Abstract
The hepatitis B and C viruses persist by evasion of T cell immunity. Persistence depends upon premature failure of CD4+ T cell help and loss of CD8+ T cell control because of epitope mutational escape and/or functional exhaustion. Powerful new immunological and transcriptomic tools provide insight into the mechanisms of T cell silencing by HBV and HCV. Similarities are apparent, including dysregulated expression of common inhibitory/immune checkpoint receptors and transcription factors. There are also differences. T cell exhaustion is uniform in HCV infection, but varies in HBV infection depending on disease stage and/or protein target. Here, we review recent advances defining similarities and differences in T cell evasion by HBV and HCV, and the potential for reversal following antiviral therapy.
Introduction
Persistent infection with the hepatitis B (HBV) and C (HCV) viruses can lead to serious chronic liver diseases including fibrosis, cirrhosis and hepatocellular carcinoma (HCC). How these liver-tropic viruses evade adaptive immune responses despite very different replication strategies and natural histories remains undefined. Accumulated evidence over the past two decades provides a compelling case that functional virus-specific T cells are required for spontaneous resolution of both infections [1,2].
A more detailed understanding of T cell immunity during acute and persistent infection remains a research priority, although the rationale differs for HBV and HCV. Chronic HBV infection can be prevented by vaccination. Nonetheless, approximately 250 million people live with chronic HBV infection globally [3]. Nucleos(t)ide analogs suppress virus replication but are not curative because the covalently closed circular DNA (cccDNA) HBV genome cannot be eliminated by this approach [4]. Long term control of virus replication after structured discontinuation of antiviral therapy is nonetheless observed in some treated individuals and may be T cell mediated. Research is therefore focused on understanding the nature of T cell failure in chronic HBV infection, with the objective of restoring responses as reviewed by Thimme in this volume [5]. HCV presents the opposite problem. Almost all chronic infections can now be cured safely with a relatively short course of direct acting antivirals (DAA) that impair the function of HCV non-structural (NS) proteins [ 6]. Therapies to restore HCV-specific T cell immunity are no longer a research or clinical priority. Attention is now focused on whether T cell immunity recovers after DAA therapy to provide protection from HCV reinfection. A second objective is to define features of acute T cell responses that contribute to spontaneous resolution observed in approximately 25% of HCV infections. This question is central to vaccine development, a major unmet need as HCV transmission continues to outpace antiviral treatment in most regions of the world [6,7].
Recently, high dimensional phenotypic and transcriptomic analyses of HBV-specific and HCV-specific T cells have emerged as powerful tools to better understand the nature of responses in acute and chronic infection of humans. They have been aided by methods to enrich for circulating virus-specific T cells that are present at low frequency in chronic HBV and HCV infection. Here, we review similarities in CD4+ and CD8+ T cell responses against HBV and HCV revealed by these studies, and also differences that are likely due to their unique replication cycles and natural histories. Finally, we summarize their functional restoration in response to antiviral therapy (Figure 1).
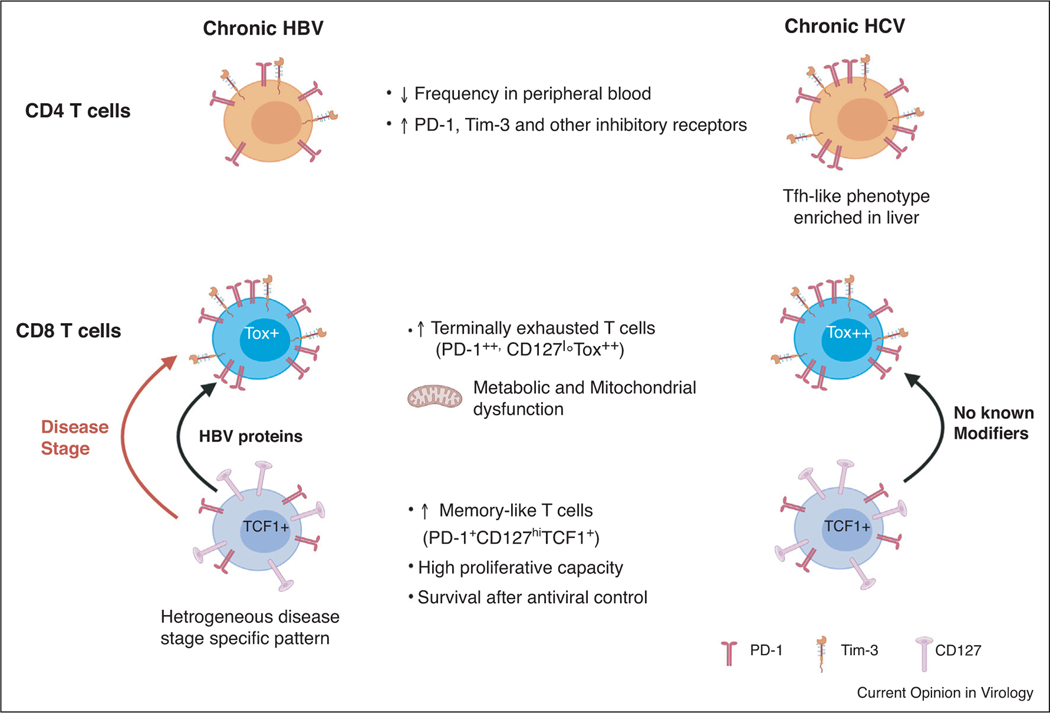
Figure 1.
CD4+ and CD8+ T cell similarities and differences in during chronic HBV and HCV infection. HBV and HCV-specific CD8+ T cells transition from a memory-like state to an exhausted state during chronic infection (indicated by arrows). HBV-specific CD8+ T cells are more heterogeneous in phenotype and function as the transition between states may be modifed by disease stage (orange arrow) and HBV protein (green arrow). There are no known modifiers of this transition in chronic HCV infection (purple arrow) and CD8+ T cell heterogeneity is not as apparent.
CD4+ T cell responses
CD4+ T cell responses leading to spontaneous resolution of HBV and HCV infections have been described as multi-specific and sustained [1,2,8]. Onset of the CD4+ T cell response in both infections typically occurs after 8–12 weeks of infection, an unusually long delay when compared with other acute virus infections. They nonetheless follow a typical differentiation program in the early phase of acute infection. For instance, HCV-specific CD4+ T cells express inhibitory/immune checkpoint receptors programmed death-1 (PD-1), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), and T cell immunoreceptor with Ig and ITIM domains (TIGIT) that are markers of activation and serve as a physiological brake on the response [9,10●,11]. Resolution of acute hepatitis C is associated with a decline in expression of the inhibitory/ immune checkpoint receptors [9,10●] and an increase in CD127, the IL-7α receptor that is a marker of memory T cells [10●]. Upregulation of PD-1 and possibly other co-inhibitory receptors almost certainly occurs during acute HBV infection although this has not yet been formally demonstrated. Many studies have established an association between resolution of HBV and HCV infections and sustained production of Th1 cytokines IFN-γ, TNF-α, and IL-2 by CD4+ T cells [1,2,8]. More recently a key role for CD4+ cell production of IL-21 has emerged. IL-21 synthesized by HCV-specific CD4+ T cells was associated with spontaneous clearance of infection and enhanced survival of HCV-specific CD8+ T cells [12] as well as seroconversion to HCV envelope proteins [13]. Circulating HCV-specific CD4+ T cells with a follicular helper (Tfh) phenotype are the most likely source of IL-21 during acute infection. They were detectable during acute resolving infections but were either undetectable or disappeared from circulation with HCV persistence, though they remain detectable in the liver [13,14]. HBV-specific CD4+ T cells that produced IL-21 were also detected during the acute but not the chronic phase of HBV infection [15,16], and in one study were identified as Tfh by expression of the subset-specific marker CXCR5 [15]. These observations are consistent with high serum IL-21 titers coinciding with a transaminase peak in humans with acute resolving hepatitis B [17].
When and how acute phase CD4+ T cell responses fail is a key unanswered question. One possibility is that CD4+ T cell responses in chronically evolving infections are inher-ently limited in breadth (i.e. the array of MHC class II epitopes targeted) or function (i.e. number and type of cytokines produced). Comparison of CD4+ T cells in the early phase of acute resolving versus persisting HCV infections suggest that this is not the case. CD4+ T cells in the early acute phase of hepatitis C targeted a broad array of epitopes [18] and produced multiple Th1 cyto-kines [10●] regardless of the infection outcome. Chronic HCV infection was instead associated with impaired proliferation of CD4+ T cells that were otherwise multi-specific and multifunctional [18]. Mechanisms causing impaired CD4+ T cell proliferation have not been defined, but may in part involve dysregulated expression of inhibitory receptors. Sustained checkpoint/inhibitory receptor expression on CD4+ T cells presages chronic HBV and HCV infection. PD-1 is the most widely distributed receptor on CD4+ T cells in chronic infection [9,10●,19,20]. Several other co-inhibitory receptors including CD160, LAG-3, TIM-3, KLRG1, and 2B4 have been visualized on HBV and HCV-specific CD4+ T cells, although they are not as widely distributed when compared with exhausted CD8+ T cells [9,10●,14]. Antibody mediated-blockade of inhibitory receptor signaling alone is not sufficient to restore function to most CD4+ T cell populations from patients with chronic HBV [20,21] and HCV [19] infections. Costimulation may be required in addition to blockade of inhibitory receptors for recovery of helper function. One recent study in HBV-infected subjects demonstrated partial restoration of IFN-γ and IL-21 production by in vitro incubation of CD4+ T cells with a combination of antibodies against PD-1 and the co-stimulatory receptor OX40 when compared with anti-PD-1 antibodies alone [21].
Together, these studies point to complex regulation of CD4+ T cell proliferation and effector function in chronic HBV and HCV infection. Transcriptional activity and epigenetic studies may shed further light on silencing mechanism(s) in acute and chronic HBV and HCV infection, although these approaches have been limited by very low frequencies of circulating virus-specific CD4+ T cells.
CD8+ T cell responses
CD8+ T cell responses are also primed in all individuals during acute HBV and HCV infection. CD8+ T cell expansion typically occurs at 8–12 weeks post-infection, in coordination with the delayed CD4+ T cell response described above [1,2,8]. CD8+ T cell immunity is compromised by abrupt disappearance of virus-specific CD4+ T helper cells in HBV and HCV infections that persist [1,2,8]. CD8+ T cells that develop with inadequate help display a gradual loss of proliferative capacity, cytokine production and cytotoxicity [1,2,8]. This process can lead to mutational escape of some HBV and HCV class I epitopes as reviewed by Neumann-Haefelin in this volume [5]. Not all class I epitopes escape recognition; CD8+ T cells targeting epitopes that remain intact transition to an exhausted state. Metabolic dysregulation of HCV-specific CD8+ T cells is apparent in the early acute phase of HCV infections that persist but not those that resolve [22,23]. Transcriptomic analysis documented dysregulation of genes involved in nucleosomal transcription, T cell differentiation, and inflammation and was linked to impairment of the HCV-specific CD4+ T cell response [22]. Transcriptome profiling also revealed mitochondrial and metabolic dysregulation in HBV-specific CD8+ T cells from patients with chronic hepatitis B [24], suggesting common cellular pathways to exhaustion in both infections. HBV and HCV-specific CD8+ T cells that are terminally exhausted express low or no CD127 [25●,26–28,29●●]. They do co-express high levels of PD-1 and other inhibitory receptors [1,30–33] as well as the TOX transcription factor that is an essential driver of exhaustion [25●,34●]. While this phenotype appears to be consistent for severely exhausted CD8+ T cells, variability in expression of PD-1, CD127, and key transcription factors has been described in both infections [27,29●●,34●,35●●,37].
Chronic HCV infection is characterized by relatively stable levels of persistent virus replication over decades without hepatitis flares or large shifts in virus replication and immune activation that characterize chronic hepatitis B [5,38]. It most closely follows the exhaustion paradigm established in mice persistently infected with LCMV, where severely exhausted CD8+ T cells are replenished from a phenotypically and transcriptionally distinct memory-like population [39]. Memory-like HCV-specific CD8+ T cells have higher CD127 and intermediate PD-1 expression when compared with exhausted CD8+ T cells that are terminally differentiated [27,37]. They are also positive for the transcription factor TCF-1 that spares proliferative capacity and is likely essential for longevity of virus-specific populations over decades of chronic infection [27,37]. A combination of methods including input bulk RNA-seq, single cell RNA-seq (scRNA-seq) and high resolution flow cytometry identified HCV-specific CD8+ T cell with CD127hi, CD127int and CD127lo expression patterns [40●●]. CD127hi cells and CD127lo had distinct molecular and phenotypic signatures of memory-like and exhausted CD8+ T cells, respectively [40●●]. This analysis also confirmed a progenitor–progeny relationship between CD127hi memory-like and CD127lo exhausted CD8+ T cells in chronic HCV infection, and demonstrated that they transition through a CD127 intermediate stage [40●●]. CD8+ T cells targeting escaped HCV class I epitopes also have reduced PD-1 intensity and increased CD127 expression, reflecting an absence of ongoing antigen stimulation [41]. Transcriptomic, metabolic and functional profiles were recently confirmed as more memory-like and less exhausted for CD8+ T cells targeting escaped versus intact HCV epitopes [22].
CD8+ T cells are considerably more heterogeneous in function and phenotype in chronic HBV versus HCV infection. A more complex natural history is significant factor contributing to this heterogeneity. Approximately 90% of vertical HBV infections and 10% of adult infections persist, resulting in chronic HBV that is classified into 5 stages defined by the presence or absence of inflammation and HBsAg and HBeAg antigenemia [ 42]. Significant shifts in the severity of liver inflammation, immune system activation, and virus replication and antigen production occur across the disease stages [38]. Recent studies demonstrated that HBV-specific CD8+ T cells are present across all stages of infection, including the so-called immune tolerant phase stage that follows perinatal infection and is defined by high HBeAg and HBV DNA titers, ALT within normal limits, and low or no liver disease activity for more than a decade [29●●,35●●,36●●,43,44]. High dimensional phenotypic profiling of CD8+ T cells targeting core and polymerase revealed disease-stage specific differences in patterns of inhibitory receptors and CD127 expression [35●●]. CD8+ T cells visualized in the immune tolerant phase were unexpectedly skewed towards a memory phenotype with lower expression of inhibitory receptors including PD-1 and increased expression of CD127 despite high levels of HBV replication and antigen production. The immune active phase characterized by lower or fluctuating HBV DNA and antigenemia, elevated ALT and liver inflammation was often associated with a severely exhausted CD8+ T cell phenotype commonly observed in HCV infection. CD8+ T cells were more memory-like during the inactive carrier state defined by low or negative HBV DNA and antigenemia. Relatively high expression of PD-1 and TIGIT during this stage may reflect functional activation and ongoing effective control of virus replication [35●●].
Heterogeneity in exhaustion profile has also been observed for CD8+ T cells targeting different HBV proteins. Phenotypic differences between core and polymerase specific CD8+ T cells were described in two studies of patients with chronic hepatitis B [29●●,36●●]. Core-specific populations underwent more robust expansion in culture when compared with polymerase-specific populations. They also displayed a less exhausted phenotype, with lower levels of PD-1 and higher CD127. Co-expression of the inhibitory receptor KLRG1 and the Eomes/T-bet transcription factor ratio was also higher in the polymerase-specific populations, indicating more profound terminal exhaustion [29●●]. This is consistent with the recent finding that TOX, a transcription factor that enforces exhaustion, was most highly expressed in populations targeting the polymerase protein [34●]. It is noteworthy that TOX was nonetheless expressed at lower levels in HBV-versus HCV-specific CD8+ T cells. Finally, the function and phenotype of core-specific CD8+ T cells did not vary with epitope sequence, a contrast with HCV infection where mutational escape favors a memory-like or less exhausted phenotype [29●●,36●●].
Recovery of exhausted T cells?
Whether exhausted T cells recover after control of virus replication to form functional, long-lived populations is of vital importance to strategies for prevention and therapy of chronic infections. Cure of persistent HCV infection by DAA-therapy provides a unique opportunity to address this question. Transcriptomic comparison of circulating CD8+ T cells taken before and after DAA-mediated HCV cure confirmed preferential maintenance of memory-like virus-specific CD8+ T cells [37]. These memory-like T cells harbored the same transcriptomic signatures [40●●] and TOX expression was sustained [25●] before and after cure, suggesting a permanent ‘exhaustion scar’ that cannot be reversed by HCV cure. These observations are consistent with an earlier study that showed exhausted CD8+ T cells in one subject with chronic HCV acquire a state-specific epigenetic landscape and chromatin accessibility similar to that observed in exhausted CD8+ T cells from LCMV infected mice [45]. HCV antigens did stimulate production of IFN-γ and TNF-α by memory-like CD8+ T cells after DAA cure, but at levels that were reduced when compared to true memory cells generated by spontaneous resolution of acute HCV infection. Whether they provide protection from HCV persistence upon re-exposure to the virus after DAA cure is not yet fully understood. In one case study of a DAA-treated chimpanzee, transient control of a second HCV infection coincided with expansion of a CD8+ T cells that targeted an escaped epitope, but not those that targeted an intact epitope [46]. Relapse in HCV infection in a DAA treated human subject resulted in re-emergence of CD8+ T cells with an exhausted phenotype [37]. Of note, the HCV genome is translated as a single polyprotein from one internal ribosomabl entry site (IRES) element. Protein-specific heterogeneity in CD8+ T cell exhaustion is therefore conceivably less likely when compared with HBV infection. Futher research is needed to determine if all CD8+ T cells targeting intact epitopes in HCV proteins exhibit the same degree of exhaustion, with poor recovery and protective capacity after DAA treatment.
Studies of CD4+ T cell recovery after DAA cure have lagged behind those of CD8+ T cells, in large measure because their frequency in blood is much lower. HCV-specific CD4+ T cells maintained an exhausted profile after DAA treatment of chronic hepatitis C, with low levels of CD127 expression, proliferation and cytokine production [47]. CD4+ T cell subset-specific differences in survival after DAA cure is a possibility, as loss of exhausted Th1 and mobilization or expansion of Tfh subsets after antiviral cure has been observed [48]. The potential for restoration of CD4+ T cell help during chronic hepatitis C was established in the unique setting of pregnancy [48]. A spontaneous multilog drop in viremia has been observed in a subset of chronically infected women approximately 2–3 months after childbirth. Significant expansion of circulating HCV-specific CD4+ T cells that produced IL-2 and IFN-γ in the post-partum period was associated with this dramatic, though often transient, control of persistent virus replication [49]. Together, these preliminary observations suggest that T cell recovery is possible after years of persistent HCV replication, but that DAA-mediated reduction or elimination of virus replication and antigen load alone may not be sufficient to achieve this goal. It is possible that impaired CD4+ and CD8+ T cells responses can be restored by vaccination after DAA cure, a concept that is now being tested in humans (ClinicalTrials.gov identifier NCT03688061).
As noted above, a sterilizing cure of chronic HBV infection with elimination of cccDNA is not possible with current direct acting antiviral therapies [4]. The ability to achieve a functional cure, defined as sustained HBsAg seronegativity after withdrawal of nucleos(t)ide therapy, is an important priority but often complicated by severe hepatic flares. Several recent studies point towards a key role for T cells in prevention of hepatic flares and control of chronic infection [16,50,51]. HBV-specific PD-1 positive T cells that proliferate in response to antigen stimulation were identified as a biomarker for safe termination of antiviral therapy [51]. An increase in CD4+ T cell proliferation was also observed in patients with sustained virus control after structured nucleos(t)ide withdrawal, and was associated with a trend towards higher PD-1 expression [50]. The importance of a change in the functional profile of HBV-specific CD4+ T cells to hepatocellular damage and virus control was underscored in study of patients with severe hepatic flares [16]. Hepatitis in these patients was associated with TNF-α synthesis by HBV-specific CD4+ T cells [16]. Virus control, on the other hand, was associated with a transition to IFN-γ production [16]. Together, these observations are consistent with functional heterogeneity of PD-1 positive T cells that may be more mildly exhausted in chronic HBV infection as described above [34●,35●●,36●●], and suggest that their effector functions are closely linked to severe hepatic flares and long-term control of virus replication. Whether HBV-specific T cells in chronic resolving HBV infection are fully recovered is uncertain, as they do continue to express the TOX transcription factor as described for HCV-specific CD8+ T cells after DAA cure [25●,29●●,34●,37].
Concluding remarks
Features of T cell immunity elicited by HBV and HCV infection are broadly similar. This is especially true in acute resolving infections, where activated PD-1hiC-D127low CD4+ and CD8+ T cells transition to durable PD-1lowCD127hi memory populations as is typical of other acute virus infections. Sustained CD4+ T cell responses are essential to control of both infections, especially IL-21 production by the Tfh subset that quite likely supports CD8+ T cell [12] and possibly B cell responses [13].
Mechanisms of CD8+ T cell silencing in the chronic phase of HBV and HCV infection are also similar, but key differences have emerged in recent studies. Severely exhausted CD8+ T cells are most similar in both infections, with intense expression of PD-1 and multiple other inhibitory receptors as well as the TOX transcription factor. Phenotypically and transcriptionally distinct memory-like CD8+ T cells that replenish the exhausted pool are evident in HBV and HCV infections, and may preferentially survive after virus antigen production is terminated or suppressed usually as a result of antiviral therapy. However, the scope of CD8+ T cell heterogeneity in expression of inhibitory receptors, transcription factors, and differentiation antigens appears to be much broader in chronic HBV infection, possible due to significant fluctuation in virus control and liver inflammation over decades of persistent infection and/or strategies for protein translation from the HBV versus HCV genome. Collectively, these observations point towards a milder or less uniform pattern of exhaustion in HBV than HCV infection. Further definition of the phenotypic, transcriptomic and epigenetic landscape in HBV and HCV-specific T cells provides an exciting pathway forward. A comparison of similarities and differences in T cell responses to these viruses is likely to provide a more holistic understanding of pathways lead to chronic infection in humans. They may also provide fundamental insights necessary to engineer strategies for prevention or reversal of T cell loss in both infections.
Acknowledgements
Our research is funded by the Canadian Institutes of Health Research (CIHR) (PJT-173467), the National Institutes of Health (U01AI131313, R01AI136533,U19AI159819,1 R01 AI 126890-01,1 R01 AI 096882, andU19 AI20123178).
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
● of special interest
●● of outstanding interest
- 1.Abdel-Hakeem MS, Shoukry NH: Protective immunity against hepatitis C: many shades of gray. Front Immunol 2014, 5:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertoletti A, Ferrari C: Adaptive immunity in HBV infection. J Hepatol 2016, 64:S71–S83. [DOI] [PubMed] [Google Scholar]
- 3.Polaris Observatory Collaborators: Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018, 3:383–403. [DOI] [PubMed] [Google Scholar]
- 4.Martinez MG, Boyd A, Combe E, Testoni B, Zoulim F: Covalently closed circular DNA: the ultimate therapeutic target for curing hepatitis B virus infections. J Hepatol 2021. [DOI] [PubMed]
- 5.Salimi Alizei Elahe, Hofmann Maike, Thimme Robert, Neumann-Haefelin: Mutational escape from cellular immunity in viral hepatitis: variations on a theme. Current Opinion in Virology 2021, 50:110–118 10.1016/j.coviro.2021.08.002 In this issue. [DOI] [PubMed] [Google Scholar]
- 6.Spearman CW, Dusheiko GM, Hellard M, Sonderup M: Hepatitis C. Lancet 2019, 394:1451–1466. [DOI] [PubMed] [Google Scholar]
- 7.Bailey JR, Barnes E, Cox AL: Approaches, progress, and challenges to hepatitis C vaccine developmen. Gastroenterology 2019, 156:418–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kemming J, Thimme R, Neumann-Haefelin C: Adaptive immune response against hepatitis C virus. Int J Mol Sci 2020, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ackermann C, Smits M, Woost R, Eberhard JM, Peine S, Kummer S, Marget M, Kuntzen T, Kwok WW, Lohse AW et al. : HCV-specific CD4+ T cells of patients with acute and chronic HCV infection display high expression of TIGIT and other co-inhibitory molecules. Sci Rep 2019, 9:10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen DY, Wolski D, Aneja J, Matsubara L, Robilotti B, Hauck G, de Sousa PSF, Subudhi S, Fernandes CA, Hoogeveen RC et al. : Hepatitis C virus-specific CD4+ T cell phenotype and function in different infection outcomes. J Clin Invest 2020, 130:768–773 •This study demonstrated that HCV-specific CD4+ T cells were broadly functional in the early phase of acute hepatitis C regardless of whether the infection ultimately resolved or persisted. It extends earlier findings that CD4+ T cells target a broad array of epitopes in acute resolving and persisting infections [17].
- 11.Binder B, Thimme R: CD4+ T cell responses in human viral infection: lessons from hepatitis C. J Clin Invest 2020, 130:595–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kared H, Fabre T, Bedard N, Bruneau J, Shoukry NH: Galectin-9 and IL-21 mediate cross-regulation between Th17 and treg cells during acute hepatitis C. PLoS Pathog 2013, 9:e1003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salinas E, Boisvert M, Upadhay A, Bédard N, Nelson SA, Bruneau J, Derdeyn C, Marcotrigiano J, Evans M, Bosinger S et al. : Early T follicular helper cell activity accelerates HCV-specific B cell expansion in acute resolving infection. J Clin Invest 2021, 131:e140590 10.1172/JCI141594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raziorrouh B, Sacher K, Tawar RG, Emmerich F, Neumann-Haefelin C, Baumert TF, Thimme R, Boettler T: Virus-specific CD4+ T cells have functional and phenotypic characteristics of follicular T-helper cells in patients with acute and chronic HCV infections. Gastroenterology 2016, 150:696–706 e693. 15. [DOI] [PubMed] [Google Scholar]
- 15.Jia Y, Zeng Z, Li Y, Li Z, Jin L, Zhang Z, Wang L, Wang FS: Impaired function of CD4+ T follicular helper (Tfh) cells associated with hepatocellular carcinoma progression. PLoS One 2015, 10:e0117458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang H, Luo H, Wan X, Fu X, Mao Q, Xiang X, Zhou Y, He W, Zhang J, Guo Y et al. : TNF-alpha/IFN-gamma profile of HBV-specific CD4 T cells is associated with liver damage and viral clearance in chronic HBV infection. J Hepatol 2020, 72:45–56. 17. [DOI] [PubMed] [Google Scholar]
- 17.Yoshio S, Mano Y, Doi H, Shoji H, Shimagaki T, Sakamoto Y, Kawai H, Matsuda M, Mori T, Osawa Y et al. : Cytokine and chemokine signatures associated with hepatitis B surface antigen loss in hepatitis B patients. JCI Insight 2018, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulze Zur Wiesch J, Ciuffreda D, Lewis-Ximenez L, Kasprowicz V, Nolan BE, Streeck H, Aneja J, Reyor LL, Allen TM, Lohse AW et al. : Broadly directed virus-specific CD4+ T cell responses are primed during acute hepatitis C infection, but rapidly disappear from human blood with viral persistence. J Exp Med 2012, 209:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raziorrouh B, Ulsenheimer A, Schraut W, Heeg M, Kurktschiev P, Zachoval R, Jung MC, Thimme R, Neumann-Haefelin C, Horster S et al. : Inhibitory molecules that regulate expansion and restoration of HCV-specific CD4+ T cells in patients with chronic infection. Gastroenterology 2011, 141:1422–1431 1431 e1421–1426. [DOI] [PubMed] [Google Scholar]
- 20.Raziorrouh B, Heeg M, Kurktschiev P, Schraut W, Zachoval R, Wendtner C, Wachtler M, Spannagl M, Denk G, Ulsenheimer A et al. : Inhibitory phenotype of HBV-specific CD4+ T-cells is characterized by high PD-1 expression but absent coregulation of multiple inhibitory molecules. PLoS One 2014, 9:e105703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobi FJ, Wild K, Smits M, Zoldan K, Csernalabics B, Flecken T, Lang J, Ehrenmann P, Emmerich F, Hofmann M et al. : OX40 stimulation and PD-L1 blockade synergistically augment HBV-specific CD4 T cells in patients with HBeAg-negative infection. J Hepatol 2019, 70:1103–1113. [DOI] [PubMed] [Google Scholar]
- 22.Wolski D, Foote PK, Chen DY, Lewis-Ximenez LL, Fauvelle C, Aneja J, Walker A, Tonnerre P, Torres-Cornejo A, Kvistad D et al. : Early transcriptional divergence marks virus-specific primary human CD8(+) T cells in chronic versus acute infection. Immunity 2017, 47:648–663.e648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barili V, Fisicaro P, Montanini B, Acerbi G, Filippi A, Forleo G, Romualdi C, Ferracin M, Guerrieri F, Pedrazzi G et al. : Targeting p53 and histone methyltransferases restores exhausted CD8+ T cells in HCV infection. Nat Commun 2020, 11:604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisicaro P, Barili V, Montanini B, Acerbi G, Ferracin M, Guerrieri F, Salerno D, Boni C, Massari M, Cavallo MC et al. : Targeting mitochondrial dysfunction can restore antiviral activity of exhausted HBV-specific CD8 T cells in chronic hepatitis B. Nat Med 2017, 23:327–336. [DOI] [PubMed] [Google Scholar]
- 25. _Alfei F, Kanev K, Hofmann M, Wu M, Ghoneim HE, Roelli P, Utzschneider DT, von Hoesslin M, Cullen JG, Fan Y et al. : TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature 2019, 571:265–269 •This study provides evidence that expression of the TOX transcription factor is associated with CD8+ T cell exhaustion in chronic HCV infection, and is complementary to a similar study in chronic HBV infection [33•].
- 26.Paley MA, Kroy DC, Odorizzi PM, Johnnidis JB, Dolfi DV, Barnett BE, Bikoff EK, Robertson EJ, Lauer GM, Reiner SL et al. : Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science 2012, 338:1220–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Utzschneider DT, Charmoy M, Chennupati V, Pousse L, Ferreira DP, Calderon-Copete S, Danilo M, Alfei F, Hofmann M, Wieland D et al. : T cell factor 1-expressing memory-like CD8(+) T cells sustain the immune response to chronic viral infections. Immunity 2016, 45:415–427. [DOI] [PubMed] [Google Scholar]
- 28.Abdel-Hakeem MS, Bedard N, Murphy D, Bruneau J, Shoukry NH: Signatures of protective memory immune responses during hepatitis C virus reinfection. Gastroenterology 2014, 147:870–881.e878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schuch A, Salimi Alizei E, Heim K, Wieland D, Kiraithe MM, Kemming J, Llewellyn-Lacey S, Sogukpinar O, Ni Y, Urban S et al. : Phenotypic and functional differences of HBV core-specific versus HBV polymerase-specific CD8+ T cells in chronically HBV-infected patients with low viral load. Gut 2019, 68:905–915 ••This study presents evidence for functional and phenotypic differences in HBV-specific CD8+ T cells targeting discrete HBV proteins. With Refs [34••] and [35••], it provides a conceptual basis for therapies to selectively restore less-exhausted CD8+ T cells populations in chronic hepatitis B.
- 30.Bengsch B, Martin B, Thimme R: Restoration of HBV-specific CD8+ T cell function by PD-1 blockade in inactive carrier patients is linked to T cell differentiation. J Hepatol 2014, 61:1212–1219. [DOI] [PubMed] [Google Scholar]
- 31.Raziorrouh B, Schraut W, Gerlach T, Nowack D, Gruner NH, Ulsenheimer A, Zachoval R, Wachtler M, Spannagl M, Haas J et al. : The immunoregulatory role of CD244 in chronic hepatitis B infection and its inhibitory potential on virus-specific CD8+ T-cell function. Hepatology 2010, 52:1934–1947. [DOI] [PubMed] [Google Scholar]
- 32.Schurich A, Khanna P, Lopes AR, Han KJ, Peppa D, Micco L, Nebbia G, Kennedy PT, Geretti AM, Dusheiko G et al. : Role of the coinhibitory receptor cytotoxic T lymphocyte antigen-4 on apoptosis-prone CD8 T cells in persistent hepatitis B virus infection. Hepatology 2011, 53:1494–1503. [DOI] [PubMed] [Google Scholar]
- 33.Gupta PK, Godec J, Wolski D, Adland E, Yates K, Pauken KE, Cosgrove C, Ledderose C, Junger WG, Robson SC et al. : CD39 expression identifies terminally exhausted CD8+ T cells. PLoS Pathog 2015, 11:e1005177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heim K, Binder B, Sagar, Wieland D, Hensel N, Llewellyn-Lacey S, Gostick E, Price DA, Emmerich F, Vingerhoet H et al. : TOX defines the degree of CD8+ T cell dysfunction in distinct phases of chronic HBV infection. Gut 2020 •Demonstration that CD8+ T cells exhaustion in chronic hepatitis B is associated with expression of TOX. Moreover, heterogeneity in TOX expression is consistent with differences in the degree of exhaustion that is disease stage and protein specific (see Refs. [28 ••,34 ••,••35]).
- 35. Cheng Y, Zhu YO, Becht E, Aw P, Chen J, Poidinger M, de Sessions PF, Hibberd ML, Bertoletti A, Lim SG et al. : Multifactorial heterogeneity of virus-specific T cells and association with the progression of human chronic hepatitis B infection. Sci Immunol 2019, 4 ••A detailed analysis of HBV-specific CD8+ T cells demonstrating disease stage-specific phenotypic heterogeneity in expression of markers associated with exhaustion, effector, and memory profiles.
- 36. Hoogeveen RC, Robidoux MP, Schwarz T, Heydmann L, Cheney JA, Kvistad D, Aneja J, Melgaco JG, Fernandes CA, Chung RT et al. : Phenotype and function of HBV-specific T cells is determined by the targeted epitope in addition to the stage of infection. Gut 2019, 68:893–904 ••This study, with Refs. [28••] and [35••], demonstrates disease and protein-specific heterogeneity in HBV-specific CD8+ T cell populations during chronic infection.
- 37.Wieland D, Kemming J, Schuch A, Emmerich F, Knolle P, Neumann-Haefelin C, Held W, Zehn D, Hofmann M, Thimme R: TCF1(+) hepatitis C virus-specific CD8(+) T cells are maintained after cessation of chronic antigen stimulation. Nat Commun 2017, 8:15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwon H, Lok AS: Hepatitis B therapy. Nat Rev Gastroenterol Hepatol 2011, 8:275–284. [DOI] [PubMed] [Google Scholar]
- 39.Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, Lynn RC, Philip M, Rao A, Restifo NP et al. : Defining ‘T cell exhaustion’. Nat Rev Immunol 2019, 19:665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hensel N, Gu Z, Sagar, Wieland D, Jechow K, Kemming J, Llewellyn-Lacey S, Gostick E, Sogukpinar O, Emmerich F et al. : Memory-like HCV-specific CD8(+) T cells retain a molecular scar after cure of chronic HCV infection. Nat Immunol 2021, 22:229–239 ••A clear demonstration that HCV-specific CD8+ T cells with a memory-like phenotype but core transcriptional signature associated with exhaustion are retained after antiviral cure of chronic infection.
- 41.Kasprowicz V, Kang YH, Lucas M, Schulze zur Wiesch J, Kuntzen T, Fleming V, Nolan BE, Longworth S, Berical A, Bengsch B et al. : Hepatitis C virus (HCV) sequence variation induces an HCV-specific T-cell phenotype analogous to spontaneous resolution. J Virol 2010, 84:1656–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.European Association for the Study of the Liver, Electronic address: easloffice@easloffice.eu: EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017, 67:370–398 Electronic address eee. [DOI] [PubMed] [Google Scholar]
- 43.Sung PS, Park DJ, Kim JH, Han JW, Lee EB, Lee GW, Nam HC, Jang JW, Bae SH, Choi JY et al. : Ex vivo detection and characterization of hepatitis B virus-specific CD8(+) T cells in patients considered immune tolerant. Front Immunol 2019, 10:1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mason WS, Gill US, Litwin S, Zhou Y, Peri S, Pop O, Hong ML, Naik S, Quaglia A, Bertoletti A et al. : HBV DNA integration and clonal hepatocyte expansion in chronic hepatitis B patients considered immune tolerant. Gastroenterology 2016, 151:986–998 e984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, Tsao HW, Godec J, LaFleur MW, Brown FD et al. : The epigenetic landscape of T cell exhaustion. Science 2016, 354:1165–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Callendret B, Eccleston HB, Hall S, Satterfield W, Capone S, Folgori A, Cortese R, Nicosia A, Walker CM: T-cell immunity and hepatitis C virus reinfection after cure of chronic hepatitis C with an interferon-free antiviral regimen in a chimpanzee. Hepatology 2014, 60:1531–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hartnell F, Esposito I, Swadling L, Brown A, Phetsouphanh C, de Lara C, Gentile C, Turner B, Dorrell L, Capone S et al. : Characterizing hepatitis C virus-specific CD4(+) T cells following viral-vectored vaccination, directly acting antivirals, and spontaneous viral cure. Hepatology 2020, 72:1541–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smits M, Zoldan K, Ishaque N, Gu Z, Jechow K, Wieland D, Conrad C, Eils R, Fauvelle C, Baumert TF et al. : Follicular T helper cells shape the HCV-specific CD4+ T cell repertoire after virus elimination. J Clin Invest 2020, 130:998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coss SL, Torres-Cornejo A, Prasad MR, Moore-Clingenpeel M, Grakoui A, Lauer GM, Walker CM, Honegger JR: CD4+ T cell restoration and control of hepatitis C virus replication after childbirth. J Clin Invest 2020, 130:748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rinker F, Zimmer CL, Honer Zu Siederdissen C, Manns MP, Kraft ARM, Wedemeyer H, Bjorkstrom NK, Cornberg M: Hepatitis B virus-specific T cell responses after stopping nucleos(t)ide analogue therapy in HBeAg-negative chronic hepatitis B. J Hepatol 2018, 69:584–593. [DOI] [PubMed] [Google Scholar]
- 51.Rivino L, Le Bert N, Gill US, Kunasegaran K, Cheng Y, Tan DZ, Becht E, Hansi NK, Foster GR, Su TH et al. : Hepatitis B virus-specific T cells associate with viral control upon nucleos(t)ide-analogue therapy discontinuation. J Clin Invest 2018, 128:668–681. [DOI] [PMC free article] [PubMed] [Google Scholar]