Abstract
Artificial control of neuronal activity enables the study of neural circuits and restoration of neural functions. Direct, rapid, and sustained photocontrol of intact neurons could overcome the limitations of established electrical stimulation such as poor selectivity. We have developed fast photoswitchable ligands of glutamate receptors (GluRs) to enable neuronal control in the auditory system. The new photoswitchable ligands induced photocurrents in untransfected neurons upon covalently tethering to endogenous GluRs and activating them reversibly with visible light pulses of a few milliseconds. As a proof of concept of these molecular prostheses, we applied them to the ultrafast synapses of auditory neurons of the cochlea that encode sound and provide auditory input to the brain. This drug-based method afforded the optical stimulation of auditory neurons of adult gerbils at hundreds of hertz without genetic manipulation that would be required for their optogenetic control. This indicates that the new photoswitchable ligands are also applicable to the spatiotemporal control of fast spiking interneurons in the brain.
Introduction
Ultrafast signaling is a feature of several important neural circuits such as those in the auditory pathway, the brainstem, the cerebellum, and the cerebrum.1−3 Such signaling builds on specialized synapses for synchronous neurotransmission as well as on suitable neural membrane properties for speedy action potential generation and propagation. The resulting neural firing features high rates and submillisecond precision. For an example, synaptic sound encoding builds on ultrafast glutamatergic transmission at specialized ribbon synapses formed by inner hair cells (IHCs) achieving firing at rates of several hundreds of hertz with submillisecond precision in spiral ganglion neurons (SGNs).4,5 Utmost precision of the neural time code of the incoming sound forms the basis of sound localization in dedicated neural circuits of the brainstem that feature powerful calyceal synapses and neurons with extremely short membrane time constants owing to their specialized set of ion channels.1,2
Dissecting the function of such time-critical neural circuitries requires ultrafast control of neuronal activity. Likewise, functional restoration, for example, following degeneration of the sensory receptor cells, needs bionic approaches that reinstate the physiological information processing as closely as possible. Restoration of hearing to the deaf currently employs electrical stimulation of SGNs by cochlear implants (CIs).6−8 Due to wide spread of current from each electrode contact, encoding of sound frequency information is heavily limited in CIs. Moreover, electrical SGN stimulation results in supernatural synchronization of neural activity and hence CIs employ high rates to generate pseudostochasticity of SGN firing. Recently, optogenetics has been proposed for improved bionic SGN stimulation, as light can be better confined in space. Rapidly gating channelrhodopsins enable optogenetically evoked firing with near-physiological temporal fidelity. Yet, genetic manipulation for expression of channelrhodopsins presents a risk to consider. Avoiding the need for gene therapy, photopharmacology could help reduce the complexity of optical SGN stimulation. Reversibility of chemical photoswitches9 makes them interesting candidates for controlling neural excitation via endogenous neuronal receptors, such as ionotropic glutamate receptors (GluRs) of the postsynaptic SGN boutons in the cochlea.
Photoswitchable tethered ligands (PTLs) are covalently attached to their receptor and seem particularly attractive for this purpose. If conjugated precisely, they can provide finer photocontrol than freely diffusible photochromic ligands. PTLs produce higher local concentrations10 and cannot diffuse away, which yields a sharper separation in biological activity between the two isomeric states.11,12 Genetic manipulation can be avoided with affinity-labeling conjugation of PTLs to target nucleophilic residues in the native protein (e.g., lysine,13 histidine14). These targeted covalent photoswitches (TCPs) can be applied to intact neurons and readily provide photocontrol for hours.13 However, they display some limitations. The standard bistable photoswitches can be isomerized in milliseconds using dual-color light flashes (UV–visible), but these wavelengths penetrate poorly in tissue and are problematic in vivo. In addition, their slow-relaxing photochromism (lifetime ∼80 min for TCPs) limits to ∼80% the fraction of trans isomer that can be achieved with light15−19 and thus the reversibility of effects. To address these shortcomings, photoswitches have been modified to isomerize with longer wavelengths and rapidly relax in the dark to 100% trans,22,26 but these compounds were designed to target genetically modified receptors instead of the native proteins,13 which has so far hindered their use in one-wavelength applications to ultrafast synapses and in vivo preparations. Thus, methods for direct, rapid, and sustained photocontrol of activity in intact neurons constitute an unmet need, both to study neuronal circuits for basic research purposes and to explore new phototherapies. Interestingly, when sensory cells or neurons are damaged or absent, fast and sustained neurotransmitter release is impaired, but the postsynaptic neurons and receptors can retain their full capacities (activation kinetics, localization, and complex formation with regulatory proteins) for extended periods of time, offering untapped potential for functional restoration.6
Here, we developed a fast-relaxing glutamate TCP (TCPfast, Figure 1) that fulfills the abovementioned requirements. We show the ability of TCPfast to produce photocurrents in naive hippocampal neurons likely via tethering to endogenous GluRs and reversibly modulating their activity with visible light pulses as short as a few milliseconds. As an original proof of concept, we demonstrate the application of this molecular tool to the ultrafast first synapses of the auditory system that mediate cochlear sound encoding. This drug-based method affords ultrafast rate stimulation of SGNs in naive adult gerbils, matching the performance of optogenetic photostimulation that requires gene therapy. In general, these photoswitches provide a fundamental resource of broad interest to spatiotemporally control endogenous receptors in intact neuronal circuits with ultrafast signaling.
Figure 1.
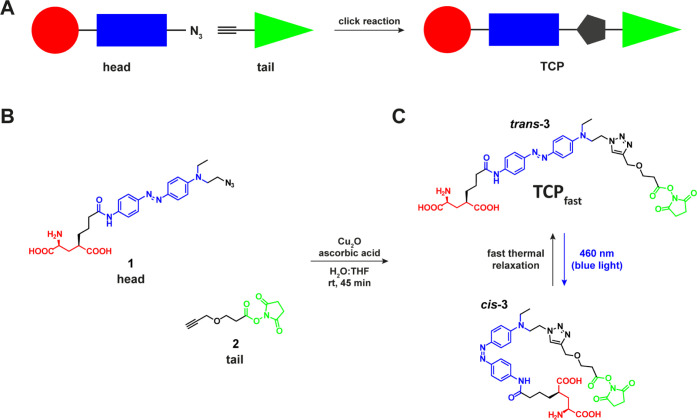
Fast-relaxing targeted covalent photoswitch of endogenous GluRs. (A) Schematic representation of the click chemistry strategy adopted for the preparation of TCP ligands. Modular parts are the ligand (red), the photoswitch (blue), and the reactive anchoring group (green). (B) Molecular design showing “head” (1) and “tail” (2) precursors of TCPfast (3) that are freshly coupled via a copper(I)-catalyzed azide–alkyne cycloaddition prior to incubation in neuronal tissue. (C) Chemical structure and photoisomerization of TCPfast between the cis (blue light, λ = 460 nm) and trans (dark, fast relaxation) conformations.
Results and Discussion
Design and Synthesis of a Fast-Switching TCP of Ionotropic GluRs
The molecular design of TCPfast was based on the recently reported TCPs13 and is shown in Figure 1A. TCPs have a modular structure obtained by combining an affinity “head” (1), which bears both the bioactive ligand (glutamate moiety) and the modified photoisomerizable unit (azobenzene), and a reactive “tail” (2) bearing the protein anchoring group (NHS ester). NHS ester-activated linkers are short-lived groups that promptly react with primary amines (e.g., lysine residues) under neutral or slightly alkaline conditions (pH 7.2–9). To avert self-reactivity, TCPs are readily generated prior to attachment to the target protein via a copper(I)-catalyzed azide–alkyne cycloaddition reaction (CuAAC, also known as “click chemistry”) (Figure 1B).
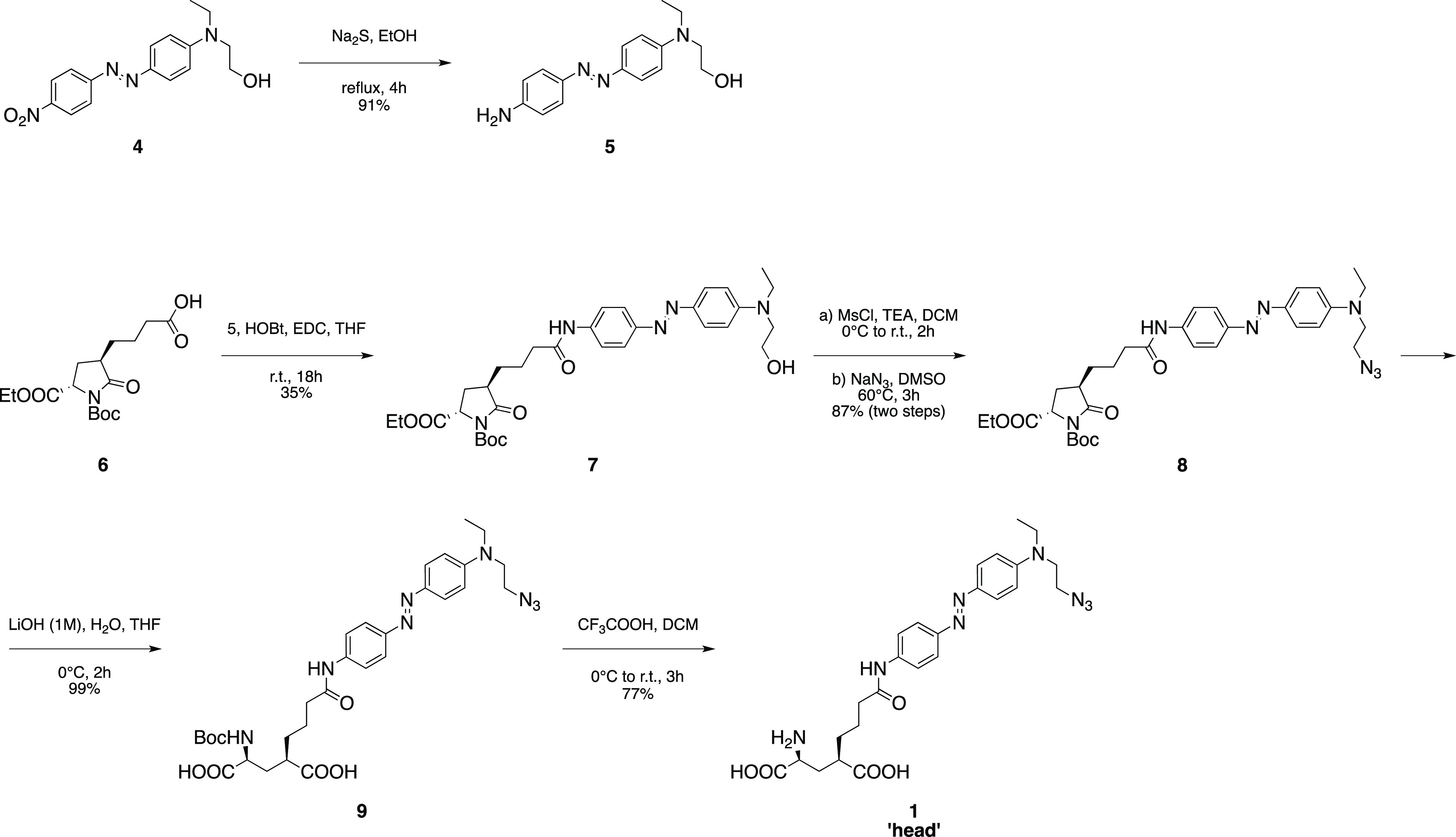
Previous TCPs featured azobenzene moieties that were photoisomerized using two different illumination wavelengths (380 and 500 nm) and characterized by slow thermal cis-to-trans relaxation.13 Azobenzenes displaying faster relaxation kinetics and single, longer wavelength switching can be obtained with minimal variation of their chemical structure by generating an electron “push–pull” system. It consists of including connected electron donating groups on one aromatic ring of the azo unit and electron withdrawing groups on the other, which lower the energy barrier of the thermal cis-to-trans isomerization,20,21 increasing the photoisomerization rate. This also results in a red-shifting of the azobenzene absorption spectrum, which is useful to reduce light scattering in tissue and phototoxicity, which is lower for longer wavelengths in applications in vivo. Thus, we designed a TCPfast head in which one of the two amide groups at the para positions of the azobenzene was replaced by a tertiary amine as the electron-donating group.22 To avoid perturbing the ligand region, we chose to introduce this modification on the opposite side of the azobenzene core (head compound 1). Compound 1 was prepared via a five-step synthesis starting from commercially available materials (Scheme 1). Azobenzene 5 was obtained by reduction of its nitro precursor 4 with sodium sulfide nonahydrate. Pyroglutamate derivative 6, prepared as previously described,15 was coupled to compound 5 using HOBt/EDC activation to give the intermediate 7 and then converted via mesylation into the corresponding azide derivative 8. Hydrolysis of the pyroglutamate moiety with concomitant saponification of the ethyl ester provided the advanced intermediate 9, which was finally converted into the desired compound 1 via removal of the tert-butoxycarbonyl protecting group under acidic conditions (see also the Supporting Information for further details). This photoswitch showed an absorption maximum at about 460 nm (blue light) in aqueous solution at neutral pH (Figure S8), as previously reported.22 Moreover, no variation of the absorption spectrum could be detected by steady-state UV–vis spectroscopy, suggesting that the cis isomer rapidly (<1 s) relaxes back to trans when the light is turned off. We used transient absorption spectroscopy to confirm photoswitching in aqueous phosphate buffer saline (PBS) solution, to measure the absorption spectrum and optimal wavelength of isomerization (450–460 nm), the relaxation lifetime in the dark (∼8 ms), and to demonstrate the high fatigue resistance of the compound (Figures S9–S11). The head (1) was coupled via CuAAC with a commercial tail (compound 2) providing a fast-relaxing and red-shifted ligand (3) with a similar length to the successful TCP9 ligand previously described13 (Figure 1C). To reach a satisfactory head-tail click coupling rate in minutes at room temperature, we replaced the conventional sodium ascorbate by ascorbic acid (see the Supporting Information for details). We hypothesized that the buffering effect of the ascorbic acid (to the tertiary amine in compound 1) could create more proper pH conditions to promote the catalytic cycle of the reaction23 as well as favor the formation of active copper(I) species from the copper(I) oxide catalyst.24 Because NHS-based ligands are constitutively short-lived, we confirmed the formation of the desired TCPfast by liquid chromatography–mass spectrometry (LC–MS) of the click reaction crudes and verified their ability to conjugate primary amine-containing biomolecules by reacting them with pure lysine as a mock protein residue (see the Supporting Information for details).
Scheme 1. Chemical Synthesis of the “Head” Module (1) Bearing the Glutamate Moiety, the Photoswitchable Unit, and the Terminal Azide.
Characterization of TCPfast in Cultured Neurons
We made use of the several GluRs subunits expressed by hippocampal neurons25 to evaluate, on dissociated neuron cultures, the ability of TCPfast to photocontrol GluR activity. TCPfast was conjugated to GluRs by using the same incubation conditions (i.e., 2 min at 25–100 μM, pH 9 to favor deprotonation and reactivity of nucleophilic residues in the receptors, followed by wash-out of physiological solution, pH 7.4) previously shown to be favorable for TCP conjugation.13 473 nm illumination did not elicit photocurrent measured by whole-cell patch-clamp recordings directly after incubation with TCPfast. However, in the additional presence of glutamate (300 μM), 473 nm illumination elicited 2.5–220 pA photocurrents (Figure 2A). As intended by the chemical design, the cis isomer of TCPfast, induced by blue light illumination, evoked inward photocurrents. thus supporting the notion that TCPfast enabled fast-one-wavelength photomodulation of GluRs (Figure 2A inset). Next, we tested the effect of light in absence of TCPfast incubation (Figure S16). The currents measured at the onset of 300 μM glutamate perfusion were of similar amplitude in both cases (793 ± 131 pA with TCPfast and 1161 ± 445 pA without TCPfast), discarding any non-specific effect of light stimulation. Direct perfusion of TCPfast in the dark indicates that trans-TCPfast can activate GluRs as a free ligand, an effect that is enhanced by light stimulation (Figure S17).
Figure 2.
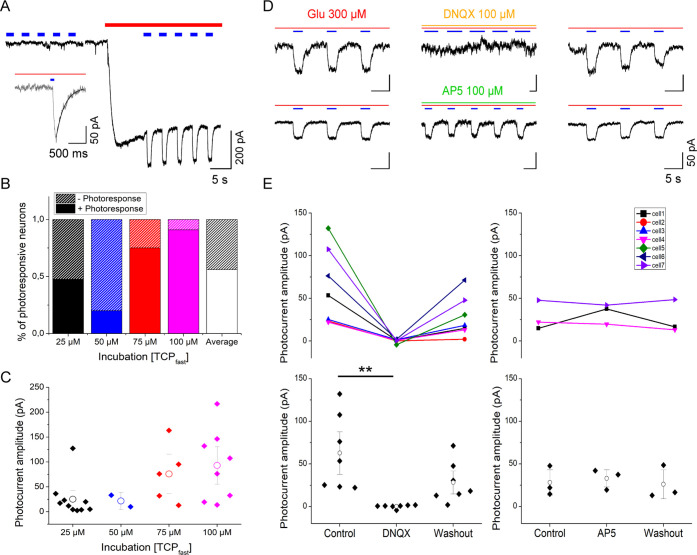
TCPfast conjugation produces photocurrents in untransfected hippocampal neurons in the presence of glutamate via endogenous non-NMDA GluRs. (A) Current recording in whole cell voltage clamp mode in dissociated hippocampal neurons after incubation in TCPfast (100 μM for 2 min at pH9). Inward current induced by bath perfusion of 300 μM glutamate (red bar) and 473 nm light (blue bars, 1 s). Time gap between traces corresponds to immediate subsequent recordings (<2s). Inset: photocurrent elicited by 100 ms light pulse. Exponential fit indicated in black correspond to Toff. (B) Increasing TCPfast concentration during incubation (2 min, pH9, 25–100 μM) leads to a higher fraction of cells displaying photoresponses. (C) Photocurrent amplitude increases relative to concentration of TCPfast incubated. Note: from the total number of cells responding to perfusion of 300 μM glutamate, 56% had light response. Per concentration, 47, 62% of cells incubated at 25 μM had light response (n = 21); 20% of cells incubated at 50 μM had a light response (n = 10); 75% of cells incubated at 75 μM had a light response (n = 8); 90% of cells incubated at 100 μM had a light response (n = 11). Means comparison by the Bonferroni test: 25 μM vs 50 μM (ns. p-value = 0.149); 25 μM vs 75 μM (ns; p-value = 0.198); 25 μM vs 100 μM (*, p-value = 0.0154); 50 μM vs 75 μM (*, p-value = 0.018); 50 μM vs 100 μM (**p-value = 2.6 × 10–4); and 75 μM vs 100 μM (ns, p-value = 0.3758). (D) Current recordings in whole cell voltage clamp mode in rat hippocampal neurons maintained 15 days in culture and incubated with TCPfast (100 μM for 2 min at pH 9). Example traces of photocurrents elicited by irradiation at λex = 473 nm (1 s, blue bars) in the presence of 300 μM glutamate (red bar). Photocurrents are reversibly blocked by perfusion of 100 μM DNQX (orange bar) and not affected by 100 μM AP5 (green bar). After washout and reperfusion of 300 μM glutamate photocurrents are recovered, indicating covalent conjugation. Note that the absence of sharp responses may be indicative of GluR desensitization as a result of the high glutamate concentration. (E) Quantification of the effect of DNQX (100 μM, n = 7, p-value = 0.008) and AP5 (100 μM, n = 3, p-value = 0.56) on the photocurrent amplitude obtained from hippocampal neurons incubated with TCPfast (25-75-100 μM for 2 min at pH9). Control and wash-out measurements were obtained after bath solution and glutamate perfusion. White dots indicate mean ± SE. Note: p-values obtained after performing Friedman test: non-parametric, data from any distribution; small samples; and related samples.
Next, we showed that the number of photosensitized hippocampal neurons (Figure 2B) and the amplitude of the photocurrent evoked by blue light (Figure 2C) increased with TCPfast concentration during the incubation (25, 50, 75, and 100 μM). At 25 μM of TCPfast, 47.62% of neurons (n = 21) had a measurable photocurrent (on average: 25 ± 12 pA), while 100 μM raised this to 90% of the neurons (n = 11) with a photocurrent of 93 ± 25 pA. In contrast, the relaxation lifetime in the dark of TCPfast-conjugated neurons was independent of TCPfast concentration and amounted to 220 ± 48 ms (Figures 2A inset and S18). As intended by our chemical design, the achieved relaxation lifetime was faster than shown for TCPs (∼80 min)13 and comparable to one-wavelength PTLs (MAG460: 710 ms,22 MAG2P: 150 ms and MAGA2P: 265 ms26). For a subset of neurons, we also found that the photocurrent increased in amplitude with the radiant flux (Figure S19) and could be evoked by light pulse as short as 3 ms (Figure S20).
Finally, we showed that the photoresponses can be reversibly blocked by the AMPA receptor antagonist DNQX (100 μM) but not by the NMDA receptor antagonist AP5 (100 μM) (Figure 2D,E). This demonstrates that TCPfast photocurrents are receptor-specific and that the photoswitchable ligands are indeed covalently tethered to GluRs (as the TCPfast-mediated photoresponses persist even after washout and competition with an antagonist).
In Vivo Photocontrol of Neural Activity in Gerbil’s Cochlea
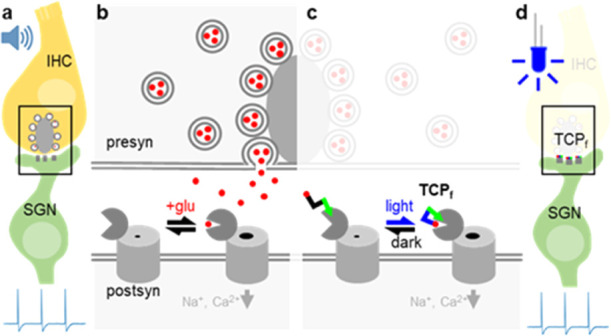
The potential of TCPfast to photosensitize SGNs in vivo was tested in the Mongolian gerbil cochlea using the same TCPfast batch produced under the same click reaction conditions as for the in vitro characterization demonstrated above. The goal was to administer TCPfast to SGNs, where it could covalently tether to the AMPA receptors in the postsynaptic density.
To do so, we surgically exposed the cochlea by performing a middle ear surgery (posterior tympanotomy) and applied TCPfast to the niche of the round window (RW, a membrane-covered bony window of the cochlea, Figure 3A,B) for passive diffusion into the cochlea. In order to evaluate the potential toxicity induced by TCPfast, we recorded cochlear mass potentials evoked by sound, before, during, and after TCPfast diffusion. These mass potentials reflect the status of three cochlear cell types: outer hair cells, inner hair cells, and SGNs (for details, see Supporting Information, Figure 3C, Figure S21A1-4). In this experiment, the acoustically evoked potentials reflecting the outer hair cells (i.e., the cochlear microphonic, Figure S21A3,B), IHCs (i.e., the summating potential, Figure S21A4,C), and SGNs (i.e., the compound action potential, CAP, Figure S21A4,D) remained largely unchanged following a 10 min application of 12.5 μM TCPfast (0.25% of organic solvent) and wash-out (n = 4, Friedman’s test). This indicates that TCPfast incubation did not interfere with cochlear physiology.
Figure 3.
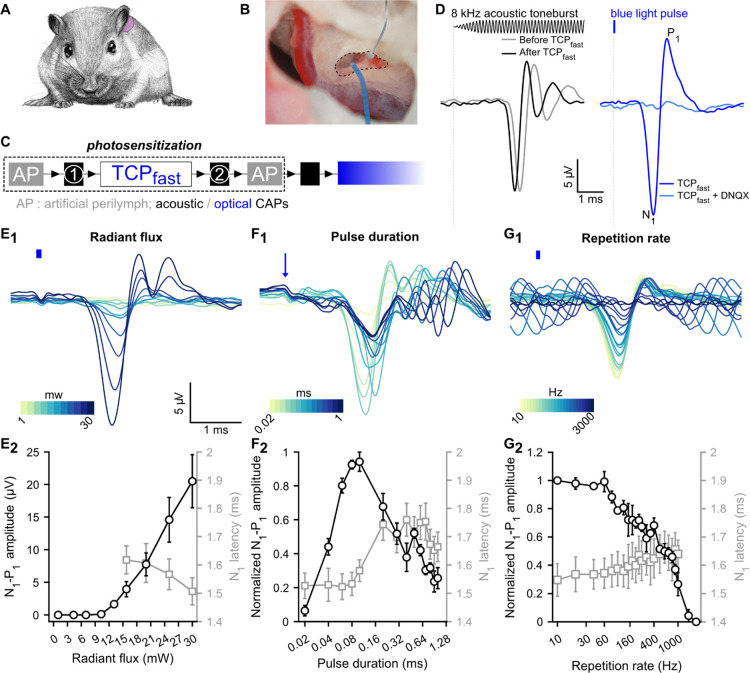
Fast photoswitchable control of SGN activity mediated by TCPfast. (A) Mongolian gerbil cochlea was exposed, through the middle ear, by performing a retro-auricular approach. (B) Silver ball electrode was placed in the RW niche (dashed line) to record CAP in response to sound or to light stimulation. The light stimulation was delivered to the cochlea via an optical fiber coupled to a 470 nm-laser. (C) Experiment time course description: TCPfast application (2.5–12.5 μM, 10 min) is preceded and followed by artificial perilymph application (AP) and acoustically CAP measurements. After the second AP application, the optical fiber is inserted in the RW and the optically evoked potentials (E–J) are recorded. (D) Representative acoustically (black, left, aCAP) and optically evoked CAP (blue, right, oCAP) following 2.5 μM application of TCPfast. aCAPs (black, 200 averages, 8 kHz toneburst, 30 dB SPL, repetition rate = 20 Hz) are similar before and after TCPfast application. oCAP (blue, 80 μs, 30 mW, repetition rate = 10 Hz) is abolished following application of competitive antagonist DNQX (1 mM). Stimuli are represented at the top. (E1,G1) Representative oCAP in response to various radiant fluxes (80 μs at 10 Hz, E1), pulse durations (27 mW at 10 Hz, F1), and repetition rates (80 μs at 27 mW, G1). In (E1,G1), the blue line indicates the light stimuli and in F1 the blue arrow indicates the beginning of the light pulse. A color scale is used to represent the variable. (E2,G2). Quantification (n = 6 cochleae) of the oCAP amplitude (N1–P1, black axis) and oCAP latency (N1, gray axis) as a function of the radiant flux (E2), the pulse duration (F2), and the repetition rate (G2). In F2 and G2, oCAP amplitudes were expressed as relative to the highest amplitude recorded for the given measure.
We then tested for a TCPfast-mediated photopharmacological SGN stimulation by inserting an optical fiber coupled to a blue laser (λ = 473 nm) into the RW. Transient optically evoked CAPs (oCAPs) were recorded from 12 out of 12 cochleae treated with TCPfast. oCAPs were similar in shape to acoustically evoked CAP and had a maximal amplitude of 40.88 ± 12.58 μV (at maximum radiant flux: 30 mW), which is equivalent to the amplitude of a 50 dB SPL tone burst (Figure S21E). oCAPs vanished within minutes of optical stimulation, which was accompanied by a disappearance of the acoustic potentials reflecting the IHC and SGNs activation. The number of synapses per IHC (i.e., the juxtaposition of pre- [CtBP2] and postsynaptic [Homer 1] markers) was similar between treated and non-treated cochleae, at all frequencies, arguing against glutamate excitotoxicity induced by TCPfast—mediated optical stimulation (Figure S21F-G and Supporting Method S2 for details). We can only speculate about a putative functional impairment of IHCs, SGNs, and their synapses, which might involve mechanisms such as phototoxic damage, for example, via generation of free radicals and desensitization and/or endocytosis of AMPA receptors. Future studies will be needed to elucidate such mechanisms. Interestingly, when we employed a lower TCPfast dose (2.5 μM, <0.05% of organic solvent), we observed stable acoustically and optically evoked cochlear potentials in 60% of the incubated cochleae (Figures 3D–G, S22A–C). The other 40% of the cochleae were similar to previously reported using the highest concentration with a loss of mass potentially (Figure S22D–F), which indicates a narrow therapeutic window.
For the positive cochleae, oCAPs were similar in shape and amplitude to aCAP evoked by a 40 dB SPL toneburst but consistently showed a shorter latency (∼1.5 ms, Figure 3D) indicating a direct optical SGN excitation bypassing the outer and middle ear transmission and inner ear mechanotransduction of sound in acoustic hearing. oCAPs could be abolished by DNQX (1 mM) application at the RW, supporting a response mediated by AMPA receptors (Figure 3D, light blue trace).
We further characterized the TCPfast-mediated optical response by measuring oCAP amplitude and latency as a function of radiant flux (n = 6) using 80 μs light pulses at a repetition rate of 10 Hz (Figure 3E1,2). oCAP threshold amounted to 12.5 ± 0.62 mW (1 ± 0.05 μJ). From there, oCAP amplitude increased linearly with radiant flux up to 20.5 ± 4.09 μV at 30 mW and latency decreased from 1.62 ± 0.05 to 1.51 ± 0.05 ms. Next, we measured the effect of the pulse duration on the oCAP (Figure 3F1,2, 30 mW, repetition rate = 10 Hz): the biggest oCAPs were recorded in response to 80 and 100 μs light pulses. From there, oCAPs decreased in amplitude for shorter and longer durations. In 50% of the cases, sizable oCAPs (i.e., > 1 μV) were measured in light pulses as short as 20 μs, corresponding to 0.6 μJ. In response to light pulses shorter than 100 μs, oCAPs were characterized by a single negative wave occurring at ∼1.54 ms. In contrast, oCAPs evoked by a longer pulse had multiple negative peaks, which might reflect the firing of multiple synchronous action potential across SGNs in response to the longer stimuli. Finally, we measured the effect of the repetition rate on oCAPs using 80 μs light pulses (Figure 3G1,2, 30 mW). The oCAPs were stable in amplitude up to 60 Hz and decreased exponentially at a higher repetition rate until not being observable for rates exceeding 1 kHz (the best performer cochlea is illustrated in Figure S23). The reported in vivo data support the concept of fast control of the AMPA receptor with TCPfast in the SGNs.
Conclusions
In this work, we developed a chemical-biological method, called TCPfast, which allows fast photoswitching of neuronal activity in native neurons. TCPfast is a photoswitchable ligand that is chemically attached to endogenous receptors at the postsynaptic side.13 The molecular design of TCPfast optimized the properties of the bistable photoisomerizable group of former TCPs,13 that is, thermal relaxation lifetime and absorption wavelength, without altering the ligand and the reactive group. TCPfast functions as a molecular prosthesis that bypasses the neurotransmitter-encoded signal by a photonic signal. Photosensitization of cochlear SGNs by locally administered TCPfast enabled temporally precise light-evoked SGN firing up to a rate of approximately 1 kHz, thus matching the fastest optogenetic SGN stimulation (≤1 kHz27). Hence, TCPfast-mediated photopharmacology might serve as an interesting alternative to the optogenetic approach6,28 for the development of an optical CI.
TCPfast elicited depolarizing photocurrents that are activated by the cis isomer (“cis-on”), which is generally preferred to avoid activation in the dark. Such behavior has been reported for similar photoswitchable ligands10,13 and has been attributed to compound lengths and conjugation sites in the protein that allow ligand binding to the active site only in a “bent” (cis) conformation.29 Molecular docking studies (Figures S25 and S26) and the cis-on agonist behavior of the freely diffusible compound in neurons (Figure S17) agree with this view. TCPfast photoresponses are reversed in the absence of illumination with a thermal relaxation lifetime ranging between 8 ms for the TCPfast head in the cuvette (Figure S9) and 220 ms in hippocampal neurons in culture (Figure S18). While slower lifetimes have been observed upon photoswitch conjugation and interaction with the target protein,26 we can currently only speculate on how such a thermal relaxation rate measured in vitro yields the ultrafast photostimulation of SGNs in vivo eliciting SGN CAPs with interstimulus intervals ranging from a few milliseconds to submillisecond. Of note, postsynaptic boutons of SGNs likely contain hundreds of AMPARs such that each light stimulus might hit a sufficient number of AMPARs with the TCPfast being in the trans state. Moreover, CAPs represent population responses and so light pulses might be recruiting variable subsets of SGNs. Finally, AMPA receptor desensitization might contribute to the transient response properties. Future work could consider to further speedup thermal relaxation potentially by replacing the current azobenzene unit in TCPfast with faster photoswitches such as (hydroxy-substituted) phenylazopyrimidine (millisecond to nanosecond range30−32). However, such accelerated back-switching might in turn result in a lower cis conversion percentage in the photostationary state and, therefore, would require higher light intensity. In addition, further increasing the wavelength for photoswitching33 might improve the overall utility of the method due to better tissue penetration and lower phototoxicity risk.
We demonstrated, in dissociated hippocampal neurons, specific photomodulation of AMPA/kainate receptors by TCPfast. Such specificity is due to the affinity-labeling mechanism of PTLs.10,13,29 In this experimental setting, TCPfast photomodulation required the presence of glutamate in the bath solution for all the tested concentrations (Figure 2C), but in the range of 0.03–3 mM free glutamate, a weak correlation with the photocurrent amplitude was observed. One explanation could be that conjugated TCPfast acts as a trans-on antagonist. However, because tethered glutamate has never been reported to act as an antagonist in GluRs and freely diffusible TCPfast acts as an agonist, we argue that the requirement of glutamate is due to incomplete conjugation of TCPfast to a heterogeneous population of endogenous GluRs. This concept may be translated into a distinctive subunit occupancy resulting in differential agonist affinity and efficacy, thus reducing the channel open probability (gating).12,34,35 Enhancing the reactivity of the electrophilic group could improve the photoswitch performance. Another possible mechanism would be the presence of native GluR heterotetramers36 with subunits displaying different TCP conjugation and photoswitching properties.12 We cannot rule out the possibility that TCPfast behaves as a positive allosteric modulator or as a partial agonist, allowing only partial receptor activation by partial closure of the ligand binding domain. We argue against this possibility because cis-TCPfast photocurrent in the presence of the orthosteric antagonist DNQX is blocked, and in the presence of glutamate is not reduced, but enhanced, indicating that it is not competing with glutamate.10,35 In any case, the effect of light in the presence of the endogenous neurotransmitter glutamate can also be envisaged as a way of potentiating physiological rather than generating totally exogenous signals, which is often preferable.
Considering the unique properties of TCPfast to modulate native GluR activity, TCPfast was tested in vivo at the synapse between IHC and SGNs of the cochlea, where excitatory responses are mainly mediated by AMPA receptors.37,38 TCPfast-mediated control of the SGNs was obtained in minutes by diffusing (and washing out) the compound into the cochlea. Interestingly, neural responses were observed in response to light pulses with energies between 0.5 and 1 μJ, which is the lower range of the optogenetic approaches applied to the adult gerbil cochlea (CatCh: 1–2 μJ,;39 f-Chrimson: ∼5 μJ).40 Additionally, maximal oCAP responses were obtained in response to light pulse as short as 80 μs, which is substantially shorter than reported for optogenetic tools in the cochlea.27,39,41,42 Finally, TCPfast enabled neural responses up to a stimulation rate of 1.5 kHz. Although this observation should be confirmed by single SGN recording, it is interesting to note that this performance is similar to what was reported using an optically evoked auditory brainstem response, for SGNs expressing the fastest opsins to date (Chronos:;27 vf-Chrimson:42), while presenting a lower activation threshold (TCPfast ∼ 1 μJ, Chronos-ES/TS and vf-Chrimson-ES/TS ∼ 6 μJ).
TCPfast provides similar or better performance than current optogenetic approaches in terms of speed and light requirements, while avoiding the gene therapy required to optogenetically modify SGNs. Yet, long-term availability, efficacy, and safety of TCPfast or more advanced photoswitches will need to be established. Moreover, neural degeneration often starts with the loss of postsynaptic structures,43,44 which may limit the usefulness of TCPfast photopharmacology for optical CIs. Future studies should focus on designing safe compound-targeting channels expressed at the surface of the surviving SGN somas, while showing similar target specificity, fast kinetics, and longer wavelength. This way, photopharmacology might establish a toolkit for photosensitization tailored to the specific neural status of the cochlea or other structures. In addition, a drug-based approach would appeal to patients and the pharmaceutical industry and facilitate regulatory processes. Photopharmacology has previously demonstrated its potential to photosensitize other sensory systems such as the retina.13,45−47 To the best of our knowledge, this is the first proof-of-concept photopharmacology study in the auditory system and opens up an avenue for auditory research and clinical applications such as in optical CIs designed to fundamentally improve hearing restoration.
Experimental Section
Click Reaction
To a 1.5 mL glass vial containing azide 1 (“head”, 1.00 mg, 1 equiv) and copper(I) oxide (0.82 mg, 3 equiv) in tetrahydrofuran (47 μL) and equipped with a magnetic stir bar was added a solution of ascorbic acid (1.34 mg, 4 equiv) in water (94 μL) and the resulting mixture was vortexed for 1 min. Then, a solution of alkyne 2 (“tail”, 0.47 mg, 1.1 equiv) in tetrahydrofuran (47 μL) was added and the resulting mixture was stirred at room temperature for 45 min. The so obtained final mixture was taken up in dimethylsulfoxide (193 μL), vortexed, centrifuged for 1 min to separate the insoluble copper(I) oxide particles, and finally divided into aliquots of the final compound stock solution (Figure 1B,C and Scheme S2).
Alternatively, the click reaction can be performed in an Eppendorf tube and stirred with a suitable mixer.
We observed that the catalytic performance of the copper(I) oxide may vary significantly from batch to batch, therefore the actual reaction time should be adjusted to obtain at least a 95% conversion of the starting material and a (TCPfast):(hydrolyzed TCPfast) ratio greater than 3 (Figure S12). In vitro and in vivo biological characterizations were done using the same batch and results were reproducible across experiments.
Rat Hippocampal Neural Primary Culture
All experiments were done in compliance with the national animal care guidelines and were approved by the board for animal welfare of the University Medical Center Goettingen and the animal welfare office of the state of Lower Saxony.
Primary hippocampal cultures were prepared from newborn P0–P3 pups Wistar rats. Brains were collected in a 10 cm Petri dish containing ice-cold dissection media (HBSS (Gibco) + 10 mM Hepes (Gibco)). Hippocampi were separated from the brain, and meninges were removed. Hippocampi were digested with 2 mL prewarmed 37 °C 0.1% trypsin–EDTA (Gibco, Germany) for 20 min at 37 °C. Trypsin was removed, and the tissue was washed three times with dissection medium. Dissection medium was replaced with 1 mL prewarmed complete DMEM (DMEM with 1× Glutamax, 10% FCS/FBS and 1% penicillin (100 U/mL)/streptomycin (100 μg/mL); all from Gibco). Tissue was triturated by gentle pipetting. The tissue suspension was filtered through a 100 μm cell strainer (BD Biosciences).
Cells were counted using the trypan blue exclusion method and cultured on 12 mm glass coverslips (Thermo Scientific) coated with poly-d-lysine (Sigma). Neurons were plated in 24-well plates at a density of 50,000 hippocampal neurons per coverslip in 500 μL of NB+ medium (Neurobasal with 2% B-27 supplement, 1% Glutamax and 1% penicillin (100 U/mL)/streptomycin (100 μg/mL); all from Gibco) at 37 °C and 5% CO2. Half of the medium in each well was changed every 3–4 days.
Electrophysiology Recording Conditions for Rat Hippocampal Neurons
Before starting the recording, neurons between 10 and 18 days in vitro (div) were incubated with TCPfast at concentrations: 25–50–75–100 μM (≤2% DMSO) for 2 min, in the absence of light and in pH 9 bath solution composed of (in mM): 100 NaCl, 1 MgCl2, 2.5 KCl, 2.5 CaCl2, 10 glucose, and 50 sodiumcarbonate/sodiumbicarbonate, 310 mOsm/kg, pH 9 adjusted with NaOH.
Voltage and current-clamp recordings under whole-cell configuration were done using an Axopatch 200B amplifier, filtered at 5 kHz, and digitized with an Axon DigiData 1440A interface (Axon Instruments). Acquisition software used was Clampex 10.5.2.6 (Axon Instruments).
Light stimulation was performed at saturating radiant flux (5–14 mW) using diode-pumped solid-state lasers (λ = 473 nm) focused into a 400 μm optic fiber. Light pulses were applied using a fast computer-controlled shutter (Uniblitz LS6ZM2, Vincent Associates, Rochester, USA). Radiant flux was adjusted by placement of density filters between the laser output and the optic fiber.
During recordings, neurons were maintained at room temperature (r.t., 25–27 °C) in a continuous perfusion of bath solution and clamped at −70 mV. Bath solution was composed of (in mM): 140 NaCl, 1 MgCl2, 2.5 KCl, 10 HEPES, 2.5 CaCl2, and 10–20 mM glucose to fix osmolarity to 310 mOsm/kg, pH 7.42 adjusted with NaOH. Borosilicate glass pipettes were pulled with a typical resistance of 3–8 MΩ for neurons. Pipette solution contained (in mM): 129 potassium gluconate, 10 HEPES, 10 KCl, 4 MgATP, and 0.3 Na3GTP. Osmolarity is adjusted at 289 mOsm/kg, and pH is adjusted to 7.2 with KOH.
Drug Preparation for In Vivo Cochlea Infusion
Artificial perilymph solution consisted of the following (in mM): 137 NaCl; 5 KCl; 2 CaCl2; 1 MgCl2; 1 NaHCO3; and 11 glucose, pH 7.4 adjusted with NaOH and osmolarity: 304 ± 4.3 mOsm/kg. Before each experiment, TCPfast was diluted in artificial perilymph to a final concentration ranging from 2.5 to 12.5 μM.
Animal Preparation for Cochlear Potential Recordings and Cochlear Pharmacology
All experiments were done in compliance with the German national animal care guidelines and were approved by the board for animal welfare of the University Medical Center Göttingen and the animal welfare office of the state of Lower Saxony (agreement 2014/1726 and 2019/3188). Experiments were performed on adult (>8 weeks old) Mongolian gerbils (Meriones unguicalatus) of both sexes.
Gerbils were anesthetized by isoflurane (5% for anesthesia induction, 1–3% for maintenance, and frequent testing for the absence of hind-limb withdrawal reflex), and analgesia was obtained by subdermal injection of buprenorphine (0.1 mg/kg body weight) and carprofen (5 mg/kg body weight). Body temperature was maintained at 37 °C using a custom-designed heat plate. The cochlea was exposed following a retroauricular approach and a bullostomy. The recording electrode was placed against the bony edge next to the RW leaving enough space to access the RW for pharmacological manipulation and optical stimulation. The pharmacological manipulation was made by filling the RW niche with the solution of interest. The RW membrane was punctured to increase the fluid exchange between the cochlea and RW niche. Between each solution, the RW niche was emptied by capillarity.
Acoustically Evoked Cochlear Potential
Acoustic cochlear potentials were obtained in response to 8 kHz tone burst (pulse duration = 8 ms, rise/fall time = 1 ms, repetition rate = 20 Hz, level: 20 to 80 dB SPL per 10 dB step, and 200 repetitions per level). The mass potentials were amplified using a custom-made physiological amplifier and sampled at a rate of 50 kHz (NI PCI-6229, National Instrument). Stimulus generation and data acquisition were made using custom-written software (MATLAB, MathWorks) employing National Instrument data acquisition cards in association with a custom-built acoustic and laser-controller.
The cochlear microphonic was extracted by averaging the band-pass filtered (cut-off frequencies = 5.6 and 11.1 kHz) mass potential recorded using the RW electrode and its amplitude defined as the RMS value. The CAP and summating potentials were obtained by averaging the low-pass filtered (cut-off frequency = 3.5 kHz) mass-potential. The CAP amplitude was defined as the amplitude between the first negative peak (N1) and the following positive peak (P1). The summating potential amplitude was defined as the difference between the plateau response (between 5 and 7 ms) and the baseline prior to the stimulation onset.
Optically Evoked Cochlear Potential
Optical cochlear potentials were obtained in response to blue light pulse delivered by a 200 μm optical fiber coupled to a 473 nm laser (MLL-FN-473-100, 100 mW, diode pumped solid state [DPSS]; Changchun New Industry Optoelectronics). Irradiance was calibrated with a laser power meter (LaserCheck; Coherent Inc). The CAP was obtained by averaging the low-pass filtered (cut-off frequency = 3.5 kHz) mass potential.
IHC Synapse Counting
Cochleae were fixated in 4% formaldehyde for about 15 min and decalcified in 0.12 M EDTA solution for about 12 h (at 4 °C). Organs of Corti were then: (i) isolated in PBS; (ii) incubated for an hour in Goat serum dilution buffer (GSDB, comprised of: 16% normal goat serum, 450 mM NaCl, 0.3% Triton X-100, 20 mM phosphate buffer, pH 7.4); (iii) treated for 2 h with the primary antibodies: mouse anti-CtBP2 (BD Biosciences, 1:200), rabbit anti-myo6 (Proteus Biosciences, 1:200), and chicken anti-Homer1 (SYsY, 1:200) for staining presynapse, IHCs, and postsynapse, respectively. After washing the primary antibodies with PBS for 20 min, the secondary antibodies were incubated: Goat-anti-Chicken 488 (Invitrogen, 1:500), Goat-anti-Rabbit 568 (Thermo Fisher, 1:500), and Goat-anti-Mouse 647 (Invitrogen, 1:500). After another washing step with PBS, samples were mounted on a glass slide in Mowiol (Carl Roth) mounting medium. Confocal images of the organ of Corti were first obtained using a 10× magnification (LSM 510 microscope, Carl Zeiss, Jena) in order to fit a Greenwood function48,49 to them and localize the tonotopic position of the IHCs. The synapses were visualized at different tonotopic regions (0.5, 1, 2, 4, 8, 16, and 32 kHz) using a 100× magnification (Abberior Instruments Expert Line STED microscope) and counted using the spot function from Imaris software (version 7.6.5).
Data Analysis and Statistics
Amplitude of photocurrents was analyzed using IgorPro (Wavemetrics). Displayed whole-cell current traces have been filtered using the infinite impulse response digital filter from IgorPro (low-pass filter with a cutoff of 50 Hz). The drift in current was corrected where appropriate with the IgorPro (WaveMetrics) software using a custom-made macro for drift correction. Statistics were done with OriginPro 8.5 (OriginLab) and Matlab (Mathworks). In vivo electrophysiological data were analyzed using custom-made Matlab routines.
Acknowledgments
This research received funding from the European Union Research and Innovation Programme Horizon 2020—Human Brain Project SG3 (945539), DEEPER (ICT-36-2020–101016787), Agency for Management of University and Research Grants/Generalitat de Catalunya (CERCA Programme; 2017-SGR-1442 and 2017-SGR-1604 projects), Fonds Européen de Développement Économique et Régional (FEDER) funds, Ministerio de Ciencia e Innovación, Agencia Estatal de Investigación and ERDF-FEDER European Fund (projects CTQ2017-89222-R and PID2020-120499RB-I00), and Fundaluce and “la Caixa” foundations (ID 100010434, agreement LCF/PR/HR19/52160010). The project Clúster Emergent del Cervell Humà (CECH, 001-P-001682) is cofinanced by the European Union Regional Development Fund within the framework of the ERDF Operational Program of Catalonia 2014–2020 with a grant of 50% of total eligible cost. A.G.-C. was supported by the fellowship BES-2014-068169 and Alexander von Humboldt Foundation. J.H. was supported by Agencia Estatal de Investigación (PID2019-106171RB-100/AEI/10.13039/501100011033) and Generalitat de Catalunya (2017 SGR00465). We thank chemical facility PCB staff (Unai Elezcano, Sònia Varon) and IBEC infrastructure staff (Isabel Oliveira, David Izquierdo). We thank Christiane Senger-Freitag, Sandra Gerke, and Sina Langer for technical support, Gerhard Hoch for his expert technical support, and Patricia Räke-Kügler for excellent administrative support. We thank Thomas Mager for providing the patch clamp setup. This work was funded by the European Research Council through the Advanced Grant “OptoHear” to T.M. under the European Union’s Horizon 2020 Research and Innovation program (grant agreement no. 670759), the Fraunhofer and Max-Planck Cooperation Program (NeurOpto grant) to T.M., was further supported by the German Research Foundation through the Priority Program 1926 “Next generation optogenetics” to A.H. and T.M., and the Leibniz Program (to T.M.) and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy—EXC 2067/1-390729940 to A.H. and T.M. In addition, this research was supported by the Fakultätinternes Forschungförderungsprogramm from the Universitätsmedizin Göttingen, Georg-August-Universität Göttingen to A.H., and Fondation Pour l’Audition (FPA RD-2020-10) to T.M. Molecular graphics and analyses were performed with UCSF Chimera, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from NIH P41-GM103311. We thank Dr. Vladimir Belov, Jurgen Bienert, and Jan Seikowski for HPLC and LC–MS measurements of final TCPfast products.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.1c12314.
General materials and methods; detailed description of the chemical synthesis and physicochemical characterization of TCPfast; biological in vitro measurements; supporting in vivo method description and measurements; and sequence alignment and molecular docking simulations (PDF)
Author Contributions
△ A.G.-C., A.H., and C.M. contributed equally to this work. T.M. and P.G. contributed equally to this work.
The authors declare the following competing financial interest(s): Prof. Dr. Tobias Moser is a co-founder and CEO of OptoGenTech company. The other authors declare no conflict of interests.
Supplementary Material
References
- Kaczmarek L. K.; Zhang Y. Kv3 Channels: Enablers of Rapid Firing, Neurotransmitter Release, and Neuronal Endurance. Physiol. Rev. 2017, 97, 1431–1468. 10.1152/physrev.00002.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe B.; Pecka M.; McAlpine D. Mechanisms of Sound Localization in Mammals. Physiol. Rev. 2010, 90, 983–1012. 10.1152/physrev.00026.2009. [DOI] [PubMed] [Google Scholar]
- Hu H.; Gan J.; Jonas P. Fast-spiking, parvalbumin + GABAergic interneurons: From cellular design to microcircuit function. Science 2014, 345, 1255263. 10.1126/science.1255263. [DOI] [PubMed] [Google Scholar]
- Huet A.; Batrel C.; Tang Y.; Desmadryl G.; Wang J.; Puel J.-L.; Bourien J. Sound Coding in the Auditory Nerve of Gerbils. Hear. Res. 2016, 338, 32–39. 10.1016/j.heares.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Moser T.; Grabner C. P.; Schmitz F. Sensory Processing at Ribbon Synapses in the Retina and the Cochlea. Physiol. Rev. 2020, 100, 103–144. 10.1152/physrev.00026.2018. [DOI] [PubMed] [Google Scholar]
- Kleinlogel S.; Vogl C.; Jeschke M.; Neef J.; Moser T. Emerging Approaches for Restoration of Hearing and Vision. Physiol. Rev. 2020, 100, 1467–1525. 10.1152/physrev.00035.2019. [DOI] [PubMed] [Google Scholar]
- Zeng F.-G. Challenges in Improving Cochlear Implant Performance and Accessibility. IEEE Trans. Biomed. Eng. 2017, 64, 1662–1664. 10.1109/TBME.2017.2718939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenarz T. Cochlear Implant – State of the Art. Laryngol., Rhinol., Otol. 2017, 96, S123–S151. 10.1055/s-0043-101812. [DOI] [PubMed] [Google Scholar]
- Ankenbruck N.; Courtney T.; Naro Y.; Deiters A. Optochemical Control of Biological Processes in Cells and Animals. Angew. Chem., Int. Ed. 2018, 57, 2768–2798. 10.1002/anie.201700171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorostiza P.; Volgraf M.; Numano R.; Szobota S.; Trauner D.; Isacoff E. Y. Mechanisms of Photoswitch Conjugation and Light Activation of an Ionotropic Glutamate Receptor. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 10865–10870. 10.1073/pnas.0701274104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüll K.; Morstein J.; Trauner D. In Vivo Photopharmacology. Chem. Rev. 2018, 118, 10710–10747. 10.1021/acs.chemrev.8b00037. [DOI] [PubMed] [Google Scholar]
- Reiner A.; Isacoff E. Y. Tethered Ligands Reveal Glutamate Receptor Desensitization Depends on Subunit Occupancy. Nat. Chem. Biol. 2014, 10, 273. 10.1038/nchembio.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Serra M.; Bautista-Barrufet A.; Trapero A.; Garrido-Charles A.; Díaz-Tahoces A.; Camarero N.; Pittolo S.; Valbuena S.; Pérez-Jiménez A.; Gay M.; García-Moll A.; Rodríguez-Escrich C.; Lerma J.; de la Villa P.; Fernández E.; Pericàs M. À.; Llebaria A.; Gorostiza P. Optical Control of Endogenous Receptors and Cellular Excitability Using Targeted Covalent Photoswitches. Nat. Commun. 2016, 7, 12221. 10.1038/ncomms12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey J. H.; Trauner D. Regulating Enzymatic Activity with a Photoswitchable Affinity Label. Chembiochem 2008, 9, 191–193. 10.1002/cbic.200700570.Regulating. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgraf M.; Gorostiza P.; Numano R.; Kramer R. H.; Isacoff E. Y.; Trauner D. Allosteric Control of an Ionotropic Glutamate Receptor with an Optical Switch. Nat. Chem. Biol. 2006, 2, 47–52. 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorostiza P.; Isacoff E. Optical Switches and Triggers for the Manipulation of Ion Channels and Pores. Mol. BioSyst. 2007, 3, 686–704. 10.1039/b710287a. [DOI] [PubMed] [Google Scholar]
- Gorostiza P.; Isacoff E. Y. Optical Switches for Remote and Noninvasive Control of Cell Signaling. Science 2008, 322, 395–399. 10.1126/science.1166022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin S.; Szobota S.; Reiner A.; Carroll E. C.; Kienzler M. A.; Guyon A.; Xiao T.; Trauner D.; Isacoff E. Y. A Family of Photoswitchable NMDA Receptors. eLife 2016, 5, e12040 10.7554/eLife.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienzler M. A.; Isacoff E. Y. Precise Modulation of Neuronal Activity with Synthetic Photoswitchable Ligands. Curr. Opin. Neurobiol. 2017, 45, 202–209. 10.1016/j.conb.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L.; Sadovski O.; Woolley G. A. A Blue-Green Absorbing Cross-Linker for Rapid Photoswitching of Peptide Helix Content. Bioconjugate Chem. 2006, 17, 670–676. 10.1021/bc050363u. [DOI] [PubMed] [Google Scholar]
- Bandara H. M. D.; Burdette S. C. Photoisomerization in Different Classes of Azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. 10.1039/c1cs15179g. [DOI] [PubMed] [Google Scholar]
- Kienzler M. A.; Reiner A.; Trautman E.; Yoo S.; Trauner D.; Isacoff E. Y. A Red-Shifted, Fast-Relaxing Azobenzene Photoswitch for Visible Light Control of an Ionotropic Glutamate Receptor. J. Am. Chem. Soc. 2013, 135, 17683–17686. 10.1021/ja408104w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C.; Wang X.; Xu J.; Zhao J.; Zhang Q.; Hu Y. Carboxylic Acid-Promoted Copper(I)-Catalyzed Azide-Alkyne Cycloaddition. J. Org. Chem. 2010, 75, 7002–7005. 10.1021/jo101495k. [DOI] [PubMed] [Google Scholar]
- Shao C.; Zhu R.; Luo S.; Zhang Q.; Wang X.; Hu Y. Copper(I) Oxide and Benzoic Acid “on Water”: A Highly Practical and Efficient Catalytic System for Copper(I)-Catalyzed Azide-Alkyne Cycloaddition. Tetrahedron Lett. 2011, 52, 3782–3785. 10.1016/j.tetlet.2011.05.061. [DOI] [Google Scholar]
- Janssens N.; Lesage A. S. J. Glutamate Receptor Subunit Expression in Primary Neuronal and Secondary Glial Cultures. J. Neurochem. 2001, 77, 1457–1474. 10.1046/j.1471-4159.2001.00369.x. [DOI] [PubMed] [Google Scholar]
- Izquierdo-Serra M.; Gascón-Moya M.; Hirtz J. J.; Pittolo S.; Poskanzer K. E.; Ferrer È.; Alibés R.; Busqué F.; Yuste R.; Hernando J.; Gorostiza P. Two-Photon Neuronal and Astrocytic Stimulation with Azobenzene-Based Photoswitches. J. Am. Chem. Soc. 2014, 136, 8693–8701. 10.1021/ja5026326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppeler D.; Merino R. M.; Lopez de la Morena D.; Bali B.; Huet A. T.; Gehrt A.; Wrobel C.; Subramanian S.; Dombrowski T.; Wolf F.; Rankovic V.; Neef A.; Moser T. Ultrafast Optogenetic Stimulation of the Auditory Pathway by Targeting-optimized Chronos. EMBO J. 2018, 37, e99649 10.15252/embj.201899649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieter A.; Keppeler D.; Moser T. Towards the Optical Cochlear Implant: Optogenetic Approaches for Hearing Restoration. EMBO Mol. Med. 2020, 12, e11618 10.15252/emmm.201911618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numano R.; Szobota S.; Lau A. Y.; Gorostiza P.; Volgraf M.; Roux B.; Trauner D.; Isacoff E. Y. Nanosculpting Reversed Wavelength Sensitivity into a Photoswitchable IGluR. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 6814–6819. 10.1073/pnas.0811899106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarero N.; Trapero A.; Pérez-Jiménez A.; Macia E.; Gomila-Juaneda A.; Martín-Quirós A.; Nevola L.; Llobet A.; Llebaria A.; Hernando J.; Giralt E.; Gorostiza P. Photoswitchable Dynasore Analogs to Control Endocytosis with Light. Chem. Sci. 2020, 11, 8981–8988. 10.1039/D0SC03820B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Amorós J.; Díaz-Lobo M.; Nonell S.; Velasco D. Fastest Thermal Isomerization of an Azobenzene for Nanosecond Photoswitching Applications under Physiological Conditions. Angew. Chem. 2012, 124, 12992–12995. 10.1002/ange.201207602. [DOI] [PubMed] [Google Scholar]
- Čechová L.; Filo J.; Dračínský M.; Slavov C.; Sun D.; Janeba Z.; Slanina T.; Wachtveitl J.; Procházková E.; Cigáň M. Polysubstituted 5-Phenylazopyrimidines: Extremely Fast Non-ionic Photochromic Oscillators. Angew. Chem., Int. Ed. 2020, 59, 15590–15594. 10.1002/anie.202007065. [DOI] [PubMed] [Google Scholar]
- Rullo A.; Reiner A.; Reiter A.; Trauner D.; Isacoff E. Y.; Woolley G. a. Long Wavelength Optical Control of Glutamate Receptor Ion Channels Using a Tetra-Ortho-Substituted Azobenzene Derivative. Chem. Commun. 2014, 50, 14613–14615. 10.1039/C4CC06612J. [DOI] [PubMed] [Google Scholar]
- Prieto M. L.; Wollmuth L. P. Gating Modes in AMPA Receptors. J. Neurosci. 2010, 30, 4449–4459. 10.1523/JNEUROSCI.5613-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R.; Banke T. G.; Mayer M. L.; Traynelis S. F.; Gouaux E. Structural Basis for Partial Agonist Action at Ionotropic Glutamate Receptors. Nat. Neurosci. 2003, 6, 803–810. 10.1038/nn1091. [DOI] [PubMed] [Google Scholar]
- Greger I. H.; Watson J. F.; Cull-Candy S. G. Structural and Functional Architecture of AMPA-Type Glutamate Receptors and Their Auxiliary Proteins. Neuron 2017, 94, 713–730. 10.1016/j.neuron.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Glowatzki E.; Fuchs P. A. Transmitter Release at the Hair Cell Ribbon Synapse. Nat. Neurosci. 2002, 5, 147–154. 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- Petitpré C.; Wu H.; Sharma A.; Tokarska A.; Fontanet P.; Wang Y.; Helmbacher F.; Yackle K.; Silberberg G.; Hadjab S.; Lallemend F. Neuronal Heterogeneity and Stereotyped Connectivity in the Auditory Afferent System. Nat. Commun. 2018, 9, 3691. 10.1038/s41467-018-06033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrobel C.; Dieter A.; Huet A.; Keppeler D.; Duque-Afonso C. J.; Vogl C.; Hoch G.; Jeschke M.; Moser T. Optogenetic Stimulation of Cochlear Neurons Activates the Auditory Pathway and Restores Auditory-Driven Behavior in Deaf Adult Gerbils. Sci. Transl. Med. 2018, 10, eaao0540 10.1126/scitranslmed.aao0540. [DOI] [PubMed] [Google Scholar]
- Huet A. T.; Dombrowski T.; Rankovic V.; Thirumalai A.; Moser T. Developing Fast, Red-Light Optogenetic Stimulation of Spiral Ganglion Neurons for Future Optical Cochlear Implants. Front. Mol. Neurosci. 2021, 14, 635897. 10.3389/fnmol.2021.635897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager T.; Lopez de la Morena D.; Senn V.; Schlotte J.; D́Errico A.; Feldbauer K.; Wrobel C.; Jung S.; Bodensiek K.; Rankovic V.; Browne L.; Huet A.; Jüttner J.; Wood P. G.; Letzkus J. J.; Moser T.; Bamberg E. High Frequency Neural Spiking and Auditory Signaling by Ultrafast Red-Shifted Optogenetics. Nat. Commun. 2018, 9, 1750. 10.1038/s41467-018-04146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bali B.; Lopez de la Morena D.; Mittring A.; Mager T.; Rankovic V.; Huet A. T.; Moser T. Utility of Red-Light Ultrafast Optogenetic Stimulation of the Auditory Pathway. EMBO Mol. Med. 2021, 13, e13391 10.15252/emmm.202013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa S. G.; Liberman M. C. Adding Insult to Injury: Cochlear Nerve Degeneration after “Temporary” Noise-Induced Hearing Loss. J. Neurosci. 2009, 29, 14077. 10.1523/jneurosci.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeyenko Y.; Lall K.; Liberman M. C.; Kujawa S. G. Age-Related Cochlear Synaptopathy: An Early-Onset Contributor to Auditory Functional Decline. J. Neurosci. 2013, 33, 13686–13694. 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc R.; Pfeiffer R.; Jones B. Retinal Prosthetics, Optogenetics, and Chemical Photoswitches. ACS Chem. Neurosci. 2014, 5, 895–901. 10.1021/cn5001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L.; Weiland J. D.; Roska B.; Humayun M. S. Retinal Stimulation Strategies to Restore Vision: Fundamentals and Systems. Prog. Retin. Eye Res. 2016, 53, 21–47. 10.1016/j.preteyeres.2016.05.002. [DOI] [PubMed] [Google Scholar]
- Tochitsky I.; Kienzler M. A.; Isacoff E.; Kramer R. H. Restoring Vision to the Blind with Chemical Photoswitches. Chem. Rev. 2018, 118, 10748–10773. 10.1021/acs.chemrev.7b00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D. D. Critical Bandwidth and the Frequency Coordinates of the Basilar Membrane. J. Acoust. Soc. Am. 1961, 33, 1344–1356. 10.1121/1.1908437. [DOI] [Google Scholar]
- Müller M. The Cochlear Place-Frequency Map of the Adult and Developing Mongolian Gerbil. Hear. Res. 1996, 94, 148–156. 10.1016/0378-5955(95)00230-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.