Abstract
Published in Cancer Research in 2014, Zhu and colleagues achieved a mechanistic leap in our understanding of cancer-associated macrophage biology with their proof-of-concept study showing that macrophage-specific targeting, via blocking colony-stimulating factor–1 (CSF1) signaling through its cognate receptor CSF1R, synergized with checkpoint immunotherapy to enhance antitumor immunity in mouse models of pancreatic cancer. Here, we reflect on the critical set of observations presented in this study and how the study’s findings fueled the subsequent efforts to translate CSF1/1R-specific and other tumor-associated macrophage modulating therapies into the clinic.
As one of the deadliest cancers, pancreatic ductal adenocarcinomas (PDAC) are extremely difficult to treat, and standard-of-care modalities fail to achieve the same level of efficacy observed for other cancer types. To date, two multi-agent cytotoxic chemotherapy regimens remain the standard of care.
Between 2000 and 2014, the first immune checkpoint agents moved from preclinical development to rapid FDA approval based on unprecedented clinical response rates in patients with deadly metastatic cancers. These agents were the first of many to activate and enhance T-cell activity in tumors. While T-cell–focused immunotherapies have offered some promise in converting the stereotypically “cold” T cell excluding PDAC tumor microenvironments (TME) into more T-cell–permissive ones, other TME barriers have significantly dampened their success. Indeed, accumulating evidence has taught us that the TME surrounding PDACs is complex, with more than one barrier to successful antitumor immune responses including the desmoplastic stroma and the presence of immunosuppressive myeloid cells.
As early as the 1980s, studies showed that the presence of tumor-associated macrophages (TAM) within the microenvironment are a negative prognosticator, implicating their importance in providing protumor signals that dampen antitumor immune responses. Subsequent studies in the early 2010s confirmed poorer cancer prognoses associated with TAMs, including pancreatic cancers (1, 2). These studies revealed that myeloid cells are recruited early in pancreatic carcinogenesis, facilitating the development of the protumor microenvironment. As a result, a critical question that remains under active investigation is whether TAMs are a necessary immunosuppressive component required for tumors to thrive, and if so, can this function be disrupted to encourage antitumor immunity?
To begin to address this critical question, Dr. David DeNardo’s team sought to elucidate the mechanisms of macrophage infiltration into PDAC by interrogating a macrophage recruiting pathway, colony-stimulating factor–1 (CSF1). CSF1, or macrophage CSF (M-CSF), is a macrophage-specific growth factor that binds CSF1 receptor [CSF1R (c-fms proto-oncogene)], a pleiotropic tyrosine kinase structurally similar to the platelet-derived growth factor (PDGF) receptor and c-kit, that is highly expressed by monocytes, macrophages, and other phagocytic cells. Seminal work by Charles Sherr, Martine Roussel, and E. Richard Stanley identified the CSF1/1R signaling axis that is now well established to promote the survival, differentiation, and migration of macrophages. mAb tools specific for characterizing the CSF1/1R pathway were also developed, all in the 1980s. The importance of CSF1/1R signaling in solid tumors was also established in the late 1980s and throughout the 1990s, especially by Barry Kacinski’s team who showed that CSF1 is expressed by breast, ovarian, and endometrial carcinomas and promotes their invasiveness.
Then, in the context of breast cancers, DeNardo and colleagues found that the use of cytotoxic chemotherapy upregulates CSF1 expression and TAM infiltration into mammary tumors in vivo (3). When anti–CSF1 antibody (clone 5a1) or a small-molecule inhibitor of CSF1R kinase (PLX3397) was administered in conjunction, response to chemotherapy was significantly augmented along with the infiltration of CD8+ T cells into the tumor. Subsequently, the DeNardo group extended these observations in mouse models of pancreatic cancer, showing that CSF1R inhibition suppressed the tumorigenicity of pancreatic cells and, as observed for mammary tumors, induced chemosensitivity and CD8+ T-cell recruitment (4).
Collectively, these findings (Fig. 1) established the rationale for further in-depth studies on how CSF1/1R targeting modulates the immunosuppressive features of PDAC-associated macrophages.
Figure 1.
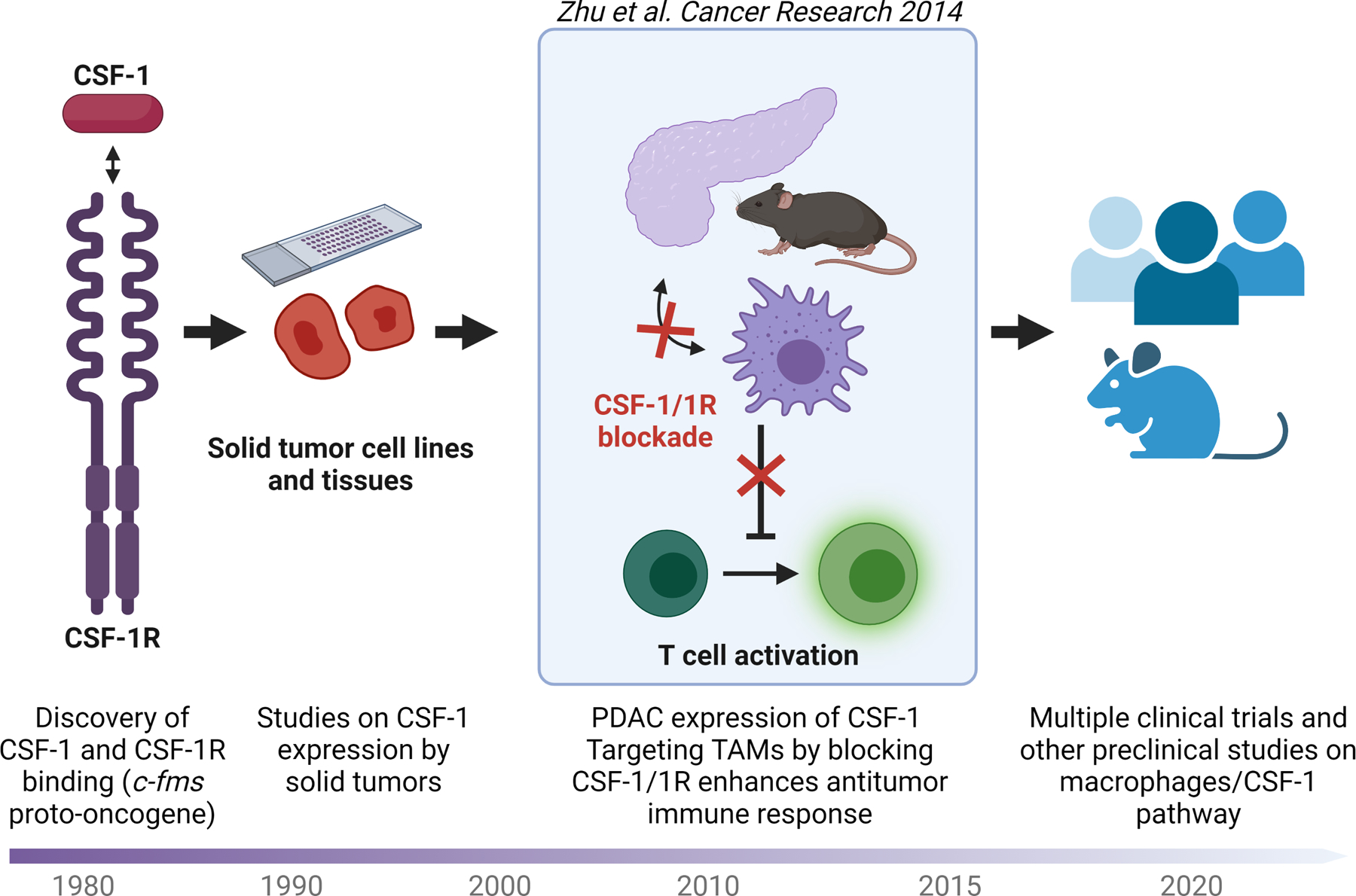
Key efforts investigating the CSF1/1R axis and its role in cancer. Timeline, research leading up to and following the landmark study published by Zhu and colleagues in Cancer Research in 2014 (5).
In a landmark study from the DeNardo group published in Cancer Research in 2014 (5), Zhu and colleagues first showed, using tissue microarrays of 77 human PDAC cases, that CSF1 expression is elevated in invasive carcinoma when compared with normal pancreatic tissue. Then, in a mouse model of pancreatic tumors arising from orthotopic injections of Kras-Ink4a/Arf mutant pancreatic cancer cell lines, the group discovered that CSF1/1R blockade upregulates proinflammatory and downregulates anti-inflammatory genes. Importantly, CSF1/1R inhibition led to the preferential depletion of immunosuppressive macrophage subtypes (CD206hi), promoted TAM antigen-presenting capacity, and enhanced antitumor T-cell activity. Notably, CSF1/1R blockade was also associated with the upregulation of PD-L1 on tumors and CTLA4 on T cells. These changes in checkpoint markers could then be further exploited by anti-PD1 and anti-CTLA4 therapies to yield much greater antitumor responses, revealing synergy between anti–CSF1/1R and checkpoint immunotherapy.
This pivotal work contributed to three important conceptual advances. First, it validated the clinical relevance of CSF1/1R signaling in PDAC and uncovered one mechanism underlying the recruitment of PDAC-promoting macrophages at a time when the role of immune-suppressive macrophages were just being recognized in cancer. Second, it showed that CSF1/1R blockade can enhance checkpoint immunotherapy, a treatment modality that was becoming increasingly relevant at the time of this initial finding. Third, and perhaps most compellingly, this report introduced for the first time the concept that TAMs can serve as targets for modulation to support antitumor immune responses against PDACs.
These foundational observations have since motivated the clinical testing of multiple antibodies against CSF1/1R in patients with PDAC. A recent phase II trial evaluated the efficacy of cabiralizumab (Five Prime), a humanized IgG4 mAb against CSF1R, together with the anti-PD1 antibody, nivolumab, in patients with advanced/metastatic PDAC who progressed after first-line chemotherapy (NCT03336216). Similarly, a multicenter phase Ib/II trial evaluated a fully human IgG2 monoclonal anti–CSF1R antibody, AMG 820 (Amgen), in combination with pembrolizumab, and enrolled 116 patients including 31 patients with metastatic PDAC (NCT02713529). Despite showing target-specific changes, e.g., depletion of monocytes, neither of these trials met their efficacy endpoints. In another phase Ib/II trial that included patients with metastatic PDAC, limited activity was observed with the anti–CSF1 antibody, lacnotuzumab (Novartis), given in combination with anti-PD1 spartalizumab (NCT02807844). In a phase I study, pexidartinib, or PLX3397 (Plexxikon), a CSF1R kinase inhibitor recently FDA-approved for tenosynovial giant cell tumor, has also been studied in combination with the anti–PD-L1 agent, durvalumab, in patients with PDAC. This trial resulted in a modest 21% clinical benefit rate (4/19 stable disease, including 2 patients with microsatellite instability-high colorectal cancers; NCT02777710).
Even though these clinical efforts could not generate the level of improvement in outcomes the field had hoped for, this early proof-of-concept seminal article by Zhu and colleagues contributed to the currently thriving field uncovering the role of myeloid cells in cancer biology. In fact, these early clinical trial results should not be considered conclusive. More recent studies in other cancer types have delineated compensatory immunosuppressive barriers, offering insights as to why the first clinical efforts evaluating CSF1/1R therapeutics might have failed. These ongoing studies are uncovering potential points of synergy to be targeted along with CSF1/1R, such as the promotion of granulocyte infiltration by the CXCL1-CXCR2 axis (6), the upregulation of 4–1BB in effector T cells (7), the production of macrophage-derived insulin-like growth factor (8), and the enhanced Treg-TAM cross-talk (9), among others. In this way, the influential work by the DeNardo group has had a lasting impact on the cancer field and will continue to guide future efforts toward developing novel strategies for treating pancreatic cancers.
Acknowledgments
Authors’ Disclosures
W.J. Ho reports other support from Rodeo/Amgen; grants from Sanofi; and other support from Exelixis outside the submitted work. E.M. Jaffee reports other support from Abmeta; personal fees from Genocea, Achilles, DragonFly, CSTONE, Candel Therapeutics, Stimit, Nextcure; personal fees and other support from Parker Institute; grants from Lustgarten, Genentech, Bristol Myers Squibb; and grants from AstraZeneca outside the submitted work.
References
- 1.Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer 2013;108:914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, et al. Significance of M2-Polarized Tumor-Associated Macrophage in Pancreatic Cancer. J Surg Res 2011;167:e211–e9. [DOI] [PubMed] [Google Scholar]
- 3.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov 2011;1:54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchem JB, Brennan DJ, Knolhoff BL, Belt BA, Zhu Y, Sanford DE, et al. Targeting tumor-infiltrating macrophages decreases tumor-initiating cells, relieves immunosuppression, and improves chemotherapeutic responses. Cancer Res 2013;73:1128–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res 2014;74:5057–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar V, Donthireddy L, Marvel D, Condamine T, Wang F, Lavilla-Alonso S, et al. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell 2017;32:654–68e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saung MT, Muth S, Ding D, Thomas DL 2nd BAB, Tsujikawa T, et al. Targeting myeloid-inflamed tumor with anti-CSF-1R antibody expands CD137+ effector T-cells in the murine model of pancreatic cancer. J Immunother Cancer 2018;6:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quail DF, Bowman RL, Akkari L, Quick ML, Schuhmacher AJ, Huse JT, et al. The tumor microenvironment underlies acquired resistance to CSF-1R inhibition in gliomas. Science 2016;352:aad3018-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez-Usatorre A, Kadioglu E, Boivin G, Cianciaruso C, Guichard A, Torchia B, et al. Overcoming microenvironmental resistance to PD-1 blockade in genetically engineered lung cancer models. Sci Transl Med 2021;13:eabd1616. [DOI] [PMC free article] [PubMed] [Google Scholar]


