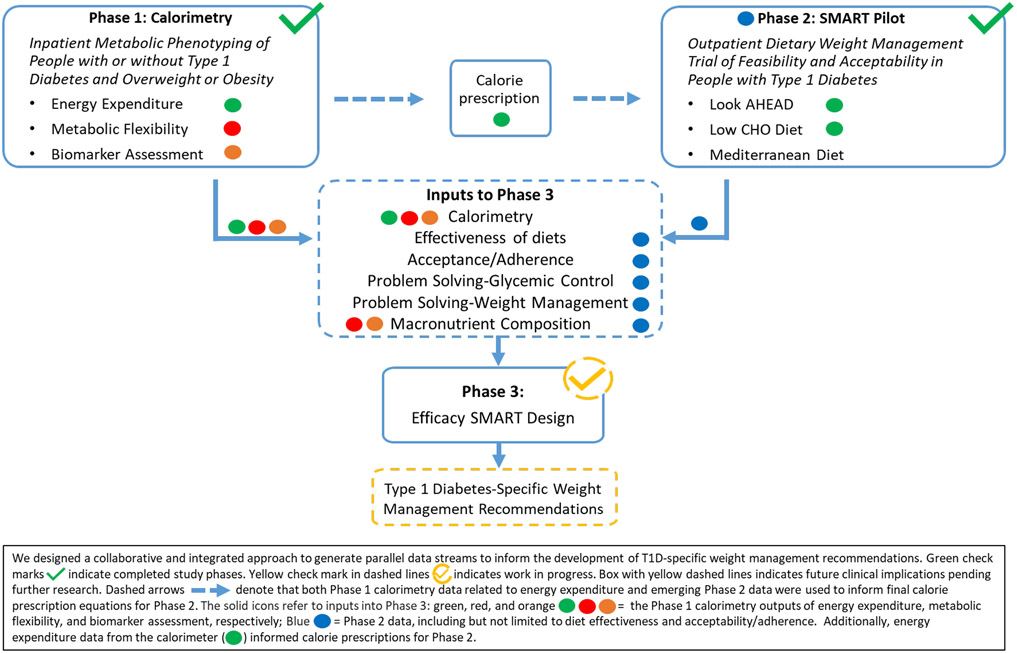
We implemented a rigorous, collaborative, multi-phase approach to generate integrated, evidence-based data for the future development of type 1 diabetes-specific weight management recommendations (Figure 1). Our transdisciplinary program involved two completed clinical trials (Phases 1 and 2) to provide key evidence for the future design (Phase 3) of a fully powered SMART. The future trial will test the efficacy of a behavioral intervention to improve weight management while optimizing glycemia, feasibility, and acceptability to enhance the likelihood of clinical effectiveness.
Figure 1.
Transdisciplinary Approach for Optimizing Weight Management and Glycemia in Young Adults with T1D
In the mechanistic Phase 1 study, we aimed to establish the rigorous scientific foundation necessary to understand metabolic parameters impacting weight management and glycemia in T1D. This study used deep metabolic phenotyping as a discovery platform to inform the design and interpretation of results from the Phase 2 behavioral study, described below. It will also inform future interventions to address the physiological defects of co-occurring obesity and T1D (13, 20, 21).
In Phase 2, we completed a pilot and feasibility SMART to identify acceptable and effective dietary strategies to optimize weight management and glycemia in young adults with T1D. The SMART design efficiently addresses practical treatment comparison questions in a clinically realistic setting and adapts dynamically to participant responses by re-randomizing those not meeting pre-specified outcome criteria to an alternate diet (22-24). We aimed to minimize adverse events (e.g., hypoglycemia). Figure 1 demonstrates how the two trials will inform Phase 3, a fully powered SMART.
The key design elements for Phases 1 and 2 are described below. For details, please see the study protocols in Supplementary Appendices 1 and 2 (NCT03651622 and NCT03379792) and CONSORT Diagrams (Supplementary Figures 1 and 2).
2.2. Core Measurements
As a matter of both rigor and reproducibility, Phases 1 and 2 included a set of core measurements (Table 1).
Table 1:
Core Measurements Common Across Studies
| Phase 1: Calorimetry |
Phase 2: SMART pilot |
|
|---|---|---|
| Primary Outcomes | ||
| HbA1c | ✓ | ✓ |
| Non-Severe Hypoglycemia (CGM) | ✓ | ✓ |
| Fat and Fat Free Mass (DXA) | ✓ | ✓ |
| BMI (height, weight) and waist circumference | ✓ | ✓ |
| Dietary Intake | ||
| Multiple 24 hour diet recalls | ✓ | ✓ |
| Food Frequency Questionnaire | ✓ | ✓ |
| Ingestive Behavior & Nutrition Knowledge | ||
| Diabetes Eating Problem Survey, Revised | ✓ | ✓ |
| Dutch Eating Behavior Questionnaire | ✓ | ✓ |
| General Impulsivity Scale | ✓ | ✓ |
| Food Craving Inventory | ✓ | ✓ |
| Weight Related Eating Questionnaire | ✓ | ✓ |
| Nutrition Knowledge Questionnaire | ✓ | ✓ |
| Physical Activity | ||
| Previous Day Physical Activity Recall | ✓ | ✓ |
| Demographics & Health History | ✓ | ✓ |
| Diabetes-Related Quality of Life | ✓ | ✓ |
| Fear of Hypoglycemia | ✓ | ✓ |
| Acceptability of Experimental Diets | ✓ | ✓ |
| Stool Collection | ✓ | ✓ |
| Plasma biobanking | ✓ | ✓ |
Abbreviations: HbA1c, hemoglobin A1c; CGM, continuous glucose monitoring; DXA, dual energy x-ray absorptiometry; BMI, body mass index.
2.2.1. Glycemia
HbA1c measured long-term glycemia. For Phase 1, participants with T1D wore an unblinded continuous glucose monitoring (CGM) system (Dexcom G4®, San Diego, CA) as a safety measure. Participants without diabetes wore the CGM for data comparison. For Phase 2, participants wore a blinded CGM (Freestyle Libre Pro, Abbott Diabetes Care Inc., CA) for 14 days following each measurement visit. We performed parallel ketone testing as needed for safety.
2.2.2. Body Composition and Weight Status
Fat and fat free mass were obtained via dual-energy x-ray absorptiometry (DXA). Outcomes included lean mass, fat mass, visceral adipose tissue, and android/gynoid fat mass ratio. Anthropometry, including height, weight, BMI, and waist circumference, followed standard procedures.
2.2.3. Dietary Intake
Two unannounced telephone 24-hour dietary recalls per data collection time point were obtained by certified interviewers from the University of North Carolina at Chapel Hill (UNC) NIH/NIDDK Nutrition Obesity Research Center (NORC) (P30DK056350) using the Nutrient Data System for Research software and the multiple pass interviewing method (25). We also assessed habitual dietary patterns using validated food frequency questionnaires (26, 27).
2.2.4. Ingestive Behavior and Nutrition Knowledge
The Diabetes Eating Problem Survey (28, 29) measured T1D-specific disordered eating. The Dutch Eating Behavior Questionnaire (30) assessed emotional eating, externality, and restrained eating behavior/cognitive restraint. The Barratt Impulsiveness Scale (31) assessed non-planning, motor impulsivity, and attention impulsivity (32). The Food Craving Inventory assessed the degree of craving for a variety of foods (33). The Nutrition Knowledge Survey, validated in people with T1D, assessed healthful eating knowledge, carbohydrate counting, blood glucose response to foods, and nutrition label reading (34).
2.2.5. Physical Activity
Physical activity was assessed with the Previous Day Physical Activity Recall (PDPAR), which was administered concurrently with the dietary recalls (35). The PDPAR queried the dominant activity and approximate intensity of each 30-minute period in the preceding day, categorized as “light,” “medium,” “hard,” or “very hard” (36, 37).
2.2.6. Other Diabetes-Specific Questionnaires
We administered the diabetes-specific quality of life (DQOL) (38) and the Fear of Hypoglycemia survey (39). The DQOL evaluated four subscales (satisfaction with oneself, impact of diabetes, diabetes worry, and social/vocational worry) which primarily measure how issues related to diabetes influence current functioning. The Fear of Hypoglycemia survey measured two subscales: behaviors to avoid hypoglycemia, and worry related to low blood sugar.
2.2.7. Demographics and Health History
Demographics and health history included age, sex (Phase 1) or gender (Phase 2), race, ethnicity, diabetes duration, highest level of education, employment status, student status, health insurance, mode and frequency of insulin delivery, concomitant medication use, diabetes self-management, medical history, and previous attempts at weight loss.
2.2.8. Exploratory Endpoints
For both phases, stool and blood samples were biobanked for assessment of the gut microbiome and circulating biomarkers, respectively.
2.3. Phase 1: The Influence of Glycemia and Obesity on Energy Balance and Metabolic Flexibility in Type 1 Diabetes
2.3.1. Overall Design
The primary objective of the Phase 1 study was to develop a comprehensive model of energy balance and metabolic flexibility in young adults with T1D, accounting for the degree of hyperglycemia, body composition, insulin use, and biological sex. The model that will be generated from the data collected in the study will address energy requirements in T1D and provide preliminary data on variability in macronutrient metabolism. This is important for designing interventions that are matched to metabolic needs in T1D and, thus, are more likely to lead to successful management of glycemia and weight.
To achieve this objective, we completed a cross-sectional study comparing the metabolic phenotype of a range of body weights in individuals with and without T1D. Briefly, participants were admitted to the Translational Research Institute Clinical Research Unit for four days for a comprehensive metabolic assessment, including measurement of body composition by DXA and whole-body magnetic resonance imaging (MRI), CGM, and 24-hour energy expenditure and substrate oxidation rates in whole room calorimeters with simultaneous measurement of 24-hour urinary glucose excretion.
2.3.2. Study Participants
This study was approved by the AdventHealth Institutional Review Board (IRB) and assessments started after participants signed informed consent. Participants were divided into three groups: those with a BMI classified as lean (BMI 18-24.9 kg/m2), overweight (BMI 25-29.9 kg/m2), and obese (BMI 30-39.9 kg/m2). The study sample consisted of 20 weight-stable young adults (8 with a BMI classified as lean, 7 with overweight, and 5 with obesity) ages 19-30 with T1D of at least 1-year duration and a range of glycemia, and nine sex- and weight-matched controls without diabetes. Participants with T1D could be on any insulin delivery method and remained on this throughout the study. The primary inclusion/exclusion criteria for both Phases 1 and 2 are in Table 2. A detailed list can be found in Supplementary Table 1.
Table 2:
Phase 1 and Phase 2 Inclusion and Exclusion Criteria for Participants with T1D.
| Phase 1: Calorimetry* |
Phase 2: SMART pilot |
|
|---|---|---|
| Inclusion criteria | ||
| Type 1 diabetes diagnosed ≥1 year ago | ✓ | ✓ |
| Age | 19 to 30 | 19 to 30 |
| Sex (Phase 1), Gender (Phase 2)** | Males and females | Any gender identity |
| HbA1c | 6.5-13% (48-113 mmol/mol) | <13% (113 mmol/mol) |
| BMI (kg/m2) | 18-39.9 | 27.0-39.9 |
| Exclusion criteria | ||
| Diagnosed eating disorder | ✓ | ✓ |
| Pregnant/lactating women | ✓ | ✓ |
| GI and bowel disorders | ✓ | ✓ |
| Dietary restrictions | ✓ | ✓ |
| DKA or severe hypoglycemia in the past 6 months | N/A | ✓ |
| Weight Instability*** | ✓ | ✓ |
Please see Supplementary Table 1 for complete list of inclusion and exclusion criteria, including for healthy control participants, for Phase 1
Potential responses for gender in Phase 2 included Male, Female, Other, or Prefer not to answer, but all recruited participants identified as either male or female.
± > 10 lb weight loss over past 6 months for Phase 2
Abbreviations: HbA1c: hemoglobin A1c; BMI: body mass index; IBD: inflammatory bowel disease; GI: gastrointestinal; DKA: diabetic ketoacidosis
2.3.3. Procedures
2.3.3.1. Physical Exam
A standard physical examination was conducted by a study physician, physician assistant, or nurse practitioner included measurement of heart rate (HR), blood pressure (BP), respirations, and temperature.
2.3.3.2. Screening Labs
Study eligibility and overall health status were assessed through complete blood count, comprehensive metabolic panel, HbA1c, lipid panel, thyroid function tests (thyroid stimulating hormone), urinalysis, urine drug test, and urine pregnancy test.
2.3.3.3. Inpatient Diet Procedures
Participants received a weight-maintaining diet of 50% of calories as carbohydrate, 35% as fat, and 15% as protein. Given that quantifying energy balance was a primary goal of the study, all meals were observed by TRI staff. Participants were asked to consume 100% of meals and compliance was tracked. In the calorimeter, macronutrient comparable units were provided or removed to achieve energy balance.
2.3.3.4. Whole Room Calorimetry
Energy expenditure and substrate oxidation (respiratory quotient [RQ] and grams of fat, carbohydrate, and protein oxidized) were calculated from O2 consumption, CO2 production, and 24-hour urinary nitrogen excretion using standard equations based on our published methods (40). Calibration, calorimetry routine, and conditions during the stay were based on published standards of calorimetry (41). Participants consumed a diet to achieve energy balance. All urine was collected for measurement of total nitrogen, creatinine, and glucose.
2.3.3.5. Activity Monitoring
Physical activity was quantified with a small, portable accelerometer (ActiGraph wGT3X+, ActiGraph, LLC, Pensacola, FL, USA) that was worn for up to seven days with the goal to collect at least four days of data to assess habitual activity. Overall physical activity levels, daily changes, amount of time spent in sedentary, moderate, vigorous intensity categories, sleep quality, and activity-associated energy expenditures were extracted.
2.3.3.6. Heart Rate Monitoring
A chest heart rate (HR) sensor and watch (Polar A300 model: wrist-worn HR monitor paired with the H7 HR Chest Strap) were worn by participants while in the calorimeter to continuously monitor HR. The watch recorded HR, time worn, steps, and energy expenditure.
2.3.3.7. Exploratory Endpoints
Given that ectopic fat deposition is associated with obesity, but not well studied in T1D, we did an exploratory evaluation of hepatic lipids with FibroScan® and MRI and spectroscopy. In addition, volumetric measurements of fat, muscle, organ, and bone were completed across the whole body with MRI.
2.3.3.8. Statistical Analysis Plan
This study Is the first, to our knowledge, to deeply phenotype people with and without T1D across a range of BMI classifications. Therefore, it was funded and designed as a pilot study to generate effect sizes needed to power future trials. Despite this, we powered the study based on our existing calorimetry data. a total sample size of 33, which allowed a power of 80% to detect a correlation of energy expenditure with glycemia of 0.4 using a type 1 error of 0.1. Since this was a pilot study, this should be sufficient power for the stated modeling and estimation goals. Similar to the work we have done previously (42, 43), we will use the calorimetry data generated from this trial to determine the 24-hour energy cost of maintaining a specific level of glycemia and body weight, body composition and activity, using exponential modeling similar to that reported by Hall et al (42). This will involve fitting a multivariate regression model, correcting for food intake, urinary glucose excretion, and energy expenditure while in the calorimeter; accounting for body composition, glycemia based on CGM while in the chamber, HbA1c, age, sex, insulin dosing, and diabetes duration; and solving for the missing energy variable. Energy modeling using variables available in the Phase 2 SMART pilot, including hyperglycemia and body composition, will also be derived to inform calorie prescriptions for a fully powered SMART in development. For research questions related to RQ, substrate utilization, and metabolic flexibility, regression modeling will be done to determine differences between T1D and controls accounting for the matched design as well as age, gender, and other important covariates.
2.4. Phase 2: SMART Pilot Dietary Intervention Study
2.4.1. Overall Design
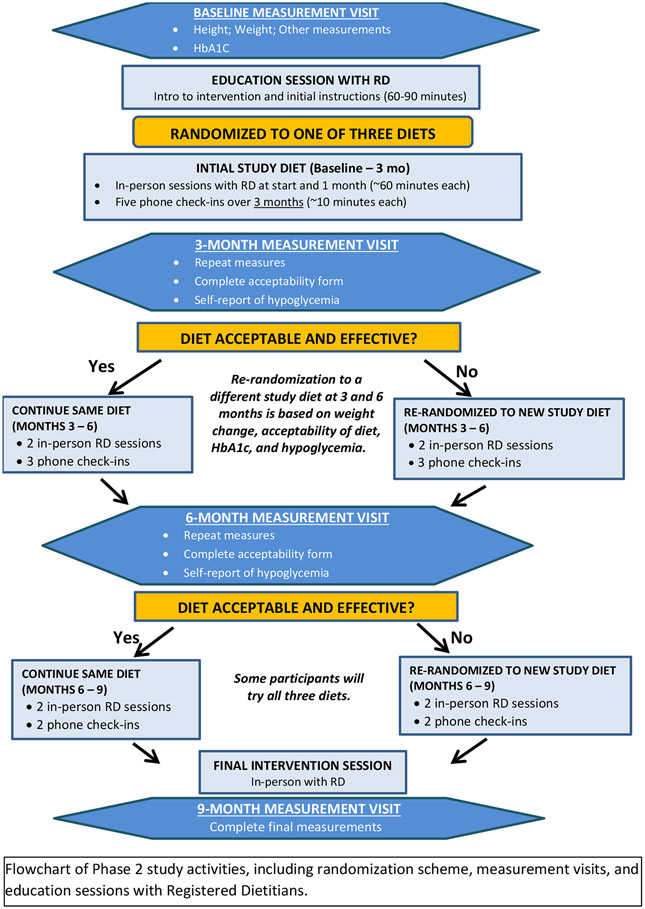
This pilot and feasibility study was conducted at UNC and Stanford used a SMART design to identify acceptable dietary strategies to optimize both weight management and glycemia among young adults with T1D. Participants were randomized according to a sequential randomization scheme following the first three measurement visits (Figure 2). A total follow-up time of approximately 10.5 months allowed for evaluation of the effect of diets on initial weight loss and its early maintenance.
Figure 2.
Flowchart of Measurement Visits, Randomization, and Education Sessions
The co-primary outcomes were weight change and glycemia (HbA1c and hypoglycemia). Secondary outcomes included body composition, assessed via a full body DXA scan, and glycemic variability, assessed via two weeks of CGM wear.
2.4.2. Study Participants
This study was approved by the UNC and Stanford IRBs and study participation did not begin until participants signed informed consent. Utilizing a two-step recruitment approach (44), the study enrolled 68 young adult males and females at UNC (57.4%) and Stanford, aged 19-30, with a T1D duration of at least one year. At screening, individuals must have had an HbA1c <13% (119 mmol/mol) and a BMI of 27-39.9 (Table 2).
2.4.3. Procedures
2.4.3.1. Experimental Diets
Behavioral counseling strategies, use of carbohydrate counting for insulin dosing, and encouragement of usual physical activity were consistent across diets. Phase 1 calorimetry data informed calorie prescriptions for the hypocaloric Look AHEAD (i.e., low fat) and Low Carbohydrate diets. Based on clinical judgment, we adjusted calorie prescriptions based on procedures from the Look AHEAD study (45). Diets were as follows:
Diet 1: Look AHEAD (hypocaloric):
The Look AHEAD “intensive lifestyle intervention” demonstrated prior efficacy for weight loss and HbA1c improvement among individuals with T2D (46). Per Look AHEAD study procedures, the energy goal for individuals who weighed <114 kg (250 lbs) was 1200-1500 kcal/day and 1500-1800 kcal/day for individuals who weighed ≥114 kg. The caloric distribution was <30% fat, <10% saturated fat, and ≥15% protein (47).
Diet 2: Low Carbohydrate (hypocaloric):
A hypocaloric Low Carbohydrate (14%) diet promoted weight loss, and improved glycemic control and variability and CVD risk factors among individuals with T2D (48). Therefore, we implemented a Low Carbohydrate diet with 15-20% of calories from carbohydrate (45-75 grams/day), and <10% of fat from saturated fat (48). Individualized calorie goals were established as noted for Diet 1.
Diet 3: Healthy (Mediterranean) Dietary Pattern (not calorically restricted):
The PREDIMED diet, supported by current ADA guidelines (49), employed Mediterranean diets without caloric restriction and resulted in reduced weight and waist circumference among people with T2D (50). Diet 3 used a Mediterranean diet approach consistent with PREDIMED dietary guidance, which included: (1) abundant use of olive oil; (2) increased consumption of fruits, vegetables, legumes and fish; (3) reduction in total meat and red or processed meat consumption; (4) preparation of home-made sauce to dress vegetables, pasta, rice and other dishes; (5) avoidance of butter, cream, fast food, sweets, pastries and sugar-sweetened beverages; and (6) in alcohol drinkers, moderate consumption of red wine (50).
2.4.3.2. Behavioral Framework for Dietary Interventions
We deployed the behavioral framework established by our FLEX study team in T1D (51) and refined it for the adaptive SMART intervention (UC4DK101132, MPI’s Mayer-Davis, Maahs, and Seid). The FLEX intervention was found to have beneficial impact on motivation, problem-solving, facets of diabetes self-management, quality of life, and the cardiovascular risk factors of blood pressure and total cholesterol (52). Furthermore, retention at 18-month study completion was 93%, which is remarkably high for a behavioral intervention (a 2005 report from the NIH defined ‘successful’ retention as ≥ 84%) (53). Applying established theories of health behavior (54, 55) and social and health psychology (56, 57), we integrated motivational interviewing (MI) and problem-solving skills training (PSST) approaches into a coherent intervention and supplemented with tools tailored to overcome specific barriers to adherence (58).
Shown in Supplementary Figure 3, the intervention was conducted by Registered Dietitians (RDs) in 23 sessions over nine months. This consisted of eight longer counseling and education sessions, and 10 shorter phone “check-in” sessions. Longer sessions provided ongoing MI and PSST related to dietary adherence and glycemia specific to diet assignment. Phone check-in sessions focused on goal attainment and problem-solving strategies. This framework is similar to the ADA guidelines for intensive lifestyle counseling for weight management (59).
RD sessions were documented including audio-recordings to assess fidelity of intervention delivery. RDs communicated with the study endocrinologist to address self-reported hypoglycemia, assessed by RDs at each encounter.
2.4.3.3. At Home Monitoring
Participants used the MyFitnessPal smartphone app (San Francisco, CA) to track food consumption and were provided with a BodyTrace© scale (Palo Alto, CA) to measure daily home weights. Food and weight tracking were not required, but were encouraged to promote dietary adherence, and based on evidence that daily self-weighing may facilitate weight management (60).
2.4.3.4. Sequential randomization (baseline, ~3 months, and ~6 months)
An advantage of the SMART design was the use of a precise diet strategy to adapt treatment to the evolving health status of each participant. Following the baseline, 3-, and 6-month data collection and a 2-week run-in, those for whom the assigned diet was not acceptable or not effective based on a priori decision rules were re-randomized to an alternate diet. The decision criteria for re-randomization (Table 3) incorporated clinical outcomes (weight change and glycemia) and self-reported diet acceptability. Calorie prescriptions for hypocaloric diets were re-calculated at each measurement visit to incorporate current body weight. If weight classified as “normal weight” (BMI <25) was achieved, then weight change was not considered for re-randomization. For these individuals, RDs advised participants regarding weight maintenance for their assigned diet. Participants for whom the diet was effective and acceptable continued their original diet assignment. At the end of the study, each participant tried one, two, or all three diets.
Table 3.
A Priori Re-Randomization Criteria
| Time after initial randomization |
Diet acceptability | Weight change | Glycemia change |
|---|---|---|---|
| ~3 and 6 months | Unacceptable based on Diet Acceptability Form | Not achieving at least 2% loss in body weight from previous measurement visit. | HbA1c increased ≥ 0.5% or self-report of increased and problematic hypoglycemia on Diet Acceptability Form. |
Re-assignment of diet occurred if one or more criteria were met.
Permuted block randomization of block size 24 was used to assign all 12 potential treatment regimens stratified by site (R statistical software). These were confidentially generated by study statisticians and revealed only at the point of diet assignment. Participants who were re-randomized at measurement visit 2 would have been able to anticipate their third diet if they met re-randomization criteria again at measurement visit 3; however, all other participants would not.
2.4.4. Feasibility and Acceptability of Experimental Diets
Pragmatic feasibility metrics for this pilot trial include those related to study recruitment and retention, qualitative assessment of feasibility of intervention delivery according to interventionist notes and participant Exit Interviews, intervention fidelity, and effect size estimation to inform the development of a fully-powered trial.
An appropriate instrument with known psychometric properties to systematically assess acceptability of specific diets could not be identified in the literature. Therefore, we developed and piloted a simple assessment for this purpose (61) and evaluated psychometric properties. The questionnaire showed excellent internal consistency throughout the diet intervention period (Cronbach’s alpha 0.89 at weeks 4 and 6) and was therefore deemed appropriate for use in Phase 2. We additionally captured open-ended participant feedback as part of an Exit Interview and in detailed notes taken by dietitian-interventionists at each study visit, both of which will be used to qualitatively assess diet acceptability as a complement to our quantitative measure.
2.4.5. COVID-19 Accommodations and Changes to Core Measures
As of April 27, 2020, in continued response to COVID-19, Phase 2 moved to a virtual format via a HIPAA-secure Zoom account, including dietary counseling and data collection; and recruitment ceased. The first virtual visit during the pandemic was conducted on June 17th, 2020. HbA1c samples were collected at home (BIO-RAD Hemoglobin Capillary Collection System for HbA1c Testing) and mailed to the Diabetes Diagnostic Lab at the University of Missouri, Columbia. Participants inserted CGM sensors at home. Weight was obtained utilizing the BodyTrace scale. Participants measured waist circumference using a study-provided flexible tape measure. Blood was not biobanked and DXA was not performed.
2.4.6. Exploratory Endpoints
Given the key role of physical activity in management of weight and glycemia (62), we piloted a voluntary assessment of physical activity with the Garmin vívosmart® 4 (Garmin Ltd., Kansas, USA) concurrent with the 14-day CGM. Additionally, participants provided a rate of perceived exertion for each bout of structured exercise.
2.4.7. Statistical analysis plan
As a pilot study, Phase 2 had sufficient data to investigate the SMART design and associated analyses, but insufficient power to provide conclusive evidence about relative intervention effectiveness. The primary comparison of treatments is the 3-month response to the three initial dietary assignments. Standard statistical procedures (t-tests and ANOVA) will be used to compare differences in weight change and glycemia (HbA1c and percent time in hypoglycemia).
Phase 2 data will be used to estimate sample size to power the primary outcomes of weight and glycemia and the design of the SMART for the next fully powered study. Key variables such as the proportion of responders to the diets will be used to ensure sufficient sample size for comparison of diet sequences. Because standard sample size formulas do not apply to the estimation of optimal personalized treatment strategies, we will rely on data from Phase 2 to obtain the needed sample size for estimation of optimal diet strategy in a fully powered SMART using a bootstrap based methodology (63) and other recent advancements (64). The sample size estimation procedure will ensure the following: 1) sufficient power for comparison of the outcome under the estimated treatment strategy against a fixed baseline treatment strategy; and 2) a high probability that the expected outcome of the estimated treatment strategy is near optimal.
Hypothesis-generating analyses based on the Phase 2 data will include estimation of deeply tailored diet strategies based on each participant’s health status and with a set of targeted outcomes in consideration (e.g., using Q-learning) (65, 66). This estimated optimal diet strategy maximizes weight loss while improving suboptimal or maintaining good glycemia and can help generate hypotheses about how to choose and adapt diets for each participant.
Discussion
We present a methodologically robust, integrated, transdisciplinary, multi-phase study design to generate evidence on metabolic, behavioral, feasibility and acceptability considerations in dietary approaches for co-management of weight and glycemia in individuals with T1D. The ACT1ON consortium is comprised of experts in the metabolic and psychosocial drivers of obesity, as well as statistical modeling approaches, thereby allowing for the systematic assessment of these drivers.
Study Strengths and Lessons Learned
The model of energy balance and metabolic flexibility from Phase 1 will be developed with data from whole room calorimetry (24-hour energy expenditure, substrate oxidation and metabolic flexibility), DXA, whole-body MRI, CGM, laboratory data, and participant demographics, among others. The unique expertise and specialized equipment required to build this model is a strength of our study design because it can reveal how the unique energy balance and metabolic flexibility parameters in people with T1D impact weight management and glycemia.
In Phase 2, we implemented three evidence-based diets and evaluated diet acceptability in the randomization process. The multi-site, prospective, randomized, and adaptive nature of our study further support the rigor of the pilot. The multidisciplinary team we assembled to design and implement a series of studies that address a spectrum of gaps in the field, from mechanistic to real-world challenges, is a model that we believe is essential for addressing a physiologically complex problem like obesity that is modulated by environmental, psychosocial, and biological factors.
Limitations and Generalizability
Although these pilot data will guide the design of a fully powered SMART trial, there are several limitations. The first is that by design, as pilot trials, Phases 1 and 2 were not powered for all analyses. These pilot and feasibility studies were tailored to young adults aged 19-30, which may limit generalizability to youth and older adults with T1D. An inherent limitation of self-reported diet and physical activity is the potential for reporting bias; however, 24-hour recalls obtained by trained interviewers are the gold-standard for self-reported dietary intake data and have been validated against doubly labeled water for assessing energy requirements (67, 68). An unforeseeable challenge was shifting Phase 2 into a virtual format due to COVID-19, which led to changes in measurements of primary outcomes, discontinuation of DXA and whole blood collection, and reduced sample size.
Future Directions and Potential Adaptations
Phase 3 will incorporate findings from the completed Phases 1 and 2 in the design of a fully powered SMART of behavioral interventions that are acceptable and feasible to optimize weight and glycemic management in young adults with T1D. Our consortium plans to conduct a separate feasibility trial of a behavioral intervention to safely increase physical activity among young adults with T1D and overweight or obesity in the context of a physical activity pilot trial will also inform a larger, fully powered SMART. Collectively, the completed and planned intervention approaches to be tested in larger, fully powered adaptive clinical trials have the potential to improve clinical practice guidelines by providing updated and tailored strategies for safely co-managing glycemia and weight in young adults with T1D.
Supplementary Material
Acknowledgements
We offer our sincerest gratitude to our study participants without whom this research would not have been possible. We also thank the UNC, Stanford, and TRI clinical operations teams, administration, and core facilities for impeccable execution of this study.
Funding
Research reported in this publication was supported by National Institute for Diabetes, Digestive and Kidney Diseases of the National Institutes of Health under award numbers 1DP3DK113358 and 1P30DK116074-01.
References
- 1.Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38(6):971–8. [DOI] [PubMed] [Google Scholar]
- 2.Maahs DM, Ogden LG, Dabelea D, Snell-Bergeon JK, Daniels SR, Hamman RF, et al. Association of glycaemia with lipids in adults with type 1 diabetes: modification by dyslipidaemia medication. Diabetologia. 2010;53(12):2518–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chillarón J, Benaiges D, Mañé L, Pedro-Botet J, Flores Le-Roux J. Obesity and type 1 diabetes mellitus management. Minerva Endocrinology. 2015;40(1):53–60. [PubMed] [Google Scholar]
- 4.Purnell JQ, Hokanson JE, Marcovina SM, Steffes MW, Cleary PA, Brunzell JD. Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT. Diabetes Control and Complications Trial. JAMA. 1998;280(2):140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Ferranti SD, De Boer IH, Fonseca V, Fox CS, Golden SH, Lavie CJ, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Circulation. 2014;130(13):1110–30. [DOI] [PubMed] [Google Scholar]
- 6.Maahs DM, Daniels SR, De Ferranti SD, Dichek HL, Flynn J, Goldstein BI, et al. Cardiovascular disease risk factors in youth with diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2014;130(17):1532–58. [DOI] [PubMed] [Google Scholar]
- 7.Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. NEJM. 1993;329(14):977–86. [DOI] [PubMed] [Google Scholar]
- 8.Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions Complications Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. NEJM. 2005;353(25):2643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purnell JQ, Zinman B, Brunzell JD. The effect of excess weight gain with intensive diabetes mellitus treatment on cardiovascular disease risk factors and atherosclerosis in type 1 diabetes mellitus: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study (DCCT/EDIC) study. Circulation. 2013;127(2):180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Purnell JQ, Braffett BH, Zinman B, Gubitosi-Klug RA, Sivitz W, Bantle JP, et al. Impact of Excessive Weight Gain on Cardiovascular Outcomes in Type 1 Diabetes: Results From the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes Care. 2017;40(12):1756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Addala A, Igudesman D, Kahkoska AR, Muntis FR, Souris KJ, Whitaker KJ, et al. The interplay of type 1 diabetes and weight management: A qualitative study exploring thematic progression from adolescence to young adulthood. Pediatric Diabetes. 2019;20(7):974–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technology & Therapeutics. 2019;21(2):66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderbro T, Amsberg S, Adamson U, Bolinder J, Lins PE, Wredling R, et al. Fear of hypoglycaemia in adults with Type 1 diabetes. Diabetic Medicine. 2010;27(10):1151–8. [DOI] [PubMed] [Google Scholar]
- 14.Association AD. 5. Facilitating Behavior Change and Well-being to Improve Health Outcomes: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(Supplement 1):S53–S72. [DOI] [PubMed] [Google Scholar]
- 15.Nair KS, Halliday D, Garrow JS. Increased energy expenditure in poorly controlled Type 1 (insulin-dependent) diabetic patients. Diabetologia. 1984;27(1):13–6. [DOI] [PubMed] [Google Scholar]
- 16.Jacob A, Salinas K, Adams-Huet B, Raskin P. Potential causes of weight gain in type 1 diabetes mellitus. Diabetes, Obesity and Metabolism. 2006;8(4):404–11. [DOI] [PubMed] [Google Scholar]
- 17.Greco AV, Tataranni PA, Mingrone G, De Gaetano A, Manto A, Cotroneo P, et al. Daily energy metabolism in patients with type 1 diabetes mellitus. Journal of the American College of Nutrition. 1995;14(3):286–91. [DOI] [PubMed] [Google Scholar]
- 18.Rigalleau V, Lasseur C, Pécheur S, Chauveau P, Combe C, Perlemoine C, et al. Resting energy expenditure in uremic, diabetic, and uremic diabetic subjects. Journal of Diabetes and its Complications. 2004;18(4):237–41. [DOI] [PubMed] [Google Scholar]
- 19.Corbin KD, Driscoll KA, Pratley RE, Smith SR, Maahs DM, Mayer-Davis EJ, et al. Obesity in type 1 diabetes: pathophysiology, clinical impact, and mechanisms. Endocrine Reviews. 2018;39(5):629–63. [DOI] [PubMed] [Google Scholar]
- 20.Treasure J, Kan C, Stephenson L, Warren E, Smith E, Heller S, et al. Developing a theoretical maintenance model for disordered eating in Type 1 diabetes. Diabetic Medicine. 2015;32(12):1541–5. [DOI] [PubMed] [Google Scholar]
- 21.Driscoll KA, Corbin KD, Maahs DM, Pratley R, Bishop FK, Kahkoska A, et al. Biopsychosocial aspects of weight management in type 1 diabetes: a review and next steps. Current diabetes reports. 2017;17(8):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kidwell KM. SMART designs in cancer research: past, present, and future. Clinical Trials. 2014;11(4):445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavori PW, Dawson R. Dynamic treatment regimes: practical design considerations. Clinical Trials. 2004;1(1):9–20. [DOI] [PubMed] [Google Scholar]
- 24.Murphy SA. An experimental design for the development of adaptive treatment strategies. Statistics in Medicine 2005;24(10):1455–81. [DOI] [PubMed] [Google Scholar]
- 25.Beaton GH, Milner J, Corey P, McGuire V, Cousins M, Stewart E, et al. Sources of variance in 24-hour dietary recall data: implications for nutrition study design and interpretation. Am J Clin Nutr. 1979;32(12):2546–59. [DOI] [PubMed] [Google Scholar]
- 26.National Institutes of Health. Epidemiology and Genomics Research Program. National Cancer Institute. Diet History Questionnaire, Version 2.0 2010. [Google Scholar]
- 27.Epidemiology and Genomics Research Program. National Cancer Institute. The Dietary Screener Questionnaire (DSQ) in the NHANES 2009-10: Data Processing and Scoring Procedure for Earlier methods. 2019. [Google Scholar]
- 28.Markowitz JT, Butler DA, Volkening LK, Antisdel JE, Anderson BJ, Laffel LM. Brief screening tool for disordered eating in diabetes: internal consistency and external validity in a contemporary sample of pediatric patients with type 1 diabetes. Diabetes Care. 2010;33(3):495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Posner BM, Smigelski C, Duggal A, Morgan J, Cobb J. Validation of two-dimensional models for estimation of portion size in nutrition research. Journal of the American Dietetic Association. 1992;92(6):738–41. [PubMed] [Google Scholar]
- 30.Van Strien T, Frijters JE, Bergers GP, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International Journal of Eating Disorders. 1986;5(2):295–315. [Google Scholar]
- 31.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51(6):768–74. [DOI] [PubMed] [Google Scholar]
- 32.Spinella M Normative data and a short form of the Barratt Impulsiveness Scale. International Journal of Neuroscience. 2007;117(3):359–68. [DOI] [PubMed] [Google Scholar]
- 33.White MA, Whisenhunt BL, Williamson DA, Greenway FL, Netemeyer RG. Development and validation of the food-craving inventory. Obesity research. 2002;10(2):107–14. [DOI] [PubMed] [Google Scholar]
- 34.Rovner AJ, Nansel TR, Mehta SN, Higgins LA, Haynie DL, Laffel LM. Development and validation of the type 1 diabetes nutrition knowledge survey. Diabetes Care. 2012;35(8):1643–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maahs DM, Mayer-Davis E, Bishop FK, Wang L, Mangan M, McMurray RG. Outpatient assessment of determinants of glucose excursions in adolescents with type 1 diabetes: proof of concept. Diabetes Technology & Therapeutics. 2012;14(8):658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keadle SK, Lyden K, Hickey A, Ray EL, Fowke JH, Freedson PS, et al. Validation of a previous day recall for measuring the location and purpose of active and sedentary behaviors compared to direct observation. International Journal of Behavioral Nutrition and Physical Activity. 2014;11(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark BK, Pavey TG, Lim RF, Gomersall SR, Brown WJ. Past-day recall of sedentary time: validity of a self-reported measure of sedentary time in a university population. Journal of Science and Medicine in Sport. 2016;19(3):237–41. [DOI] [PubMed] [Google Scholar]
- 38.Reliability and validity of a diabetes quality-of-life measure for the diabetes control and complications trial (DCCT). The DCCT Research Group. Diabetes Care. 1988;11(9):725–32. [DOI] [PubMed] [Google Scholar]
- 39.Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care. 1987;10(5):617–21. [DOI] [PubMed] [Google Scholar]
- 40.Corbin KD, Krajmalnik-Brown R, Carnero EA, Bock C, Emerson R, Rittmann BE, et al. Integrative and quantitative bioenergetics: Design of a study to assess the impact of the gut microbiome on host energy balance. Contemp Clin Trials Commun. 2020;19:100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen KY, Smith SR, Ravussin E, Krakoff J, Tanaka S, Murgatroyd P, et al. Room Indirect Calorimetry Operating and Reporting Standards (RICORS 1.0). Obesity (Silver Spring, Md). 2020;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hall KD, Chen KY, Guo J, Lam YY, Leibel RL, Mayer LE, et al. Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr. 2016;104(2):324–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whytock KL, Carnero EA, Vega RB, Tillner J, Bock C, Chivukula K, et al. Prolonged Glucagon Infusion Does Not Affect Energy Expenditure in Individuals with Overweight/Obesity: A Randomized Trial. Obesity (Silver Spring, Md). 2021;29(6):1003–13. [DOI] [PubMed] [Google Scholar]
- 44.Standiford DA, Morwessel N, Bishop FK, Thomas JM, Smith E, Crandell J, et al. Two-step recruitment process optimizes retention in FLEX clinical trial. Contemporary clinical trials communications. 2018;12:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Look AHEAD Research Group. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14(5):737–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.American Diabetes Association. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Look AHEAD Research Group. Long term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes: four year results of the Look AHEAD trial. Archives of Internal Medicine. 2010;170(17):1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tay J, Luscombe-Marsh ND, Thompson CH, Noakes M, Buckley JD, Wittert GA, et al. Comparison of low-and high-carbohydrate diets for type 2 diabetes management: a randomized trial. The American Journal of Clinical Nutrition. 2015;102(4):780–90. [DOI] [PubMed] [Google Scholar]
- 49.Evert AB, Boucher JL, Cypress M, Dunbar SA, Franz MJ, Mayer-Davis EJ, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2013;36(11):3821–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lasa A, Miranda J, Bulló M, Casas R, Salas-Salvadó J, Larretxi I, et al. Comparative effect of two Mediterranean diets versus a low-fat diet on glycaemic control in individuals with type 2 diabetes. European Journal of Clinical Nutrition. 2014;68(7):767–72. [DOI] [PubMed] [Google Scholar]
- 51.Mayer-Davis E, Seid M, Crandell J, Dolan L, Lagarde W, Letourneau L, et al. Flexible Lifestyles for Youth (FL 3X) behavioural intervention for at-risk adolescents with Type 1 diabetes: a randomized pilot and feasibility trial. Diabetic Medicine. 2015;32(6):829–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayer-Davis EJ, Maahs DM, Seid M, Crandell J, Bishop FK, Driscoll KA, et al. Efficacy of the Flexible Lifestyles Empowering Change intervention on metabolic and psychosocial outcomes in adolescents with type 1 diabetes (FLEX): a randomised controlled trial. The Lancet Child & Adolescent Health. 2018;2(9):635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coday M, Boutin-Foster C, Sher TG, Tennant J, Greaney ML, Saunders SD, et al. Strategies for retaining study participants in behavioral intervention trials: retention experiences of the NIH Behavior Change Consortium. Annals of Behavioral Medicine. 2005;29(2):55–65. [DOI] [PubMed] [Google Scholar]
- 54.Wade S, Weil C, Holden G, Mitchell H, Evans R III, Kruszon-Moran D, et al. Psychosocial characteristics of inner-city children with asthma: a description of the NCICAS psychosocial protocol. National Cooperative Inner-City Asthma Study. Pediatric Pulmonology. 1997;24(4):263–76. [DOI] [PubMed] [Google Scholar]
- 55.Wigal JK, Stout C, Brandon M, Winder JA, McConnaughy K, Creer TL, et al. The Knowledge, Attitude, and Self-Efficacy Asthma Questionnaire. Chest. 1993;104(4):1144–8. [DOI] [PubMed] [Google Scholar]
- 56.Leroyer C, Lebrun T, Proust A, Lenne X, Lucas E, Rio G, et al. Knowledge, self-management, compliance and quality of life in asthma: a cross-sectional study of the French version of the Asthma Quality of Life Questionnaire. Quality of Life Research. 1998;7(3):267–72. [DOI] [PubMed] [Google Scholar]
- 57.Hilton S, Sibbald B, Anderson HR, Freeling P. Controlled evaluation of the effects of patient education on asthma morbidity in general practice. Lancet. 1986;1(8471):26–9. [DOI] [PubMed] [Google Scholar]
- 58.Kichler JC, Seid M, Crandell J, Maahs DM, Bishop FK, Driscoll KA, et al. The Flexible Lifestyle Empowering Change (FLEX) intervention for self-management in adolescents with type 1 diabetes: Trial design and baseline characteristics. Contemporary Clinical Trials. 2018;66:64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Obesity Management for the Treatment of Type 2 Diabetes. Diabetes Care. 2016;39(Supplement 1):S47–S51. [DOI] [PubMed] [Google Scholar]
- 60.Pacanowski CR, Bertz FC, Levitsky DA. Daily Self-Weighing to Control Body Weight in Adults: A Critical Review of the Literature. SAGE open. 2014;4(4):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.MacIntosh B, Ramsden C, Honvoh G, Faurot K, Palsson O, Johnston A, et al. Diet intervention methodology for investigating modifications in omega-3 and omega-6 fatty acids for ameliorating chronic migraine pain. Clinical Nutrition. 2020. [Google Scholar]
- 62.Riddell MC, Gallen IW, Smart CE, Taplin CE, Adolfsson P, Lumb AN, et al. Exercise management in type 1 diabetes: a consensus statement. The lancet Diabetes & endocrinology. 2017;5(5):377–90. [DOI] [PubMed] [Google Scholar]
- 63.Rose EJ, Laber EB, Davidian M, Tsiatis AA, Zhao Y-Q, Kosorok MR. Sample size calculations for SMARTs. arXiv preprint arXiv:190606646. 2019. [Google Scholar]
- 64.Laber EB, Zhao YQ, Regh T, Davidian M, Tsiatis A, Stanford JB, et al. Using pilot data to size a two-arm randomized trial to find a nearly optimal personalized treatment strategy. Statistics in Medicine. 2016;35(8):1245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schulte PJ, Tsiatis AA, Laber EB, Davidian M. Q- and A-learning Methods for Estimating Optimal Dynamic Treatment Regimes. Statistical Science: a Review Journal of the Institute of Mathematical Statistics. 2014;29(4):640–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watkins CJCH, Dayan P. Q-learning. Machine Learning. 1992;8(3):279–92. [Google Scholar]
- 67.Johnson RK, Driscoll P, Goran MI. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. Journal of the American Dietetic Association. 1996;96(11):1140–4. [DOI] [PubMed] [Google Scholar]
- 68.Tran KM, Johnson RK, Soultanakis RP, Matthews DE. In-person vs telephone-administered multiple-pass 24-hour recalls in women: validation with doubly labeled water. Journal of the American Dietetic Association. 2000;100(7):777–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.