Abstract
Purpose:
Dual inhibition of glucose and glutamine metabolism results in synergistic anti-cancer effects in solid tumor models. Telaglenastat, an investigational, small molecule, glutaminase inhibitor, exhibits modest single agent activity in RCC patients. This phase 1b trial evaluated telaglenastat plus cabozantinib or everolimus, agents known to impair glucose metabolism in patients with metastatic RCC (mRCC).
Experimental Design:
mRCC patients received escalating doses of telaglenastat (400–800mg per os [PO] twice daily) in a 3+3 design, plus either everolimus (10mg daily PO; TelaE) or cabozantinib (60mg daily PO; TelaC). Tumor response (RECISTv1.1) was assessed every 8 weeks. Endpoints included safety (primary) and anti-tumor activity.
Results:
27 patients received TelaE, 13 received TelaC, with median 2 and 3 prior therapies, respectively. Treatment-related adverse events were mostly grade 1–2, most common including decreased appetite, anemia, elevated transaminases, and diarrhea with TelaE, and diarrhea, decreased appetite, elevated transaminases, and fatigue with TelaC. One dose-limiting toxicity occurred per cohort: grade 3 pruritic rash with TelaE and thrombocytopenia with TelaC. No MTD was reached for either combination, leading to a recommended phase 2 dose of 800mg telaglenastat BID with standard doses of E or C. TelaE disease control rate (DCR; response rate + stable disease) was 95.2% (20/21, including 1 partial response [PR]) among 21 patients with clear cell histology and 66.7% (2/3) for papillary. TelaC DCR was 100% (12/12) for both histologies (5/10 PRs as best response [3 confirmed] in clear cell).
Conclusions:
TelaE and TelaC showed encouraging clinical activity and tolerability in heavily pre-treated mRCC patients.
Keywords: Renal cell carcinoma, glutaminase inhibitor, tumor metabolism, phase 1
Introduction
Renal cell carcinoma (RCC) is the most common type of kidney cancer in adults, accounting for approximately 3% of adult malignancies and 90–95% of tumors in the kidney.1 The incidence of RCC has increased in recent years due to advances in diagnostics. Although death rates have fallen due to introduction of new therapeutic approaches, metastatic RCC (mRCC) has a high mortality, with a 5-year survival rate of only 12%.2 Furthermore, despite wide availability of targeted therapies for mRCC, including tyrosine kinase inhibitors (TKI), anti-PD-1 antibodies, and mTOR inhibitors, many patients develop resistance to these treatments, underscoring a pressing need for new therapeutic approaches and combination therapies.
The pathogenesis of clear cell RCC is attributed to loss-of-function mutation or epigenetic silencing of the von Hippel-Lindau (VHL) tumor suppressor gene,3 which results in accumulation of hypoxia-inducible factors (HIF)-1α/2α. HIF-1α/2α regulate the cellular hypoxia response, including activation of genes involved in energy metabolism. Their overexpression in RCC results in a metabolic switch to a glutamine-maintained tricarboxylic acid cycle (TCA), increasing tumor cell dependency on glutamine for growth and proliferation.4,5 Glutaminase is a critical enzyme of glutamine-dependent pathways, converting glutamine to glutamate within the mitochondria. Glutaminase overexpression is driven by oncogenic transformation. Inhibition of glutaminase activity with glutaminase inhibitors leads to depletion of glutamate, glutathione, several TCA cycle intermediates, and other metabolic intermediates associated with glutamate production—which ultimately inhibit tumor cell proliferation.6
Telaglenastat is an investigational, small molecule, reversible oral inhibitor of glutaminase. In a prior phase 1 in patients with advanced solid tumors, single agent telaglenastat was well tolerated at active doses of 600 and 800 mg BID and demonstrated a favorable pharmacokinetic/pharmacodynamic profile with potent inhibition of glutaminase in platelets and tumors of patients with solid tumors.7 An efficacy signal was observed in heavily pre-treated patients with RCC (50% [14/28] disease control rate [DCR = overall response rate + stable disease], including 1 partial response [PR]).7
Dual metabolic targeting of glutamine and glucose utilization pathways via inhibition of both glutaminase and growth factor signaling pathways is hypothesized to synergize to suppress tumor cell proliferation in patients with RCC (Supplementary Figure S1). Cabozantinib, a VEGFR2/MET/AXL inhibitor, is currently approved alone and in combination with nivolumab for first-line treatment of clear cell RCC. The mTOR inhibitor, everolimus, is an approved agent in the second line setting.2,8 Each agent has demonstrated single agent activity in metastatic clear cell RCC, with overall response rates (ORR) of 17% with cabozantinib and 3% with everolimus and median PFS of 7.4 months and 3.9 months, respectively.9 Preclinical studies have demonstrated that telaglenastat synergizes with cabozantinib and with everolimus, both in vitro and in mouse xenograft models of RCC.10 To determine whether preclinical synergistic activity of those combinations would translate to the clinical setting, we evaluated safety and efficacy from two phase 1b cohorts of telaglenastat in combination with cabozantinib or everolimus in patients with mRCC.
Methods
Study Design
This was an open-label, multicenter, phase 1 trial (NCT02071862) that included dose escalation and dose expansion of telaglenastat, both as a single agent and in combination with different anticancer therapies in patients with advanced or metastatic and/or refractory solid tumors (Supplementary Figure S2). The single agent dose-escalation and dose expansion parts of the study were recently reported.7 Here we report findings from the combination cohorts of telaglenastat plus everolimus (TelaE) or telaglenastat plus cabozantinib (TelaC) in patients with mRCC.
The study was conducted in accordance with the ethics principles of the Declaration of Helsinki and the International Council of Harmonization Guidelines on Good Clinical Practice. The study protocol and amendments were approved by an institutional review board or ethics committee for each center. All patients provided written, informed consent.
Patient Selection
Eligible patients were ≥18 years of age, had Eastern Cooperative Oncology Group (ECOG) performance status 0–1, measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1,11 adequate organ function, and histologically confirmed diagnosis of locally-advanced and inoperable or metastatic RCC. Patients were not eligible if they had prior cytotoxic chemotherapy, TKI (or other targeted anti-cancer agent), radiation therapy, hormone therapy within 14 days prior to the first day of treatment; immunotherapy, biologic agent, systemic steroid therapy, or investigational agent within 21 days. Patients with unstable or compromised cardiac function, past small bowel resection or gastric bypass surgery, major surgery within 28 days (within 3 months or unhealed surgical wounds for TelaC cohort), or untreated brain metastases or central nervous system disease were excluded. Patients with clear cell RCC must have had at least 1 line of therapy with a vascular endothelial growth factor receptor (VEGFR) inhibitor (such as sunitinib or pazopanib). Patients with non-clear cell variants of RCC were eligible without prior therapy since there were no agents of proven benefit at the time the study was initiated.
TelaE:
For dose escalation, no more than 4 prior lines of therapy for metastatic disease were allowed. For dose expansion, patients with clear cell RCC had to have 1–3 prior treatments for advanced/metastatic disease, including ≥1 VEGFR-TKI. Patients with papillary RCC could not have received >3 prior treatments for advanced/metastatic disease. Patients with uncontrolled diabetes mellitus or hyperlipidemia were excluded. In dose expansion, patients with prior mTOR inhibitors (e.g., everolimus/temsirolimus) were excluded.
TelaC:
Patients were excluded for recent hemorrhage or the risk thereof, myocardial or cerebral infarction or other serious thromboembolic event within 6 months, or concurrent use of strong CYP3A4 inhibitors or inducers within 14 days of study entry.
Additional details of patient selection are provided in the Data Supplement.
Treatment
For the TelaE cohort, patients received telaglenastat 400, 600, or 800 mg twice daily (BID) with food, plus standard dose everolimus 10 mg per os (PO) route once daily (QD) on a 28-day cycle. The first telaglenastat dose was taken in the morning with breakfast. The second dose of telaglenastat was taken in the evening with dinner. Everolimus could be administered at the same time as telaglenastat.
For the TelaC cohort, which was added as a protocol amendment after initial results had been collected at lower telaglenastat doses and informed starting doses for additional cohorts, patients received telaglenastat 600 or 800 mg BID, with food, plus standard dose cabozantinib (60 mg PO QD) in 28-day cycles. Cabozantinib was taken on an empty stomach at least 1 hour before and 2 hours after ingestion of food.
Patients in both cohorts were enrolled in a traditional 3+3 design: a minimum of 3 patients were assigned to the 400 or 600 mg BID dose for the TelaE and TelaC cohorts, respectively, escalating to a maximum dose of 800 mg BID. If 1 of 3 patients experienced a dose-limiting toxicity (DLT), the dose level would be expanded to include an additional 3 patients.
Dose Limiting Toxicities
Patients must have received at least 75% of planned doses (both telaglenastat and cabozantinib/everolimus) in the first treatment cycle to be considered evaluable for a dose-limiting toxicity (DLT), unless the patient had the study drug held for an AE that may herald a DLT or if the patient experienced a DLT. DLTs were defined as any AE that could not be determined to be unrelated to study treatment, occurs within the first treatment cycle, and meets at least one of the following criteria: Any grade ≥3 clinically significant non-hematologic toxicity per the Common Toxicity Criteria for Adverse Events (CTCAE) v.4, except nausea/vomiting/diarrhea lasting <48 hours and controlled with antiemetic/antidiarrheal therapy, grade 3 hyperglycemia lasting <72 hours with standard anti-diabetic therapy, laboratory abnormalities reversible to grade ≤1 or baseline status within 72 hours with outpatient care and/or monitoring, or that are considered not clinically significant by the investigator; Grade 4 neutropenia (ANC < 0.5 × 109/mL); Grade 3 febrile neutropenia (ANC < 1.0 × 109/L with temperature ≥ 38.3°C); Grade 4 thrombocytopenia (platelet count < 25.0 × 109/L) lasting >4 days or requiring platelet transfusion; Grade ≥3 thrombocytopenia (platelet count <50.0 × 109/L) associated with grade ≥3 bleeding; any other AE that is felt to be treatment-limiting in the medical opinion of the principal investigator and medical monitor.
For the TelaC cohort, DLTs included any AE expected to occur with cabozantinib monotherapy and unlikely to be worsened by combination with telaglenastat. DLTs also included any grade ≥3 event expected with cabozantinib, including but not limited to palmar-plantar erythrodysesthesia syndrome, deep vein thrombosis/pulmonary embolism, mucositis, hypothyroidism, hypophosphatemia, diarrhea, and hypertension.
Dose Adjustments
For the dose escalation part of the study, dose reductions of telaglenastat were permitted during the first 28 days in the event of a DLT. If a patient experienced a DLT, treatment continuation at a lower telaglenastat dose was permitted as long as the toxicity returned to grade ≤1 or baseline within 14 days. Patients who did not recover within 14 days were not eligible to resume treatment. Patients could miss up to 7 doses of the combination therapy during Cycle 1, based upon the discretion of the Investigator. Dose adjustments for everolimus or cabozantinib were permitted according to the package insert and dose reduction guidelines. See Data Supplement for full dose modification guidelines.
Endpoints
The primary objectives were to evaluate the safety and tolerability of teleglenastat in combination with everolimus or cabozantinib for the treatment of advanced RCC and to identify the recommended phase 2 dose (RP2D) for combination therapy. Other objectives included the evaluation of anti-tumor activity in RCC. RP2D for the combination regimens was to be selected based on the clinical data and not to exceed the maximum tolerated dose (MTD).
Safety and Efficacy Evaluation
Safety evaluations occurred on days 1, 8, and 15 of cycle 1, and on day 1 of each cycle thereafter and included adverse events (AEs), laboratory test results (hematology, coagulation, serum chemistry, and urinalysis), electrocardiogram (ECG), weight, and vital signs. AEs were pooled across doses, based on target inhibition observed at 400, 600, and 800 mg BID doses in the monotherapy study.7 AE’s were summarized according to system organ class and preferred term according to the most recent version of the Medical Dictionary for Drug Regulatory Activities (MedDRA) and tallied for overall frequency, worst reported severity (NCI-CTCAE v4.03), and relationship to study drug. All AEs were recorded from first dose of study drug up to 28 days after the last dose. Laboratory variables, ECG, weight, and vital signs were summarized by changes from baseline to scheduled points.
Response to treatment was based on Investigator assessment using RECIST v1.1.11 Radiographic evaluation of tumor burden (diagnostic computed tomography or magnetic resonance imaging) occurred at screening and approximately every 8 weeks (±7 days) after study initiation, or more frequently as clinically indicated. For each patient with objectively measurable disease, response to therapy, DOR, and progression-free survival (PFS) were calculated. Progression per RECIST criteria, clinical progression, and death were all counted as events for the PFS analysis.
Statistical Analysis
For the safety analysis, the population included all patients who received at least one dose of telaglenastat, everolimus, or cabozantinib in the respective cohorts. Results were tabulated by frequency and relationship to study treatment. Safety data were examined on an ongoing basis to ensure safety of patients and compliance with trial rules. Descriptive statistics were used to analyze the data, and summary statistics for continuous variables (mean, standard deviation, median, range). Categorical variables are presented as frequency counts and percentages.
For the efficacy analysis, all patients who completed at least 1 post-baseline tumor assessment or who discontinued study medication early due to drug-related toxicity were considered evaluable; patients who discontinued due to disease-related death had to have received at least 32 doses of telaglenastat and 16 doses of cabozantinib or everolimus to be considered evaluable for efficacy. The Kaplan-Meier method was used to estimate median duration of response (DOR) and PFS.
Data Availability Statement
The data generated in this study are available within the article and its supplementary data files.
Results
Patients
From July 2015 to November 2018, 27 patients were enrolled in the TelaE cohort (22 clear cell; 3 papillary; 2 other), and from September 2016 to March 2019, 13 patients were enrolled in the TelaC cohort (11 clear cell, 2 papillary). In the TelaE cohort, one patient with adenocarcinoma was initially categorized in the “other” histological group, but was later determined to have clear cell RCC and subsequently reclassified accordingly. Another patient initially identified as having papillary RCC was determined to have a mutation in the fumarate hydratase (FH) gene, resulting in a phenotype which is recognized as distinctly different from papillary type 2 RCC, and was recategorized into the “other” histology. Median age was 60 years (range, 32–80) in the TelaE cohort and 59 years (27–71) in the TelaC cohort. Both cohorts had a higher proportion of male patients: 85% and 82% in the TelaE and TelaC cohorts, respectively. Most patients were receiving third-line or later of systemic therapies, including 8 patients who had received ≥4 prior lines of therapy (Table 1). All patients with clear cell histology had received prior TKI therapy, and >90% had undergone prior nephrectomy. In TelaE and TelaC cohorts, 55% and 64%, respectively, had received prior immunotherapy.
Table 1.
Demographics & Baseline Characteristics
| Telaglenastat + Everolimus | Telaglenastat + Cabozantinib | ||||
|---|---|---|---|---|---|
| Clear Cell (n-22) | Papillary (n=3) | Other (n=2) | Clear Cell (n=11) | Papillary (n=2) | |
| Median age, y (range) | 61.5 (39–80) | 56 (32–60) | 55.5 (49 – 62) | 59 (44–71) | 43 (27–59) |
| Sex, n (%) | |||||
| Female | 4 (18.2) | 0 | 0 | 2 (18.2) | 2 (100) |
| Male | 18 (81.8) | 3 (100) | 2 (100) | 9 (81.8) | 0 |
| Race, n (%) | |||||
| White | 22 (100) | 2 (66.7) | 2 (100) | 10 (90.9) | 2 (100) |
| Black or African American | 0 | 1 (33.3) | 0 | 0 | 0 |
| Other | 0 | 0 | 0 | 1 (9.1) | 0 |
| ECOG Score, n (%) | |||||
| 0 | 6 (27.3) | 0 | 1 (50.0) | 2 (18.2) | 0 |
| 1 | 16 (72.7) | 3 (100) | 1 (50.0) | 9 (81.8) | 2 (100) |
| MSKCC risk score, n (%) | |||||
| Favorable | 8 (36.4) | 0 | 0 | 2 (18.2) | 1 (50.0) |
| Intermediate | 12 (54.5) | 2 (66.7) | 2 (100) | 9 (81.8) | 1 (50.0) |
| Poor | 2 (9.1) | 1 (33.3) | 0 | 0 | 0 |
| Median time from metastatic/advanced disease, months (range) | 33.3 (7.0–100.2) | 7.0 (1.4–25.2) | 13.0 (3.2 −22.8) | 24.9 (6.5−117.9) | 12.5 (0.5−24.5) |
| Prior treatments, n (%) | |||||
| ≥ 1 Prior nephrectomy | 20 (90.9) | 1 (3.3) | 2 (100) | 10 (90.9) | 1 (50.0) |
| ≥ 1 Prior radiotherapy | 12 (54.5) | NA | 0 | 6 (54.5) | 0 |
| ≥ 1 Prior systemic therapy | 22 (100) | 2 (66.7) | 2 (100) | 11 (100) | 1 (50.0) |
| 0 | 0 | 2 (66.7) | 2 (100) | 0 | 1 (50.0) |
| 1 | 1 (4.5) | 1 (3.3) | 0 | 2 (18.2) | 1 (50.0) |
| 2 | 11 (50.0) | 2 (66.7) | 1 (50.0) | 1 (9.1) | 0 |
| 3 | 5 (22.7) | 0 | 1 (50.0) | 4 (36.4) | 0 |
| 4–10 | 5 (22.7)a | 0 | 0 | 4 (36.4) | 0 |
| Prior immunotherapy | 12 (54.5) | 1 (33.3) | 0 | 7 (63.6) | 0 |
| Prior TKI | 22 (100) | 1 (33.3) | 2 (100) | 11 (100) | 1 (50.0) |
| Prior mTOR | 1 (4.5) | 0 | 1 (100) | 3 (27.3) | 0 |
| Telaglenastat dose, n (%) | |||||
| 400 mg BID | 3 (13.6) | 2 (66.7) | 2 (100) | 0 | 0 |
| 600 mg BID | 12 (54.5) | 1 (33.3) | 0 | 5 (45.5) | 1 (50.0) |
| 800 mg BID | 7 (31.8) | 0 | 0 | 6 (54.5) | 1 (50.0) |
Abbreviation: BID, twice daily; ECOG, Eastern Cooperative Oncology Group, MSKCC, Memorial Sloan Kettering Cancer Center; mTOR, mammalian target of rapamycin; NA, not available; TKI, tyrosine kinase inhibitor.
No patient had >4 prior systemic therapies per eligibility criteria.
Among 17 patients who enrolled and received TelaE in dose escalation, 1 patient discontinued therapy due to an AE, 1 due to physician decision, 14 for radiographic progressive disease (PD), and 1 due to clinical progression. Among 10 patients who enrolled in the TelaE dose expansion, 2 patients discontinued therapy due to AEs, 1 patient withdrew consent, 6 patients had PD, and 1 patient had clinical progression.
Among 13 patients who enrolled in the TelaC cohort, 9 patients discontinued therapy due to PD, 1 patient discontinued for poor tolerance, and 1 patient died; 2 patients were still on study at study closure, one of whom continued on the same dosing regimen (telaglenastat 800 mg BID).
Safety
In the TelaE cohort, 15 (56%) patients received 5 or more cycles of treatment; median cumulative dose was 167,400 mg telaglenastat and 1090 mg everolimus, and mean relative dose intensity was 93.1% and 93.9%, respectively. The most common treatment-related AEs occurring in two or more patients included decreased appetite (37%), anemia (33%), mucosal inflammation (33%), thrombocytopenia (33%), diarrhea (26%), increased transaminases (26%), nausea (22%), and fatigue (22%) (Table 2). Most common treatment-related grade ≥3 AEs were fatigue (11%), anemia (7%), hyperglycemia (7%), and hypertension (7%). Thirteen patients experienced 1 or more serious AEs (SAEs: gastrointestinal hemorrhage [4 events], anemia [2 events], cough, diarrhea, dermatitis, dyspnea, hypoxia, myalgia, pain, pneumonia, pyrexia, renal failure acute, small intestinal obstruction, supraventricular tachycardia, syncope); of these pyrexia and dermatitis (both occurring in the same patient) were considered possibly related to study treatment. One DLT of grade 3 pruritic rash occurred at the 400 mg telaglenastat dose. Telaglenastat doses were reduced in four patients due to AEs, and everolimus doses reduced in 9 patients due to AEs. Three patients had AEs that led to withdrawal from the study: one patient each with dermatitis and fatigue (both considered related to everolimus), and one patient with two events of gastrointestinal haemorrhage and acute renal failure (considered unrelated to either telaglenastat or everolimus).
Table 2.
Treatment-related AEs in ≥2 Patients: Telaglenastat + Everolimus
| MedDRA Preferred Term, n (%) | Telaglenastat + Everolimus (n=27) | |
|---|---|---|
| All Grades | Grade ≥3 | |
| Patients with ≥1 treatment-related AE | 27 (100) | 14 (52) |
| Decreased appetite | 10 (37) | 0 |
| Anemia | 9 (33) | 2 (7) |
| Mucosal inflammationa | 9 (33) | 1 (4) |
| Thrombocytopeniab | 9 (33) | 1 (4) |
| Diarrhea | 7 (26) | 0 |
| Increased transaminasesc | 7 (26) | 0 |
| Nausea | 6 (22) | 0 |
| Fatigue | 6 (22) | 3 (11) |
| Blood creatinine increased | 5 (19) | 0 |
| Rash | 5 (19) | 0 |
| Dysgeusia | 5 (19) | 0 |
| Proteinuria | 5 (19) | 0 |
| Hyperglycemia | 4 (15) | 2 (7) |
| Dermatitis acneiform | 4 (15) | 0 |
| Epistaxis | 4 (15) | 0 |
| Photophobia | 4 (15) | 0 |
| Myalgia | 4 (15) | 0 |
| Vomiting | 3 (11) | 0 |
| Weight decreased | 3 (11) | 0 |
| White blood cell count decreased | 3 (11) | 0 |
| Pyrexia | 3 (11) | 0 |
| Pruritus | 3 (11) | 0 |
| Rash maculopapular | 3 (11) | 1 (4) |
| Cough | 3 (11) | 0 |
| Dry mouth | 2 (7) | 0 |
| Dyspepsia | 2 (7) | 0 |
| GGT increased | 2 (7) | 0 |
| Neutropenia | 2 (7) | 1 (4) |
| Chills | 2 (7) | 0 |
| Edema peripheral | 2 (7) | 1 (4) |
| Hypertriglyceridemia | 2 (7) | 1 (4) |
| Dyspnea | 2 (7) | 0 |
| Dizziness | 2 (7) | 0 |
| Headache | 2 (7) | 0 |
| Hypertension | 2 (7) | 2 (7) |
NOTE: Other Grade ≥3 AEs: cystitis, hypophosphatemia, edema localized, lymphocyte count decreased, pharyngeal inflammation, rash pruritic (n=1 each).
Abbreviation: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyltransferase; MedDRA, Medical Dictionary for Drug Regulatory Affairs.
Combined terms: mucosal inflammation and stomatitis
Combined terms: thrombocytopenia and platelet count decreased
Combined terms: alanine transaminase increased and aspartate transaminase increased
In the TelaC cohort, 9 (69%) patients received 5 or more cycles of treatment, with a median cumulative dose of 301,600 mg telaglenastat and 9240 mg cabozantinib, and mean relative dose intensity was 82.7% and 87.3%, respectively. The most common treatment-related AEs with TelaC (in three or more patients) were diarrhea (62%), decreased appetite (46%), increased transaminases (46%), fatigue (38%), nausea (31%), rash (31%), dehydration (23%), mucosal inflammation (23%), proteinuria (23%), and vomiting (23%) (Table 3). Treatment-related grade ≥3 AEs occurred in 30.8% of patients and included one each of diarrhea, hypertension, thrombocytopenia, and weight loss. Three patients experienced 5 SAEs (chest pain, ophthalmic herpes zoster, hip fracture, cerebrovascular accident, hallucination), none which were considered related to study treatment. One DLT (thrombocytopenia) occurred at the 600 mg telaglenastat dose. Telaglenastat doses were reduced in 2 patients due to AEs, and cabozantinib doses reduced in seven patients due to AEs. No patients withdrew due to AEs.
Table 3.
Treatment-related AEs by Preferred Term in ≥2 Patients: Telaglenastat + Cabozantinib
| MedDRA Preferred Term, n (%) | Telaglenastat + Cabozantinib (n=13) | |
|---|---|---|
| All Grades | Grade ≥3 | |
| Patients with ≥1 treatment-related AE | 13 (100) | 4 (31) |
| Diarrhea | 8 (62) | 1 (8) |
| Decreased appetite | 6 (46) | 0 |
| Increased transaminasesa | 6 (46) | 0 |
| Fatigue | 5 (38) | 0 |
| Nausea | 4 (31) | 0 |
| Rash | 4 (31) | 0 |
| Dehydration | 3 (23) | 0 |
| Mucosal inflammation | 3 (23) | 0 |
| Proteinuria | 3 (23) | 0 |
| Vomiting | 3 (23) | 0 |
| Abdominal pain | 2 (15) | 0 |
| Anemia | 2 (15) | 0 |
| Dysgeusia | 2 (15) | 0 |
| Hypertension | 2 (15) | 1 (8) |
| Muscle spasms | 2 (15) | 0 |
| Palmar-plantar erythrodysesthesia syndrome | 2 (15) | 0 |
| Platelet count decreased | 2 (15) | 1 (8) |
| Pruritus | 2 (15) | 0 |
| Weight decreased | 2 (15) | 1 (8) |
NOTE: Other Grade ≥3 AEs: photophobia (n=1)
Abbreviation: ALT, alanine aminotransferase; AST, aspartate aminotransferase
Combined terms: alanine transaminase increased and aspartate transaminase increased
No MTD was reached for telaglenastat for either TelaE or TelaC combinations, leading to RP2D selection of 800 mg BID (based on findings from the telaglenastat monotherapy study7) plus standard doses of everolimus or cabozantinib.7
Clinical Activity
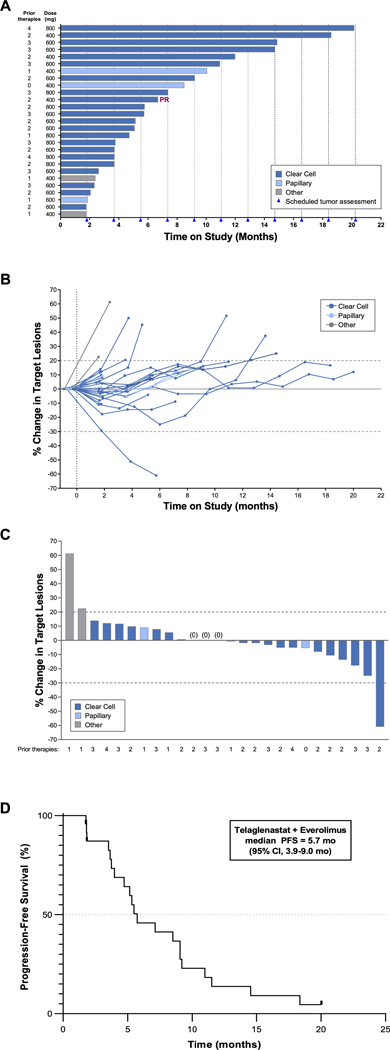
Twenty-four patients in the TelaE cohort were evaluable for efficacy (21 clear cell, 3 papillary). Of the 22 patients with clear cell RCC enrolled, 1 was not evaluable for response because of premature discontinuation due to an unrelated AE. Among the remaining 21 patients with clear cell RCC, 1 experienced a confirmed PR and 19 had stable disease (SD), for a disease control rate (DCR) of 95% (Table 4). Median PFS for clear cell RCC was 5.5 months (95% confidence interval [CI], 3.7–9.1). In patients with papillary RCC (n=3), 2 patients had SD for a DCR of 67%, and median PFS was 8.5 months (95% CI, 1.8–not reached). Median PFS for all histologies combined was 5.7 months (95% CI 3.9–9.0 months) (Figure 1).
Table 4.
Efficacy Summary in Evaluable RCC Patients Receiving Telaglenastat + Everolimus or Telaglenastat + Cabozantinib by Histology
| Telaglenastat + Everolimus | Telaglenastat + Cabozantinib | |||
|---|---|---|---|---|
| Clear Cell RCC (n-21) | Papillary RCC (n=3) | Clear Cell RCC (n=10) | Papillary RCC (n=2) | |
| Best response, n (%) | ||||
| Partial response | 1 (4.8) | 0 | 5 (50.0))b | 0 |
| Stable disease | 19 (90.1) | 2 (66.7) | 5 (50.0) | 2 (100) |
| Progressive disease | 1 (4.8) | 1 (33.3) | 0 | 0 |
| Best overall response rate, n (%)a | 1 (4.8) | 0 | 5 (50.0)b | 0 |
| Disease control rate, n (%)a | 20 (95.2) | 2 (66.7) | 10 (100) | 2 (100) |
| Median duration of response, months, (95% CI) | 1.9 (NA, NA) | NA | 5.3 (2.0, 17.8) | NA |
| Median PFS, months (95% CI) | 5.5 (3.7, 9.1) | 8.5 (1.8, NR) | 6.2 (3.7, NR) | 7.7 (3.6, NR) |
Abbreviations: CI, confidence interval; CR, complete response; NA, not available; NR, not reached; PFS, progression-free survival; RCC, renal cell carcinoma.
DCR = complete response + partial response + stable disease in evaluable patients (at least one evaluable post-baseline tumor assessment per RECIST v1.1); not confirmed
3/10 (30%) confirmed
Figure 1. Telaglenastat + Everolimus Efficacy Outcomes.
(A) Time on study; (B) Tumor burden over time by patient; (C) Best response for target lesions; (D) Kaplan-Meier estimate of PFS.
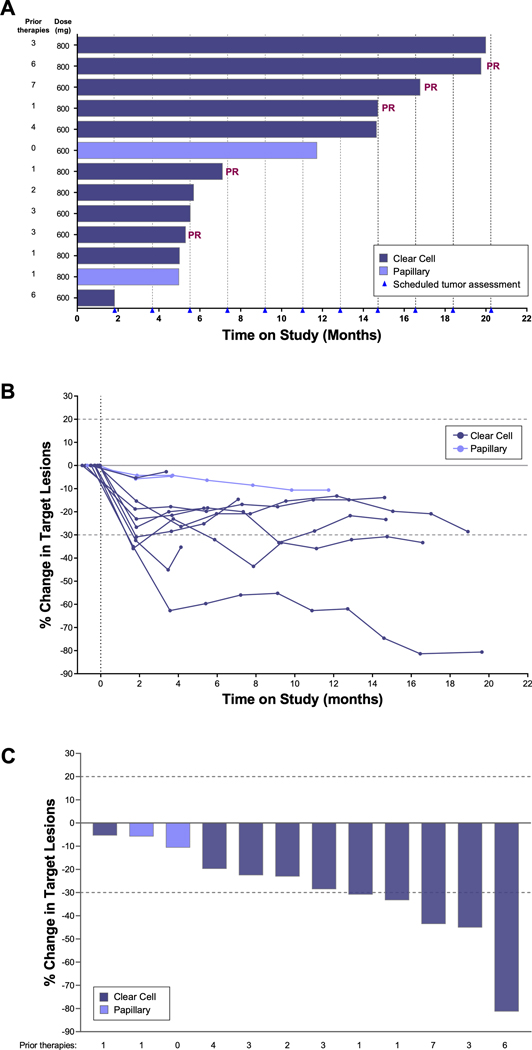
Of 12 evaluable patients receiving TelaC (10 clear cell, 2 papillary), 5 patients (42%) had best response of PR and 7 (58%) had SD for a DCR of 100% (Table 4). All 5 PRs occurred in patients with clear cell RCC (n=10), for an ORR of 50%, and median duration of response was 5.3 months (95% CI, 2.0–17.8). Three of 5 PRs were confirmed, one of which was confirmed following a dose interruption due to hospitalization from an unrelated event of ocular shingles. Both patients with papillary RCC had SD. Target lesions decreased over time in all patients (Figure 2). Median PFS was 6.2 months in patients with clear cell RCC and 7.7 months in patients with papillary RCC (Table 4), but meaningful conclusions cannot be drawn from these data due to the small sample sizes.
Figure 2. Telaglenastat + Cabozantinib Efficacy Outcomes.
(A) Time on study; (B) Tumor burden over time by patient; (C) Best response for target lesions.
Discussion
Despite recent improvements in kidney cancer mortality rates, survival outcomes remain poor for patients with metastatic disease who are resistant to current therapies. Our findings from the cohorts of patients with metastatic RCC receiving the glutaminase inhibitor telaglenastat with everolimus or cabozantinib followed initial reports of an encouraging safety and efficacy profile of single agent telaglenastat in patients with heavily pre-treated, advanced solid tumors.7 Telaglenastat monotherapy was well tolerated, with manageable side effects. Observations of potential activity that appeared to be amplified in RCC, including a PR lasting for 7.4 months in a patient on fourth-line therapy,7 led to prioritization of RCC for further study. RCC tumor growth is known to be particularly dependent on elevated glucose and glutamine metabolism4, thus the combination of telaglenastat, an inhibitor of glutamine metabolism, with SOC agents that attenuate glucose metabolism was explored in this study as a means to exploit the unique disease biology of RCC. In our study, telaglenastat in combination with everolimus or cabozantinib was well tolerated and showed encouraging activity, including DCRs of 95% and 100%, respectively, in patients with mRCC.
While the study was enrolling, cabozantinib and nivolumab were approved and became preferred post-first-line regimens, with everolimus as an acceptable alternative in regions where lenvatinib plus everolimus is not approved.2,8 In the METEOR study, everolimus monotherapy in patients with metastatic RCC was associated with an ORR of 3% and median PFS of 3.9 months.9 In our study, the combination of telaglenastat with everolimus resulted in an ORR of 5% and median PFS of 5.7 months. Overall, the SD rate of 90% (19/21) plus 5% PR (1/21) resulted in a combined 95% DCR. Although it is very difficult to compare single arm PFS data between studies, the median PFS in our study was somewhat longer than either the historic everolimus monotherapy or collective third-line targeted therapies (axitinib, sorafenib, sunitinib, everolimus, temsirolimus, pazopanib, bevacizumab) compiled from 25 international cancer centers (3.9 months for third-line metastatic RCC). 9,12 These observations were consistent with preclinical studies demonstrating enhanced or synergistic anti-proliferative effects of telaglenastat and everolimus in several RCC cell lines and in RCC tumor-bearing xenograft mice.10 TelaE was well tolerated, with safety profiles generally similar to that reported for everolimus alone, with the exception of photophobia.9, 13 No MTD was reached at doses up to 800 mg BID.
Cabozantinib monotherapy was previously reported to lead to an ORR of 17% with cabozantinib.9 In our study, with the combination of telaglenastat plus cabozantinib, five (50%) of the patients with clear cell RCC had a PR as best response. The activity observed with the combination may reflect synergy seen in preclinical studies in vitro and in xenograft models of RCC.10 TelaC was also well tolerated, with safety profiles generally similar to cabozantinib alone,9,13and no MTD reached at doses up to 800 mg BID. RP2D was therefore at established 800 mg BID—the same as for telaglenastat monotherapy—for both TelaC and TelaE.
Limitations of this study include those inherent to phase 1 trials, such as the small sample size, absence of a control treatment arm and correlative studies. During much of the study enrollment period, immune checkpoint inhibitor (ICI) therapy had not become standard of care for clear cell RCC, so only 54.5% of the clear cell RCC patients in the everolimus cohort and 63.6% of the patients in the cabozantinib cohort had received prior ICI. Although no pharmacokinetics are reported herein, given the lack of metabolic effects by CYP enzymes (data not shown), there is no expectation of DDI between agents.
In conclusion, the glutaminase inhibitor telaglenastat, in combination with everolimus or cabozantinib, showed encouraging clinical activity and tolerability in heavily pre-treated patients with metastatic RCC. Given the overall positive findings from addition of everolimus or cabozantinib to telaglenastat in combination cohorts of this early study, randomized studies were warranted to determine the extent of benefit of the TelaE and TelaC combinations in metastatic RCC. Two phase 2, randomized, placebo-controlled trials were initiated. First, ENTRATA, which compared TelaE vs. everolimus + placebo, met its primary endpoint, demonstrated improved PFS with TelaE over the placebo arm (median PFS, 3.8 vs. 1.9 months; HR=0.64 [95% CI, 0.34–1.20, 1-sided P=0.079), supporting proof of concept for the combination of GLS inhibition with mTOR inhibition.14 The second trial, CANTATA, which compared TelaC vs. cabozantinib + placebo, showed no significant differences between treatment arms in outcomes of PFS or responses.15 In both studies, telaglenastat appeared to be well tolerated, with safety profiles consistent with known risks of combination partners from each study.14,15 The divergent findings from ENTRATA and CANTATA, despite overall encouraging results from the present study, present obvious questions about the mechanism responsible for the differential activity, the understanding of which will help in the selection of combination partners for telaglenastat or other glutaminase inhibitors. Detailed findings of each study will be reported and discussed in separate publications.
Supplementary Material
Translational Relevance:
Dysregulated metabolism is a hallmark of cancer and represents an emerging target for therapeutic intervention. Dual inhibition of glucose and glutamine metabolism pathways is a promising approach for highly metabolic tumors such as renal cell carcinoma (RCC). Preclinical studies in RCC models have shown synergistic anticancer effects by telaglenastat in combination with everolimus (mTOR inhibitor) or cabozantinib (VEGFR2/MET/AXL inhibitor), two approved agents that have inhibitory effects on glucose metabolism. In this phase 1b study, patients with metastatic RCC received the glutaminase inhibitor telaglenastat in combination with either everolimus or cabozantinib. Both combinations resulted in encouraging clinical activity and tolerability in heavily pre-treated patients with metastatic RCC, supporting proof of concept of the combination of glutaminase inhibition with mTOR or VEGFR2/MET/AXL inhibition. These findings have prompted further evaluation of the combinatorial potential of telaglenastat with other anticancer agents.
Acknowledgments
We thank the investigators and site staff and patients and their families for their participation in the study. Editorial support was provided by Ingrid Koo, PhD, and funded by Calithera Biosciences, Inc. This study was funded by Calithera. Additional support was obtained by the NIH Clinical Translational Science Award 1UL1TR003167, and MD Anderson Cancer Center support grant P30 CA016672. This study was also supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research
Conflict-of-Interest Disclosures
FMB has received consulting fees for Aduro BioTech Inc., Alkermes, AstraZeneca, DebioPharm, eFFECTOR Therapeutics, F. Hoffman-La Roche Ltd., Genentech Inc., IBM Watson, Jackson Laboratory, Kolon Life Science, OrigiMed, PACT Pharma, Parexel International, Pfizer Inc., Samsung Bioepis, Seattle Genetics Inc., Tyra Biosciences, Xencor, Zymeworks; participated on advisory committees for Immunomedics, Inflection Biosciences, Mersana Therapeutics, Puma Biotechnology Inc., Seattle Genetics, Silverback Therapeutics, Spectrum Pharmaceuticals, Zentalis; received research grants from Aileron Therapeutics, Inc. AstraZeneca, Bayer Healthcare Pharmaceutical, Calithera Biosciences Inc., Curis Inc., CytomX Therapeutics Inc., Daiichi Sankyo Co. Ltd., Debiopharm International, eFFECTOR Therapeutics, Genentech Inc., Guardant Health Inc., Klus Pharma, Millennium Pharmaceuticals Inc., Novartis, Puma Biotechnology Inc., Taiho Pharmaceutical Co., honoraria from Chugai Biopharmaceuticals, Mayo Clinic, Rutgers Cancer Institute of New Jersey; and travel-related expenses from Beth Israel Deaconess Medical Center
NMT has consulted for Novartis, Exelixis, Bristol-Myers Squibb, Nektar, Pfizer, Eisai Medical Research, Ono Pharmaceutical, Oncorena, Surface Oncology, Neoleukin Therapeutics, Ipsen, Merck Sharp & Dohme, Calithera Biosciences; research funding from Bristol-Myers Squibb, Exelixis, Pfizer, Nektar Therapeutics, Calithera Biosciences, Lilly, Mirati Therapeutics, Arrowhead Pharmaceuticals, Takeda, Epizyme, Eisai; received honoraria from Pfizer, Novartis, Bristol-Myers Squibb, Exelixis, Nektar, Eisai Medical Research, Ono Pharmaceutical, Eli Lilly, Oncorena, Ipsen, Surface Oncology, Neoleukin Therapeutics, Merck Sharp & Dohme, Calithera Biosciences; and travel-related expenses from Pfizer, Nektar, Bristol-Myers Squibb, Eisai, Medical Research, Surface Oncology, Lilly Oncology, Ipsen, Calithera Biosciences.
OI has consulted for Merck.
RJL has received research funding from Janssen and has consulted for Bayer, Dendreon, Exelixis, and Janssen.
MT reports an advisory role for AbbVie, Aduro, AstraZeneca, Blueprint Medicines, Celgene, Daiichi Sankyo, Guardant, Genentech, G1 Therapeutics, Immunomedics, Lilly, Merck & Co.., Natera, OncoSec Medical, and Pfizer; institutional research funding from AbbVie, AstraZeneca, Bayer, Biothera, Calithera, EMD Serono, Genentech, Merck & Co., OncoSec Medical, Pfizer, PharmaMar, Tesaro, Vertex.
ACF has consulted for Duke Clinical Research Institute (Verily Baseline Study), research funding from NCI, DOD, Conquer Cancer Foundation, Calithera Biosciences, Filtricine Inc., Earli Inc, Vortex Biosciences, advisory board for Dendreon Inc, honoraria from the Medical Educator Consortium, and has founder’s equity in Molecular Decisions, Inc.
NH has consulted with Eisai, Aveo and Genentech.
MRP has served on advisory boards for Genentech, Exelixis, Pfizer,EMD serono, Bayer, Celgene, Pharmacyclics, Janssen, and Abbvie; and received institutional research funding from Acerta Pharma, ADC Therapeutics, Agenus, Aileron Therapeutics, Artios Pharma, AstraZeneca, Bicycle Therapeutics, BioNTech, Boehringer Ingelheim, Calithera, Celgene, Checkpoint Therapeutics, Ciclomed, Clovis, Curis, Cyteir Therapeutics, Daiichi Sankyo, Effector Therapeutics, Eli Lilly, EMD Serono, Evelo Biosciences, Forma Therapeutics, Genentech/Roche, Gilead, GlaxoSmithKline, H3 Biomedicine, Hengrui, Hutchinson MediPharma, Ignyta, Incyte, Jacobio, Janssen, Jounce Therapeutics, Klus Pharma, Kymab, Loxo Oncology, LSK Biopartners, Lycera, Mabspace,Macrogenics, Merck, Millennium Pharmaceuticals, Mirati Therapeutics, ModernaTX, ORIC Pharmaceuticals, Pfizer, Phoenix Molecular Designs, Placon Therapeutics, Portola Pharmaceuticals, Prelude Therapeutics, Qilu Puget Sound Biotherapeutics, Revolution Medicines, Ribon Therapeutics, Seven and Eight Biopharmaceuticals, Syndax, Synthorx, Stemline Therapeutics, Taiho, Takeda, Tesaro, TopAlliance, Vedanta, Verastem, Vigeo, and Xencor
JJH has received research funding from Bristol Myers Squibb and has consulted for Bristol Myers Squib, Merck, Eisai, Exelexis, Eli Lilly, QED, Zymework, ImVax, and Cytomx.
MHV has received commercial research grants from Bristol-Myers Squibb, Pfizer and Genentech/Roche; honoraria from Novartis and Bristol-Myers Squibb; travel/accommodation fees from AstraZeneca, Eisai, Novartis, and Takeda; and has served as a consultant/advisory board member for Alexion Pharmaceuticals, Aveo, Bayer, Calithera Biosciences, Corvus Pharmaceuticals, Exelixis, Eisai, GlaxoSmithKline, Merck, Natera; Onquality Pharmaceuticals; Novartis, and Pfizer.
TKO serves on the Data Safety Monitoring Committee for a Calithera-sponsored trial. He received payment for advisory board participation for Novartis Oncology, AstraZeneca, Bayer, Eisai, Amgen, Takeda, Debiopharma, Ipsen, Beigene, Lilly, Jannssen, Genentech, Merck, EMD Serono and Exelixis.
BC has no conflicts of interests to declare.
RS reports funding to CCR, NCI by Calithera Biosciences, Peloton/Merck partly defrayed the costs of clinical studies conducted in collaboration with these entities.
JCB has received institutional research funding from Gilead, Genentech/Roche, BMS, Five Prime, Lilly, Merck, MedImmune, Celgene, EMD Serono, Taiho, Macrogenics, GSK, Novartis, OncoMed, LEAP, TG Therapeutics, AstraZeneca, BI, Daiichi Sankyo, Bayer, Incyte, Apexigen, Koltan, SynDevRex, Forty Seven, AbbVie, Array, Onyx, Sanofi, Takeda, Eisai, Celldex, Agios, Cytomx, Nektar, ARMO, Boston Biomedical, Ipsen, Merrimack, Tarveda, Tyrogenex, Oncogenex, Marshall Edwards, Pieris, Mersana, Calithera, Blueprint, Evelo, FORMA, Merus, Jacobio, Effector, Novocare, Arrys, Tracon, Sierra, Innate, Arch Oncology, Prelude Oncology, Unum Therapeutics, Vyriad, Harpoon, ADC, Amgen, Pfizer, Millennium, Imclone, Acerta Pharma, Rgenix, Bellicum, Gossamer Bio, Arcus Bio, Seattle Genetics, TempestTx, Shattuck Labs, Synthorx, Inc, Revolution Medicines, Inc., Bicycle Therapeutics, Zymeworks, Relay Therapeutics, Scholar Rock, NGM Biopharma, Stemcentrx, Beigene, CALGB, Cyteir Therapeutics, Foundation Bio, Innate Pharma, Morphotex, OncXerna, NuMab, AtlasMedx, Treadwell Therapeutics, IGM Biosciences, Mabspace, Hutchinson MediPharma, REPARE Therapeutics, NeoImmune Tech, Regeneron, and PureTech Health; consulting/advisory roles (institutional) for Gilead, Genentech / Roche, BMS, Five Prime, Lilly, Merck, MedImmune, Celgene, Taiho, Macrogenics, GSK, Novartis, OncoMed, LEAP, TG Therapeutics, AstraZeneca, BI, Daiichi Sankyo, Bayer, Incyte, Apexigen, Array, Sanofi, ARMO, Ipsen, Merrimack, Oncogenex, FORMA, Arch Oncology, Prelude Therapeutics, Phoenix Bio, Cyteir, Molecular Partners, Innate, Torque, Tizona, Janssen, Tolero, Amgen, Seattle Genetics, Moderna Therapeutics, Tanabe Research Laboratories, Beigene, Continuum Clinical, Agios, Bicycle Therapeutics, Relay Therapeutics, Evelo, Pfizer, Samsung Bioepios, Fusion Therapeutics; and travel/food/beverage from Gilead, Genentech/Roche, BMS, Lilly, Merck, MedImmune, Celgene, Taiho, Novartis, OncoMed, BI, ARMO, Ipsen, Oncogenex, FORMA
YJ, SW, KO, and the late MKB are current or former employees and have held or currently hold ownership interest in Calithera Biosciences, Inc.
TMB has held consulting or advisory roles for Guardant Health, Loxo, Pfizer, Exelixis, Blueprint Medicines, Foundation Medicine, Bayer, AstraZeneca; has served on Speakers’ Bureaus for Bayer, Bristol-Myers Squibb, and Lilly; travel/accommodations/expenses from Astellas Pharma, AstraZeneca, celgene, Clovis Oncology, EMD Serono, Genentech, Lilly, Merck, Novartis, Pharmacyclics, Sysmex, and Pfizer. TMB reports institutional research funding from Daiichi Sankyo, Medpacto, Inc., Incyte, Mirati Therapeutics, MedImmune, Abbvie, AstraZeneca, Leap Therapeutics, MabVax, Stemline Therapeutics, Merck, Lilly, GlaxoSmithKline, Novartis, Pfizer, Genetech/Roche, Deciphera, Merrimack, Immunogen, Millennium, Ignyta, Calithera Biosciences, Koltan Pharmaceuticals, Principa Biopharma, Peleton, Immunocore, Roche, Aileron, Bristol-Myers Squibb, Amgen, Moderna Therapeutics, Sanofi, Boehringer Ingelheim, Astellas Pharma, Five Prime Therapeutics, Jacobio, Top Alliance BioScience, Loxo, Janssen, Clovis Oncology, Takeda, Karyopharm Therapeutics, Onyx, Phosplatin Therapeutics, Foundation Medicine, and ARMO Biosciences; and consulting/advisory roles (institutional) for Ignyta, Moderna Therapeutics, and Pfizer.
References
- 1.American Cancer Society: Cancer Facts & Figures 2019. Date accessed: January 10, 2020. URL: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf
- 2.National Comprehensive Cancer Network: Kidney Cancer (Version 2.2020). Date accessed: January 10, 2020. URL: https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf
- 3.Rathmell WK, Chen S: VHL inactivation in renal cell carcinoma: implications for diagnosis, prognosis and treatment. Expert Rev Anticancer Ther 8:63–73, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okazaki A, Gameiro PA, Christodoulou D, et al. : Glutaminase and poly(ADP-ribose) polymerase inhibitors suppress pyrimidine synthesis and VHL-deficient renal cancers. J Clin Invest 127:1631–1645, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoerner CR, Chen VJ, Fan AC: The ‘Achilles Heel’ of Metabolism in Renal Cell Carcinoma: Glutaminase Inhibition as a Rational Treatment Strategy. Kidney Cancer 3:15–29, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parlati F, Demo SD, Gross MI, et al. : CB-839, a novel potent and selective glutaminase inhibitor, has broad antiproliferative activity in cell lines derived from both solid tumors and hematological malignancies. Cancer Research 74:Abstract 1416, 2014. 24390735 [Google Scholar]
- 7.Harding J, Telli ML, Munster PN, et al. : A Phase 1 Dose-Escalation and Expansion Study of Telaglenastat in Patients With Advanced or Metastatic Solid Tumors. Clinical Cancer Research. 27:4994–5003, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escudier B, Porta C, Schmidinger M, et al. : Renal cell carcinoma: ESMO Clinical PracticeGuidelines for diagnosis, treatment and follow-up. Annals of Oncology 30:706–720, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Choueiri TK, Escudier B, Powles T, et al. : Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncology 17:917–927, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Emberley E, Bennett MK, Chen J, et al. : CB-839, a Selective Glutaminase Inhibitor, has Anti-Tumor Activity in Renal Cell Carcinoma and Synergizes with Cabozantinib and Everolimus, Keystone Symposia on Tumor Metabolism. Whistler, Canada, March 5–9, 2017 [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–47, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Wells JC, Stukalin I, Norton C, et al. : Third-line Targeted Therapy in Metastatic Renal Cell Carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. European Urology 71:204–209, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Choueiri TK, Escudier B, Powles T, et al. : Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. New England Journal of Medicine 373:1814–1823, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motzer RJ, Lee C, Emamekhoo H, et al. ENTRATA: Randomized, double-blind, phase 2 study of telaglenastat (tela; CB-839) + everolimus (E) vs. placebo (pbo) + E in patients (pts) with advanced/metastatic renal cell carcinoma (mRCC). Ann Oncol 30:v851–v934, 2019 [Google Scholar]
- 15.Tannir NM, Agarwal N, Porta C, et al. : CANTATA: Primary analysis of a global, randomized, placebo (Pbo)-controlled, double-blind trial of telaglenastat (CB-839) + cabozantinib versus Pbo + cabozantinib in advanced/metastatic renal cell carcinoma (mRCC) patients (pts) who progressed on immune checkpoint inhibitor (ICI) or anti-angiogenic therapies. J Clin Oncol 39(15 suppl):4501, 2021 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available within the article and its supplementary data files.