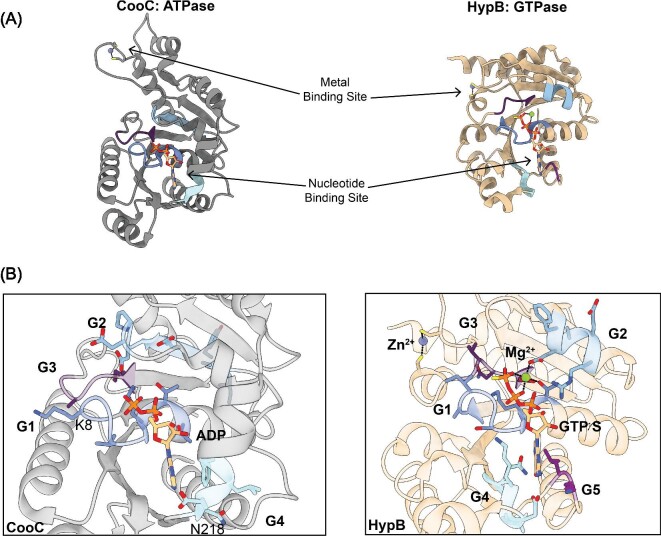
Fig. 5.
Comparison of the ATPase CooC and the GTPase HypB. (A) The ATPase metallochaperone CooC from C. hydrogenoformans (PBD 3KJI) (left, gray)60 and the GTPase metallochaperone HypB from M. jannaschii (PBD 2HF8) (right, tan)37 contain the conserved overall fold found in P-loop NTPases consisting of repeating α–β units. The metal-binding site and the nucleotide-binding site are spatially separated but involved in controlling the oligomeric states of the NTPases. Only one monomer for each metallochaperone is shown for simplicity. (B) (Left) A closer view of the ATP-binding site of CooC bound to ADP indicates that it contains the conserved G1–G3 motifs implicated in interacting with the phosphates of the nucleotide and the Mg2+ ion if present. The G1 residues (dark blue), also known as the Walker A motif, interact with the α and β phosphates and the bound Mg2+ ion. CooC contains the deviant Walker A motif with a highly conserved lysine residue (K8 in CooC) in the second position of the sequence instead of the second to last position. The G2 residues (light blue), also known as switch I, change conformation based on the nucleotide state and the conserved aspartate residue coordinates the bound Mg2+ ion. The G3 residues (dark purple), also known as the Walker B motif, coordinate the γ-phosphate and the Mg2+ ion if present. CooC only retains the first asparagine residue (N218 in CooC) of the G4 motif (cyan) that confers nucleotide specificity. An alpha helix, which is only present when a nucleotide is bound, interacts with the adenine base. (Right) A closer view of the GTP-binding site of HypB bound to GTPγS and a Mg2+ ion reveals the canonical G1–G5 motifs described in Fig. 2. Unlike CooC, HypB contains the conserved G4 motif (cyan) that confers nucleotide specificity by interacting with the guanosine base. The G5 residues (light purple) are involved in nucleotide dissociation and are not conserved in CooC.