Abstract
Verotoxigenic Escherichia coli (VTEC) strains were isolated from food and animal fecal samples by using PCR to screen for the presence of VTEC after broth enrichment and then filtering VTEC-positive cultures through hydrophobic-grid membrane filters (HGMFs) which were incubated on MacConkey agar. The filters were probed with a digoxigenin-labeled PCR product generated by amplification of a conserved verotoxin gene sequence. Replication of the growth on filters allowed probe-positive colonies to be picked. When ground beef samples were inoculated with VTEC strains, 100% of the strains were recovered, and the detection limit was 0.1 CFU per g. Similar results were obtained with seven types of artificially contaminated vegetables. A survey of 32 packages of vegetables and 23 samples of apple cider obtained at the retail level did not reveal the presence of VTEC. However, the intestinal fecal contents of a moose, 1 of 35 wild mammals and birds examined, contained E. coli O157:H7. The DNA hybridization-HGMF method was also used in a prevalence survey of 327 raw and 744 ready-to-eat products; VTEC strains were recovered from 4.9% of the raw products and 0.7% of the ready-to-eat products. No serotype O157:H7 strains were detected. This method is particularly suited for surveys in which low numbers of VTEC-positive samples are expected and isolates are required.
More than 200 serotypes of verotoxigenic Escherichia coli (VTEC), also known as Shiga toxin-producing E. coli, have been isolated from cattle (12), and approximately 50 of these serotypes have been identified as causative agents of hemorrhagic colitis (HC) and hemolytic uremic syndrome (HUS) (1, 3, 11, 13, 25, 26, 36). The E. coli strains which are implicated in HC and HUS in humans are called enterohemorrhagic E. coli (EHEC) strains, but for most serotypes of VTEC it is not known whether the organisms can cause HC and HUS in humans. The serotype most frequently associated with disease outbreaks is E. coli O157:H7, but members of other serotypes, including O26, O104, O111, and O145, have also been responsible for serious illnesses and deaths resulting from consumption of contaminated food or water. Non-O157 VTEC strains are found in cattle in Ontario at a higher frequency than O157 strains are found (13). Strains of Citrobacter freundii containing verotoxin (VT) genes have also been implicated in food-borne disease (4, 33, 40). VT or Shiga toxins are the main virulence factors in VTEC strains that cause HC and HUS (14), and there is presently no marker other than serotype that can be used to predict the virulence of an isolate (2–4, 12, 13, 18, 20). Therefore, there is a need to identify all VT-positive organisms, not just E. coli O157:H7 strains, as all VT-positive organisms need to be treated as potential pathogens. Potential virulence factors, such as the 60-MDa plasmid, Eae, and EHEC hemolysin, have been investigated as virulence markers. Although these factors are correlated with virulence, they cannot be used to assess whether an isolate has the potential to cause disease, as some pathogenic VTEC may lack these genes and proteins (12, 17, 20, 32).
PCR methods for detection of VT genes (21–23, 42) can detect VTEC in mixed cultures, but confirmation of positive results by isolating VTEC is challenging. Broth cultures must be streaked onto selective agar, and individual colonies or colony sweeps must be picked and tested for VT production (7). This is a time-consuming process without any guarantee of success, especially if the food and environmental samples being tested have low concentrations of VTEC. In this paper we describe a DNA probe hybridization procedure in which we used hydrophobic-grid membrane filters (HGMFs), hereinafter called the DNA-HGMF method. This procedure has the advantage that the HGMFs can be mechanically replicated so that individual bacterial colonies in hydrophobic-grid cells on a replicate correspond to colonies on the original filter (34). This procedure was successfully used to isolate E. coli O157 and Salmonella strains from food and environmental samples with specific monoclonal antibodies (6, 34, 37–39). A similar method in which a DNA probe hybridization procedure is used with HGMFs to isolate Listeria monocytogenes has also been described (19). With these methods, not only can specific pathogens be isolated, but there is the additional advantage that multiple strains can be picked, because each HGMF consists of 1,600 separate cells in a hydrophobic grid.
Whereas it has been proven that monoclonal antibodies can be used to detect specific pathogens, such antibodies are not always available or may be licensed to a supplier. In contrast, the DNA sequences of specific primers are usually published, and PCR products that are used for probes can be made in a standard manner. In this paper we describe a method in which a DNA hybridization procedure is used in combination with HGMFs to isolate VTEC from food and from animal intestinal and fecal samples. This technique can be used in surveys in combination with PCR, a Vero cell assay, or other screening tests and is particularly valuable when low frequencies of positive samples are expected. In this assay samples are screened for VTEC by PCR, and the positive isolates are identified on HGMFs.
MATERIALS AND METHODS
Media.
The following media were purchased from Difco Laboratories, Detroit, Mich.: tryptic soy broth, tryptic soy agar (TSA), brain heart infusion (BHI) broth, BHI agar, MacConkey agar, and MacConkey broth. Other media were prepared from ingredients obtained from Difco Laboratories; these media included HC broth (20 g of tryptone, 1.12 g of bile salts no. 3, 5 g of NaCl, 10 g of sorbitol, 0.015 g of bromcresol purple, 1 liter of distilled water; (pH 7.0), Institut Pasteur maintenance medium (5 g of beef extract, 10 g of peptone, 3 g of NaCl, 2 g of Na2HPO4, 10 g of agar, 1 liter of distilled water; (pH 7.0), and TSA-Nal (TSA supplemented with 30 μg of nalidixic acid/ml).
Bacterial strains and culture conditions.
The strains used for inoculation of foods were obtained from the research laboratory culture collection of the Health Protection Branch, Health Canada, and were maintained on TSA slants or Institut Pasteur maintenance medium at room temperature (38). For routine use, strains were inoculated into BHI broth, incubated overnight at 35°C, and diluted to a concentration of approximately 101 CFU/ml in 0.1% peptone water before they were added to foods. Master libraries on HGMFs were prepared as described by Todd et al. (38) and were probed directly as described below. These libraries contained 50 E. coli O157:H7 strains, 3 E. coli O157:NM (nonmotile) strains, 19 other VTEC strains, 2 Shigella dysenteriae strains, and 268 non-VTEC gram-negative bacterial strains (members of Citrobacter, Enterobacter, Klebsiella, Proteus, Salmonella, Shigella, and Yersinia spp.). The E. coli strains used to generate the VTEC probe (H30, E32511, 412, H.I.8, and 933W), as described by Yee et al. (42), were obtained from the Laboratory Services Division, University of Guelph (formerly the Ontario Ministry of Agriculture, Food and Rural Affairs). E. coli H30 and ATCC 25922 were used as positive and negative controls on the HGMFs, respectively. Nalidixic acid-resistant O157:H7 VTEC strain E318 from the Health of Animals Laboratory (now Guelph Laboratory, Health Canada), Guelph, Ontario, Canada, was used in the limit-of-detection experiment.
Artificial inoculation of food samples.
Artificially inoculated foods were prepared and tested with the DNA-HGMF method. In order to establish the lowest level of detection (limit of detection) for the assay, locally purchased freshly ground beef, a sample of which had previously tested negative for VTEC as described below, was spiked with VTEC. A total of 111 25-g samples were inoculated individually with between 9 and 18 CFU of 99 different VTEC strains, 10 non-VT-producing E. coli strains, and two S. dysenteriae strains (Table 1) that had been grown overnight at 35°C in BHI broth. The 25-g samples were placed in sterile plastic bags, and the bags were kneaded by hand to distribute the bacteria evenly throughout the meat; then the samples were placed in Mason jars containing 225 ml of BHI broth and processed as described below. In order to determine the inoculum size, aliquots of 10-fold dilutions of each culture were filtered through HGMFs (ISO-GRID; Gelman Scientific, Montreal, Quebec, Canada). The HGMFs were incubated overnight on MacConkey agar at 35°C to allow cells to grow into isolated colonies, and the numbers of colonies were counted electronically with a model MI-100 HGMF interpreter (Richard Brancker Research Ltd., Ottawa, Ontario, Canada). An additional 29 25-g samples were similarly inoculated with 29 different VTEC strains, wrapped in plastic, and stored at −20°C for 7 to 14 days in order to simulate typical retail products. Each sample was later thawed, added to 225 ml of BHI broth, and processed just as the inoculated fresh ground beef was processed.
TABLE 1.
Strains of VTEC or S. dysenteriae that were positive as determined by the DNA-HGMF method (102 CFU/25 g of ground beef)
| Taxon | Serotype(s) or strain | No. of positive samplesa |
|---|---|---|
| E. coli | O157:H7 | 75 |
| O157:NM | 10 | |
| O26:H11 | 2 | |
| O145:NM | 1 | |
| K-12 (plasmid-encoded VT) | 1 | |
| O121:H19, O126:B16, O26:B6, O26:H11, O138:K81, O128:B12, O68:H12, O145:H2, O45:H2, O113:H7 | 1 each | |
| S. dysenteriae type 1 | NAb | 2 |
All of the samples tested were positive.
NA, not applicable.
Samples of seven other foods (ham, Cheddar cheese, bread, carrots, potatoes, broccoli, and cabbage) were prepared like the beef samples without a freeze-thaw step, and each type of food was inoculated separately with four VTEC strains at levels between 19 and 26 CFU/25 g.
Survey of retail food samples, meat samples, and animal fecal samples.
Ten samples of meat, 32 samples of fresh vegetables (with soil particles when possible), and 23 samples of unpasteurized apple cider without preservatives were obtained during the 1993–1994 winter season from retail stores across Canada by employees of the Health Protection Branch of Health Canada in Halifax, Montreal, Toronto, Winnipeg, and Vancouver. These samples were shipped at 4 to 8°C to the Bureau of Microbial Hazards Laboratory, Health Protection Branch, in Ottawa, Canada.
Samples of raw and ready-to-eat processed meat products were collected from meat-processing plants during May to October and were shipped at 4 to 8°C to the Laboratory Services Division, University of Guelph. A total 1,071 of samples were tested; 327 of these samples were raw meat samples, and 744 were processed meat samples.
In order to determine whether there was an environmental reservoir of VTEC in the animal population, intestinal contents of 35 recently killed wild animals were obtained through the cooperation of the National Capital Commission, Ottawa, Canada, and the Ontario Ministry of Natural Resources, Cornwall, Ontario, Canada. Three culled seagulls were from the airport in Trenton, Ontario, Canada. The other 32 samples were obtained mostly during the winter from animals that were killed on the road or were shot.
VTEC isolation procedure.
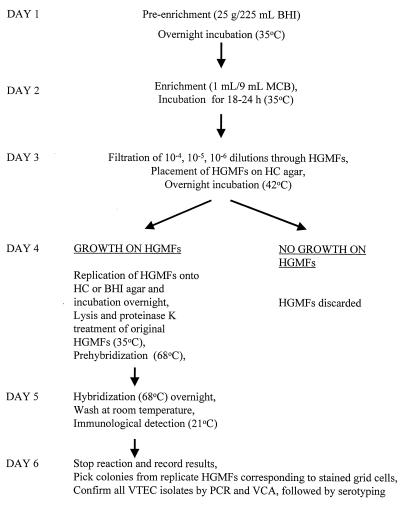
The flow diagram in Fig. 1 shows the procedure used for isolating VTEC from food or animal samples. Each sample (25 g) was added to BHI preenrichment broth (225 ml) and incubated overnight at 35°C with shaking at 150 rpm (model G76 gyrotory water bath shaker; New Brunswick Scientific, Edison, N.J.). Aliquots of the preparations were used to screen samples (see below). One milliliter of each broth culture was transferred to 9 ml of enrichment MacConkey broth and incubated for 18 to 24 h at 35°C. The next day, 10−4, 10−5, and 10−6 dilutions were prepared from each MacConkey broth preparation and pipetted through prefilters (FiltaTips; Filtaflex Ltd., Almonte, Ontario, Canada) to remove debris (34), and 1-ml aliquots of each sample were filtered through HGMFs. Positive and negative control strains were applied to the HGMFs by using sterile toothpicks (upper left and right corners of each filter) in order to verify assay performance. The filters were then incubated overnight on hemorrhagic colitis (HC) agar at 42°C. All HGMFs with bacterial growth were replicated with a model R-100 replicator (Richard Brancker Research Ltd.), and each replicate was incubated overnight on HC agar or BHI agar. The HGMFs which exhibited no bacterial growth after 24 h were discarded and were considered negative. HGMFs with bacterial growth were prepared for hybridization with the DNA probe.
FIG. 1.
Flow chart for the VTEC DNA-HGMF method. MCB, MacConkey broth; VCA, Vero cell assay.
Screening samples for VTEC.
The primary BHI broth preparations obtained from food samples that had been artificially inoculated with E. coli O157 strains were screened by performing an enzyme-linked antibody assay (35). Isolates obtained from the primary BHI broth cultures prepared from the food and animal fecal samples, both artificially inoculated and naturally contaminated, were screened for the presence of VTEC by using the PCR protocol of Read et al. (23), with the following modification: the initial denaturation step consisted of 5 min at 99°C and was followed by 30 cycles consisting of 1 min at 94°C, 1 min at 49°C, and 30 s at 72°C. PCR procedures were carried out with a model 480 thermocycler (Perkin-Elmer, Norwalk, Conn.). The PCR procedure of Pollard et al. (21, 22) was later used to confirm the presence of verotoxin genes VT1 and VT2 in pure cultures of the isolates.
Preparation of the DNA probes.
PCR products that were used as probes for detection of VT genes were generated by the PCR protocol of Read et al. (23). These products were prepared from overnight BHI broth cultures of strains H30, E32511, 412, H.I.8, and 933W, as described by Yee et al. (42). One-milliliter aliquots of overnight BHI broth cultures of the strains were centrifuged, the sediments were washed, boiled, and centrifuged again, and the supernatants were used as templates for PCR. The PCR products were combined, purified with a Microcon 30 DNA concentrator (Amicon, Bedford, Mass.), and end labeled by using a digoxigenin labeling kit (Boehringer Mannheim, Indianapolis, Ind.). Prehybridization, hybridization, and immunological detection of the labeled probe were carried out as recommended in the instructions of the Boehringer Mannheim kit. The concentration of the DNA probe was estimated by the Dipstick procedure (Invitrogen Inc., San Diego, Calif.).
HGMF pretreatment.
HGMFs exhibiting bacterial growth in grid cells were placed on Whatman no. 1 filter paper discs (diameter, 7.0 cm) soaked with pretreatment solution (45 ml of 10 mM Na2PO4 [pH 6.0] [1.42 g/liter], 9 ml of 1 M NaHCO3, 135 μl of Lugalvan G35 detergent [3 ml/filter; BASF, Toronto, Ontario, Canada]) and incubated at 35°C for 30 min. These preparations were then transferred to fresh filter paper soaked with a lysis solution (150 mM NaOH in 70% ethanol [3 ml of 5 N NaOH in 73.7 ml of 95% ethanol]) (3 ml/filter) and heated in a microwave oven for 30 s on the high setting. The heat-treated HGMFs were then gently shaken in a proteinase K solution (0.01% proteinase K [Sigma Chemical Co., St. Louis, Mo.] in 2× SSC [1× SSC is 0.15 M sodium chloride plus 0.15 M sodium citrate] supplemented with 0.1% sodium dodecyl sulfate [SDS]) (50 ml/filter) for at least 60 min at 35°C. Subsequently, the preparations were rinsed with vigorous shaking in 5× SSC–1.0% SDS for 10 min and then in 2× SSC for 1 h. Any remaining cellular material visible on the HGMFs was removed by vigorous shaking for 1 min or by pummelling in a stomacher for 15 s at high speed. The HGMFs were then placed on blotting paper and air dried (30 min to overnight) before they were exposed to 125 mJ of UV light.
Prehybridization.
The HGMFs were placed in 5-ml portions of hybridization solution (100 μg of herring sperm DNA [Sigma] per ml in 6× SSC supplemented with 0.5% SDS) (8 ml/filter) in heat-sealable pouches and incubated for at least 30 min (or overnight) in a 68°C water bath with gentle shaking. Any empty grid cells were blocked by using the blocking solution (Boehringer Mannheim) to reduce background staining in grid cells.
Hybridization.
The hybridization solution used for prehybridization was discarded and replaced with fresh hybridization solution containing 120 ng of denatured probe/HGMF. The HGMFs were incubated at 68°C for 2 h (or overnight) with gentle shaking, and hybridization and immunological detection were carried out by using the protocol provided with a digoxigenin (DIG) DNA labeling and detection kit (Boehringer Mannheim). The HGMFs were then washed at 68°C with 2× SSC.
Confirmation of VTEC on HGMFs.
For each HGMF containing purple-stained grid cells, bacterial colonies in the corresponding cells of the replicate were removed with a sterile toothpick and streaked onto MacConkey agar (or BHI agar). Up to 20 colonies per HGMF were obtained for each positive sample. Subsequently, pure colonies were confirmed to be VTEC colonies by PCR (21–23) and by the Vero cell assay for VT production (7). The VTEC serotype was determined by O and H typing by workers at the Laboratory Centre for Disease Control, Ottawa, Canada.
Limits of detection.
One milliliter of BHI broth was inoculated with a colony of the nalidixic acid-resistant strain E. coli E318 and incubated overnight at 35°C. Serial 10-fold dilutions of this culture were prepared with 900 μl of sterile 0.85% NaCl. Six stomacher bags containing 25 g of ground beef and 225 ml of BHI broth were inoculated with 100 μl of the E318 dilutions containing 106 to 101 cells/ml. The bags were then massaged with a stomacher for 60 s and incubated at 35°C overnight. In addition, 100-μl portions of the same E318 dilutions were plated onto TSA-Nal plates to determine colony counts. The following day, colony counts were obtained, and 1 ml of each BHI broth sample was transferred to 9 ml of MacConkey broth. MacConkey broth was also inoculated with controls, including a VT-positive E. coli strain and a VT-negative E. coli strain. After overnight incubation at 35°C, 100 μl of each MacConkey broth sample was transferred to 1 ml of BHI broth and incubated overnight at 35°C.
The next day, a Vero cell assay was performed, and the results were read 48 h later in order to determine the extent of cell death (cytotoxicity) (7, 15). For Vero cell assay-positive samples, 10−3 and 10−4 dilutions of MacConkey broth were filtered through HGMFs, which were then placed on TSA-Nal plates. After overnight incubation at 35°C, the HGMFs were replicated once, and the replicates were grown overnight at 35°C on MacConkey agar plates. Cells growing on the original HGMFs were lysed, hybridized, and identified as VTEC cells as described above and shown in the flow chart in Fig. 1. For probe-positive colonies, the corresponding growth on the replicate HGMFs was confirmed by performing a Vero cell assay and PCR.
Characterization of VTEC strains. (i) PCR Amplification of virulence genes.
All of the putative virulence factors except the EHEC virulence plasmid were identified by PCR amplification of gene sequences. Template DNA was prepared by a boiling method described above. For the EHEC hemolysin A (ehxA) and alpha-hemolysin A (hlyA) genes, the PCR amplification protocol used was the protocol described by Sandhu et al. (31); for the EAF plasmid and bfpA, amplification reactions were performed as described by Sandhu (29); and for eae, amplification reactions were performed as described by Sandhu et al. (30). The EAF and bfpA sequence reactions were performed together; the other reactions were performed individually. PCR products were detected after electrophoresis by staining agarose gels with ethidium bromide. The enteropathogenic strain E. coli 2348, supplied by M. A. Karmali (Hospital for Sick Children, Toronto, Canada), was used as a positive control for EAF and bfpA. EHEC strain E32511 (O157:H7), supplied by Lothar Beutin (E. coli Reference Laboratory, Robert Koch Institut, Berlin, Germany), was used as a positive control for EHEC hemolysin. Two E. coli O26:H11 strains and two E. coli O157:H7 strains were used as positive controls for eae.
For VT1 and VT2, PCR amplification was performed in a multiplex reaction as follows. Each 25-μl reaction mixture contained Stoffel buffer, each deoxynucleoside triphosphate at a concentration of 0.2 mM, 3 mM MgCl2, each primer at a concentration of 0.10 μM, 1.25 U of Stoffel fragment (Perkin-Elmer), and 5 μl of template. Amplification was performed for 30 cycles consisting of 94°C for 1 min, 55°C for 2 min, and 72°C for 1 min; this was followed by a final extension step consisting of 72°C for 1 min. Bands (130 bp for VT1 and 346 bp for VT2) were visualized on 2% agarose gels after staining with ethidium bromide.
(ii) Plasmid DNA isolation.
The presence of a 60-MDa plasmid (pO157) has been associated with colonization in EHEC strains. The isolates were screened for the presence of this plasmid by using an alkaline detergent method for large plasmids (9). Bacteria were grown on TSA plates for 18 h at 37°C and lysed for 30 min at 50°C. After electrophoresis and ethidium bromide staining, plasmid sizes were determined by comparison with plasmids of known sizes by using the Bio-Image software (Millipore, Ann Arbor, Mich.). Plasmids from E. coli V517, pDT36g, and pDT285 and Salmonella typhimurium pSLT2 supplied by Cornelius Poppe (Guelph Laboratory, Health Protection Branch, Health Canada, Guelph, Canada) were used as plasmid size markers.
(iii) Detection of hemolysin activity.
To detect EHEC hemolysin, bacteria were grown on tryptose blood agar (Difco) supplemented with 10 mM CaCl2 and 5% defibrinated sheep erythrocytes that had been washed three times with phosphate-buffered saline (pH 7.2) (31). The plates consisted of a bottom layer of medium (15 ml) without erythrocytes overlaid with a second layer of medium (10 ml) containing washed sheep erythrocytes. To detect alpha-hemolysin, horse blood agar plates (PML Microbiologicals) were inoculated, and the results were determined after overnight incubation at 37°C.
RESULTS
Specificity of the DNA probe assay for VTEC and other bacterial strains in pure culture on HGMFs.
All 50 E. coli O157:H7 strains, 3 O157:NM strains, 19 strains of other VTEC serotypes, and 2 S. dysenteriae strains tested with the master libraries in pure culture were positive as determined by the DNA-HGMF method. In contrast, 60 non-VT-producing E. coli strains, 52 Yersinia enterocolitica strains, 40 Salmonella strains, 33 Shigella sonnei, Shigella flexneri, and Shigella boydii strains, 16 Enterobacter spp. strains, 15 Proteus strains, 12 Citrobacter strains (VT-negative strains), 12 Pseudomonas spp. strains, 7 Klebsiella spp. strains, 5 Hafnia alvei strains, 5 Serratia spp. strains, 4 Aeromonas hydrophila strains, 2 Achromobacter spp. strains, 2 Alcaligenes faecalis strains, 2 Providencia strains, and 1 Bordetella bronchiseptica strain were all negative as determined by the hybridization method.
Detection of VTEC in artificially inoculated samples.
All 101 meat samples that were freshly inoculated with 99 different VTEC strains (E. coli O157 and non-O157) and two S. dysenteriae strains were determined to be positive by the DNA-HGMF method (Table 1). The 29 VTEC strains were recovered from 29 frozen and thawed samples of ground meat. No isolates were obtained from the 10 samples inoculated with non-VT-producing E. coli strains. The four VTEC strains (O111:NM, O26:H11, O111:H8, and O157:H7) added separately to bread, ham, Cheddar cheese, broccoli, carrots, potatoes, and cabbage were all recovered by the DNA-HGMF method.
Detection of VTEC in retail food and animal fecal samples.
Fifty-five samples of vegetables and cider and 10 refrigerated ground meat samples obtained from retail stores in five Canadian cities were all negative for VTEC. Intestinal fecal contents of recently killed birds (three ring-billed gulls, two caspian terns, two starlings, one cowbird, one common grackle, one crow, one mourning dove, and one mallard duck) and mammals (12 beavers, 3 white-tailed deer, 2 mule deer, 2 moose, 1 squirrel, 1 ground hog, 1 red fox, and 1 raccoon) were tested for the presence of VTEC. Only one sample was found to be positive for VTEC. An O157:H7 strain was isolated from fecal contents extracted from the intestine of a moose killed in a collision with a car.
Detection of VTEC in raw and ready-to-eat meat samples.
Of the 327 raw meat samples (both beef and pork), 19 (5.8%) were found to contain VTEC. Of the 744 ready-to-eat meat products, 6 (0.8%) were positive for VTEC. The VTEC-positive meat products were pepperoni (two samples), smoked ham, salami, Kielbasa sausage, and beef jerky (Table 2). No VTEC strains of the O157:H7 serotype were detected. On six separate occasions, the DNA-HGMF method detected VTEC, whereas no VTEC strains were recovered when 30 random colonies from each of the six samples tested were assayed for cytotoxicity.
TABLE 2.
Sources and characteristics of VTEC isolates
| Serotype | Source | Toxin type(s) | 60-MDa plasmid | EHEC hemolysina |
|---|---|---|---|---|
| O2:H29b | Pork | VT2 | − | − |
| O5:NMb | Salami (RTE)c | VT1 | − | + |
| O8:H9 | Beef | VT2 | − | − |
| O8:H19b | Beef | VT1 | + | + |
| O8:H19b | Beef | VT1, VT2 | − | − |
| O8:H? | Beef jerky (RTE) | VT2 | − | − |
| O15:H27 | Beef | VT2 | − | − |
| O15:NMb | Beef | VT2 | − | − |
| O22:H8b | Kielbasa (RTE) | VT1, VT2 | + | + |
| O88:H25 | Beef | VT2 | − | + |
| O91:H14b | Pork | VT1 | + | − |
| O91:H14b | Pork | VT1 | + | + |
| O91:H14b | Pork | VT2 | + | − |
| O91:H21b | Smoked ham (RTE) | VT1, VT2 | − | + |
| O91:H21b | Pork | VT2 | − | − |
| O91:NMb | Pepperoni (RTE) | VT1 | + | + |
| O91:NMb | Beef | VT1 | + | − |
| O103:H2bd | Beef | VT1 | − | + |
| O142:H38 | Beef | VT1 | − | − |
| O153:H25b | Beef | VT2 | + | + |
| O163:NM | Pork | VT1 | + | + |
| O163:NM | Pepperoni (RTE) | VT1 | + | − |
| O?:H7 | Beef | VT2 | − | + |
| O?:H19b | Pork | VT1 | + | + |
| O?:H19b | Beef | VT2 | + | − |
Level of detection of the DNA-HGMF method.
Experiments to determine the limits of detection were conducted on three separate occasions. In the three experiments we used nalidixic acid-resistant VTEC strain E318, and the demonstrated levels of detection were 1.5, 5.2, and 2.7 cells per 25 g (mean, 3.1 cells per 25 g).
Characterization of VTEC strains.
Of the 25 VTEC strains recovered, 11 belonged to serotypes that have been associated with human disease. These serotypes included O5:NM, O91:H14, O91:H21, O91:NM, O103:H2, and O153:H25 (Table 2). Most of the VTEC isolates were recovered from raw beef or pork, but three strains (O5:NM, O91:NM, and O91:H21 strains) were isolated ready-to-eat products (salami, pepperoni, and smoked ham, respectively). One isolate (serotype O103:H2) was positive for the eae gene. No serotype O157:H7 VTEC strains were recovered. None of the VTEC strains was positive as determined by the PCR for the EAF plasmid and the bfpA gene, which have been associated with enteropathogenic E. coli rather than VTEC. PCR amplification of the ehxA gene revealed that 11 of the 25 isolates had the gene. All 11 isolates which were positive as determined by the PCR expressed the EHEC hemolysin on washed sheep erythrocyte agar. A 60-MDa plasmid was detected in 7 of the 12 isolates with the ehxA gene and in five isolates that lacked this gene (Table 2). Two of the 25 isolates (serotype O8:H9 and O142:H38 strains) had an alpha-hemolytic phenotype and, as determined by the PCR, were positive for the hlyA gene but not the ehxA gene.
DISCUSSION
Although many methods for detecting E. coli O157:H7 have been described, much less attention has been given to methods for isolating all VTEC serotypes from clinical, environmental, and food samples. Studies of disease outbreaks (5, 13, 36) caused by VTEC O157:H7 and by non-O157 serotypes have suggested that the source of contamination is contact with animals or their feces or manure. The continuing weakness of the methods used to isolate food-borne bacterial pathogens is their inability to isolate bacteria from samples that are positive as determined by very sensitive screening assays (6, 7, 19). In this study, we developed a digoxigenin-labeled DNA probe to detect conserved sequences found in VT1 and VT2 genes (23). The probe hybridized with all strains of E. coli O157:H7 and O157:NM, other serotypes of VTEC, and S. dysenteriae type 1 but not with non-VT-producing E. coli and other strains tested. The DNA-HGMF isolation procedure was used to isolate all 99 VTEC strains, including E. coli O157:H7 and O157:NM and S. dysenteriae strains, from fresh and frozen ground beef inoculated with as little as 9 CFU/25 g. VTEC strains were also isolated from bread, cheese, ham, and vegetable products artificially inoculated with as little as 19 CFU/25 g. Since the two strains of S. dysenteriae type 1 that possessed the Shiga toxin genes (stx) were also detected with the probe, the method detects this pathogen but not other Shigella spp. in food, animal specimens, or the environment. When the nalidixic acid-resistant strain was used, the limit of detection of the assay was found to be 3.1 cells/25 g of ground beef, equivalent to 0.1 CFU/g.
No isolates were obtained from 65 uninoculated ground beef, cider, and vegetable samples, but this is a small number of samples and contamination rates may be lower in the winter than in the summer. In the prevalence survey which involved 327 raw and 744 processed meat products, the DNA-HGMF method combined with a Vero cell assay screening step resulted in identification of VTEC in 5.8% of the raw meat samples and 0.8% of the ready-to-eat meat samples. This protocol was more effective than random testing of isolated colonies and colony sweeps of BHI broth cultures (7). Pierard et al. (20) obtained results similar to our results in a study in which they found that 4.6% of 2,440 raw meat samples were positive for VTEC as determined by PCR. In the present study, no O157:H7 isolates were detected, but 11 of the VTEC isolates, were members of serotypes previously implicated in human illnesses (Table 2). The fact that VTEC were found in fermented ready-to-eat products (pepperoni, salami, and beef jerky) is of particular concern because these meat products require no further cooking before consumption and therefore VTEC contamination of these types of products poses a hazard to the consumer. A non-O157 VTEC-related outbreak was attributed to O111-contaminated mettwurst in South Australia in 1995 (3, 5). Additional quality control steps may be required when fermented ready-to-eat products are manufactured in order to reduce the risk of VTEC contamination. This possibility was suggested by Glass and her colleagues (10) in a study in which they demonstrated that serotype O157:H7 survived in fermented sausage. Additional studies regarding this category of ready-to-eat products are urgently needed.
Samadpour et al. (27) used a colony blot procedure involving radiolabeling to identify VTEC in several different kinds of foods (surimi-based salad, raw goat milk, and blueberries). In a later study, Samapadour et al. (28) found that 17% of 294 food samples contained VT1 and/or VT2 genes; these samples mainly came from beef, veal, pork, and lamb, but they also came from chicken, turkey, fish, and shellfish. The limit of detection of the method used by Samadpour et al. is ≤1.3 CFU/g, which is higher than the limit of detection of the DNA-HGMF method (0.1 CFU/g). It is important to be able to detect very low levels of VTEC because outbreaks caused by ingestion of doses estimated to be 100 or fewer cells have occurred (13, 16). Two other advantages of our method are the fact that a nonradioactively labeled probe is used and the fact that bacterial growth on HGMFs can be replicated, which allows probe-positive colonies to be isolated.
Among VTEC, serotype O157:H7 is well established as a pathogen, as are a few other serotypes, such as O26:NM, O111:H8, and O111:NM, because they have been implicated in several outbreaks of HC and HUS (13, 14). However, there are many serotypes of VTEC that have been isolated from animals and foods of animal origin whose pathogenic potential is unknown (20). The Eae protein (intimin) is involved in close attachment of pathogenic VTEC to the intestine and is produced by the serotypes of VTEC most frequently implicated in human disease (2). In the present study, only 1 of 25 isolates (a serotype O103:H2 isolate) had the eae gene. The presence of this gene has been associated with more severe disease (4). However, isolates which lack this gene may also cause HC and HUS (12, 14). This gene is also found in non-VTEC of bovine origin (12). The 60-MDa virulence plasmid found in O157:H7 and other serotypes frequently associated with HC and HUS encodes the EHEC hemolysin; it has been suggested that this hemolysin is a virulence marker. The presence of a 60-MDa plasmid did not correlate well with the presence of the EHEC hemolysin A gene, possibly because considerable variation has been observed in the size of the plasmid which encodes the EHEC hemolysin and isolates may contain 60-MDa plasmids that are not related to the virulence plasmid. Twelve of the isolates were positive for EHEC hemolysin (Table 2). Of the 19 serotypes that were isolated, at least 11 have been reported to be implicated in human disease.
On the basis of our limited study of intestinal contents of wild birds and mammals, the occurrence of VTEC appears to be rare in this population. VTEC were not found in avian samples, and E. coli O157:H7 was the only VTEC serotype found in mammals (it was found in one moose). These findings suggest that VTEC are present only occasionally in birds and mammals in the area around Ottawa, Canada, but additional studies are necessary to confirm this, especially as our samples were obtained largely in the winter months. Two samples from the Lethbridge, Alberta, Canada, area were also negative. A more complete prevalence study performed in different parts of the country in different seasons would give a more realistic indication of VTEC levels. However, our results are consistent with the results of a recent study of Wallace et al. (41) in which HGMF culture methods were used to isolate E. coli O157. An examination of 691 freshly voided fecal specimens obtained from 349 urban landfill sites and 342 intertidal sediment samples in Morecambe, England, for VTEC O157 revealed a low contamination rate, 1.9%, and most of the positive samples were sediment samples. The authors recognized, however, that even a low carriage rate can result in contaminated fields because gulls roost on nearby farmland. Non-O157:H7 VTEC have been found in meat samples obtained from domestic and wild animals (20, 24, 27, 28), but the number of samples was small. The seroprevalence of neutralizing antibodies to VT1 was examined in an Italian study of animals (8). For domestic cattle, sheep, and goats, the seroprevalence values ranged from 42 to 71%; for pigs the value was only 2%; and no horse or dog sample was positive. Among wild animals, positive results were obtained with 8% of the deer samples and 1% of the fox samples. Thus, it appears that domestic ruminants are a major reservoir of VTEC, but wild animals are also exposed to VTEC and may be sources of these organisms as well.
There is no marker that can predict the virulence of a VTEC isolate, but serotype analysis is valuable for determining whether an isolate belongs to a serotype previously implicated in disease. Thus, until a suitable marker becomes available, all VT-producing E. coli strains should be considered potential pathogens. The method described above is applicable not only to foods but also to feces and environmental samples and has the potential to be used for surveys and outbreak investigations involving apple cider, fruit juices, lettuce, and sprouts, as well as soil and manure samples.
With the increasing demand for Hazard Analysis and Critical Control Point (HACCP) plans, risk assessments, and health hazard evaluations, quantitative procedures for assessing VTEC contamination will be required. For this purpose, the DNA-HGMF method could be modified to obtain direct counts of VTEC by eliminating the enrichment step, although the limit of detection would be increased.
ACKNOWLEDGMENTS
We acknowledge the assistance of cooperative students from Simon Fraser University and the University of Victoria (Victoria, British Columbia, Canada), Suzanne Chong, Lisa Dubey, Marie Evangelista, Cory Giesbrecht, David Granville, Doug Halverson, Jaswinder Khattra, Edward Leong, and Leo Wong. We thank Wendy Johnson, National Enteric Reference Centre, L.C.D.C., Ottawa, Canada, for supplying non-O157 VTEC cultures and serotyping strains that are positive as determined by the DNA-HGMF method. Finally, we thank Health Protection Branch inspectors across the country for gathering ground beef, unpreserved apple cider, and fresh vegetable products; workers at the Ontario Ministry of Agriculture, Food and Rural Affairs for supplying raw and ready-to-eat meat samples; and workers at the National Capital Commission, Ottawa, Canada, the Ontario Ministry of Natural Resources, Cornwall, Ontario, Canada, and the Canadian Wildlife Service, Hull, Quebec, Canada, for providing wildlife specimens.
REFERENCES
- 1.Allerberger F, Rossboth D, Dierich M P, Aleksic S, Schmidt H, Karch H. Prevalence and clinical manifestations of Shiga toxin-producing Escherichia coli infections in Austrian children. Eur J Clin Microbiol Infect Dis. 1996;15:545–550. doi: 10.1007/BF01709361. [DOI] [PubMed] [Google Scholar]
- 2.Barrett T J, Kaper J B, Jerse A E, Wachsmuth I K. Virulence factors in Shiga-like toxin-producing Escherichia coli isolated from humans and cattle. J Infect Dis. 1992;165:979–980. doi: 10.1093/infdis/165.5.979. [DOI] [PubMed] [Google Scholar]
- 3.Bettelheim K A. Enterohaemorrhagic Escherichia coli: a new problem, an old group of organisms. Austr Vet J. 1996;73:20–26. doi: 10.1111/j.1751-0813.1996.tb09948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beutin L, Zimmermann S, Gleier K. Human infections with Shiga toxin-producing Escherichia coli other than serogroup O157 in Germany. Emerg Infect Dis. 1998;4:635–639. doi: 10.3201/eid0404.980415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron S, Walker C, Beers M, Rose N, Akear E. Enterohaemorrhagic Escherichia coli outbreak in South Australia associated with the consumption of mettwurst. Commun Dis Intelligence Aust. 1995;19:70–71. [Google Scholar]
- 6.Cerqueira-Campos M L, Peterkin P I, Sharpe A N. Improved immunological membrane filter method for detection of food-borne Salmonella strains. Appl Environ Microbiol. 1986;52:124–127. doi: 10.1128/aem.52.1.124-127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke R C, McEwen S A, Gannon V P, Lior H, Gyles C L. Isolation of verotoxin producing Escherichia coli from milk filters in southwestern Ontario. Epidemiol Infect. 1989;102:253–260. doi: 10.1017/s0950268800029927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conedera G, Zuin A, Marangon S. Recent Advances in Verocytotoxin Producing Escherichia coli Infections: Proceedings of the 2nd International Symposium and Workshop on Verocytotoxin (Shiga-like toxin)-Producing Escherichia coli Infections, Bergamo, Italy, June 27–30, 1994. Amsterdam, The Netherlands: Elsevier Science Publishers B. V.; 1994. Seroprevalence of neutralising antibodies to Escherichia coli verocytotoxins in domestic and wild animals in Italy; pp. 81–84. [Google Scholar]
- 9.Crosa J H, Tolmasky M E, Actis L A, Falkow S. Plasmids. In: Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C.: ASM Press; 1994. p. 368. [Google Scholar]
- 10.Glass K A, Loeffelholz J M, Ford J P, Doyle M J. Fate of Escherichia coli O157:H7 as affected by pH or sodium chloride and in fermented, dry sausage. Appl Environ Microbiol. 1992;58:2513–2516. doi: 10.1128/aem.58.8.2513-2516.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldwater P N, Bettelheim K A. New perspectives on the role of Escherichia coli O157:H7 and other enterohaemorrhagic E. coli serotypes in human disease. J Med Microbiol. 1998;47:1039–1045. doi: 10.1099/00222615-47-12-1039. [DOI] [PubMed] [Google Scholar]
- 12.Gyles C, Johnson R, Gao A, Ziebell K, Pierard D, Aleksic S, Boerlin P. Association of enterohemorrhagic Escherichia coli hemolysin with serotypes of Shiga-like-toxin-producing Escherichia coli of human and bovine origins. Appl Environ Microbiol. 1998;64:4134–4141. doi: 10.1128/aem.64.11.4134-4141.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson R P, Clarke R C, Wilson J B, Read S C, Rahn K, Renwick S A, Sandhu K A, Alves D, Karmali M A, Lior H, McEwen S A, Spika J S, Gyles C L. Growing concerns and recent outbreaks involving non-O157:H7 serotypes of verotoxigenic Escherichia coli. J Food Prot. 1996;59:1112–1122. doi: 10.4315/0362-028X-59.10.1112. [DOI] [PubMed] [Google Scholar]
- 14.Karmali M A. Infections by verotoxin producing E. coli. Clin Microbiol Rev. 1989;12:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konowalchuk J, Speirs J, Stavric S. Vero response to a cytotoxin of Escherichia coli. Infect Immunol. 1977;18:775–779. doi: 10.1128/iai.18.3.775-779.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meng J, Doyle M P. Microbiology of Shiga toxin-producing Escherichia coli in foods. In: Kaper J B, O’Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C.: ASM Press; 1998. pp. 92–108. [Google Scholar]
- 17.Meng J, Zhao S, Doyle M P. Virulence genes of Shiga toxin-producing Escherichia coli isolated from food, animals and humans. Int J Food Microbiol. 1998;45:229–235. doi: 10.1016/s0168-1605(98)00163-9. [DOI] [PubMed] [Google Scholar]
- 18.Paton J C, Paton A W. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev. 1998;11:450–479. doi: 10.1128/cmr.11.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterkin P I, Idziak E S, Sharpe A N. Detection of Listeria monocytogenes by direct colony hybridization on hydrophobic grid-membrane filters by using a chromogen-labeled DNA probe. Appl Environ Microbiol. 1991;57:586–591. doi: 10.1128/aem.57.2.586-591.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pierard D, Van Damme L, Moriau L, Stevens D, Lauwers S. Virulence factors of verocytotoxin-producing Escherichia coli isolated from raw meats. Appl Environ Microbiol. 1997;63:4585–4587. doi: 10.1128/aem.63.11.4585-4587.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pollard D R, Johnson W M, Lior H, Tyler S D, Rozee K R. Differentiation of Shiga toxin and verocytotoxin type 1 genes by polymerase chain reaction. J Infect Dis. 1990;162:1195–1198. doi: 10.1093/infdis/162.5.1195. [DOI] [PubMed] [Google Scholar]
- 22.Pollard D R, Johnson W M, Lior H, Tyler S D, Rozee K R. Rapid and specific detection of verotoxin genes in Escherichia coli by polymerase chain reaction. J Clin Microbiol. 1990;28:540–545. doi: 10.1128/jcm.28.3.540-545.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Read S C, Clarke R C, Martin A, DeGrandis S A, Hii J, McEwen S, Gyles C L. Polymerase chain reaction for detection of verocytotoxigenic Escherichia coli isolated from animal and food sources. Mol Cell Probes. 1992;6:153–161. doi: 10.1016/0890-8508(92)90060-b. [DOI] [PubMed] [Google Scholar]
- 24.Read S C, Gyles C L, Clarke R C, Lior H, McEwen S. Prevalence of verotoxigenic Escherichia coli in ground beef, pork and chicken in southwestern Ontario. Epidemiol Infect. 1990;105:11–20. doi: 10.1017/s0950268800047592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reilly W J. Proceedings of the Meeting of the Society for Veterinary Epidemiology and Preventive Medicine, 27 to 29 March 1996, Glasgow, Scotland. Belfast, Northern Ireland: Society for Veterinary Epidemiology and Preventive Medicine; 1996. Verotoxigenic Escherichia coli O157 in Scotland; pp. 60–71. [Google Scholar]
- 26.Rivas M, Voyer L E, Tous M, De Mana M F, Leardini N, Wainsztein R, Callejo R, Quadri B, Corti S, Prado V. Verocytotoxin-producing Escherichia coli infection in family members of children with hemolytic uremic syndrome. Medicina (Buenos Aires) 1996;56:119–123. [PubMed] [Google Scholar]
- 27.Samadpour M, Liston J, Ongerth J E, Tarr P I. Evaluation of DNA probes for detection of Shiga-like toxin-producing Escherichia coli in food and calf fecal samples. Appl Environ Microbiol. 1990;56:1212–1215. doi: 10.1128/aem.56.5.1212-1215.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samadpour M, Ongerth J E, Liston J, Tran N, Nguyen D, Whittam T S, Wilson R A, Tarr P I. Occurrence of Shiga-like toxin-producing Escherichia coli in retail fresh seafood, beef, lamb, pork, and poultry from grocery stores in Seattle, Washington. Appl Environ Microbiol. 1994;60:1038–1040. doi: 10.1128/aem.60.3.1038-1040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandhu K S. Virulence attributes of bovine verotoxigenic Escherichia coli in relation to intestinal colonization. Ph.D. thesis. Guelph, Ontario, Canada: University of Guelph; 1996. [Google Scholar]
- 30.Sandhu K S, Clarke R C, McFadden K, Brouwer A, Louie M, Wilson J, Lior H, Gyles C L. Prevalence of the eaeA gene in verotoxigenic Escherichia coli strains from dairy cattle in Southwest Ontario. Epidemiol Infect. 1996;116:1–7. doi: 10.1017/s095026880005888x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandhu K S, Clarke R C, Gyles C L. Hemolysin phenotypes and genotypes of eaeA-positive and eaeA-negative bovine verotoxigenic Escherichia coli. Adv Exp Biol Med. 1997;412:295–302. doi: 10.1007/978-1-4899-1828-4_49. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt H, Geitz C, Tarr P I, Frosch M, Karch H. Non-O157:H7 pathogenic Shiga toxin-producing Escherichia coli: phenotypic and genetic profiling of virulence traits and evidence for clonality. J Infect Dis. 1999;179:115–123. doi: 10.1086/314537. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt H, Montag M, Bockemühl J, Heesemann J, Karch H. Shiga-like toxin II-related cytotoxins in Citrobacter freundii strains from humans and beef samples. Infect Immun. 1993;61:534–543. doi: 10.1128/iai.61.2.534-543.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharpe A N, Peterkin P I. Membrane filter food microbiology. New York, N.Y: John Wiley & Sons, Inc.; 1988. [Google Scholar]
- 35.Szabo R A, Todd E, MacKenzie J, Parrington L, Armstrong A. Increased sensitivity of the rapid hydrophobic grid membrane filter enzyme-linked antibody procedure for Escherichia coli O157 detection in foods and bovine feces. Appl Environ Microbiol. 1990;56:3546–3549. doi: 10.1128/aem.56.11.3546-3549.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas A, Cheasty T, Frost J A, Chart H, Smith H R, Rowe B. Vero cytotoxin-producing Escherichia coli, particularly serogroup O157, associated with human infections in England and Wales: 1992–4. Epidemiol Infect. 1996;117:1–10. doi: 10.1017/s0950268800001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Todd E C D, Szabo R A, Peterkin P I, Sharpe A N, Parrington L J, Bundle D, Gidney M A J, Perry M B. Rapid hydrophobic grid membrane filter–enzyme-labeled antibody procedure for identification and enumeration of Escherichia coli O157 in foods. Appl Environ Microbiol. 1988;54:2536–2540. doi: 10.1128/aem.54.10.2536-2540.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Todd E C D, MacKenzie J M, Parrington L J, Sharpe A N, Peterkin P I, Diotte M P, Gidney M A J, Nielson A, Fraser A, Rahn K, Tiffin A I, Paterson G, Gehle W. Evaluation of Salmonella antisera for an optimum enzyme-linked antibody detection of Salmonella using hydrophobic grid membrane filters. Food Microbiol. 1991;8:311–324. [Google Scholar]
- 39.Todd E C D, MacKenzie J M, Peterkin P I. Development of an enzyme-linked antibody hydrophobic grid membrane filter method for the detection of Salmonella in foods. Food Microbiol. 1993;10:87–99. [Google Scholar]
- 40.Tschäpe H, Prager R, Streckel W, Fruth A, Tietze E, Böhme G. Verotoxinogenic Citrobacter freundii associated with severe gastroenteritis and cases of haemolytic uraemic syndrome in a nursery school: green butter as the infection source. Epidemiol Infect. 1995;114:441–450. doi: 10.1017/s0950268800052158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wallace J S, Cheasty T, Jones K. Isolation of Vero cytotoxin-producing Escherichia coli O157 from wild birds. J Appl Bacteriol. 1997;82:399–404. doi: 10.1046/j.1365-2672.1997.00378.x. [DOI] [PubMed] [Google Scholar]
- 42.Yee A J, De Grandis S, Gyles C L. Mitomycin-induced synthesis of a Shiga-like toxin from enteropathogenic Escherichia coli H.I.8. Infect Immun. 1993;61:4510–4513. doi: 10.1128/iai.61.10.4510-4513.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]