Abstract
Active crosstalk between the nervous system and breast cancer cells has been experimentally demonstrated in vitro and in animal models. However, low frequencies of peripheral nerve presence in human breast cancers reported in previous studies (~30% of cases) potentially negate a major role of the nervous system in breast cancer development and progression. This study aimed to clarify the incidence of nerves within human breast cancers and to delineate associations with clinicopathological features. Immunohistochemical staining was conducted in formalin‐fixed paraffin‐embedded breast cancer tissue sections using antibodies against the pan‐neuronal markers protein gene product 9.5 and growth‐associated protein 43, and the sympathetic nerve‐specific marker tyrosine hydroxylase. Nerve trunks and isolated nerve fibers were quantitated. The chi‐squared test was used to determine the associations between nerve counts and clinicopathological parameters. The log‐rank test was used to compare differences in patient progression‐free survival (PFS) and overall survival (OS). The overall frequency of peripheral nerves in breast cancers was 85%, a markedly higher proportion than reported previously. Of note, most nerves present in breast cancers were of the sympathetic origin. While high density of nerve trunks or isolated nerve fibers was associated with poor PFS and OS of patients, high nerve trunk density appeared also to predict poor patient PFS independently of lymph node metastasis. Innervation of breast cancers is a common event correlated with poor patient outcomes. These findings support the notion that the nervous system plays an active role in breast cancer pathogenesis.
Keywords: breast cancer, cancer neuroscience, innervation, nerves, tumor microenvironment
1. INTRODUCTION
Perineural invasion (PNI), a process in which cancer cells grow around existing nerves and/or invade the perineural space of nerves, has long been known to be associated with metastasis and poor patient outcomes in many cancer types. 1 , 2 , 3 , 4 However, the active crosstalk between the nervous system and cancer cells as well as other types of cells in the tumor microenvironment has not been appreciated until recently. 4 , 5 , 6 On the one hand, neurotransmitters and growth factors secreted by nerves activate signal pathways that promote cancer cell proliferation, invasion and metastasis. 4 , 5 , 6 , 7 On the other hand, cancer cells produce neurotrophins that stimulate neural invasion of the tumor microenvironment. 4 , 5 , 6 , 7 Moreover, signals generated by nerves can modulate the tumor microenvironment through regulating angiogenesis and infiltrating immune cells. 4 , 5
Through secreting neurotrophins such as nerve growth factor (NGF), breast cancer cells induce neurite outgrowth of neuronal cells in vitro. 8 , 9 , 10 , 11 Indeed, denervation causes regression of established breast cancer in ex vivo models, 12 providing direct evidence that nerve supply is necessary for breast cancer growth. Consistently, chronic neural activity can be recorded within the tumor mass of mouse breast cancer models. 13 Of interest, sympathetic and parasympathetic nerves appear to have opposite effects on breast cancer growth 14 : sympathetic neurostimulation accelerates, whereas parasympathetic neurostimulation decelerates the progression of both human breast cancer xenografts and spontaneous breast cancer models in mice. 14 Intriguing clinical evidence supporting the role of the sympathetic nervous system in breast cancer also comes from several population‐based studies involving beta‐blockers, competitive antagonists that block interactions between epinephrine and norepinephrine with adrenergic beta‐receptors, reducing breast cancer progression and improving patient outcomes. 15 , 16 , 17
Despite these advances in understanding of the role of the nervous system in breast cancer progression, information about innervation of human breast cancers in vivo is still limited. The reported incidence of nerves in breast cancers varied widely and was generally low, with frequencies ranging from 28% to 61%, 8 , 18 , 19 potentially negating a major role of the nervous system in this disease. Moreover, whether nerve fiber innervation of breast cancers is associated with patient outcomes remains unclear. 8 , 15 , 16 In view of these, we have carried out immunohistochemistry (IHC) analysis of breast cancer innervation in a patient cohort. Here, we report that both nerve trunks that are comprised of many nerve fibers/axons and isolated nerve fibers are present in a markedly larger proportion of breast cancers than previously described, and that high density of nerve trunks or isolated nerve fibers is associated with poor patient progression‐free survival (PFS) and overall survival (OS). Moreover, we demonstrate that high nerve trunk density is potentially a predictor of poor patient PFS independently of lymph node involvement. Our results also reveal that nerves infiltrating breast cancers are predominantly of the sympathetic origin.
2. MATERIALS AND METHODS
2.1. Patients and tissue specimens
The archival formalin‐fixed paraffin‐embedded (FFPE) breast cancer tissue blocks from 126 female patients (median age 50, ranging from 26 to 81) who underwent surgery at the Department of Breast Surgery of Shanxi Bethune Hospital, Taiyuan, China, during the period from March 2012 to December 2014 were retrieved from the Department of Pathology of the hospital. Hematoxylin and eosin (H&E) stained sections of all 126 cases were reviewed and pathological diagnoses were confirmed by two independent pathologists (LN Hu and L Li). Estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) positivity were determined using immunohistochemistry (IHC) at the time of pathological examination of surgical specimens and the results were retrieved from medical records. The clinicopathological characteristics of the patients are included in Table 1 and Tables S1–S6. All included patients were regularly followed up for a minimal 66‐month period. The study was approved by the Human Ethics Review Committee of Shanxi Bethune Hospital. Informed consents were obtained from all patients during their hospitalization.
TABLE 1.
The relationship between the density of nerve fibers and clinicopathological parameters in breast cancer (the high quartile nerve fiber count as the cut‐off)
| Parameter | Low nerve fiber counts (n = 92) | High nerve fiber counts (n = 34) | p‐value a |
|---|---|---|---|
| Tumor size b | 0.1837 | ||
| 1 (n = 56) | 45 (80.4%) | 11 (19.6%) | |
| 2 (n = 61) | 42 (68.9%) | 19 (31.1%) | |
| 3 (n = 4) | 3 (75.0%) | 1 (25.0%) | |
| 4 (n = 5) | 2 (40.0%) | 3 (60.0%) | |
| Patient age c | 0.0865 | ||
| ≤50 (n = 64) | 51 (79.7%) | 13 (20.3%) | |
| >50 (n = 62) | 41 (66.1%) | 21 (33.9%) | |
| Lymph node involvement d | 0.0312 | ||
| 0 (n = 71) | 56 (78.9%) | 15 (21.1%) | |
| 1 (n = 31) | 24 (77.4%) | 7 (22.6%) | |
| 2 (n = 10) | 6 (60.0%) | 4 (40.0%) | |
| 3 (n = 14) | 6 (42.9%) | 8 (57.1%) | |
| HER2 e | 0.4858 | ||
| HER2 − (n = 91) | 68 (74.7%) | 23 (25.3%) | |
| HER2 + (n = 35) | 24 (68.6%) | 11 (31.4%) | |
| ER e | 0.4304 | ||
| ER − (n =44) | 34 (77.3%) | 10 (22.7%) | |
| ER + (n = 82) | 58 (70.7%) | 24 (29.3%) | |
| PR e | 0.8779 | ||
| PR − (n = 57) | 42 (73.7%) | 15 (26.3%) | |
| PR + (n = 69) | 50 (72.5%) | 19 (27.5%) | |
| Molecular subtype f | 0.7351 | ||
| Luminal A (n = 69) | 50 (72.5%) | 19 (27.5%) | |
| Luminal B (n = 15) | 10 (66.7%) | 5 (33.3%) | |
| HER−2 positive (n = 20) | 14 (70.0%) | 6 (30.0%) | |
| Triple negative (n = 22) | 18 (81.8%) | 4 (18.2%) | |
| Pathological subtype g | 0.4957 | ||
| IDC (n = 116) | 85 (73.3%) | 31 (26.7%) | |
| ILC (n = 6) | 5 (83.3%) | 1 (16.7%) | |
| Others (n = 4) | 2 (50.0%) | 2 (50.0%) | |
| Menopause | 0.0235 | ||
| Pre‐menopause (n = 69) | 56 (81.2%) | 13 (18.8%) | |
| Post‐menopause (n = 57) | 36 (63.2%) | 21 (36.8%) | |
| The age of menarche h | 0.0065 | ||
| ≤15 (n = 73) | 60 (82.2%) | 13 (17.8%) | |
| >15 (n = 53) | 32 (60.4%) | 21 (39.6%) |
Chi‐squared test, a p value ≤0.05 was considered statistically significant.
Tumor sizes were scored according to the TNM staging system.
Patients were arbitrarily divide into two groups according to the median age at diagnosis age 50.
Lymph node involvement was scored according to the TNM staging system.
HER2, ER and PR positivity defined using immunohistochemistry was recorded in the pathological report of surgically removed breast cancer tissues.
Molecular subtypes were defined as luminal A: ER+and/or PR+/HER2−; luminal B: ER+and/or PR+/HER2+; HER2+; TNBC: ER−/PR−/HER2−.
IDC: Invasive ductal carcinomas; ILC: Invasive lobular carcinomas; Others including micropapillary carcinomas, metaplastic carcinomas and mucinous adenocarcinomas.
Patients were arbitrarily divided into two groups according to the median age of menarche age 15.
Bold values represent the p value is less than 0.05, and the difference is statistically significant.
2.2. Immunohistochemistry (IHC) and assessment of IHC staining
Serial four‐micrometer‐thick sections were prepared from each FFPE tissue block before deparaffinization and rehydration following standard procedures. Heat‐induced epitope retrieval was carried out in a citrate‐based low pH buffer (Vector Laboratories) using a decloaking chamber (Biocare) at 95°C for 20 min. IHC was then carried out using an automated immunohistochemistry system (Ventana BenchMark XT, Roche, Switzerland). Briefly, endogenous peroxidase activity was blocked with 0.03% hydrogen peroxidase and Fc receptors blocked with 10% normal horse serum. Antibodies (Abs) and controls were purchased from Abcam (Abcam, Shanghai, China), including rabbit anti‐human protein gene product 9.5 (PGP9.5) monoclonal Ab (Cat: ab108986), rabbit anti‐human growth associated protein 43 (GAP43) monoclonal Ab (Cat: ab75810), rabbit anti‐human tyrosine hydroxylase (TH) polyclonal AB (Cat: ab75875), and non‐immune rabbit IgG control (Cat: ab188776). ImmPRESS HRP anti‐rabbit IgG (peroxidase) (Cat: NC9294174) was then applied, and staining revealed with DAB peroxidase substrate solution (Cat: 34002). Sections were finally counterstained with Harris hematoxylin (Cat: ab220365).
The identity of positively stained nerves was readily confirmed by comparison with serial sections applied with the non‐immune rabbit IgG control. The counts of nerve trunks (comprised of many nerve fibers/axons) and isolated nerve fibers (positively stained cells with or without typical morphology of axons outside definable nerve trunks; hereafter referred to as nerve fibers for simplicity) were derived at high (×400) magnification from 10 random fields using an Olympus BLISS High‐Definition Virtual Microscope (Olympus, Japan). Each slide was examined by two independent pathologists and counts of nerve trunks and isolated nerve fibers, respectively, were recorded as averages.
2.3. Statistical analysis
Statistical analysis was carried out using GraphPad Prism 9 and IBM SPSS Statistics 27. Progression‐free survival (PFS) and overall survival (OS) were calculated using the Kaplan–Meier univariate estimates. Differences in PFS and OS between patients with high or low nerve fiber/trunk numbers upon various cut‐offs were compared using the log‐rank (Mantel‐Cox) test. The univariate analysis was followed by multivariate analysis according to the Cox Proportional Hazards Model to assess independent prognostic factors. The correlation between GAP43 and PGP9.5 or TH and PGP9.5 staining was compared using simple linear regression. Simple unadjusted associations between nerve trunks and nerve fibers and other pathological variables were performed using the chi‐squared (χ2) test. A p value <0.05 was considered statistically significant.
3. RESULTS
3.1. Breast cancers are frequently innervated by nerve trunks and isolated nerve fibers
We initially carried out studies in FFPE breast cancer tissue sections from 50 patients using immunohistochemistry with Abs against the two pan‐neuronal makers, PGP9.5 and GAP43. 17 , 18 The results showed that both Abs identified nerve trunks and fibers with identical patterns and frequencies, although PGP9.5 staining intensity was commonly weaker, albeit moderately, than GAP43 (Figure 1A). Nerve trunks were present in 38 cases (76%), whereas nerve fibers, 39 cases (78%). There was virtually perfect correlation between the counts of nerve trunks and fibers stained by the two Abs (Figure 1B). We thus employed only the anti‐PGP9.5 Ab for further investigation.
FIGURE 1.
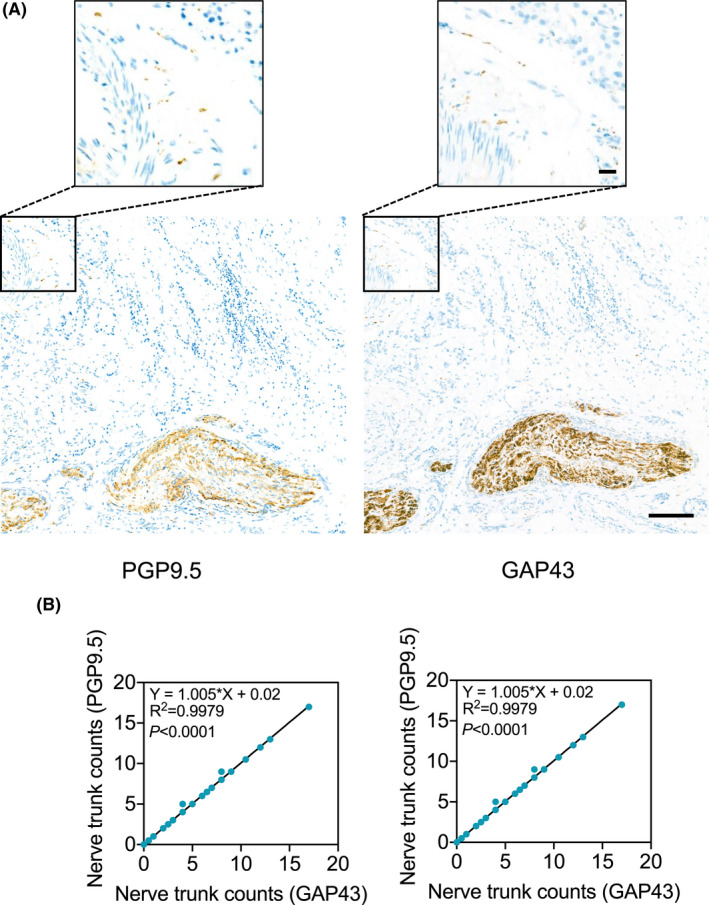
IHC staining of PGP9.5 and GAP43 identified nerve trunks and fibers with similar patterns and frequencies in breast cancer tissues. (A) Representative microphotographs of IHC staining of PGP9.5 and GAP43 in serial breast cancer tissue sections. Scale bar, 100µm. (B) The positive correlation in the counts of nerve trunks (left) or fibers (right) identified by staining of PGP9.5 and GAP43 with IHC (regression analysis)
We extended the IHC study using the anti‐PGP9.5 Ab to a total of 126 breast cancers. Nerve trunks were detected in 95 (75%) cases, and nerve fibers in 98 (77%) cases. The presence of nerve trunks, fibers or the co‐existence of nerve trunks and fibers occurred in 107 cases (85%), demonstrating that innervation of breast cancer is a common event. Nerve fibers were most frequently observed around cancer cell nests or alongside blood vessels in the tumor stroma (Figure 2A–D), consistent with previous results. 8 Noticeably, nerve fibers infiltrating into cancer cell nests were also observed (Figure 2E), supporting the notion that cancer cells chemoattract nerve fibers to support their malignancy. 4 , 5 , 6
FIGURE 2.
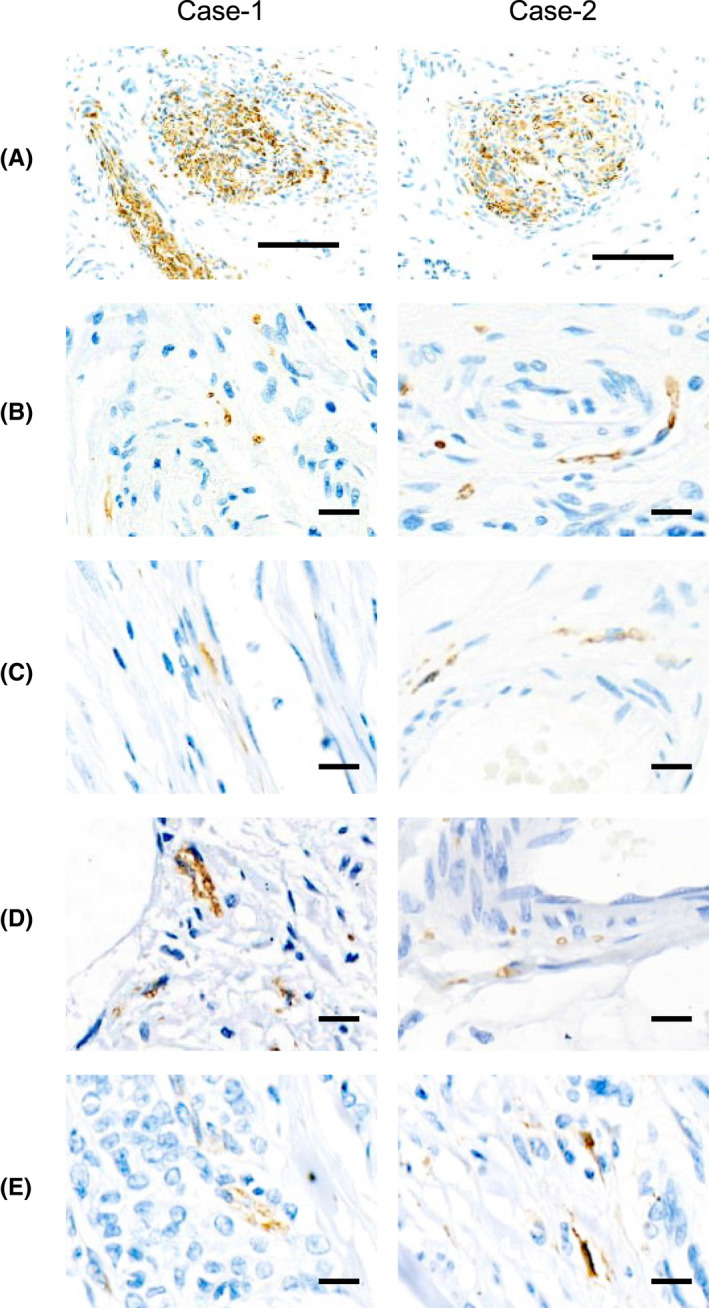
Representative microphotographs of IHC staining of PGP9.5 showing nerve trunks (A) and nerve fibers around cancer cell nests (B), alongside blood vessels (C), next to adipocytes and infiltrating into cancer nests (D)
To further characterize the nerve trunks and nerve fibers identified, we stained breast cancer tissue sections from 20 patients that were positive for PGP9.5 using an anti‐tyrosine hydroxylase (TH) Ab, a marker of sympathetic neurons.22 Indeed, most nerve trunks and fibers positive for PGP9.5 were also positive for TH (Figure 3A,B). The counts of nerve trunks identified by the anti‐PGP9.5 Ab were positively correlated with those identified with the anti‐TH Ab (Figure 3B). Similarly, TH‐positive nerve fiber counts were correlated with those positive for PGP9.5. Thus, nerves present in breast cancers are predominantly of the sympathetic origin.
FIGURE 3.
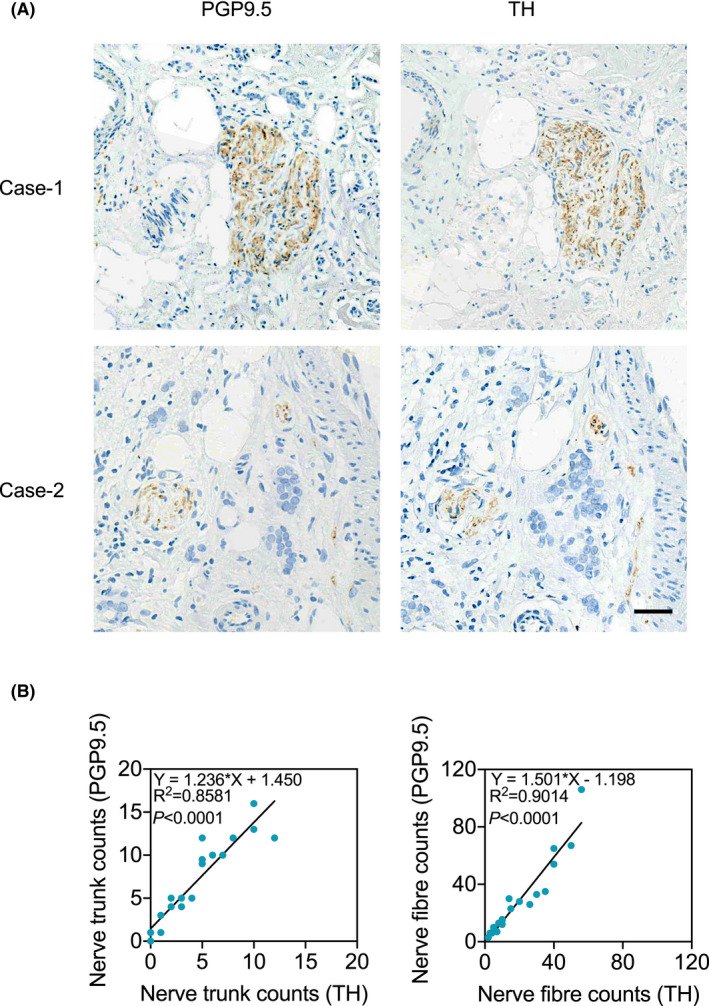
Nerve trunks and fibers present in breast cancer tissues are predominantly of the sympathetic nervous system. (A) Representative microphotographs of IHC staining of PGP9.5 and TH in serial breast cancer tissue sections. Scale bar, 100µm. (B) The positive correlation in the counts of nerve trunks (left) or fibers (right) identified by staining of PGP9.5 and TH using IHC (regression analysis)
3.2. Nerve presence in breast cancer is associated with poor patient PFS
To test for associations between nerve presence and other clinicopathological characteristics, we classified the 126 breast cancers into nerve positive and nerve negative groups, i.e. cases containing nerve trunks or fibers, alone or in combination, or cases lacking nerve trunks and fibers. Chi‐squared analysis showed that there were no significant differences in the frequencies of nerves among breast cancers of different clinicopathological groups defined by tumor size, lymph node involvement, pathological and molecular subtype, patient age, and menopausal status (Table S1). Similarly, no significant differences were found in the frequencies of nerves among breast cancers with and without estrogen receptor (ER), progestogen receptor, or HER2 expression (Table S1). Interestingly, nerves were present more frequently in breast cancers of patients with relatively late menarche (Table S1). The presence of nerves was observed in 79.5% of breast cancers of patients with menarche occurring before or at age 15 (the median age at menarche of the 126 patients), whereas 92.5% of breast cancers from those with late menarche exhibited nerve presence (p = 0.0441) (Table S1).
We then analyzed whether nerve presence is associated with patient outcomes. The Log‐rank test revealed that the presence of nerves is significantly related to poor PFS of patients [hazard ratio (HR) = 4.064, 95% confidence interval (CI) = 1.813 to 9.111, p = 0.0351] (Figure 4A). However, it was not significantly associated with patient OS, although there was a consistent trend that patients with breast cancers containing nerves had shorter OS (HR = 4.378, 95% CI = 1.406 to 13.13, p = 0.1136) (Figure 4B).
FIGURE 4.
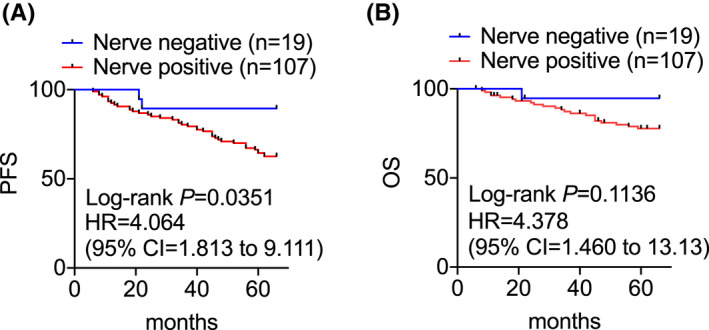
Log‐rank analysis of the probability of PFS (A) and OS (B) of patients of breast cancers with or without the presence of nerves
3.3. High nerve fiber density is associated poor patient PFS and OS in breast cancer
We next asked whether the presence of nerve fibers alone is associated with breast cancer patient outcomes. When the 126 breast cancers were classified into nerve fiber positive and negative groups, it was found that patients with breast cancers displaying nerve fibers tended to have poorer PFS (HR = 2.401, 95% CI = 1.188 to 4.850, p = 0.0567) and OS (HR = 3.367, 95% CI = 1.298 to 8.734, p = 0.0806), although the differences were not statistically significant (Figure 5A,B).
FIGURE 5.
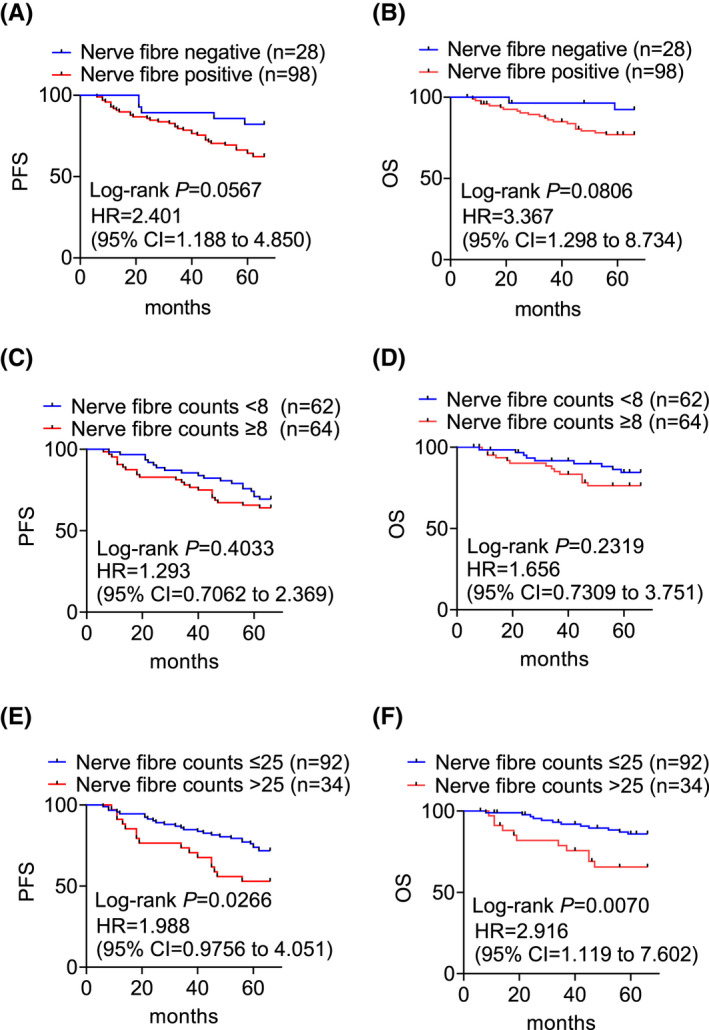
Log‐rank analysis of the probability of PFS and OS of patients of breast cancers with or without the presence of nerve fibers (A, B), with high or low densities of nerve fibers defined with the median (C, D) or high quartile (E, F) of nerve fiber counts as the cut‐off (C, D)
Similarly, when the cases were stratified using the median of nerve fiber counts as cut‐off, there were no significant differences between breast cancers containing high and low densities of nerve fibers for either PFS (HR = 1.293, 95% CI = 0.7062 to 2.369, p = 0.4033) or OS (HR = 1.656, 95% CI = 0.7309 to 3.751, p = 0.2319) between patients with breast cancers containing high and low densities of nerve fibers (Figure 5C,D). Nonetheless, when the high quartile of nerve fiber counts was chosen as the cut‐off, the high occurrence of denser nerve fibers was associated with significantly worse PFS (HR = 1.988, 95% CI = 0.976 to 4.051, p = 0.0266) and OS (HR = 2.916, 95% CI = 1.119 to 602, p = 0.0070) (Figure 5E,F). Therefore, high nerve fiber density in breast cancer is associated with compromised patient PFS and OS.
We also analyzed the relationship between nerve fiber presence and other clinicopathological characteristics. The occurrence of nerve fibers was not associated with tumor size, lymph node involvement, pathological and molecular subtype, patient age, and menopausal status, estrogen and progestogen receptor statuses, HER2 positivity and age of menarche (Table S2). However, when cases were stratified into high and low nerve fiber groups using a median nerve fiber count cut‐off, later menarche compared to early menarche patients (at or before age 15) displayed higher nerve fiber densities in breast cancer tissues (62.3% versus 42.5%, respectively; p = 0.0282) (Table S3). Intriguingly, ER positive breast cancers appeared to have significantly more nerve fibers than non‐ER cases (p = 0.0176) (Table S3).
Analyses performed using the high quartile nerve fiber counts as the cut‐off showed that a significantly larger proportion of patients with late menarche exhibited higher density of nerve fibers compared with those with early menarche (p = 0.0065) (Table 1). Furthermore, breast cancers with increased lymph node involvement displayed a higher density of nerve fibers (p = 0.0312) (Table 1). Only 21.1% of breast cancers without lymph node involvement displayed high density of nerve fibers compared with 57.1% of N3‐staged breast cancers (Table 1).
3.4. High nerve trunk density is associated with poor patient PFS and OS in breast cancer
While there was a clear trend between nerve fibers and patient outcomes (Figure 5), analyses stratified by nerve trunks alone indicated their presence was significantly related to patient PFS (HR = 2.755, 95% CI = 1.395 to 5.439, p = 0.0258) and OS (HR = 3.845, 95% CI = 1.528 to 9.674, p = 0.0494) (Figure 6A,B). No statistically significant differences were seen in either PFS or OS when cases were analyzed as high or low nerve trunk counts based on median counts as the cut‐off (PFS HR = 1.822, 95% CI = 0.9917 to 3.348, p = 0.0517 and OS HR = 1.601, 95% CI = 0.7040 to 3.643, p = 0.2574) (Figure 6C,D). Nevertheless, the same tendency remained that patients with breast cancers containing more nerve trunks had worse outcomes. Indeed, setting the high quartile of nerve trunk counts as the cut‐off, the association between high nerve trunk density and poorer patient PFS was significant (HR = 2.684, 95% CI = 1.235 to 5.834, p = 0.0011), with the trend of association with OS almost reaching statistical significance (HR = 2.274, 95% CI = 0.8023 to 6.445, p = 0.0530) (Figure 6E,F).
FIGURE 6.
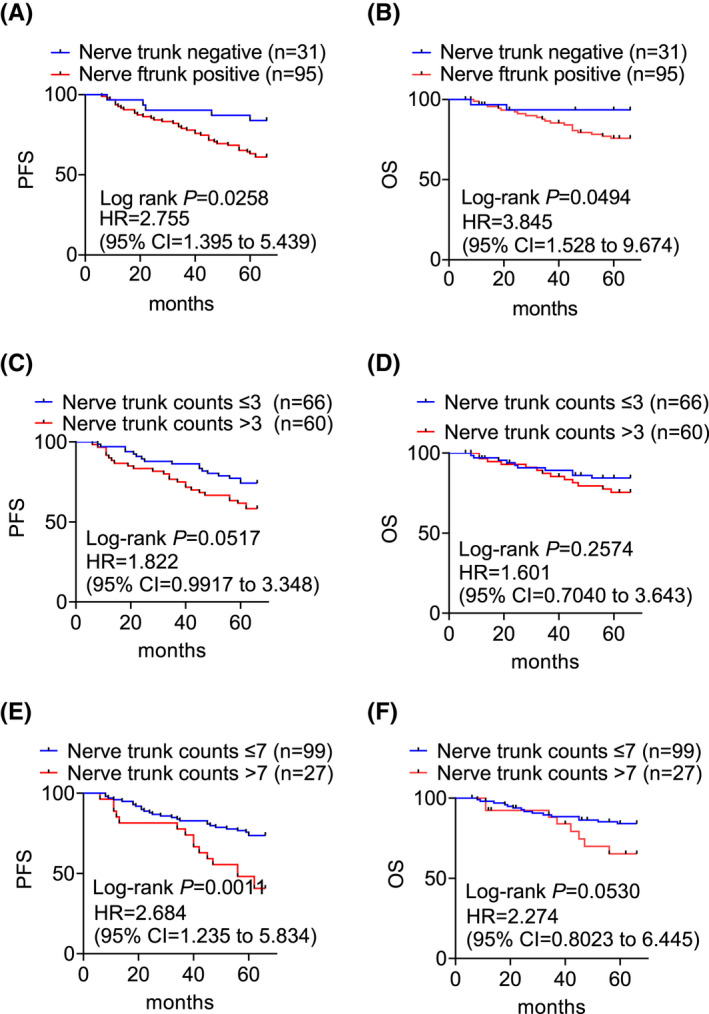
Log‐rank analysis of the probability of PFS and OS of patients of breast cancers with or without the presence of nerve trunks (A, B), with high or low densities of nerve trunks defined with the median (C, D) or high quartile (E, F) of nerve trunk counts as the cut‐off (C, D)
Analysis of the relationship between nerve trunks and other clinicopathological characteristics indicated nerve trunks were present in a larger proportion of breast cancers cases with late (86.8%) compared to early menarche (67.1%) (p = 0.0114) (Table S4). Notably, more breast cancers from post‐menopausal patients (84.2%) showed nerve trunk presence than those from pre‐menopausal patients (68.1%) (p = 0.0368) (Table S4). However, there were no significant differences in the frequency of nerve trunks among breast cancers grouped according to tumor size, lymph node involvement, pathological and molecular subtype, patient age, and estrogen and progestogen receptor status (Table S4). Analysis of the data using cut‐off values according to either median or high quartile nerve trunk counts showed no significant differences amongst the different clinicopathological variables (Tables S5 and S6).
3.5. High nerve trunk density in breast cancer may predict poorer patient PFS independently of lymph node involvement
Based on the preceding findings, we assessed the value of nerve fiber and trunk innervation as an independent predictive factor of breast cancer outcomes, directly comparing this with lymph node involvement, the strongest known prognostic factor in breast cancer. 19 , 20 , 21 Strikingly, the high density of nerve trunks defined using the high quartile of nerve trunk counts in breast cancers appeared to predict poorer patient PFS (HR = 2.281, 95% CI = 1.209 to 4.301, p = 0.011) independently of lymph node involvement (HR = 1.667, 95% CI = 1.279 to 2.172, p = 0.000), although it did not appear to be associated with OS (Table 2). In contrast, nerve fiber or trunk presence, high nerve fiber density defined using the median or high quartile of counts as the cut‐off, or high nerve trunk density defined using the median of counts as the cut‐off, did not appear to have an independent prognostic significance.
TABLE 2.
High abundance of nerve trunks is a predictive factor of PFS independently of lymph node involvement at surgery (Multivariate Cox regression analysis).
| Factors | Progression‐free survival | Overall survival | ||
|---|---|---|---|---|
| HR (95%CI) | p value a | HR (95%CI) | p value | |
| High nerve trunk counts | 2.281 (1.209–4.301) | 0.011 | 1.832 (0.764–4.394) | 0.175 |
| Lymph Node involvement | 1.667 (1.279–2.172) | 0.000 | 1.848 (1.302–2.623) | 0.001 |
Abbreviations: CI: confidence intervalHR: Hazard Ratio.
Multivariate Cox regression analysis with the P value obtained from two‐sided log‐rank test. A p value ≤0.05 was considered statistically significant.
4. DISCUSSION
Active crosstalk between the nervous system and cancer cells as well as other types of cells in the tumor microenvironment has been experimentally demonstrated in an increasing variety of cancers such as pancreatic, gastric, colon, prostate, ovary, skin and breast cancer. 12 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 However, the varying incidence of innervation in breast cancers, particularly those reports indicating a low frequency, have cast doubts as to whether nerves play a major role in the development and progression of the disease. 8 , 15 , 16 We demonstrated in this study that nerves are present in 85% of breast cancers, a proportion markedly larger than previously observed, 12 indicating that innervation is a common event in breast cancers. The reason of the discrepancy between this and previous studies is not entirely clear, but we used the entire section from each tissue block, whereas the previous studies employed tissue microarrays (TMAs) that might generate bias in tissue sampling and thus underrepresent nerve presence. 8 Moreover, we used two independent pan‐neuronal makers in our initial study that exhibited virtually the same expression pattern and density, indicating that the high frequency of nerves we observed was not caused by non‐specific staining of other types of cells. Our results therefore provide strong evidence supporting the frequent occurrence of neuronal involvement in breast cancer and establish a rational basis for active interactions between the nervous system and human breast cancer.
Different types of nerves may exert cancer type‐specific functions. 4 , 5 For example, cholinergic signaling generated from parasympathetic nerves inhibits the growth and progression of pancreatic adenocarcinoma and breast cancer but has a strong oncogenic effect on gastric adenocarcinoma. 30 We found that the vast majority of nerves infiltrating into breast cancer tissues are of the sympathetic origin, consistent with the promoting role of sympathetic nerves demonstrated in animal models of breast cancer. 14 In accordance, increased sympathetic nerve density in breast cancers is associated with poor patient prognosis, whereas beta‐blocker intake inhibits breast cancer progression. 14 Although how sympathetic nerves promote breast cancer progression is not fully understood, their presence has been linked to high expression of immune checkpoint molecules such as PD‐1 and PD‐L1 in the breast cancer microenvironment, 14 pointing to a role sympathetic nerves in regulating the interaction between breast cancer cells and the immune system.
An important finding of this study was that the presence of nerves identified using a pan‐neuronal maker was similarly associated with poor PFS of patients. Moreover, our quantitative analysis revealed that when the high quartile of counts was used as the cut‐off, high density of nerve trunks or isolated nerve fibers was associated with poor PFS as well as poor OS of patients. The presence of nerves was previously shown to be associated with lymph node metastasis and consistently we also found that high density of isolated nerve fibers was related to increased lymph node involvement. 8 These results substantiate the role of the nervous system in promoting breast cancer progression and further suggest that pathological assessment of innervation status could provide additional prognostic information. However, this approach would require some practical considerations as nerve trunks may not always be observed due to small size of tumors and bias in sampling of specimens. On the other hand, as we observed in this study, isolated nerve fibers are dispersed throughout breast cancer tissues, either within the tumor stroma or often around or infiltrating into cancer cell nests, mirroring the neurite outgrowth induced by cancer cells in vitro. 8 Nevertheless, the density of nerve trunks but not nerve fibers was found to predict poor patient PFS independently of lymph node involvement, the most powerful prognostic factor in breast cancer. 19 , 20 , 21 This implies that that nerve trunks and not isolated nerve fibers are more strongly associated with breast cancer progression. Indeed, cancer cells use nerves in addition to lymphatics and blood vessels as routes of metastasis and PNI are known to associate with poor outcomes in many cancer types including breast cancer. 15 , 16
Another pertinent consideration involves the potential links between cancer neuroscience and psycho‐oncology, another rapidly growing interdisciplinary field. 31 , 32 , 33 , 34 Many clinical studies have established links between psychological stress and breast cancer progression and treatment resistance, with associations with poor patient prognosis. 31 , 32 , 33 , 35 Although the molecular mechanism(s) responsible remains to be fully elucidated, it is known that psychological stress triggers alterations in neuronal secretions, 34 , 36 , 37 whereas a number of neurotransmitters such as dopamine and norepinephrine can regulate the migration of the breast cancer cells. 38 Moreover, stress may stimulate angiogenesis directly through sympathetic nerve activation within the cancer microenvironment. 26 , 39 , 40 , 41 Our findings that innervation is common in breast cancer and high nerve density is associated with poor patient outcome support the notion that there is a close association between psychological stress and breast cancer pathogenesis and progression.
It is intriguing that breast cancers from patients with later menarche commonly contain more nerves, implying that these patients may have worse prognosis than those whose menarche occurs at younger age. However, early menarche is a well‐established breast cancer risk factor and is also associated with the risk of lymph node metastasis and poor patient prognosis. 42 , 43 Similarly, our results showed that ER+ breast cancers displayed higher densities of nerve fibers, implicating worse outcomes of these patients. However, ER+ breast cancer patients tend to have better survival outcomes related to benefits associated with treatment efficacy and the long‐term tolerability of endocrine therapy. 44 , 45 Moreover, we also found that breast cancers of post‐menopausal patients tended to have high densities of nerves, suggestive of poorer prognosis of these patients. Indeed, older breast cancer patients commonly have worse survival than those diagnosed at younger ages. 46 , 47 Nevertheless, we did not identify differences in breast cancer innervation among different age groups. What causes these paradoxes is unknown, but our results suggest that the innervation status be considered in stratifying patients in future studies. Large cohorts of breast cancers need to be analyzed to draw more explicit conclusions about the clinical usefulness of qualitative and quantitative analysis of nerve trunks and fibers in breast cancer tissues. Furthermore, it would be interesting to interrogate whether the presence of nerves is associated with breast cancer responses to systematic treatments.
In conclusion, our results indicate that innervation of breast cancers is a common event and reveal a correlation between high nerve density in breast cancers and poor patient outcomes. These findings support the notion that the nervous system plays an active role in breast cancer pathogenesis and call for further exploration of approaches to disrupt the effects of nerves on breast cancer cells and the tumor microenvironment for the treatment of the disease.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
J.N.G. and L.L designed research; D.L., S.M.Z., L.N.H., X.J.Z., X.J., and Z.H.Z. performed research; D.L., T.L., L.W., L.Y.W., and Y.C.F. analyzed data; X.D.Z., D.L., T.L., R.F.T., and H.H. wrote/edited the paper. All authors have read and agreed to the published version of the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Shanxi Province “136 Revitalization Medical Project Construction Funds” (Grant No. 2021YZ10) and the Key Research and Development Plan of Shanxi Province, China, which was funded by Shanxi Science and Technology Department (Grant No. 201903D421025).
Li D, Hu LN, Zheng SM, et al. High nerve density in breast cancer is associated with poor patient outcome. FASEB BioAdvances. 2022;4:391–401. doi: 10.1096/fba.2021-00147
Dong Li and Li Na Hu are contributed equally to this work.
This article is part of the Neuroscience of Cancer, Regeneration, and Immunity special collection.
Contributor Information
Li Li, Email: limuzi73@163.com, Email: sxjinnan@aliyun.com.
Jin Nan Gao, Email: sxjinnan@aliyun.com.
REFERENCES
- 1. Marchesi F, Piemonti L, Mantovani A, Allavena P. Molecular mechanisms of perineural invasion, a forgotten pathway of dissemination and metastasis. Cytokine Growth Factor Rev. 2010;21:77‐82. [DOI] [PubMed] [Google Scholar]
- 2. Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11:695‐707. [DOI] [PubMed] [Google Scholar]
- 3. Wang H, Zheng Q, Lu Z, et al. Role of the nervous system in cancers: A review. Cell Death Discov. 2021;7:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Faulkner S, Jobling P, March B, Jiang CC, Hondermarck H. Tumor neurobiology and the war of nerves in cancer. Cancer Discov. 2019;9:702‐710. [DOI] [PubMed] [Google Scholar]
- 5. Monje M, Borniger JC, D'Silva NJ, et al. Roadmap for the emerging field of cancer neuroscience. Cell. 2020;181:219‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boilly B, Faulkner S, Jobling P, Hondermarck H. Nerve dependence: From regeneration to cancer. Cancer Cell. 2017;31:342‐354. [DOI] [PubMed] [Google Scholar]
- 7. Jobling P, Pundavela J, Oliveira SM, Roselli S, Walker MM, Hondermarck H. Nerve‐cancer cell cross‐talk: A novel promoter of tumor progression. Cancer Res. 2015;75:1777‐1781. [DOI] [PubMed] [Google Scholar]
- 8. Pundavela J, Roselli S, Faulkner S, et al. Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Mol Oncol. 2015;9:1626‐1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hondermarck H. Neurotrophins and their receptors in breast cancer. Cytokine Growth Factor Rev. 2012;23:357‐365. [DOI] [PubMed] [Google Scholar]
- 10. Campbell JP, Karolak MR, Ma Y, et al. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol. 2012;10:e1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Adriaenssens E, Vanhecke E, Saule P, et al. Nerve growth factor is a potential therapeutic target in breast cancer. Cancer Res. 2008;68:346‐351. [DOI] [PubMed] [Google Scholar]
- 12. Kappos EA, Engels PE, Tremp M, et al. Denervation leads to volume regression in breast cancer. J Plast Reconstr Aesthet Surg. 2018;71:833‐839. [DOI] [PubMed] [Google Scholar]
- 13. McCallum GA, Shiralkar J, Suciu D, et al. Chronic neural activity recorded within breast tumors. Sci Rep. 2020;10:14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kamiya A, Hayama Y, Kato S, et al. Genetic manipulation of autonomic nerve fiber innervation and activity and its effect on breast cancer progression. Nat Neurosci. 2019;22:1289‐1305. [DOI] [PubMed] [Google Scholar]
- 15. Huang D, Su S, Cui X, et al. Nerve fibers in breast cancer tissues indicate aggressive tumor progression. Medicine (Baltimore). 2014;93:e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhao Q, Yang Y, Liang X, et al. The clinicopathological significance of neurogenesis in breast cancer. BMC Cancer. 2014;14:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Day IN. Enolases and pgp9.5 as tissue‐specific markers. Biochem Soc Trans. 1992;20:637‐642. [DOI] [PubMed] [Google Scholar]
- 18. Benowitz LI, Perrone‐Bizzozero NI, Neve RL, Rodriguez W. Gap‐43 as a marker for structural plasticity in the mature cns. Prog Brain Res. 1990;86:309‐320. [DOI] [PubMed] [Google Scholar]
- 19. Fisher B, Bauer M, Wickerham DL, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer. 1983;52:1551‐1557. [DOI] [PubMed] [Google Scholar]
- 20. Chang J, Clark GM, Allred DC, Mohsin S, Chamness G, Elledge RM. Survival of patients with metastatic breast carcinoma: Importance of prognostic markers of the primary tumor. Cancer. 2003;97:545‐553. [DOI] [PubMed] [Google Scholar]
- 21. Largillier R, Ferrero JM, Doyen J, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008;19:2012‐2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Renz BW, Tanaka T, Sunagawa M, et al. Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. Cancer Discov. 2018;8:1458‐1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. [DOI] [PubMed] [Google Scholar]
- 24. Zhao CM, Hayakawa Y, Kodama Y, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6;250ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liebl F, Demir IE, Rosenberg R, et al. The severity of neural invasion is associated with shortened survival in colon cancer. Clin Cancer Res. 2013;19:50‐61. [DOI] [PubMed] [Google Scholar]
- 26. Sloan EK, Priceman SJ, Cox BF, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042‐7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Albo D, Akay CL, Marshall CL, et al. Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer. 2011;117:4834‐4845. [DOI] [PubMed] [Google Scholar]
- 28. Peterson SC, Eberl M, Vagnozzi AN, et al. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell. 2015;16:400‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Allen JK, Armaiz‐Pena GN, Nagaraja AS, et al. Sustained adrenergic signaling promotes intratumoral innervation through bdnf induction. Cancer Res. 2018;78:3233‐3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hayakawa Y, Sakitani K, Konishi M, et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell. 2017;31:21‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chida Y, Hamer M, Wardle J, Steptoe A. Do stress‐related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466‐475. [DOI] [PubMed] [Google Scholar]
- 32. Giese‐Davis J, Collie K, Rancourt KM, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: A secondary analysis. J Clin Oncol. 2011;29:413‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kornblith AB, Herndon JE 2nd, Weiss RB, et al. Long‐term adjustment of survivors of early‐stage breast carcinoma, 20 years after adjuvant chemotherapy. Cancer. 2003;98:679‐689. [DOI] [PubMed] [Google Scholar]
- 34. Andersen BL, Farrar WB, Golden‐Kreutz DM, et al. Psychological, behavioral, and immune changes after a psychological intervention: A clinical trial. J Clin Oncol. 2004;22:3570‐3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Su F, Ouyang N, Zhu P, et al. Psychological stress induces chemoresistance in breast cancer by upregulating mdr1. Biochem Biophys Res Commun. 2005;329:888‐897. [DOI] [PubMed] [Google Scholar]
- 36. Ulrich‐Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939‐944. [DOI] [PubMed] [Google Scholar]
- 38. Drell Tl, Joseph J, Lang K, Niggemann B, Zaenker KS, Entschladen F. Effects of neurotransmitters on the chemokinesis and chemotaxis of mda‐mb‐468 human breast carcinoma cells. Breast Cancer Res Treat. 2003;80:63‐70. [DOI] [PubMed] [Google Scholar]
- 39. Zahalka AH, Arnal‐Estapé A, Maryanovich M, et al. Adrenergic nerves activate an angio‐metabolic switch in prostate cancer. Science. 2017;358:321‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hanoun M, Maryanovich M, Arnal‐Estapé A, Frenette PS. Neural regulation of hematopoiesis, inflammation, and cancer. Neuron. 2015;86:360‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dantzer R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol Rev. 2018;98:477‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abubakar M, Sung H, Bcr D, et al. Breast cancer risk factors, survival and recurrence, and tumor molecular subtype: analysis of 3012 women from an indigenous Asian population. Breast Cancer Res. 2018;20:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2(3):133‐140. [DOI] [PubMed] [Google Scholar]
- 44. McAndrew NP, Finn RS. Management of ER positive metastatic breast cancer. Semin Oncol. 2020;47:270‐277. [DOI] [PubMed] [Google Scholar]
- 45. Bergen ES, Berghoff AS, Medjedovic M, et al. Continued endocrine therapy is associated with improved survival in patients with breast cancer brain metastases. Clin Cancer Res. 2019;25:2737‐2744. [DOI] [PubMed] [Google Scholar]
- 46. Brandt J, Garne JP, Tengrup I, Manjer J. Age at diagnosis in relation to survival following breast cancer: a cohort study. World J Surg Oncol. 2015;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tzikas AK, Nemes S, Linderholm BK. A comparison between young and old patients with triple‐negative breast cancer: Biology, survival and metastatic patterns. Breast Cancer Res Treat. 2020;182:643‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


